Abstract
Cell-laden microgels with highly uniform sizes have significant potential in tissue engineering and cell therapy due to the capability to provide physiologically relevant three-dimensional (3D) microenvironment for living cells. In this work, we present a simple and efficient microfluidic approach to produce monodisperse cell-laden microgels through the use of double emulsion drops with an ultra-thin oil shell as the sacrificial template. Specifically, the thin oil shell in double emulsion spontaneously dewets upon polymerization of the innermost precursor drop and subsequent transfer into an aqueous solution, resulting in direct dispersion of microgels in an aqueous phase. Compared to conventional single emulsion-based techniques for cell encapsulation, this one-step approach prevents prolonged exposure of cells to the oil phase, leading to high-throughput cell encapsulation in microgels without compromising the cell viability. Moreover, this approach allows us to culture cells within a 3D microgel which mimics the extracellular matrix, thus enabling long-term cell functionality. This microfluidic technique represents a significant step forward in high-throughput cell microencapsulation technology and offers a potentially viable option to produce cell-laden microgels for widespread applications in tissue engineering and cell therapies.
Introduction
Hydrogels consisting of biocompatible and biodegradable polymeric matrix provide a physiologically relevant three-dimensional (3D) microenvironment for living cells.1–5 However, cells embedded within macroscopic hydrogels suffer from restricted intracellular communications and nutrient exchange due to limited diffusion rate and distance of extracellular molecules through the crosslinked network.6 In contrast, microgels on a microscopic scale are more suitable carriers for cell encapsulation and 3D culture, as they ease mass transport and facilitate higher control over the environmental cues of extracellular matrices.7–9 Thus, high-throughput encapsulation of cells in microgels can potentially enable major advances in tissue engineering and cell therapy strategies.1, 7 Such microgels loaded with living cells can serve as building blocks that allow assembly into complex tissue mimics,10 or can act as carriers for controlled delivery in cell therapy.11 However, it is critical to control both the size and size distribution of the delivery vehicles as they can affect bioavailability; moreover, they also determine the behavior of the encapsulated cells.5, 12 This demands techniques to produce microgels that are both highly controlled in size and structure, and in which cells can be easily encapsulated while retaining very high viability.
Several microfabrication techniques to produce the cell-laden microgels have been proposed. These approaches make use of photolithography,13 micromolding9, 14 and centrifuge-based drop maker,15 providing many advantages such as scalability, and precise control over particle size and shape. However, these conventional techniques are limited by the inherent nature of batch processing, resulting in low throughput. Recent advances in microfluidic techniques enable precise control of immiscible multiphase flows, providing continuous and rapid production of monodisperse microgels with controllable sizes. For example, water-in-oil (w/o) emulsion drops that are formed using a T-junction16 or through flow-focusing,17 can be used as templates for producing monodisperse microgels with a variety of polymerization schemes.18–20 However, this approach is not efficient due to use of a continuous oil phase, which can significantly decrease the viability of immobilized cells due to prolonged exposure to the oil and surfactants that can be potentially cytotoxic.8, 21 In addition, extra washing step is needed to break the emulsion and transfer the resultant microgel particles into an aqueous medium for use, which makes this approach time and labor-consuming. Alternatively, water-in-water emulsion drops, an aqueous two phase system, can be used as a template to produce microgels in an aqueous medium, avoiding the extra washing steps.22, 23 However, this system is limited to specific combinations of two immiscible aqueous solutes, such as dextran and polyethylene glycol, and this precludes widespread use of this technique for cell encapsulation. Thus, it still remains a challenge to produce monodisperse microgels that can effectively encapsulate cells while retaining their viability, and new techniques to accomplish this are required.
In this work, we report a simple and efficient microfluidic approach to produce monodisperse microgels by utilizing an ultra-thin oil shell of double emulsion drops as a sacrificial template. Using a glass capillary device, we form a coaxial flow of an aqueous prepolymer solution surrounded by an oil phase that are subsequently emulsified into continuous aqueous phase, resulting in monodisperse double emulsion drops, as shown schematically in Figure 1a. Upon UV exposure, the innermost drops comprising of prepolymer solution are selectively solidified, and the drops are then immediately transferred to an aqueous solution (Figure 1b). The surrounding thin oil shell in double emulsion spontaneously dewets upon polymerization of the innermost drop and subsequent transfer into an aqueous solution, thereby resulting in thorough separation of the oil phase from the microgels and direct dispersion of the microgels in the aqueous solution. We further demonstrate that this one-step approach can be extended to achieve scalable production of cell-laden microgels while retaining high cell viability by preventing prolonged exposure of cells to the oil phase. By utilizing biocompatible and biodegradable polymer as the extracellular matrix, we provide the encapsulated cells with a physiologically relevant 3D microenvironment and enable long-term cell functionality within the gel matrix.
Fig. 1.
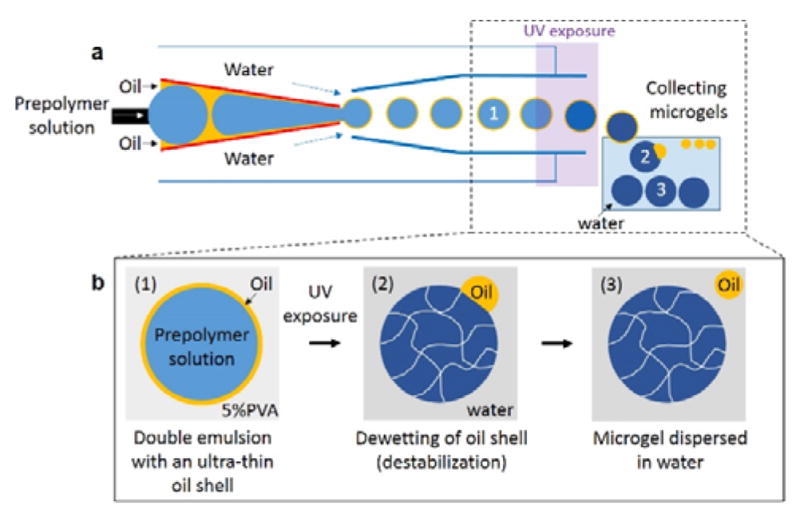
One-step production of microgels through use of double emulsion drops with an ultra-thin oil layer. (a) Schematic illustration showing glass capillary microfluidic device for preparation of double emulsion drops. The innermost drops are solidified to form microgels upon UV exposure. (b) Schematic illustration showing the detailed procedure to form solid microgels from double emulsion drops through a dewetting process of the oil layer.
Results and Discussion
Formation of Double Emulsion Drops with an Ultra-thin Sacrificial Oil Shell
To make double emulsion drops with an ultra-thin sacrificial oil layer, we use a glass capillary microfluidic device comprised of two tapered circular capillaries inserted into a square capillary.24 We use n-octadecyltrichlorosilane to make the circular injection capillary wall hydrophobic. In addition, a small tapered capillary is inserted into the injection capillary to facilitate simultaneous injection of two immiscible fluids. Another circular capillary is inserted into the square capillary at the other side to confine the flow near the injection tip, thereby increasing the flow velocity; this is treated with PEG-silane to make the capillary wall hydrophilic. The assembly of the capillary microfluidic device is illustrated schematically in Figure 1a.
An aqueous prepolymer solution is injected through the small tapered capillary to form the innermost drop, while an oil phase is injected through the injection capillary. The coinjection of these two immiscible fluids leads to a coaxial flow that consists of an ultra-thin oil layer surrounding the innermost fluid of prepolymer phase due to the strong affinity of the oil phase to the hydrophobic wall of the injection capillary. Additional aqueous phase is injected through the interstices of the square and injection capillaries from the same side with the injection capillary. The coaxial flow from the injection capillary is emulsified in the dripping regime by the continuous aqueous phase at the exit of the injection capillary, resulting in the formation of monodisperse double emulsion drops with an ultra-thin oil layer, as shown in Figure 1b-1. Upon exposure to UV illumination, the innermost drops, containing the prepolymer solution, are selectively solidified, forming microgels in the core as shown in Figure 1b-2. Collecting these microgels in an aqueous solution without any surfactants causes the ultra-thin oil layer to dewet from the surface of the microgels, resulting in the formation of cell-laden microgels directly in water, without the additional washing step, as shown in Figure 1b-3.
Production of Monodisperse Microgels through Dewetting of the Oil Shell
To produce the microgels, we prepare monodisperse double emulsion drops with an ultra-thin oil layer using a capillary microfluidic device operating in the dripping regime,25 as shown in optical images of Figure 2a. To accomplish this, we use an aqueous solution of 10% polyethyleneglycol diacrylate (PEG-DA) for the innermost prepolymer phase, mineral oil with 0.5% Span80 as the middle oil phase and aqueous solution of 5% PVA as the continuous phase. The stream of double emulsion drops is exposed to UV illumination; this leads to selective polymerization of the innermost prepolymer drops. To visualize how the microgels are formed from the double emulsions, we label the ultra-thin oil layer with an oil-soluble fluorescent dye (Nile red). Close examination of the microgels upon collection in an aqueous solution without any surfactants reveals that the ultra-thin oil layer completely engulfs the microgels. However, after 1 min, the thin oil layer starts to gradually dewet from the surface of the microgels, leading to the segregation of oil drops on the surface of the microgels and ultimately to the separation of the oil drops from the microgels, as shown in Figure 2b. We attribute the dewetting process to significant change in the interfacial tension among the fluids comprising the emulsions when they are transferred into an aqueous solution in the absence of surfactants. Specifically, lack of surfactants causes the oil layer in double emulsions to destabilize, making it separate from the innermost phase and thus forming oil-free microgels in a consistent manner. The separated oil drops immediately migrate to the upper region of the vial due to buoyancy (ρmineral oil =0.840 and ρ10% PEG-DA aq. =1.01), as shown in Figure 2c; this facilitates collection of microgels, as shown in Figure 2d. The resulting microgels are monodisperse with coefficient of variation of 3%, as shown by the size distribution curve in Figure 2e.
Fig. 2.
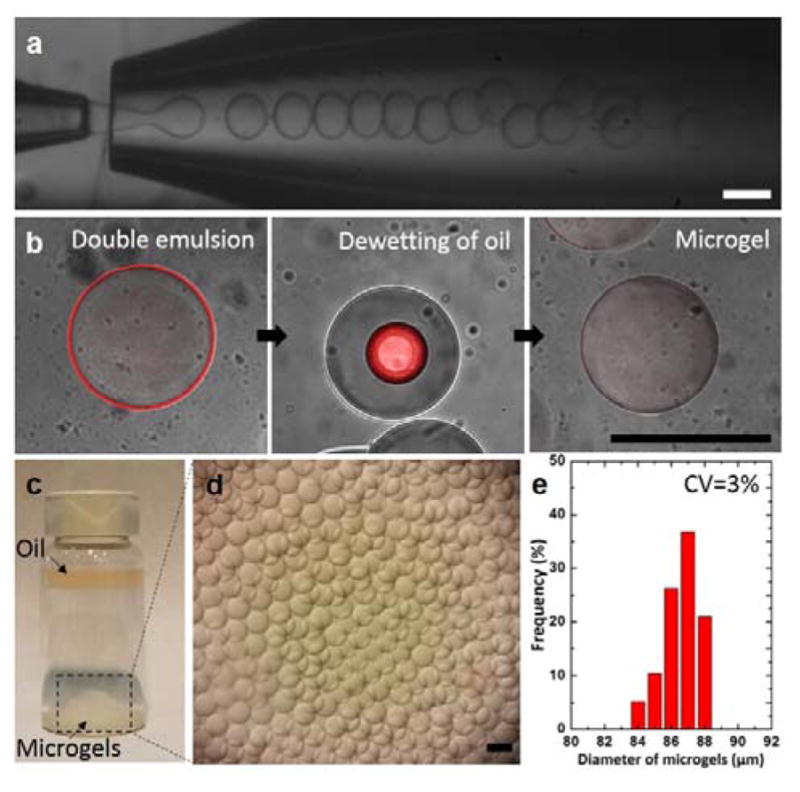
Monodisperse microgels produced by glass capillary microfluidic device. (a) Optical images showing continuous formation of double emulsion drops in a dripping mode. (b) A series of confocal images showing dynamic behavior of the oil layer as the polymerized drops are transferred in an aqueous solution (DI water). Estimated thickness of the oil shell is approximately 1 μm using image analysis. (c–d) Optical images showing the resulting microgels on the bottom surface and the oil layer collected on the top surface after the dewetting process. (e) Size distribution of the resulting microgels. All scale bars represent 100 μm.
Encapsulation of Mammalian Cells with High Viability
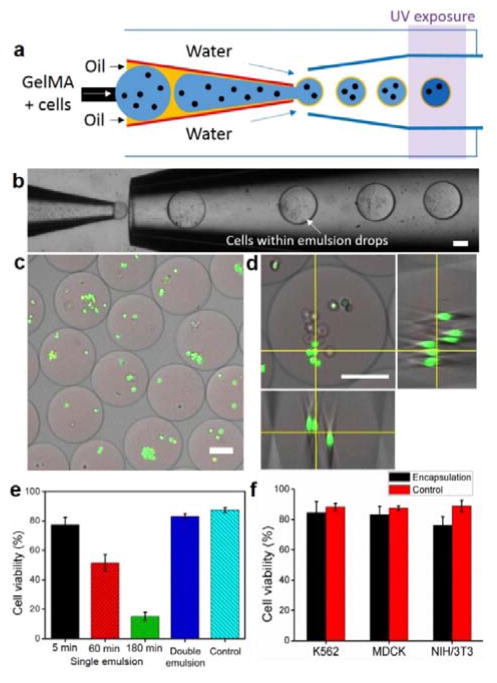
Compared to conventional single-emulsion techniques for microgel preparation, this thin-shell double emulsion approach avoids the time- and labor-consuming washing step, and achieves direct transfer of resultant microgels from the oil phase into the aqueous phase; this leads to one-step production of microgels in a continuous manner. Importantly, this approach significantly reduces the residence time of the microgels within the oil phase from hours to minutes. This improves the viability of encapsulated cells by avoiding prolonged exposure of the cells to the potentially harmful oil and surfactants21. To demonstrate this, we compare the viability of encapsulated cells using conventional single emulsion technique with the current one-step thin-shell double emulsion one. We encapsulate living Madin-Darby canine kidney epithelial (MDCK) cells using biocompatible polymer (polyethyleneglycol diacrylate, PEGDA, or gelatin methacrylate, GelMA) as the microgel material. To prepare these microgels, we first dissolve the hydrogel precursor and photoinitiator (Irgacure 2959) into cell culture medium (i.e. DMEM). Then, we disperse the cells into this solution with a concentration of 1×106 cells/ml. For cell encapsulation, we use mineral oil containing 0.5% Span 80 surfactants or fluorinated oil (HFE 7500) containing 0.5% krytox-PEG-krytox surfactants as the middle oil phase. An aqueous solution of 5% PVA is used as the continuous phase to form double emulsion drops. A schematic illustration of cell encapsulation procedure based on double emulsions with a sacrificial oil shell is shown in Figure 3a. We successfully demonstrate the feasibility of this technique for cell encapsulation as shown in the optical images of Figure 3b. Upon UV illumination (λ ~ 365 nm for 2 sec), cells are immobilized as the gel matrix is solidified. Collection of the cell-loaded microgels into an aqueous solution of cell culture media (DMEM) leads to spontaneous dewetting of oil phase on the surface of microgels, allowing autonomous transfer of microgels into aqueous phase. The resulting microgels contain multiple cells and have an average diameter of 244 μm, as shown in the fluorescent microscopic images of Figure 3c and d. The number of encapsulated cells per microgel and the size of microgels can be modulated by varying the concentration of the cell suspension, the nozzle size of the capillary, and the fluid flow rates8, 26. We observe reduced monodispersity of cell-laden microgels (see Supplementary Information, Figure S1) compared to cell-free ones; this can be attributed to the inhomogeneity of the cell suspension, leading to variability in the drop size during emulsification and subsequent break-up.
Fig. 3.
Production of cell-laden microgels. (a) Schematic illustration showing encapsulation of living mammalian cells using the thin-shell double emulsion drops formed with photo-crosslinkable polymer. (b) Optical image showing cell encapsulation within double emulsion drops. (c) Representative fluorescent microscopic image showing cells embedded within microgels. Cells were stained by calcein-AM (green-fluorescent dye for live cells) and ethidium homodimer (red-fluorescent dye for dead cells). (d) Representative fluorescent microscopic image of single microgels containing multiple cells. Orthogonal view showing three-dimensional cell distribution within the microgels. (e) Survival rates of MDCK cells encapsulated in microgels prepared by two-step single emulsion approach in comparison to cells encapsulated by the proposed one-step thin shell double emulsion approach. The effect of the conventional two-step approach on cell viability was evaluated by incubating the encapsulated cells within the emulsion containing mineral oil and Span80 for different periods of time (5, 60 and 180 min), and then determining the survival rates of the cells. The fraction of cell viability was determined from LIVE/DEAD staining. The control group is cells that have been dispersed in the polymer precursor solutions followed by 2D culture on tissue culture plate. (f) Survival rates of different cell types after microfluidic encapsulation using thin-shell double emulsion method using HEF and Krytox-PEG-Krytox as the oil phase. All scale bars represent 100 μm.
The rapid dewetting allows spontaneous transfer of the resultant cell-laden microgels into cell-culture media, therefore avoiding extensive exposure of living cells to the oil phase which can depress the viability and metabolic activity of the cells, and providing unperturbed nutrient exchange through the hydrogel layer. Indeed, we observe that cells encapsulated in microgels using one-step double emulsion technique exhibit survival rates comparable to that of cells cultured on a tissue culture plate (polystyrene) as evidenced by the Live/Dead staining assay performed after 3 hr of microfluidic cell encapsulation (Figure. 3c and d). In contrast, cell viability within microgels from the single emulsion approach significantly reduces as residence time of the cells within oil phase increases. As shown in Figure 3e, long-term exposure (3 hr) of cell-laden microgels to oil phase (herein mineral oil containing 0.5% Span 80 surfactants) results into less than 20% survival rate of the cells. This cytotoxicity of conventional method can be related to i) the prolonged exposure of encapsulated cells to the surfactants which could potentially lead to rupture of cell membrane,21 ii) the cytotoxicity of photo-initiator,27, 28 and limited nutrient/gas exchange in the oil phase. Further, we use fluorinated oil (HFE 7500) containing 0.5% krytox-PEG-krytox surfactants as the middle oil phase instead of mineral oil and Span80; this could improve the cyto-compatibility of the oil phase because of the negligible solubility of fluorinated oil in water (3 ppm) and its high gas permeability.29 We observe that all three cell types tested (i.e. myelogenous leukemia cells (K562), MDCK cells and NIH/3T3 fibroblast cells) exhibit desirable survival rates after encapsulation (Figure 3f), confirming the biocompatibility of our approach for cell encapsulation for a variety of cell types. These results indicate that conventional single-emulsion approach is not suitable for scale-up production of cell-laden microgels since cells have to be trapped within the oil phase for a period of time to collect enough samples. By contrast the double emulsion method enables continuous cell encapsulation without compromising cell viability, which can serve as a cost-effective tool for scalable 3D cell encapsulation.
Three-dimensional (3D) cell culture within microgels
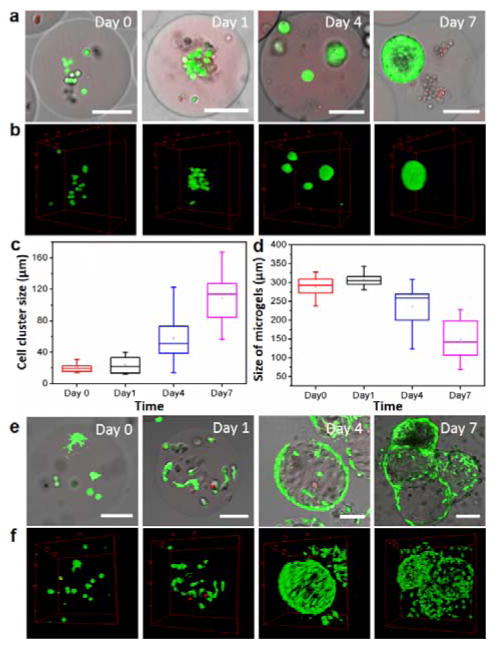
We further demonstrate the biocompatibility of this encapsulation technique by culturing the mammalian cells within the three dimensional microenvironment. We encapsulate and subsequently culture MDCK and NIH/3T3 cells (both are anchorage-dependent cells) using biodegradable GelMA microgels. Gelatin matrices allow cells to adhere and proliferate three-dimensionally due to the presence of cell-attachment site (i.e. Arg-Gly-Asp, RGD sequence) in gelatin molecules30, and maintain a healthy and viable state to proceed their normal functionality. Indeed, we observe continuous growth of both cell types within the gelatin matrices (Figure 4). MDCK cells reproduce and aggregate with each other to form stratified cell spheroids after 4 days (Figure 4a and b). The size of the spheroidal cellular constructs increases gradually (Figure. 4c), which eventually leads to the formation of specific architecture resembling simple epithelial tissues after 7 days (see Supplementary Information, Figure S2). Such a cyst structure with a multi-cell thick shell and a central lumen is representative of 3D culture of MDCK cells in collagen matrix,31 confirming the biocompatibility of our approach.
Fig. 4.
3D cell culture in GelMA migrogels. (a) Representative fluorescent microscopic images of the cross-section of the cell-laden microgels and (b) corresponding 3D reconstruction showing the growth of MDCK cells embeded within microgels at different time points during 3D culture. Cells were stained by calcein (green-fluorescent dye for live cells) and ethidium homodimer (red-fluorescent dye for dead cells). (c) The size of cell clusters (characterized by the diameter of cell spheroids) formed within GelMA microgels at different time points during 3D cell culture. (d) The diameter of cell-laden microgels (characterized by the diameter of the microgels) at different time points during 3D cell culture. (e) Representative fluorescent microscopic images of the cross-section of the cell-laden microgels and (f) corresponding 3D reconstruction showing NIH/3T3 fibroblast cells embedded within microgels at different time point during 3D culture. All scale bars represent 100 μm.
Unlike epithelial MDCK cells that form coherent, densely packed cellular organization, NIH/3T3 fibroblasts exhibit completely different behavior after being encapsulated into GelMA microgels. Remarkably, we observe cell attachment and spreading within the 3D matrix three hours after encapsulation, as evidenced by the penetration of pseudopodia throughout the matrix (Figure 4e and f). The majority of encapsulated cells egress out from the internal space of the microgels, and attach to the surface of the gels after 4 days. Interestingly, the GelMA microgels severely deforms from original spherical shape and considerably reduces the size of microgels (Figure 4d), most likely due to the compressive forces exerted by cell adhesion to the hydrogel matrices. Moreover, the growth of fibroblasts eventually bridges and aggregates the neighboring microgels. These results confirm that our approach could provide an efficient platform to create these cell-laden microgels that can serve not only as a microscopic platform for the study of long-term cell functionality in 3D microenvironment, but also as building blocks for bottom-up assembly of complex cellularized constructs with tissue-specific architecture.
Experimental
Preparation of double emulsions with a sacrificial ultra-thin oil shell
To produce double emulsion drops, we inject 8–10% aqueous solution of polyethyleneglycol diacrylate (PEG-DA, Mn 700, Sigma-Aldrich) and gelatin-methacrylate (GelMA) through the small tapered capillary with a typical flow rate of 1000 μL hr−1. A mineral oil (Sigma-Aldrich) containing 0.5% Span80 (Sigma-Aldrich) as a non-ionic surfactant or HFE-7500 (3M) containing 0.5% Krytox-PEG-Krytox (Ran Biotechnologies, Inc.) is simultaneously supplied through the injection capillary with a typical flow rate of 1000 μL hr−1. An aqueous solution of 5% poly(vinyl alcohol) (PVA) (Mw 13 000–23 000, Sigma-Aldrich) is injected through the interstices of the square and collection capillary with a typical flow rate of 15000 μL hr−1. These rates ensure that drop formation is restricted to the dripping regime.
Preparation of microfluidic device and drop generation
We use a glass capillary microfluidic device to produce double emulsion drops with an ultra-thin oil layer. We prepare an injection capillary by tapering a 560 μm inner diameter cylindrical glass capillary (1B100–6, World Precision Instruments, Inc.) to 50 μm inner diameter; to make the inner wall hydrophobic or fluorophilic, we treat it with n-octadecyltrimethoxyl silane (Aldrich) or Heptadecafluoro-1,1,2,2 tetrahydrodecyl trichlorosilane (Gelest, Inc.), respectively, for 10 minute and subsequently wash with ethanol. We insert the injection capillary into a square capillary (AIT Glass) whose inner width (1.05 mm) is slightly larger than that of the outer diameter of the injection capillary (1 mm). Next, we prepare a small tapered glass capillary (10 μm inner diameter) by heating and pulling a cylindrical capillary by hand using a gas torch; this capillary is inserted into the injection capillary for simultaneous injection of two immiscible fluids. Finally, a cylindrical collection capillary is inserted into the square capillary from the other end; we also treat this collection capillary with 2-[methoxy(polyethyleneoxy)propyl] trimethoxy silane (Gelest, Inc.) to make the capillary wall hydrophilic. During drop generation, the volumetric flow rate is controlled by syringe pumps (Harvard Apparatus) and the production of emulsion drops is observed using an inverted microscope equipped with a high-speed camera (Phantom V9.0). The experimental setup is shown in Figure S3.
Cell encapsulation and 3D culture
Myelogenous leukemia cells (K562), Madin-Darby canine kidney epithelial (MDCK) cells and NIH/3T3 fibroblast cells are obtained from ATCC®, and are cultured for 6 days in proliferation medium (Dulbecco’s modified Eagle medium (DMEM, Sigma-Aldrich), supplemented with 10% v/v fetal bovine serum (FBS, Gibco), at 37°C, 95% relative humidity and 5% CO2. The medium is refreshed every three days of culture. At confluence, cells are washed twice with PBS, detached using trypsin/EDTA (0.25 % w/v trypsin/0.02 % EDTA) for 5 min and resuspended in cell culture media. GelMA and photo-initiator (Irgacure 2959) are dissolved in DMEM followed by sterilization and filtration. All devices are sterilized prior to use by exposing to ultraviolet illumination (λ ~ 254 nm) for 60 min. Cell-laden microgels are prepared by encapsulating the cells suspended in DMEM containing 10 % w/v of PEG-DA or GelMA and 1% w/v photoinitiator in double emulsion drops. Then these double emulsion drops are in-situ photopolymerized in UV for 2 sec to form cell-laden microgels. The cell-laden microgels are collected using a cell strainer followed by re-dispersion in cell culture media. The medium is refreshed every three days of culture. Cell survival is determined using a LIVE/DEAD Viability/Cytotoxicity Kit (Molecular Probes). To this end, gels are washed in sterile PBS for 30 min at 37°C prior to incubation for 30 min at room temperature with 2 mM calcein-AM (red-fluorescent dye for dead cells) and 4 mM ethidium homodimer (green-fluorescent dye for live cells) in PBS solution. After incubation, gels are rinsed in PBS and examined using a Leica SP5 confocal laser scanning microscope with a 40× water-immersion objective.
Conclusions
In this paper, we present a simple microfluidic approach to produce monodisperse microgels by utilizing double emulsion drops with an ultra-thin oil shell as a sacrificial template. This approach allows the production of microgels in a simple and efficient manner, avoiding the extra washing procedure required for the conventional single emulsion based approaches. In addition, we demonstrate the advantages of this method by encapsulating and culturing living mammalian cells in three dimensional microgel matrices; we achieve high cell viability by avoiding potential cytotoxicity from prolonged exposure to oil and surfactants. We anticipate that the cell-laden microgels with tunable sizes and compositions fabricated using this microfluidic platform are not limited to UV-induced polymerization but can also be formed using other polymerization schemes such as chemical diffusion, and temperature-induced solidification. Moreover, this approach allows us to manipulate cells at the microscopic scale based on microfluidics and to observe long-term cell functionality within the microgel matrices. This microfluidic technique represents a significant step forward in high-throughput cell microencapsulation technology, and offers a potentially viable option to produce cell-laden microgels for widespread applications in tissue engineering and cell therapy.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Health (R01 EB014703 and P01GM096971), Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2013R1A6A3A03065122), Rubicon postdoctoral fellowship from the Netherlands Organization for Scientific Research (No. 825.12.018), and the National Natural Science Foundation of China (No. 51503208). C.-H Choi and H. Wang contributed equally to this work. J. H. Kim is currently affiliated with Buckingham Browne and Nichols.
Footnotes
Electronic Supplementary Information (ESI) available: See DOI: 10.1039/x0xx00000x
References
- 1.Discher DE, Mooney DJ, Zandstra PW. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drury JL, Mooney DJ. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 3.Hoffman AS. Adv Drug Deliv Rev. 2002;54:3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 4.Klouda L, Mikos AG. Eur J Pharm Biopharm. 2008;68:34–45. doi: 10.1016/j.ejpb.2007.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seliktar D. Science. 2012;336:1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 6.Nicodemus GD, Bryant SJ. Tissue Eng Part B Rev. 2008;14:149–165. doi: 10.1089/ten.teb.2007.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khademhosseini A, Langer R. Biomaterials. 2007;28:5087–5092. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 8.Tsuda Y, Morimoto Y, Takeuchi S. Langmuir. 2010;26:2645–2649. doi: 10.1021/la902827y. [DOI] [PubMed] [Google Scholar]
- 9.Yeh J, Ling Y, Karp JM, Gantz J, Chandawarkar A, Eng G, Blumling J, Iii, Langer R, Khademhosseini A. Biomaterials. 2006;27:5391–5398. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Yanagawa F, Kaji H, Jang YH, Bae H, Yanan D, Fukuda J, Qi H, Khademhosseini A. J Biomed Mater Res Part A. 2011;97A:93–102. doi: 10.1002/jbm.a.33034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oh JK, Drumright R, Siegwart DJ, Matyjaszewski K. Prog Polym Sci. 2008;33:448–477. doi: 10.1016/j.progpolymsci.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allazetta S, Lutolf MP. Curr Opin Biotech. 2015;35:86–93. doi: 10.1016/j.copbio.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Du Y, Lo E, Ali S, Khademhosseini A. Proc Natl Acad Sci USA. 2008;105:9522–9527. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tekin H, Tsinman T, Sanchez JG, Jones BJ, Camci-Unal G, Nichol JW, Langer R, Khademhosseini A. J Amer Chem Soc. 2011;133:12944–12947. doi: 10.1021/ja204266a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maeda K, Onoe H, Takinoue M, Takeuchi S. Adv Mater. 2012;24:1340–1346. doi: 10.1002/adma.201102560. [DOI] [PubMed] [Google Scholar]
- 16.Tan WH, Takeuchi S. Adv Mater. 2007;19:2696–2701. [Google Scholar]
- 17.Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RMA, Walker GC, Kumacheva E. J Amer Chem Soc. 2006;128:12205–12210. doi: 10.1021/ja0635682. [DOI] [PubMed] [Google Scholar]
- 18.Rossow T, Heyman JA, Ehrlicher AJ, Langhoff A, Weitz DA, Haag R, Seiffert S. J Amer Chem Soc. 2012;134:4983–4989. doi: 10.1021/ja300460p. [DOI] [PubMed] [Google Scholar]
- 19.Choi CH, Jung JH, Rhee Y, Kim DP, Shim SE, Lee CS. Biomed Microdevices. 2007;9:855–862. doi: 10.1007/s10544-007-9098-7. [DOI] [PubMed] [Google Scholar]
- 20.Choi CH, Jung JH, Hwang TS, Lee CS. Macromol Res. 2009;17:163–167. [Google Scholar]
- 21.Dehghan Noudeh G, Khazaeli P, Mirzaei S, Sharififar F, Nasrollahosaiani S. J Biol Sci. 2009;9:423–430. [Google Scholar]
- 22.Song Y, Chan YK, Ma Q, Liu Z, Shum HC. ACS Appl Mater Interfaces. 2015;7:13925–13933. doi: 10.1021/acsami.5b02708. [DOI] [PubMed] [Google Scholar]
- 23.Ziemecka I, van Steijn V, Koper GJM, Rosso M, Brizard AM, van Esch JH, Kreutzer MT. Lab Chip. 2011;11:620–624. doi: 10.1039/c0lc00375a. [DOI] [PubMed] [Google Scholar]
- 24.Kim SH, Kim JW, Cho JC, Weitz DA. Lab Chip. 2011;11:3162–3166. doi: 10.1039/c1lc20434c. [DOI] [PubMed] [Google Scholar]
- 25.Utada AS, Fernandez-Nieves A, Stone HA, Weitz DA. Phys Rev Lett. 2007;99:094502. doi: 10.1103/PhysRevLett.99.094502. [DOI] [PubMed] [Google Scholar]
- 26.Utech S, Prodanovic R, Mao AS, Ostafe R, Mooney DJ, Weitz DA. Adv Healthc Mater. 2015;4:1628–1633. doi: 10.1002/adhm.201500021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panda P, Ali S, Lo E, Chung BG, Hatton TA, Khademhosseini A, Doyle PS. Lab Chip. 2008;8:1056–1061. doi: 10.1039/b804234a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams CG, Malik AN, Kim TK, Manson PN, Elisseeff JH. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 29.Lowe KC. J Fluor Chem. 2002;118:19–26. [Google Scholar]
- 30.Kang HW, Tabata Y, Ikada Y. Biomaterials. 1999;20:1339–1344. doi: 10.1016/s0142-9612(99)00036-8. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien LE, Zegers MMP, Mostov KE. Nat Rev Mol Cell Biol. 2002;3:531–537. doi: 10.1038/nrm859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.