Highlights
-
•
We investigated the effect of P. nattereri venom on the isolated perfused kidney.
-
•
We study the mechanism of cytotoxicity of venom on renal epithelial cells.
-
•
We demonstrated P. nattereri venom is composed for 86.3% of proteins.
-
•
The venom of is capable of changing the kidney functional parameters.
Keywords: Philodryas nattereri, Protein content, Renal failure, Cytotoxic activity
Chemical compounds studied in this article: Beta-mercaptoethanol (PubChem CID: 1567), Serum albumin (PubChem CID: 16132), MMT tetrazolium (PubChem CID: 64965), Tris hydrochloride (PubChem CID 93573), 2-Propenamide (PubChem CID: 6579), Saline (PubChem CID 5234)
Abstract
The venom of the snake Philodryas nattereri is a mixture of proteins and toxic peptides with several important local and systemic actions, which are similar to those occurring in Bothrops snake bites. The mechanisms involved in the local and systemic actions of this venom are unknown. The aims of the work were to initial characterization of P. nattereri venom and investigate the effects of the poison in the renal perfusion system and in cultured renal tubular cells of the type MDCK (Madin–Darby canine kidney). The P. nattereri venom is composed majority of proteins (86.3%) and this poison promoted changes in all the evaluated renal parameters, mainly decreasing renal perfusion pressure (PP) and renal vascular resistance (RVR) and increasing urine flow (UF) and glomerular filtration rate (GFR). The most relevant result was that this venom was highly detrimental to the renal tubules independent of the PP reduction, which was shown by a decrease in sodium (Na+), potassium (K+) and chloride (Cl−) electrolyte transport in the studied concentrations. The glomeruli and tubules contain protein bodies and blood extravasation, which were observed by histological analysis. The venom of P. nattereri reduced viability of the MDCK cells only at high concentrations (50 and 100 μg/mL) with an IC50 of 169.5 μg/mL.
1. Introduction
Philodryas nattereri Steindachner, 1870 of the family Dipsadidae [1], commonly called the brown racer, has an olive green coloration with the final portion of its body colored brown. This snake is 1.20–1.60 m long, has large eyes with round pupils, is fast and has an intense daily activity (Vitt and Colli [27]). The snakes’ habitat is related to the environment's physical structure, food availability, presence of predators and the physiology of these snakes, which are diurnal, arboreal and semi-arboreal. These snakes feed on small mammals, birds and lizards [25], are oviparous and lay from 6 to 20 eggs. The snakes’ dentition is opisthoglyphous and connected to the Duvernoy's gland.
P. nattereri is distributed in arid and semiarid regions of South America and is most common in northeastern Brazil (Ceará and Rio Grande do Norte).
During evolution, snakes have specialized in affecting the vital functions of their prey by releasing a large number of toxins (enzymes, proteins and peptides) through venom that destabilize the physiological levels of hormones, alter the activity of enzymes, receptors or ion channels, and promote cardiovascular and nervous system imbalance in their prey. The use of snake toxins as pharmacological tools and prototypes for drug development is increasing [2].
It is also important to emphasize that the severity of symptoms after poisoning is related to the amount of venom inoculated, which depends on the snake's size, age and time it was fed. Poisoning caused by species of Philodryas is characterized by local symptoms such as pain, swelling, erythema, bruising, renal failure and regional lymphadenopathy with normal coagulation [3].
The pathogenesis of the renal alterations following envenomation by Philodryas species is not well defined. Thus, this study aimed to evaluate the renal effects of P. nattereri venom in a perfusion system using different concentrations of venom, to characterize possible histological alterations promoted by venom in isolated rat kidneys and to study venom-induced changes in culture of Madin–Darby canine kidney (MDCK).
2. Material and methods
2.1. Animals and venom extraction
P. nattereri snakes were captured on Aroeiras Farm in the municipality of Upanema (5°38′32″ S and 37°15′27″ W), state of Rio Grande do Norte and transported to NUROF (Ophiology Regional Nucleus of Ceará).
The animals were maintained in individual cages with free access to water and fed with 15 g mice every 30 days. Venom pools were made from more than 40 individual snakes and collected from the venom gland into capillary tubes to prevent contamination with saliva. After the outflow of the venom into the capillary tube, the venom was frozen and lyophilized.
2.2. Analysis of protein content
The venom of P. nattereri (1 mg) was lyophilized and resuspended in 1 mL of saline solution. An aliquot of venom (100 μL) was taken for the quantification of proteins by the method of Bradford [4] using Bio-Rad reagents and bovine serum albumin (BSA) as a standard.
2.3. Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE)
Sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli [5]. SDS-PAGE was carried out in a 2 mm vertical slab gel (10 cm × 8 cm) consisting of stacking gel mix, 5% total acrylamide, and main running gel mix, 17.5% acrylamide, prepared in 3.0 M Tris-HCl, pH 8.8. Samples (20 μg) were dissolved in Tris–HCl 0.0625 M, pH 6.8, containing 1% SDS and 1% β-mercaptoethanol and incubated at 100 °C for 10 min. Electrophoresis was carried out at 20 mA per plate for 1.5 hours. Protein markers employed were myosin (212 kDa), β-galactosidase (116 kDa), phosphorylase B (97.4 kDa), BSA (66.2 kDa), egg albumin (45 kDa), carbonic anhydrase (31 kDa), soybean trypsin inhibitor (21.4/19.7 kDa) and lysozyme (14.2 kDa) (AMRESCO Inc., Ohio, USA) [6], [28].
2.4. Nuclear magnetic resonance spectroscopy (NMR)
For 1H NMR analysis, spectra were recorded on a Bruker DRX-300 MHz FT NMR spectrometer. Venom samples of P. nattereri (5 mg/mL) were lyophilized and prepared using dimethyl sulphoxide (DMSO) as a solvent. The spectra were obtained at 85 °C using a relaxation delay of 1 s and a pulse width of 90° to reach the conditions of quantitative analysis, according with Ahmad et al. [7]. Silica Gel 60 (Merck, 70–230 mesh) was used for analytical TLC. Column chromatographies were performed over silica gel (Merck, 60 F254 230–400 mesh).
2.5. Kidney perfusion
Adult male Wistar rats (260–320 g) were fasted for 24 h with free access to water. The rats were anesthetized with sodium pentobarbitone (50 mg/kg, i.p.) and after careful dissection of the right kidney; the right renal artery was cannulated via the mesenteric artery without interrupting the blood flow as described by Bowman [8].
The perfusion fluid consisted of modified Krebs–Henseleit solution (MKHS) of the following composition (in mmol/L): 114.00 NaCl, 4.96 KCl, 1.24 KH2PO4, 0.5 MgSO4·7H2O, 2.10 CaCl2 and 24.99 NaHCO3. Bovine serum albumin (BSA 6 g%; fraction V), urea (0.075 g), inulin (0.075 g) and glucose (0.15 g) were added to the solution, resulting in a final perfusate volume of 100 mL. The pH was adjusted to 7.4. In each experiment, 100 mL of MKHS were recirculated for 120 min. The perfusion pressure (PP) was measured at the tip of the stainless steel cannula in the renal artery. Samples of urine and perfusion fluid were collected at 10 min intervals for analysis of the sodium, potassium and chloride levels by ion-selective electrodes (Rapid chem 744, Bayer Diagnostic, UK); inulin, as described by Walser et al. [9] and modified by Fonteles et al. [10]; and osmolality, which was measured in vapor pressure osmometer (Wescor 5100C, USA). The venom of P. nattereri (10 mg/mL) was added to the system 30 min after the beginning of each perfusion.
The perfusion pressure (PP), renal vascular resistance (RVR), urinary flow (UF), glomerular filtration rate (GFR), the percentage of sodium (%TNa+), potassium (%TK+) and chloride (%TCl−) tubular transport were determined [11]. The results were compared to the control group, at 30 min early in each experiment (n = 6). The experimental procedures were conducted according to guidelines for the care and use of laboratory animals as approved by the Ethical Committee (68/08) from Federal University of Ceará (UFC).
2.6. Renal histological evaluation
After the renal perfusion experiment, both right and left kidneys were removed and fixed in 10% formaldehyde for histological processing. Kidney tissue was embedded in paraffin, cut into 5 μm sections, stained with hematoxylin–eosin and further processed for light microscopy (Olympus BX41, USA). The photomicrographs were taken by means of a digital camera (Nikon Coolpix 885, Japan).
2.7. Cell culture
Epithelial Madin–Darby Canine Kidney (MDCK) was cultivated in RPMI 1640 medium (MEM) supplemented with 10% fetal bovine serum, 1% penicillin (10 000 IU/mL) and streptomycin (10 mg/mL). For each experiment, cells were removed and incubated with trypsin-EDTA (0.25/0.02%, v/v) at 37 °C at about 5 min.
After this, the cells were counted in a Neubauer chamber and suspended in culture medium (1 × 105 cells) and 24 h later used for the experiments.
2.8. Cytotoxic assay
Cell viability was assessed by MTT (4,5-dimetilazil-2-il)-2,5 diphenyl tetrazolium) assay as described by Mosmann [12]. The MDCK cells are plated in 96-well plates at a density of 105 cells and treated with different concentrations of P. nattereri venom (1.56, 3.12, 6.25, 12.5, 25, 50, 100 μg/mL). After 24 h of treatment, the cells were incubated with 0.5 mg of MTT/mL for 4 h. The formazan crystals that resulted from MTT reduction were dissolved by adding SDS (10%) to each well followed by incubation for 17 h. The absorbance was read at 570 nm in a microplate reader, and cell viability was calculated by comparing the resulting absorbances with the mean absorbance of the control wells (without venom, considered to be 100% viable).
2.9. Statistical analysis
The results were expressed as means ± SEM (n = 6). Statistical evaluation was determined by analysis of variance (ANOVA) and corrected by the Bonferrni test. Statistical significance was set at 5%. The programs used to perform the statistical analysis were Microsoft Excel 2007 and GraphPad Prism 5.0.
3. Results
3.1. Protein content of venom
In the present study, the value of total protein of the venom from P. nattereri was of 863.9 μg/mg of venom, corresponding in 86.3% of total content of venom this species. In comparison with venom of others species of the genera Philodryas, such as P. olfersii (923 μg/mg), P. patagoniensis (814 μg/mg) and P. nattereri (847 μg/mg) and Bothrops jararaca (799 μg/mg) [13], [14]. The venom of P. nattereri was similar among the others venoms.
3.2. SDS-PAGE and NMR spectrum
SDS-polyacrylamide gel electrophoresis was carried out according to Laemmli [5] and showed distinct protein patterns among the venoms of P. nattereri and B. jararaca. The venom of P. nattereri showed multiple protein bands, ranging from 45 kDa to 100 kDa, while in B. jararaca have protein bands ranging from 45 to 210 kDa (Fig. 1).
Fig. 1.
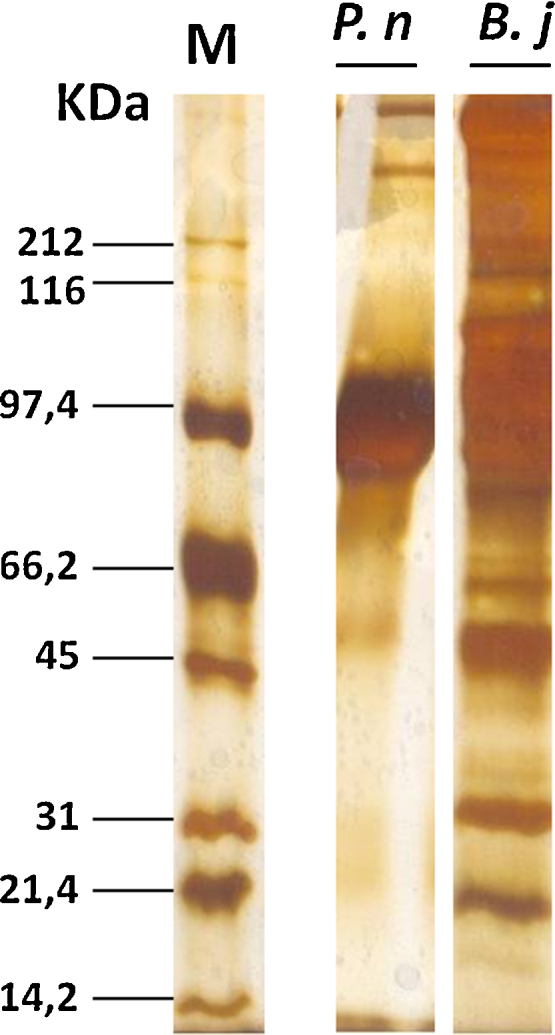
Electrophoretic profiles of P. nattereri (P.n.) and B. jararaca (B.j.) venoms. Each lane was loaded with 20 μL of venom. Proteins were silver stained.
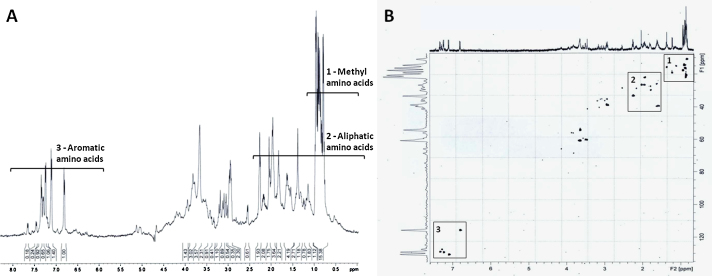
The 1H NMR spectrum of P. nattereri venom showed the presence of peptides, amino acids simple (phenolic, aromatics and aliphatic) and amino acid derivatives represented the major components of this venom (Fig. 2). The peaks generated correspond to amino acids of the protein constituents of the poison, which comprise 86.3% of this.
Fig. 2.
Detailed 1H NMR-spectroscopic (500 MHz, CD3OD) analysis of P. nattereri venom. (A) The 1H NMR spectrum revealing a highly complex composition with different groups of amino acids (B) Cozy spectrum (CD3OD) with different groups of amino acids.
3.3. Effects of the P. nattereri venom in the isolated rat kidney
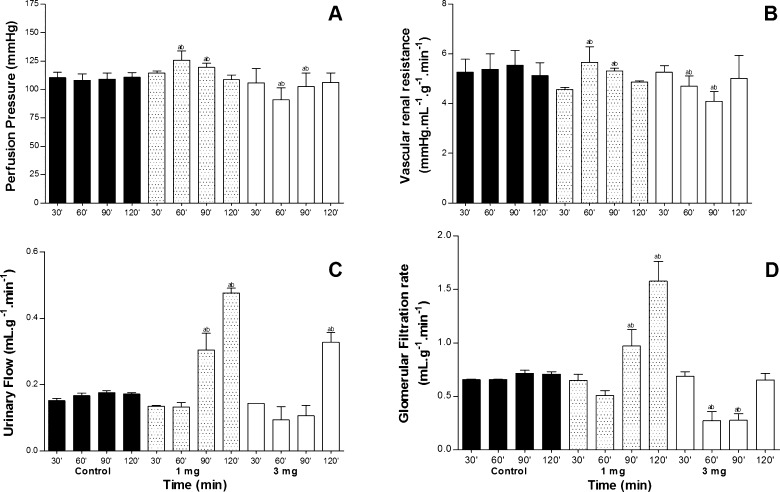
Physiological renal changes were observed after injecting doses of 1 and 3 mg/mL of P. nattereri venom. There were a significant increase of pressure perfusion (PP) and renal vascular resistance (RVR) at 60 and 90 min for the 1 mg/mL concentration. For the 3 mg/mL concentration, PP and RVR were significantly reduced at 60 and 90 min, after which it returned to values close to the control group at 120 min (Fig. 3A and B). The effect on urinary flow (UF) and glomerular filtration rate (GFR) were a significantly increase at 90 and 120 min for the 1 mg/mL concentration and to the 3 mg/mL reduced significantly at 60 and 90 min for both parameters, but at 120 min increase significantly in UF, while GFR returned to normal perfusion (Fig. 3C and D).
Fig. 3.
Effects of P. nattereri venom in concentrations of 1 and 3 mg/mL on perfusion pressure (A), renal vascular resistance (B), urinary flow (C) and glomerular filtration rate (D). Data are expressed as mean ± SEM from six different animals. *p < 0.05 compared to the corresponding control group for each interval. (ANOVA and Bonferroni test). The venom was added to the system 30 min after the beginning of each perfusion.
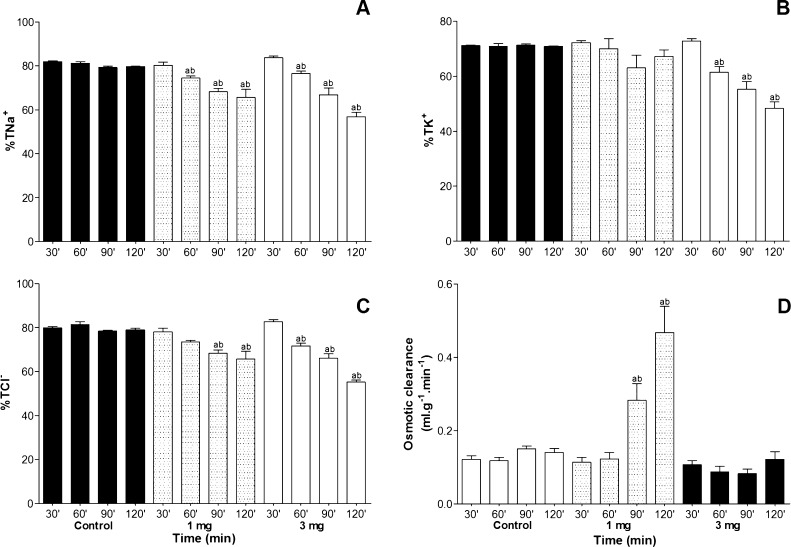
Na+, K+, Cl− electrolyte transport was altered in the perfused kidney, regardless of the venom concentration. Regarding the percent of sodium tubular transport (%TNa+) was reduced at 60, 90 and 120 min for 1 and 3 mg/mL concentrations of venom and percent of potassium tubular transport (%TK+) was reduced at 60, 90 and 120 min only in 3 mg/mL concentration, when compared in control group (Fig. 4A and B).
Fig. 4.
Effects of P. nattereri venom in concentrations of 1 and 3 mg/mL on sodium (A) potassium (B) chloride (C) tubular transport percent and osmotic clearance. Data are expressed as mean ± SEM from six different animals. *p < 0.05 compared to the corresponding control group for each interval (ANOVA and Bonferroni test). The venom was added to the system 30 min after the beginning of each perfusion.
The percent of chloride tubular transport (%TCl−) was reduced at 90 and 120 min for 1 mg/mL, while in 3 mg/mL concentration at 60, 90 and 120 min. Osmotic clearance showed increase at 90 and 120 min in 1 mg/mL concentration of venom of P. nattereri (Fig. 4C and D).
3.4. Renal histological evaluation
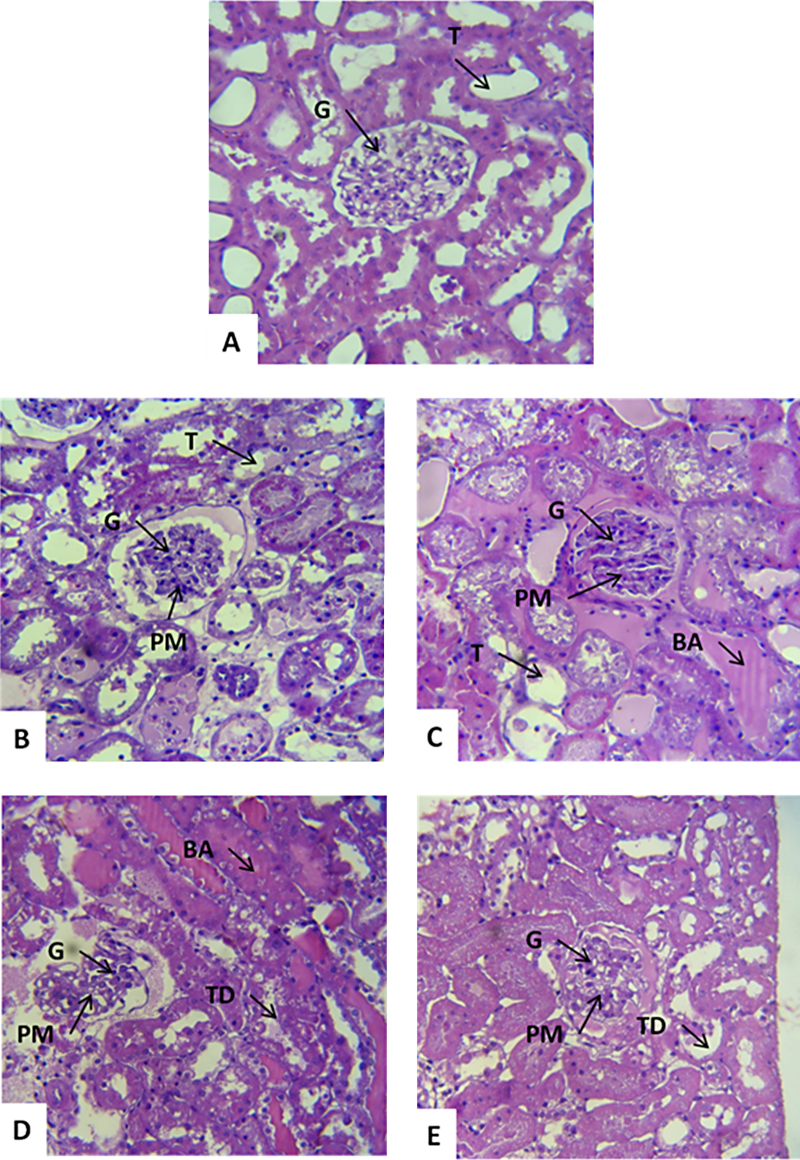
In the control group composed of left kidneys perfused with Krebs solution (MKHS), the kidney showed normal structures (glomerulus, tubule, vessels and interstitium) (Fig. 5A).
Fig. 5.
Renal cortical sections of rats stained with hematoxylin–eosin (n = 6). (A) The sections were obtained from the left kidney incubated with Krebs solution (control) for 120 min showing a normal appearance for tubules (T) and glomerulus (G). (B) and (C) The right kidney perfused with 1 mg/mL of venom indicating blood accumulation, glomerulus and tubules with proteinaceous material (PM) in a greater quantity and in a diffuse manner in the tubules. (D) and (E) The right kidney perfused with 3 mg/mL of venom indicating. There is evident tubular degeneration (TD), blood accumulation (BA) and proteinaceous material (PM) within the tubules and Bowman spaces. H. E. staining 400×.
The right kidney perfused with 1 mg/mL of venom showed tubular dilation, mainly in the distal convoluted tubule and Henle's loop, proteinaceous material (PM) and blood accumulation (BA) within the tubules, normal vessels and interstices (Fig. 5B and C).
Kidneys perfused with 3 mg/mL of venom showed an obvious tubutar degeneration, presence of proteinaceous material within the tubules and the Bowman spaces, glomeruli with slight alterations and tubules with moderate dilatation (TD) (Fig. 5D and E).
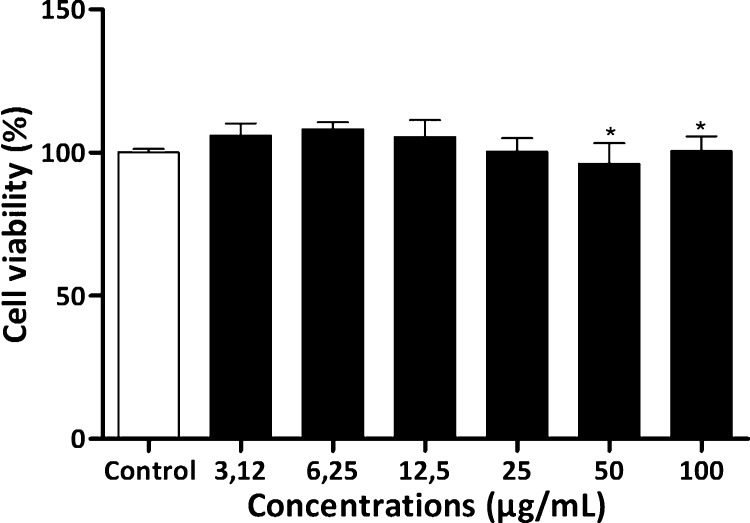
3.5. Cytotoxic effect of the P. nattereri venom on MDCK cells
The P. nattereri cytotoxicity was assessed in the renal tubular cells (MDCK cell culture) after 24 h of exposure to various venom concentrations (3.125, 6.25, 12.5, 25, 50 and 100 μg/mL). The venom significantly reduced the viability of the MDCK cells in the 50 and 100 μg/mL concentrations compared to the control with an IC50 of 169.5 μg/mL (Fig. 6).
Fig. 6.
Viability of MDCK cells incubated with different concentrations of P. nattereri venom for 24 h.
4. Discussion
To initial characterization of venom from P. nattereri, we analyzed total protein content and profile by SDS-PAGE and NMR analysis of lyophilized venom. The poison of P. nattereri exhibited 86.3% of total protein content. This value is similar to that found by Zelanis et al. [14] for others species of the genera Philodryas, such as P. olfersii (923 μg/mg), P. patagoniensis (814 μg/mg) and P. nattereri (847 μg/mg), and Bothrops jararaca (Viperidae family) with 799 μg/mg [13].
Regarding SDS-PAGE analysis, The venom of P. nattereri showed a less complex profile in comparison to the other two congeneric species (P. olfersii and P. patagoniensis) [14], with major protein bands ranging from 45 kDa to 100 kDa, while in B. jararaca have a large number of protein bands ranging from 45 to 210 kDa.
These results may strengthen the hypotheses of close similarity regarding the actions and activities of venom from the families Viperidae and Dipsadidae and may corroborate the eletrophoretic analysis performed by Rocha and Furtado [26].
To investigate the effect of the P. nattereri venom in the kidney without interference of systemic factors, we used perfusion in the rat kidney. In the present study, we observed a decrease in perfusion pressure, renal vascular resistance, urinary flow and glomerular filtration rate, as well as a decrease in sodium transport and chloride after the kidney was perfused by the venom, whereas the clearance osmotic was higher compared with control. This agrees with findings for Bothrops marajoensis [15], Bothrops insularis [24], Bothrops jararaca [16] and Bothrops jararacussu [17].
Bothrops venom is characterized for promoting hypotension through mechanisms that promote systemic vasorelaxation [18]. These effects are likely identical to those affecting renal PP, as we observed in this study for P. nattereri venom.
However, the reduction in almost renal parameters observed in the experiments cannot be related to a specific component because the overall composition of crude P. nattereri venom is unknown. Despite this limitation, mediators, such as tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), IL-6, IL-10 and gamma interferon (IFN-Y), can be involved in renal parameters decreases (PP, GFR, RVR, TNa+, TK+ and TCL−), because venom typically induces the release of these substances, as has been previously reported [19]. In addition, there might be compounds that exert direct action on kidney tissues. These inflammatory mediators, with relaxation potential, may have contributed to the RVR reduction observed in this study.
The histological alterations promoted by P. nattereri venom in two concentrations of 1 and 3 mg/mL, respectively showed initially blood accumulation, glomerulus, tubules with proteinaceous material and dilatation tending toward degeneration, reversible lesions and the accumulation of proteins material released into the damaged cells cytoplasm reflected the early stages of venom toxic agression and the histological analysis corroborate the results for the genus Bothrops [17], [20], [24].
The cytotoxic potential of P. nattereri venom was evaluated using MDCK cells, which is a cell line with similar morphological and functional characteristics to cells in the distal collecting tubules of mammals [21].
Th results of cytotoxic effects showed that the venom was toxic to cells because it significantly reduced viability in concentrations of 50 and 100 mg/mL compared to the control with an IC50 of 169.5 μg/mL. This value for IC50 is higher in relation of others species of snakes, such as Bothrops leucurus and Crotalus durissus cumanensis with 1.25 and 5.38 μg/mL, respectively [22], [23].
5. Conclusions
The P. nattereri venom was composed and caused toxicity in kidney isolated and induced cell death on cultured MCDK cells. We demonstrated P. nattereri venom is composed for proteins (86.3%) and capable of changing the kidney functional parameters (PP, RVR, GFR, UF, osmotic clearance, percent of sodium, potassium and chloride transport) in the isolated rat kidneys. Furthermore, the venom promoted morphological alterations in the renal tubules, such as blood accumulation, glomerulus, tubules with proteinaceous material. It is supposed that this renal change occurs probably by damaging both vascular and glomerular sites. Regarding the effects of venom in viability of the MDCK cells was observed only at high concentrations (50 and 100 μg/mL of venom) with an IC50 of 169.5 μg/mL. These findings may be important aspects of the process of toxicity mediated by P. nattereri venom.
Transparency document
Footnotes
Available online 2 October 2014
References
- 1.Zaher H., Grazziotin F.G., Cadle J.E., Murphy R.W., Moura-Leite J.C., Bonatto S.L. Molecular phylogeny of advanced snakes (Serpentes, Caenophidia) with an emphasis on South American Xenodontines: a revised classification and descriptions of new taxa. Pap. Avulsos Zool. 2009;49:115–153. [Google Scholar]
- 2.Paioli S.F. Inter-Unit Biotechnology Program, University of São Paulo; São Paulo: 2011. Cytotoxic Effect of Crotoxin on Murine Melanoma Cells and Fibroblasts. (Dissertation) 39 pp. [Google Scholar]
- 3.Ribeiro L.A., Puorto G., Jorge M.T. Bites by the colubrid snake Philodryas olfersii: a clinical and epidemiological study of 43 cases. Toxicon. 1999;37:943–948. doi: 10.1016/s0041-0101(98)00191-3. [DOI] [PubMed] [Google Scholar]
- 4.Bradford M.M. A rapid and sensitive method for the quantifications of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 5.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad S., Moinuddin H.S., Shahab U., Alam K., Ali A. Autoimmune response to AGE modified human DNA: implications in type 1 Diabetes mellitus. J. Clin. Transl. Endocrinol. 2014;1:66–72. doi: 10.1016/j.jcte.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad S., Moinuddin S.U., Khan M.S., Habeeb S., Alam K., Ali A. Glyco-oxidative damage to human DNA-Neo-antigenic epitopes on DNA molecule could be a possible reason for autoimmune response in type 1 diabetes. Glycobiology. 2013;24:281–291. doi: 10.1093/glycob/cwt109. [DOI] [PubMed] [Google Scholar]
- 8.Bowman R.H. Gluconeogenesis in the isolated perfused rat kidney. J. Biol. Chem. 1970;245:1604–1612. [PubMed] [Google Scholar]
- 9.Walser M., Davidson D.G., Orloff J. The renal clearance of alkali stable inulin. J. Clin. Invest. 1955;34:1520–1523. doi: 10.1172/JCI103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fonteles M.C., Cohen J.J., Black J., Wherthein S.J. Support of kidney function by long-fatty acids derived from renal tissue. Am. J. Physiol. 1983;244:235–246. doi: 10.1152/ajprenal.1983.244.3.F235. [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Maldonado M., Opava-Stitzer R. Free water clearance curves during saline, mannitol, glucose and urea diuresis in the rat. J. Physiol. 1978;280:487–497. doi: 10.1113/jphysiol.1978.sp012396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 13.Prianti A.C.G., Jr., Ribeiro W., Lopes-Martins R.A.B., Lira-Da-Silva R.M., Prado-Franceschi J., Rodrigues-Simioni L., Cruz-Höfling M.A., Leite G.B., Hyslop S., Cogo J.C. Effect of Bothrops leucurus venom in chick biventer cervicis preparations. Toxicon. 2003;41:595–603. doi: 10.1016/s0041-0101(03)00002-3. [DOI] [PubMed] [Google Scholar]
- 14.Zelanis A., Rocha M.M.T., Furtado M.F.D. Preliminary biochemical characterization of the venoms of five Colubridae species from Brazil. Toxicon. 2010;55:666–669. doi: 10.1016/j.toxicon.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 15.Evangelista I.L., Martins A.M., Nascimento N.R., Havt A., Evangelista J.S., Norões T.B. Renal and cardiovascular effects of Bothrops marajoensis venom and phospholipase A2. Toxicon. 2010;55:1061–1070. doi: 10.1016/j.toxicon.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 16.Monteiro H.S.A., Fonteles M.C. The effect of Bothrops jararaca venom on rat kidney after short-term exposure: preliminary results. Pharmacol. Toxicol. 1999;85:198–200. doi: 10.1111/j.1600-0773.1999.tb00092.x. [DOI] [PubMed] [Google Scholar]
- 17.Havt A., Fonteles M.C., Monteiro H.S.A. The renal effects of Bothrops jararacussu venom and the role of PLA2 and PAF blockers. Toxicon. 2001;39:1841–1846. doi: 10.1016/s0041-0101(01)00146-5. [DOI] [PubMed] [Google Scholar]
- 18.Soares A.M., Fontes M.R.M., Giglio J.R. Phospholipases A2 myotoxins from Bothrops snake venoms: structure–function relationship. Curr. Org. Chem. 2004;8:1677–1690. [Google Scholar]
- 19.Petricevich V.L., Teixeira C.F.P., Tambourgi D.V., Gutiérrez J.M. Increment in serum cytokine and nitric oxide in mice injected with Bothrops asper and Bothrops jararaca snake venoms. Toxicon. 2000;38:1253–1266. doi: 10.1016/s0041-0101(99)00227-5. [DOI] [PubMed] [Google Scholar]
- 20.Burdmann E.A., Woronik V., Prado E.B., Abdulkader R.C., Saldanha L.B., Barreto O.C., Marcondes M. Snakebite-induced acute renal failure. An experimental model. Am. J. Trop. Med. Hyg. 1993;48:82–88. doi: 10.4269/ajtmh.1993.48.82. [DOI] [PubMed] [Google Scholar]
- 21.Collares-Buzato C.B., De Paula L.S.L., Cruz-Höfling M.A. Impairment of the cell-to-matrix adhesion and cytotoxicity induced by Bothrops moojeni snake venom in cultured renal tubular epithelia. Toxicol. Appl. Pharmacol. 2002;181:124–132. doi: 10.1006/taap.2002.9404. [DOI] [PubMed] [Google Scholar]
- 22.Pereira T.P., Bezerra M.R.R.P.P., Torres A.F.C., Brito T.S., Batista-Lima F.J., Vinhote J.F.C., Sousa D.F., Ximenes R.M., Toyama M.H., Diz-Filho E.B.S., Magalhães P.J.C., Monteiro H.S.A., Martins A.M.C. Renal and vascular effects of Crotalus durissus cumanensis venom and its crotoxin fraction. J. Venom. Anim. Toxins Incl. Trop. Dis. 2011;17:333–347. [Google Scholar]
- 23.De Morais I.C., Torres A.F., Pereira G.J. Bothrops leucurus venom induces nephrotoxicity in the isolated perfused kidney and cultured renal tubular epithelia. Toxicon. 2013;61:38–46. doi: 10.1016/j.toxicon.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Braga M.D., Martins A.M., Amora D.N., de Menezes D.B., Toyama M.H., Toyama D.O. Purification and biological effects of C-type lectin isolated from Bothrops insularis venom. Toxicon. 2006;15:859–867. doi: 10.1016/j.toxicon.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 25.FUNASA, Ministério da Saúde. Fundação Nacional da Saúde. Manual de Diagnóstico e Tratamento de Acidentes por Animais Peçonhentos [Manual of Diagnosis and Treatment of Snake Bites]. Brasília, October 2001.
- 26.Rocha M.M.T., Furtado M.F.D. Caracterização individual do veneno de Bothrops alternatus Duméril, Bibron & Duméril em função da distribuição geográfica no Brasil (Serpentes, Viperidae) Rev. Bras. Zool. 2005;22:383–393. [Google Scholar]
- 27.Vitt L.J., Colli G.R. Geographical ecology of a neotropical lizard: Ameiva ameiva (Teiidae) in Brazil. Can. J. Zool. 1994;72:1986–2008. [Google Scholar]
- 28.Blum H., Beier H., Gross J. Improved silver staining of plant proteins, RNA and DNA in polyacrylamide gels. Electrophoresis. 1987;8:93–99. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.