Highlights
-
•
We examine the toxicological profile of Tepary Bean lectins by oral route.
-
•
Tepary bean lectins showed digestion resistance up to 72 h.
-
•
Tepary bean lectins induce granulocyte increase after 24 h treatment.
-
•
A reduction in body weight gain was observed after 6 weeks treatment.
-
•
No toxicity was observed for Tepary bean lectins after 6 weeks.
Keywords: Antinutritional factors, Lectins toxicity, Phaseolus acutifolius, Tepary bean
Abstract
Our previous studies have shown that a lectin rich fraction (TBLF) extracted from Tepary bean seeds differentially inhibits cancer cells proliferation in vitro. Before testing the in vivo anticancer effect, the acute and subchronic toxicological assays in rats were conducted, where an oral dose of 50 mg/body weight kg was determined as the NOAEL. This study evaluated the resistance to digestion and complete blood count (CBC) after 24 h of the orally administered 50 mg/kg TBLF. The digestion resistance test showed lectins activity retention after 72 h and the CBC study showed a high level of eosinophils, suggesting an allergic-like response. Tolerability was assayed after 6 weeks of treatment by dosing with an intragastric cannula every third day per week. It was observed a transient reduction in food intake and body weight in the first weeks, resulting in body weight gain reduction of 10% respect to the control group at the end of the study. Additionally, organs weight, histopathological analysis and blood markers for nutritional status and for liver, pancreas and renal function were not affected. Our results suggest that 50 mg/kg TBLF administered by oral route, exhibit no toxicity in rats and it was well tolerated. Further studies will focus on long-term studies.
1. Introduction
Lectins include a group of proteins from non-immune origin that share the property of binding specifically and reversibly to carbohydrates [1]. Many plant lectins have attracted the attention due to their effects on proliferation and differentiation of animal cells, including lymphocytes and cancer cells. The in vitro and the in vivo antitumor effects of plant lectins are apparently associated with their ability to modulate growth, differentiation, proliferation and apoptosis [2], [3], [4].
Toxicity of lectins must be considered before used as medical tools, mainly because they are considered antinutritional factors. It has been shown that binding lectins to intestinal epithelium can interfere with nutrient absorption, reduction of nitrogen retention, increased urine nitrogen excretion and reduction of insulin production in rats [5], [6], [7], [8]. Antinutritional and negative effects on digestion and absorption have been described for lectins from different sources [9], [10], [11], [12]. Studies with common bean (Phaseolus vulgaris L.) lectins show that they can interfere with bowel function, causing changes in systemic metabolism and affecting the growth in rats, decrease in glucose, lipids, vitamin B12 and nitrogen uptake [13], [14]. Adverse effects in organs are produced by some diet lectins, which included Phaseolus vulgaris. Rats fed with navy beans showed morphological changes that include increased weight of kidney and heart, pancreatic acinar atrophy, fatty liver and multiple histological lesions as thymus atrophy respect to control healthy rats. Death by lectin administration can be produced by different mechanisms including the reduced availability of nitrogen in the body, the induction of bacterial overgrowth in the small intestine, the effects on blood cells or acting as ribosome inactivating proteins [15], [16].
Some studies showed that intraperitoneal administration of Tepary bean (Phaseolus acutifolius) crude extract presented toxic effects as weight loss, negative efficiency on protein ratio, negative net protein utilization, poor digestion of proteins and death of rats and mice after 10 days treatment, however, after autoclaving the crude extract, the toxic effects were lost [17]. Studies on the toxicity of semipure lectins from Tepary bean intraperitoneally administrated in CD-1 mice, found a lethal dose (LD50) of 1100 and 1120 mg/kg body weight for males and females, respectively [18].
A semipure lectin fraction from Tepary bean seeds (TBLF) obtained by a molecular weight exclusion chromatography protocol exhibits in vitro antiproliferative differential effect on cancer and normal cells [19]. Before testing the in vivo anticancer effect, we studied the acute toxicity of TBLF using intragastric doses from 5 to 2000 mg/body weight kg suggesting a secure dose of 50 mg/kg. The intragastric 50 mg/kg TBLF dose was assayed for subchronic toxicity (daily dosing for 28 days) where no toxic or adverse effects were observed, therefore 50 mg/kg TBLF was determined as the NOAEL [20]. Here we present a short-term assay in order to know the digestion resistance of lectins and the effect on complete blood count (CBC) after 24 h of 50 mg/kg TBLF single-dose administration. The anti-nutritional effects and toxic parameters of a 6-week schedule study (intragastric administration every third day) were studied; where food intake, body weight, biochemical blood markers and histopathological analysis were included.
2. Materials and methods
2.1. Experimental animals
Sprague Dawley (SD) rats were purchased from Institute of Neurobiology, Universidad Nacional Autonoma de Mexico (INB-UNAM) and placed in individual cages with ad libitum water and rodent chow food (Rodent Laboratory Chow 5001, Saint Louis, MO, USA). The animals remained 1 week for acclimatization where the circadian cycle was adjusted to 12 h light/12 h darkness, at 22 °C and a relative humidity of 30%. The animals were sacrificed by decapitation at the end of the experiments. The experimental protocol was based on the Mexican official standard [21] and approved by the INB-UNAM ethics committee.
2.2. Tepary bean lectin fraction (TBLF)
We have performed a standardized method for TBLF obtaining [19]. Some modifications were done in order to improve the lectin enrichment. Briefly, Tepary bean seeds were grinded (A-10 Analytical Tekmar mill) and degreased with chloroform-methanol 2:1 in a 4:1 w/v proportion, stirring for 15 min and then vacuum filter; this process was repeated 2 more times and flour was dried at room temperature in a fume hood. In order to obtain the crude extract, 100 g of degreased flour were dissolved in 500 mL of 50 mM Tris–HCl pH 8 with stirring for 12 h at 4 °C and centrifuging at 39,200 × g for 60 min (Bekman JA-20 centrifuge). A sequential precipitation was done using 40% ammonium sulphate saturation with slow stirring for 30 min, equilibrating for 30 min at 4 °C and centrifuging at 39,200 × g for 45 min. The supernatant was precipitated with 60% ammonium sulfate saturation and treated as previously described, but in this case the precipitate was recovered and the supernatant was discarded. The fraction was resuspended in a minimum volume of deionizer water, dialyzed through a 3-kDa pore size membrane and centrifuged, loading 4 mL aliquots on a Sephadex G-75 gel filtration 167 cm × 1.7 cm column (Pharmacia Biotech, Uppsala, Switzerland). The column was equilibrated with 0.01 M ammonium bicarbonate buffer pH 7.8. The experiment was performed at 4 °C collecting 0.3 mL/min fractions with the same buffer. Protein was monitored at 280 nm in a Beckman DU-65 spectrophotometer. Agglutination activity [22] was determined by microscopic counting using glutaraldehyde-fixed type A+ human erythrocytes [23]. Specific activity was determined using protein concentration [24]. Electrophoretic profile was obtained by 10% polyacrylamide SDS-PAGE [25]. Glycoproteins were confirmed by periodic acid-Schiff staining (PASS) [26]. Additionally, lectins were observed by western blot using an anti-phytohemagglutinin antibody from Phaseolus vulgaris (Vector Laboratories Inc. Burlingame, CA, USA. cat. No. AS-2300). The fraction was dialyzed against deionized water, lyophilized and stored at −20 °C until use.
2.3. Digestion-resistance assay and complete blood count (CBC) determination after a single dose of TBLF
Five-week old male SD rats were divided into 2 groups (n = 8 per group). After fasting for 24 h, the treated group received a single dose of the lyophilized 50 mg/kg TBLF dissolved in standard saline solution (0.9% NaCl in deionized water) using an intragastric cannula [20], while the control group received saline solution. Autoclaving (121 °C for 15 min) was necessary for denature food lectins. Feeding was restarted with ad libitum water and autoclaved chow food (Rodent Laboratory Chow 5001. Saint Louis, MO, USA). Feces form 4 rats per group were collected at 0, 24, 48, 72, 96 and 120 h, fecal protein was extracted in PBS, filtered through a 0.22 mm membrane and agglutination specific activity was determined by microscopic counting [22]. Other 4 rats per group were sacrificed at 24 h in order to recover blood for CBC (CellDyn® 1600) and a commercial kit for differential blood cells staining was used for cell counting from blood smear (Hycel, Mexico; cat. number 548). Erythrocytes, neutrophils, eosinophils, basophils, lymphocytes, monocytes and platelets were counted using a 100× microscope objective. Results are expressed as absolute blood counts or percentage respect to control animals.
2.4. Tolerability study
Fifteen-week old male SD rats were randomly selected in 2 groups (n = 12 per group). Treated rats were dosed with 50 mg/kg TBLF dissolved in saline solution and control group was administered with saline solution by using an intragastric cannula. For CBC determination, 4 rats per group were daily administered for 30 days and sacrificed. For the tolerability assessment, treated group was administered with the 50 mg/kg TBLF in saline solution every third day for 6 weeks and control group administered with saline solution. This administration schedule was defined from the digestion resistance data and it will be used in further studies, i.e. against colon cancer. Food intake was determined twice a week and body weight weekly. After the 6-week administration schedule, rats were sacrificed by decapitation. Blood was collected in vacutainer tubes without anticoagulant and serum was recovered by centrifugation at 5000 × g for 5 min and stored at −80 °C until use for clinical chemistry parameters determination as described below. Liver, kidney, stomach, pancreas, small intestine, colon, thymus and spleen were dissected, weighted and fixed in 10% buffered formalin. A veterinary pathologist conducted the histopathological analyses for liver, kidney, small intestine and colon using Hematoxylin-Eosin staining and analyzed by microscopy (Olympus, model BX51, Evolution MP).
Commercial kits (Diagnostic Chemicals Limited, Canada) were used for determination of liver function using aspartate aminotransferase (AST) (Catalog No. 319-10), alanine aminotransferase (ALT) (Catalog No. 318-10) and total bilirubin (Catalog No. 243-17) kits. Urea (Catalog No. 283-17) and α-amylase (Catalog No. 341-10) were measured as renal and pancreas function markers, respectively. Serum creatinine (Catalog No. 221-30), total protein (Catalog No. 200-55), glucose (SL ELITech, Clinical Systems, France. Catalog No. B01-4509-01), and albumin (SL ELITech, Clinical Systems, France. Catalog No. ALBU-0600) were determined as nutritional status markers.
2.5. Statistical analysis
Differences between TBLF treated rats against control rats were calculated by the t-student test (p ≤ 0.05) using the SPSS 17 software.
3. Results and discussion
3.1. TBLF profile
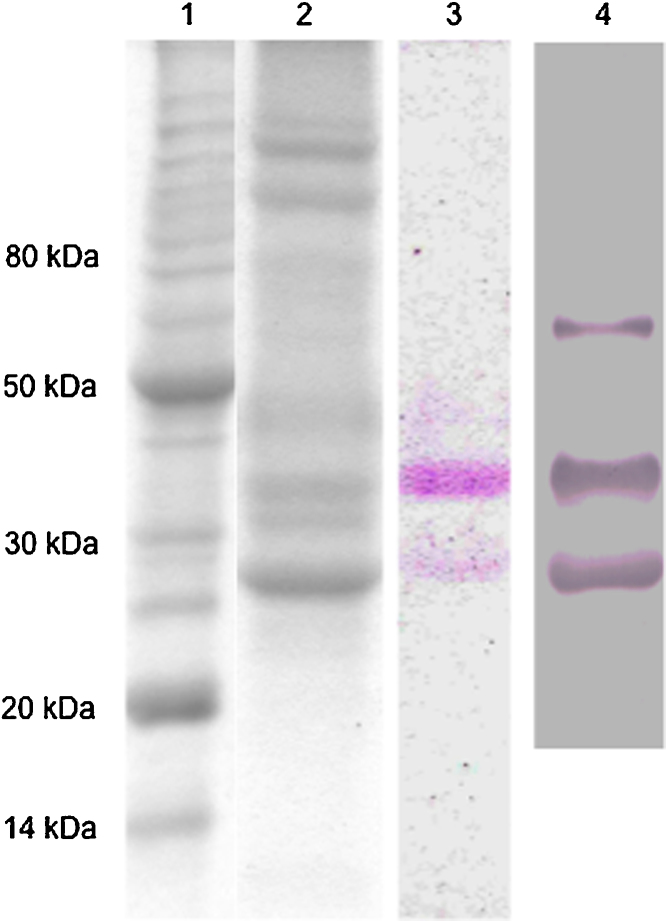
The molecular weight exclusion chromatography protocol shows a reproducible profile for TBLF obtainment. The method allows observing the two main lectins (Fig. 1), similar than the observed profile previously obtained [19]. The presence of lectins was confirmed by PASS and western blot. The specific agglutination activity for the TBLF was 5566 AU/protein mg.
Fig. 1.
TBLF electrophoretic profile and lectins identification. TBLF was obtained by molecular weight exclusion chromatography. Monodimensional SDS-PAGE was performed. (1) Molecular weight markers, (2) TBLF, (3) glycoprotein PASS, (4) western blot using anti-phytohemagglutinin from Phaseolus vulgaris.
3.2. Lectins from TBLF resist digestion up to 72 h
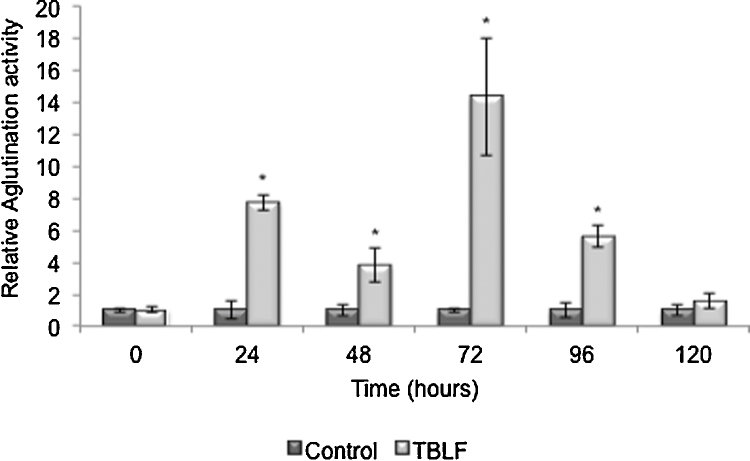
Some lectins exhibit high resistance to digestion by proteolytic enzymes in mammals, allowing them to effectively bind to intestinal epithelial cells. Lectins can also resist bacterial degradation and can remain in their biological and immunological intact forms [5], [6], [7]. It has been reported that this kind of proteins can be recovered with their intact biological activity after passing through the digestive tract of mice over a period of 24 h as Pisum sativum and Kintoki bean lectins [27], [28], [29]. In order to establish the resistance to gastric digestion of TBLF, agglutination activity was monitored through 120 h in feces after a 50 mg/kg TBLF single dose (Fig. 2). Agglutination activity in TBLF-treated rats was 8 and 4 times higher than in control rats after 24 and 48 h, respectively. However, instead of diminishing, it increased 15 times after 72 h and then gradually diminished until basal levels at 120 h. This result suggests that no retained lectin was excreted within the first 48 h and retained lectin releases after 72 h maintaining its biological activity.
Fig. 2.
Agglutination activity from rat feces administrated with TBLF. Rats were administrated with a single dose of TBLF (50 mg/kg) and feces were recollected every 24 h for 5 days. Agglutination activity was determined using A+ human erythrocytes. Asterisks show statistical significant difference (t test p ≤ 0.05).
3.3. TBLF exhibits an allergic-like response after 24 h
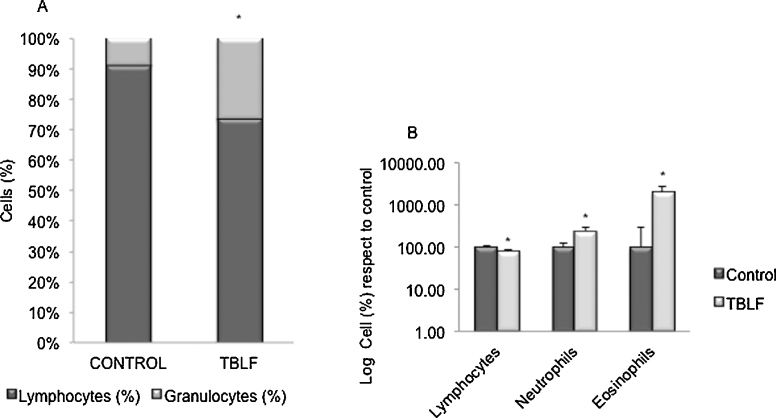
CBC is shown in Table 1 where only granulocytes count showed difference (p = 0.001) with an increase of 3.86 times in TBLF-treated animals respect to control rats. The proportion of granulocytes and lymphocytes was different respect to control animals, mainly due to an increment of granulocytes (Fig. 3A). Blood smears were used to differential counting of cells (Fig. 3B). Lymphocytes decreased 20% while neutrophils and eosinophils increased 2.4 and 20 times, respectively. Basophils, monocytes, erythrocytes, and platelets did not show significant changes (data not shown). This result suggests an allergic-like response, mainly indicated by the eosinophils increase.
Table 1.
CBC after 24 h of a single-dose TBLF oral administration.
| Blood parameter | Control | TBLF |
|---|---|---|
| White blood cells (103/μL) | 5.16 ± 1.35 | 6.78 ± 1.56 |
| Lymphocytes (103/μL) | 4.70 ± 1.27 | 5.00 ± 1.44 |
| Granulocytes (103/μL) | 0.46 ± 0.21 | 1.78 ± 0.53* |
| Red blood cells (106/μL) | 6.73 ± 0.20 | 6.55 ± 1.59 |
| Platelets (103/μL) | 874.40 ± 84.97 | 737.75 ± 216.71 |
| Hemoglobin (g/dL) | 12.50 ± 0.37 | 12.24 ± 2.88 |
| Hematocrit (%) | 42.20 ± 1.06 | 40.76 ± 9.52 |
| Mean corpuscular volume (fL) | 62.80 ± 2.77 | 62.40 ± 1.14 |
| Mean corpuscular hemoglobin (pg) | 18.60 ± 0.99 | 18.74 ± 0.33 |
| Mean corpuscular hemoglobin concentration (g/dL) | 29.62 ± 0.77 | 30.00 ± 0.48 |
Statistically significant difference (t test, p = 0.001).
Fig. 3.
White blood cells counting after 24 h of a single dose of TBLF. CBC was determined and blood cells were counted from blood smears by differential staining. (A) Percentage of granulocytes and lymphocytes were determined by automated counting. (B) White cells count was determined by microscopic counting. Results are expressed as percentage respect to control animals in a logarithmic scale. Asterisks show statistical significant difference (t test p ≤ 0.05).
3.4. Six weeks TBLF administration provoked partial reduction of food intake and decrease in body weight gain
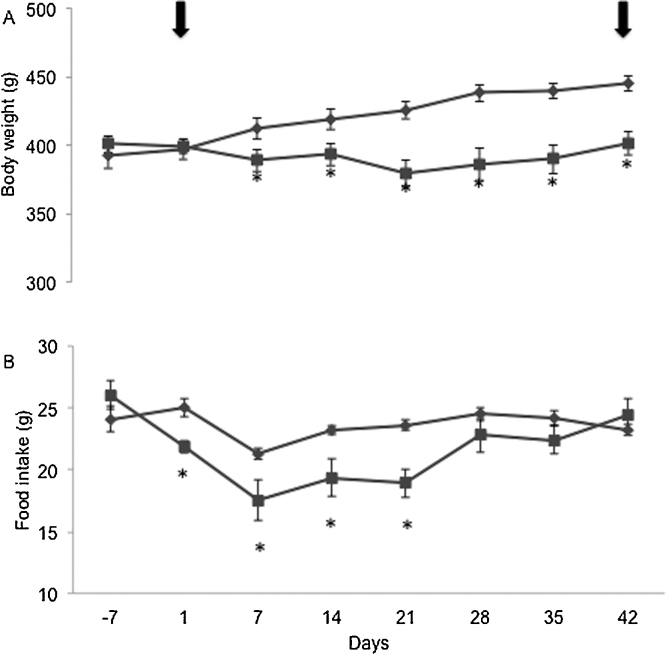
Fifty mg/kg TBLF dose was administrated via intragastric cannula every third day for 6 weeks. Significant decreased in food consumption was observed from the first week of administration until the fourth week respect to control group (p ≤ 0.05). However, on the fifth week, food consumption was the same than the control group (Fig. 4A), maybe as the result of compensatory mechanisms where the treated animals overcame the negative effects of the lectins administration. Rats body weight also showed significant changes (p ≤ 0.05) between the two groups (Fig. 4B). Treated animals presented a transient decrease of body weight in the first weeks (5.25% respect to the start of dosing) however; at the end of the study, a recovery of weight was observed resulting in a reduction in body weight gain of 10% respect to the control group. It is known that lectins can provoke nonspecific interference with nutrient absorption, causing changes in animal nutrition status. Our results show that TBLF administration causes antinutritional effects at the beginning of the experiment with a final recovery, which resulted in a reduction in body weight gain.
Fig. 4.
Food intake and body weight changes after TBLF oral administration. Rats were orally administrated with TBLF (50 mg/kg) every third day for 6 weeks after an adaptation period (7 days). Dosing was done to complete 6 weeks, from day 1 to day 42 (arrows indicate the start and end of treatment). (A) Net food intake, (B) Net body weight changes. Asterisks show statistical significant difference (t test p ≤ 0.05).
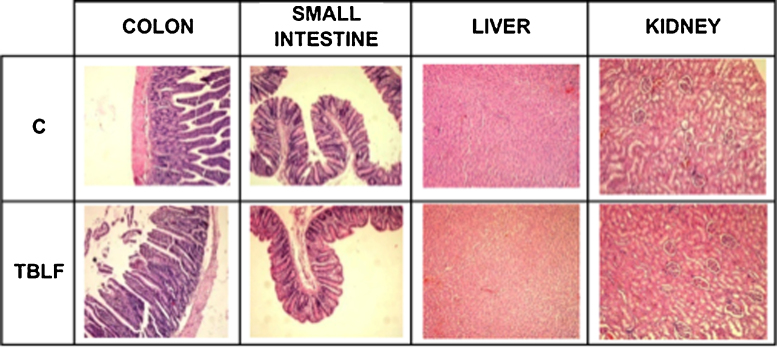
3.5. Six weeks TBLF administration did not cause organs or blood markers alterations
The effect of TBLF on organs and blood markers is shown in Table 2. No significant differences were observed in spleen, heart, liver, kidney, stomach, thymus, pancreas, small intestine and colon weight. Small intestine and colon length were also determined and no significant differences were found with respect to the control group. No histopathological alterations were observed in colon, small intestine, liver and kidney (Fig. 5). A strong association between changes in the morphology and structure of the intestine and the ingestion of lectins have been observed, such changes may result from the decrease in intestinal permeability as shown with Con A, wheat agglutinin and navy bean lectin. The intestinal growth is maybe the result of compensatory process in order to maintain nutrient uptake, since some lectins directly stimulate intestinal cells causing hyperplasia and hypertrophy [9]. However, TBLF at the tested dose din not provoked macroscopic effects or histological changes on the intestinal tract.
Table 2.
Organs weight or length after TBLF subchronic oral administration.
| Organs | Control | TBLF |
|---|---|---|
| Colon weight (g) | 2.94 ± 0.40 | 2.68 ± 0.40 |
| Colon length (cm) | 19.38 ± 1.14 | 19.60 ± 0.81 |
| SI weight (g) | 8.06 ± 0.23 | 8.28 ± 0.52 |
| SI length (cm) | 125.96 ± 2.40 | 122.02 ± 5.08 |
| Spleen (g) | 0.84 ± 0.03 | 0.83 ± 0.05 |
| Heart (g) | 1.65 ± 0.06 | 1.52 ± 0.06 |
| Liver (g) | 11.98 ± 0.42 | 10.57 ± 0.37 |
| Kidneys (g) | 3.31 ± 0.12 | 3.18 ± 0.15 |
| Stomach (g) | 2.21 ± 0.08 | 2.14 ± 0.07 |
| Thymus (g) | 0.45 ± 0.04 | 0.48 ± 0.05 |
| Pancreas (g) | 1.46 ± 0.27 | 1.73 ± 0.12 |
No statistically significant difference was founded (t test, p ≥ 0.05).
Fig. 5.
Histopatological analysis after TBLF oral administration. Rats were orally administrated with TBLF (50 mg/kg) every third day for 6 weeks. Colon, small intestine, liver and kidney were collected after sacrifice, stained and observed (10×).
Different blood markers were determined in order to study hepatotoxicity (AST and ALT), renal injury (urea, creatinine), pancreatic damage (α-amylase), and nutritional status (albumin, total protein, creatinine and glucose). No differences between groups were found, suggesting that the oral TBLF administration exhibit no toxicity at the end of the treatment (Table 3). Urea levels were found out of range, according to reference values for SD rats [30] but the high values applied to both, control and treated animals. To find out if blood parameters could change during the treatment, in a separated experiment total protein, albumin, creatinine and ALT were measured every 2 weeks and CBC was determined after 4 weeks. No significant differences between groups were found, suggesting no adverse effects after long-term treatment (data not shown).
Table 3.
Blood markers after TBLF subchronic oral administration.
| Blood markers | Control | TBLF |
|---|---|---|
| Glucose (mg/dL, RV: 50–160) | 93.5 ± 7.10 | 96.9 ± 6.69 |
| TP (mg/dL, RV: 5.9–7.9) | 8.3 ± 0.30 | 8.4 ± 0.44 |
| ALB (mg/dL, RV: 3.8–4.8) | 3.2 ± 0.02 | 2.9 ± 0.13 |
| AST (U/L, RV: 39–262) | 186.7 ± 21.50 | 160.1 ± 10.90 |
| ALT (U/L, RV: 35–80) | 65.5 ± 3.10 | 66.8 ± 4.93 |
| Urea (mmol/L, RV: 9.28–22.13) | 29.5 ± 4.03 | 30.8 ± 5.07 |
| Serum creatine (mg/dL, RV: 0.5–1.5) | 0.9 ± 0.02 | 0.9 ± 0.02 |
| α-Amylase (U/L, RV: NA) | 2.0 ± 0.04 | 1.9 ± 0.07 |
No statistically significant difference was founded (t test, p ≥ 0.05).
TP: Total protein, ALB: Albumin, AST: Aspartate aminotransferase, ALT: Alanine aminotransferase, RV: Reference values (16), NA: Data not available.
Other studies have observed that intragastric administration of saline extract of P. acutifolius to rats caused intestinal cells microvilli destruction, as well as breaking of endoplasmic reticulum outline [31]. These authors attributed the toxicity and poor nutritional value of P. acutifolius to high concentrations of phytohemagglutinin, however, it is known that Tepary bean protease inhibitor is also present in crude protein extracts [17]. TBLF does not contain the protease inhibitor form Tepary bean as a result of the chromatographic procedure [19]. Therefore, the results obtained in the present work could be attributed to the lectins contained in TBLF.
4. Conclusions
Here we report that after subchronic oral administration, TBLF provoked antinutritional effects in rats resulting in a transient decrease of food intake and body weight in he first weeks. The final result was observed as a reduction in body weight gain respect to the control group. The digestion assay suggests that lectins from Tepary bean can remain intact into the digestive tract up to 72 h. CBC at 24 h post-administration showed an allergic-like response that disappeared after 4 weeks of treatment. Blood markers suggest no toxic effects and no alterations in the evaluated organs. Taking together, our results showed that TBLF provoke a reduction in body weight gain with no other remaining effects, suggesting compensatory mechanisms and good tolerability. More studies are needed to determine effects on nutrient availability and intestinal integrity after TBLF administration, especially in long-term assays and in different development stages.
Conflict of interest
The authors declare that they do not have any conflict of interest.
Transparency document
Acknowledgments
We would like to acknowledge to Veronica Andrade-Portillo, Josue Lopez-Martinez, Evelyn Flores, Omar Perez-Segura, Miguel Angel Ortiz-Aguilar and Adin Meraz-Perez for their technical assistance. This work was supported by CONACYT, Ciencia Basica under grant number 82349; CONCYTEQ, FOMIX under grants numbers QRO-2007-C01-78221 and QRO-2011-C02-175340. Publication was possible with the founding of PIFI.
References
- 1.Sharon N. Lectins: past, present and future. Biochem. Soc. Trans. 2008;36:1457–1460. doi: 10.1042/BST0361457. [DOI] [PubMed] [Google Scholar]
- 2.González de Mejía E., Prisecaru V. Lectins as bioactive plant proteins: a potencial in cancer treatment. Crit. Rev. Food Sci. Nutr. 2005;45:425–445. doi: 10.1080/10408390591034445. [DOI] [PubMed] [Google Scholar]
- 3.Ferriz-Martinez R.A., Torres-Arteaga I.C., Blanco-Labra A., Garcia-Gasca T. New Approaches in the Treatment of Cancer. Nova Science Publishers, Inc.; Hauppauge, NY: 2010. The role of plant lectins in cancer treatment; pp. 71–89. [Google Scholar]
- 4.Chan Y.S., Ng T.B.A. Lectin with highly potent inhibitory activity toward breast cancer cells from edible tubers of Dioscorea opposita cv. nagaimo. PLOS ONE. 2013;8(1):e54212. doi: 10.1371/journal.pone.0054212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pusztai A., Bardocz S. Biological effects of plant lectins on the gastrointestinal tract: metabolic consequences and applications. Trends Glycosci. Glicotechnol. 1996;8:149–165. [Google Scholar]
- 6.Rhodes M. Beans means lectins. Gut. 1999;44:593–594. doi: 10.1136/gut.44.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lajolo F., Genovese M. Nutritional significance of lectins and enzyme inhibitors from legumes. J. Agric. Food Chem. 2002;50:6592–6598. doi: 10.1021/jf020191k. [DOI] [PubMed] [Google Scholar]
- 8.Czerwinski J., Leontowicz M., Gralak M. Response of rats to a moderate intake of soybean lectin. J. Anim. Feed Sci. 2005;14:37–540. [Google Scholar]
- 9.Figueroa M., Mancini J., Lajolo F. Antinutritional effect of phytohemagglutinins of Phaseolus Vulgaris. Arch. Lat. Nutr. 1984;34:488–499. [PubMed] [Google Scholar]
- 10.Pusztai A., Greer F., Silva M., Prouvost-Danon A., King T. Effect of wheat germ agglutinin on the interleukin pathway of the human T lymphocyte activation. J. Immunol. 1985;134:314–323. [PubMed] [Google Scholar]
- 11.Pusztai A., Ewen S.W.B., Grant G., Brown D.S., Stewart J.C., Peumans W.P., Van Damme E.J.M., Bardocz S. Antinutritive effects of wheat-germ agglutinin and other N-acetylglucosamine-specific lectins. Br. J. Nutr. 1993;70:313–321. doi: 10.1079/bjn19930124. [DOI] [PubMed] [Google Scholar]
- 12.Sanae H., Hideo O., Setsuko M., Yuki I., Kumiko S., Setsuko S., Yukihiko H., Yoshio Y. Soybean protein isolate and soybean lectin inhibit iron absorption in rats. J. Nutr. 1992;122:1190–1196. doi: 10.1093/jn/122.5.1190. [DOI] [PubMed] [Google Scholar]
- 13.Donatucci D., Lienner I., Gross C. Binding of navy bean (Phaseolus vulgaris) lectin to the intestinal cells of the rat and its effect on the absorption of glucose. J. Nutr. 1987;117:2154–2160. doi: 10.1093/jn/117.12.2154. [DOI] [PubMed] [Google Scholar]
- 14.Marzo F., Alonso R., Urdaneta E., Arricibita F.J., Ibáñez F. Nutritional quality of extruded kidney bean (Phaseolus vulgaris L. var, Pinto) and its effects on growth and skeletal muscle nitrogen fractions in rats. J. Anim. Sci. 2002;80:875–879. doi: 10.2527/2002.804875x. [DOI] [PubMed] [Google Scholar]
- 15.Greer F., Putzai A. Toxicity of kidney bean (Phaseolus vulgaris) in rats: changes in intestinal permeability. Digestion. 1985;32:42–46. doi: 10.1159/000199215. [DOI] [PubMed] [Google Scholar]
- 16.Pusztai A., Bardocz S. vol. 6. Taylor and Francis e-Library; London: 2005. p. 93. (Lectins, biomedical perspectives). [Google Scholar]
- 17.Osman M., Reid P., Weber C. The effect of feeding Tepary bean (Phaseolus acutifolius) proteinase inhibitors on the growth on pancreas of young mice. J. Nutr. 2003;2:111–115. [Google Scholar]
- 18.Reynoso Camacho R., González de Mejía E., Loarca Piña G. Purification and acute toxicity of a lectin extracted from tépari bean (Phaseolus acutifolius) Food Chem. Toxicol. 2003;41(1):21–27. doi: 10.1016/s0278-6915(02)00215-6. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Gasca T., Garcia-Cruz M., Hernandez-Rivera E., Lopez-Martinez J., Castañeda-Cuevas A., Yllescas-Gasca L., Rodríguez-Mendez J., Mendiola-Olaya E., Castro-Guillen J., Blanco-Labra A. Effects of Tepary beans (Phaseolus acutifolius) protease inhibitor and semipure lectin fraction on cancer cells. Nutr. Cancer. 2012;64(8):1269–1278. doi: 10.1080/01635581.2012.722246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez-Sanchez C., Lopez-Martinez F.J., Castañeda-Cuevas A., Yllescas-Gasca L., Ferriz-Martinez R., Torres-Arteaga I., Gallegos-Corona M.A., Rodríguez-Mendez A.J., Mendiola-Olaya E., Blanco-Labra A., Garcia-Gasca T. Evaluación de la toxicidad in vitro e in vivo de lectinas de frijol Tépari. Ciencia@UAQ. 2010;3:3–13. Available from: http://www.uaq.mx/investigacion/revista_ciencia@uaq/ArchivosPDF/v3-n1/Evaluacion.pdf. [Google Scholar]
- 21.NOM-062-ZOO . 1999. Norma Oficial Mexicana. Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio. Available from: http://148.243.71.63/default.asp?doc=743. [Google Scholar]
- 22.Jaffe W.G. Hemagglutinins. In: Liener I.E., editor. Toxic Constituents of Plant Foodstuffs. Academic Press; New York: 1980. pp. 69–101. [Google Scholar]
- 23.Turner R., Liener I. The use of glutaraldehyde-treated erythrocytes for assaying the agglutinating activity of lectins. Anal. Biochem. 1975;68:651–653. doi: 10.1016/0003-2697(75)90663-6. [DOI] [PubMed] [Google Scholar]
- 24.Bradford M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 25.Shagger H., Von Jagow G. Tricine-sodium dodecyl suphate-polyacrilamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal. Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 26.Gerard C. Purification of glycoproteins. In: Deutscher M.P., editor. Guide to Protein Purification. Academic Press; San Diego, CA: 1990. pp. 535–536. [Google Scholar]
- 27.Hara T., Mukunoki Y., Tsukamoto I., Miyoshi M., Hasegawa K. Susceptibility of Kintoki bean lectin to digestive enzymes in vitro and its behavior in the digestive organs of mouse in vivo. J. Nutr. Sci. Vitaminol. 1984;30:381–394. doi: 10.3177/jnsv.30.381. [DOI] [PubMed] [Google Scholar]
- 28.Nakata S., Kimura T. Effect to a moderate intake of ingested toxic bean lectins on the gastrointestinal tract in the rat. J. Nutr. 1985;115:1621–1629. doi: 10.1093/jn/115.12.1621. [DOI] [PubMed] [Google Scholar]
- 29.Le Gall L., Quillien B., Séve J., Guéguen J., Lallès J.P. Weaned piglets display low gastrointestinal digestion of pea (Pisum sativum L.) lectin and pea albumin 2. J. Anim. Sci. 2007;85:2972–2981. doi: 10.2527/jas.2006-795. [DOI] [PubMed] [Google Scholar]
- 30.Canadian Council on Animal Care . 1993. Guide to the Care and Use of Experimental Animals. Available from: http://www.ccac.ca/Documents/Standards/Guidelines/Experimental_Animals_Vol1.pdf. [Google Scholar]
- 31.Sotelo A.A., Gonzales M.T., Gonzales E., Velasco E., Fieria A. Ultrastructural change of epithelial intestinal cells induced by ingestion of raw Phaseolus acutifolius. Nutr. Rep. Int. 1983;27:329–337. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.