Abstract
Background
FOXP3 has been discovered to be expressed in tumor cells and participate in the regulation of tumor behavior. Herein, we investigated the clinical relevance and biological significance of FOXP3 expression in human hepatocellular carcinoma (HCC).
Methods
Expression profile of FOXP3 was analyzed using real-time RT-PCR, western blotting and immunofluorescence on HCC cell lines, and immunostaing of a tissue microarray containing of 240 primary HCC samples. The potential regulatory roles of FOXP3 were dissected by an integrated approach, combining biochemical assays, analysis of patient survival, genetic manipulation of HCC cell lines, mouse xenograft tumor models and chromatin immunoprecipitation (ChIP) sequencing.
Results
FOXP3 was constitutively expressed in HCC cells with the existence of splice variants (especially exon 3 and 4 deleted, Δ3,4-FOXP3). High expression of FOXP3 significantly correlated with low serum α-fetoprotein (AFP) level, absence of vascular invasion and early TNM stage. Survival analyses revealed that increased FOXP3 expression was significantly associated with better survival and reduced recurrence, and served as an independent prognosticator for HCC patients. Furthermore, FOXP3 could potently suppress the proliferation and invasion of HCC cells in vitro and reduce tumor growth in vivo. However, Δ3,4-FOXP3 showed a significant reduction in the tumor-inhibiting effect. The inhibition of FOXP3 on HCC aggressiveness was acted probably by enhancing the TGF-β/Smad2/3 signaling pathway.
Conclusion
Our findings suggest that FOXP3 suppresses tumor progression in HCC via TGF-β/Smad2/3 signaling pathway, highlighting the role of FOXP3 as a prognostic factor and novel target for an optimal therapy against this fatal malignancy.
Electronic supplementary material
The online version of this article (10.1186/s12885-017-3633-6) contains supplementary material, which is available to authorized users.
Keywords: FOXP3, Hepatocellular carcinoma, TGF-β, Prognosis, ChIP
Background
FOXP3 is a member of the forkhead family of transcription factors, and initially thought to be restricted to hematopoietic tissues and act as a “master switch” for the development and function of regulatory T Cells (Treg) [1]. Genome-wide analysis of FOXP3+ T cells indicated that FOXP3 can bind to and up- or down-regulate a large number of genes and microRNAs, indicating a dual role of FOXP3 as both transcriptional activator and repressor [2, 3]. Recently, reports have demonstrated that FOXP3 is also expressed in tumor cells, suggesting that FOXP3 may have a broader role in cancer than initially thought [4, 5]. However, the biological function and clinical relevance of FOXP3 in tumor cells remain controversial. Data have suggested that FOXP3 expression in tumor cells could be a poor prognostic factor in breast cancer [6], colorectal cancer [4], and bladder cancer [7], indicating FOXP3 significantly contributed to tumor progression. Functional experiments have indicated that melanoma cells could have FOXP3-dependent suppressive effects on T cells [8], and FOXP3 play an important role in progression of cervical cancer cells [9], thus suggesting that FOXP3 expression in cancer cells might trigger a mechanism of immune evasion and tumor progression. In contrast with these data, FOXP3 was demonstrated to be a transcriptional repressor of two breast cancer oncogenes, SKP2 and HER2, acting as a potential tumor suppressor gene [10, 11]. Similar results were reported in prostate cancer [12] and gastric cancer [13]. Thus, it seems that the exact function of FOXP3 acts in a cancer type-specific manner.
Hepatocellular carcinoma (HCC), epidemic to Asia and Africa with an increasing incidence in western countries, is one of the most common and aggressive cancers worldwide [14]. Previously, we and others have demonstrated that high-density of FOXP3+ Treg infiltration was associated with tumor aggressiveness and poor clinical outcome in HCC [15, 16]. In our precious studies [15], immunohistochemical staining for FOXP3+ Treg in human HCC tissue also revealed positive FOXP3 staining in HCC cells. Thus, an interesting issue is whether FOXP3 gene is a friend or a foe for HCC cells. Interestingly, investigation of heterozygous Scurfy mice Foxp3sf/+ revealed that they also harbored hepatoma, in addition to mammary tumors, suggesting that FOXP3 may have an impeditive role in hepatocarcinogenesis [10]. However, the expression and the molecular biological functions of FOXP3 have not been investigated deeply.
In this study, we showed that FOXP3 was consistently expressed in HCC cell lines and was further identified as an independent predictor for better prognosis in HCC patients. FOXP3 reduced tumor growth and invasion in vitro and in vivo, however, the splice variant of FOXP3 led to impaired protective function. In addition, the tumor inhibition of FOXP3 might be regulated by TGF-β signaling pathway via Smad2/3.
Methods
Cell lines and transfection
The human HCC cell lines MHCC97H (97H) and MHCC97L (97 L) were established in our laboratory [17, 18]. Huh7 (JCRB0403) was obtained from the Japanese Cancer Research Bank; PLC/PRF/5 (PLC, CRL-8024), HepG2 (HB-8065) and Hep3B (HB-8064) were purchased from the American Type Culture Collection; SMMC-7721 (7721, TCHu52), and human hepatocytes HL-7702 (L02, GNHu 6) were obtained from Cell Bank (Shanghai, China).
Full-length FOXP3 and Δ3,4-FOXP3 cDNA were amplified by PCR from cDNA of 97H cells. Then, the FOXP3 constructs were subcloned into pWPXL vector (Addgene). Lentiviral stocks were prepared by co-transfecting HEK-293 T cells with FOXP3 expression constructs and the corresponding empty vectors, and standard virus packaging systems [19]. Knock-down of FOXP3 was accomplished using shRNA (5′-GCA CAT TCC CAG AGT TCC T-3′), targeting FOXP3 or a non-target shRNA control in PGLV3-H1 vector (Shanghai, China). Target cells were infected with filtered lenti-virus plus 6 μg/mL polybrene (Sigma-Aldrich) to generate stable cell lines [20].
Isolation of CD4+CD25+ T cells and CD4−CD25− T cells
Heparin-treated blood was obtained from 3 donors and peripheral blood mononuclear cells (PBMC) PBMC were isolated by centrifugation over lymphocyte separation medium (Sigma-Aldrich). CD4+CD25+ T cells and CD4−CD25− T cells which were sorted by magnetic beads (Miltenyi Biotec) from PBMC were used as positive and negative controls and cultured in RPMI-1640 (Invitrogen) at 37 °C in a humidified atmosphere containing 5% CO2.
Laser capture microdissection
Immunohistochemical staining was performed on frozen sections of 15 HCC patients who underwent primary and curative resection in Liver Cancer Institute, Zhongshan Hospital of Fudan University to identify and isolate tumor cells from other immunocytes, allowing a more precise microdissection. Microdissection was performed by a PixCell laser capture microscope with an infrared diode laser (Arcturus Engineering, Santa Clara). Primary HCC cells were captured for RNA extraction by focal melting of the membrane through laser activation.
Real-time RT-PCR analysis
Real-time RT-PCR was performed as described in Additional file 1: Supplementary Methods.
Western blotting analysis
Western blotting was performed as described previously [21] with recommended concentrations of antibodies. More details were in Additional file 1: Supplementary Methods.
Immunofluorescence
The expression of FOXP3 in Hep3B and 97H cells was detected by immunofluorescence, which was performed as previously described [22], using mouse anti-human FOXP3 Ab (1:200 dilution, Santa Cruz Biotechnology) and a goat anti-mouse IgG-FITC antibody (Invitrogen). Images were acquired using a LSM510 Confocal Laser Scanning Microscope (Carl Zeiss).
Tissue samples
Paraffin-embedded tissue samples were selected from 240 HCC patients who underwent primary and curative resection for their tumor in Liver Cancer Institute, Zhongshan Hospital of Fudan University (Shanghai, China) between 2002 and 2006, as previously described [23]. Follow-up procedures and post-operative treatments according to a uniform guideline were described previously [24]. Tumor differentiation was graded using the Edmondson grading system. Clinical staging was according to the 7th edition of AJCC/UICC TNM classification system. Conventional clinicopathologic variables are detailed in Table 1. Overall survival (OS) and time to recurrence (TTR) were calculated from the date of surgery to the date of the first recurrence and death, respectively. Data were censored at the last follow-up for patients without relapse, or death. The median follow-up period was 39.0 months (range, 1.5–95.0; SD, 22.7).
Table 1.
Correlation between FOXP3 expressin in tumor cells and clinicopathologic characteristics (n = 240)
| Variables | FOXP3 staining | P | |
|---|---|---|---|
| Low (n = 125) | High (n = 115) | ||
| n (%) | n (%) | ||
| Age (years) | |||
| ≤ 50 | 59 (47.2) | 53 (46.1) | 0.897 |
| > 50 | 66 (52.8) | 62 (53.9) | |
| Gender | |||
| Male | 103 (82.4) | 101 (87.8) | 0.280 |
| Female | 22 (17.6) | 14 (12.2) | |
| HBsAg | |||
| Negative | 10 (8.0) | 7 (6.1) | 0.622 |
| Positive | 115 (92.0) | 108 (93.9) | |
| Liver cirrhosis | |||
| No | 12 (9.6) | 16 (13.9) | 0.321 |
| Yes | 113 (90.4) | 99 (86.1) | |
| Serum AFP (ng/mL) | |||
| ≤ 20 | 34 (27.2) | 48 (41.7) | 0.021 |
| > 20 | 91 (72.8) | 67 (58.3) | |
| Tumor size (cm) | |||
| ≤ 5 | 57 (45.6) | 60 (52.2) | 0.366 |
| > 5 | 68 (54.4) | 55 (47.8) | |
| Tumor number | |||
| Single | 98 (78.4) | 86 (74.8) | 0.543 |
| Multiple | 27 (21.6) | 29 (25.2) | |
| Tumor encapsulation | |||
| Yes | 58 (46.4) | 61 (53.0) | 0.366 |
| No | 67 (53.6) | 54 (47.0) | |
| Vascular invasion | |||
| No | 53 (42.4) | 72 (62.6) | 0.002 |
| Yes | 72 (57.6) | 43 (37.4) | |
| Tumor differentiation | |||
| I/II | 71 (56.8) | 64 (55.7) | 0.897 |
| III/IV | 54 (43.2) | 51 (44.3) | |
| BCLC | |||
| 0/A | 54 (43.2) | 60 (52.2) | 0.196 |
| B/C | 71 (56.8) | 55 (47.8) | |
| TNM stage | |||
| I | 46 (36.8) | 60 (52.2) | 0.019 |
| II/III | 79 (63.2) | 55 (47.8) | |
Tissue microarray construction and immunohistochemistry
Tissue microarrays (TMAs) were generated as previously described [21], and immunohistochemistry for FOXP3 was carried out on TMAs as previously described [12]. More details were in Additional file 1: Supplementary Methods.
Cell proliferation and migration assays
Cell proliferation and migration assays were described in the Supplementary Methods. All tests and analyses were carried out in triplicate.
Xenograft tumor models
We performed subcutaneous tumorigenicity assays in BALB/c nude mice as described in Additional file 1: Supplementary Methods.
Chromatin immunoprecipitation (ChIP)
For FOXP3 ChIP, goat polyclonal Ab to recombinant Foxp3 protein (ChIP Grade, Abcam) was used. ‘Flow-through’ IgG devoid of FOXP3 antibodies served as a negative control. Antibodies specific for different histone modifications were from Upstate Biotechnology. ChIP was carried out as described elsewhere [25]. Relative abundance of regions of interest in precipitated DNA was measured by qPCR using Power SYBR Green PCR master mix (Applied Biosystems).
Statistical analysis
All statistical analyses were performed with SPSS version 16.0 software. The data were expressed as mean ± standard deviation (SD). The association between variables was analyzed using Student’s t-test, Mann–Whitney U test or Fisher’s exact test, as appropriate. Survival curves were obtained by the Kaplan–Meier method using a log-rank test. Cox regression analysis was used to evaluate the prognostic significance. P-values <0.05 were considered to be statistically significant.
Results
FOXP3 Expression in HCC cell lines and tissues
FOXP3 mRNA expression was clearly detectable in all HCC cell lines and a normal liver cell line L-02, using CD4+CD25+ T cells as a positive control and CD4+CD25− T cells as the negative control (Fig. 1a).
Fig. 1.
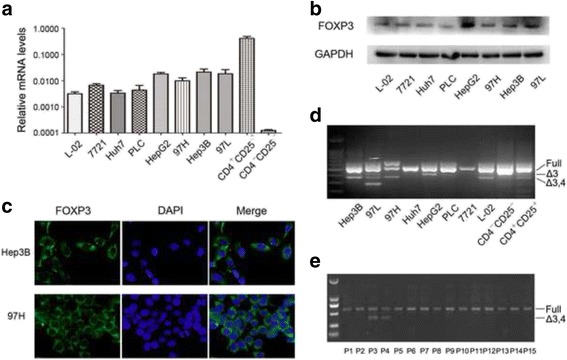
Expression profile of FOXP3 in human HCC. a Real-time RT-PCR analysis of FOXP3 expression in a normal liver cell line and HCC cell lines, using T cells as control. b Western blotting analysis of the protein levels of FOXP3 in a normal liver cell line and HCC cell lines. c Immunofluorescence images of FOXP3 expression Hep3B and 97H cells. d FOXP3 splice variants were detected in HCC cell lines by PCR analysis. Full, full-length; Δ3, the splice variant with exons 3 deleted; Δ3,4, the splice variant with exons 3 and 4 deleted. e FOXP3 splice variant with exons 3 and 4 deleted was found in 2/15 HCC patients
Then, Western blotting revealed obvious FOXP3 protein expression in HCC cell lines and normal liver cell L-02 (Fig. 1b). Notably, the immunofluorescence assays proved the expression of FOXP3 in Hep3B and 97H cells as examples and FOXP3 staining was localized predominantly in the cytoplasm (Fig. 1c).
The FOXP3 transcripts in HCC cell lines were amplified using primers spanning exons 1–12, and then the PCR products were sequenced. As shown in Fig. 1d, Huh7, PLC and 7721 only expressed the full-length FOXP3, while other tumor cell lines also expressed the splice variants as observed in the T cells. In addition, three tumor cell lines (Hep3B, 97 L and 97H) as well as L-02 cells expressed a splice variant with both exons 3 and 4 deleted, similar to but slightly different to that identified in breast cancers and melanoma [10, 26]. The alternative deletion mutation resulted in a frame shift beginning with codon 70 and a premature termination at codon 151. Importantly, the splice variant lacking both exons 3 and 4 were detected in 13.3% (2/15 cases) of laser captured microdissection of primary HCC cells (Fig. 1e).
Evaluation of FOXP3 expression as a prognostic factor in human HCC
We next evaluated the value of tumor cell FOXP3 expression in predicting clinical outcome in a cohort of 240 HCC patients by immunohistochemistry. The relationship between FOXP3 expression and clinicopathological features showed that high expression of FOXP3 correlated significantly with low serum AFP level (P = 0.021), absence of vascular invasion (P = 0.002) and early TNM stage (P = 0.019), suggesting an tumor-inhibiting effect of FOXP3 in HCC (Table 1). In our cohort, only 16 patients have hepatitis C infection (6.7%), thus, we exclude the hepatitis C infection from clinicopathologic characteristics.
In univariate analysis, conventional clinicopathological features which correlated with prolonged OS and TTR were low serum AFP level, small tumor size(<5 cm), single tumor, complete tumor capsule, absence of vascular invasion and early tumor stage, while the absence of liver cirrhosis and well differentiation were significantly associated just with better OS (Table 2). In particular, FOXP3-high patients showed significantly increased OS and TTR than FOXP3-low patients (Estimated mean OS 60.0 versus 44.8 months, P = 0.002; Estimated mean TTR 56.1 versus 34.9 months, P = 0.001, respectively; Fig. 2a and b).
Table 2.
Univariate and multivariate analyses of factors associated with OS and TTR (n = 240)
| Variables | OS | TTR | ||||
|---|---|---|---|---|---|---|
| Multivariate | Multivariate | |||||
| Univariate P | HR (95.0% CI) | P | Univariate P | HR (95.0% CI) | P | |
| Age, >50 vs. ≤50 years | 0.169 | NA | 0.289 | NA | ||
| Gender, Male vs. Female | 0.259 | NA | 0.084 | NA | ||
| HBsAg, Positive vs. negative | 0.264 | NA | 0.171 | NA | ||
| Liver cirrhosis, Yes vs. No | 0.019 | 1.77 (0.89–3.52) | 0.104 | 0.150 | NA | |
| Serum AFP, >20 vs. ≤20 ng/mL | <0.001 | 1.23 (0.80–1.88) | 0.350 | 0.002 | 1.31 (0.88–1.94) | 0.181 |
| Tumor size, >5 vs. ≤ 5 cm | <0.001 | 1.16 (0.56–2.43) | 0.692 | <0.001 | 1.57 (0.75–3.26) | 0.231 |
| Tumor number, Multiple vs. Single | 0.001 | 1.78 (1.13–2.80) | 0.013 | <0.001 | 2.34 (1.48–3.70) | <0.001 |
| Tumor encapsulation, No vs. Yes | 0.011 | 1.06 (0.73–1.53) | 0.770 | <0.001 | 1.54 (1.07–2.22) | 0.021 |
| Vascular invasion, Yes vs. No | <0.001 | 4.42 (2.26–8.63) | <0.001 | <0.001 | 4.28 (2.30–7.97) | <0.001 |
| Tumor differentiation, III/IV vs. I/II | 0.002 | 1.74 (1.20–2.53) | 0.004 | 0.077 | NA | |
| TNM stage, II /III vs. I | <0.001 | 0.72 (0.35–1.51) | 0.389 | <0.001 | 0.73 (0.37–1.45) | 0.368 |
| BCLC stage, B/C vs. 0/A | <0.001 | 1.30 (0.59–2.86) | 0.521 | <0.001 | 0.76 (0.35–1.64) | 0.478 |
| FOXP3, High vs. Low | 0.003 | 0.64 (0.44–0.92) | 0.017 | 0.001 | 0.66 (0.46–0.95) | 0.027 |
Fig. 2.
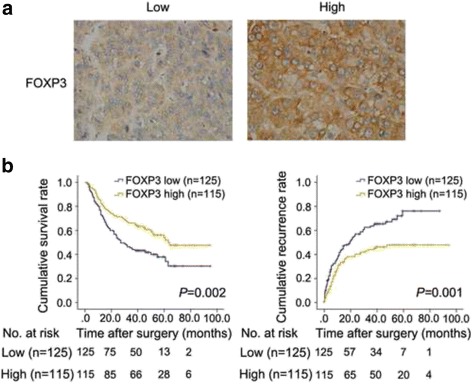
FOXP3 predicted a favorable prognosis in HCC patients. a The representative low expression and high expression of FOXP3 in HCC tissue. b The OS and TTR for the low and high FOXP3 expression groups were significantly different (by the 2-sided log-rank test) in the cohort of 240 patients. The absolute number of patients at risk is listed below each curve
Multivariate Cox regression analysis was performed using significant factors (P < 0.05) in univariate analysis along with FOXP3 expression status. The results proved FOXP3 as an independent predictor of both OS [hazard ratio (HR) = 0.64; 95% confidence interval (CI) = 0.44–0.92; P = 0.017] and TTR (HR = 0.66, 95% CI = 0.46–0.95; P = 0.027; Table 2). Survival analysis was clearly indicated that the expression of FOXP3 was a significant and independent predictor of HCC patient outcome.
FOXP3 Reduces proliferation, migration and invasion of HCC cells in vitro, as well as tumor growth in vivo
To substantiate tumor suppressive activity of FOXP3, we knocked down and over-expressed FOXP3 in HCC cell lines Hep3B (Hep3B-shFOXP3 and Hep3B-FOXP3, respectively) and 97H (97H–shFOXP3 and 97H–FOXP3, respectively). Previous studies have indicated that artificially designed deletions of the N-terminal portion, C-terminal portion, zinc finger domain or leucine zipper domain of FOXP3 resulted in reduced tumor suppressive activities. However, the exact significance and biological relevance of the naturally detected Δ3,4-FOXP3 remains elusive. We thus cloned and transfected Δ3,4-FOXP3 cDNA into HCC cell lines Hep3B and 97H (Hep3B-Δ3,4-FOXP3 and 97H-Δ3,4-FOXP3, respectively). We found that knock-down of FOXP3 in Hep3B and 97H cells led to obviously enhanced tumor proliferation (*P < 0.05, **P < 0.01; Fig. 3a) and cell migration (P = 0.009 and P = 0.002 for Hep3B and 97H, respectively; Fig. 3b) compared to control cells in vitro. In contrast, over-expression of FOXP3 in Hep3B and 97H cells resulted in significantly reduced tumor proliferation (*P < 0.05, **P < 0.01; Fig. 3a) and cell migration (P = 0.003 and P = 0.014 for Hep3B and 97H, respectively; Fig. 3b) compared to control cells. Additionally, over-expression of Δ3,4-FOXP3 showed a significantly reduced inhibition on tumor growth (*P < 0.05, **P < 0.01; Fig. 3a) and migration (P = 0.018 and P = 0.039 for Hep3B and 97H, respectively; Fig. 3b) than full-length FOXP3. The results further indicated that FOXP3 played a critical role in suppressing HCC growth and invasion.
Fig. 3.
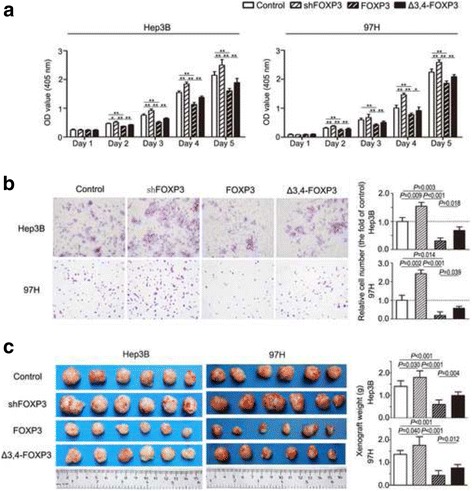
FOXP3 and FOXP3 splice variant inhibited HCC proliferation and invasion. a Proliferation assays were visualized as histogram. *P < 0.05, **P < 0.01; Δ3,4-FOXP3, the FOXP3 splice variant with exons 3 and 4 deleted. b The invasion of cancer cells was measured by matrigel-coated transwell assay. c Morphologic characteristics of subcutaneous tumor xenografts. Significant differences in tumor weight were revealed between the experimental and control groups (n = 6)
To evaluate the impact of FOXP3 on tumorigenicity in vivo, transfected tumor cell lines and their respective controls were subcutaneously injected into nude mice. The tumor cells with down-regulation of FOXP3 grew to a significantly heavier weight (P = 0.030 and P = 0.040 for Hep3B and 97H, respectively; Fig. 3c) compared with controls. Meanwhile, up-regulation of FOXP3 in Hep3B and 97H inhibited their subcutaneous tumorigenesis (P < 0.001 and P < 0.001 for Hep3B and 97H, respectively; Fig. 3c). However, over-expression of Δ3,4-FOXP3 showed a remarkable loss of tumor suppression than full-length FOXP3 (P = 0.004 and P = 0.012 for Hep3B and 97H, respectively; Fig. 3c).
FOXP3 Targeted genes in HCC
Using ChIP sequencing, we evaluated FOXP3 DNA binding in pcDNA-FOXP3 Hep3B and 97H, from immunoprecipitated DNA fragments. The DNA precipitated by control-IgG was used as a control. Given that most of the FOXP3-binding sites reside within 2 kb of TSS [27], we defined direct target genes with FOXP3 binding within 2 kb of their TSS in both Hep3B and 97H cells and identified a common gene pool of 2853 genes. Importantly, Gene ontology (GO) analysis indicated that FOXP3 may exert tumor inhibition effects by affecting TGF-β receptor binding activity, and pathway analysis showed that FOXP3-induced gene regulation may be controlled by TGF-β signaling pathway (Fig. 4a and b). To gain insight into the mechanism by which FOXP3 inhibited HCC growth and invasion, a panel of signaling pathways that may relate to HCC progression and especially TGF-β pathway were examined by western blotting. The phosphorylation of TGF-β RI was reduced when FOXP3 was knocked down, and enhanced remarkably as FOXP3 was over-expressed (Fig. 4c). Immunoblotting further revealed that FOXP3 deficiency decreased the phosphorylation of Smad2/3, and over-expression of FOXP3 triggered the phosphorylation of Smad2/3 (Fig. 4c).
Fig. 4.
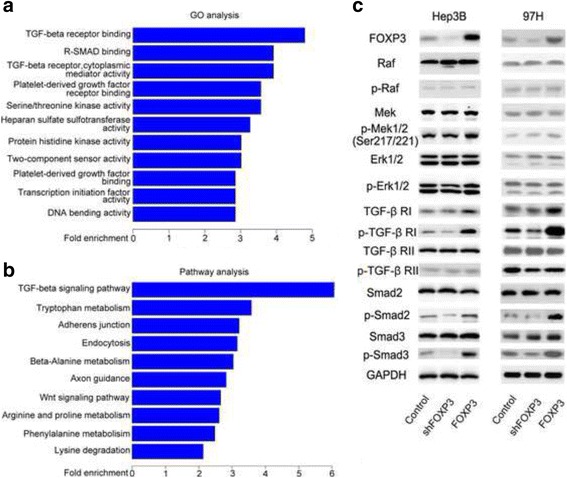
FOXP3 inhibited HCC progression by enhancing TGF-β signaling pathway. a and b TGF-β signaling pathway was screened out by Gene ontology (GO) analysis and Pathway analysis as a candidate pathway which was correlated with FOXP3. c The expression and phosphorylation of indicated molecules were detected by Western blotting
In conclusion, our studies suggested that FOXP3 inhibited HCC progression by enhancing the phosphorylation of TGF-β RI and activating TGF-β/Smad2/3 signaling pathway.
Discussion
FOXP3 is a member of the forkhead/winged-helix gene family of transcription factors associated with activation and differentiation of Treg cells [28]. Recently, the expression of FOXP3 has been recently reported in non-hematopoietic derived cells, including normal epithelial cells and some tumor cells from different tissue origins [29]. In this study, the expression of FOXP3 was detected in HCC cell lines and tumor tissues, although the expression was obviously lower than CD4+CD25+ cells. Immunostaining of FOXP3 in Hep3B and 97H cells indicated that FOXP3 staining was localized predominantly in the cytoplasm. The cytoplasmic localization of FOXP3 was detected in several human cancers, including human breast carcinoma [30]. Foxp3 molecule includes a winged-helix/forkhead (FKH) domain that carries a supposed nuclear localization signal. Mutations of this domain were observed in the Scurfy mouse and in the corresponding human IPEX syndrome, leading to a cytoplasmic accumulation of FOXP3 [31].
Previous data derived from several in vitro studies increasingly pointed to the critical role of FOXP3 as a tumor suppressor, including repressing tumor cell proliferation, promoting apoptosis and reducing cell invasion in at least breast, prostate and ovarian carcinoma models [10, 12, 32]. However, there were conflicting data suggesting a strong correlation of elevated FOXP3 expression in cancer cells with poor survival and appearance of lymph node metastases in gastric tumor, urinary bladder cancer and esophageal squamous carcinoma [33–35]. The reason for this discrepancy remains unclear, but Triulzi et al. considered it might be explained by a loss of oncosuppressive function of FOXP3 in human tumors due to breaking-function mutations [5]. Our tissue microarray data showed that high expression of FOXP3 in tumor tissue significantly correlated with low serum AFP level, absence of vascular invasion and early TNM stage, suggesting a negative regulatory role of FOXP3 in HCC progression. Although over half of the patients in advanced HCC with BCLC B/C stadium expressed low FOXP3, the inverse correlation of FOXP3 and BCLC stadium was insignificant (P = 0.196) probably due to small sample size (Table 1). Coincidently, survival analysis revealed that patient with high level of FOXP3 in tumor cells was remarkably and independently associated with improved survival and reduced recurrence, regarding FOXP3 as an independent prognostic factor of HCC patient outcome. Furthermore, over-expression of FOXP3 obviously inhibited tumor proliferation and invasion, while FOXP3 down-regulation resulted in enhanced tumor growth and invasion. Additionally, we detected the deletion mutation of FOXP3 in HCC cell lines and tissue samples. With the data of our cell and animal models, we found that Δ3,4-FOXP3 showed a moderate inhibition on tumor growth and migration than full-length FOXP3, indicating a restricted tumor-suppressive function probably caused by exon loss. Previously, FOXP3 was identified as a key player in mediating Treg inhibitory functions. Hence, our study provided additional information on the anti-tumor effect of FOXP3 in human HCC and was the first to demonstrate FOXP3 as a putative tumor suppressor in HCC progression.
Although the mechanism underlying the tumor suppression function of FOXP3 is not fully characterized, several genes and pathways have been found to be closely related. FOXP3 was found to inhibit tumor cell growth, serving as an important repressor for breast cancer oncogene SKP2 and HER2 [10, 11], and FOXP3–miR-146–NF-kB Axis has been suggested in leading to apoptosis during tumor initiation and tumor suppression in prostate cancer [36]. In our study, TGF-β signaling pathway was elucidated to correlate with FOXP3 by pathway analysis, and furthermore the FOXP3-related regulation of TGF-β/Smad2/3 was confirmed by western blotting assays. Smad2 and Smad3 are receptor-associated R-Smads which form the classical TGF-β signaling cascade [37]. It is widely accepted that TGF-β plays a bifunctional roles in tumor suppression and tumor promotion [38]. In a Japanese study, TGF-β acted as a tumor suppressor by transmitting a signal through pSmad3C (phosphorylate Smad3 at the COOH-terminal) and participating in the cytostatic response by repressing c-Myc gene, and on the other hand, pSmad2/3 L (phosphorylate Smad2 and Smad3 at the linker regions) signaling had oncogenic potential on tumor growth and invasion via up-regulation of c-Myc and MMP [39]. Their results indicated the reversibility of Smad-dependent signaling between tumor suppression and oncogenesis. Interestingly, a recent study showed that TGF-β could drive tumor suppression in pancreatic cancer cells which underwent a lethal epithelial-mesenchymal transition (EMT) by converting TGF-β-induced Sox4 from an enforcer of tumorigenesis into a promoter of apoptosis [40]. All these studies provided us novel ideas in searching for further understandings of tumor inhibition via FOXP3/TGF-β/Smad2/3 pathway.
Conclusions
In summary, we demonstrated that FOXP3 was expressed in tumor cells and an increased expression of FOXP3 was associated with better survival and reduced recurrence. In mechanism, FOXP3 exerted its tumor inhibition effects probably via the regulation of TGF-β/Smad2/3 pathway. Thus, there is a strong tendency to believe FOXP3 as a potential tumor suppressor in HCC. Although this is a single-center study and the FOXP3’s exact role in cancer cells remains open-ended, the identification of molecules related to FOXP3 expression and function in tumor cells would provide additional information on understanding the biological behavior of HCC.
Additional files
Supplementary Methods. (DOC 37 kb)
The additional file contains 6 sub-files. (ZIP 2185 kb)
Acknowledgements
Not applicable
Funding
The design of this study and cell transfections were supported by National Natural Science Foundation of China (Nos. 81,572,292 and 81,401,928), while proliferation and migration assays were supported by Nos. 81,502,028, 81,372,648 and 81,272,730. TMAs construction and immunostaining were sponsored by National Program for Special Support of Eminent Professionals (for Qiang Gao) and the follow-up was supported by Key Program (QYZDB-SSW-SMC036). The animal experiments were funded by External Cooperation Program (No. GJHZ201312) and Interdisciplinary Innovation Team from Chinese Academy of Sciences.
Availability of data and materials
The raw data of relative mRNA levels of FOXP3 in cell lines, CCK8 experiment, follow-up data and ChIP-seq test including GO and Pathway analysis were uploaded as Additional file 2.
Abbreviations
- AFP
α-fetoprotein
- ChIP
Chromatin immunoprecipitation
- CI
Confidence interval
- EMT
Epithelial-mesenchymal transition
- FOXP3
Forkhead box P3
- HCC
Hepatocellular carcinoma
- OS
Overall survival
- PBMC
Peripheral blood mononuclear cell
- SD
Standard deviation
- TGF-β
Transforming growth factor-β
- TMA
Tissue microarray
- TTR
Time to recurrence
Authors’ contributions
JYS, LJM, XYW, and QG conceived and performed most of the experiments; JWZ and LXY performed cell transfections and proliferation & migration assays; MD and ZBD provided clinical specimens, constructed TMAs and completed immunostaining; JYS and QG performed survival analyses; YJZ and XMZ performed subcutaneous tumorigenicity assays in BALB/c nude mice; and YC, JZ and JF critically reviewed the manuscript, improved the text structure and polished the language. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was conducted according to the ethical approval and animal use guidelines from Zhongshan Hospital Ethics Committee (Reference number Y2014–028), and patient written informed consents were obtained under institutional reviewer board protocols.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (10.1186/s12885-017-3633-6) contains supplementary material, which is available to authorized users.
Contributor Information
Jie-Yi Shi, Email: shi.jieyi@zs-hospital.sh.cn.
Li-Jie Ma, Email: 14211210033@fudan.edu.cn.
Ji-Wei Zhang, Email: zhangjiwei985@163.com.
Meng Duan, Email: ilovegoogle01@163.com.
Zhen-Bin Ding, Email: ding.zhenbin@zs-hospital.sh.cn.
Liu-Xiao Yang, Email: yang.liuxiao@zs-hospital.sh.cn.
Ya Cao, Email: ycao98@vip.sina.com.
Jian Zhou, Email: zhou.jian@zs-hospital.sh.cn.
Jia Fan, Email: fan.jia@zs-hospital.sh.cn.
Xiaoming Zhang, Email: xmzhang@ips.ac.cn.
Ying-Jun Zhao, Email: zhaoyingjun@fudan.edu.cn.
Xiao-Ying Wang, Email: wang.xiaoying1@zs-hospital.sh.cn.
Qiang Gao, Phone: +86-021-64037181, Email: gao.qiang@zs-hospital.sh.cn.
References
- 1.Zheng Y, Rudensky AY. Foxp3 In control of the regulatory T cell lineage. Nat Immunol. 2007;8:457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 2.Sadlon TJ, Wilkinson BG, Pederson S, Brown CY, Bresatz S, Gargett T, et al. Genome-wide identification of human FOXP3 target genes in natural regulatory T cells. J Immunol. 2010;185:1071–1081. doi: 10.4049/jimmunol.1000082. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y, Josefowicz SZ, Kas A, Chu TT, Gavin MA, Rudensky AY. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 4.Kim M, Grimmig T, Grimm M, Lazariotou M, Meier E, Rosenwald A, et al. Expression of Foxp3 in colorectal cancer but not in Treg cells correlates with disease progression in patients with colorectal cancer. PLoS One. 2013;8:e53630. doi: 10.1371/journal.pone.0053630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Triulzi T, Tagliabue E, Balsari A, Casalini P. FOXP3 Expression in tumor cells and implications for cancer progression. J Cell Physiol. 2013;228:30–35. doi: 10.1002/jcp.24125. [DOI] [PubMed] [Google Scholar]
- 6.Merlo A, Casalini P, Carcangiu ML, Malventano C, Triulzi T, Menard S, et al. FOXP3 Expression and overall survival in breast cancer. J Clin Oncol. 2009;27:1746–1752. doi: 10.1200/JCO.2008.17.9036. [DOI] [PubMed] [Google Scholar]
- 7.Winerdal ME, Marits P, Winerdal M, Hasan M, Rosenblatt R, Tolf A, et al. FOXP3 And survival in urinary bladder cancer. BJU Int. 2011;108:1672–1678. doi: 10.1111/j.1464-410X.2010.10020.x. [DOI] [PubMed] [Google Scholar]
- 8.Niu J, Jiang C, Li C, Liu L, Li K, Jian Z, et al. Foxp3 Expression in melanoma cells as a possible mechanism of resistance to immune destruction. Cancer Immunol Immunother. 2011;60:1109–1118. doi: 10.1007/s00262-011-1025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng C, Yao Y, Jie W, Zhang M, Hu X, Zhao Y, et al. Up-regulation of Foxp3 participates in progression of cervical cancer. Cancer Immunol Immunother. 2013;62:481–487. doi: 10.1007/s00262-012-1348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zuo T, Wang L, Morrison C, Chang X, Zhang H, Li W, et al. FOXP3 Is an X-linked breast cancer suppressor gene and an important repressor of the HER-2/ErbB2 oncogene. Cell. 2007;129:1275–1286. doi: 10.1016/j.cell.2007.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zuo T, Liu R, Zhang H, Chang X, Liu Y, Wang L, et al. FOXP3 Is a novel transcriptional repressor for the breast cancer oncogene SKP2. J Clin Invest. 2007;117:3765–3773. doi: 10.1172/JCI32538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Liu R, Li W, Chen C, Katoh H, Chen GY, et al. Somatic single hits inactivate the X-linked tumor suppressor FOXP3 in the prostate. Cancer Cell. 2009;16:336–346. doi: 10.1016/j.ccr.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma GF, Chen SY, Sun ZR, Miao Q, Liu YM, Zeng XQ, et al. FoxP3 Inhibits proliferation and induces apoptosis of gastric cancer cells by activating the apoptotic signaling pathway. Biochem Biophys Res Commun. 2013;430:804–809. doi: 10.1016/j.bbrc.2012.11.065. [DOI] [PubMed] [Google Scholar]
- 14.Maluccio M, Covey A. Recent progress in understanding, diagnosing, and treating hepatocellular carcinoma. CA Cancer J Clin. 2012;62:394–399. doi: 10.3322/caac.21161. [DOI] [PubMed] [Google Scholar]
- 15.Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol. 2007;25:2586–2593. doi: 10.1200/JCO.2006.09.4565. [DOI] [PubMed] [Google Scholar]
- 16.Yuan CH, Sun XM, Zhu CL, Liu SP, Wu L, Chen H, et al. Amphiregulin activates regulatory T lymphocytes and suppresses CD8+ T cell-mediated anti-tumor response in hepatocellular carcinoma cells. Oncotarget. 2015;6:32138–32153. doi: 10.18632/oncotarget.5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Y, Tang ZY, Ye SL, Liu YK, Chen J, Xue Q, et al. Establishment of cell clones with different metastatic potential from the metastatic hepatocellular carcinoma cell line MHCC97. World J Gastroenterol. 2001;7:630–636. doi: 10.3748/wjg.v7.i5.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian J, Tang ZY, Ye SL, Liu YK, Lin ZY, Chen J, et al. New human hepatocellular carcinoma (HCC) cell line with highly metastatic potential (MHCC97) and its expressions of the factors associated with metastasis. Br J Cancer. 1999;81:814–821. doi: 10.1038/sj.bjc.6690769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinz S, Pagerols-Raluy L, Oberg HH, Ammerpohl O, Grussel S, Sipos B, et al. Foxp3 Expression in pancreatic carcinoma cells as a novel mechanism of immune evasion in cancer. Cancer Res. 2007;67:8344–8350. doi: 10.1158/0008-5472.CAN-06-3304. [DOI] [PubMed] [Google Scholar]
- 20.Shi JY, Yang LX, Wang ZC, Wang LY, Zhou J, Wang XY, et al. CC chemokine receptor-like 1 functions as a tumour suppressor by impairing CCR7-related chemotaxis in hepatocellular carcinoma. J Pathol. 2015;235:546–558. doi: 10.1002/path.4450. [DOI] [PubMed] [Google Scholar]
- 21.Gao Q, Zhao YJ, Wang XY, Qiu SJ, Shi YH, Sun J, et al. CXCR6 Upregulation contributes to a proinflammatory tumor microenvironment that drives metastasis and poor patient outcomes in hepatocellular carcinoma. Cancer Res. 2012;72:3546–3556. doi: 10.1158/0008-5472.CAN-11-4032. [DOI] [PubMed] [Google Scholar]
- 22.Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, et al. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5994–6005. doi: 10.1158/1078-0432.CCR-12-3497. [DOI] [PubMed] [Google Scholar]
- 23.Xu YF, Yi Y, Qiu SJ, Gao Q, Li YW, Dai CX, et al. PEBP1 Downregulation is associated to poor prognosis in HCC related to hepatitis B infection. J Hepatol. 2010;53:872–879. doi: 10.1016/j.jhep.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 24.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, et al. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clinical cancer research: an official journal of the American Association for Cancer Research. 2009;15:971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 25.Valouev A, Johnson DS, Sundquist A, Medina C, Anton E, Batzoglou S, et al. Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nat Methods. 2008;5:829–834. doi: 10.1038/nmeth.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ebert LM, Tan BS, Browning J, Svobodova S, Russell SE, Kirkpatrick N, et al. The regulatory T cell-associated transcription factor FoxP3 is expressed by tumor cells. Cancer Res. 2008;68:3001–3009. doi: 10.1158/0008-5472.CAN-07-5664. [DOI] [PubMed] [Google Scholar]
- 27.Katoh H, Qin ZS, Liu R, Wang L, Li W, Li X, et al. FOXP3 Orchestrates H4K16 acetylation and H3K4 trimethylation for activation of multiple genes by recruiting MOF and causing displacement of PLU-1. Mol Cell. 2011;44:770–784. doi: 10.1016/j.molcel.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 29.McInnes N, Sadlon TJ, Brown CY, Pederson S, Beyer M, Schultze JL, et al. FOXP3 And FOXP3-regulated microRNAs suppress SATB1 in breast cancer cells. Oncogene. 2012;31:1045–1054. doi: 10.1038/onc.2011.293. [DOI] [PubMed] [Google Scholar]
- 30.Takenaka M, Seki N, Toh U, Hattori S, Kawahara A, Yamaguchi T, et al. FOXP3 Expression in tumor cells and tumor-infiltrating lymphocytes is associated with breast cancer prognosis. Mol Clin Oncol. 2013;1:625–632. doi: 10.3892/mco.2013.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes JE, Torgerson TR, Schubert LA, Anover SD, Ocheltree EL, Ochs HD, et al. Analysis of FOXP3 reveals multiple domains required for its function as a transcriptional repressor. J Immunol. 2006;177:3133–3142. doi: 10.4049/jimmunol.177.5.3133. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HY, Sun H. Up-regulation of Foxp3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 2010;287:91–97. doi: 10.1016/j.canlet.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 33.Xue L, Lu HQ, He J, Zhao XW, Zhong L, Zhang ZZ, et al. Expression of FOXP3 in esophageal squamous cell carcinoma relating to the clinical data. Dis Esophagus. 2010;23:340–346. doi: 10.1111/j.1442-2050.2009.01013.x. [DOI] [PubMed] [Google Scholar]
- 34.Hao Q, Li W, Zhang C, Qin X, Xue X, Li M, et al. TNFalpha induced FOXP3-NFkappaB interaction dampens the tumor suppressor role of FOXP3 in gastric cancer cells. Biochem Biophys Res Commun. 2013;430:436–441. doi: 10.1016/j.bbrc.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 35.Phe V, Roupret M, Cussenot O, Chartier-Kastler E, Game X, Comperat E. Forkhead box protein P3 (Foxp3) expression serves as an early chronic inflammation marker of squamous cell differentiation and aggressive pathology of urothelial carcinomas in neurological patients. BJU Int. 2015;115(Suppl 6):28–32. doi: 10.1111/bju.13044. [DOI] [PubMed] [Google Scholar]
- 36.Liu R, Yi B, Wei S, Yang WH, Hart KM, Chauhan P, et al. FOXP3-miR-146-NF-kappaB Axis and therapy for precancerous lesions in prostate. Cancer Res. 2015;75:1714–1724. doi: 10.1158/0008-5472.CAN-14-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schon HT, Weiskirchen R. Immunomodulatory effects of transforming growth factor-beta in the liver. Hepatobiliary Surg Nutr. 2014;3:386–406. doi: 10.3978/j.issn.2304-3881.2014.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morikawa M, Derynck R, Miyazono K. TGF-beta and the TGF-beta family: context-dependent roles in cell and tissue physiology. Cold Spring Harb Perspect Biol. 2016;8:a021873. doi: 10.1101/cshperspect.a021873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sekimoto G, Matsuzaki K, Yoshida K, Mori S, Murata M, Seki T, et al. Reversible Smad-dependent signaling between tumor suppression and oncogenesis. Cancer Res. 2007;67:5090–5096. doi: 10.1158/0008-5472.CAN-06-4629. [DOI] [PubMed] [Google Scholar]
- 40.David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, et al. TGF-beta tumor suppression through a lethal EMT. Cell. 2016;164:1015–1030. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods. (DOC 37 kb)
The additional file contains 6 sub-files. (ZIP 2185 kb)
Data Availability Statement
The raw data of relative mRNA levels of FOXP3 in cell lines, CCK8 experiment, follow-up data and ChIP-seq test including GO and Pathway analysis were uploaded as Additional file 2.


