Abstract
Ginger [Zingiber officinale Roscoe (Zingiberaceae)] and turmeric [Curcuma longa Linn (Zingiberaceae)] rhizomes have been reportedly used in folk medicine for the treatment of hypertension. However, the prevention of its complication such as male infertility remains unexplored. Hence, the aim of the present study was to investigate the preventive effects of ginger and turmeric rhizomes on some biomarkers of male reproductive function in L-NAME-induced hypertensive rats. Male Wistar rats were divided into seven groups (n = 10): normotensive control rats; induced (L-NAME hypertensive) rats; hypertensive rats treated with atenolol (10 mg/kg/day); normotensive and hypertensive rats treated with 4% supplementation of turmeric or ginger, respectively. After 14 days of pre-treatment, the animals were induced with hypertension by oral administration of L-NAME (40 mg/kg/day). The results revealed significant decrease in serum total testosterone and epididymal sperm progressive motility without affecting sperm viability in hypertensive rats. Moreover, increased oxidative stress in the testes and epididymides of hypertensive rats was evidenced by significant decrease in total and non-protein thiol levels, glutathione S-transferase (GST) activity with concomitant increase in 2′,7′-dichlorofluorescein (DFCH) oxidation and thiobarbituric acid reactive substances (TBARS) production. Similarly, decreased testicular and epididymal NO level with concomitant elevation in arginase activity was observed in hypertensive rats. However, dietary supplementation with turmeric or ginger efficiently prevented these alterations in biomarkers of reproductive function in hypertensive rats. The inhibition of arginase activity and increase in NO and testosterone levels by both rhizomes could suggest possible mechanism of action for the prevention of male infertility in hypertension. Therefore, both rhizomes could be harnessed as functional foods to prevent hypertension-mediated male reproductive dysfunction.
Keywords: Ginger, Turmeric, Hypertension, Male fertility, L-NAME
1. Introduction
Hypertension is a chronic medical condition in which the blood pressure (BP) in the arteries is elevated [3]. It is considered a major public health epidemic and affects more than 25% of the general population, with its prevalence increasing with age [64]. Evidence suggests that hypertension is associated with an impairment of male sexual function [26] but their pathophysiological pathways are yet to be clearly elucidated.
The association of hypertension with increased incidence of male sexual dysfunction includes problems related to libido, erection and ejaculation [67], [25]. In addition, it has also been linked to cause male infertility via a decrease in blood flow to the testis [45], [72]. A reduction of blood flow to various vital organs of the body as a result of vasoconstriction of the arterial vessels is one of the principal manisfestation of hypertension.
Several studies have reported induction of hypertension in rats by oral administration of N-nitro-l-arginine methyl ester hydrochloride (an inhibitor of nitric oxide biosynthesis) [42], [31], [30], [22], [21]. Nitric oxide (NO) deficiency has been suggested as a contributory factor in pre-eclampsia [29], [60], while the vasodilatory properties of NO are essential for cavernosal smooth muscle action in achieving penile erection [20]. NO can act as a free radical scavenger, inactivating and even inhibiting production of superoxide anions [10], [46], [24] which cause lipid peroxidation, a process which leads to functional impairment of spermatozoa [39]. This suggest a beneficial role for NO in the male reproductive system [2].
The inhibition of NO synthesis (by L-NAME administration), can result in a very low concentration of NO-mediated vasodilatation, an increase in vasoconstriction, and subsequently an increase in systemic vascular resistance, which contributes to BP elevation and its complications [19]. The activity of endothelial nitric oxide synthase (eNOS) is competitively inhibited by arginase, which catalyzes the hydrolysis of l-arginine to form l-ornithine and urea, thereby making NO unavailable. Arginase reciprocally regulates eNOS and NO production by competing for l-arginine [66]. In various pathological disorders, arginase has been shown to regulate vascular cell functions primarily through impairment of NO production [66]. Therefore, suppressing high activity of arginase will favor eNOS which in turn leads to increase production of NO. Recent findings have shown phenolic phytochemicals to have promising potential as well in mitigating this process [43].
Zingiber officinale (Ginger, Family Zingiberaceae) roots are commonly used as culinary spice and used medicinally for its antioxidant [59]. It is a plant that is used in folk medicine from south-east Asia, and also in Africa, China, India and Jamaica, the India also cultivate the rhizomes for medicinal purpose [59]. The important active compounds of the ginger root are thought to be volatile oils and pungent phenol compounds such as gingerols, shogaols, zingerone, and gingerols [59]. Recently, ginger rhizomes are used in traditional medicine as therapy against several cardiovascular diseases such as hypertension [33]. It has been reported that ginger lowers blood pressure (BP) through blockade of voltage-dependent calcium channels [32]. Zancan et al. [70] reported the roots and leaves of ginger exhibited antioxidant activity. In addition, Yang et al. [73] concluded that antioxidants can protect sperm DNA and other important molecules from cell damage induced by oxidation, improve sperm quality and increase reproductive efficiency of men. In rats, Khaki et al. [74] reported that ginger has a protective effect against DNA damage induced by H2O2 and may be promising in enhancing healthy sperm parameters. Traditionally, ginger rhizome was used in Iran for enhancing male sexuality, regulating female menstrual cycle, and also reducing painful menstrual periods [36].
Other notable member of this plant family (Zingiberaceae) is turmeric otherwise called red ginger (Curcuma longa). It is a rhizomatous herbaceous perennial plant, in the ginger family, employed as a dye source and food colorant due to its characteristic yellow color [23]. Turmeric is one of the main ingredients for curry powder, and used as an alternative to medicine and can be made into a drink to treat colds and stomach complaints [23]. Like ginger, it is cultivated for used in folk medicine from south-east Asia, and also in Africa, China, India and Jamaica [59]. The curcuminoids compound are the major phytochemicals of the turmeric responsible for the characteristic yellow color and has been investigated to containing biological activities, such as antioxidant, anti-hypertensive, anti-inflammatory, anticarcinogenic, thrombus suppressive, hypoglycaemic and antiarthritic properties [13], [41], [5], [7]. In folk medicine, turmeric has been used in lowering blood pressure and as tonic and blood purifier [65]. Traditional Indian medicine claims the use of its powder against biliary disorders, cough, diabetic wounds, hepatic disorder and rheumatism [12]. [56] observed that curcumin is capable of scavenging oxygen free radicals such as superoxide anions and hydroxyl radicals, which are the initiators of lipid peroxidation.
Both rhizomes are useful in folk medicine against hypertension and are considered safe herbal medicines because no significant side effect has yet been described using 2–4% dietary supplementation [33], [8], [9] and their effect on NO production has been published [9].
Thus, considering the association of hypertension with male infertility, the ethnopharmacological actions of both rhizomes with limited information on their promising potential in improving male fertility in hypertensive individuals, the aim of the present study therefore was to evaluate the preventive effect of dietary supplementation of ginger and turmeric, on some biomarkers of reproductive function in L-NAME-induced hypertensive male rats.
2. Materials and methods
2.1. Chemicals
The substrate l-arginine, as well as urea, N-(l-naphthyl)ethylene-diamine dihydrochloride, Tris–HCl buffer, HEPES, L-NAME, and Coomassie brilliant blue G were obtained from Sigma Chemical Co. (St. Louis, MO, USA) and bovine serum albumin, nitrate, vanadium chloride (VCl3) from Reagen (Colombo, PR, Brazil). All the other chemicals used in this experiment were of the analytical grade, while the water was glass distilled. All the kits used for the bioassay were sourced from Randox Laboratories Ltd. (Crumlin, Northern Ireland, United Kingdom).
2.2. Sample collection
The fresh samples of ginger (Z. officinale) and turmeric (C. longa) rhizomes were obtained from a farmland at Akure metropolis, Nigeria. Authentication of the plants was carried out at the Department of Biology, Federal University of Technology, Akure, Nigeria.
2.3. Animals
Adult male Wistar rats (200–300 g) from the Central Animal House of the Federal University of Santa Maria were used in this experiment. The animals were maintained at a constant temperature (22 ± 2 °C) on a 12 h light/dark cycle with free access to food and water. The animals were used according to the guidelines of the National Council for Animal Experiments Control (CONCEA) and are in accordance with international guidelines. The research project was approved by the ethics committee of the Federal University of Santa Maria—Brazil by the number 23/2011.
2.4. Experimental protocol
The rats were acclimatized for two weeks and randomly divided into seven groups of ten animals each (n = 10). Group 1: (Control) serve as the normotensive control group placed on a basal diet; Group 2: (Induced) serve as the hypertensive (L-NAME) group placed on a basal diet plus L-NAME; Group 3: (L-NAME + AT) serve as the positive control placed on a basal diet plus L-NAME plus atenolol (10 mg/kg/day); Group 4: (RG Normal) serve as the normotensive diet group placed on a diet supplemented with (4%); Group 5: (RG + L-NAME) serve as the hypertensive diet group placed on a diet supplemented with (4%) plus L-NAME; Group 6: (WG Normal) serve as the normotensive diet group placed on a diet supplemented with ginger (4%); and Group 7: (WG + L-NAME) serve as the hypertensive diet group placed on a diet supplemented with ginger (4%) plus L-NAME. The rats were placed on their respective diet for two weeks before induction of hypertension (Table 1). Daily feed intake was monitored and body weight was taken both at the beginning and at end of the experiment. In the hypertensive groups, hypertension was induced by the oral administration of the nitric oxide synthase (NOS) inhibitor L-NAME (40 mg/kg/day) by gavage for ten days [31]. In the normotensive groups, the animals received water by gavage throughout the experiment. All the animals were sacrificed 24 h after the last treatment under ketamine and xylazine anesthesia.
Table 1.
Diet formulation for basal and supplemented diets for control and test groups.
| Treatment | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Gourp 6 | Group 7 |
|---|---|---|---|---|---|---|---|
| Skimmed milk | 39.4 | 39.4 | 39.4 | 39.4 | 39.4 | 39.4 | 39.4 |
| Oil | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 | 10.0 |
| Vitamin premix | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 |
| Corn starch | 46.6 | 46.6 | 46.6 | 42.6 | 42.6 | 42.6 | 42.6 |
| Ginger | – | – | – | 4.0 | 4.0 | 4.0 | 4.0 |
| Total | 100 g | 100 g | 100 g | 100 g | 100 g | 100 g | 100 g |
Note: Skimmed milk = 32% protein; The vitamin premix (mg or IU/g) h was the following composition; 3200 IU vitamin A, 600 IU vitamin D3, 2.8 mg vitamin E, 0.6 mg vitamin K3, 0.8 mg vitamin B1, 1 mg vitamin B2, 6 mg niacin, 2.2 mg pantothenic acid, 0.8 mg vitamin B6, 0.004 mg vitamin B12, 0.2 mg folic acid, 0.1 mg biotin H2, 70 mg choline chloride, 0.08 mg cobalt, 1.2 mg copper, 0.4 mg iodine, 8.4 mg iron, 16 mg manganese, 0.08 mg selenium, 12.4 mg zinc, 0.5 mg antioxidant.
Group 1: (Control) serve as the normotensive control group placed on a basal diet; Group 2: (Induced) serve as the hypertensive (L-NAME) group placed on a basal diet plus L-NAME; Group 3: (L-NAME + AT) serve as the positive control placed on a basal diet plus L-NAME plus atenolol (antihypertensive drug); Group 4: (RG Normal) serve as the normotensive diet group placed on a diet supplemented with turmeric (4%); Group 5: (RG + L-NAME) serve as the hypertensive diet group placed on a diet supplemented with turmeric (4%) plus L-NAME; Group 6: (WG Normal) serve as the normotensive diet group placed on a diet supplemented with ginger (4%); and Group 7: (WG + L-NAME) serve as the hypertensive diet group placed on a diet supplemented with ginger (4%) plus L-NAME.
2.5. Diet formulation
The diets were freshly formulated according to a modified method of Akinyemi et al. [7] (Table 1).
2.6. Hemodynamic parameter determination
In all rats, systolic blood pressure (SBP) was measured in awaken animals, by tail-cuff plethysmography (Kent Scientific; RTBP1001 Rat Tail Blood Pressure System for rats and mice, Litchfield, USA). Rats were conditioned with the apparatus before measurements were taken. SBP was recorded at the end of experiment (last treatment week).
2.7. Sperm motility and viability assays
The sperm were collected immediately after a rat was sacrificed. Briefly, epididymal sperm was obtained by cutting the epididymis with surgical blades and released onto a sterile clean glass slide. The sperm was subsequently diluted with 2.9% sodium citrate dehydrate solution, mixed thoroughly and covered with a 24 × 24 mm coverslip before examination under a phase contrast microscope at 200× magnification to evaluate the sperm progressive motility. The data were expressed as percentage of sperm progressive motility. Sperm viability was determined by staining with 1% eosin and 5% nigrosine in 3% sodium citrate dehydrate solution according to established procedures [68], [4].
2.8. Serum testosterone
The serum total testosterone level was measured by ELISA method using DRG Elisa testosterone kit (ELISA EIA-1559, 96 Wells kit, DRG Instruments, GmbH, Marburg, Germany) according to the standard protocol supplied by the kit manufacturer.
2.9. Measurement of nitric oxide (NO)
NO content in testes and epididymis supernatant was estimated in a medium containing 400 mL of 2% vanadium chloride (VCl3) in 5% HCl, 200 mL of 0.1% N-(l-naphthyl)ethylene-diamine dihydrochloride, 200 mL of 2% sulfanilamide (in 5% HCl). After incubating at 37 °C for 60 min, nitrite levels, which corresponds to an estimative of levels of NO, were determined spectrophotometrically at 540 nm, based on the reduction of nitrate to nitrite by VCl3 [48]. Testes and epididymis nitrite and nitrate levels were expressed as nanomole of NO/milligram of protein.
2.10. Arginase activity assay
The arginase activity of testes and epididymis from normotensive and hypertensive rats were assay as described by Mendez et al. [47]. Briefly, tissue lysate (50 μL) was added into 75 μL of Tris–HCl (50 mmol/L, pH 7.5) containing 10 mmol/L MnCl2. Heating the lysate at 55–60 °C for 10 min activated arginase. The hydrolysis reaction of l-arginine by arginase was performed by incubating the mixture containing activated arginase with 50 μL of l-arginine (0.5 mol/L, pH 9.7) at 37 °C for 1 h and was stopped by adding 400 μL of the acid solution mixture (H2SO4:H3PO4:H2O = 1:3:7). For calorimetric determination of urea, α-isonitrosopropiophenone (25 μL, 9% in absolute ethanol) was then added and the mixture was heated at 100 °C for 45 min. After placing the sample in the dark for 10 min at room temperature, the urea concentration was determined spectrophotometrically by the absorbance at 550 nm measured with a microplate reader. The amount of urea produced, after normalization with protein, was used as an index for arginase activity.
2.11. Estimation of antioxidant status
The right testes and epididymis of each rat were homogenized in 50 mM Tris–HCl buffer (pH 7.4). The resulting homogenate was centrifuged at 10,000 × g for 15 min at 4 °C and the supernatant was subsequently collected for estimation of antioxidant status. Protein was measured by the Coomassie blue method according to [18] using serum albumin as standard.
2.11.1. Determination of glutathione-S-transferase activity
Glutathione-S-transferase activity was determined according to the method of [35] using 1-chloro-2,4-dinitrobenzene (CDNB) as a substrate. The assay reaction mixture consisted of 270 μL of a solution containing (20 mL of 0.25 M potassium phosphate buffer, pH 7.0, 10.5 mL of distilled water, and 500 μL of 0.1 M GSH at 25 °C), 20 μL of sample (1:50 dilution), and 10 μL of 25 mM CDNB. The reaction was monitored for 5 min (30 s intervals) at 340 nm in a SpectraMax plate reader (Molecular Devices, CA, USA) and the data were expressed as μmol/min/mg protein using the molar extinction coefficient (ϵ) of 9.6 mM−1 cm−1 for CDNB conjugate.
2.11.2. Total thiol (T-SH) determination
Total thiol content was determined according to the method previously described by [27]. Briefly, the reaction mixture consisted 40 μL of testicular or epididymal homogenate, 10 μL of 10 mM DTNB and 0.1 M potassium phosphate buffer (pH 7.4) in a final volume of 200 μL. The mixture was incubated for 30 min at ambient temperature and then read the absorbance at 412 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). A standard curve was plotted for each measurement using cysteine as a standard and the results expressed as μmol/mg protein.
2.11.3. Determination of non-protein thiols (NPSH)
NPSH levels were determined by the method of [27]. Briefly, an aliquot of testicular or epididymal homogenate was mixed (1:1) with 10% trichloroacetic acid. Subsequent to precipitation of protein, the resulting solution was centrifuged at 10,000 × g for 5 min at 4 °C and the free —SH groups were determined in the supernatant. The reaction mixture consisting 50 μL of sample, 450 μL phosphate buffer and 1.5 mL of 0.1 mM of 5′,5′-dithiobis 2-nitro benzoic acid was incubated for 10 min at 37 °C. The absorbance was measured at 412 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). NPSH levels were expressed as μmol/mg of protein.
2.11.4. Reactive oxygen species (ROS) detection
ROS production was quantified by the 2′,7′-dichlorodihydrofluorescin diacetate (DCFH-DA) method based on the ROS-dependent oxidation of DCFH-DA to DCF [71], [44]. Briefly, 50 μL of testes and epididymides homogenates, Tris–HCl buffer (10 mM; pH 7.4) and DCFH-DA solution at final concentration of 50 μM were incubated in the dark for 30 min to allow the probe to be incorporated into any membrane-bound vesicles, and the diacetate groups cleaved by esterases. Fluorescence of the samples was monitored at an excitation wavelength of 488 nm and an emission wavelength of 525 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). Background fluorescence was corrected by inclusion of parallel blanks. DCF levels were expressed as percentage of control.
2.11.5. Lipid peroxidation
Lipid peroxidation was determined as the formation of thiobarbituric acid reactive substances (TBARS) during an acid-heating reaction according to previously published study [54]. Briefly, the reaction mixture consisting 200 μL of testes and epididymides homogenates or standard (0.03 mM MDA), 200 μL of 8.1% sodium dodecyl sulfate (SDS), 500 μL of 0.8% TBA and 500 μL of acetic acid solution (2.5 M HCl, pH 3.4) was heated at 95 °C for 1 h. The absorbance was measured at 532 nm using a SpectraMax plate reader (Molecular Devices, CA, USA). TBARS tissue levels were expressed as μmol MDA/mg of protein.
2.12. Statistical analysis
The statistical analysis used was one-way ANOVA, followed by Duncan’s multiple range tests, p < 0.05 was considered to represent a significant difference in both analyses used. All data were expressed as mean ± S.E.M.
3. Results
3.1. Systolic blood pressure
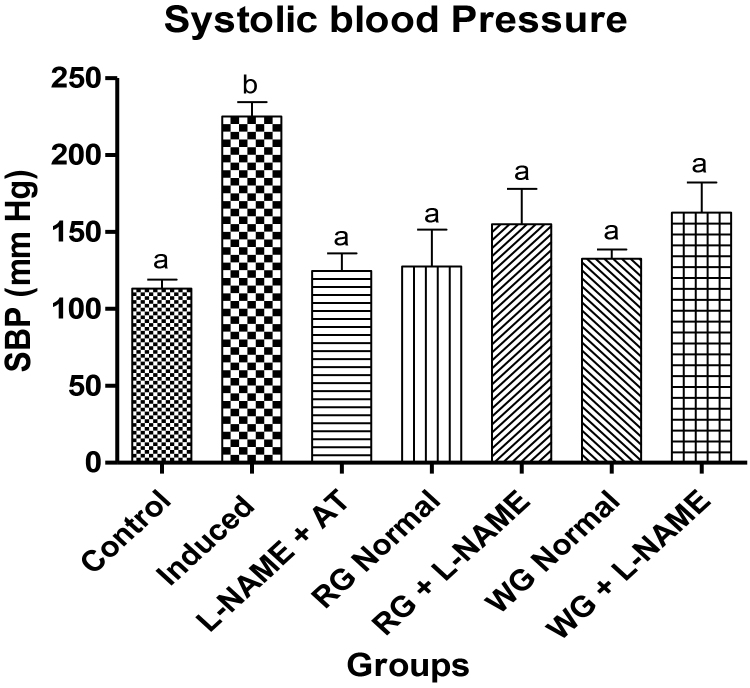
In this study, the oral administration of L-NAME by gavage was associated with a significant rise in the final systolic blood pressure (SBP) when compared with the normotensive control rats, validating the hypertensive model. However, we could observe that dietary supplementation of both turmeric and ginger as well as anti-hypertensive drug (atenolol) clearly possesses hypotensive effect, causing a significant reduction of SBP in the L-NAME-induced hypertensive rats (Fig. 1).
Fig. 1.
Effects of dietary supplementation of turmeric and ginger on the final systolic blood pressure (SBP) measurements in control and L-NAME-induced hypertensive rats. Data are presented as mean + SEM (n = 10). Bars with different letters are statistically different (p < 0.05). Control: Normotensive control rats placed on basal diet; Induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
3.2. Body weight, absolute and relative organ weights
The body weight, absolute and relative organ weights of control and L-NAME exposed rats are presented in Table 2. Following induction of hypertension with L-NAME, there was a significant decrease in the body weight of induced group (hypertensive rats) whereas no significant different was observed in testes and epididymides weights when compare to the control. However, pre-treatment with dietary supplementation of both rhizomes as well as anti-hypertensive drug (atenolol) prevented a significant decrease in the body weight and did not alter testes and epididymides weights.
Table 2.
Body weight, absolute and relative organ weights of L-NAME induced hypertensive rats treated with dietary supplementation with red and white ginger.
| Treatment groups | Final body weight (g) | Absolute testis weight (g) | Absolute epididymis weight (g) | Relative testis weight (g) | Relative epididymis weight (g) |
|---|---|---|---|---|---|
| Control | 365.0 ± 31.6a | 1.68 ± 0.13a | 0.27 ± 0.02a | 0.50 ± 0.06a | 0.08 ± 0.006a |
| L-NAME | 345.6 ± 32.9b | 1.51 ± 0.12a | 0.26 ± 0.02a | 0.45 ± 0.06a | 0.07 ± 0.01a |
| L-NAME + AT | 373.7 ± 11.9a | 1.56 ± 0.09a | 0.26 ± 0.02a | 0.42 ± 0.03a | 0.07 ± 0.007a |
| RG Normal | 363.3 ± 16.9a | 1.76 ± 0.05a | 0.30 ± 0.03a | 0.48 ± 0.03a | 0.08 ± 0.008c |
| RG + L-NAME | 352.0 ± 20.9a | 1.88 ± 0.10a | 0.32 ± 0.02a | 0.52 ± 0.05a | 0.09 ± 0.006a |
| WG Normal | 355.3 ± 23.3a | 1.74 ± 0.17a | 0.25 ± 0.03a | 0.49 ± 0.04a | 0.07 ± 0.007a |
| WG + L-NAME | 363.6 ± 19.7a | 1.69 ± 0.11a | 0.27 ± 0.03a | 0.46 ± 0.04a | 0.07 ± 0.01a |
The results are presented as mean ± SEM (n = 10). Values with the same superscript letter on the same column are not significantly different (p < 0.05).
Control: Normotensive control rats placed on basal diet; induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
3.3. Sperm progressive motility and viability
The motility of epididymal sperm in the hypertensive rats decreased significantly as compared to the control (normotensive rats). However, pre-treatment with dietary supplementation of both turmeric and ginger rhizomes and atenolol caused a significant recovery in the sperm motility when compared with the L-NAME-induced hypertensive rats (Table 3). However, sperm viability was not affected in all treatment groups.
Table 3.
Sperm progressive motility, sperm viability and testosterone level in L-NAME induced hypertensive rats treated with dietary supplementation with red and white ginger.
| Treatment groups | Sperm motility (%) | Sperm viability (%) | Testosterone (ng/dl) |
|---|---|---|---|
| Control | 70.0 ± 6.3a | 82.3 ± 18.2a | 103.7 ± 10.4a |
| L-NAME | 43.3 ± 8.2b | 65.3 ± 2.9a | 34.8 ± 6.2b |
| L-NAME + AT | 60.0 ± 16.2a | 75.0 ± 16.6a | 123.1 ± 6.1a |
| RG Normal | 56.7 ± 8.6a | 75.0 ± 16.7a | 232.9 ± 13.0c |
| RG + L-NAME | 60.0 ± 11.9a | 66.7 ± 16.7a | 73.8 ± 5.4a |
| WG Normal | 56.7 ± 11.7a | 75.0 ± 16.7a | 135.4 ± 6.7a |
| WG + L-NAME | 57.5 ± 4.3a | 80.0 ± 16.7a | 126.8 ± 9.2a |
The results are presented as mean ± SEM (n = 10). Values with the same superscript letter on the same column are not significantly different (p < 0.05).
Control: Normotensive control rats placed on basal diet; Induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
3.4. Testosterone concentration, Nitric oxide (NO) level and arginase activity
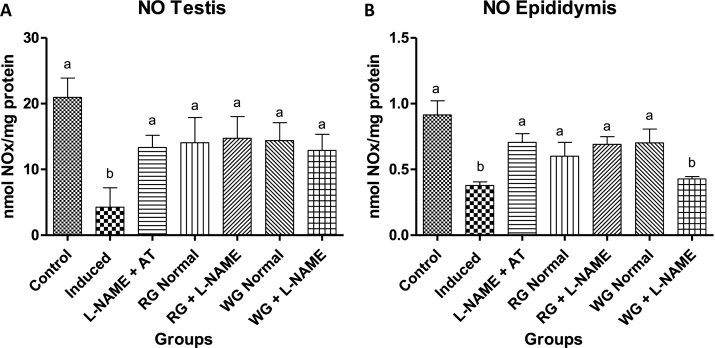
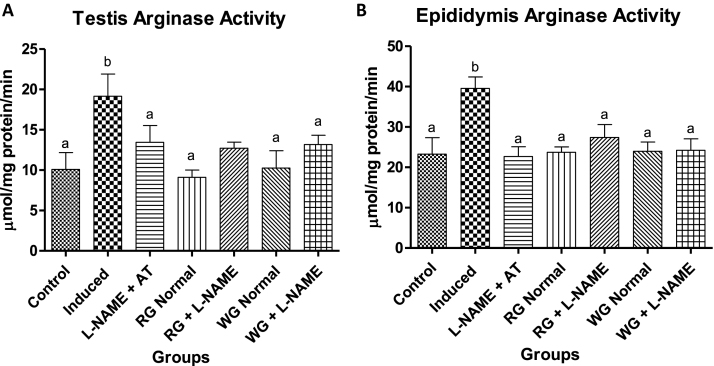
Serum testosterone concentration significantly decreased in L-NAME hypertensive animals. However, dietary supplementation with turmeric and ginger significantly increased the testosterone level when compared with the hypertensive group (Table 2). Nitric oxide (NO) level in the testes and epididymis were decreased in induced group (hypertensive rats) when compared with the control (normotensive) group. In the diet-supplemented hypertensive group the levels of NO were clearly elevated compared to the induced group (hypertensive rats) but were not significantly different from the control (normotensive animals) as presented in Fig. 2. Testiscular and epididymal arginase activity of hypertensive rats increased significantly when compared to the normotensive control rats (Fig. 3). However, pre-treatment with dietary supplementation of ginger and turmeric rhizomes as well as positive control drug caused a significant decrease in the arginase activity when compared with induced group (hypertensive rats).
Fig. 2.
Effects of dietary supplementation of turmeric and ginger on the testicular and epididymal nitric oxide (NO) level in control and L-NAME-induced hypertensive rats. Data are presented as mean + SEM (n = 10). Bars with different letters are statistically different (p < 0.05). Control: Normotensive control rats placed on basal diet; Induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
Fig. 3.
Effects of dietary supplementation of turmeric and ginger on the testicular and epididymal arginase activity in control and L-NAME-induced hypertensive rats. Data are presented as mean + SEM (n = 10). Bars with different letters are statistically different (p < 0.05). Control: Normotensive control rats placed on basal diet; Induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
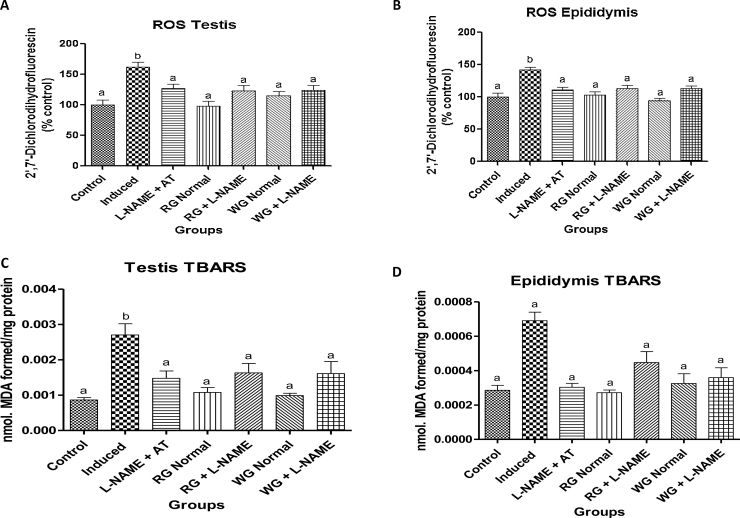
3.5. Testicular and epipidymal antioxidant status
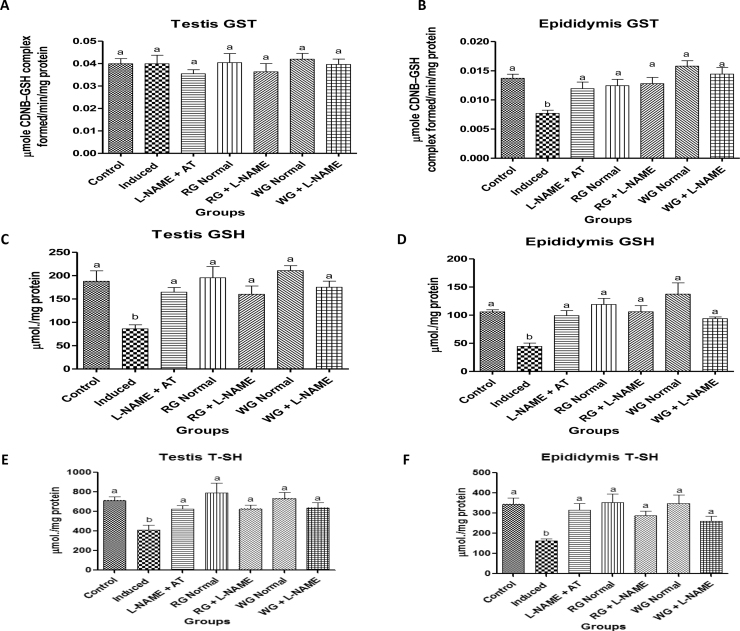
The antioxidant status of the testes and epididymis in normotensive and L-NAME induced hypertensive rats is presented in Fig. 4, Fig. 5. Oral administration of L-NAME drug resulted in a significant decrease in the GST activities as well as in GSH and T-SHs levels with concomitant elevation in the ROS and TBARS production in the testes and epididymis of L-NAME-induced hypertensive rats when compared with control (normotensive rats). However, pre-treatment with dietary supplementation of turmeric and ginger as well as positive control drug caused a significant increase in the GST activities as well as in GSH and T-SHs levels with concomitant decrease in the ROS and TBARS production when compared with hypertensive rats.
Fig. 4.
Effects of dietary supplementation of turmeric and ginger on the testicular and epididymal glutathione S-transferase (GST) activity, total thiol (T-SHs) and non-protein thiol (NPSH) or reduced glutathione (GSH) level in control and L-NAME-induced hypertensive rats. Data are presented as mean + SEM (n = 10). Bars with different letters are statistically different (p < 0.05). Control: Normotensive control rats placed on basal diet; Induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
Fig. 5.
Effects of dietary supplementation of turmeric and ginger on the testicular and epididymal reactive oxygen species (ROS) and thiobarbituric acid reactive substances (TBARS) level in control and L-NAME-induced hypertensive rats. Data are presented as mean + SEM (n = 10). Bars with different letters are statistically different (p < 0.05). Control: Normotensive control rats placed on basal diet; Induced: Hypertensive rats placed on basal diet; L-NAME + AT: Hypertensive rats placed on basal diet + atenolol (10 mg/kg/day); RG Control: Normotensive rats placed on basal diet supplemented with 4% turmeric; RG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% turmeric; WG Control: Normotensive rats placed on basal diet supplemented with 4% ginger; WG + L-NAME: Hypertensive rats placed on basal diet supplemented with 4% ginger.
4. Discussion
In the present study, we observed a significant rise in systolic blood pressure after treatment with L-NAME (40 mg/kg bwt/day) by oral gavage. This result is in agreement with previously described studied by Balbinott et al. [16]. However, dietary supplementation with ginger and turmeric as well as treatment with a positive control drug (atenolol) caused a significant reduction of SBP in the hypertensive rats. This clearly indicates that both ginger varieties possess hypotensive effect. This is in agreement with [32], where they reported hypotensive effect of aqueous extract of ginger in normotensive rats. The association of hypertension with increased incidence of male infertility has been reported [26], [45], [72], [15] but their pathophysiological pathways are yet to be clearly elucidated. The marked decrease in sperm motility and sperm count with concomitant elevated sperm abnormalities observed in the present study indicates an adverse effect of oxidative damage on male reproductive function in vivo as a result of oral administration of L-NAME drug to induced hypertension. The chronic inhibition of NO can affect sperm function and hence, availability of NO is an essential mediator in the male reproductive tracts [2]. Also, NO can act as a free radical scavenger, inactivating and even inhibiting production of superoxide anions [10], [46], [24] which cause lipid peroxidation, a process which leads to functional impairment of spermatozoa [39].
The balance between antioxidant defense system and ROS generation in the male reproductive system is required to maintain the regulation of normal sperm function/fertility [6], [14]. Moreover, we observed an imbalance between antioxidant defense system and ROS generation in testes and epididymis of hypertensive male rats. This clearly is an indication of oxidative stress which has been linked to cause male infertility [14]. There was a significant elevation in the testicular and epididymal ROS and TBARS production in the hypertensive rats with a concomitant decrease in GST activity, GSH and TSH levels. These observations could result in the inadequacy of the testes and epididymis antioxidant status to effectively mitigate induction of oxidative stress in hypertensive rats. The damage due to oxidative stress in testes and epididymis in hypertensive rats was evident by the elevated production of ROS and TBARS in the induced rats. Excessive generation of TBARS from lipid peroxidation may cause over utilization of GSH. The level of GSH and T-SHs as well as GST activity was decreased in the testes and epididymis in the present study. The decrease in the GSH level suggests overutilization in the detoxification process in other to cope with oxidative stress while the decrease in GST activity may result from decrease substrate GSH or inhibition by increased free radicals in the testes and sperm of L-NAME treated rats. However, dietary supplementation with both ginger rhizomes effectively prevented the decrease in GST activity, GSH and TSH levels thereby resulting in a significant reduction in ROS and TBARS levels in testes and epididymides of L-NAME hypertensive rats. This observation may be due to the protective role of phenolic acids and flavonoid compounds on testicular androgenesis and spermatogenesis [1], [38] which have already been characterized in the present plant study as reported by Akinyemi et al. [9]. Also, the antioxidant effect and ability to prevent lipid peroxidation by the rhizomes [52] could be a possibility to prevent the induction of oxidative stress in the testis and epididymis of rats treated with L-NAME compound.
In male reproductive system, leydig cells are predominantly responsible for the biosynthesis and secretion of testosterone which is vital in the initiation and maintenance of spermatogenesis by affecting Sertoli cell androgen receptors [37], [62]. The reduction in the serum concentration of testosterone in the present study may result from oxidative damage in the testes of the L-NAME hypertensive rats. Low levels of testosterone adversely affect spermatogenesis and can lead to Sertoli cells dysfunction [69]. However, pre-treatment with dietary ginger and turmeric rhizomes respectively caused an increase in testosterone hormone. The increase in serum testosterone level, reported in this study, were in agreement with those obtained by Morakino et al. [49] and Moselhy et al. [50] where extract of Z. officinale possess pro-fertility properties in male rats which might be a product of both its potent antioxidant properties and androgenic activities. Moreover, Khaki et al. [74] reported that administration of 100 mg/kg/day of ginger significantly increased sperm concentration, viability, motility and serum total testosterone in H2O2 induced male infertility. This suggested that ginger varieties may be promising in enhancing male infertility induced by hypertensive condition.
Previous studies have demonstrated that reductions in blood flow to the testis could play an important role in the pathogenesis of male infertility via formation of hypo-spermatogenesis with consequent compromise in reproductive capability. The NO-cGMP pathway has been implicated to plays a key role in the male sexual function via production of NO, a potent vasodilator [40]. Endothelium nitric oxide synthase (eNOS) utilizes L-arginine and oxygen as substrates to produce nitric oxide (NO) and citrulline. l-Arginine is also utilized by another enzyme arginase, a metalloenzyme that catalyzes the hydrolysis of l-arginine to produce l-ornithine and urea. The key role of arginine as a substrate for both nitric oxide synthase and arginase serves as a potential point of regulation for the NO/cGMP pathway such that an up-regulation of one enzyme leads to the down-regulation of the other. In the present study, there was a significant increase in the arginase activity in the testes and epididymides of L-NAME-treated rats when compared with control without L-NAME administration (Fig. 3). This result is contrary to what has been previously published by Reisser et al. [57], where L-NAME inhibited arginase activity in vivo. The difference in arginase activity could be due to experimental model or organ differences. L-NAME was previously demonstrated to inhibit the activity of arginase in lysates from rat colon cancer cells and liver in vitro which was confirmed by in vivo in tumor nodules and liver [57]. Nevertheless, the increase in arginase activity could be due to the inhibition of eNOS activity as a result of L-NAME treatment (potent inhibitor of NO production) thereby favouring arginase pathway. Furthermore, our result was accompanied by significant decrease in the NO level in both the testes and epididymides of hypertensive rats. The reduction in the NO level has been shown to be associated with elevated vascular superoxide anion production and consequently, impairment of vasodilation [55]. However, dietary ginger rhizomes treatment were able to cause an inhibition of arginase activity leading to an increase in NO production in genital tissues in hypertensive rats, thus affecting male sexual function. The ability of the rhizomes to inhibit arginase activity in the present study is in line with Akinyemi et al. [8], where dietary supplementation of two ginger varieties inhibits arginase activity in hypercholesterolemic rats showing that both rhizomes have inhibitory effects on arginase activity under pathological state. Nitric oxide (NO) is a potent vasodilator that plays a vital physiological/pharmacological impact in several diseases associated with vasoconstriction. Hence, the implication of the pharmacological benefits of ginger and turmeric rhizomes in the prevention of male infertility in hypertensive rats.
Thus, in mechanistic term, ginger rhizomes supplementation clearly ameliorated hypertension-mediated reproductive dysfunction via enhancement of NO bioavailability and diminished ROS formation which are associated with vascular endothelial dysfunction and hypertension. The significant reduction in oxidative stress has been shown to prevent the activation of various molecular mechanisms involved in vascular remodelling associated with hypertension, particularly NOXs in angiotensin signaling [58].
5. Conclusion
In conclusion, this study demonstrated that L-NAME-induced hypertension resulted in male reproductive dysfunction via alterations in the anti-oxidant status in the testes and epididymides, testosterone level and sperm motility. However, dietary supplementation with turmeric or ginger rhizome was associated with restoration of systolic blood pressure, sperm motility, testosterone level and improvement of antioxidant status in the epididymides and testes of L-NAME-induced hypertensive rats. Therefore, we can suggest that both rhizomes could be harnessed as functional foods to prevent hypertension-mediated male reproductive dysfunction.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgments
Ayodele Jacob Akinyemi is a beneficiary of 2014CNPq/TWAS sandwich postgraduate fellowship. The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and the Academy of Sciences for the Developing World (TWAS) for their support towards this study.
Contributor Information
Ganiyu Oboh, Email: goboh2001@yahoo.com.
Maria Rosa Chitolina Schetinger, Email: mariachitolina@gmail.com.
References
- 1.Abarikwu S.O., Pant A.B., Farombi E.O. The dietary antioxidant, quercetin protects Sertoli-germcell co-culture from atrazine-induced oxidative damage. J. Biochem. Mol. Toxicol. 2012;26:477–485. doi: 10.1002/jbt.21449. [DOI] [PubMed] [Google Scholar]
- 2.Adams M.L., Nock B., Truong R., Cicero T.J. Nitric oxide control of steroidogenesis: endocrine effects of NG-nitro-l-arginine and comparisons to alcohol. Life Sci. 1992;50:PL35–PL40. doi: 10.1016/0024-3205(92)90384-2. [DOI] [PubMed] [Google Scholar]
- 3.Adebayo A., Olamide A., Helen A., Oluwaseun H.I., Olusegun S., Selimot H.A. Toxicity effects of amlodipine on the testis histology in adult Wistar rats. Am. J. Med. Med. Sci. 2012;2:36–40. [Google Scholar]
- 4.Adedara I.A., Farombi E.O. Chemoprotection of ethyleneglycol monoethyl ether induced reproductive toxicity in malerats by kolaviron, isolated biflavonoid from Garcinia kola seed. Hum. Exp. Toxicol. 2012;31:506–517. doi: 10.1177/0960327111424301. [DOI] [PubMed] [Google Scholar]
- 5.Aggarwal B.B., Harikumar K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplasic diseases. Int. J. Biochem. Cell. 2009;41:40–59. doi: 10.1016/j.biocel.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aitken R.J. The Amoroso lecture. The humanspermatozoon—a cell in crisis? J. Reprod. Fertil. 1999;115:1–7. doi: 10.1530/jrf.0.1150001. [DOI] [PubMed] [Google Scholar]
- 7.Akinyemi A.J., Ademiluyi A.O., Oboh G. Inhibition of angiotensin-1-converting enzyme activity in high cholesterol fed diet in rats by two varieties of ginger (Zingiber officinale) J. Med. Food. 2014;17(3):317–323. doi: 10.1089/jmf.2012.0264. [DOI] [PubMed] [Google Scholar]
- 8.Akinyemi A.J., Thome G.R., Morsch V.M., Stefanello N., Goularte J.F., Bello-Klein A., Oboh G., Schetinger M.R.C. Effect of dietary supplementation of ginger and turmeric rhizomes on angiotensin-1 converting enzyme (ACE) and arginase activities in L-NAME induced hypertensive rats. J. Funct. Foods. 2015;17:792–801. [Google Scholar]
- 9.Akinyemi A.J., Oboh G., Ademiluyi A.O., Boligon A.A., Athayde M.L. Effect of two ginger varieties on arginase activity in hypercholesterolemic rats. J. Acupunct. Meridian Stud. 2015 doi: 10.1016/j.jams.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez J.G., Touchstone J.C., Blasco L., Storey B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide in human spermatozoa. J. Androl. 1987;8:338–348. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 12.Ammon H.P.T., Anazoda M.I., Safayhi H., Dhawan B.N., Srimal R.C. Curcumin: a potent inhibitor of Leukotriene B4 formation in rat peritoneal polymorphonuclear neutrophils (PMNL) Planta Med. 1992;58:26–28. doi: 10.1055/s-2006-961438. [DOI] [PubMed] [Google Scholar]
- 13.Arun N., Nalini N. Efficacy of turmeric on blood sugar and polyol pathway in diabetic albino rats. Plant Foods Hum. Nutr. 2002;57:41–52. doi: 10.1023/a:1013106527829. [DOI] [PubMed] [Google Scholar]
- 14.Aybek H., Aybek Z., Rota S., Sxen N., Akbulut M. The effects of diabetes mellitus, age, and vitamin E on testicular oxidative stress. Fertil. Steril. 2008;90:755–760. doi: 10.1016/j.fertnstert.2007.01.101. [DOI] [PubMed] [Google Scholar]
- 15.Azu O.O. Testicular morphology in spontaneously hypertensive rat model: oxidant status and stereological implications. Andrologia. 2015;47:123–137. doi: 10.1111/and.12233. [DOI] [PubMed] [Google Scholar]
- 16.Balbinott A.W., Irigoyen M.C., Brasileiro-Santos M.S. Dose-dependent autonomic dysfunction in chronic L-NAME hypertensive diabetic rats. J. Cardiovasc. Pharmacol. 2005;46:563–569. doi: 10.1097/01.fjc.0000179433.80631.9f. [DOI] [PubMed] [Google Scholar]
- 18.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Briones A.M., Touyz R.M. Oxidative stress and hypertension: current concepts. Curr. Hypertens. Rep. 2010;12:135–142. doi: 10.1007/s11906-010-0100-z. [DOI] [PubMed] [Google Scholar]
- 20.Burnett A.L., Ricker D.D., Chamness S.L. Localization of nitric oxide synthase in the reproductive organs of male rat. Biol. Reprod. 1995;52:1–7. doi: 10.1095/biolreprod52.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Cardoso A.M., Abdalla F.H., Bagatini M.D., Martins C.C., Fiorin F.S., Baldissarelli J., da Costa P., Mello F.F., Fiorenza A.M., Serres J.D., Gonçalves J.F., Chaves H., Royes L.F., Belló-Klein A., Morsch V.M., Schetinger M.R. Swimming training prevents alterations in acetylcholinesterase and butyrylcholinesterase activities in hypertensive rats. Am. J. Hypertens. 2014;27:522–529. doi: 10.1093/ajh/hpt030. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso A.M., Bagatini M.D., Martins C.C., Abdalla F.H., Zanini D., Schmatz R., Gutierres J., Pimentel V.C., Thomé G., Leal C.A., Vieira J.M., Stefanello N., da Silva F.F., Baldissareli J., Royes L.F., Klein A.B., Morsch V.M., Schetinger M.R. Exercise training prevents ecto-nucleotidases alterations in platelets of hypertensive rats. Mol. Cell. Biochem. 2012;371:147–156. doi: 10.1007/s11010-012-1431-7. [DOI] [PubMed] [Google Scholar]
- 23.Chan E.W.C., Lim Y., Wong S. Effects of different drying methods on the antioxidant properties of leaves and tea of ginger species. Food Chem. 2009;113:166–172. [Google Scholar]
- 24.Clancy R.M., Lesczynska-Pikiak L., Abramson S.B. Nitric oxide, an endothelial cell relaxation factor, inhibits neutrophil superoxide production via a direct action on the NADPH oxidase. J. Clin. Invest. 1992;90:1116–1121. doi: 10.1172/JCI115929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark J.T. Sexual function in altered physiological states: comparison of effects of hypertension, diabetes, hyperprolactinemia, and others to “normal” aging in male rats. Neurosci. Biobehav. Rev. 1995;19:279–302. doi: 10.1016/0149-7634(94)00058-9. [DOI] [PubMed] [Google Scholar]
- 26.Dusing R. Effect of the angiotensin II antagonist valsartan on sexual function in hypertensive men. Blood Press. Suppl. 2003;12:29–34. doi: 10.1080/08038020310021967. [DOI] [PubMed] [Google Scholar]
- 27.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 29.Fickling S.A., Williams D., Vallance P., Nussey S.S., Whitley G.S. Plasma concentrations of endogenous inhibitor of nitric oxide synthesis in normal pregnancy and preeclampsia. Lancet. 1993;41:1447–1451. doi: 10.1016/0140-6736(93)92335-q. [DOI] [PubMed] [Google Scholar]
- 30.Furstenau C.R., Ramos D.B., Vuaden F.C., Casali E.A., Monteiro P.S., Trentin D.S., Gossenheimer A.N., Bogo M.R., Bonan C.D., Barreto-Chaves M.L., Sarkis J.J., Wofchuk S.T. L-NAME treatment alters ectonucleotidase activities in kidney membranes of rats. Life Sci. 2010;87:325–332. doi: 10.1016/j.lfs.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Furstenau C.R., Trentin D.S., Gossenheimer A.N., Ramos D.B., Casali E.A., Barreto-Chaves M.L., Sarkis J.J. Ectonucleotidase activities are altered in serum and platelets of L-NAME-treated rats. Blood Cells Mol. Dis. 2008;41:223–229. doi: 10.1016/j.bcmd.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Ghayur M.N., Gilani A.H. Ginger lowers blood pressure through blockade of voltage-dependent calcium channels. J. Cardiovasc. Pharmacol. 2005;45:74–80. doi: 10.1097/00005344-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 33.Ghayur M.N., Gilani A.H., Afridi M.B. Cardiovascular effects of ginger aqueous extract and its phenolic constituents are mediated through multiple pathways. Vascul. Pharmacol. 2005;43:234–241. doi: 10.1016/j.vph.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferase: the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 36.Hafez D.A. Effect of extracts of ginger roots and cinnamon bark on fertility of male diabetic rats. J. Am. Sci. 2010;6:940–947. [Google Scholar]
- 37.Hancock K.D., Coleman E.S., Tao Y.X., Morrison E.E., Braden T.D., Kemppainen B.W., Akingbemi B.T. Genistein decreases androgen biosynthesis in rat Leydig cells by interference with luteinizing hormone-dependent signaling. Toxicol. Lett. 2009;184:169–175. doi: 10.1016/j.toxlet.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 38.Huang J., Roby K.F., Pace J.L. Cellular localization and hormonal regulation of inducible nitric oxide synthase in mouse uterus. J. Leukoc. Biol. 1995;57:27–35. doi: 10.1002/jlb.57.1.27. [DOI] [PubMed] [Google Scholar]
- 39.Jeyendran R., van der Ven H., Perez-Pelaez M. Development of an assay to assess the functional integrity of human sperm membranes and its relationship to other semen characteristics. J. Reprod. Fertil. 1984;70:219–228. doi: 10.1530/jrf.0.0700219. [DOI] [PubMed] [Google Scholar]
- 40.Kim S.W., Cuong T.D., Hung T.M., Ryoo S., Lee J.H., Min B.S. Arginase II inhibitory activity of flavonoid compounds from Scutellaria indica. Arch. Pharm. Res. 2013;36:922–926. doi: 10.1007/s12272-013-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kowluru R.A., Kanwar M. Effects of curcumin on retinal oxidative stress and inflammation in diabetes. Nutr. Metab. 2007;4(1):8. doi: 10.1186/1743-7075-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lerman L.O., Chade A.R., Sica V., Napoli C. Animal models of hypertension: an overview. J. Lab. Clin. Med. 2005;146:160–173. doi: 10.1016/j.lab.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Lim C.J., Cuong T.D., Hung T.M., Ryoo S., Lee J.H., Kim E.H. Arginase ii inhibitory activity of phenolic compounds from Saururus chinensis. Bull. Korean Chem. Soc. 2012;33:3079–3082. [Google Scholar]
- 44.Lovato F.L., Adedara I.A., Barbisan F., Moreira K.L.S., da Rocha M.I.U.M., da Cruz I.B. Quercetin ameliorates polychlorinated biphenyls-induced testicular DNA damage in rats. Andrologia. 2015 doi: 10.1111/and.12417. [DOI] [PubMed] [Google Scholar]
- 45.Mallick C., Mandal S., Barik B., Bhattacharya A., Ghosh D. Protection of testicular dysfunctions by MTEC, a formulated herbal drug, in streptozotocin induced diabetic rat. Biol. Pharm. Bull. 2007;30:84–90. doi: 10.1248/bpb.30.84. [DOI] [PubMed] [Google Scholar]
- 46.McCall T.B., Broughton-Smith N.K., Palmer R.M.J. Synthesis of nitric oxide from l-arginine by neutrophils. Biochem. J. 1989;261:293–296. doi: 10.1042/bj2610293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendez J.D., Sosa A., Palomar-Morales M. Effect of l-arginine on arginase activity in male accessory sex glands of alloxan-treated rats. Reprod. Toxicol. 2002;16:809–813. doi: 10.1016/s0890-6238(02)00057-6. [DOI] [PubMed] [Google Scholar]
- 48.Miranda K.M., Espay M.G., Wink D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 49.Morakino A.O., Adeniyi O.S., Arikawe A.P. Effects of Zingiber officinale on reproductive functions of the male rat. Afr. J. Biomed. Res. 2008;2:329–339. [Google Scholar]
- 50.Moselhy W.A., Helmy N.A., Abdel-Halim B.R., Nabil T.M., Abdel-Hamid M.I. Role of ginger against the reproductive toxicity of aluminium chloride in albino male rats. Reprod. Domet. Anim. 2012;47:335–343. doi: 10.1111/j.1439-0531.2011.01878.x. [DOI] [PubMed] [Google Scholar]
- 52.Oboh G., Ademiluyi A.O., Akinyemi A.J. Inhibition of acetylcholinesterase activities and some pro-oxidant induced lipid peroxidation in rat brain by two varieties of ginger (Zingiber officinale) Exp. Toxicol. Pathol. 2012;64:315–319. doi: 10.1016/j.etp.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 54.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 55.Oktem F., Kirbas A., Armagan A., Kuybulu A.E., Yilmaz H.R., Ozguner F., Uz E. Lisinopril attenuates renal oxidative injury in L-NAME-induced hypertensive rats. Mol. Cell. Biochem. 2011;352:247–253. doi: 10.1007/s11010-011-0760-2. [DOI] [PubMed] [Google Scholar]
- 56.Pulla Reddy A.C.H., Lokesh B.R. Studies on spice principles as antioxidants in the inhibition of lipid peroxidation of rat liver microsomes. Mol. Cell. Biochem. 1992;111:117–124. doi: 10.1007/BF00229582. [DOI] [PubMed] [Google Scholar]
- 57.Reisser D., Onier-Cherix N., Jeannin J.F. Arginase activity is inhibited by L-NAME, both in vitro and in vivo. J. Enzyme Inhib. Med. Chem. 2002;17:267–270. doi: 10.1080/1475636021000006252. [DOI] [PubMed] [Google Scholar]
- 58.Santillo M., Colantuoni A., Mondola P., Guida B., Damiano S. NOX signaling in molecular cardiovascular mechanisms involved in the blood pressure homeostasis. Front. Physiol. 2015;6:194–201. doi: 10.3389/fphys.2015.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sekiwa Y., Kubota K., Kobayashi A. Isolation of novel glycosides from ginger and their antioxidative activity. J. Agric. Food Chem. 2000;8:373–379. doi: 10.1021/jf990674x. [DOI] [PubMed] [Google Scholar]
- 60.Seligman S.P., Abramson S.B., Young B.K., Buyon J.P. The role of nitric oxide in the pathogenesis of pre-eclampsia. Am. J. Obstet. Gynaecol. 1994;172:944–948. doi: 10.1016/s0002-9378(94)70064-8. [DOI] [PubMed] [Google Scholar]
- 62.Sherrill J.D., Sparks M., Dennis J., Mansour M., Kemppainen B.W., Bartol F.F., Morrison E.E., Akingbemi B.T. Developmental exposures of male rats to soy isoflavones impact Leydig cell differentiation. Biol. Reprod. 2010;83:488–501. doi: 10.1095/biolreprod.109.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sowers J.R., Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy: an update. Hypertension. 1995;26:869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 65.The Wealth of India, A dictionary of Indian raw materials and industrial products, first supplement series vol. II, New Delhi: National Institute of Science Communication, CSIR, 2001, pp. 264–293.
- 66.Vanhoutte P.M. Arginine and arginase: endothelial NO synthase double crossed? Circ. Res. 2008;102:866–868. doi: 10.1161/CIRCRESAHA.108.175570. [DOI] [PubMed] [Google Scholar]
- 67.Virag R., Bouilly P., Frydman D. Is impotence an arterial disorder? A study of arterial risk factors in 440 impotent men. Lancet. 1985;1:181–184. doi: 10.1016/s0140-6736(85)92023-9. [DOI] [PubMed] [Google Scholar]
- 68.Wells M.E., Awa O.A. New technique for assessing acrosomal characteristics of spermatozoa. J. Dairy Sci. 1970;53(2):227–232. doi: 10.3168/jds.S0022-0302(70)86184-7. [DOI] [PubMed] [Google Scholar]
- 69.Yoshida S., Hiyoshi K., Ichinose T., Takano H., Oshio S., Sugawara I., Takeda K., Shibamoto T. Effect of nanoparticles on the male reproductive system of mice. Int. J. Androl. 2009;32:337–342. doi: 10.1111/j.1365-2605.2007.00865.x. [DOI] [PubMed] [Google Scholar]
- 70.Zancan K.C., Marques M.O., Petenate A.J., Meireles M.A. Extraction of ginger (Zingiber officinale Roscoe) oleoresin with CO2 and co-solvents: a study of the antioxidant action of the extracts. J. Supercrit. Fluids. 2002;24:57–67. [Google Scholar]
- 71.Zapolska-Downar D., Zapolski-Downar A., Naruszewicz M., Siennicka A., Krasnodebska B., Kołdziej B. Protective properties of artichoke (Cynara scolymus) against oxidative stress induced in cultured endothelial cells and monocytes. Life Sci. 2002;71:2897–2908. doi: 10.1016/s0024-3205(02)02136-7. [DOI] [PubMed] [Google Scholar]
- 72.Zhao H., Xu S., Wang Z., Li Y., Guo W., Lin C., Gong S., Li C., Wang G., Cai L. Repetitive exposures to low-dose X-rays attenuate testicular apoptotic cell death in streptozotocin-induced diabetes rats. Toxicol. Lett. 2010;192:356–364. doi: 10.1016/j.toxlet.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 73.Yang H.S., Han D.K., Kim J.R., Sim J.C. Effect of alpha-tocopherol on cadmium induced-toxicity in rat testis and carcinogenesis. Korean Med. J. 2006;21:445–451. doi: 10.3346/jkms.2006.21.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Khaki A., Fatemek F., Mohammad N., Amir A.K., Chelar C.O., Marefat N., Mohammad H. The effects of ginger on spermatogenesis and sperm parameters. Iran J. Reprod. Med. 2009;7:7–12. [Google Scholar]