Abstract
The distribution of non-essential trace elements in some vital organs of 11 fish species from Aiba Reservoir, Iwo, Nigeria was assessed between November 2010 and June 2011. The fish species belong to seven families; family Mormyridae, family Cyprinidae, family Hepsetidae and family Channidae each with one species; family Bagridae and family Clariidae each with two species; and family Cichlidae with three species. All families, except Clariidae and Channidae, are common in the daily catch from the reservoir. Atomic absorption spectrophotometry was used to determine the levels of cadmium, mercury and lead in fish organs. The concentration of toxic trace metals in fish ranged from 0.001 to 0.100 ppm (Cd), 0.000–0.067 ppm (Hg) and 0.001–0.125 ppm (Pb) dry weight. This study shows similarity (p > 0.05) in the distribution of Cd, Hg and Pb among fish species; and a non-uniform distribution of toxic trace metals within fish organs with Kidney > Liver > Gill ≥ Intestine ≥ Muscle. Canonical variate analysis shows clear discrimination of Clarias macromystax and Channa obscura for gill trace metal levels of Cd, Hg and Pb while Labeo senegalensis and Oreochromis niloticus were discriminated for liver trace metal values of Cd and Pb only when compared to other fish species studied. The discrimination of some fish species based on trace metals in the gills and liver suggests different regulatory strategies for trace metal accumulation. Variation due to comparison among different fish species from the same water body suggests that accumulation may be species dependent. Differential accumulation of toxic trace metals in fish organs makes them good bioindicators of freshwater contamination.
Keywords: Toxic metals, Bio-indicator, Tropical reservoir, Environmental contamination, Cichlids
1. Introduction
Trace metals are accumulated by aquatic organisms and persist in food webs [1]. The quantification of potential contaminants in biological tissues can be an important part of water quality assessment programs because it can provide information about threats to human life and its distribution in the environment [2]. Biota such as fish are used as indicators of trace metal contamination of aquatic environments because they are large and easily identified [3]; have longer life-span and high position in the aquatic food chain [4]; and have been recommended as valuable biological indicators in aquatic environmental pollution assessment [5].
Two main ways by which trace metals enter the aquatic food chain are by direct consumption of water and food through the digestive tract and non-dietary routes across permeable membranes such as the skin and gills [1]. Therefore levels in fish usually reflect levels found in sediment and water of the particular aquatic environment from which they are sourced [6]; and time of exposure [7]. Fish have the ability to accumulate heavy metals in their tissues by absorption along gill surface and gut tract wall to higher levels than environmental concentration [7]. Accumulation of trace metals by organisms may be passive or selective; and differences in accumulation of trace metals by organisms could be as a result of differences in assimilation, egestion or both [8].
Non-essential trace metals such as Cadmium (Cd), Mercury (Hg) and Lead (Pb) have no known essential role in living organisms; exhibit extreme toxicity even at very low (trace) exposure levels and have been regarded as the main threats to all forms of life especially human health [9], [10]. Toxic effects occur when excretory, metabolic, storage and detoxification mechanisms are no longer able to counter uptake [11]; eventually resulting in physiological and histopathological changes [1], [12], [13], [14]. These changes can also be altered by water physicochemistry [7]. Entry of heavy metals into the organs of a fish mainly takes place by adsorption and absorption; the rate of accumulation is a function of uptake and depuration rates [7]. Non-essential metals, aside from being toxic and persistent, are bioaccumulated and internally regulated using different strategies such as active excretion and storage [15]. Significant variations in the levels of non-essential trace elements have been reported between organs and species of fish inhabiting the same freshwater body: Lake Balaton, Hungary [16]; İskenderun Bay, Turkey [17]; Three Gorges Reservoir, China [18].
Elevated levels of toxic trace metals have been reported from areas experiencing increasing settlement, traffic and agricultural activities [7], [8]. The levels of non-essential trace elements in fish are important because fish is an important source of food for the general human population; fish from freshwater bodies receiving industrial effluents have been reported to be unfit for human consumption because of high tissue levels of some trace metals [11], [19], [20], [21], [22]. In order to protect aquatic biota, it is necessary to determine contamination levels of trace elements through chemical biomonitoring and evaluation of biomarkers that represent early indicators of biological effects [7]. Certain fish species may be better bioindicators of specific trace metal contamination compared to others [23], [24].
Aiba Reservoir is located in Iwo Local Government Area of Osun State, Nigeria. It is mainly an agrarian society. The reservoir is the main source of potable water and secondary source of food fish for the local populace. With recent increase in population of Iwo due to increased development; the demand for this precious resource has increased, thereby resulting in point and non-point anthropogenic contamination of this water body. This study, therefore, assesses the levels of three toxic metals (Cadmiun, Mercury and Lead) in some vital organs of different fish species inhabiting Aiba Reservoir, Iwo, Nigeria, in order to determine their distribution and to suggest indicator fish species.
2. Experimental
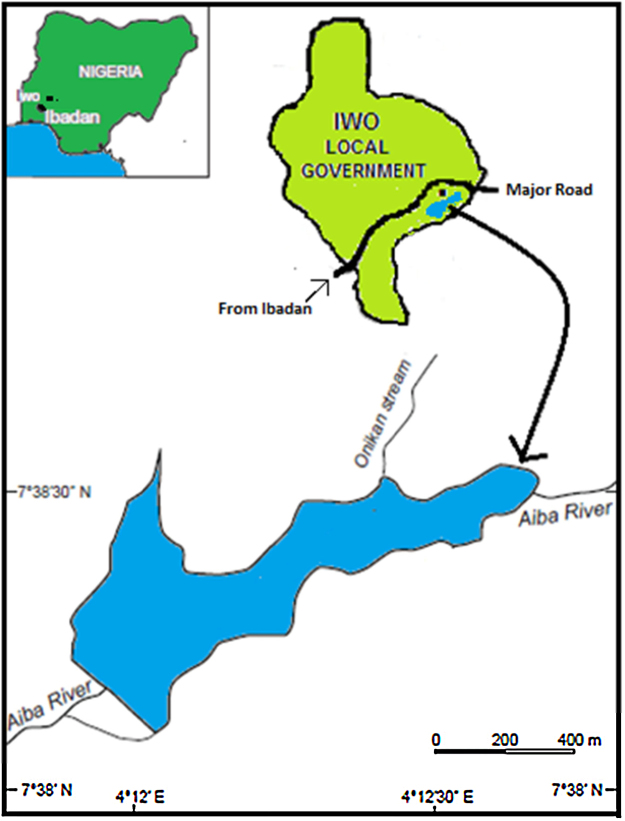
Aiba Reservoir (Fig. 1), a small tropical reservoir with a mean depth of 0.75 m (maximum depth = 7.5 m), is State-owned and is located in Iwo town. It lies between longitudes 004°11′–004°13′E and latitudes 07°38′ – 07°39′N. Iwo town is located almost equidistant between two state capitals; Ibadan in Oyo State and Osogbo in Osun State, Nigeria. Earlier studies [25], [26] show seasonal (mainly run-off from the catchment area during the wet season and a short harmattan spell during the dry season) as well as spatial (mainly anthropogenic) influence on the reservoir. Anthropogenic sources of contamination include encroachment of residential and business (furniture, auto repair, etc.) structures at the northern portion of the reservoir and contamination (point and non-point sources) at the southern portion of the reservoir such as run-off from agricultural areas and other human related activities such as swimming, bathing and washing of domestic wares. Although potable water is the main purpose for its creation in 1957; artisanal fishing is a common and daily activity in the reservoir.
Fig. 1.
Map showing location of Aiba Reservoir, Iwo, Nigeria.
A total of eleven fish species (Table 1) caught with gill nets (mesh size = 25 mm) between November 2010 and June 2011 to spread over the dry (November to March) and wet (April to October) seasons were used in the analysis. All except the Clariidae and Channidae (caught once only during the dry season) are common in the daily catch of artisanal fishermen of Aiba Reservoir. Fish species were bought from fishermen at their landing sites and taken to the laboratory in a cool box. In the laboratory, each fish was identified to species level using identification guide [27]. The fish samples were measured to the nearest 0.1 cm with ruler and wooden board; and weighed (wet) to the nearest 0.1 g with a digital balance. Eighty-six samples of individual fish were used for trace metal analysis: Marcusenius senegalensis (n = 8; standard length range = 10.1–17.5 cm; weight range = 18.7–82.2 g); Labeo senegalensis (n = 7; standard length range = 10.7–13.9 cm; weight range = 24.8–58.2 g); Hepsetus odoe (n = 10; standard length range = 16.4–24.5 cm; weight range = 56.0–208.1 g); Chrysichthys auratus (n = 10; standard length range = 12.4–16.5 cm; weight range = 36.1–98.7); Chrysichthys nigrodigitatus (n = 8; standard length range = 9.6–22.7 cm; weight range = 162.2–228.8 g); Clarias ebriensis (n = 1; standard length = 18.2 cm; weight = 61.0 g); Clarias macromystax (n = 1; standard length = 18.7 cm; weight = 73.8 g); Channa obscura (n = 1; standard length = 17.5 cm; weight = 69.4 g); Tilapia zillii (n = 15; standard length range = 7.6–16.8 cm; weight range = 18.2–200 g); Sarotherodon galilaeus (n = 15; standard length range = 8.4–17.5 cm; weight range = 23.1–201.8 g) and Oreochromis niloticus (n = 10; standard length range = 7.4–13.9 cm; weight range = 15.6–91.4 g). Each fish sample was dissected with stainless steel forceps, scalpels and scissors in a dissecting tray to bring out different organs. Pooled organ samples of fish (n > 1) caught at each sampling period were analysed. Fish organs were dried in labelled pre-washed crucibles at 70 °C for 24 h in an oven. Each dry sample was then pulverised, homogenized with porcelain mortar and pestle and stored in labelled pre-treated specimen bottles. Each sample was transferred to pre-weighed crucibles and dried again in the oven until constant weight was obtained. One gram of the dry sample, placed in a Teflon beaker, was digested with 10 mL analytical grade nitric acid (HNO3) and hydrogen peroxide (H2O2) (1:1) on a hot plate at 100 °C to near dryness when a clear solution was obtained. The solution was then diluted up to 25 mL with distilled water. The concentrations of trace elements in each sample were determined using atomic absorption spectrophotometer. Blank experiments were run to check for background contaminants by the reagents and apparatus used. The values obtained from running blank experiments were subtracted from the analyte values as applicable. The Atomic Absorption Spectrophotometer (AAS) used was calibrated to evaluate the response of the analytical procedure with respect to known quantities of the standards of the trace metals so that the response to unknown quantities in the samples could be reliably estimated. The calibration of the AAS was done with 10, 8, 6, 4, 2 and 0 ppm solution of the metals obtained by serial dilution of 1000 ppm of the stock metal solution. Analysis of each sample was carried out in duplicate and results expressed in microgram per gram (or ppm).
Table 1.
Classification and the main feeding habits of eleven fish species sampled from Aiba Reservoir, Iwo.
| S/no | Species | Family | Order | Main feeding habit |
|---|---|---|---|---|
| 1 |
Marcusenius senegalensis (Steindachner, 1807) |
Mormyridae | Mormyriformes | Substrate feeder (insect larvae) |
| 2 |
Labeo senegalensis (Cuvier and Valenciennes,1842) |
Cyprinidae | Cypriniformes | Substrate feeder (epipelic algae) |
| 3 | Hepsetus odoe (Block, 1794) | Hepsetidae | Cypriniformes | Carnivorous (piscivorous) |
| 4 | Chrysichthys auratus (Geoffrey, 1809) | Bagridae | Siluriformes | Omnivorous (mesopredators) |
| 5 |
Chrysichthys nigrodigitatus (Lacepede, 1803) |
Bagridae | Siluriformes | Omnivorous (mesopredators) |
| 6 | Clarias (Anguilloclarias) ebriensis (Pellegrin, 1920) | Clariidae | Siluriformes | Omnivorous (bottom feeder) |
| 7 | Clarias (Claroides) macromystax (Gunther, 1864) | Clariidae | Siluriformes | Omnivorous (bottom feeder) |
| 8 | Channa obscura (Smith, 1873) | Channidae | Perciformes | Omnivorous (small fish and insects) |
| 9 | Tilapia zillii (Gervais, 1848) | Cichlidae | Perciformes | Herbivorous |
| 10 | Sarotherodon galilaeus (Artedi, 1757) | Cichlidae | Perciformes | Herbivorous |
| 11 | Oreochromis niloticus (Linnaeus, 1757) | Cichlidae | Perciformes | Omnivorous |
Recovery analysis was conducted to assess the error levels arising from contamination and also to ascertain the precision of the analytical procedures used in this study. Two fish samples, each weighing 1 g, were placed into different Teflon beakers. One of the samples was spiked with 100 μg/g of the mixed metal standard while the other was left unspiked. The two samples were subjected to the same sample digestion procedures. The digested solution was quantitatively added to a 25 mL volumetric flask. The levels of Cd, Hg and Pb in the two samples were determined using the AAS. The percentage recovery (%R) for each metal was calculated using the relationship: where A = concentration of a metal in the spiked sample, B = concentration of a metal in the unspiked sample; and C = the amount of metal (ppm) used for spiking.
The metal content of all the extracts in the centrifuged and digested solutions were determined using Buck model 205 Flame Atomic Absorption Spectrophotometer, available at the International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. Similarly, the accuracy of the method was assessed through a calibration standard of 10 ppm, which was run as a check after every five batch of the samples analysed to ensure that less than 20% variation was found from the initial calibration standard.
Non-parametric Kruskal–Wallis test was carried out on the datasets since they do not satisfy the assumptions of parametric test. Data sets that were significantly different among groups were further subjected to multivariate Canonical Variance analysis to give a graphical discrimination among fish species. Non-parametric test was carried out with SPSS software (SPSS 14.0 for Windows Evaluation version). Multivariate graphical illustrations of datasets were made using the GenStat Software (GenStat-Tenth Edition (DE 4), Service Pack 1, version 10.3.0.0; 2011).
3. Results and discussion
3.1. Results
The standard calibration curves obtained from the recovery analysis results of trace metals analysed for fish samples from Aiba Reservoir shows high linearity level with r2 values of 0.9819 (Cd), 0.9856 (Hg) and 0.9891 (Pb). Recoveries of trace metals in fish are: 89.79 ± 4.35% (Cd), 84.72 ± 4.02% (Hg) and, 95.39 ± 3.86% (Pb). These values are adjudged acceptable therefore the results obtained are reliable.
The overall mean ± standard error for all fish samples combined are 0.012 ± 0.002 ppm (0.001 – 0.0125 ppm) for Cd; 0.006 ± 0.001 ppm (0.000–0.067 ppm) for Hg; and 0.010 ± 0.001 ppm (0.001 – 0.100 ppm) for Pb (Table 2). L. senegalensis had the highest mean value (0.020 ± 0.001 ppm) for Cd, while C. nigrodigitatus recorded the lowest (0.004 ± 0.001 ppm); O. niloticus had the highest mean (0.011 ± 0.005 ppm) for Hg, while C. nigrodigitatus recorded the lowest (0.002 ± 0.000 ppm); however, T. zillii had the highest mean value (0.024 ± 0.011 ppm) for Pb, while C. auratus, C. nigrodigitatus and M. senegalensis had the lowest (0.005 ± 0.001 ppm). Kruskal–Wallis One Way Analysis of Variance of the 11 fish species shows no significant difference among species for Cd ( = 6.25, df = 10, p = 0.794), Hg ( = 12.59, df = 10, p = 0.247) and Pb ( = 7.67, df = 10, p = 0.794).
Table 2.
Overall mean ± Standard Error (SE), range and Kruskal–Wallis One-Way Analysis of Variance test of toxic metals (Cd, Hg and Pb) measured for eleven fish species from Aiba Reservoir, Iwo, Nigeria.
| Fish species | Cd (ppm) Mean ± SE |
Hg (ppm) Mean ± SE |
Pb (ppm) Mean ± SE |
|---|---|---|---|
| Marcusenius senegalensis | 0.007 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 |
| Labeo senegalensis | 0.020 ± 0.001 | 0.008 ± 0.004 | 0.020 ± 0.008 |
| Hepsetus odoe | 0.007 ± 0.001 | 0.005 ± 0.001 | 0.007 ± 0.001 |
| Chrysichthys auratus | 0.006 ± 0.001 | 0.004 ± 0.001 | 0.005 ± 0.001 |
| Chrysichthys nigrodigitatus | 0.004 ± 0.001 | 0.002 ± 0.000 | 0.005 ± 0.001 |
| Clarias ebriensis | 0.013 ± 0.005 | 0.008 ± 0.003 | 0.009 ± 0.003 |
| Clarias macromystax | 0.008 ± 0.003 | 0.006 ± 0.003 | 0.008 ± 0.003 |
| Channa obscura | 0.012 ± 0.004 | 0.008 ± 0.003 | 0.014 ± 0.006 |
| Tilapia zillii | 0.013 ± 0.006 | 0.007 ± 0.004 | 0.024 ± 0.011 |
| Sarotherodon galilaeus | 0.009 ± 0.003 | 0.004 ± 0.002 | 0.021 ± 0.009 |
| Oreochromis niloticus | 0.022 ± 0.008 | 0.011 ± 0.005 | 0.021 ± 0.008 |
| Total | |||
| Mean ± SE (ppm) | 0.012 ± 0.002 | 0.006 ± 0.001 | 0.010 ± 0.001 |
| Range (ppm) | 0.001–0.125 | 0.000–0.067 | 0.001–0.100 |
| Species number (S) | 11 | 11 | 11 |
| Sample number (n) | 168 | 168 | 168 |
| (df = 10) | 6.25 | 12.59 | 7.67 |
| P-value | 0.794 | 0.247 | 0.794 |
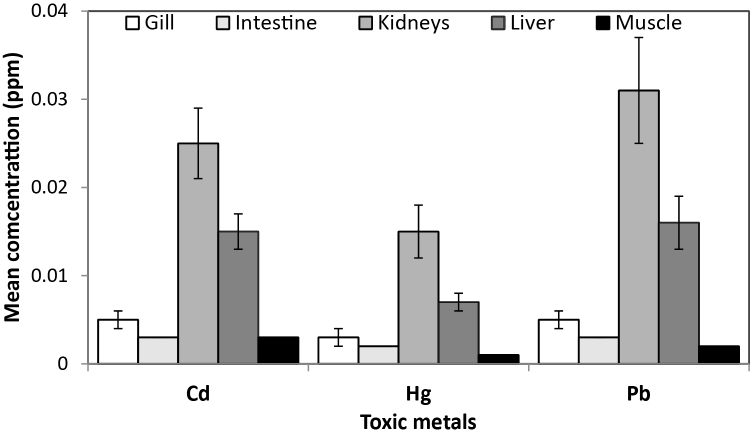
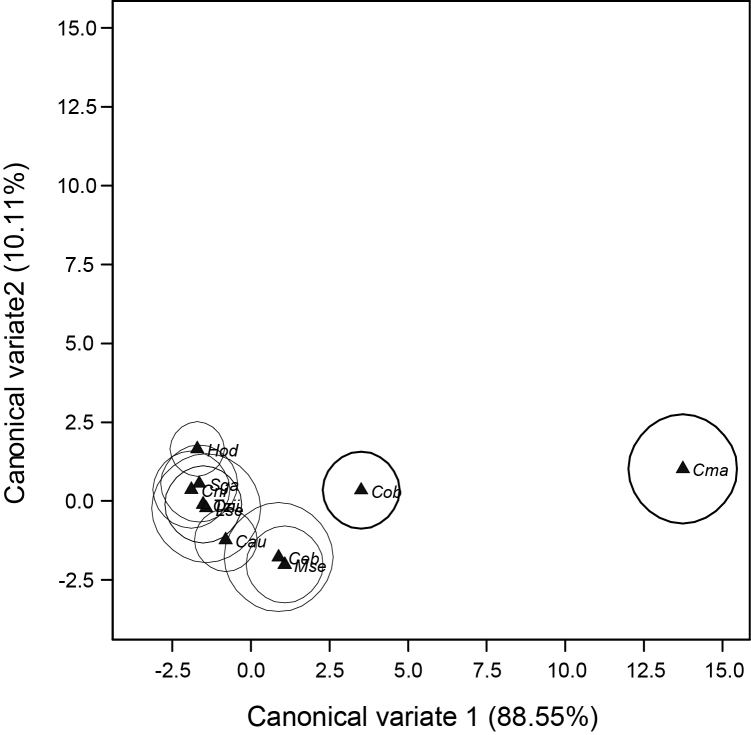
The pattern of mean values for the five fish organs showed Kidney > Liver > Gill > Intestine = Muscle for Cd; Kidney > Liver > Gill ≥ Intestine > Muscle for Hg; and Kidney > Liver > Gill > Intestine > Muscle for Pb (Fig. 2). Kruskal–Wallis One-Way ANOVA showed significant difference in mean gill concentration of trace metals for Cd ( = 30.78, df = 10, p = 0.001), Hg( = 28.21, df = 10, p = 0.002) and Pb( = 27.67, df = 10, p = 0.001) for all fish species studied (Table 3). C. macromystax had a significantly higher mean Cd (0.024 ± 0.004 ppm), Hg (0.017 ± 0.004 ppm) and Pb (0.024 ± 0.0004 ppm) values followed by C. obscura with mean Cd (0.010 ± 0.003 ppm), Hg (0.007 ± 0.002 ppm) and Pb (0.010 ± 0.0003 ppm) values compared with the three cichlid species, two bagrid species, H. odoe and L. senegalensis. Further analysis with canonical variates plot shows that C. macromystax and C. obscura are distinctly separated, with the latter having a lesser separation, from the other fish species with regards to the distribution of Cd, Hg and Pb (Fig. 3). The first two canonical variates accounted for 98.66% of the total variation.
Fig. 2.
Overall mean values (with standard error bars) to show the pattern of cadmium (Cd), mercury (Hg) and lead (Pb) accumulation in five organs of all fish species caught from Aiba Reservoir.
Table 3.
Non-parametric Kruskal–Wallis One-Way Analysis of Variance Test on mean values of Cd, Hg and Pb for the gills of eleven fish species from Aiba Reservoir, Iwo, Nigeria.
| Fish species | Cd (ppm) Mean ± SE |
Hg (ppm) Mean ± SE |
Pb (ppm) Mean ± SE |
|---|---|---|---|
| Marcusenius senegalensis | 0.006 ± 0.001 | 0.004 ± 0.001 | 0.004 ± 0.001 |
| Labeo senegalensis | 0.002 ± 0.001 | 0.001 ± 0.001 | 0.002 ± 0.001 |
| Hepsetus odoe | 0.003 ± 0.000 | 0.002 ± 0.000 | 0.004 ± 0.000 |
| Chrysichthys auratus | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.003 ± 0.001 |
| Chrysichthys nigrodigitatus | 0.004 ± 0.000 | 0.003 ± 0.000 | 0.004 ± 0.000 |
| Clarias ebriensis | 0.007 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 |
| Clarias macromystax | 0.024 ± 0.004 | 0.017 ± 0.004 | 0.024 ± 0.004 |
| Channa obscura | 0.010 ± 0.003 | 0.007 ± 0.002 | 0.010 ± 0.003 |
| Tilapia zillii | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.002 ± 0.000 |
| Sarotherodon galilaeus | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.003 ± 0.000 |
| Oreochromis niloticus | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.002 ± 0.000 |
| (df = 10) P-value |
30.78 0.001 |
28.21 0.002 |
27.67 0.002 |
Fig. 3.
Canonical variate analysis plot of gill concentration of Cd, Hg and Pb against sampled fish species with 95% confidence regions of means.
The Canonical Variate mean (dark triangle) and 95% confidence region (circle) of toxic trace metals (Cd, Hg and Pb) liver concentration for fish species from Aiba Reservoir. The first two Canonical Variates accounted for 98.66% of the total variation.
Key:
Cau = Chrysichthys auratus; Ceb = Clarias ebriensis; Cma = Clarias macromystax; Cni = Chrysichthys nigrodigitatus; Cob = Channa obscura; Hod = Hepsetus odoe; Lse = Labeo senegalensis; Mse = Marcusenius senegalensis; Oni = Oreochromis niloticus; Sga = Sarotherodon galilaeus; Tzi = Tilapia zillii.
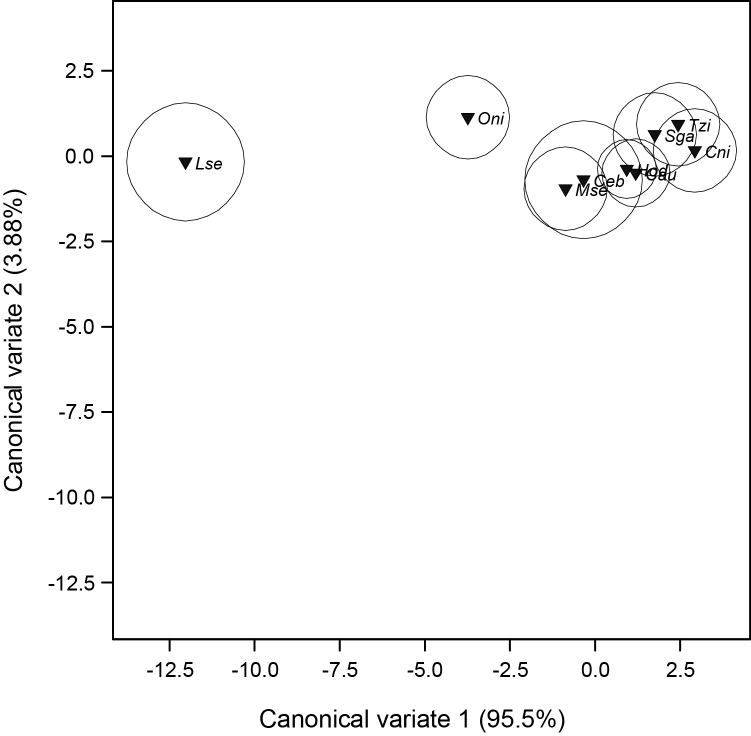
There was no significant difference (p > 0.05) in the distribution of Cd, Hg and Pb in intestine of five fish species recorded for the study (Table 4). Only Pb recorded a significant ( = 17.14, df = 9, p = 0.047) mean contamination for fish kidneys with T. zillii having a very high level of Pb in the kidney(0.113 ± 0.013 ppm) compared to other species(Table 5). There was significant difference in the distribution of Cd ( = 17.80, df = 8, p = 0.023) and Pb( = 22.17, df = 8, p = 0.005) for liver among fish species(Table 6). Canonical variates analysis for the distribution of trace metal levels for liver among fish species shows that L. senegalensis and O. niloticus have distinct separation (as both species recorded very high mean levels of Cd and Pb) from the other fish species with the latter having a lesser separation (Fig. 4). The first two canonical variates accounted for 99.38% of the total variation (CV1 = 95.5% and CV2 = 3.88%). The mean levels of trace metals in muscle did not show any significant difference among fish species studied for Cd ( = 14.40, df = 10, p = 0.155), Hg( = 14.40, df = 10, p = 0.155) and Pb( = 15.75, df = 10, p = 0.107)(Table 7).
Table 4.
Non-parametric Kruskal–Wallis One-Way Analysis of Variance Test on mean values of Cd, Hg and Pb for the intestines of five fish species from Aiba Reservoir, Iwo, Nigeria.
| Fish species | Cd (ppm) Mean ± SE |
Hg (ppm) Mean ± SE |
Pb (ppm) Mean ± SE |
|---|---|---|---|
| Marcusenius senegalensis | 0.002 ± 0.001 | 0.001 ± 0.001 | 0.002 ± 0.001 |
| Hepsetus odoe | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.003 ± 0.001 |
| Chrysichthys auratus | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.004 ± 0.001 |
| Clarias ebriensis | 0.004 ± 0.001 | 0.003 ± 0.001 | 0.004 ± 0.001 |
| Clarias macromystax | 0.005 ± 0.001 | 0.003 ± 0.001 | 0.005 ± 0.001 |
| (df = 4) P-value |
7.24 0.124 |
6.20 0.185 |
7.24 0.124 |
Table 5.
Non-parametric Kruskal–Wallis One-Way Analysis of Variance Test on mean values of Cd, Hg and Pb for the kidneys of ten fish species from Aiba Reservoir, Iwo, Nigeria.
| Fish species | Cd (ppm) Mean ± SE |
Hg (ppm) Mean ± SE |
Pb (ppm) Mean ± SE |
|---|---|---|---|
| Marcusenius senegalensis | 0.010 ± 0.002 | 0.006 ± 0.002 | 0.010 ± 0.002 |
| Labeo senegalensis | 0.025 ± 0.005 | 0.015 ± 0.005 | 0.025 ± 0.005 |
| Hepsetus odoe | 0.016 ± 0.003 | 0.011 ± 0.003 | 0.017 ± 0.002 |
| Chrysichthys auratus | 0.012 ± 0.002 | 0.008 ± 0.002 | 0.010 ± 0.003 |
| Chrysichthys nigrodigitatus | 0.006 ± 0.002 | 0.003 ± 0.001 | 0.007 ± 0.003 |
| Clarias ebriensis | 0.050 ± 0.010 | 0.030 ± 0.010 | 0.030 ± 0.010 |
| Channa obscura | 0.035 ± 0.005 | 0.025 ± 0.005 | 0.045 ± 0.005 |
| Tilapia zillii | 0.063 ± 0.013 | 0.038 ± 0.013 | 0.113 ± 0.013 |
| Sarotherodon galilaeus | 0.022 ± 0.010 | 0.008 ± 0.006 | 0.061 ± 0.030 |
| Oreochromis niloticus | 0.046 ± 0.023 | 0.028 ± 0.015 | 0.031 ± 0.013 |
| (df = 9) P-value |
16.25 0.062 |
16.09 0.065 |
17.14 0.047 |
Table 6.
Non-parametric Kruskal–Wallis One-Way Analysis of Variance Test on mean values of Cd, Hg and Pb for the liver of nine fish species from Aiba Reservoir, Iwo, Nigeria.
| Fish species | Cd (ppm) Mean ± SE |
Hg (ppm) Mean ± SE |
Pb (ppm) Mean ± SE |
|---|---|---|---|
| Marcusenius senegalensis | 0.015 ± 0.002 | 0.010 ± 0.002 | 0.008 ± 0.001 |
| Labeo senegalensis | 0.050 ± 0.017 | 0.017 ± 0.017 | 0.050 ± 0.017 |
| Hepsetus odoe | 0.019 ± 0.003 | 0.007 ± 0.002 | 0.009 ± 0.004 |
| Chrysichthys auratus | 0.008 ± 0.001 | 0.005 ± 0.001 | 0.005 ± 0.001 |
| Chrysichthys nigrodigitatus | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.006 ± 0.002 |
| Clarias ebriensis | 0.013 ± 0.003 | 0.008 ± 0.003 | 0.008 ± 0.003 |
| Tilapia zillii | 0.012 ± 0.003 | 0.005 ± 0.002 | 0.022 ± 0.008 |
| Sarotherodon galilaeus | 0.011 ± 0.005 | 0.005 ± 0.003 | 0.019 ± 0.008 |
| Oreochromis niloticus | 0.038 ± 0.010 | 0.016 ± 0.007 | 0.049 ± 0.021 |
| (df = 8) P-value |
22.17 0.005 |
10.77 0.215 |
17.80 0.023 |
Fig. 4.
Canonical variate analysis plot of liver concentration of Cd, Hg and Pb against sampled fish species with 95% confidence regions of means.
The Canonical Variate mean (dark triangle) and 95% confidence region (circle) of toxic trace metals (Cd, Hg and Pb) liver concentration for fish species from Aiba Reservoir. The first two Canonical Variates accounted for 99.38% of the total variation.
Key:
Cau = Chrysichthys auratus; Ceb = Clarias ebriensis; Cma = Clarias macromystax; Cni = Chrysichthys nigrodigitatus; Cob = Channa obscura; Hod = Hepsetus odoe; Lse = Labeo senegalensis; Mse = Marcusenius senegalensis; Oni = Oreochromis niloticus; Sga = Sarotherodon galilaeus; Tzi = Tilapia zillii.
Table 7.
Non-parametric Kruskal–Wallis One-Way Analysis of Variance Test on mean values of Cd, Hg and Pb for the muscle of eleven fish species from Aiba Reservoir, Iwo, Nigeria.
| Fish species | Cd (ppm) Mean ± SE |
Hg (ppm) Mean ± SE |
Pb (ppm) Mean ± SE |
|---|---|---|---|
| Marcusenius senegalensis | 0.003 ± 0.000 | 0.002 ± 0.000 | 0.003 ± 0.000 |
| Labeo senegalensis | 0.002 ± 0.000 | 0.001 ± 0.001 | 0.002 ± 0.001 |
| Hepsetus odoe | 0.003 ± 0.000 | 0.002 ± 0.000 | 0.003 ± 0.000 |
| Chrysichthys auratus | 0.003 ± 0.000 | 0.002 ± 0.000 | 0.003 ± 0.000 |
| Chrysichthys nigrodigitatus | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.004 ± 0.000 |
| Clarias ebriensis | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.004 ± 0.001 |
| Clarias macromystax | 0.003 ± 0.001 | 0.002 ± 0.001 | 0.003 ± 0.001 |
| Channa obscura | 0.003 ± 0.000 | 0.002 ± 0.000 | 0.002 ± 0.000 |
| Tilapia zillii | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.004 ± 0.001 |
| Sarotherodon galilaeus | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.004 ± 0.001 |
| Oreochromis niloticus | 0.002 ± 0.000 | 0.001 ± 0.000 | 0.003 ± 0.001 |
| (df = 10) P-value |
14.40 0.155 |
14.40 0.155 |
15.75 0.107 |
3.2. Discussion
Trace metals studied were above detection levels in fish organs. The mean level of trace metal concentration recorded for this study is in the order Cd > Pb > Hg; and ranged between 0.001 and 0.125 ppm for Cd, 0.001–0.100 for Pb and 0.000–0.067 for Hg. The low levels of these trace elements in fish suggest low trace metal levels in the reservoir water and sediments; which may be attributed to level of development of the town as there is no major industry compared to other surrounding cities in southwest Nigeria. Fish from a freshwater body receiving industrial effluent has been regarded as unfit for human consumption because of high tissue levels of toxic trace metals such as Cd and Pb [22]. However, observed contamination of fish suggests anthropogenic influence from point and non-point sources on the reservoir such as atmospheric deposition, run-off from agricultural areas and the influence of municipal wastes through the catchment area. The inability to discriminate between fish species studied for Cd, Hg and Pb when all organs were combined suggests large intraspecific variation in fish, which may be due to uptake, age, sex [28] and individual metabolic response to detoxification [1].
The distribution of trace metal studied for fish organs show similar pattern with Kidney > Liver > Gill ≥ Intestine ≥ Muscle. Kidney and liver recorded significantly higher trace metal levels for all fish species studied compared to gill, intestine and muscle. Cadmium has been reported to bioaccumulate most significantly in the kidney followed by liver and gills [29]. The strong affinity for the non-essential trace elements Cd and Pb by the kidney suggests tolerance of organs to chronic Pb and Cd intoxication [30]; thereby exhibiting great nephrotoxic potentials [31].
Kidney is the gateway for heavy metal detoxification in the body [32]. There was no clear discrimination (p ≥ 0.05) among fish species for trace metal accumulation in the kidney except for Pb with T. zillii having a relatively high concentration. The result shows some level of accumulation of trace metals in fish kidney; and suggests similar metal detoxification ability by fish species studied. Laboratory experiments have shown that cadmium exposure results in observed differential behavioural anomalies in different fish species [33]. Dissolved cadmium in freshwater can rapidly cause physiological changes in the gill and kidney of fish by affecting osmotic control and enzyme activity [34].
L. senegalensis and O. niloticus were clearly discriminated from other fish species studied based on the distribution of Cd and Pb in liver. This is in agreement with the work of Benson et al. [35] and Yilmaz [36] that reported significantly higher liver trace metal values for O. niloticus from different aquatic ecosystems within the Niger Delta region in Nigeria and Anguilla anguilla from Köycegiz Lake in Turkey, respectively. Jirsa et al. [37] reported also that the liver of a predominantly herbivorous fish (Chondrostoma nasus) is the preferred organ for Cd and Pb storage. Benson et al. [35] regarded O. niloticus as a good bioindicator of Hg contamination of aquatic environments. However, for this study, despite high liver mean Hg levels recorded for L. senegalensis and O. niloticus, mercury did not discriminate significantly among the fish species. Maximum background level of Hg in uncontaminated freshwater fish is reported to be about 0.2 μg/g [38]; and a recommended limit of 1.0 ppm total mercury was set by USFDA [39]. Most Hg in fish tissue is methylmercury due to its preferential uptake, ability to be transferred among tissues and slow depuration; and the main depuration pathway of Hg is through the liver and kidney in fish [40]. Trophic status, dietary patterns and activity of a fish have been reported to be important in the bioaccumulation of total mercury by fish [40], [41], [42]; consequently higher Hg values have been recorded from the organs of predator and non-sedentary (less active) fish [16], [23], [43], [44], [45], [46]. Conversely, Black et al. [47] reported lower than average mercury concentrations in fish from tropical Africa; with piscivorous fish recording lower mean values than non-piscivorous fish. Higher levels of trace metal accumulation in liver have been attributed to the presence of metallothionein proteins that enables fish to detoxify trace metals [48].
The gill is an important site for the entry of trace metals [32]; and is the first target organ for exposure in fish. Gills of C. macromystax and C. obscura showed significantly higher levels of trace metal studied compared to gills of other fish species in the reservoir; suggesting that their gills could accumulate trace metals from the environment. C. macromystax and C. obscura are both omnivorous feeders (the former being a bottom feeder) and are rarely caught in the reservoir except occasionally during the dry season when the water level is low. High gills trace metal levels have been reported for catfish (Clarias gariepinus) from Ikpoba and Ogba Rivers [11]; A. anguilla from Köycegiz Lake in Turkey [36]; and in Cyprinus carpio [32].
Trace metals in the intestine of fishes is related to the diet and feeding behaviour of fishes [4], [11]. Trace metals levels recorded in the intestine of fish studied shows no discrimination with regard to fish species. This may support the view that intestinal levels reflect diet, as three of the five species analysed have similar diets being omnivorous except for M. senegalensis and H. odoe whose major diets are insect larvae and fish respectively. The low level of trace metals detected in the intestine may suggest that food source plays a less important role in trace metal uptake for fish species in Aiba Reservoir.
Muscle, an edible portion of fish that plays an important role in human nutrition, has been reported to have the lowest concentration of metals except for mercury [4] compared to other organs [17], [18], [28], [36], [48], [49], [50], [51]. Fish muscle exhibited the least mean concentration of all fish organs studied. This contrasts with a study on three fish species from Lake Balaton, Hungary [16], where muscle levels of Hg were higher than for gills and liver. Mbabazi and Wasswa [52] explained that the low level of Pb in fish muscle compared to ambient water is the relatively low rate of its binding to –SH groups and the low solubility of lead salts that restrict movement across cell membranes.
The result shows that the edible portion (muscle) of all fish studied have low levels of trace metals, although noticeable levels especially of Cd and Pb were recorded from kidney and liver tissues. This study supports earlier reports that some trace metals are rarely distributed uniformly within fish body tissues and are therefore accumulated by particular target organs [7] such as liver and kidneys [9]. Due to the nutritional benefits of fish, periodic monitoring of aquatic environments and its biota has been suggested to avoid excessive intake of trace metals by humans from consumption of fish and other aquatic biota [19], [53], [54]. Abolude et al. [55] also suggested reduction in farming activities around reservoirs in order to reduce excessive discharge into the water body.
Variation in the level of trace metals among different fish species inhabiting the same aquatic environment has been attributed to differences in foraging habits [17], behaviour and habitat [56]. Although, Mokhtar et al. [57] opined that it is difficult to compare similar tissue concentration of heavy metals from different species due to factors such as physiological tolerance, body reaction and regulatory mechanisms, however, differences in the levels of trace metals in different tissues of organisms may be attributed to their tendency to bind to specific cellular molecular groups and to the metabolic characteristics of such tissues [48]. This study suggests that such ability may be species specific in some instances.
Differential accumulation of trace metals in fish organs from a single freshwater body regardless of ambient levels may suggest different regulatory strategies by fish; consequently making fish good indicators of trace metal contamination of freshwaters. Moreso, reduction of accumulation of non-essential trace metals in some taxonomic groups may be a physiological mechanism for metal resistance and adaptation. Although sample size for this study is rather small, this paper suggests that rarely caught fish may be better indicators of freshwater contamination through the gills while L. senegalensis (Cyprinidae) and O. niloticus (Cichlidae) accumulate trace metals in the liver through strategies not necessarily related to taxonomic distinctness and foraging habits.
Acknowledgement
The authors are grateful to Bowen University, Iwo, who provided funds for this study through the research grant reference number BUI/CRLCF/01/10.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.06.003.
Contributor Information
Oluwatosin Ebenezer Atobatele, Email: tosine.ben@gmail.com.
Godwin Oladele Olutona, Email: delog2@gmail.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Oliveira Ribeiro C.A., Vollaire Y., Sanchez-Chardi A., Roche H. Bioaccumulation and the effects of organochlorine pesticides, PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat. Toxicol. 2005;74:53–69. doi: 10.1016/j.aquatox.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Deacon J.R., Stephens V.C. Trace elements in streambed sediment and fish liver at selected sites in the upper Colorado River Basin, Colorado, 1995–1996. US Geol. Surv. Water-Res. Invest. Rep. 1998;98–4124:22. [Google Scholar]
- 3.Kumolu-Johnson C.A., Ndimele P.E., Akintola S.L., Jibuike C.C. Copper, zinc and iron concentrations in water, sediment and Cynothrissa mento (Regan 1917) from Ologe Lagoon, Lagos, Nigeria: a preliminary survey. Afr. J. Aquat. Sci. 2010;35:87–94. [Google Scholar]
- 4.Farkas A., Salánki J., Specziár A., Varanka I. Metal pollution as health indicator of Lake ecosystems. Int. J. Occup. Med. Environ. Health. 2001;14(2):163–170. [PubMed] [Google Scholar]
- 5.Naigaga I., Kaiser H., Muller W.J., Ojok L., Mbabazi D., Magezi G., Muhumuza E. Fish as bioindicators in aquatic environmental pollution assessment: a case study in Lake Victoria wetlands, Uganda. Phys. Chem. Earth. 2011;36:918–928. [Google Scholar]
- 6.Nhiwatiwa T., Barson M., Harrison A.P., Utete B., Cooper R.G. Metal concentrations in water, sediment and sharptooth catfish Clarias gariepinus from three peri-urban rivers in the upper Manyame catchment, Zimbabwe. Afr. J. Aquat. Sci. 2011;36:243–252. [Google Scholar]
- 7.Annabi A., Said K., Messaoudi I. Cadmium: bioaccumulation, histopathology and detoxifying mechanisms in fish. Am. J. Res. Commun. 2013;1:60–79. [Google Scholar]
- 8.Egila J.N., Daniel V.N. Trace metals accumulation in freshwater and sediment insects of Liberty Dam, Plateau State Nigeria. Int. J. Basic Appl. Sci. 2011;11:128–140. [Google Scholar]
- 9.Eisler R. Cadmium hazard to fish, wildlife and invertebrates: a synoptic review. U.S Fish Wildl. Serv. Biol. Rep. 1985;85(12):1–30. [Google Scholar]
- 10.Järup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 11.Obasohan E.E., Oronsaye J.A.O., Eguavoen O.I. A comparative assessment of the heavy metal loads in the tissues of a common catfish (Clarias gariepinus) from Ikpoba and Ogba Rivers in Benin City, Nigeria. Afr. Sci. 2008;9:13–23. [Google Scholar]
- 12.Vinodhini R., Narayanan M. Heavy metal induced histopathological alterations in selected organs of the Cyprinus carpio L. (Common carp) Int. J. Environ. Res. 2009;3:95–100. [Google Scholar]
- 13.Rajamanickam V., Muthuswamy N. Effect of heavy metals on the level of vitamin, total lipid and glycogen reserves in the liver of common carp (Cyprinus carpio L.) Maejo Int. J. Sci. Technol. 2008;2:391–399. [Google Scholar]
- 14.Georgieva E., Velcheva I., Yancheva V., Stoyanova S. Trace metal effects on gill epitheliumof common carp Cyprinus carpio L. (cyprinidae) Acta Zoologica Bulgarica. 2014;66:277–282. [Google Scholar]
- 15.DeForest D.K., Brix K.V., Adams W.J. Assessing metal bioaccumulation in aquatic environments: the inverse relationship between bioaccumulation factors, trophic transfer factors and exposure concentration. Aquat. Toxicol. 2007;84:236–246. doi: 10.1016/j.aquatox.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 16.Farkas A., Salánki J., Varanka I. Heavy metal concentrations in fish of Lake Balaton. Lakes Reservoirs: Res. Manag. 2000;5:271–279. [Google Scholar]
- 17.Yilmaz A.B. Comparison of heavy metal levels of grey mullet (Mugil cephalus L.) and sea bream (Sparus aurata L.) caught in İskenderun Bay (Turkey) Turk. J. Vet. Animal Sci. 2005;29:257–262. [Google Scholar]
- 18.Zhang Z., He L., Li J., Wu Z. Analysis of heavy metals of muscle and intestine tissue in fish – in Banan section of Chongquing from three Gorges Reservoir. China. Pol. J. Environ. Stud. 2007;16:949–958. [Google Scholar]
- 19.Obasohan E.E., Oronsaye J.A.O., Obano E.E. Heavy metal concentrations in Malapterurus electricus and Chrysichthys nigrodigitatus from Ogba River in Benin City, Nigeria. Afr. J. Biotechnol. 2006;5:974–982. [Google Scholar]
- 20.Obasohan E.E. Heavy metals concentrations in the offal gill muscle And liver Of A freshwatermudfish (Parachanna obscura) from Ogba River in Benin City, Nigeria. Afr. J. Biotechnol. 2007;6:2620–2627. [Google Scholar]
- 21.Maitera O.N., Ogugbuaja V.O., Barminas J.T. Determination of trace metal levels in water and sediments of River Benue in Adamawa State, Nigeria. J. Ecol. Nat. Environ. 2012;3:149–156. [Google Scholar]
- 22.Tyokumbur E., Okorie T. Toxic trace metal contamination (Arsenic, Cadmium and Lead) of Sarotherodon melanotheron (Rupell, 1852) from Alaro Stream in Ibadan. J. Food Nutr. Sci. 2014;2:258–261. [Google Scholar]
- 23.Burger J., Gochfeld M. Mercury and selenium levels in 19 species of saltwater fish from New Jersey as a function of species, size, and season. Sci. Total Environ. 2011;409:1418–1429. doi: 10.1016/j.scitotenv.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Soto D.X., Roig R., Gacia E., Catalan J. Differential accumulation of mercury and other trace metals in the food web components of a reservoir impacted by chlo-alkali plant (Flix, Ebro River, Spain): implications for biomonitoring. Environ. Pollut. 2011;159:1481–1489. doi: 10.1016/j.envpol.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 25.Atobatele O.E., Ugwumba O.A. Seasonal variation in the physicochemistry of a small tropical reservoir (Aiba Reservoir, Iwo, Osun, Nigeria) Afr. J. Biotechnol. 2008;7:1962–1971. [Google Scholar]
- 26.Atobatele O.E. Pelagic phytoplankton succession pattern in a tropical freshwater reservoir (Aiba Reservoir, Iwo, Osun, Nigeria) Biorem. Biodivers. Bioavailability. 2013;7:81–84. [Google Scholar]
- 27.Adesulu E.A., Sydenham D.H.J. Macmillan Nigeria Publishers Limited; Nigeria: 2007. The Freshwater Fishes and Fisheries of Nigeria; p. 397. ISBN 978-978-018-362-2. [Google Scholar]
- 28.Protasowicki M., Morsy G. Preliminary studies on heavy metal contents in aquatic organisms found from the Hornsund area, with a particular reference to the arctic charr (Salvelinus alpinus L.) Acta Ichthyologica et Piscatoria. 1993;23(Suppl):115–132. [Google Scholar]
- 29.Pretto A., Loro V.L., Baldisserotto B., Pavanato M.A., Moraes B.S., Menesez C., Cattaneo R., Clasen B., Finamor I.A., Dressler V. Effects of water cadmium concentrations on bioaccumulation and various oxidative stress parameters in Rhamida quelen. Arch. Environ. Contam. Toxicol. 2011;60:309–318. doi: 10.1007/s00244-010-9586-2. [DOI] [PubMed] [Google Scholar]
- 30.Yabe J., Nakayama S.M.M., Ikenaka Y., Muzandu K., Choongo K., Mainda G., Kabeta M., Isshizuka M., Umemura T. Metal distribution in tissues of free-range chickens near lead-zinc mine in Kabwe, Zambia. Environ. Toxicol. Chem. 2013;32:189–192. doi: 10.1002/etc.2029. [DOI] [PubMed] [Google Scholar]
- 31.Gonick H.C. Nephrotoxicity of cadmium & lead. Indian J. Med. Res. 2008;128:335–352. [PubMed] [Google Scholar]
- 32.Vinodhini R., Narayanan M. Bioaccumulation of heavy metals in organs of freshwater fish Cyprinus carpio (Common carp) Int. J. Environ.l Sci. Technol. 2008;5:179–182. [Google Scholar]
- 33.Triputhi D.R. Sensitivity evaluation in two commonly occurring freshwater fishes after intoxication with cadmium. IOSR J. Environ. Sci. Toxicol. Food Technol.(IOSR–JESTFT) 2014;8:102–105. [Google Scholar]
- 34.da Silva A.O.F., Martinez C.B.R. Acute effects of cadmium on osmoregulation of the freshwater teleost Prochilodus lineatus: Enzyme activity and plasma ions. Aquat. Toxicol. 2014;156:161–168. doi: 10.1016/j.aquatox.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Benson N.U., Essien J.P., Williams A.B., Bassey D.E. Mercury accumulation in fishes from tropical aquatic ecosystems in the Niger Delta, Nigeria. Curr. Res. 2007;92:781–785. [Google Scholar]
- 36.Yilmaz F. The comparison of heavy metal concentrations (Cd, Cu, Mn, Pb, and Zn) in tissues of three economically important fish (Anguilla Anguilla Mugil cephalus and Oreochromis niloticus) inhabiting Köycegiz Lake-Mugla (Turkey) Turk. J.Sci. Technol. 2009;4:7–15. [Google Scholar]
- 37.Jirsa F., Leodolter-Dorak M., Krachler R., Frank C. Heavy metals in the nase, Chondrostoma nasus (L. 1958), and its intestinal parasite Caryophyllaeus laticeps (Pallas 1781) from Austrian Rivers: bioindicative aspects. Arch. Environ. Contam. Toxicol. 2008;55:619–626. doi: 10.1007/s00244-008-9154-1. [DOI] [PubMed] [Google Scholar]
- 38.Ullrich S.M., Tanton T.W., Abdrashitova S.A. Mercury in the aquatic environment: a review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol. 2001;31:241–293. [Google Scholar]
- 39.Nauen C.E. Compilation of legal limits for hazardous substances in fish and fishery products. FAO Fish. Circ. 1983;764:102. [Google Scholar]
- 40.Beckvar N., Field J., Salazar S., Hoff R. Contaminants in aquatic habitats at waste sites: Mercury. NOAA Tech. Memorandum NOS ORCA Seattle Hazard. Mater. Response Assess. Div. Nat. Oceanic Atmos. Administration. 1996:74. [Google Scholar]
- 41.Campbell L., Dixon D.G., Hecky R.E. A review of mercury in Lake Victoria, East Africa: implications for human and ecosystem health. J. Toxicol. Environ. Health, Part B: Crit. Rev. 2003;6:325–356. doi: 10.1080/10937400306474. [DOI] [PubMed] [Google Scholar]
- 42.Kwaansa-Ansah E.E., Adimado A.A., Ephraim J.H. Distribution of mercury in water,sediment and fish from the Volta Lake and its major tributaries. J. Environ. Occup. Sci. 2012;1:27–36. [Google Scholar]
- 43.Studnicka M. Studies on mercury content in muscles of fishes from some natural Polish water bodies. Acta Ichthyologica et Piscatoria. 1981;11:59–73. [Google Scholar]
- 44.Kasper D., Palermo E.F.A., Dias A.C.M.I., Ferreira G.L., Leitão R.P., Branco C.W.C., Malm O. Mercury distribution in different tissues and trophic levels of fish from a tropical reservoir, Brazil. Neotrop. Ichthyol. 2009;7:751–758. [Google Scholar]
- 45.Marrugo-Negrete J., Benítez L.N., Olivero-Verbel J., Lans E., Gutierrez F.V. Spatial and seasonal mercury distribution in the Ayapel Marsh, Mojana region, Colombia. Int. J. Environ. Health Res. 2010;20:451–459. doi: 10.1080/09603123.2010.499451. [DOI] [PubMed] [Google Scholar]
- 46.Chodiniecki A., Kurpios M., Protasowicki M., Ociepa A., Juran J. Studies on mercury content in selected fish species from Pomeranian Gulf and Szczecin Firth. Acta Ichthyologica Et Piscatoria. 1975;5:51–57. [Google Scholar]
- 47.Black F.J., Bokhutlo T., Somoxa A., Maethamako M., Modisaemang O., Kemosedile T., Cobb-Adams C., Mosepele K., Chimbari M. The tropical African mercury anomaly: lower than expected mercury concentrations in fish and human hair. Sci. Total Environ. 2011;409:1967–1975. doi: 10.1016/j.scitotenv.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 48.El-Sadaawy M.M., El-Said G.F., Sallam N.A. Bioavailability of heavy metals in freshwater Tilapia nilotica (Oreochromis niloticus Linnaeus, 1758): potential risk to fishermen and consumers. J. Environ. Sci. Health, Part B. 2013;48:402–409. doi: 10.1080/03601234.2013.742719. [DOI] [PubMed] [Google Scholar]
- 49.Al-Weher S.M. Levels of heavy metal Cd, Cu and Zn in three fish species collected from the Northern Jordan Valley, Jordan. Jordan Journal Of Biological Sciences. 2008;1:41–46. [Google Scholar]
- 50.Krywult M., Klich M., Szarek-Gwiazda E. Metal concentrations in chub, Leuciscus cephalus, from a submontane river. Acta Ichthyologica Et Piscatoria. 2008;38:47–53. [Google Scholar]
- 51.Reynders H., Bervoets L., Gelders M., De Coen W.M., Blust R. Accumulation and effects of metals in caged carp and resident roach along a metal pollution gradient. Sci. Total Environ. 2008;391:82–95. doi: 10.1016/j.scitotenv.2007.10.056. [DOI] [PubMed] [Google Scholar]
- 52.Mbabazi J., Wasswa J. Contamination by heavy metals in silver fish (Rastreneobola argentea) caught from Lakes Kyoga and Victoria, Uganda. Int. J. Environ. Stud. 2010;67:543–556. [Google Scholar]
- 53.Olowu R.A., Ayejuyo O.O., Adewuyi G.O., Adejoro I.A., Denloye A.A.B., Babatunde A.O., Ogundajo A.L. Determination of heavy metals in fish tissues, water and sediment from Epe and Badagry Lagoons, Lagos, Nigeria. E-J. Chem. 2010;7:215–221. [Google Scholar]
- 54.Mol S., Özden O., Oymak S.A. Trace metal contents in fish species from Atarturk Dam Lake (Euphrates, Turkey) Turk. J. Fish. Aquat. Sci. 2010;10:209–213. [Google Scholar]
- 55.Abolude D.S., Davies O.A., Ayong D.W. Level of heavy metals in freshwater crab (Cardisoma guahumi) obtained from Ahmadu Bello University Reservoir, Zaria, Nigeria. Int. J. Animal Vet. Adv. 2009;1:54–58. [Google Scholar]
- 56.Mwashote B.M. Levels of cadmium and lead in water, sediments and selected fish species in Mombasa, Kenya. Western Indian Ocean Journal Of Marine Science. 2003;2:25–34. [Google Scholar]
- 57.Mokhtar M.B., Aris A.Z., Munusamy V., Praveena S.M. Assessment level of heavy metals in Penaeus monodon and Oreochromis spp in selected aquaculture ponds of high densities development area. Eur. J. Sci. Res. 2009;30:348–360. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.