Abstract
Purpose
This study is aimed to the investigation of the features of morphological changes in the urinary bladder of mature rats.
Results
Received results shown that the experimental group of rats that had the intake of heavy metal salts (HMS) mixture during 30 and 90 days were observed histological and immunohistochemical changes in all structures of the urinary bladder. Depending on the period of influence of heavy metal ions on the wall of the organ, the pathological changes developed in cellular layers of the wall can lead to degenerative and later to atrophic and sclerotic changes.
Conclusions
This study demonstrates that high concentrations of HMS can significantly influence the body. The histological and immunohistochemical studies showed that the influence of the HMS combination leads to deep morphological changes in all structures of the urinary bladder. These changes depend on the period of intake of HMS. Analysis of the obtained results demonstrates the dependence of expression of morphological changes in the urinary bladder on the experiment duration. The final result of these changes may lead to the disorders of bladder’s functions.
Keywords: heavy metal salts, urinary bladder, histological studies, immunohistochemical studies, Ki-67, Hps90α, environment
Introduction
Anthropogenic impact on the environment is one of the threatening factors for planet ecosystem, which has negative impact on the living organisms [1, 2]. A significant portion of the exogenous factors that enters into biosphere is comprised of heavy metal salts (HMS). The prevalence and toxicity of these metals pose a significant problem to many countries [3, 4]. Unfortunately, because of the increase in day-to-day pollution by various emissions, ecological disasters, and industrial wastes, the number of HMS in water, soil, and atmospheric air steadily increases [5], which leads to its penetration and further accumulation in living organisms [6, 7]. Heavy metal ions in their physiological concentrations are involved in various vital processes, such as regulation of the fermentation activity, transportation of electrolytes, and regulation of redox reactions. Although these ions are contained in various vitamins, hormones, and others, the excessive intake of HMS leads to the metabolic disorders that have negative impact on the structure and functioning of tissues, organs, and systems [8–10].
The main function of the urogenital system is to excrete the large amount of toxic substances from the body. Urinary bladder plays important role by reserving, retaining, and excreting the urine. Finally, the metabolic end products and toxins are excreted together with the urine from the body. Additional intake of these exogenous substances (including HMS) may result in disorders of the urinary bladder functioning [11, 12].
From recent studies, some information about the impact of these urinary bladder pollutants [13, 14] is available. But studies on the combined effect of these HMS on the urogenital system are lacking.
Hence, in this study, we aimed to determine the morphological changes of the urinary bladder wall in mature rats under excessive intake of HMS.
Materials and Methods
The experiment was carried out on mature male Wistar albino rats, weighing 180–220 g, in the laboratory setting. The rats (n = 24) were divided into two groups: control group and experimental group. The rats in the control group received normal drinking water, while the rats in the experimental group received drinking water with the combination of HMS [zinc (ZnSO4 × 7H2O) – 5 mg/L, copper (CuSO4 × 5H2O) – 1 mg/L, iron (FeSO4) – 10 mg/L, manganese (MnSO4 × 5H2O) – 0.1 mg/L, lead (Pb(NO3)2) – 0.1 mg/L, and chromium (K2Cr2O7) 0.1 mg/L]. The laboratory rats were then kept in vivarium in accordance with the regulations adopted by European Convention for the Protection of Vertebrate Animals used for Scientific Purposes, principles of General Assembly World Medical Association Declaration of Helsinki, and general ethical principles on animal experiments.
To observe the changes in the urinary bladder, the rats were decapitated under ether narcosis (six animals from each group) on the 30th and 90th days. After midline laparotomy, the urinary bladder was removed, fixed in 10%-buffered formalin solution (24 h), dried in alcohol, and embedded with paraffin. The 5-μm-thick sections were made by the rotary microtome. To study the general structural features of the urinary bladder, the samples were stained with hematoxylin and eosin. Van Gieson’s method was used to study experimentally the characteristics of the collagen fibers in the bladder wall. Proliferative potential of the epithelial cells was immunohistochemically assessed by incubation with rabbit monoclonal Ki-67 antibodies (SP6 clone) at 1:100 dilution. The maximum Ki-67 expression was observed in active mitosis phases (G1-, S-, G2-, and M-phase) excluding phase G0. The reaction was considered as positive when the number of positively stained nuclei was more than 1% per 10 fields at magnification (400×). The presence of Ki-67 in the cells’ nuclei was assessed as follows: 1%–10%, weak positive; 10%–20%, medium positive; and more than 20%, strong positive reaction. To study the role of heat shock proteins, the expression level of nuclear receptor Hsp90α was evaluated. A semi-quantitative method was used to measure the staining intensity and the amount of cells. The reaction was scored as follows: 0, negative; 1, weak positive; 2, medium positive, and 3, strong positive reaction.
Obtained tissue specimen and the cells immune response were studied under microscope (Carl Zeiss Primo Star; Germany) with digital camera (Zeiss AxioCam ERc 5s; Germany) and photographed with a digital image output system (ZEN 2; blue edition; Germany).
Mathematical calculations were done using Microsoft excel 2010 with AtteStat 12.0.5.
Results
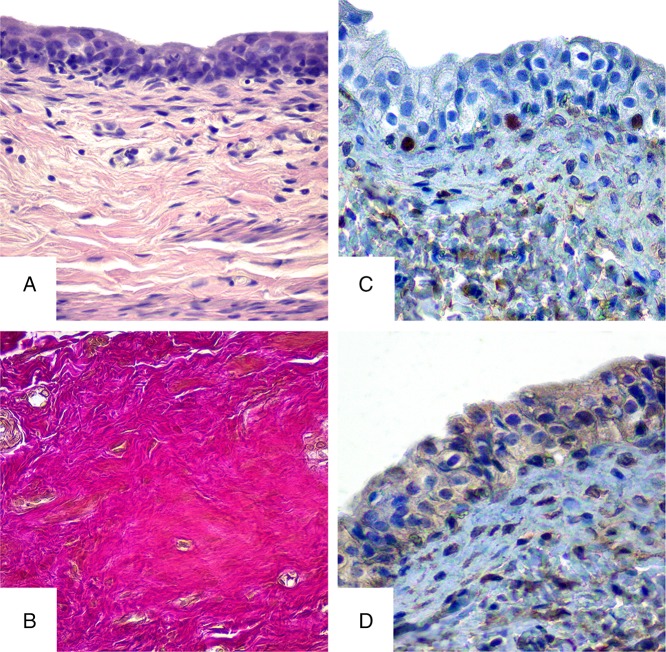
Histological study of the urinary bladder walls from the control group rats during the whole period (30 and 90 days) showed normal structure of all the membranes. Clear separation of the layers on mucous, submucous, muscular, and serous membranes was observed with close interconnections between them. The mucous membrane of the urinary bladder is lined with transitional epithelium-urothelial cells with numerous wrinkles. It is structured with three cell layers – surface (umbrella), intermediate, and basal. The surface layer cells are flat, horizontal, tightly interconnected and have one or more nucleuses. Basal and intermediate cells are much smaller and have one nucleus. The intermediate layer is structured with several cell layers compared with basal cell layer that is presented by single layer of epithelial cells. The urothelium layer consists of 4–5 cell layers. The two lower rows of cells have isolated lymphocytes (Fig. 1A). The mucous membrane is located on the loose submucous layer, tightly connected to the muscular membrane, where all layers of smooth myocytes are observed that are represented by outer, middle circular, and inner longitudinal layers. Connective tissue with blood vessels is present between them and muscular fascicles. Serous membrane is formed by loose connective tissue that is outermost layer of the urinary bladder. Vascular net of the urinary bladder wall has a typical structure with well-developed microvasculature.
Fig. 1.
Histological and immunohistochemical studies of the bladder in rats of control group. A – hematoxylin and eosin staining, B – Van Gieson stain, C – immunohistochemical study of Ki-67, and D – immunohistochemical study of receptors prior to Hsp90α. Magnification 400×
Van Gieson staining of the bladder wall of rats in the control group reveals sufficient bright red collagen fibers, which indicate their maturity. The fibers are round, flatten, spiroid, and wave shaped of different lengths and are present in different locations. Collagen fibers are network-forming compounds to ensure normal histoarchitecture and the ratio of plain muscle to connective tissue elements in the bladder wall (Fig. 1B).
In epithelial tissue of the urinary bladder of control group animals, the low expression of Ki-67 is observed, which is represented in isolated cells. Positive reaction was observed in the basal layer of the epithelium. Average index of Ki-67-positive cells was 5%–6% (Fig. 1C).
Hsp90α expression in the bladder urothelium of the control group was weak and appeared in the cytoplasm of sporadic cells, which indicated the normal condition (Fig. 1D).
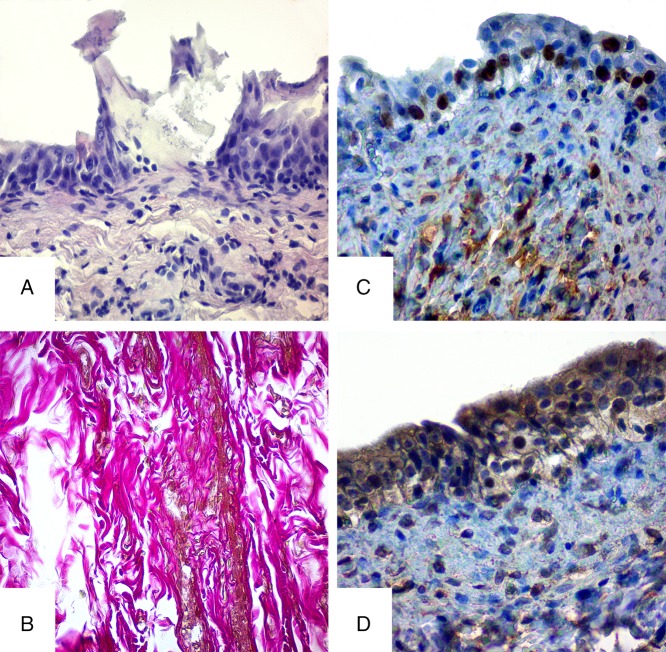
The histological examination of the urinary bladder in the experimental animals demonstrated the significant morphological changes on 30th day. Swelling and degenerative changes were observed in epithelium. The apical contour of the cells was indistinct, their cytoplasm was enlightened, and chromatin condensation and margination were observed in the nucleuses. Hydropic degeneration and coagulative necrosis as well as karyopyknosis and nucleorrhexis were observed in some cells of urothelium. In intermediate and basal layers of the urothelium, the increased number of lymphocytes was observed. Features of epithelium destruction and ecdysis of the surface layer were present on significant part of mucosa membrane of the urinary bladder. In place of ecdysis, there were depressions on the surface of the basal membrane, where the basal cells were located. Submucosa swelling with diffuse mixed cellular inflammatory infiltration depraves the visualization of transitional epithelium wrinkles. Structural layer disturbances, swelling, and thinning of smooth myocytes were present in muscular membrane of the organ. Histological changes in connective tissue were presented by swelling, histocytic, and lymphocytic infiltration. Venous congestion and stasis, focal perivascular hemorrhages, moderate lymphohysteocytic infiltration of connective tissue around the blood vessels were observed in the urinary bladder vessels.
Thus, all urinary bladder wall structures are damaged under the influence of HMS, but the most significant damages were observed in the mucosa and submucosa membranes (Fig. 2A).
Fig. 2.
Histological and immunohistochemical studies of the bladder in rats of experimental group on the 30th day of HMS combination intake. A – hematoxylin and eosin staining, B – Van Gieson stain, C – immunohistochemical study of Ki-67, and D – immunohistochemical study of receptors prior to Hsp90α. Magnification 400×
The bladder obtained from the laboratory control group rats, fed with HMS, showed changes in collagen fibers histoarchitecture with evidences of disintegration. Swellings and a lesion of fiber layers separation were present. Progressive dystrophy of the collagen fibers included increase in the diameter, disorientation, disorganization, and denaturation of collagen in the bladder layers. Lesions were also observed in immature under-stained fibers (Fig. 2B).
Thirty days of HMS intake resulted in excessive number of Ki-67-positive cells (up to 14%–16%) in basal layer of epithelium in urinary bladder urothelium. The growth of these indicators compared with the control rates indicates the regenerative processes (epithelial healing) (Fig. 2C).
Immunohistochemistry of the Hsp90α expression in the bladder urothelium of the experimental group (30 days of HMS intake) showed the increasing induction of the protein synthesis, characterized by strong positive reaction with cytoplasmic and partly nuclear compartments (Fig. 2D). These results indicate participation of the protein in the heavy metals (HM) stress response in the bladder cells and their participation in compensation for the intracellular homeostasis changes.
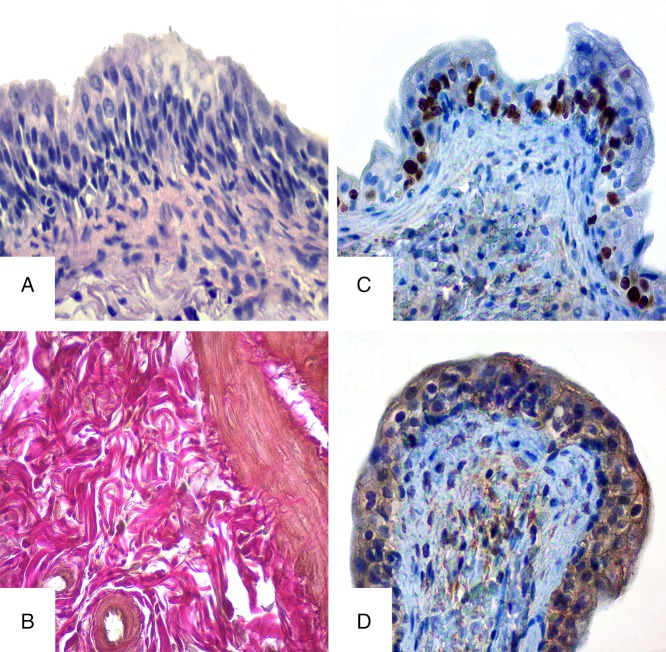
Study results of the experimental group of rats that had the intake of HMS mixture during 90 days differ significantly from the results of control and experimental group of rats that drank HMS for 30 days. The features of urothelium atrophy were observed on urinary bladder’s mucosa. Transitional epithelium was unevenly thinned in some areas, its surface and intermediate cells were flattened. Desquamation in some areas, squamous metaplasia, and inflammatory anabrosis were observed in mucosa. But besides the above-mentioned changes, proliferative activity was observed in urothelium, expressed by the changes in number of layers of intermediated cells that indicate the compensatory and adaptive changes (Fig. 3A). Fibrotic changes were observed in lamina propria of mucous membrane because of swelling. Moderate swelling and mixed cellular infiltration were observed in submucous layer. Dystrophic and atrophic changes, features of smooth and muscular depletion, and significant diffuse fibrosis were present in muscular membrane. Development of adaptive processes expressed by compensatory hypertrophy of the survived cells should be mentioned. Blood vessels were with irregular blood supply and sclerosis in their walls. Diffused mixed cellular infiltration represented by lymphocytes and numerous histiocytes were observed in urinary bladder membranes.
Fig. 3.
Histological and immunohistochemical studies of the bladder in rats of experimental group on the 90th day of HMS combination intake. A – hematoxylin and eosin staining, B – Van Gieson stain, C – immunohistochemical study of Ki-67, and D – immunohistochemical study of receptors prior to Hsp90α. Magnification 400×
On the 90th day of the experiment, the bladder wall, affected with HMS for a long time, showed swelling, layer separation, and collagen fiber displacement. Although the revealed changes were present as lesions and less obvious than the changes in the rats of the previous group, the number of collagen fibers and their thickening were increasing, which might indicate the rat bladder’s adaptive and age peculiarities (Fig. 3B).
Immunohistochemical study of receptors for Ki-67 in rats from experimental group on 90th day showed focal increase in the number of Ki-67-positive cells (up to 25%–27%), throughout all epithelium, indicating the long-lasting impact of HMS and active proliferative activity (Fig. 3C).
The experimental group presented medium Hsp90α expression in urothelium of rat bladder (Fig. 3D). Such results might indicate prolonged effect of heavy metals and formation of adaptive response to the disturbing factor for the bladder.
Discussion
Excessive and long-term HMS intake leads to numerous negative consequences [15, 16]. Excessive concentrations of salts can generate reactive oxygen species [superoxide anion (O2−), hydrogen peroxide (H2O2), hydroxyl radical (OH), organic hydroperoxide (ROOH), peroxynitrite (ONOO−), peroxy radicals (ROO), and other] and interrupt the redox systems, which in turn leads to the development of pathologies at the cellular level [8, 17]. Antioxidative systems that are present in cells serve as a protection from free radicals that can cause lipid peroxidation, DNA damage, protein depletion, acidification of sulfhydryl groups, and other pathologies. Many studies showed that the imbalance between free radicals formation and antioxidants generation causes the oxidative stress in living cells [18, 19]. High concentration of free radicals can lead to the cell structures, proteins, lipids, membranes, and nucleus acid damages, which results in stress development at the cellular level [17, 20].
In this study, the influence of ions such as lead, chromium, iron, manganese, zinc, and copper on the urinary bladder of the rats was observed. The results indicate the negative impact of the high concentration of these salts on the bladder’s wall. Histological and immunohistochemical studies of the bladder membranes indicate deep morphologic transformations in all structures of organ wall. From the results, it is clear that the excessive intake of HMS during different periods has negative impact on the urinary bladder. Depending on the period of influence of heavy metal ions on the wall of the organ, the pathological changes developed in cellular layers of the wall can lead to degenerative and later to atrophic and sclerotic changes.
The assessment of Ki-67 expression in the bladder epithelium showed increased proliferation. Thus, the medium positive reaction on the 30th day of the experiment indicated the beginning of reparative response to the negative impact of heavy metal ions. The expression of Ki-67 continued to increase and was assessed as strong positive reaction on the 90th day of the experiment. Results from the histological and immunohistochemical studies may indicate the strengthening of reparation processes because of the adaptive response.
It was proved that the heat shock protein (Hps90α) was also involved in the stress response of bladder epithelial cells on the heavy metal ions. In the urothelium of control group rats, the weak expression of Hsp90α was observed, which is normal. However, on the 30th day of the HMS intake, the level of these proteins increased significantly that is why the reaction was assessed as strong positive. Medium positive reaction, expressed on the 90th day, indicated the adaptive response in the bladder epithelium under the damaging factor, expressed as a decrease of stress proteins in the cell.
The immunohistochemical study of bladder epithelium showed that the expression of Ki-67 and Hsp90α directly depends on the period of intake of the water with elevated concentration of HMS.
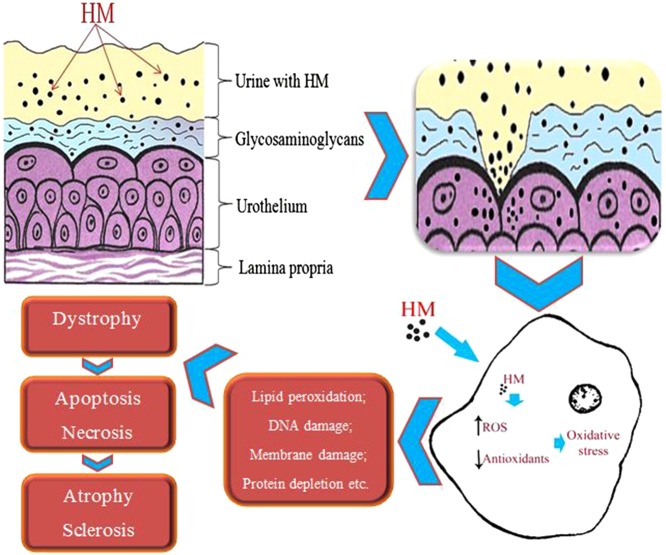
It is proved that the mucous membrane of the urinary bladder is protected by layer of glycosaminoglycans, lines the surface of urothelium and has protective properties [21]. Umbrella cells from the urothelium surface that are tightly connected by thin fibrils also serve as the barrier [22]. The majority of toxic substances that circulate in the body are excreted by the kidneys with the urine. Taking into account that the bladder’s main function is to reserve and evacuate, the urine with toxins (including HMS) is retained in the bladder and the toxic substances are accumulated on the walls of the bladder, which has direct influence on the bladder and leads to accumulation of toxic substances in the body (Fig. 4).
Fig. 4.
Scheme of HM impact on the cell
Several studies have shown that HMS affects various organs and systems by penetrating into blood–vascular system [16, 23]. As the urinary bladder belongs to those few organs that suffer from bilateral influence (indirect hematogenous and direct contact), the changes in the urinary bladder can be easily observed. Hence, the study of the heavy metals impact resulting from urine and direct contact with the urinary bladder epithelium is of special interest.
Accumulation of certain heavy metals leads to negative consequences that affect both the urinary bladder (morphological changes) and the body as a whole. To prevent the environmentally caused diseases, it is important to monitor and study the metal concentrations, their toxicity, and impact on the body. The search of protective tools will help to reduce the accumulation of heavy metals in the body and can be perspective for understanding and reducing of environmentally dependent diseases and pathologies.
Conclusions
Thus, the results of this study demonstrate that high concentrations of HMS can significantly influence the body. The histological and immunohistochemical studies showed that the influence of the HMS combination leads to deep morphological changes of all structures in the urinary bladder. These changes depend on the period of intake of HMS. Analysis of the obtained results demonstrates the dependence of expression of morphological changes in the urinary bladder on the experiment duration. The final result of these changes may lead to the disorders of bladder’s functions.
Funding Statement
Funding sources: No financial support was received for this study.
Authors’ contribution
AR, VlSik, ML, and AP: made the critical review; YuL, VlSik, and NG: prepared the article, prepared figures, and prepared the manuscript; AK, ML, VolSik, and VlSm: made literature review and prepared figures; AP, AR, VolSik, and NG: made literature review; ML, VlSm, and AP: prepared figures. All authors agreed to be accountable for all aspects of the work, ensured accuracy and integrity, and approved the final version of this manuscript.
Confict of interest
The authors declare that they have no conflict of interest.
References
- 1.Dinis MAP: Environment and human health. J Environ Pollut Human Health 4, 52–59 (2016) [Google Scholar]
- 2.Shakir SK, Azizullah A, Murad W, Daud MK, Nabeela F, Rahman H, Ur Rehman S, Häder DP: Toxic metal pollution in Pakistan and its possible risks to public health. Rev Environ Contam Toxicol 242, 1–60 (2016) [DOI] [PubMed] [Google Scholar]
- 3.Kong IC, Bitton G, Koopman B, Jung KH: Heavy metal toxicity testing in environmental samples. Rev Environ Contam Toxicol 142, 119–147 (1995) [DOI] [PubMed] [Google Scholar]
- 4.Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. (2012): Heavy metals toxicity and the environment. In: Molecular, Clinical and Environmental Toxicology, ed Luch A, Springer Basel, Basel, pp. 133–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid A, Riaz H, Akhtar S, Ahmad SR: Heavy metal contamination in vegetables, soil and water and potential health risk assessment. Am Eurasian J Agric Environ Sci 16, 786–794 (2016) [Google Scholar]
- 6.Singh R, Gautam N, Mishra A, Gupta R: Heavy metals and living systems: An overview. Indian J Pharmacol 43, 246–253 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jallad KN: Heavy metal exposure from ingesting rice and its related potential hazardous health risks to humans. Environ Sci Pollut Res 22, 15449–15458 (2015) [DOI] [PubMed] [Google Scholar]
- 8.Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN: Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7, 60–72 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin S, Griswold W: Human health effects of heavy metals. Environ Sci Technol Briefs Citiz 15, 1–6 (2009) [Google Scholar]
- 10.Wang YP, Shi JY, Lin Q, Chen XC, Chen YX: Heavy metal availability and impact on activity of soil microorganisms along a Cu/Zn contamination gradient. J Environ Sci (China) 19, 848–853 (2007) [DOI] [PubMed] [Google Scholar]
- 11.Hickling DR, Sun TT, Wu XR: Anatomy and physiology of the urinary tract: Relation to host defense and microbial infection. Microbiol Spectr 3 (2015), doi:10.1128/microbiolspec.UTI-0016-2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Groat WC, Yoshimura N: Anatomy and physiology of the lower urinary tract. Handb Clin Neurol 130, 61–108 (2015) [DOI] [PubMed] [Google Scholar]
- 13.Luster MI, Simeonova PP: Arsenic and urinary bladder cell proliferation. Toxicol Appl Pharmacol 198, 419–423 (2004) [DOI] [PubMed] [Google Scholar]
- 14.Mao S, Huang S: Zinc and copper levels in bladder cancer: A systematic review and meta-analysis. Biol Trace Elem Res 153, 5–10 (2013) [DOI] [PubMed] [Google Scholar]
- 15.Järup L: Hazards of heavy metal contamination. Br Med Bull 68, 167–182 (2003) [DOI] [PubMed] [Google Scholar]
- 16.Romaniuk A, Lyndin M: Immune microenvironment as a factor of breast cancer progression. Diagn Pathol 10, 79 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flora SJ, Mittal M, Mehta A: Heavy metal induced oxidative stress & its possible reversal by chelation therapy. Indian J Med Res 128, 501–523 (2008) [PubMed] [Google Scholar]
- 18.Sharma B, Singh S, Siddiqi NJ: Biomedical implications of heavy metals induced imbalances in redox systems. BioMed Res Int 2014, 26 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Potapov S, Sidorenko R, Galata D, Stratiy N, Gargin V: Peculiarities of catenin activity in the embryonal testicular carcinoma. Georgian Med News 261, 65–70 (2016) [PubMed] [Google Scholar]
- 20.Mathew BB, Tiwari A, Jatawa SK: Free radicals and antioxidants: A review. J Pharm Res 4, 4340–4343 (2011) [Google Scholar]
- 21.Cervigni M: Interstitial cystitis/bladder pain syndrome and glycosaminoglycans replacement therapy. Transl Androl Urol 4, 638–642 (2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis SA: Everything you wanted to know about the bladder epithelium but were afraid to ask. Am J Physiol Renal Physiol 278, F867–F874 (2000) [DOI] [PubMed] [Google Scholar]
- 23.Alissa EM, Ferns GA: Heavy metal poisoning and cardiovascular disease. J Toxicol 2011, 21 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]