Abstract
Few studies have explored the role of microRNAs (or miRNAs) in Amyotrophic Lateral Sclerosis (ALS) muscle, possibly because of the difficulty in obtaining samples and because this is a rare disease.
We measured the expression levels of muscle-specific miRNAs (miRNA-1, miRNA-206, miRNA-133a, miRNA-133b, miRNA-27a) and inflammatory/angiogenic miRNAs (miRNA-155, miRNA-146a, miRNA-221, miRNA-149*) in the muscles of 13 ALS patients and controls. To highlight differences, patients were subdivided according to their gender, age at onset of symptoms, and disease duration.
A significant over-expression of all miRNAs was observed in ALS patients versus controls, in male patients versus females, in patients with early onset versus patients with late onset, and in patients with long disease duration versus patients with short duration.
A differential expression of miRNAs according to gender could be explained by the hormonal regulation which determines the body muscle mass. The course of the disease might reflect differential degree of muscle atrophy and signaling at miRNA levels. An evident role is also played by inflammatory/angiogenetic factors as shown by the observed miRNA changes.
Abbreviations: ALS, amyotrophic lateral sclerosis; MiRNA, micro-RNA; NF-kB, nuclear factor kappa light chain enhancer of activated B cells; PCR, polymerase chain reaction
Keywords: ALS, miRNA, Myo-miRNA, Inflammatory miRNA
Highlights
-
•
MyomiRNAs (especially miRNA-206) are up-regulated in ALS muscle than in controls.
-
•
Inflammatory miRNA-(especially miRNA-221) is up-regulated in ALS than in controls.
-
•
There is gender difference in expression of myo-miRNAs and inflammatory miRNAs.
-
•
MiRNAs levels differ according to age at onset and disease duration.
1. Introduction
Amyotrophic lateral sclerosis (ALS) is a progressive, neurodegenerative disorder caused by selective degeneration of upper and lower motorneurons. The disease process leads to progressive muscle weakness, atrophy, respiratory failure, and death three to five years after onset [1], [2]. ALS phenotypes are frequently classified on the basis of symptoms at onset: two-thirds of patients have limb-onset (spinal ALS), while one-third of patients present bulbar-onset (bulbar ALS), since they first manifest symptoms of dysarthria and dysphagia [3].
The frequency of ALS appears to be higher in male than in female patients (3:1) [2], [4], but this difference decreases with age. Gender appears to influence the clinical features of the disease: men are more likely to present limb-onset, whereas women have more frequently a bulbar-onset, which usually occurs at an older age [5]. The pathogenesis of ALS is attributed to a complex interaction between impaired molecular pathways and epigenetic regulation [6]. However, the variability in clinical features of the disease (age of onset, spinal or bulbar involvement, rapidity of progression, etc.) remains poorly understood, as well as the role of modifier genes and epigenetic factors.
In the present study we investigated microRNAs (miRNAs) expression levels in muscles of ALS patients. MiRNAs are small non-coding RNA molecules, which regulate the expression of a network of genes at the post-transcriptional level, usually binding the 3′-untranslated region of mRNA sequence. MiRNAs are involved in many biological processes in response to extracellular signals and stress.
A group of muscle-specific miRNAs (called “myo-miRNAs”) including miRNA-1, miRNA-206, and miRNA-133a/b, are involved in the processes of proliferation, regeneration, plasticity, and repair of muscle (Table 1), where they act as muscle mass regulators and aid the maintenance and function of homeostasis [7], [8]. MiRNA-206 is specific to skeletal muscle, while miRNA-1 and miRNA-133a/b are also found in the heart muscle [9]. Another possible myo-miRNA (miRNA-27a) promotes myoblast proliferation by targeting myostatin, an inhibitor of skeletal myogenesis [10].
Table 1.
Role of miRNAs in muscle, neuromuscular junction, lymphocytes or macrophages.
| miRNAs | Biological process involved | Findings |
|---|---|---|
| miR-206 | Muscle proliferation and differentiation | Up-regulated following myoblasts differentiation into myotubes and regeneration |
| miR-31, miR-335, miR-34c, miR-449, miR-494 | Muscle proliferation and differentiation | Up-regulated following myoblasts differentiation into myotubes and regeneration |
| miR-1, miR-133a, miR-133b, miR-29c, miR-135a | Degeneration and atrophy | Interfere with ability of muscle to adapt to exercise: down-regulated during hypertrophy, physical inactivity, fiber loss, atrophy |
| miR-27a | Muscle proliferation | Promotes myoblasts proliferation |
| miR-155 | Inflammation | Promotes pro-inflammatory pathways |
| miR-221, miR-223, miR-21 | Inflammation | Correlated with presence of inflammatory cells |
| miR-146a | Inflammation | Inhibits pro-inflammatory cytokines |
| miR-149* | Inflammation | Inhibits pro-inflammatory cytokines and innate responses |
Our hypothesis is that myo-miRNAs might play a role in modulating the disease course in ALS, where degeneration of both upper and lower motorneurons leads to muscle atrophy. Indeed, we expected miRNAs to exert their action by regulating muscle atrophy and wasting, possibly under the effect of sexual steroid hormones. Furthermore, since miRNAs have a role both in hypoxia and immune stimulation [11], we also investigated whether immune system-related and angiogenic-related miRNAs [12] have a role in ALS pathology.
In the muscle of 13 ALS patients we measured the levels of 5 myo-miRNAs and 4 miRNAs implicated in the inflammatory/angiogenic process. To determine whether gender and age at onset might be relevant factors in the modulation of miRNAs expression in muscle, we compared their results in subgroups of ALS patients divided according to their gender and the age at onset of symptoms.
2. Materials and methods
2.1. Patients selection
We selected 13 sporadic patients affected with ALS (6 males and 7 females) who were diagnosed according to the revised El Escorial Criteria [3] and followed at the IRCCS San Camillo Hospital in Venice, Italy. None of these patients showed mutations in SOD1 or TDP43 genes. Clinical data are summarized in Table 2.
Table 2.
Clinical data of ALS patients.
| Patient N. | Gender | Type of onset | Age at onset (years) | Age at biopsy (years) | Disease duration (months) | Clinical features | Associated disorders |
|---|---|---|---|---|---|---|---|
| 1 | M | Spinal | 66 | 69 | 36 | ||
| 2 | F | Spinal | 46 | 46 | 0 | Spasticity, tetraplegia | |
| 3 | F | Spinal | 70.5 | 72 | 18 | Diabetes mellitus | |
| 4 | M | Spinal | 63 | 63 | 0 | ||
| 5 | F | Spinal | 30 | 30.5 | 6 | ||
| 6 | M | Spinal | 58 | 58 | 0 | Lung cancer | |
| 7 | M | Spinal | 51.5 | 53 | 18 | Monoclonal gammopathy | |
| 8 | M | Spinal | 45 | 46.5 | 18 | ||
| 9 | F | Bulbar | 63.5 | 66 | 30 | RIG | Monoclonal gammopathy |
| 10 | M | Bulbar | 65.5 | 68 | 18 | PEG, tracheostomy | |
| 11 | F | Bulbar | 71 | 71.5 | 6 | PEG | |
| 12 | F | Bulbar | 40 | 41 | 12 | PEG, NIV | Cystic teratoma |
| 13 | F | Bulbar | 53 | 53 | 0 | ||
| Mean 55.6 | Mean 56.7 | Mean 12.4 |
M: male. F: female. RIG: radiologically inserted gastrostomy. PEG: percutaneous endoscopic gastrostomy. NIV: non-invasive ventilation.
For the purpose of the study, the patients were subdivided in subgroups according to their gender (males, females), the age at onset of symptoms (early onset: < 55 years, late onset: > 55 years), and the duration of the disease (short duration: 0–6 months, long duration: 7–36 months).
2.2. Muscle biopsies
Soon after onset of symptoms and diagnosis, ALS patients underwent a quadriceps femoris muscle biopsy (following written informed consent and ethical committee approval) as a part of the diagnostic process and to exclude other disorders which may mimic ALS at onset, such as inclusion body myositis, motor polyneuropathies, multifocal motor neuropathy, chronic proximal spinal muscular atrophy, and hereditary spastic paraplegia with polyneuropathy.
Immediately after collection, muscle biopsy samples were frozen in isopentane, chilled in liquid nitrogen, and then stored at − 80 °C at the Telethon Genetic Biobank Network (TGBN) in Padova. As healthy controls we used quadriceps muscle samples from five age-matched individuals who had no neuromuscular disorders.
2.3. RNA extraction and miRNA quantification
Muscle RNA isolation was performed with the miRNeasy Mini Kit (Qiagen, Hilden, Germany) following the instructions of the manufacturer. RNA was reverse transcribed using TaqMan microRNAs reverse transcription kit (Applied Biosystems, Carlsbad, CA, USA) according to the manufacturer's protocol and specific probes for each miRNA. Primers sequences are indicated in Supplementary Table 1. Quantitative PCR (real-time-PCR) experiments were conducted in duplicate and repeated at least twice. MiRNA expression levels were normalized on miRNA-39-3p of C. elegans used as an internal control and to measure the reverse transcription efficiency. After median Ct values normalization, miRNA expression level was calculated using the 2− ΔΔCt method, and normalized to the control mean.
2.4. Statistical analysis
Values are expressed as mean ± standard deviation, and differences in various groups were analysed using two-tailed Student t-tests. The level of significance was set at p < 0.05.
3. Results
Our study involved 13 ALS patients whose symptoms at onset were either bulbar (5 cases) or spinal (8 cases), and disease signs occurred at ages ranging from 30 to 71 years (mean 55.6) (Table 2). Age at biopsy ranged from 30.5 to 72 years (mean 56.7), and disease duration ranged from 0 to 36 months (mean 12.4) (Table 2). Patients underwent a clinical follow-up during a rehabilitation treatment at IRCCS San Camillo Hospital: three patients underwent a percutaneous endoscopic gastrostomy, one underwent radiologically inserted gastrostomy, one presented spasticity and tetraplegia, one required tracheostomy, one required non-invasive ventilation, and five presented other associated disorders (two had monoclonal gammopathy with abnormal immunophenotype). These two latter patients actually had dysproteinemia (which is a purely quantitative change due to increase in the normal components of gamma-globulines, i.e. monoclonal gammopathy) and not paraproteinemia, which is due to abnormal plasma cells proliferation.
When subdividing the patients according to their gender, the two subgroups had similar age at onset, age at biopsy, and disease duration (Table 3). Conversely, when we subdivided the patients according to the age at onset of symptoms or according to disease duration, early onset and late onset patients significantly differed for age at biopsy, whereas short duration and long motorneuron (MND) disease duration patients had similar age at onset and age at biopsy.
Table 3.
Clinical data of ALS patients subdivided according to gender, age at onset, and disease duration.
| Patients | Gender (M, F) | Age at onset (years) | Age at biopsy (years) | Disease duration (months) |
|---|---|---|---|---|
| Males | 6 M | 58.0 ± 8.4 n.s. | 62.2 ± 6.8 n.s. | 15.0 ± 13.5 n.s. |
| Females | 7 F | 53.4 ± 15.7 | 54.3 ± 16.1 | 10.3 ± 10.8 |
| Early onset (< 55 years) | 2 M, 4 F | 42.5 ± 7.2⁎⁎ | 43.4 ± 7.5⁎⁎ | 9.0 ± 8.3 n.s. |
| Late onset (> 55 years) | 4 M, 3 F | 65.3 ± 4.5 | 66.7 ± 4.9 | 15.4 ± 14.2 |
| Short duration (0–6 months) | 2 M, 4 F | 53.5 ± 14.3 n.s. | 53.6 ± 14.2 n.s. | 2.0 ± 3.1⁎⁎ |
| Long duration (7–36 months) | 4 M, 3 F | 57.4 ± 11.8 | 59.3 ± 12.3 | 21.4 ± 8.3 |
p < 0.001 in early onset group vs late onset group, and in short duration group vs long duration group. N.s.: non-significant difference.
3.1. Myo-miRNAs and inflammatory miRNAs in ALS versus controls
The expression levels of both myo-miRNAs and inflammatory/angiogenic miRNAs were significantly higher in ALS than in controls, with the exception of miRNA-149* (Supplementary Table 2, Fig. 1A).
Fig. 1.
Histograms showing the expression levels of myo-miRNAs and inflammatory/angiogenic miRNAs in ALS patients (black bars) and controls (white bars) (Panel A), and of male patients (black bars) and female patients (white bars) (Panel B). Significantly higher levels were found in ALS patients versus controls, and between males versus females (** = p < 0.005).
3.2. Gender differences
The expression levels of myo-miRNAs and inflammatory/angiogenic miRNAs (with the exception of miRNA-149*) were significantly higher in male patients than in females (Supplementary Table 2, Fig. 1B).
3.3. Different age of onset and disease duration
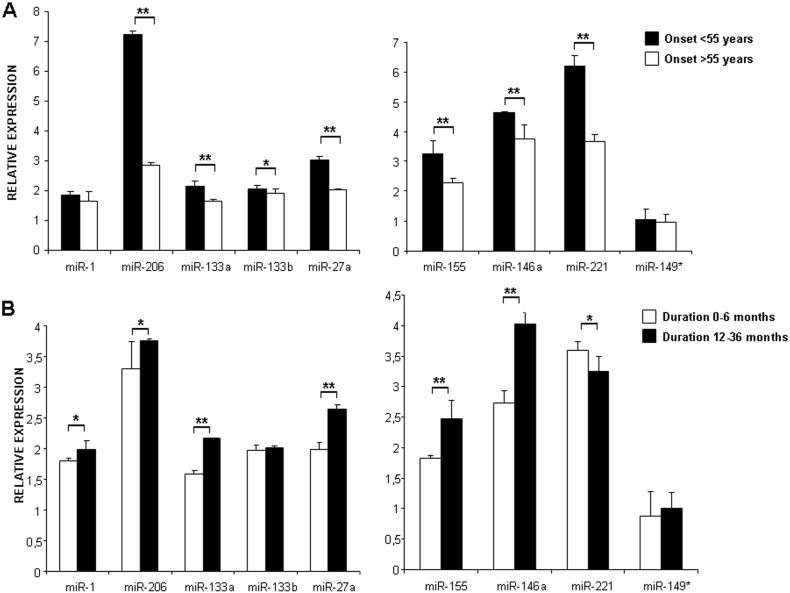
The expression levels of myo-miRNAs (except for miRNA-133b) were significantly higher in early onset than late onset patients, while the levels of inflammatory miRNAs (except for miRNA-149*) were down-regulated in patients with early compared to late onset (Supplementary Table 2, Fig. 2A). The expression levels of myo-miRNAs (except for miRNA-133b) were significantly lower in patients with short versus long disease duration, and the levels of inflammatory/angiogenic miRNAs (except for miRNA-149* and miR-221) were significantly down-regulated in patients with short compared to long disease (Supplementary Table 2, Fig. 2B).
Fig. 2.
Histograms showing the expression levels of myo-miRNAs and inflammatory/angiogenic miRNAs in ALS patients with different age at onset (Panel A) and with different disease duration (Panel B). Patients with onset before 55 years (black bars) showed a significant up-regulation of all miRNAs versus patients with onset occurring later than 55 years (white bars) (* = p < 0.05, ** = p < 0.005). Patients with short disease duration (white bars) showed a significant down-regulation of most miRNAs versus patients with long disease duration (black bars) (* = p < 0.05, ** = p < 0.005), with the exception of miR-133b, miRNA149*, and miR-221 (this latter being up-regulated).
These results suggest a modification of molecular pathways involving miRNAs during the course of the disease.
4. Discussion
The role of miRNAs and their abnormal expression has been documented in various muscle disorders including Duchenne dystrophy [13], [14], [15], myotonic dystrophy [16], [17], [18] and inflammatory myopathies [19]. However, few studies have so far focused on miRNAs action in ALS muscle.
Several studies in mouse models of both ALS [20], [21], [22] and spinal muscular atrophy [23] have demonstrated an increase in miRNA-206 expression level, which strongly parallels the onset of the disease and muscle atrophy, and a prominent disorganization of the neuromuscular junction (NMJ), which might slow down the reinnervation of denervated muscle [20]. These data suggest that muscle signaling might contribute to neuronal degeneration and play a role in the progression of ALS disease.
Furthermore, an up-regulation of miRNA-206 and variable changes of other myo-miRNAs have been documented in rodent muscle after nerve injury or denervation [8], [21], [24], suggesting that the underlying mechanism is not specific of MND but it is shared between various conditions in which NMJ function is impaired. Indeed, miRNA-206 is a key regulator of bidirectional signaling between muscle fibers and neurons, since it is able of sensing damage or loss of motorneurons and promoting regeneration of functional NMJ to attenuate muscle injury [8], [22], [25] and delay the progression of the disease [20], [26].
Our hypothesis is that the molecular mechanisms involved in the growth and maintenance of muscle are dys-regulated in ALS and that these changes might contribute to progression of the disease. Since the loss of muscle mass and weakness reduce the capacity for exercise rehabilitation, and contribute to disease progression, investigations into the molecular pathways involved in muscle atrophy have important clinical consequences in ALS.
The serum of ALS patients showed different miRNA levels in patients with spinal-onset as compared with bulbar-onset [27], and the more rapidly evolving clinical group (bulbar-onset) showed a higher degree of muscle fiber atrophy. Few studies have investigated the expression of miRNAs in muscle of ALS patients, providing non-concordant results [28], [29].
The over-expression of myo-miRNAs we found in ALS patients supports the notion that they regulate muscle plasticity or active remodelling process [30], which includes atrophy of some fibers, compensatory hypertrophy of other fibers to overcome the loss of muscle fibers, reinnervation occurring after denervation in MND (as demonstrated by fiber type grouping in chronic and prolonged ALS muscle), and occurrence of target or targetoid muscle fibers. Indeed, miRNA-1 and miRNA-133a are down-regulated in muscle hypertrophy [31] and miRNA-206, which is widely expressed in newly-formed muscle fibers, has a crucial role in the regeneration and maturation of muscle during atrophy [32]. Therefore, the high levels of miRNA-206 we found in ALS muscles are important because this miRNA is involved in the regulation of muscle mass [28], [26].
Another original field of research in ALS is the potential involvement of the immune system. Interestingly, we found a significant over-expression of inflammatory/angiogenic miRNAs, which was particularly evident in patients with longer disease duration.
In this context, it is worth noting that miRNA-155, which regulates both innate and adaptative immune response [33], [34], [35], was found to be two-fold increased in the spinal cord of ALS patients [33] and its inhibition was able to promote a significant extension in survival time of ALS animal model.
MiRNA-221 has a role both in the maintenance of skeletal muscle differentiation [36], in inflammatory responses (by regulating the expression of pro-inflammatory cytokines) [37], and in endothelial inflammation (by inhibiting nitric oxide production and activated NF-kB signaling in endothelial cells) [38].
MiRNA-146a (a pro-inflammatory miRNA regulated by NF-kB [39]) is involved in innate immunity and negatively regulates the secretion of pro-inflammatory cytokines preventing aggressive inflammation [40], [41], [42]. NF-kB plays a key role in skeletal muscle, leading to atrophy through classical pathway or promoting skeletal muscle homeostasis by alternative pathways [43]. Therefore, both miRNA-146a and miRNA-221 might have a role in ALS pathogenesis due to their link between muscle atrophy and inflammation via NF-kB action. The increased expression of inflammatory miRNAs we observed especially in ALS with long disease duration might be attributed to the activation of NF-kB that is known to cause both loss of muscle mass and production of inflammatory cytokines which have found to be elevated also in sarcopenic condition [43].
Another novel and interesting result from our study is the differential miRNAs expression in ALS muscle from male patients as compared to females, in agreement with the previous suggestion of a different disease course related to gender [5], [44]. It is well known that normal male and female skeletal muscles display considerable differences in their metabolic properties and gene expression patterns [45]. Furthermore, the aged muscle transcriptome differs between healthy men and women in immune activation, extracellular matrix remodelling and lipid component [46]. Earlier studies comparing man and women in response to disuse atrophy suggested that there are gender differences in muscle strength loss and atrophy. A gender difference in muscle inflammation following muscle exercise showed that women have less inflammation [47]. Another factor enhancing the degree of atrophy may be the initial muscle mass, suggesting that a smaller initial muscle mass in females attenuates gender-specific muscle loss. Recent studies have focused on the gender differences in ALS patients [44]: the incidence of the disease was found to be higher in men than in women, and there was a gender difference between age of onset and spinal- or bulbar-onset [48]. Here we provided further evidence that miRNAs might play a regulatory role leading to different clinical features of ALS according to gender. The lower expression of miRNAs levels we found in female patients might be related to different hormonal control, implying a slower course in females. A positive association of a longer survival of ALS women suggested that a longer exposure to female sexual hormones might have a neuro-protective effect [49]. Furthermore, miRNA-206 could have a positive regulatory effect in men, promoting the formation of new synapses, while miRNA-133a/b and miRNA-133b might delay the onset of ALS in women [50].
The present study offers the novel observation that there is an up-regulation of myo-miRNAs and of inflammatory miRNAs in ALS patients with earlier age at onset and in patients with longer disease duration, and as such could be a useful diagnostic marker at the onset of the disease. These differences might reflect an active remodelling at NMJ level in the early-onset cases, and concomitant atrophic and inflammatory processes due to active tissue inflammation in the disease course. This result is in agreement with observation on consecutive ALS muscle biopsies showing an increased inflammation after 12 weeks [29].
5. Conclusions
Although the mechanism underlying ALS pathogenesis is poorly understood, it is likely that multi-factorial components are at play. We demonstrated a significant up-regulation of myo-miRNAs (especially miRNA-206) and of inflammatory miRNAs (especially miRNA-221) in the patients with earlier age at onset of symptoms. This difference may suggest a possible role of miRNAs in the progression of events modulating the disease, including reinnervation or inflammation.
Our findings show differential miRNAs expression in muscle from males and females ALS patients, which could be attributed to hormonal regulation that controls body muscle mass, differential response to muscle atrophy, and sarcopenia.
The differential expression in patients with various disease durations might reflect different degree of plasticity or inflammatory cell response in the disease course.
The following are the supplementary data related to this article.
Primer sequences of miRNA probes.
Expression levels of miRNAs in ALS patients and controls. **: p < 0.005 in total patients vs controls, in males vs females, in eraly onset vs late onset, in short duration vs long duration. *: p < 0.05 in males vs females, in eraly onset vs late onset, in short duration vs long duration.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing financial interests
The authors declare no competing financial interests.
Acknowledgments
We acknowledge the Telethon Genetic BioBank Network (TGBN), Biobank of Rare Disease for Neurorehabilitation of BBMRI italian node and the EuroBioBank Network of Padova for providing samples of muscle biopsies. We acknowledge A.S.L.A. for support.
References
- 1.Gordon P.H. Amyotrophic Lateral Sclerosis: an update for 2013 clinical features, pathophysiology, management and therapeutic trials. Aging Dis. 2013;4:295–310. doi: 10.14336/AD.2013.0400295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harms M.B., Baloh R.H. Clinical neurogenetics: amyotrophic lateral sclerosis. Neurol. Clin. 2013;31:929–950. doi: 10.1016/j.ncl.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brooks B.R., Miller R.G., Swash M., Munsat T.L. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Motor Neuron Disord. 2000;1:293–299. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 4.Rosen A.D. Amyotrophic lateral sclerosis. Clinical features and prognosis. Arch. Neurol. 1978;35:638–642. doi: 10.1001/archneur.1978.00500340014003. [DOI] [PubMed] [Google Scholar]
- 5.Blasco H., Guennoc A.M., Veyrat-Durebex C., Gordon P.H., Andres C.R., Camu W., Corcia P. Amyotrophic lateral sclerosis: a hormonal condition? Amyotroph. Lateral Scler. 2012;13:585–588. doi: 10.3109/17482968.2012.706303. [DOI] [PubMed] [Google Scholar]
- 6.Paez-Colasante X., Figueroa-Romero C., Sakowski S.A., Goutman S.A., Feldman E.L. Amyotrophic lateral sclerosis: mechanisms and therapeutics in the epigenomic era. Nat. Rev. Neurol. 2015;11:266–279. doi: 10.1038/nrneurol.2015.57. [DOI] [PubMed] [Google Scholar]
- 7.Diniz G.P., Wang D.Z. Regulation of skeletal muscle by microRNAs. Compr. Physiol. 2016;6:1279–1294. doi: 10.1002/cphy.c150041. [DOI] [PubMed] [Google Scholar]
- 8.Mitchelson K.R., Qin W.Y. Roles of the canonical myo-miRs miR-1, -133, and -206 in cell development and disease. World J. Biol. Chem. 2015;6:162–208. doi: 10.4331/wjbc.v6.i3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Townley-Tilson W.H., Callis T.E., Wang D. MicroRNAs-1, 133, and 206: critical factors of skeletal and cardiac muscle development, function, and disease. Int. J. Biochem. Cell Biol. 2010;42:1252–1255. doi: 10.1016/j.biocel.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang Z., Chen X., Yu B., He J., Chen D. MicroRNA-27a promotes myoblast proliferation by targeting myostatin. Biochem. Biophys. Res. Commun. 2012;423:265–269. doi: 10.1016/j.bbrc.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 11.Leung A.K.L. The whereabouts of microRNAs actions: cytoplasm and beyond. Trends Cell Biol. 2015;25:601–610. doi: 10.1016/j.tcb.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murdock B.J., Bender D.E., Segal B.M., Feldman E.L. The dual roles of immunity in ALS: injury overrides protection. Neurobiol. Dis. 2015;77:1–12. doi: 10.1016/j.nbd.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Cacchiarelli D., Legnini I., Martone J., Cazzella V., D'Amico A., Bertini E., Bozzoni I. miRNAs as serum biomarkers for Duchenne muscular dystrophy. EMBO Mol. Med. 2011;3:258–265. doi: 10.1002/emmm.201100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Greco S., De Simone M., Colussi C., Zaccagnini G., Fasanaro P., Pescatori M., Cardani R., Perbellini R., Isaia E., Sale P., Meola G., Capogrossi M.C., Gaetano C., Martelli F. Common micro-RNA signature in skeletal muscle damage and regeneration induced by Duchenne muscular dystrophy and acute ischemia. FASEB J. 2009;23:3335–3346. doi: 10.1096/fj.08-128579. [DOI] [PubMed] [Google Scholar]
- 15.Zaharieva I.T., Calissano M., Scoto M., Preston M., Cirak S., Feng L., Collins J., Kole R., Guglieri M., Straub V., Bushby K., Ferlini A., Morgan J.E., Muntoni F. Dystromirs as serum biomarkers for monitoring the disease severity in Duchenne muscular dystrophy. PLoS One. 2013;8 doi: 10.1371/journal.pone.0080263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gambardella S., Rinaldi F., Lepore S.M., Viola A., Loro E., Angelini C., Vergani L., Novelli G., Botta A. Overexpression of microRNA-206 in the skeletal muscle from myotonic dystrophy type 1 patients. J. Transl. Med. 2010;8:48. doi: 10.1186/1479-5876-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perfetti A., Greco S., Bugiardini E., Cardani R., Gaia P., Geatano C., Meola G., Martelli F. Plasma microRNAs as biomarkers for myotonic dystrophy type 1. Neuromuscul. Disord. 2014;24:509–515. doi: 10.1016/j.nmd.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Koutsoulidou A., Kyriakides T.C., Papadimas G.K., Christou Y., Kararizou E., Papanicolau E.Z., Phylactou L.A. Elevated muscle-specific miRNAs in serum of myotonic dystrophy patients relate to muscle disease progress. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georgantas R.W., Streicher K., Greenberg S.A., Greenlees L.M., Zhu W., Brohawn P.Z., Higgs B.W., Czapiga M., Morehaouse C.A., Amato A., Richman L., Jallal B., Yao Y., Ranade K. Inhibition of myogenic microRNAs-1, 133, and 206 by inflammatory cytokines links inflammation and muscle de generation in adult inflammatory myopathies. Arthritis Rheum. 2014;66:1022–1033. doi: 10.1002/art.38292. [DOI] [PubMed] [Google Scholar]
- 20.Williams A.H., Valdez G., Moresi V., Qi X., McAnally J., Elliott J.L., Bassel-Duby R., Sanes J.R., Olson E.N. MicroRNA-206 delays ALS progression and promotes regeneration of neuromuscular synapses in mice. Science. 2009;326:1549–1554. doi: 10.1126/science.1181046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang X.H. MicroRNA in myogenesis and muscle atrophy. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:258–266. doi: 10.1097/MCO.0b013e32835f81b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma G., Wang Y., Li Y., Cui L., Zhao Y., Zhao B., Li K. MiR-206, a key modulator of skeletal muscle development and disease. Int. J. Biol. Sci. 2013;11:345–352. doi: 10.7150/ijbs.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valsecchi V., Boido M., De Amicis E., Piras A., Vercelli A. Expression of muscle-specific miRNA-206 in the progression of disease in a murine SMA model. PLoS One. 2016;10:e0128560. doi: 10.1371/journal.pone.0128560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiberg R., Jonsson S., Novikova L.N., Kingham P.J. Investigation of the expression of myogenic transcription factors, microRNAs and muscle-specific E3 ubiquitin ligases in the medial gastrocnemius and soleus muscles following peripheral nerve injury. PLoS One. 2015;10:e0142699. doi: 10.1371/journal.pone.0142699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdez G., Heyer M.P., Feng G., Sanes J.R. The role of muscle microRNAs in repairing the neuromuscular junction. PLoS One. 2014;9:e93140. doi: 10.1371/journal.pone.0093140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toivonen J.M., Manzano R., Oliván S., Zaragoza P., García-Redondo A., Osta R. MicroRNA-206: a potential circulating biomarker candidate for amyotrophic lateral sclerosis. PLoS One. 2014;9:e89065. doi: 10.1371/journal.pone.0089065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tasca E., Pegoraro V., Merico A., Angelini C. Circulating microRNAs as biomarkers of muscle differentiation and atrophy in ALS. Clin. Neuropathol. 2016;35:22–30. doi: 10.5414/NP300889. [DOI] [PubMed] [Google Scholar]
- 28.Russell A.P., Wada S., Vergani L., Hock M.B., Lamon S., Léger B., Ushida T., Cartoni R., Wadley G.D., Hespel P., Kralli A., Sorarù G., Angelini C., Akimoto T. Disruption of skeletal muscle mitochondrial network genes and miRNAs in amyotrophic lateral sclerosis. Neurobiol. Dis. 2012;49C:107–117. doi: 10.1016/j.nbd.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 29.Jensen L., Jorgensen L.H., Bech R.D., Frandsen U., Schroder H.D. Skeletal muscle remodelling as a function of disease progression in amyotrophic lateral sclerosis. Biomed. Res. Int. 2016;2016:5930621. doi: 10.1155/2016/5930621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potthoff M.J., Olson E.N., Bassel-Duby R. Skeletal muscle remodelling. Curr. Opin. Rheumatol. 2007;19:542–549. doi: 10.1097/BOR.0b013e3282efb761. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy J.J., Esser K.A. MicroRNA-1 and microRNA-133a expression are decreased during skeletal muscle hypertrophy. J. Appl. Physiol. 2007;102:306–313. doi: 10.1152/japplphysiol.00932.2006. [DOI] [PubMed] [Google Scholar]
- 32.Yuasa K., Hagiwara Y., Ando M., Nakamura A., Takeda S., Hijikata T. MicroRNA-206 is highly expressed in newly formed muscle fibers: implications regarding potential for muscle congenital on and maturation in muscular dystrophy. Cell Struct. Funct. 2008;33:163–169. doi: 10.1247/csf.08022. [DOI] [PubMed] [Google Scholar]
- 33.Koval E.D., Shaner C., Zhang P., du Maine X., Fischer K., Tay J., Chau B.N., Wu G.F., Miller T.M. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 2013;22:4127–4135. doi: 10.1093/hmg/ddt261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez A., Vigorito E., Clare S., Warren M.V., Couttet P., Soond D.R., van Dongen S., Grocock R.J., Das P.P., Miska E.A., Vetrie D., Okkenhaug K., Enright A.J., Dougan G., Turner M., Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science. 2007;316:608–611. doi: 10.1126/science.1139253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O'Connell R.M., Rao D.S., Chaudhuri A.A., Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 2010;10:111–122. doi: 10.1038/nri2708. [DOI] [PubMed] [Google Scholar]
- 36.Cardinali B., Castellani L., Fasanaro P., Basso A., Alema S., Martelli F., Falcone G. MicroRNA-221 and microRNAs-222 modulate differentiation and maturation of skeletal muscle cells. PLoS One. 2009;4:e7607. doi: 10.1371/journal.pone.0007607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia L., Zhang Y., Dong T. Inhibition of microRNAs-221 alleviates neuropathic pain through targeting suppressor of cytokine signaling 1. J. Mol. Neurosci. 2016;59:411–420. doi: 10.1007/s12031-016-0748-1. [DOI] [PubMed] [Google Scholar]
- 38.Chen C.F., Huang J., Li H., Zhang C., Huang X., Tong G., Xu Y.Z. MicroRNA-221 regulates endothelial nitric oxide production and inflammatory response by targeting adiponectin receptor 1. Gene. 2015;565:246–251. doi: 10.1016/j.gene.2015.04.014. [DOI] [PubMed] [Google Scholar]
- 39.Taganov K.D., Boldin M.P., Chang K.J., Baltimore D. NF-kB-dependent induction of microRNAs miR-146a, an inhibitor targeted to signaling proteins of innate immune responses. Proc. Natl. Acad. Sci. U. S. A. 2006;103:12481–12486. doi: 10.1073/pnas.0605298103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dai R., Ahmed S.A. MicroRNA, a new paradigm for understanding immunoregulation, inflammation, and autoimmune diseases. Transl. Res. 2011;157:163–179. doi: 10.1016/j.trsl.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xie Y.F., Shu R., Jiang S.Y., Liu D.L., Zhang X.L. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J. Inflamm. 2013;10:20. doi: 10.1186/1476-9255-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cheng H.S., Sivachandran N., Lau A., Boudreau E., Zhao J.L., Baltimore D., Delgado-Olguin P., Cybulsky M.I., Fish J.E. MicroRNA-146 represses endothelial activation by inhibiting pro-inflammatory pathways. EMBO Mol. Med. 2013;5:1017–1034. doi: 10.1002/emmm.201202318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li H., Malhotra S., Kumar A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 2008;86:1113–1126. doi: 10.1007/s00109-008-0373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCombe P.A., Henderson R.D. Effects of gender in amyotrophic lateral sclerosis. Gend. Med. 2010;7:557–570. doi: 10.1016/j.genm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Welle S., Tawil R., Thornton C.A. Sex-related differences in gene expression in human skeletal muscle. PLoS One. 2008;3:e1385. doi: 10.1371/journal.pone.0001385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D., Sartor M.A., Nader G.A., Pistilli E.E., Tanton L., Lilly C., Gutmann L., Iglay Reger H.B., Visich P.S., Hoffman E.P., Gordon P.M. Microarray analysis reveals novel features of the muscle aging process in men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2013;68:1035–1044. doi: 10.1093/gerona/glt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stupka N., Lowther S., Chorneyko K., Bourgeois J.M., Hogben C., Tarnoplolsky M.A. Gender differences in muscle inflammation after eccentric exercise. J. Appl. Physiol. 2000;89:2325–2332. doi: 10.1152/jappl.2000.89.6.2325. [DOI] [PubMed] [Google Scholar]
- 48.Beghi E., Logroscino G., Chiò A., Hardiman O., Millul A., Mitchell D., Swingler R., Traynor B.J. Amyotrophic lateral sclerosis, physical exercise, trauma and sports: results of a population-based pilot case-control study. Amyotroph. Lateral Scler. 2010;11:289–292. doi: 10.3109/17482960903384283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Jong S., Huisman M., Sutedja N., van der Kooi A., de Visser M., Schelhaas J., van der Schouw Y., Veldink J., van den Berg L. Endogenous female reproductive hormones and the risk of amyotrophic lateral sclerosis. J. Neurol. 2013;260:507–512. doi: 10.1007/s00415-012-6665-5. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen S., Hvid T., Kelly M., Lindegaard B., Dethlefsen C., Winding K., Mathur N., Scheele C., Pedersen B.K., Laye M.J. Muscle specific miRNAs are induced by testosterone and independently upregulated by age. Front. Physiol. 2013;4:394. doi: 10.3389/fphys.2013.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences of miRNA probes.
Expression levels of miRNAs in ALS patients and controls. **: p < 0.005 in total patients vs controls, in males vs females, in eraly onset vs late onset, in short duration vs long duration. *: p < 0.05 in males vs females, in eraly onset vs late onset, in short duration vs long duration.