Highlights
-
•
Kerosene supplementation increases serum testosterone levels in rat.
-
•
Increased testosterone levels were associated with increased aggression.
-
•
Kerosene supplementation had varied effects on blood parameters, notably, increased WBC counts.
-
•
Supplementation resulted in active/chronic gastritis in the stomach of our rat model.
Abbreviations: ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; EDTA, ethylenediaminetetraacetate; ELISA, enzyme linked immunosorbent assay; HCT, hematocrit concentration; LFT, liver function tests; RBC, red blood cells; RDW, red cell distribution width; RDW, red cell distribution width; RFT, renal function tests; T, testosterone; WBC, white blood cell
Keywords: Crude kerosene, Testosterone, Sex drive, Aggression, Toxicity, Gastritis
Chemical compounds studied in this article: Testosterone (PubChem CID: 6013), Picrate (PubChem CID: 62496), Bromocresol green (PubChem CID: 6451), Creatinine (PubChem CID: 588), Hematoxylin (PubChem CID: 442514), Formaldehyde (PubChem CID: 712), Eosin (PubChem CID: 11048), Ethylenediaminetetraacetic acid (PubChem CID: 6049)
Abstract
The use of crude kerosene as a dietary supplement in boarding schools has been a common practice in east Africa and other countries for many years, with the belief of it reducing the sex drive (libido) at the pubertal stage. There is however no scientific basis for this belief. The present study aimed at using a rat animal model to investigate the effects of crude kerosene on serum testosterone levels, aggression and its possible toxic effects. Fifteen male albino rats of approximately similar age and average weights were put into three groups of five animals each; the control group (placebo), low kerosene dose (10 μl/day) group and high kerosene dose (300 μl/day) group. ELISA was used to determine the serum testosterone levels. During treatment, changes in aggression were observed and noted. Liver toxicity was determined using enzyme assays, total protein and albumin while renal toxicity was monitored using serum creatinine levels. A full hemogram was conducted to determine hematological effects. Various tissue biopsies were obtained and examined using histopathological techniques for evidence of toxicity. Contrary to the common belief, our findings showed an overall increase of serum testosterone levels of up to 66% in the low dose and 75% in the high dose groups, with an increasing trend by the end of the study. The high dose group showed significantly increased levels of white blood cells (WBC) (p = 0.036), red blood cells (RBC) (p = 0.025), hematocrit (HCT) (p = 0.03), red cell distribution width (p = 0.028) and platelets (p = 0.017). The histological results of the stomach indicated chronic gastritis.
1. Introduction
Kerosene is a distillate of crude petroleum that contains aliphatic, aromatic and a variety of other branched saturated and unsaturated hydrocarbons [1]. The use of crude kerosene has been a common practice in east Africa and other countries for many years, with the belief of it reducing the sex drive (libido) at the pubertal stage. In the course of daily meals consumption students are exposed to doses of kerosene as a dietary supplement, usually without their consent.
The process of puberty results in the release of some specific hormones which are primarily responsible for the development of secondary sex characteristics and for the emergence of reproductive capabilities in boys [2]. During this stage an increase in testosterone causes an increase in the sex drive (libido), enlargement of the reproductive organs such as the penis and testes, the production of sperm, increase of muscle mass and lowering of the voice, increased frequency of erection, and the growth of facial, chest, nipple and pubic hair among boys [3]. The link between testosterone (T) levels and the sexual drive was demonstrated in a study done using adolescent boys with the findings indicating that the adolescent boys who had higher levels T levels also reported higher levels of sexual activity (i.e. coitus) [4], [5], [6], [7]. From the studies by Brooks-Gun and Halpern [5], [6] it can be inferred that hormones may enhance feelings of sexual arousal in adolescents but how they act on those feelings is very much determined by multiple internal and external variables.
From the study conducted by Olweus et al. [4], [8] it was noted that adolescent boys with higher T levels were more likely to engage in aggressive behavior. Under conditions of threat or unfair treatment, [9] they were shown to be aggressive. They further showed a link between higher T level and a lower tolerance for frustration. Further to these, they also observed that when no provoking situation occurred, T levels did not predict aggression. Various animal studies conducted on mice demonstrated the link between aggressive behavior and increased T levels [10], [11]. In a study on mice exposed to jet kerosene continuously for 90 days, there was an observed increased incidence in the fighting of the test group mice [12].
There is increasing trend regarding the percentage of teenagers reporting sexual initiation at younger ages [13]. This early sexual initiation (before age 16) is likely to involve sexual risk-taking and expose young people to unwanted sex, sexually transmitted infections, and teenage pregnancy. This may be attributed to exposure to a highly sexualized media environment that may represent a primary source of sexual socialization [14], [15]. Middle-childhood problems, poor parenting and peer pressure have also been shown to contribute to early sexual behavior [16], [17]. Due to the dire consequences of early sexual activity [18], there have been efforts toward finding effective remedies to tame teenage sexual hyperactivity. In many Kenyan boarding schools, especially high schools, one such remedy that has been used traditionally is crude kerosene. In a recent survey that we conducted using structured questionnaires at a Public University admitting students from all over the country, (data not shown) we found out that 68% female and 76% male first year, random respondents from 28 of 47 counties in Kenya, reported that at least one of their main meals (lunch or dinner) was supplemented with kerosene on daily basis during their high school years. Interestingly, over 60% of respondents in the above category gave why they thought kerosene was included in their diets as being to reduce their desire for sex. The remainder (40%) did not know why it was added.
Kerosene is readily available and at fairly low costs throughout the country. The primary use is for lighting and in cooking stoves. Whether or not kerosene supplementation is effective in reducing libido has not been scientifically tested. Further, the dietary use of kerosene in schools to tame sexual drive occurs with little or no care at all on its possible hazardous effects on the health status these students. Although some information is currently available on the effect of dietary kerosene supplementation in animals and/or humans [12], [19], such studies have failed to provide comprehensive information on effects on T levels, link to aggression and body tissue toxicity.
The present study was designed to monitor the effects on serum T levels, hematological, biochemical and histopathological changes in rats exposed to crude kerosene as a dietary supplement at doses that are comparable to those commonly used in Kenyan boarding schools.
2. Materials and methods
2.1. Ethics statement
All the animal protocols and experiments were approved by the Institution animal care and use committee of the University of Eldoret (Protocol No. UOE/001/14).
2.2. Animals
Male Wistar rats (Rattus norvegicus) of approximately the same age (six weeks old) corresponding to early adolescent boys [20] and similar body weights were obtained from the University of Eldoret animal facility. They were acclimatized and given free access to water and standard rodent chow diet (Unga Farmcare East Africa Limited, Nakuru, Kenya) for two weeks prior to initiation of the experimental diet. The rats were housed and maintained at ambient temperature of 25 °C under a photoperiod of 12 h of light and 12 h of darkness. The animals were assorted into three groups of five rats each with all groups having similar average serum testosterone levels.
2.3. Sample size determination
The sample size was determined according to the formula by Charan et al. [21].
E = total number of animals − total number of groups (if the value of E is between 10 and 20 then it is considered as an adequate, for this study, E = 12).
2.4. Animal treatment
Animal groups consisted of the control (placebo group – distilled water) low dose (10 μl kerosene) and high dose (300 μl kerosene). All animals were maintained on regular rodent chow diet throughout the study. Kerosene (National Oil Corporation, Eldoret, Kenya) was delivered orally on a daily basis. Blood samples from animals in all groups (control and treatment) were collected from the tail under local anesthesia at baseline, day 7 and day 14. Since T levels in young male rats have been shown to vary with time of the day [22], [23], all blood collections were done between 12.00 noon and 1.00 pm at all time points. Animals were also observed for changes in behavior on daily basis during and after treatment. At the end of study (day 28) blood samples were collected via cardiac puncture under chloroform anesthesia. The stomach and the brain and esophagus tissues were dissected and fixed in 10% formalin and used for histological analysis. Whole blood was collected in EDTA vacutainers for full hemogram while blood samples for serum were collected in plain tubes and serum obtained after centrifugation at 3000 × g for 10 min at 4 °C and kept at −20 °C until use for determination of the biochemical markers. Animal weights were monitored on weekly basis.
2.5. Changes in animal aggression
To evaluate the effect of kerosene supplementation on our experimental animal behavioral changes, an observational method was used. In brief, rats were monitored for observable changes in behavior following dietary kerosene supplementation and also after bleeding. Aggressive behavior was defined as burrowing (mechanical removal/moving of bedding material by rats within their cages) and fighting (chasing after other animals, voluntary attacks by one animal on another including biting and/or scratching) within a period of 20 min post supplementation among animals in the same group. Level of aggression was rated in terms of proportion of animals per group engaged in burrowing and fighting following kerosene supplementation. Comparisons in behavioral changes were made between the various groups to determine the relative aggression.
2.6. Biochemical/hematological analyses
Serum testosterone levels were determined by Enzyme linked immunoassay kit, (Human Diagnostics worldwide, Wiesbaden, Germany); ALT and AST activity were measured using reagent kits (Human Diagnostics worldwide, Wiesbaden, Germany; total proteins by biuret methods (Human Diagnostics worldwide, Wiesbaden, Germany); albumin by bromocresol green reagent (Human Diagnostics worldwide, Wiesbaden, Germany) according to the manufacturer's instructions. Renal functions were monitored using serum creatinine levels by alkaline picrate method (Biosystems, Barcelona, Spain) according to the manufacturer's instructions.
The hematological parameters were determined using the ADVIA 120D hematology system (Global Siemens Healthcare, Henkestrasse, Germany) according to the manufacturer's instructions.
2.7. Histopathological assays
The stomach, brain and esophagus tissues were excised and fixed in 10% neutral buffered formalin at room temperature and the representative tissues were taken from the stomach, brain and esophagus then processed using Automated Vacuum Tissue Processor Leica ASP6025 (Biosystems, Barcelona, Spain). They were embedded using paraffin wax and the blocks were sectioned into 5 μm thick slices, and stained with Hematoxylin and Eosin. Specimens were examined for morphological changes under light microscope (Olympus 6V20WHAL) (Olympus Imaging America Inc., PA, USA) and images were captured using a Smartphone digital camera obtained by its autofocus and automatic exposure control.
2.8. Statistical analysis
Statistical analysis was conducted using Student's t tests. Values are expressed as means ± SD or SEM and p < 0.05 was considered significant.
3. Results
3.1. Crude kerosene supplementation and associated effects among high school students
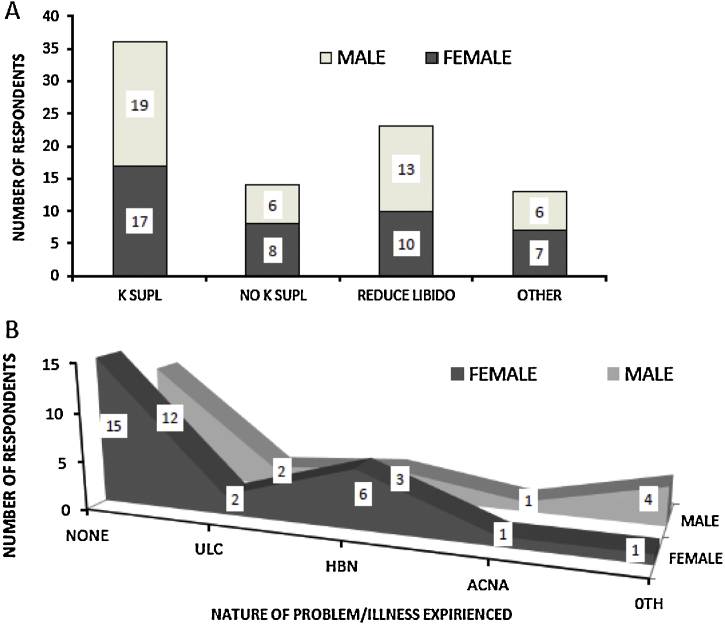
To determine the extent of kerosene dietary supplementation in Kenyan high schools; a pre-study survey was undertaken prior to our animal studies. Out of a total of 50 (half from either gender) fresh high school graduates who had recently enrolled at a local University taking part in our pre-study survey, 72% of respondents indicated that kerosene was routinely added to their school diets with slightly higher number of male (76%) than female students (68%) (Fig. 1A). Most of the respondents in the supplementation category thought that the reason for kerosene supplementation was intended to tame sex drive among students. Among students where kerosene was added to their diets, 46% reported having experienced at least one of the various diet related stomach problems with stomach ulcers, heart burns and stomach ache and/or nausea collectively comprising 47.8% of these problems (Fig. 1B).
Fig. 1.
Kerosene supplementation and associated effects among high school students. Structured questionnaires were used to conduct a pre-study survey. (A) The graph shows proportion of respondents who indicated that kerosene was included in their high school diet against those who stated otherwise and their supposed reason for inclusion. (B) Shows data for diet related problems experienced by students where kerosene was included in the diet. K SUPL: kerosene supplementation; ULC: stomach ulcers; HBN: heart burns; ACNA: stomach ache and or nausea; OTH: other.
3.2. Crude kerosene supplementation had no effect on animal body weights
Body weights were monitored regularly from start to end of study. No differences were seen at all time points in the body weights among the three groups (data not shown) (p > 0.05).
3.3. Crude kerosene increased serum testosterone levels
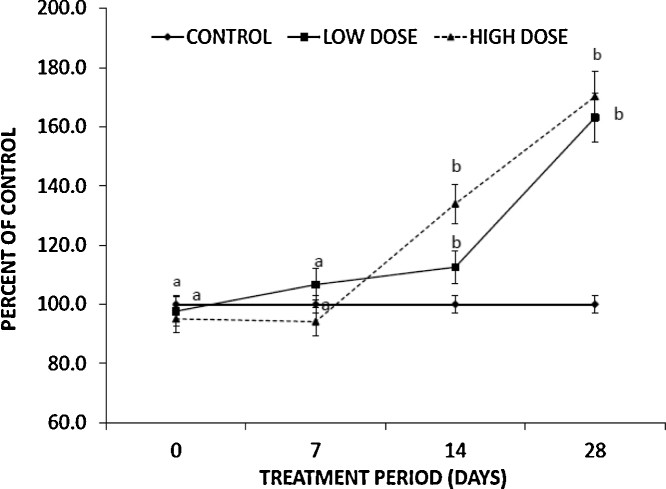
The average initial T values for the animals in our study were 3.05 ng/ml, 2.98 ng/ml and 2.9 ng/ml for control, low dose and high dose groups, respectively. The levels of T in our study animals are comparable to those obtained by other earlier studies conducted in rat where T levels were measured [24], [25]. Although the T levels in the control group showed a gradual decline in the course of our study duration, however this decline did not reach statistical significance (2.20 ng/ml at day 28).
The ELISA results at the various time points (Fig. 2) indicated that kerosene supplementation caused a marked increase in the rat's serum testosterone levels in the test groups relative to the control group. Both the low and high dose groups had an upward trend with an overall increase of 66% in the low dose and 75% in the high dose group with a continuing upward trend at the end of the study duration. There was however, no significant change in the T levels following acute (1st seven days) supplementation.
Fig. 2.
Effect of dietary kerosene supplementation on serum testosterone levels. Blood samples were collected from the tail at baseline (day 0), day 7 and day 14. At the end of study (day 28) blood samples were collected via cardiac puncture under chloroform anesthesia and analyzed for T levels by ELISA. The values represent means ± SEM of five animals per group. Data that share the same letters indicate values that are not significantly different (p < 0.05). T: testosterone; ELISA: enzyme linked immunosorbent assay.
3.4. Crude kerosene supplementation resulted in increased aggressive behavior
Crude kerosene supplementation resulted in increased incidences of aggression among the test group animals. This increased aggression (including fighting and burrowing) was specifically observed during and immediately after treatment administration and following bleeding at the various time points. Among the control animals, there were no observable changes in behavior before and after supplementation throughout the study. Incidences of burrowing and fighting were also minimal (attributable to normal behavior in rats). However, among the treatment groups, a progressive increase in number of animals engaged in burrowing and fighting was noted during the study period. It was also noted that the ease of handling during dosing became increasingly difficult in these groups. Although difficult to quantify, it was also observed that as the study progressed an increasingly higher proportion of animals in the high dose category displayed aggressive tendencies as compared to the low dose animals.
3.5. Crude kerosene had no effect on the hepatic and renal functions
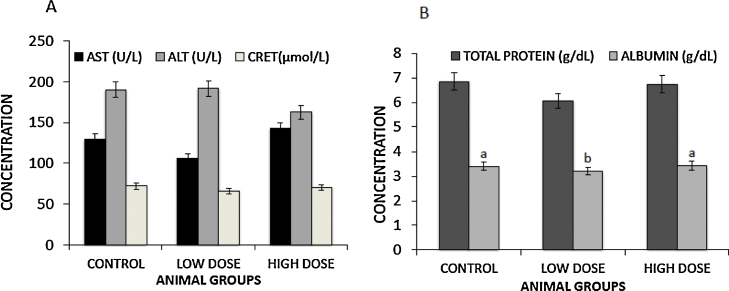
We investigated the potential toxic effects of the kerosene supplementation rat liver. Our results showed no statistically significant effects on the liver enzymes (AST and ALT) for both doses tested (Fig. 3A). Total proteins showed a decreasing trend but it did not reach statistical significance (Fig. 3B) (low dose p = 0.064, high dose p = 0.068). Serum albumin levels showed a significant decrease (Fig. 3B) (p = 0.038) for the low dose group. Kerosene supplementation did not significantly affect the kidney's ability to eliminate creatinine from blood (Fig. 3A).
Fig. 3.
Effects of dietary kerosene supplementation on hepatic and renal functions. At day 28, blood was collected via cardiac puncture following an overnight fast and analyzed for hepatic and renal functions. Serum was obtained and analyzed for AST, ALT, total protein and albumin to determine hepatic functions while creatinine levels were used to determine the renal function. Data show an average of five animals per group ±SEM. Different letters show significantly different values (p < 0.05); ALT: alanine transaminase; AST: aspartate transaminase; CRET: creatinine.
3.6. Effects of crude kerosene on different blood parameters
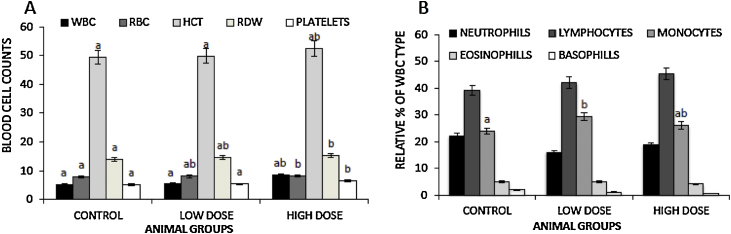
Crude kerosene supplementation increased white blood cells (WBC), red blood cells (RBC), platelets, hematocrit concentration (HCT) and the red cell distribution width (RDW) counts in a dose depended manner (Fig. 4A). Although there were increases in the counts for low dose group, the values did not reach statistical significance. The animals on a high dose kerosene supplementation had a significant increase in the WBC (p = 0.036, RBC (p = 0.025), HCT (p = 0.029), RDW (0.029) and platelets (p = 0.018) as compared to the untreated controls. WBC differential count showed a significant increase in the levels of monocytes in the low dose group relative to the control group (Fig. 4B). Differential counts of the other types of WBC remained essentially unaltered between all the groups.
Fig. 4.
Effects of dietary kerosene supplementation on hematological parameters. At day 28, whole blood was collected via cardiac puncture following an overnight fast. Hematological parameters were determined using the ADVIA 120D hematology system. (A) The graph shows counts of various blood cells and (B) shows the relative percentages of the different types of white blood cell. Data show ±SEM. Different letters show significantly different values with p < 0.05. WBC: white blood cells; RBC: red blood cells; HCT: hematocrit concentration; RDW: red cell distribution width.
3.7. Crude kerosene supplementation resulted in chronic gastritis
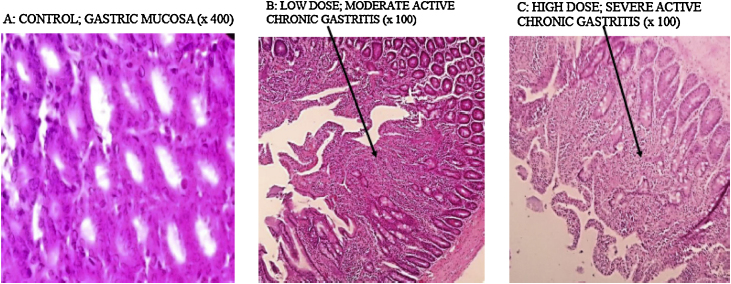
Kerosene supplementation resulted in an active chronic gastritis in the stomach in both test group animals. This effect was demonstrated by the infiltration of the eosinophils, lymphocytes and plasma cells present on the gastric mucosa and sub-mucosa. Despite being on similar diets and environmental condition, the control animals showed no signs of gastritis (Fig. 5).
Fig. 5.
Light micrograph images of different sections of the rat's stomach: (A) gastric mucosa from the control group animals (×400), (B) gastric mucosa from low dose group shows moderate inflammation by the eosinophils, lymphocytes and plasma cells which has extended to the sub mucosa (×100) and (C) gastric mucosa from high dose group shows severe active chronic gastritis with marked infiltration of both the mucosa and sub mucosa by eosinophils, lymphocytes and plasma cells.
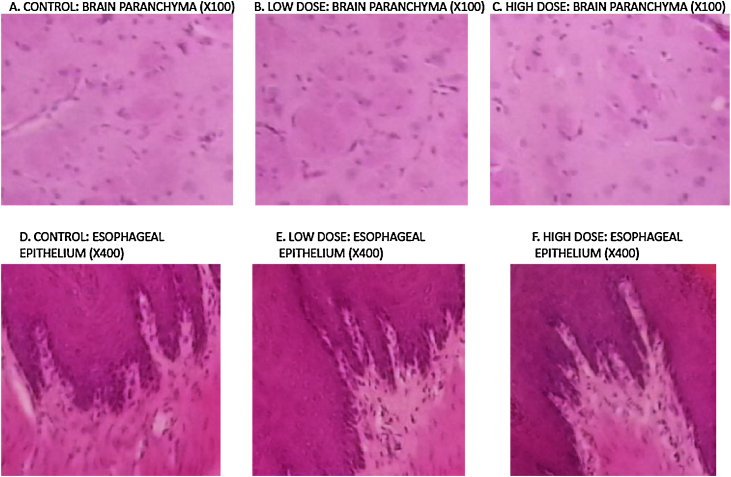
3.8. Crude kerosene supplementation resulted in no pathological effects on the brain and esophagus
There were no morphological changes in the brain (Fig. 6A–C) and the esophagus (Fig. 6D–F) for the animals in the various groups including control and treatment. This is indicative that the kerosene supplementation at our experimental doses had no toxic effects on both the brain and the esophagus.
Fig. 6.
Light micrograph images of different sections of the brain and esophagus. (A–C) Brain section showing the absence of parenchymal abnormalities. Similar findings (lack of pathology) were also observed in the brain stem and cerebellum. (D–F) Esophageal section showing epithelium with no sign of pathology.
4. Discussion
Kenyan boarding schools have for a long time continued to supplement student meals with crude kerosene with the belief that this would restrain their sexual desires that predispose them to high risk behaviors. Such risky behaviors lead to an increase in the incidences of STDs (including HIV), unsafe and illegal abortion, adolescent pregnancy and motherhood, single mother child/abandoned child, juvenile delinquency and many more [26]. The use of kerosene for the above purpose has however not been backed scientifically. Several studies have shown that accidental ingestion of kerosene results in toxic effects [27], [28], [29], [30].
Since T is known to regulate libido [6], [31], we hypothesized that if kerosene indeed reduces libido, then it might mediate its effects through modulation of T levels. A reduction in plasma levels has been associated with reduced sexual drive [7]. Further, increase in T has also been associated with aggressive tendencies [32], [6], [33]. We therefore investigated the effects of dietary crude kerosene supplementation on the plasma levels of this hormone and aggression behavior in a rat animal model.
Our results indicate that there was no change in the T level following acute (1st seven days) supplementation (Fig. 2). However, the trend changed drastically following continued prolonged (chronic) administration. Both the low dose and high dose groups showed an upwards trend with an overall increase of serum T levels of up to 66% in the low dose and 75% increase in the high dose groups, respectively, by the end of the treatment period (Fig. 2). The levels were on an upward trajectory even at the end of study suggesting that longer durations of supplementation are more likely to result in even higher increases in T levels. It can be inferred therefore that initial (acute) dietary supplementation with kerosene in boarding school has no effects on blood T levels among students. On the contrary, prolonged (chronic) use over the extended schooling years may with time result in elevated levels of T among students with the concomitant increase in desire for sexual activity.
This result associating kerosene supplementation to increase levels of serum T may in part explain the rising cases of premature sexual activity leading to high cases of sexually transmitted infections, unwanted sex and teenage pregnancy [14], [15]. As indicated earlier, evidence has shown that high levels of T are also associated with aggressive tendencies [32], [6], [33]. It was interestingly observed that animals on kerosene supplementation displayed increasing aggression over the study period. The higher kerosene dose group displayed even higher levels of aggression during and immediately after either kerosene supplementation or bleeding. This corroborates the findings by Olweus et al. [34], [35] in a study where it was noted that adolescent boys with higher T levels were not only more likely to engage in aggressive behavior but under conditions of threat or unfair treatment (provocation), they were shown to be more likely to be aggressive. These results may provide a partial link between kerosene supplementation in boarding schools and the ever increasing teenage sexual hyperactivity, teenage pregnancy and aggressive behavior such as riots. For our animals, aggressive behavior was observed during and after treatment and also during and after blood collection from the tails. These events may mimic provocative conditions that have often led to student unrests. Studies conducted earlier however showed that given acutely, over a few hours, no abnormal neurologic signs or behavior were notable in baboons [36]. This is in agreement with our findings where we noted no significant alteration in testosterone levels for the first week of supplementation. This means that it may require chronic kerosene supplementation to see both increase in T levels in blood and the T mediated effects on behavior such as increased aggressive tendencies. The mechanism through which the kerosene results in the increase of T remains to be elucidated.
Various studies have shown that ingestion or inhalation of kerosene could lead to various toxic effects [27], [37], [38]. Reported clinical effects of accidental ingestion or suicide attempt are quite varied ranging from mild to fatal. The severities of the effects appear to be largely dependent on the quantity ingested, the age and interaction with drugs (such as metformin) that the victim might be using at the time of ingestion [39], [40]. The common effects include cough with difficulty in breathing, vomiting, fever, central nervous system involvement, severe lactic acidosis and acute renal failure, pyopneumothorax and deaths [39], [40], [41]. It is important to note that effects reported on accidental ingestion or intended suicide are acute effects occurring within a short period of time post ingestion and are usually due to ingestion of large quantities. We therefore postulated that chronic dietary kerosene supplementation albeit at lower doses than above (accidental or suicide attempt) may also be harmful to body tissues. We thus investigated the potential toxic effects of kerosene on the liver, kidney, blood and the brain, esophagus and stomach lumen.
It was notable from our findings that there was a uniform steady rate of increase in the body weights from all the three groups with no significant difference (p > 0.05) among the three groups.
Regarding potential toxic effects to the liver, relative to the control group, kerosene supplementation showed little to no effects (Fig. 3A and B). The liver enzymes remained unchanged (ALT, p = 0.97 and p = 0.35, AST, p = 0.11 and p = 0.34 for low and high dose groups, respectively. Similarly, kerosene supplementation did not have a significant effect on the serum total proteins. Although results depicted a decreasing trend, it did not reach statistical significance (low dose p = 0.064, high dose p = 0.068). Serum albumin levels showed a significant decrease of p = 0.038 for the low dose however for the high dose there was no significant effect (p = 0.77). This finding may require further investigations. Overall, for the period of study, kerosene supplementation resulted in minimal signs of liver toxicity.
Further, no toxic effects were observed with respect to kidneys. Kerosene supplementation did not significantly affect the kidneys ability to eliminate creatinine (Fig. 3A). It was interestingly observed that on the contrary to our expectation, the kidneys in the treated groups relative to the control group appeared to be eliminating creatinine from the blood more efficiently as shown by their lower serum creatinine levels (Fig. 3A). In their earlier studies, Starek et al. observed signs of liver and kidney respiratory toxicity by kerosene in rats, however effects were noted mainly in rats acutely poisoned, while in sub-chronic poisoning they were less pronounced [10]. This may suggest a possible adaptation over time as minimal toxic effects were also seen in our chronic study.
Unlike the other effects noted so far, kerosene supplementation appeared to have a possible dose related effects with respect to the WBC, RBC, platelets, HCT and the RDW. Relative to the control group there was an increasing trend in these cell counts (Fig. 4A) which appeared to be dose-dependent. Although there were increases in the counts for low dose group, the values did not reach statistical significance (Fig. 4A). The animals on a high dose kerosene supplementation had a significant increase in the WBC (p = 0.036) corroborating findings by Dede et al. [37], RBC (p = 0.025), HCT (p = 0.029), RDW (0.029) and platelets (p = 0.018). This RBC and HCT increase may be beneficial since it may lead to increased oxygen carrying capacity of the blood. The initial increase in the platelets may be beneficial but continued increase could be toxic if it goes beyond the limit of the normal ranges as then it could lead to increased incidences of clotting disorders such as stroke. RDW is used as a measurement of the red blood cells variation in size and an increase is commonly used in humans as a prognostic marker of either a cardiovascular event, or a metabolic inflammatory event [42], [43], [44], [45]. What was interesting to note is that kerosene supplementation at the doses used in our study did not cause anemia as is commonly observed in petroleum products toxicity reported earlier [46], [47], [48]. This might be explained by the relatively high doses used in these studies, i.e. 6 ml/kg which are over four times higher than the high doses used in our study (low dose = 0.05 ml/kg, high dose = 1.3 ml/kg).
As noted earlier, there was an overall increase in the WBC counts in test groups (Fig. 4A), the reason for this observed increase was suggested by Krishan Veena [49] to be due to a defensive mechanism triggered by the immune system. The low dose group showed a marginal (6%) increase while the high dose group had a significant increase of 61%. Similar findings were obtained by Dede [37] and Krishan Veena [49]. WBC differential count showed a significant increase in the levels of serum monocytes in the low dose group relative to the control group (p = 0.023), (Fig. 4B).
Kerosene supplementation had an inflammatory effect on the stomach lumen in all the test groups. This effect was demonstrated by the active and chronic inflammation observed histologically (Fig. 5A–C). From these findings, it can be concluded that kerosene supplementation causes gastritis. The inflammation was observed to be more pronounced at the gastro duodenal junction of the stomach. Although studies have shown that Helicobacter pylori is the chief cause of gastritis in Kenya [50], there may be need to re-examine the contribution of dietary kerosene supplementation especially among school going children. From data obtained during an earlier pre-animal study survey (Fig. 1B), 47.8% of respondents with kerosene supplementation reported that they had experienced either ulcers or heart burns. This points to the role that kerosene supplementation in Kenyan schools may have in the high number of cases of students with gastritis.
There were no significant morphological changes on the brain (Fig. 6A–C) with the parenchyma, brain stem and cerebellum all showing lack of abnormalities (pathology). Similarly, images were obtained from the esophagus from all three groups (Fig. 6D–F) also indicates lack of abnormalities. The kerosene doses used in our study were therefore found not to be toxic to the brain and the esophagus.
5. Conclusion
This study established for the first time that kerosene supplementation results in increased serum T levels which have been shown to be directly associated with higher sex drive (libido). Based on these findings therefore, crude kerosene supplementation is ineffective in controlling sexual hyperactivity in boarding schools. Our findings also demonstrate the relationship between increased serum T levels with increased aggression. Kerosene supplementation in boarding schools may result to similar effects. These findings may explain the increase in the numbers of teenage pregnancies, rebellion to authority and violence as seen in school going teenage children. The findings from the present study further show that crude kerosene supplementation caused gastritis in our animal model. Kerosene supplementation in schools thus may be a contributing factor in the increasing cases of gastritis and ulcers among students.
We recommend that alternative, effective and safe ways to control sexual hyperactivity that are scientifically proven need be sought as a replacement to kerosene dietary supplementation.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for profit sector
Conflict of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research findings reported.
Transparency document
Acknowledgments
The authors thank Edwin Kirwa for helping with animal care and treatment and Winfridah Cheriro for assistance during the running of hormone assays.
References
- 1.Anderson J.N., Neff J.M., Acox B., Tatem H.E., Hightower G.M. Characteristics of dispersion and water soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Mar. Biol. 1974;27:75–88. [Google Scholar]
- 2.Collaer M.L., Hines M. Human behavioral sex differences: a role for gonadal hormones during early development? Psychol. Bull. 1995;118(1):55–107. doi: 10.1037/0033-2909.118.1.55. [DOI] [PubMed] [Google Scholar]
- 3.Mandal A. 2014. Testosterone physiological effects. Available from: http://www.news-medical.net/health/Testosterone-Physiological-Effects.aspx (accessed 20.11.14) [Google Scholar]
- 4.Olweus D. Testosterone, aggression, physical, and personality dimensions in normal adolescent males. Psychosom. Med. 1980;42(2):253–269. doi: 10.1097/00006842-198003000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Halpern C.T., Udry J.R., Suchindran C. Monthly measures of salivary testosterone predict sexual activity in adolescent males. Arch. Sex. Behav. 1998;27(5):445–465. doi: 10.1023/a:1018700529128. [DOI] [PubMed] [Google Scholar]
- 6.Corona G.I.A., Buvat J., Aversa A., Rastrelli G., Hackett G., Rochira V., Sforza A., Lenzi A., Mannucci E., Maggi M. Testosterone supplementation and sexual function: a meta-analysis study. J. Sex. Med. 2014;11(6):1577–1592. doi: 10.1111/jsm.12536. [DOI] [PubMed] [Google Scholar]
- 7.Snyder P.J. The testosterone trials: seven coordinated trials of testosterone treatment in elderly men. Clin. Trials. 2014;11(3):362–375. doi: 10.1177/1740774514524032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olweus D. Circulating testosterone levels and aggression in adolescent males: a causal analysis. Psychosom. Med. 1988;50(3):261–272. doi: 10.1097/00006842-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Scaramella T.J., Brown W.A. Serum testosterone and aggressiveness in hockey players. Psychosom. Med. 1978;40(3):262–265. doi: 10.1097/00006842-197805000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Starek A., Vojtisek M. Effects of kerosene hydrocarbons on tissue metabolism in rats. Pol. J. Pharmacol. Pharm. 1986;38(5–6):461–469. [PubMed] [Google Scholar]
- 11.Rao G.S. Subcutaneous kerosene toxicity in albino rats. Environ. Res. 1984;35(2):516–530. doi: 10.1016/0013-9351(84)90158-0. [DOI] [PubMed] [Google Scholar]
- 12.Mattie D.R., Marit G.B., Flemming C.D., Cooper J.R. The effects of JP-8 jet fuel on male Sprague-Dawley rats after a 90-day exposure by oral gavage. Toxicol. Ind. Health. 1995;11(4):423–435. doi: 10.1177/074823379501100405. [DOI] [PubMed] [Google Scholar]
- 13.Ramiro L., Reis M., de Matos M.G., Diniz J.A. Trends in adolescent sexual behavior, impact of information, and attitudes about HIV/AIDS in Portugal. Psychol. Health Med. 2013;19(5):614–624. doi: 10.1080/13548506.2013.845299. [DOI] [PubMed] [Google Scholar]
- 14.Parkes A., Wight D., Hunt K., Henderson M., Sargent J. Are sexual media exposure, parental restrictions on media use and co-viewing TV and DVDs with parents and friends associated with teenagers’ early sexual behaviour? J. Adolesc. 2013;36(6):1121–1133. doi: 10.1016/j.adolescence.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landry M., Gonzales F.A., Wood S., Vyas A. New media use and sexual behavior among Latino adolescents. Am. J. Health Behav. 2013;37(3):422–430. doi: 10.5993/AJHB.37.3.15. [DOI] [PubMed] [Google Scholar]
- 16.Parkes A., Waylen A., Sayal K., Heron J., Henderson M., Wight D., Macleod J. Which behavioral, emotional and school problems in middle-childhood predict early sexual behavior? J. Youth Adolesc. 2014;43:507–527. doi: 10.1007/s10964-013-9973-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parkes A., Henderson M., Wight D., Nixon C. Is parenting associated with teenagers’ early sexual risk-taking, autonomy and relationship with sexual partners? Perspect. Sex. Reprod. Health. 2011;43(1):30–40. doi: 10.1363/4303011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayer E. Rosen Publishing Group; 2000. It's Okay to Say No: Choosing Sexual Abstinence (Teen Pregnancy Prevention Library) Revised edition (January 2000) [Google Scholar]
- 19.Dede E.B., Kaglo H.O. Aqua-toxicological effects of water soluble fraction (WSF) of diesel fuel on Oreochromis niloticus fingerlings. J. Appl. Sci. Environ. Manag. 2001;5:93–96. [Google Scholar]
- 20.Sengupta P. The laboratory rat: relating its age with human's. Int. J. Prev. Med. 2013;4(6):624–630. [PMC free article] [PubMed] [Google Scholar]
- 21.Charan J., Kantharia N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013;4(4):303–306. doi: 10.4103/0976-500X.119726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kashiwagi B. Changes in testosterone and dihydrotestosterone levels in male rat accessory sex organs, serum, and seminal fluid after castration: establishment of a new highly sensitive simultaneous androgen measurement method. J. Androl. 2005;26(5):586–591. doi: 10.2164/jandrol.04164. [DOI] [PubMed] [Google Scholar]
- 23.Chacon F. 24-Hour changes in ACTH, corticosterone, growth hormone, and leptin levels in young male rats subjected to calorie restriction. Chronobiol. Int. 2005;22(2):253–265. doi: 10.1081/cbi-200053522. [DOI] [PubMed] [Google Scholar]
- 24.Bartke A. Fluctuations in plasma testosterone levels in adult male rats and mice. Endocrinology. 1973;92(4):1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- 25.Ibukun P., Oyeyipo Y.R., Adeyombo F.B. Nicotine alters male reproductive hormones in male albino rats: the role of cessation. J. Hum. Reprod. Sci. 2013;6(1):40–44. doi: 10.4103/0974-1208.112380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dave V.R., Makwana N.R., Yadav B.S., Yadav S. A study on high-risk premarital sexual behavior of college going male students in Jamnagar City of Gujarat, India. Int. J. High Risk Behav. Addict. 2013;2(3):112–116. doi: 10.5812/ijhrba.11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbas S.K., Tikmani S.S., Siddiqui N.T. Accidental poisoning in children. J. Pak. Med. Assoc. 2012;62(4):331–334. [PubMed] [Google Scholar]
- 28.Achuba F.I., Osakwe S.A. Petroleum-induced free radical toxicity in African catfish (Clarias gariepinus) Fish Physiol. Biochem. 2003;29:97–103. [Google Scholar]
- 29.Brata Ghosh V. Common childhood poisonings and their outcome in a tertiary care center in Delhi. Indian J. Pediatr. 2013;80(6):516–518. doi: 10.1007/s12098-012-0879-5. [DOI] [PubMed] [Google Scholar]
- 30.Chhetri U.D., Ansari I., Shrestha S. Pattern of pediatric poisoning and accident in Patan Hospital. Kathmandu Univ. Med. J. (KUMJ) 2012;10(39):39–43. doi: 10.3126/kumj.v10i3.8016. [DOI] [PubMed] [Google Scholar]
- 31.Hashizume K. The relationship between serum hormone levels and reproductive ability in aging male rats. Jikken Dobutsu. 1984;33(2):159–163. doi: 10.1538/expanim1978.33.2_159. [DOI] [PubMed] [Google Scholar]
- 32.Halpern C.T., Udry J.R., Suchindran C. Monthly measures of salivary testosterone predict sexual activity in adolescent males. Arch. Sex. Behav. 1998;5:445–465. doi: 10.1023/a:1018700529128. [DOI] [PubMed] [Google Scholar]
- 33.Koo K.C., Ahn J.H., Hong S.J., Lee J.W., Chung B.H. Effects of chemical castration on sex offenders in relation to the kinetics of serum testosterone recovery implications for dosing schedule. J. Sex. Med. 2014;11(5):1316–1324. doi: 10.1111/jsm.12492. [DOI] [PubMed] [Google Scholar]
- 34.Olweus D., Mattsson A., Schalling D., Low H. Testosterone, aggression, physical and personality dimensions in normal adolescent males. Psychosom. Med. 1980;42:253–269. doi: 10.1097/00006842-198003000-00003. [DOI] [PubMed] [Google Scholar]
- 35.Olweus D., Mattsson A., Schalling D., Low H. Circulating testosterone levels and aggression in adolescent males a casual analysis. Psychosom. Med. 1988;50:261–272. doi: 10.1097/00006842-198805000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Mann M.D., Pirie D.J., Wolfsdorf J. Kerosene absorption in primates. J. Pediatr. 1977;91(3):495–498. doi: 10.1016/s0022-3476(77)81334-6. [DOI] [PubMed] [Google Scholar]
- 37.Dede E.B., Igboh N.M., Ayalogu O.A. Chronic toxicity study of the effect of crude petroleum (bonny light), kerosine and gasoline on rats using haematological parameters. J. Appl. Sci. Environ. Manag. 2002;6(1):60–63. [Google Scholar]
- 38.Krishan A.G., Veer G. Investigation on the toxicity of seawater extracts of three crude oils on eggs of Cod (Gadus Morhua L.) Beridt wise Komm. Meeress Forsch. 1980;23:165–180. [Google Scholar]
- 39.Belonwu R.O., Adeleke S.I. A seven-year review of accidental kerosene poisoning in children at Aminu Kano Teaching Hospital, Kano. Niger. J. Med. 2008;17(4):380–382. doi: 10.4314/njm.v17i4.37415. [DOI] [PubMed] [Google Scholar]
- 40.Rathnapala A., Matthias T., Jayasinghe S. Severe lactic acidosis and acute renal failure following ingestion of metformin and kerosene oil: a case report. J. Med. Case Rep. 2012;6:18. doi: 10.1186/1752-1947-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verma S.K. Pyopneumothorax following suicidal kerosene ingestion. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-007795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felker G.M.A.L., Pocock S., Shaw L.K., McMurray V., Pfeffer M.A., Swedberg K., Yusuf S., Michelson E.L., Granger C.B. Red cell distribution width as a novel prognostic marker in heart failure. Am. Coll. Cardiol. 2007;50:40–47. doi: 10.1016/j.jacc.2007.02.067. [DOI] [PubMed] [Google Scholar]
- 43.Tonell M.S.F., Arnold M., Moye L., Davis B., Pffer M. Relation between red blood cell distribution and cardiovascular event rate in people with coronary disease. Circulation. 2008;117:163–168. doi: 10.1161/CIRCULATIONAHA.107.727545. [DOI] [PubMed] [Google Scholar]
- 44.Lippi G., Targher G., Montagnana T.G., Salvagno G.L., Zoppini G., Guidi G.C. Relationship between red cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch. Pathol. Lab. Med. 2009;133:628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
- 45.Van Guilder G.P., Hoetzer G.L., Greiner J.J., Staufer B.L., Desouza C.A. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammatory in obese adults. Obesity. 2006;(14):2127–2131. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 46.Becki L. General chemical information and toxicity of crude petroleum. Chemicals. 2007;26:6–19. [Google Scholar]
- 47.Suzanne I.B. Therapeutic and nutritional information on crude oil. Chemicals. 2003;6:4–11. [Google Scholar]
- 48.Ita S.O., Udofia U.A. Comparative study of some haematological parameters in rats following ingestion of crude oil (Nigerian bonny light), petrol, kerosene and diesel. Asian J. Biol. Sci. 2011;4:498–505. [Google Scholar]
- 49.Krishan A.G., Veena G. 2,3,4-Triaminoazo benzene-induced hemato biochemical anomalies in fish (Channa puntatus) Bull. Environ. Contam. Toxicol. 1980;25:136–141. doi: 10.1007/BF01985501. [DOI] [PubMed] [Google Scholar]
- 50.Kalebi A. Histopathological profile of gastritis in adult patients seen at a referral hospital in Kenya. World J. Gastroenterol. 2007;13(30):4117–4121. doi: 10.3748/wjg.v13.i30.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.