Abstract
This study was conducted to investigate the possible protective role of thymoquinone (TQ) and l-cysteine on the reproductive toxicity of male rats induced by cadmium chloride (CdCl2). Forty rats were divided into four even groups. The first group served as untreated control. The second, third and fourth groups received CdCl2, CdCl2 and TQ, and CdCl2 and l-cysteine, respectively for 56 days. Cd exposure caused spermatological damage (decrease sperm count and motility and increased the rates of sperm abnormalities), decrease serum testosterone level and increased oxidative stress. Histological alterations were also observed in the form of vascular and cellular changes in CdCl2 treated rats. The vascular changes were congestion of the blood vessels with interstitial edema in the testes, epididymis, seminal vesicle and prostate. The cellular changes were in the form of degenerative changes with presence of multinucleated giant cells in the lumen of seminiferous tubules, vacuolation and sloughing of the lining epithelium of the epididymis, seminal vesiculitis and prostatitis. Co-administration of TQ and l-cysteine with CdCl2 increased glutathione (GSH), superoxide dismutase (SOD), catalase (CAT) and testosterone and reduced lipid peroxidation (LPO) activity. In conclusion, our results showed that TQ and l-cysteine can ameliorate the deleterious effects of CdCl2 probably by activating testicular endocrine and antioxidant systems.
Keywords: Cadmium, Thymoquinone, l-cysteine, Histopathology, Antioxidants
1. Introduction
Cadmium (Cd) is one of the most toxic industrial and environmental heavy metals. It acts as an endocrine disruptor and oxidative stress inducer in humans and animals [1]. Cadmium is widely used in industrial processes, e.g., as an anticorrosive agent, as a color pigment, a neutron absorber in nuclear power plants and in the fabrication of nickel–cadmium batteries. Phosphate fertilizers also show a big cadmium load [2]. It has been demonstrated that Cd is extremely toxic to the testicular tissues of rats and mice, and several morphological and biochemical changes in the mammalian testis [3].
Different doses of cadmium chloride (CdCl2) (2, 4, and 8 mg/kg body weight) induced testicular oxidative stress is mediated through generation of reactive oxygen species (ROS), depletion of reduced glutathione (GSH), elevated lipid peroxidation (LPO) and altered antioxidant enzymes, which can ultimately result in infertility of male mice [4]. The testis of adult rats is very sensitive to CdCl2 (1 or 1.2 mg/kg body weight) which causes profound testicular damage and irreversible infertility [5]. Exposure of Wistar rats to 1 mg CdCl2 resulted in suppressed sperm count, motility and increased the percentage of sperm abnormalities. These effects were associated with a reduction in serum testosterone level [6]. Cadmium has also been reported to affect testicular spermatogenic and steroidogenic functions, impair male fertility, degrade semen quality and cause testicular degeneration, seminiferous tubular (ST) damage and ultimately, reproductive failure [7], [8]. Cadmium has long been known to damage the hepatic, respiratory and reproductive systems [9].
Thymoquinone (TQ), a main active constituent of the volatile oil extracted from the black seed (Nigella sativa L.), was shown to inhibit tissue inflammation and oxidative stress [10]. Alenzi et al. [11] found that the administration of TQ led to decreased malondialdehyde (MDA) levels in different toxicity studies. Also TQ Orally administered TQ increased the activities of CAT and GSH-Px enzymes in studies carried out by Fouda et al. [12].
l-cysteine and it derivatives are large group of exogenous antioxidant drugs that protects against oxidative tissue injury. This effect may be directly related to the drug itself (e.g., inactivation of hydroxyl radicals) or to the secondary induction of glutathione (GSH) production [13]. Ahmed et al. [14] evaluated the antioxidant activity of vitamin C, N,N0-diphenyl-p-phenylenediamine (DPPD) and l-cysteine against cisplatin (CP) induced testicular oxidative damage in rats. They founded that l-cysteine reduced the testicular damage effect of CP on in rats. l-cysteine had a protective effect similar to that of DPPD on LPO, nitric oxide (NO), superoxide anion and superoxide dismutase (SOD). l-cysteine significantly reduced the elevation in LPO and reduced the effect of CP on superoxide anion and antioxidant enzymes and also on the antioxidants contents in rats.
The aim of the present study was to investigate the possible protective role of thymoquinone (TQ) and l-cysteine on the reproductive toxicity of male Sprague-Daweley (SD) rats induced by cadmium chloride.
2. Materials and methods
All experimental procedures in this study were done in accordance with the guidelines of the European Union Council (86/609/EU). In which, 40 adult male Sprague-Daweley (SD) rats (130–150 g) were obtained and maintained in The Assiut University Animal Nutrition and Care House. Animals were maintained under standard conditions with 12-h light/dark cycles and 22 °C and 60% humidity. The food in the form of dry chow pellets and water were available ad libitum. Cadmium chloride (CdCl2) and 5,5-dithiobis-(2-nitrobenzoic acid) (DTNB, Ellman's reagent) and thymoquinone and l-cysteine were purchased from Sigma–Aldrich (Germany).
2.1. Experimental design
After 2 weeks of acclimatization, rats were randomly divided into 4 equal groups (10 rats each). The first group served as a control and received daily normal saline by stomach tube (0.5 ml/rat). The second, third and fourth groups received CdCl2, CdCl2 and TQ and CdCl2 and l-cysteine, respectively, for 56 days. The 56-day treatment period was chosen based on the time necessary to complete a spermatogenic cycle in rats [15]. CdCl2 was dissolved by normal saline and given by stomach tube (1 mg/kg body weight) [5]. TQ was given twice weekly at a dose of 10 mg/kg body weight in corn oil (0.5 ml/rat) by stomach tube [16]. l-cysteine was given twice weekly at a dose of 100 mg/kg body weight dissolved in1 M HCl (0.5 ml/rat) by stomach tube [14].
2.2. Sampling
At the end of experiment, the rats were deprived of food overnight, individually weighed and anesthetized with diethyl ether. Blood was collected from the retro-orbital plexus into anticoagulant-free test tubes, and the serum was removed and stored at −20 °C until determination of testosterone hormone and lipid peroxidation and glutathione, superoxide dismutase and catalase. The rats were then immediately sacrificed by cervical decapitation. The testes, epididymis and accessory sex organs (seminal vesicles and prostate glands) were excised. One testis, epididymis, prostate and seminal vesicle from each rat were removed, thoroughly washed with physiological saline (NaCl 0.9%), blotted on filter paper, rapidly fixed in modified Davidson's fluid for 48 h and stored in 70% alcohol until further processing [17].
2.3. Biochemical estimations
Serum lipid peroxidation (LPO): products as thiobarrbaturic acid reactive substance (TBARS) were determined according to the method of Ohkawa et al. [18].
Glutathione (GSH): determined using the method of Beutler et al. [19]. Briefly, in ependorff tube 200 μl sample was added to 300 μl distilled water and 300 μl precipitating solution (1.67 gm glacial meta-phosphoric acid, 0.2 gm EDTA and 30 gm NaCl). The solution was left for 5 min and then was centrifuged at 5000 rpm for 10 min. In cuvette, 200 μl of the supernatant, 800 μl of the phosphate solution (0.3 M Na2HPo4) and 100 μl DTNP [0.04 gm 5,5 dithiobis-(2-nitrobenzoeix acid)/100 ml of 0.1% sodium citrate] was added and measured at 412 nm against blank consists of 1 ml phosphate solution, 250 μl diluted precipitating solution (3:2 distilled water) and 125 μl DTNB.
Superoxide dismutase (SOD): activity in serum was determined according to its ability to inhibit the autoxidation of epinephrine at alkaline medium according to the method of Misra and Fridovich [20].
Catalase (CAT): activity in serum was determined according to the procedure of Luck [21] based on its ability to decompose hydrogen peroxide, which can be measured at 240 nm.
Determination of the serum testosterone hormone: level measured by quantitative enzyme immunoassay using commercially formulated kits (Immunometrics Ltd., London, UK). The enzymes were detected by color and the absorbance was measured at 450 nm by ELIZA reader.
2.4. Assessment of sperm production
Epididymal spermatozoa were counted using a modified method of Yokoi et al. [22]. Briefly, the cauda epididymis was minced in 5 ml saline, placed in a rocker for 10 min and incubated at room temperature for 2 min. The supernatant was diluted 1:100 in a solution containing 5 g NaHCO3, 1 ml formalin 35% and 25 mg eosin per 100 ml distilled water. Approximately, 10 μl of the diluted semen was transferred to each counting chamber of an improved Neubaur haemocytometer (Depth 0.1 mm; LABART, Munich, Germany) and was allowed to stand for 5 min before counting under a light microscope at 9200 magnification.
2.5. Evaluation of sperm motility and morphology
Sperm progressive motility was evaluated microscopically within 2–4 min of isolation from the cauda epididymis, as previously described [23]. Fluid was obtained from the cauda epididymis with a pipette and diluted to 2 ml with Tris buffer solution. The percentage of motile sperm was evaluated at 9400 magnification. The percentage of morphologically abnormal spermatozoa was determined after staining with eosin–nigrosin stain [24]; a total of 300 sperm were counted on each slide under alight microscope at 9400 magnification.
2.6. Histopathology
Specimens were fixed in 10% neutral buffered formalin, dehydrated in a graded alcohol series, cleared with methyl benzoate and embedded in paraffin wax. Sections of 5 μm were cut and stained with hematoxylin/eosin stain for light microscopic examination [25]. Stained sections were examined by light microscope and photographed using digital camera.
2.7. Statistical analysis
Data are expressed as mean ± SE for all parameters. The data were analyzed using GraphPad Prism data analysis program (GraphPad Software, Inc., San Diego, CA, USA). For multiple comparisons, One-way analysis of variance (ONE-WAY-ANOVA) test.
3. Results
3.1. Biochemical results
Table 1 represents the levels of LPO, GSH, SOD, CAT and testosterone rat the serum of rats. CdCl2 exposure leads to a significant increase in LPO levels. However, GSH, SOD and CAT levels were significantly decreased. Additionally, it was shown that treatment with TQ and l-cysteine improved values of serum LPO, GSH, SOD and CAT and testosterone become similar to control group. It was observed that the mean level of testosterone control group and Cd + l-cysteine were extremely significantly increased (P ≤ 0.001) when compared with CdCl2 group.
Table 1.
LPO, antioxidants and testosterone levels in the experimental groups (Mean ± SE).
| LPO nmol/mg | GSH μg/mg | SOD U/mg | Catalase U/mg | Testosterone Ng/ml | |
|---|---|---|---|---|---|
| Control | 2.48 ± 0.26b | 2.4 ± 0.4a | 0.5 ± 0.05a | 0.04 ± 0.003a | 2.62 ± 0.07a |
| CdCl2 | 4.95 ± 0.41a | 0.81 ± 0.2c | 0.14 ± 0.02c | 0.01 ± 0.003c | 1.16 ± 0.08d |
| Cd + TQ | 2.06 ± 0.26b | 2.8 ± 0.08a | 0.8 ± 0.05b | 0.06 ± 0.008a | 2.40 ± 0.05a |
| Cd + l-cys | 2.76 ± 0.45b | 2.3 ± 0.2a | 0.6 ± 0.05a | 0.04 ± 0.005a | 2.20 ± 0.11b |
Means bearing different superscripts within same column were significantly different (P ≤ 0.001).
3.2. Sperm parameters
Table 2 represents the sperm counts, motility and abnormality in the experimental groups. There was significant decrease in the sperm count in the Cd-treated rats when compared with control. There was no significance difference of sperm count between Cd-treated rats and rats treated with TQ and l-cysteine. Also, sperm motility and sperm abnormalities (small head, large head, double head, double tail and abnormal middle-piece) were significantly impaired in Cd-treated rats and significantly improved in rats treated with TQ and l-cysteine.
Table 2.
Sperm count, motility and incidence of abnormalities in rats semen of the experimental groups.
| Count × (106 ml−1) | Motility (%) | Abnormalities (%) | |
|---|---|---|---|
| Control | 200.00 ± 11.54a | 85.00 ± 2.88a | 21.66 ± 1.66c |
| CdCl2 | 86.66 ± 8.81b | 53.33 ± 3.33d | 50.33 ± 0.88a |
| Cd + TQ | 110.00 ± 5.77b | 75.00 ± 2.89b | 30.00 ± 2.88b |
| Cd + l-cys | 100.00 ± 5.77b | 65.00 ± 2.88c | 35.00 ± 2.88b |
All values are expressed as mean ± SE, different superscripts within same column were significantly different (P ≤ 0.001).
3.3. Histopathology
Histopathological findings of testes, epididymis, seminal vesicle and prostate gland were evaluated by light microscopy. Incidence of lesions in the experimental groups is summarized in (Table 3).
Table 3.
Incidence of histopathological lesions in testes, epididymis, seminal vesicle and prostate of the experimental groups.
| Lesions | Control | CdCl2 | Cd + TQ | Cd + l-Cys |
|---|---|---|---|---|
| Testes: | ||||
| Congestion | – | +++ | – | – |
| Interstitial edema | – | +++ | – | + |
| Degeneration | – | +++ | – | – |
| Intratubular giant cell formation | – | ++ | – | – |
| Epididymis: | ||||
| Congestion | – | +++ | – | ++ |
| Interstitial edema | – | +++ | – | ++ |
| Vacuolation of germinal epithelium | – | ++ | – | – |
| Sloughing of germinal cells | – | +++ | – | – |
| Perivascular lymphocytic infiltration | – | +++ | – | – |
| Proliferation of fibrous tissue | – | ++ | – | – |
| Seminal vesicle: | ||||
| Congestion | – | +++ | – | + |
| Interstitial edema | – | +++ | – | + |
| Hemorrhage | – | ++ | – | – |
| Perivascular lymphocytic infiltration | – | +++ | – | – |
| Prostate: | ||||
| Interstitial edema | – | +++ | + | + |
| Lymphocytic infiltration | – | +++ | – | – |
| Prostatitis | – | ++ | – | – |
| Tubular dilatation | – | ++ | + | ++ |
– no lesions; + lesions found in 1–3 rats; ++ lesions found in 4–6 rats; +++ lesions found in 7–10 rats.
3.3.1. Testicle
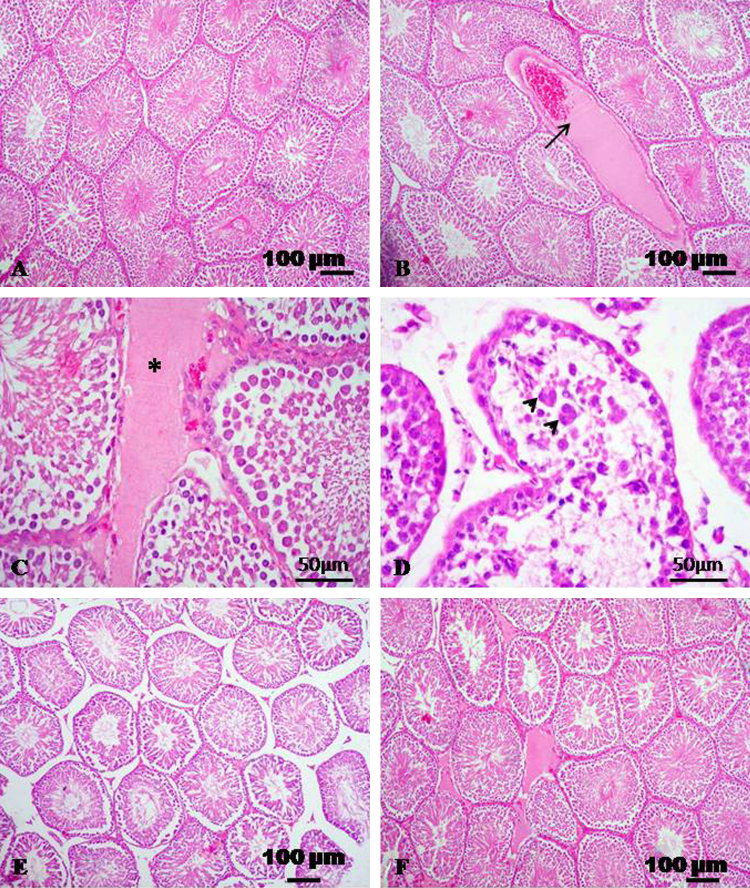
Testicular sections of control rats had normal histoarchitecture (Fig. 1A). Histopathological examination of HE-stained sections revealed that the treatment of male rats received CdCl2 produced vascular and cellular changes. The vascular changes were congestion of the blood vessels with (Fig. 1B) and interstitial edema with faint eosinophilic material (Fig. 1C). The seminiferous tubules suffered from degenerative changes with presence of multinucleated spermatid giant cells in the lumen of seminiferous tubules (Fig. 1D). Examination of testicular sections of CdCl2 + TQ treated group and CdCl2 + l-cysteine treated group showing normal histological appearance (Fig. 1E and F).
Fig. 1.
Representative micrograph of testis stained with HE. (A) Testicular section of control group showing normal histological appearance. (B–D) Testicular sections treated with CdCl2 showing congestion (arrow), interstitial edema (asterisk), degeneration with intratubular giant cell formation (arrowheads). (E) CdCl2 + TQ treated group showing normal histological appearance. (F) CdCl2 + l-cysteine treated group showing normal histological appearance.
3.3.2. Epididymis
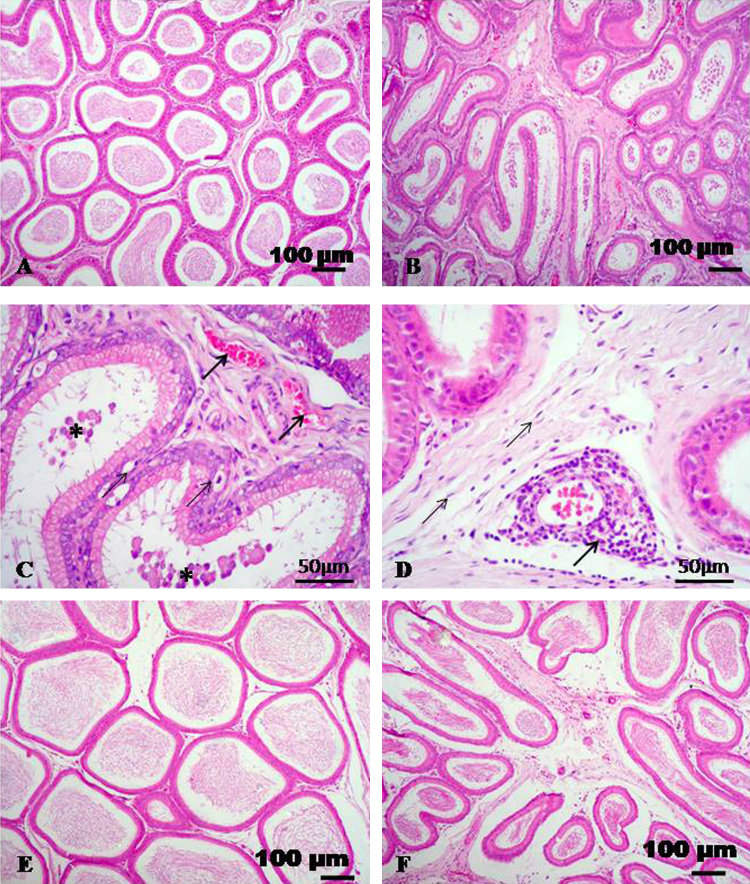
Control rats showed normal epididymal histological architecture with normal sperm density (Fig. 2A). In rats received CdCl2 showed histopathological alterations of the epididymis. These alterations were consisted of low density of spermatozoa, congestion, interstitial edema and fibrosis. Moreover, the epididymal epithelium showed vacuolation and sloughing of the lining epithelium was evident in some ducts (Fig. 2B–D). The group of rats received CdCl2 + TQ showed normal histological appearance with normal sperm density (Fig. 2E). The group of rats received CdCl2 + l-cysteine showed nearly normal histoarchitecture with interstitial edema in some cases (Fig. 2F).
Fig. 2.
Representative micrograph of epididymis stained with HE. (A) Epididymal section of control group showing normal histological appearance. (B and C) Epididymis of rats treated with CdCl2 showing edema, congestion (thick arrows), vacuolation of germinal epithelial cells (thin arrows) and sloughing of some germinal cells (asterisks). (D) Epididymis of rats treated with CdCl2 showing perivascular lymphocytic infiltration (arrow) and proliferation of fibrous tissue (thin arrows). (E) CdCl2 + TQ treated group showing normal histological appearance. (F) CdCl2 + l-cysteine treated group showing nearly normal structure with interstitial edema.
3.3.3. Seminal vesicle
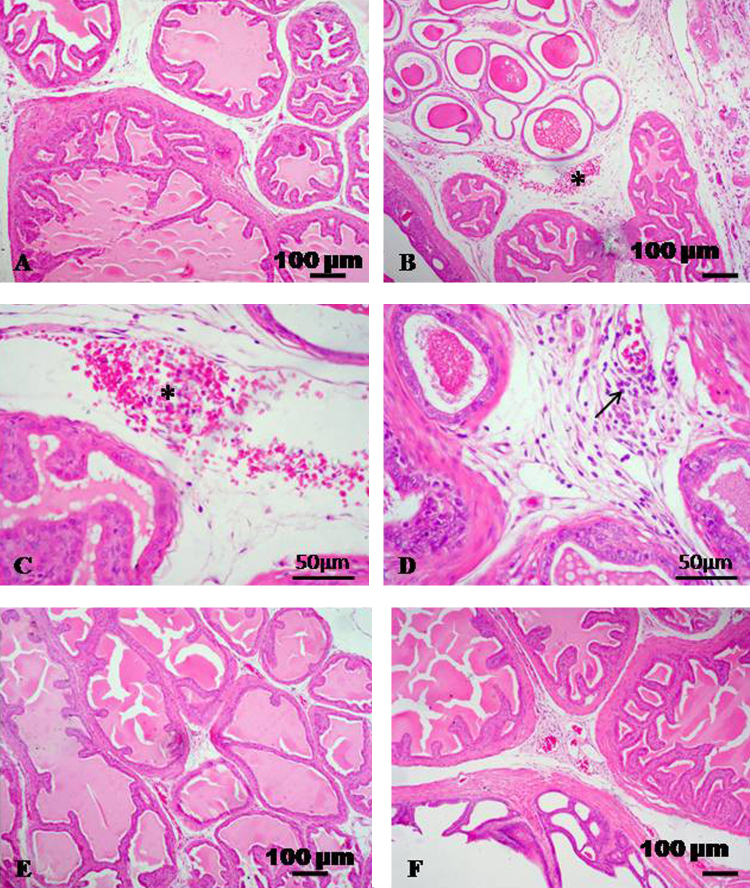
The seminal vesicle of control rats revealed normal histological appearance (Fig. 3A). In rats received CdCl2 showed histopathological alterations of the seminal vesicle. Such alterations were consisted of interstitial edema, congestion, hemorrhage, perivascular lymphocytic infiltration and degeneration of epithelial lining (Fig. 3B–D). The groups of rats received CdCl2 + TQ and CdCl2 + l-cysteine showed normal histological appearance with moderate congestion of some blood vessels (Fig. 3E and F).
Fig. 3.
Representative micrograph of seminal vesicle stained with HE. (A) Seminal vesicle of control group showing normal histological appearance. (B–D) Seminal vesicle treated with CdCl2 showing interstitial edema, congestion, hemorrhage (asterisks) and perivascular lymphocytic infiltration (arrow). (E and F) CdCl2 + TQ treated group and CdCl2 + l-cysteine treated group showing normal histological appearance with moderate congestion of some blood vessels.
3.3.4. Prostate
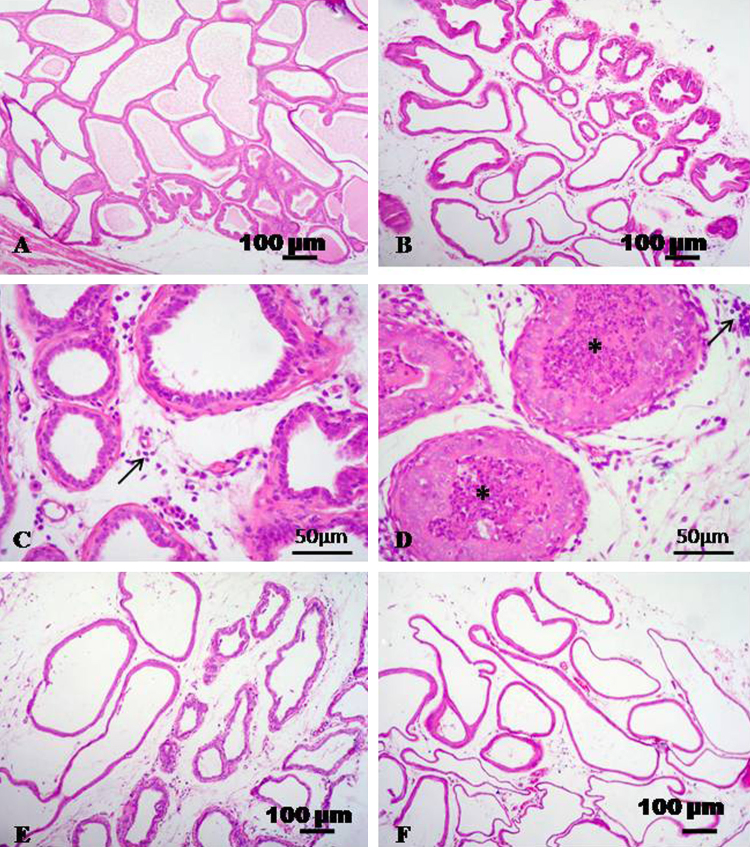
Prostate gland of control rats showed normal histological appearance (Fig. 4A). Prostatitis was obvious in CdCl2 treated group. There were interstitial edema, congestion and lymphocytic infiltration. Some glandular acini appeared necrosed with desquamated epithelium admixed with leucocytes (Fig. 4B–D). Prostate of rats treated with CdCl2 + TQ and CdCl2 + l-cysteine appeared nearly normal with edema and moderate dilatation in some cases (Fig. 4E and F).
Fig. 4.
Representative micrograph of prostate stained with HE. (A) Prostate of control group showing normal histological structure. (B–D) Prostate treated with CdCl2 showing interstitial edema, lymphocytic infiltration (arrows), desquamation of the glandular epithelium and presence of leucocytes (asterisks). (E and F) CdCl2 + TQ treated group and CdCl2 + l-cysteine treated group showing edema and moderate dilatation.
4. Discussion
Cadmium is a heavy metal and classified as an environmental and industrial pollutant that causes oxidative stress in several tissues such as testis. It plays a serious role in male infertility through alteration in histopathology, sperm characteristics and lipid peroxidations [26], [5]. This study was designed to assess the effects of TQ and l-cysteine intake on cadmium cadmium-induced reproductive toxicity in male rats after long-term exposure to low doses of this metal.
The results of this study clearly demonstrated that the mean level of testosterone was decreased significantly in CdCl2 when compared with other groups, while there was no significance between control and Cd + TQ treated group. Moreover, these were accompanied by reduction in the sperm motility and sperm abnormalities in the Cd-treated rats and improvement in TQ and l-cysteine groups. No significance difference of sperm count between Cd-treated rats and rats-treated with TQ and l-cysteine. Similarly, El-Neweshy et al. [6] reported that Cd suppressed sperm count, motility and increased the percentage of sperm abnormalities with a reduction in serum testosterone levels. Siu et al. [7] reported that prolonged exposure to cadmium resulted in declining fertility in man and animals by reducing sperm count, testis function and testosterone synthesis. Previous studies have shown that Cd can modify hormone levels by affecting the hypothalamic–pituitary–testicular axis in different aspects, not only via its effects on Leydig cells. For instance, Cd affected the circadian pattern release of noradrenaline, a regulator of hypothalamus hormone secretion, which resulted in changes in the daily pattern of plasma testosterone and luteinizing hormone (LH) levels [27].
It was supposed that there is a correlation between the histological and oxidative statuses induced by CdCl2. Testis is the major target organ for oxidative stress because of its high content of polyunsaturated membrane lipids [28]. In the present study, it was well recognized that CdCl2 exposure induced LPO via a significant increase in TBARS level and is regarded as an indicator of LPO and leads to irreversible cell damage in testis tissue.
Our results revealed that the mean level of LPO was increased significantly associated with a reduction in antioxidant defense systems that protect tissues against oxidative damage through a decrease in GSH, SOD and CAT levels in CdCl2 treated group. In spite of that, there was significance decrease of LPO values in Cd + TQ treated group when compared with CdCl2 treated group. Similar results obtained by [11], [29], [30], [31] who found that the administration of TQ led to decreased MDA levels in different toxicity studies. Inci et al. [10] also reported that increase in LPO levels and histological damage caused by E. Coli was improved markedly with TQ treatment through its restored antioxidant defense against generation of reactive oxygen species (ROS) in different tissues. Fouda et al. [12] reported that orally administered TQ increased the activities of CAT and GSH-Px enzymes in rats. So in our results, the rats treated with TQ and l-cysteine revealed improvement of oxidants/antioxidant mechanism. Helal [31] attributed these effects of TQ to its anti-inflammatory, anti-oxidant and anti-apoptotic properties. Also, Ahmed et al. [14] reported that the co-administration of cisplastin with antioxidants l-cysteine reduced the effect of CP on LPO, total peroxides, superoxide anion, and antioxidant enzymes.
Our previous study [12], [14] showed that TQ and l-cysteine are potent antioxidative compounds, and because of this property, it strongly protects tissues against the oxidative stress. Also, Zafeer et al. [32] revealed that TQ treatment particularly prevents oxidative stress in hepatic tissue of rat caused by CdCl2, and this result was confirmed by our findings. Our histopathological findings showed that CdCl2 exposure leads to major structural changes in testis tissue compared to the control group. On the other hand, it was determined that TQ treatment alleviated the toxic effects of CdCl2 on testis histology, when given together with CdCl2.
The testis is extremely sensitive to Cd toxicity [7]. In the present study, histopathological findings in the testicle of rats treated with CdCl2 revealed vascular changes in the form of congestion and interstitial edema and degenerative changes with presence of multinucleated spermatid giant cells. Similarly, Li and Heindel [33] reported that in vivo acute exposure to Cd causes blood testes barrier (BTB) disruption, germ cell loss, testicular edema, hemorrhage, necrosis, and sterility in several mammalian species (e.g., rodents, rabbit, dog, calf, stallion). In vitro studies have illustrated that Cd induced damage to testicular cells. Also, Fouda et al. [12] and Obianime and Roberts [34] studied the histopathological effect of Cd on the testes of the rat. These were characterized by vascular congestion, vacuolation, destruction of the germinal epithelial layers, focal necrosis and edema of the seminiferous tubules and reduction of spermatogenesis. In parallel, Monsefi et al. [35] showed a severe necrosis and atrophy in the testis of male mice that received 50 mg/kg of cadmium chloride in 0.5 ml distilled water for 45 days. On the other hand, it was determined that TQ and l-cysteine treatment improved the toxic effects of CdCl2 on testis histology, when given together with CdCl2.
The epididymis of rats received CdCl2 showed congestion, interstitial edema and fibrosis. vacuolation and sloughing of the lining epithelium. Similarly results obtained by Sucheela and Kumar [36] who studied the effect of chronic fluoride toxicity in rabbits. They found that 18 or 29 months of toxicity induced loss of cilia on the epithelial cells lining the lumen of the ductuli efferentes of the caput epididymis. Arabi and Heydarnejad [37] reported that Cd induced membrane impairments, lowered motility, DNA breaks and a decreased rate in the acrosome reaction of bull spermatozoa, leading to sperm dysfunction.
Accessory sex glands such as seminal vesicles and prostate glands require androgen for differentiation, development and maintenance of epithelial cells [38]. Congestion, edema, necrosis and desquamation of some tubuloalveolar glandular epithelial cells of the prostates and the seminal vesicles of CdCl2-treated rats could be attributed to either the decreased serum testosterone level or the CdCl2-induced LPO. Also, decreased prostate secretions may be due to the decreased serum testosterone level. The picture of prostatitis and seminal vesiculitis in rats could be related to immunosuppression [39].
In conclusion, long-term exposure to low doses of cadmium alters the antioxidant enzymatic profile and induced oxidative damage, spermatological damage and histopathological alterations in testes, epididymis and accessory glands. The co-administration with TQ showed beneficial effect of all parameters. On the other hands, l-cysteine showed less improvement especially in the histopathology of rats reproductive organs.
Transparency document
Footnotes
Available online 13 August 2014
References
- 1.Takiguchi M., Yoshihara S. New aspects of cadmium as endocrine disruptor. Environ. Sci. 2006;13:107–116. [PubMed] [Google Scholar]
- 2.Jarup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 3.Acharya U.R., Mishra M., Patro J., Panda M.K. Effect of vitamins C and E on spermatogenesis in mice exposed to cadmium. Reprod. Toxicol. 2008;25:84–88. doi: 10.1016/j.reprotox.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Bu T., Mi Y., Zeng W., Zhang C. Protective effect of quercetin on cadmium-induced oxidative toxicity on germ cells in male mice. Anat. Rec. (Hoboken) 2011;294:520–526. doi: 10.1002/ar.21317. [DOI] [PubMed] [Google Scholar]
- 5.de Souza Predes F., Diamante M.A., Dolder H. Testis response to low doses of cadmium in Wistar rats. Int. J. Exp. Pathol. 2010;91:125–131. doi: 10.1111/j.1365-2613.2009.00692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Neweshy M.S., El-Maddawy Z.K., El-Sayed Y.S. Therapeutic effects of date palm (Phoenix dactylifera L.) pollen extract on cadmium-induced testicular toxicity. Andrologia. 2013;45:369–378. doi: 10.1111/and.12025. [DOI] [PubMed] [Google Scholar]
- 7.Siu E.R., Mruk D.D., Porto C.S., Cheng C.Y. Cadmium induced testicular injury. Toxicol. Appl. Pharmacol. 2009;238:240–249. doi: 10.1016/j.taap.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandya C., Pillai P., Nampoothiri L.P., Bhatt N., Gupta S. Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 9.WHO . Cadmium–environmental aspects. In: Dobson S., editor. Vol. 135. World Health Organization; Geneva, Switzerland: 1992. p. 156. (Environmental Health Criteria). [Google Scholar]
- 10.Inci M., Davarci M., Motor S., Yalcinkaya F.R., Nacar E., Aydin M., Sefil N.K., Zararsız I. Anti-inflammatory and antioxidant activity of thymoquinone in a rat model of acute bacterial prostatitis. Hum. Exp. Toxicol. 2012;18:1–8. doi: 10.1177/0960327112455068. [DOI] [PubMed] [Google Scholar]
- 11.Alenzi F.Q., El-Bolkiny Y.S., Salem M.L. Protective effects of Nigella sativa oil and thymoquinone against toxicity induced by the anticancer drug cyclophosphamide. Br. J. Biomed. Sci. 2010;67:20–28. doi: 10.1080/09674845.2010.11730285. [DOI] [PubMed] [Google Scholar]
- 12.Fouda A.M., Daba M.H., Dahab G.M., Sharaf El-Din O.A. Thymoquinone ameliorates renal oxidative damage and proliferative response induced by mercuric chloride in rats. Basic Clin. Pharmacol. Toxicol. 2008;103:109–118. doi: 10.1111/j.1742-7843.2008.00260.x. [DOI] [PubMed] [Google Scholar]
- 13.Kerksick C., Willoughby D. The antioxidant role of glutathione and N-acetyl cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2005;2:38–44. doi: 10.1186/1550-2783-2-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed E.A., Omar M.O., Abdelghaffar S.K., Ragb S.M.M., Nasser A.Y. The antioxidant activity of vitamin C, DPPD and l-cysteine against cisplatin-induced testicular oxidative damage in rats. Food Chem. Toxicol. 2011;49:1115–1121. doi: 10.1016/j.fct.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Russell L.D., Ettlin R.A., Hikim A.P., Clegg E.D., Russell L.D. The classification and timing of spermatogenesis. In: Russell L.D., Ettlin R.A., Hikim A.P., Clegg E.D., editors. Histological and Histopathological Evaluation of the Testis. Cache River Press; Clearwater: 1990. pp. 41–58. [Google Scholar]
- 16.Radad K., Hassanein K., Al-Shraimb M., Moldzio R., Rausch W. Thymoquinone ameliorates lead-induced brain damage in Sprague Dawley rats. Exp. Toxicol. Pathol. 2014;66:13–17. doi: 10.1016/j.etp.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 17.Latendresse J.R., Warbrittion A.R., Jonassen H., Creasy D.M. Fixation of testes and eyes using a modified Davidson's fluid: comparison with Bouin's fluid and conventional Davidson's fluid. Toxicol. Pathol. 2002;l30:524–533. doi: 10.1080/01926230290105721. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissue by thiobarbaturic acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 20.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 21.Luck H. Catalase. In: bergmer H-U., editor. Methods of Enzematic Analysis. Academic Press; New York: 1963. pp. 885–888. [Google Scholar]
- 22.Yokoi K., Uthus E.O., Nielsen F.H. Nickel deficiency diminishes sperm quantity and movement in rats. Biol. Trace Elem. Res. 2003;93:141–154. doi: 10.1385/BTER:93:1-3:141. [DOI] [PubMed] [Google Scholar]
- 23.Sönmez M., Türk G., Yüce A. The effect of ascorbic acid supplementation on sperm quality, lipid peroxidation and testosterone levels of male Wistar rats. Theriogenology. 2005;63:2063–2072. doi: 10.1016/j.theriogenology.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Evans G., Maxwell M.C. Handling and examination of semen. In: Maxwell W.M.C., editor. Salamon's Artificial Insemination of Sheep and Goats. Butterworths; Sydney, NSW, Australia: 1987. p. p93. [Google Scholar]
- 25.Bancroft T.D., Stevens A., Turner D.R. 4th ed. Churchill; Livingstone, New York: 1996. Theory and Practice of Histological Technique. [Google Scholar]
- 26.El-Demerdash F.M., Yousef M.I., Kedwany F.S., Baghdadi H.H. Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem. Toxicol. 2004;42:1563–1571. doi: 10.1016/j.fct.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Lafuente A., Gonzalez-Carracedo A., Romero A., Cano P., Esquifino A.I. Cadmium exposure differentially modifies the circadian patterns of norepinephrine at the median eminence and plasma LH, FSH and testosterone levels. Toxicol. Lett. 2004;146:175–182. doi: 10.1016/j.toxlet.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Chainy G.B.N., Samantaray S., Samanta L. Testosterone induced changes in testicular antioxidant system. Andrologia. 1997;29:343–349. doi: 10.1111/j.1439-0272.1997.tb00328.x. [DOI] [PubMed] [Google Scholar]
- 29.Ola-Mudathir K.F., Suru S.M., Fafunso M.A., Obioha U.E., Faremi T.Y. Protective roles of onion and garlic extracts on cadmium induced changes in sperm characteristics and testicular oxidative damage in rats. Food Chem. Toxicol. 2008;46:3604–3611. doi: 10.1016/j.fct.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Ognjanović B.I., Markovic S.D., Dordević N.Z., Trbojević I.S., Stajn A.S., Saiić Z.S. Cadmium-induced lipid peroxidation and changes in antioxidant defense system in the rat testes: protective role of coenzyme Q10 and vitamin E. Reprod. Toxicol. 2010;29:191–197. doi: 10.1016/j.reprotox.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 31.Helal G.K. Thymoquinone supplementation ameliorates acute endotoxemia-induced liver dysfunction in rats. Pak. J. Pharm. Sci. 2010;23:131–137. [PubMed] [Google Scholar]
- 32.Zafeer M.F., Waseem M., Chaudhary S., Parvez S. Cadmium-induced hepatotoxicity and its abrogation by thymoquinone. J. Biochem. Mol. Toxicol. 2012;26:199–205. doi: 10.1002/jbt.21402. [DOI] [PubMed] [Google Scholar]
- 33.Li L., Heindel J. Sertoli cell toxicants. In: Reproductive, Korach K., editors. Reproductive and Developmental Toxicology. Marcel Dekker; New York: 1998. pp. 655–691. [Google Scholar]
- 34.Obianime A.W., Roberts I.I. Antioxidants, cadmium-induced toxicity, serum biochemical and the histological abnormalities of the kidney and testes of the male Wistar rats. Niger. J. Physiol. Sci. 2009;24:177–185. doi: 10.4314/njps.v24i2.52910. [DOI] [PubMed] [Google Scholar]
- 35.Monsefi M., Alaee S., Moradshahi A., Rohani L. Cadmium-induced infertility in male mice. Environ. Toxicol. 2010;25:94–102. doi: 10.1002/tox.20468. [DOI] [PubMed] [Google Scholar]
- 36.Sucheela A.K., Kumar A. A study of the effect of high concentration of fluoride on the reproductive organs of male rabbits, using light and scanning electron microscopy. J. Reprod. Fertil. 1991;92:353–360. doi: 10.1530/jrf.0.0920353. [DOI] [PubMed] [Google Scholar]
- 37.Arabi M., Heydarnejad M.S. Mechanism of the dysfunction of the bull spermatozoa treated with cadmium. Zhonghua Nan. KeXue. 2007;13:291–296. [PubMed] [Google Scholar]
- 38.Ono Y., Suzuki K., Kashiwagi B., Shibata Y., Ito K., Fukabori Y., Yamakana H. Role of androgen on blood flow and capillary structure in rat seminal vesicles. Tohoku. J. Exp. Med. 2004;202:193–201. doi: 10.1620/tjem.202.193. [DOI] [PubMed] [Google Scholar]
- 39.Creasy D., Bube A., de Rijk E., Kandori H., Kuwahara M., Masson R., Nolte T., Reams R., Regan K., Rehm S., Rogerson P., Whitney K. Proliferative and nonproliferative lesions of the rat and mouse male reproductive system. Toxicol. Pathol. 2012;40:40S–121S. doi: 10.1177/0192623312454337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.