Abstract
Carbon tetrachloride (CCl4), a hepatotoxic agent is widely used to study the toxic mechanisms in experimental animals. We have investigated whether oxidative stress is induced in the brain at a single hepatotoxic dosage (1 ml/kg bw) of CCl4. Increased lipid peroxidation (LPO), protein carbonyls (PC) content and glutathione (GSH) depletion were observed in the brain regions of rats treated with CCl4 which was higher than that of liver. A drastic reduction in the activity of glutathione-S-transferase (GST) was seen in the brain regions which was higher than that of liver. Similarly, activities of glutathione peroxidase (GPx), glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), NADH- and NADPH-dehydrogenase were reduced in the brain regions similar to that of liver. Higher induction of oxidative stress in the brain compared to that of liver implies vulnerability of the brain for CCl4 neurotoxicity. Our study shows that a single hepatotoxic dose of CCl4 is equally neurotoxic to rats.
Keywords: Carbon tetrachloride, GSH, ROS, Neurotoxicity, Oxidative stress, Antioxidant enzymes
1. Introduction
Oxidative stress (OS) results as a consequence of imbalance between ROS and cellular antioxidant defenses. ROS is implicated in the etiology of many disease conditions including cardiovascular diseases, neurodegenerative disorders, cancer and aging [1], [2], [3]. Major source of free radicals in vivo is autoxidation of flavin thiols, activity of electron transport chain, oxidases, cyclooxygenases, peroxidases etc. [4]. Environmental sources of OS include xenobiotics, organic solvents, pesticides, tobacco smoke, anesthetics, drugs and radiation [5].
Carbon tetrachloride (CCl4) is widely used to induce hepatotoxicity in experimental animals. CCl4 hepatotoxicity is characterized by hepatocellular necrosis with fat deposition. At acute toxic doses of CCl4, when hepatocellular necrosis exceeds the regenerative capacity of the liver, fatal liver failure often ensues. High doses of CCl4 results in nonspecific toxicity, including central nervous system depression and respiratory failure resulting in death [6]. The free radicals generated from CCl4 and the parent molecule by itself, damage endoplasmic reticulum (ER), which leads to accumulation of lipids, reduced protein synthesis and mixed function oxidases activity [7]. CCl4 belongs to the class of hepatotoxins, which act after metabolic activation. It is metabolized in the endoplasmic reticulum by cytochrome p450 enzymes (mostly CYP2E1) to the highly reactive trichloromethyl radical (CCl3•). CCl3• rapidly reacts with oxygen to form the highly reactive trichloromethylperoxyl radical (CCl3OO•), which rapidly reacts with lipids to form lipid peroxidation products [8]. Polyunsaturated fatty acids or PUFA of the ER and mitochondria are more susceptible to oxidation by the free radicals. The free radical mediated lipid peroxidation is one of the main mechanisms of hepatic injury by CCl4 [7].
The brain is highly vulnerable to OS than other organs of the body in view of the unusually high rate of oxygen consumption, being rich in PUFA, and low levels of antioxidant enzymes coupled with high amount of non-haem iron [9], [10]. Neurotoxic compounds induce OS by inducing lipid peroxidation and altering the antioxidant defenses in the brain [11], [12], [13]. In view of this, we hypothesized that brain could be a vulnerable target organ for the action of CCl4. Although the hepatotoxicity of CCl4 is well understood, reports on the effects of CCl4, on the brain are sketchy [14]. In this study, we have investigated whether a single sublethal, hepatotoxic dose of CCl4 causes OS in the brain of the laboratory rat.
2. Material and methods
2.1. Chemicals
Nicotinamide adenine dinucleotide phosphate reduced (NADPH), nicotinamide adenine dinucleotide reduced (NADH), 1-chloro-2,4-dinitrobenzene (CDNB), thiobarbituric acid (TBA), glutathione reduced (GSH), oxidised glutathione (GSSG), 2,4-dinitrophenylhydrazine (DNPH), and pyrogallol were purchased from Sigma Chemical Co. (St. Louis, MO, USA). 5,5′dithiobis (2-nitrobenzoic acid) (DTNB), nitrobluetetrazolium (NBT), Triton-X, Tris base and other reagents were purchased from Sisco Research Laboratories, Mumbai, India
2.2. Animals and treatment
Ninety days old adult male Wistar rats (200–230 g) were divided into two groups of four each. CCl4 was dissolved in sunflower oil. To the control group, only the vehicle (sunflower oil) was administered. A single dose of CCl4 (1 ml/kg bw), the dose at which hepatotoxicity occurs [15] was administered by oral gavage and, animals were sacrificed by ether anesthesia after 24 h. Liver and brain were removed and the brain dissected on ice to get the different regions, viz, forebrain, midbrain, and hindbrain, which were processed immediately for biochemical assays. The institute animal ethical guidelines were followed for the animal experiments.
2.3. Lipid peroxidation
Lipid peroxidation (LPO) in tissue homogenate was measured by estimating the formation of thiobarbituric acid reactive substances (TBARS) [16]. Tissue homogenate (10% w/v in 1.15% KCl) was mixed with 1.5 ml 20% TCA and 1.5 ml 1.34% TBA and, boiled for 30 min, cooled, which was followed by the addition of 2.5 ml butanol and centrifuged at 2000 rpm for 5 min. The upper layer was read at 535 nm. TBARS as malondialdehyde (MDA) content of the sample was calculated using the molar extinction coefficient 1.43 × 10−3 M−1 cm−1 and expressed as nmol of MDA formed/mg protein.
2.4. Protein carbonyls
Protein carbonyls were estimated by preparing 10% w/v tissue homogenates in 20 mM Tris–HCl buffer (pH 7.4), centrifuged at 3000 rpm for 20 min at 4 °C and 1 ml of the supernatant was precipitated with an equal volume of 20% TCA and centrifuged. The pellet was suspended in 10 mM DNPH and allowed to stand at room temperature for 60 min with occasional vertexing. This was followed by adding 0.5 ml 20% TCA to the mixture and centrifuged, the pellet obtained was washed three times with ethanol:ethylacetate (1:1) and solubilized in 2% SDS (in 20 mM Tris–HCl, 0.1 M NaCl, pH 7.4). Protein carbonyl (PC) content was measured by the method of Levine et al. [17]. The absorbance was read at 360 nm in UV–vis spectrophotometer and the carbonyl content was calculated using a molar extinction coefficient of 22,000 M−1 CM−1 and expressed as μg/mg protein.
2.5. Glutathione
Glutathione (GSH) content was estimated by the method of Ellman et al. [18], 10% tissue homogenates were prepared in 5% w/v TCA, centrifuged at 2000 rpm for 10 min and supernatant (GSH) was mixed with 10 mM DTNB in 0.1 M phosphate buffer (pH 8.0). The mixture was incubated for 10 min at room temperature and the color was read at 412 nm. Glutathione content was calculated by a standard curve and expressed as μg/mg protein.
2.6. Antioxidant enzymes
Tissues were homogenized (10% w/v) in ice cold Tris buffer (50 mM, pH 7.4) and centrifuged at 3000 rpm for 20 min at 4 °C. The supernatant was used for biochemical assays.
Glutathione-S-transferase (GST) activity was assayed by the method of Warholm et al. [19] with CDNB as the substrate. The reaction mixture contained 20 mM GSH and the enzyme (supernatant) in 0.1 M phosphate buffer (pH 7.4). The reaction was started by adding 30 mM CDNB and change in the absorbance at 344 nm was monitored in spectrophotometer. The enzyme activity was expressed as nmol CDNB conjugate/min/mg protein.
Glutathione peroxidase (GPx) activity was assayed by the indirectcoupled oxidation method using glutathione reductase [20]. Cumenehydroperoxide (1 mM) and glutathione (0.25 mM) were used as substrates and coupled oxidation of NADPH by glutathione reductase (0.25 U) in Tris buffer (50 mM, pH 7.6) was monitored at 340 nm.
Glutathione reductase (GR) activity was measured by the method of Calberg and Mannervick [21]. The reaction mixture contained 20 mM GSSG and 2 mM NADPH in 100 mM phosphate buffer (pH 7.4). The reaction was started by adding the enzyme and the absorbance change was measured at 340 nm for 3 min.
Superoxide dismutase (SOD) activity was measured using pyrogallol (2 mM) autooxidation as described by Marklund and Marklund [23]. The reaction mixture contained pyrogallol in 0.1 M Tris buffer (pH 8.2) and the enzyme. The reaction was started by adding the substrate and the absorbance was read at 420 nm for 3 min at an interval of 1 min.
Catalase (CAT) activity was assayed by the method of Aebi [22]. The reaction mixture contained 3% H2O2 in 0.05 M phosphate buffer (pH 7.0). The reaction was started by the addition of 100 μl of enzyme and the change in absorbance was read at 240 nm for 3 min and activity was expressed as n mole H2O2/min/mg protein.
NADH-dehydrogenase activity was assayed in a reaction mixture containing 1 mM NADH, 1 mM NBT and enzyme supernatant in 0.02 M Tris buffer (pH 7.4) for 20 min at 37 °C, followed by the addition of 1% Triton X-100. The color developed due to reduction of tetrazolium (formazan) was read at 560 nm.
NADPH-dehydrogenase activity was assayed by the method of Dawson et al. [24]. The reaction mixture containing the enzyme, NADPH (0.5 mM), EDTA (5 mM), NBT (0.5 mM), in 50 mM Tris buffer (pH 7.4) was incubated for 15 min at 37 °C and the color was read at 560 nm as described above.
2.7. Protein estimation
Protein estimation was determined by the method of Lowry et al. [25] using bovine serum albumin as the standard.
2.8. Statistics
The data were expressed as mean ± standard error (S.E) (n = 4) and significance was evaluated by Independent sample “T” test using SPSS version 14 (*p < 0.05, **p < 0.01, ***p < 0.005).
3. Results
3.1. Oxidative stress markers
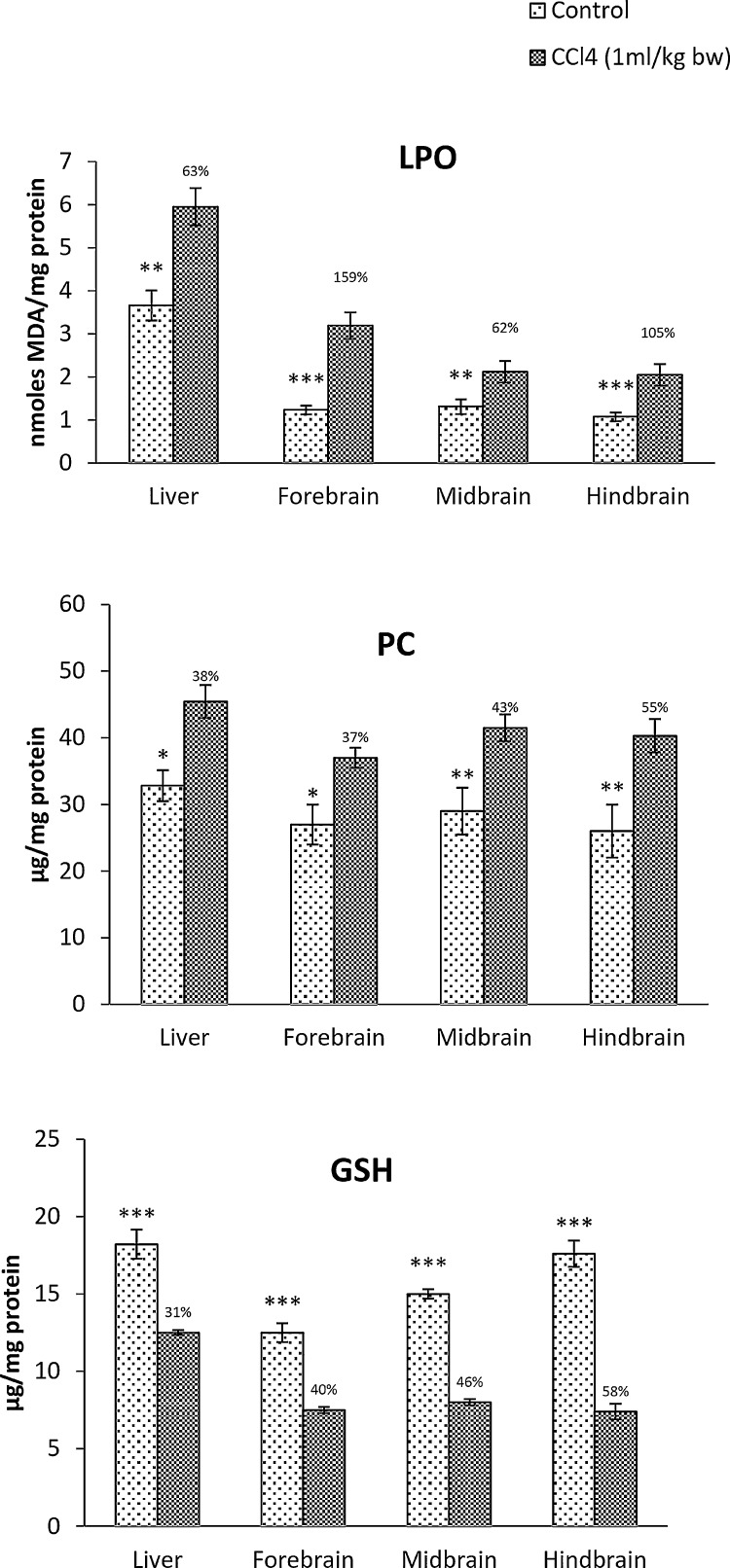
Marked increase in lipid peroxidation was seen in all the brain regions of CCl4 treated rats, being highest in the forebrain (159%) followed by hindbrain (105%) and midbrain (62%), whereas liver showed 63% increase (Fig. 1). Significant increase in protein carbonyl content was observed in the brain regions of CCl4 treated rats which was higher than that of liver. Reduction of GSH content was seen in the brain regions of CCl4 treated rats which was highest in the hindbrain (58%) followed by midbrain (46%) and forebrain (40%). GSH depletion in the liver was 31% which was lower than that of brain. Overall, brain showed higher oxidative stress than liver (Fig. 1).
Fig. 1.
CCl4-induced oxidative stress in the rat brain in comparison with the liver. LPO: Lipid peroxidation; PC: Protein carbonyls; GSH: Glutathione. Each bar represents the mean ± S.E, (n = 4). Data were analyzed by Independent Sample T test using SPSS version 14, (*p ˂ 0.05, ** p ˂ 0.01, ***p ˂ 0.005). The numbers above the dark bar represent the percentage difference between control and treated groups.
3.2. Antioxidant enzymes
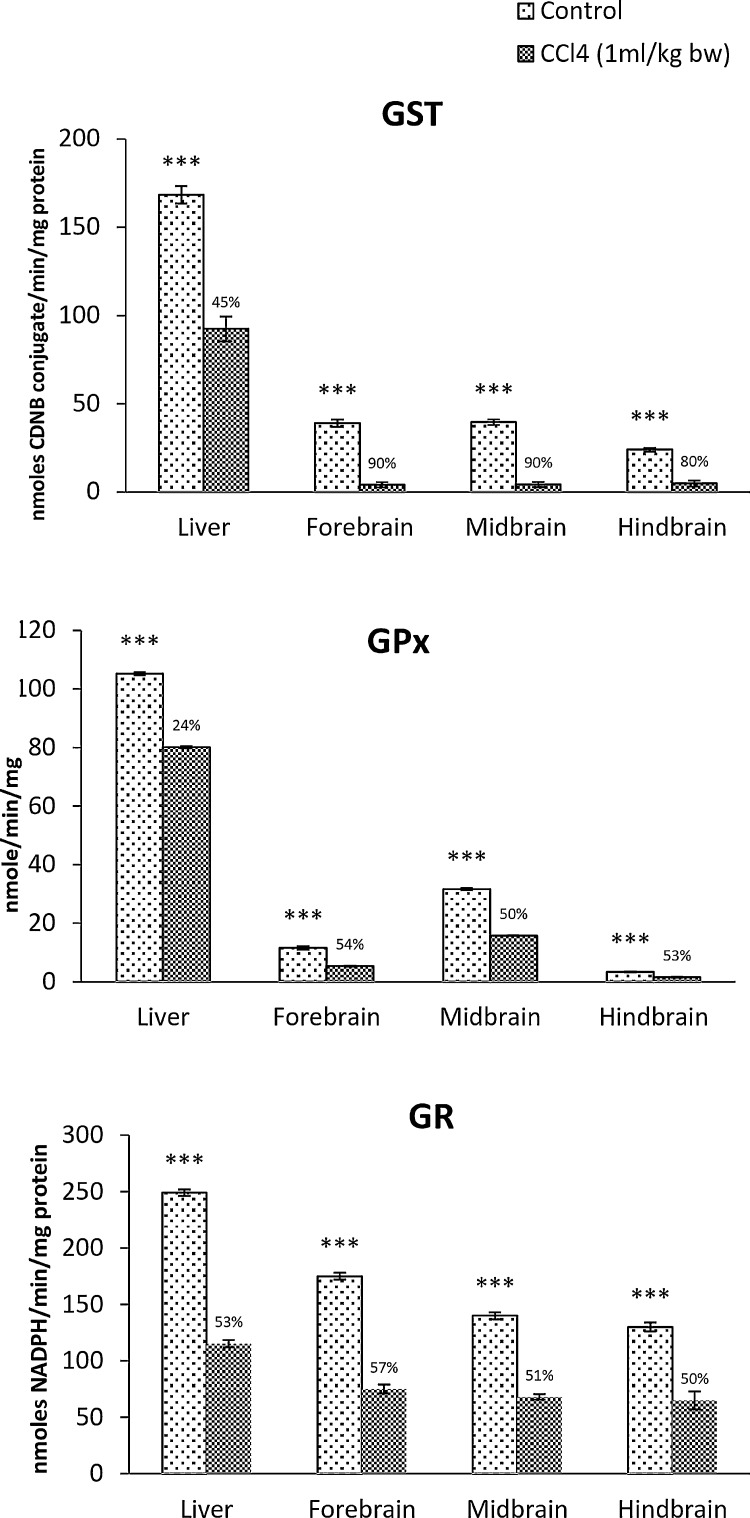
Effect of CCl4 on the antioxidant enzymes was marked and showed distinct regional variation in the brain. A marked reduction in the activity of GST was observed in all the brain regions compared to that of liver in CCl4 treated rats (Fig. 2). Forebrain and midbrain showed almost 90% reductions in the enzyme activity while hindbrain showed 80% reduction, whereas, in the liver, it was 45% reduction. GPx and GR activity were reduced by about 50% in all the brain regions, which was comparable to that of liver (Fig. 2).
Fig. 2.
CCl4-induced oxidative stress in the rat brain in comparison with the liver. GST: Glutathione-S-transferase; GPx: Glutathione peroxidase; GR: Glutathione reductase. Each bar represents the mean ± S.E, (n = 4). Data were analyzed by Independent Sample T test using SPSS version 14, (*p ˂ 0.05, ** p ˂ 0.01, ***p ˂ 0.005). The numbers above the dark bar represent the percentage difference between control and treated groups.
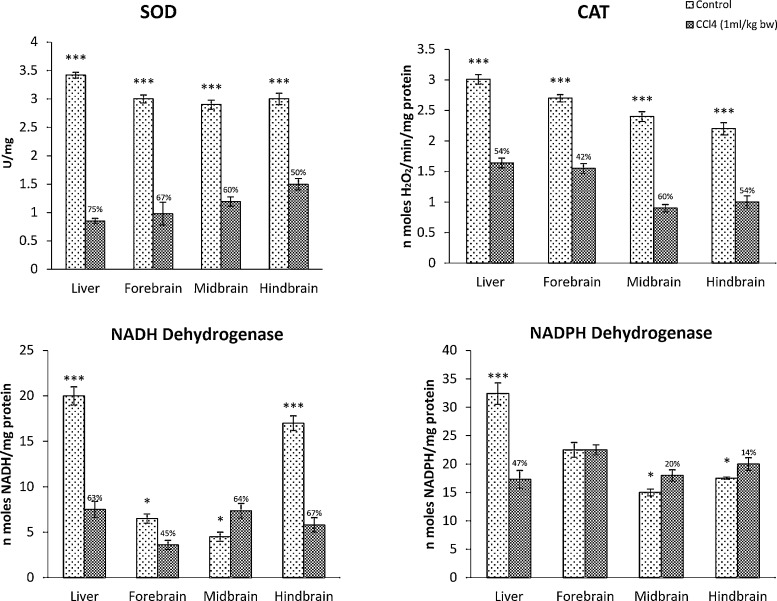
SOD activity in the liver was decreased by 75%, while in the brain regions, it ranged from 50% to 67%. (Fig. 3). Catalase activity was also markedly decreased from 60% (midbrain) to 42% (forebrain), in the brain of CCl4treated rats, whereas, in the liver, the reduction was 54% (Fig. 3). NADH-dehydrogenase showed significant reduction in the activity in hindbrain (67%) followed by forebrain (45%) of CCl4 treated rats whereas, liver showed 52% reduction (Fig. 3). NADPH-dehydrogenase, on the other hand, was not significantly affected in the brain in contrast to that of liver which showed 42% reduction in CCl4 treated rats (Fig. 3).
Fig. 3.
CCl4-induced oxidative stress in the rat brain in comparison with the liver. SOD: Superoxide dismutase; CAT: Catalase; NADH-dehydrogenase; NADPH-dehydrogenase. Each bar represents the mean ± S.E, (n = 4). Data were analyzed by Independent Sample T test using SPSS version 14, (*p ˂ 0.05, ** p ˂ 0.01, ***p ˂ 0.005). The numbers above the dark bar represent the percentage difference between control and treated groups.
4. Discussion
CCl4, being lipophilic, easily crosses cell membranes and gets distributed in tissues. CCl4 is rapidly taken up by both liver and brain, but its toxic effects on the brain are poorly understood compared to that of liver [14]. CCl4 exerts its toxic effects by generation of free radical CCl3• which leads to membrane lipid peroxidation [6]. Hepatic injury by a single dose of CCl4 (1 ml/kg bw) has been reported by us and others which involves induction of OS (15). In this study, we have shown that the dose at which CCl4 causes hepatotoxicity causes OS in the brain. Our results show a marked increase in TBARS, an index of LPO, in the brain of treated rats which was higher than that of liver. Free radical mediated LPO is one of the main pathogenic mechanisms of hepatic injury by CCl4, and therefore, a similar mechanism could cause neurotoxic injury to the brain. Higher LPO in the brain could be attributed to higher lipid content especially in the white matter which is rich in myelin with lower PUFA than grey matter [26]. The midbrain is relatively heavily myelinated, and therefore, is expected to be more resilient to OS [27].
Reactive oxygen species is constantly produced in cells which is scavenged by cellular the antioxidant enzyme defenses during normal cell metabolism [28]. A delicate balance exists between the rate of formation of H2O2 via dismutation of superoxide by SOD activity and the rate of its removal by catalase and GPx. Any impairment in this pathway will affect the activities of other enzymes in the cascade [29], [30]. Induction of antioxidant enzyme activities enhances metabolic detoxification and antioxidant defenses of the tissue, while their reduction results in OS [31], [32]. In our study, CCl4 administration to rats led to reduced antioxidant capacity of the brain as evident from the decreased activity of all the antioxidant enzymes. Many neurotoxic chemicals including ethanol cause OS in the brain [33].
LPO is often the consequence of lowered antioxidant defenses. Our results show increased PC content in the brain which indicates oxidative modifications of proteins, with functional consequences including loss of enzyme activity [34], [35]. Oxidative modifications to proteins such as glutathione synthetase and mitochondrial aconitase in the brain, resulting in the loss of enzymatic activity have been reported in ischemia-induced brain damage and Alzheimer’s disease [36].
Glutathione, a cytosolic tripeptide, ubiquitously present in all cell types at millimolar concentration is the major non-enzymatic regulator of intracellular redox homeostasis [37]. Our result shows that CCl4 administration led to marked depletion of GSH level in the brain which was higher than that of liver. GSH depletion predisposes the brain to OS due to compromised redox balance. GSH also serves as a substrate for GPx and GST, primarily involved in the detoxification of toxic electrophilic metabolites via formation of GSH-conjugate [38]. During the enzymatic reaction catalyzed by GPx, GSH is oxidized to GSSG which is reduced back to GSH by GR. GR, therefore plays a central role in maintaining the cellular GSH level. Reduction in GR activity by CCl4 in the brain, as seen in our results, affects the regeneration of GSH, thus accentuating the effects of GSH depletion [28]. GST plays an important role in protecting cells against ROS mediated injury by detoxification of lipid hydro peroxides formed due to oxidative damage [39]. Our results show drastic reduction in the activity of GST in all the brain regions by CCl4 which could compromise the biochemical antioxidant defenses of the brain. This is significant particularly since the effect of CCl4 on the hepatic GST is relatively lessmarked compared to that of brain. Marked depletion of GSH and its dependent enzymes in the brain regions by CCl4 indicates a major deleterious effect on the brain. NAD(P)H dehydrogenases belongs to a class of enzymes that transfer electrons from NAD(P)H to small acceptor molecules such as quinines. NAD(P)H dehydrogenases are important in the redox balance but their precise role in cell injury and tissue damage is not clear. However, these dehydrogenases may be involved in the antioxidant defense system in relation to protection of cells against electrophilic toxicity [40]. Our results show that CCl4 administration decreased the activity of NADH dehydrogenase in the brain suggesting altered redox status of the cells.
Our results are consistent with earlier observations on the regional differences in the antioxidant enzymes in the brain regions and add to the evidence that the vulnerability to OS of the brain is region-specific [10], [13], [41]. Regional variation in the response, the brain regions to CCl4 implies its differential distribution in the brain and/or its metabolism. Another contributing factor for the regional differences in the OS could also involve differences in the neurotransmitter profile [42], [43]. Our study has demonstrated that CCl4 induces OS in the brain regions by marked alteration of antioxidant balance in both enzymatic and non-enzymatic status. Significant reduction of antioxidant enzymes, along with increased LPO and PC content by a single dose of CCl4 could be considered a deleterious effect of CCl4 on the brain. The overall OS response to CCl4 in the brain was higher than that of liver. Although CCl4 is well known as a hepatotoxic agent, our results show that it is equally a neurotoxic chemical that causes oxidative damage to the brain. Further studies are needed to ascertain the consequences of CCl4 action on the brain with regard to cellular damage and neurotoxic consequences.
Conflict of interest
The authors declare that there are no conflicts of interest.
Acknowledgements
This work was carried out at Central Food technological Research Institute, Mysore, India. The authors wish to thank the Director of the institute for his keen interest in this study. The first author acknowledges the University Grants Commission, New Delhi for awarding the research fellowship.
References
- 1.Halliwell B., Gutteridge J. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michael T.L., Beal M.F. Mitochondrial dysfunction and oxidative stress in neuro degenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 3.Nathan C., Bussel A.C. Beyond oxidative stress on immunologists guide to reactive oxygen species. Nat. Rev. Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman H.J., Maiorino M., Ursini F. Signaling function of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokke H., Ragas M.J., Holmstrup M. Tools and perspectives for assessing chemical mixtures and multiple stressors. Toxicology. 2013;313:73–82. doi: 10.1016/j.tox.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Recknagel R.O., Glende E.A., Dolak J.A., Waller R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 7.Weber L.W., Boll M., Stamp A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. CRC Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 8.Risal P. Hispidin analogue davallialactone attenuates carbon tetrachloride induced hepatotoxicity in mice. J. Nat. Prod. 2012;75:1683–1689. doi: 10.1021/np300099a. [DOI] [PubMed] [Google Scholar]
- 9.Chong Z.Z., Li F., Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Progress Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Somani S., Husain K., Diaz-Phillips L., Lanzotti D., Kareti K., Trammell G. Interaction of exercise and ethanol on antioxidant enzymes in brain regions of the rat. Alcohol. 1996;13 doi: 10.1016/s0741-8329(96)00075-4. [DOI] [PubMed] [Google Scholar]
- 11.Verma R.S., Srivastava N. Chlorpyrifos induced alterations in levels of thiobarbituric acid reactive substances and glutathione in rat brain. Indian J. Exp. Biol. 2001;39:174–177. [PubMed] [Google Scholar]
- 12.Latini A., Scussiato K., Rosa R.B. D-2-hydroxyglutaric acid induces oxidative stress in cerebral cortex of young rats. Eur. J. Neurosci. 2003;17:2017–2022. doi: 10.1046/j.1460-9568.2003.02639.x. [DOI] [PubMed] [Google Scholar]
- 13.Srivastava A., Shivanandappa T. Hexachlorocyclohexane differentially alters the antioxidant status of the brain regions in rat. Toxicology. 2005;214:123–130. doi: 10.1016/j.tox.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Szymonik-Lesiuk S., Czechowska G., Stryjecka-Zimmer M. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. J. Hepato-Biliary-Pancreatic Surg. 2003;10:309–315. doi: 10.1007/s00534-002-0824-5. [DOI] [PubMed] [Google Scholar]
- 15.Srivastava A., Shivanandappa T. Hepatoprotective effect of the root extract of Decalepis hamiltonii against carbon tetrachloride-induced oxidative stress in rat. Food Chem. 2010;118(2):411–417. [Google Scholar]
- 16.Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 17.Levine R.L., Garland D., Oliver C.N. Determination of carbonyl content in oxidatively modified proteins. Method Enzymol. 1990;18:6–46. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 18.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophy. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 19.Warholm M., Guthenberg C., Von Bahr C., Mannervik B. Glutathionetransferases from human liver. Method Enzymol. 1985;113:499–504. doi: 10.1016/s0076-6879(85)13065-x. [DOI] [PubMed] [Google Scholar]
- 20.Mannervik B. Glutathione peroxidase. In: Meister A., editor. vol. 113. Academic Press; Florida: 1985. pp. 490–495. (Methods in Enzymology). [Google Scholar]
- 21.Carlberg I., Mannervik B. Glutathione reductase. Method. Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 22.Aebi H. Catalase. In: Bergneyer H.U., editor. Methods of Enzymatic Analysis. Verlag Chemie; New York,Weinheim: 1983. pp. 276–286. [Google Scholar]
- 23.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 24.Dawson T.M., Bredt D.S., Fotuhi M., Hwang P.M., Snyder S.H. Nitric oxide synthase and neuronal NADPH diaphorases are identical in brain and peripheral tissues. Proc. Natl. Acad. Sci. U. S. A. 1991;88:7797–7801. doi: 10.1073/pnas.88.17.7797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Svennerholm L. Distribution and fatty acid composition of phosphoglycerides in normal human brain. J. Lipid Res. 1968;9:570–579. [PubMed] [Google Scholar]
- 27.MacEvilly C.J., Muller D.P. Lipid peroxidation in neural tissues and fractions from vitamin E-deficient rats. Free Radic. Biol. Med. 1996;20:639–648. doi: 10.1016/0891-5849(95)02147-7. [DOI] [PubMed] [Google Scholar]
- 28.Martinez-Cayuela M. Oxygen free radicals and human disease. Biochimie. 1995;77:147–161. doi: 10.1016/0300-9084(96)88119-3. [DOI] [PubMed] [Google Scholar]
- 29.Sinet P.M., Garber P. Inactivation of the human CuZn superoxide dismutase during exposure to O-2 and H2O2. Arch. Biochem. Biophys. 1981;212:411–416. doi: 10.1016/0003-9861(81)90382-9. [DOI] [PubMed] [Google Scholar]
- 30.Kono Y., Fridovich I. Superoxide radical inhibits catalase. J. Biol. Chem. 1982;257:5751–5754. [PubMed] [Google Scholar]
- 31.Nomura T., Yamaoka K. Low-dose γ-ray irradiation reduces oxidative damage induced by CCl4 in mouse liver. Free Radic. Biol. Med. 1999;27:1324–1333. doi: 10.1016/s0891-5849(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 32.Cao Z., Li Y. Chemical induction of cellular antioxidants affords marked protection against oxidative injury in vascular smooth muscle cells. Biochem. Biophys. Res. Commun. 2002;292:50–57. doi: 10.1006/bbrc.2002.6614. [DOI] [PubMed] [Google Scholar]
- 33.Houze P., Rouach H., Gentil M., Orfanelli M., Nordmann R. Effect of allopurinol on the hepatic and cerebellar iron, selenium, zinc and copper status following acute ethanol administration to rats. Free Radic. Res. 1991;13:663–668. doi: 10.3109/10715769109145844. [DOI] [PubMed] [Google Scholar]
- 34.Pigeolet E., Corbisier P., Houbion A. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990;51:283–297. doi: 10.1016/0047-6374(90)90078-t. [DOI] [PubMed] [Google Scholar]
- 35.Bowling A.C., Beal M.F. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56:1151–1171. doi: 10.1016/0024-3205(95)00055-b. [DOI] [PubMed] [Google Scholar]
- 36.Shacter E. Quantification and significance of protein oxidation in biological samples. Drug Metabol. Rev. 2000;32:307–326. doi: 10.1081/dmr-100102336. [DOI] [PubMed] [Google Scholar]
- 37.Meister A., Anderson M.E. Glutathione. Annu. Rev. Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- 38.Hayes J.D., Flanagan J.U., Jowsey I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Cheng J.Z., Singhal S.S. Role of glutathione S-transferases in protection against lipid peroxidation overexpression of HGSTA2-2 in K562 cells protects against hydrogen peroxide-induced apoptosis and inhibits JNK and caspase 3 activation. J. Biol. Chem. 2001;276:19220–19230. doi: 10.1074/jbc.M100551200. [DOI] [PubMed] [Google Scholar]
- 40.Benson A.M., Hunkeler M.J., Talalay P. Increase of NAD (P) H: quinonereductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc. Natl. Acad. Sci. U. S. A. 1980;77:5216–5220. doi: 10.1073/pnas.77.9.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baek B.S., Kwon H.J., Lee K.H. Regional difference of ROS generation, lipid peroxidaton, and antioxidant enzyme activity in rat brain and their dietary modulation. Arch. Pharm. Res. 1999;22(4):361–366. doi: 10.1007/BF02979058. [DOI] [PubMed] [Google Scholar]
- 42.Sunol C., Tusell J., Gelpi E., Rodriguez-Farre E. Regional concentrations of GABA, serotonin and noradrenaline in brain at onset of seizures induced by lindane (γ-hexachlorocyclohexane) Neuropharmacology. 1988;27:677–681. doi: 10.1016/0028-3908(88)90075-5. [DOI] [PubMed] [Google Scholar]
- 43.Weber G. The pathophysiology of reactive oxygen intermediates in the central nervous system. Med. Hypotheses. 1994;43:223–230. doi: 10.1016/0306-9877(94)90070-1. [DOI] [PubMed] [Google Scholar]