Abstract
Hepatocellular carcinoma (HCC) is the third most frequent cause of cancer deaths throughout the world. This study was aimed to analyze oxidative stress and cell damage in a multistage model of liver carcinogenesis induced by diethylnitrosamine (DEN) in rats. Male Wistar rats weighing 145–150 g were divided into three groups: control, precancerous lesions (PL) (which received 100 mg DEN once a week every 6 weeks up to 28 weeks), and advanced HCC (50 mg DEN once/twice per week up to 19 weeks). Lipid peroxidation (TBARS), superoxide dismutase (SOD) activity, and expression of transforming growth factor-1 beta (TGF)-1β, endothelial and inducible nitric oxide syntahese (eNOS, iNOS), NADPH quinone oxireductase (NQO)-1, nuclear factor erythroid 2-related factor (NrF)2, kelch-like ECH-associated protein (Keap)1 and heat shock protein (HSP)70 were measured. TBARS concentration was augmented in the PL and advanced HCC groups. SOD activity, TGF-1β and Nrf2 expression were higher in animals with precancerous lesions. In advanced HCC, expression of NQO1 and iNOS increased while there was a decrease in HPS70 expression. Data obtained provide evidence for the differential activation of proteins involved in oxidative stress and cell damage during progression of carcinogenesis in an animal model of HCC.
Abbreviations: 2-AAF, 2-acetylaminofluorene; ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase; DEN, diethylnitrosamine; EDTA, ethylenediamine tetraacetic acid; eNOS, endothelial nitric oxide synthase; GGT, gamma-glutamyl transferase; HCC, hepatocellular carcinoma; HSC, hepatic stellate cells; HSP70, heat shock 70-kDa protein; iNOS, inducible nitric oxide synthase; Keap1, kelch-like ECH-associated protein 1; MDA, malonaldehyde; NQO1, NADPH quinone oxireductase-1; NO, nitric oxide; Nrf2, nuclear factor erythroid 2-related factor 2; PVDF, polyvinylidene fluoride; SOD, superoxide dismutase; TBARS, thiobarbituric acid reactant substances; TGF-1β, transforming growth fator-1 beta; TTBS, Tris-buffered containing 0.05% Tween 20; UV, ultra violet
Keywords: Hepatocarcinoma, Diethylnitrosamine, Oxidative stress, Nuclear factor erythroid 2-related factor 2, Nitric oxide synthase, Heat shock protein
1. Introduction
The most common histological type of primary liver cancer is hepatocellular carcinoma (HCC). In 2008, there were approximately 694,000 deaths from HCC, making it the third most common cause of cancer death worldwide [1]. Chronic liver diseases are risk factors that predispose to HCC, as any agent or factor that chronically and slowly damages the hepatocytes induces mitosis and makes the DNA of these cells more susceptible to genetic alterations [2]. Such diseases include alcoholic cirrhosis, hepatitis B or C virus infection, α1-antitrypsin deficiency, hemochromatosis and tyrosinemia. In HCV-positive patients, for example, HCC appears on average 30 years after infection, almost exclusively in those with cirrhosis [3]. The development of HCC is a complex process, involving accumulation of genetic and epigenetic alterations, which passes through stages of initiation, promotion and progression, and numerous experimental observations have shown that viral products may contribute to the malignant transformation of hepatocytes [4].
Curative therapeutic approaches for HCC involve liver transplantation, or surgical and radiofrequency ablation, but these treatments are not yet effective [5]. Although surgical resection can sometimes be curative, few patients have resectable tumors because of the presence of cirrhosis or distant metastases; moreover, even after resection, preexisting liver cirrhosis persists and may cause other tumors in the remaining tissue. Orthotopic liver transplantation is the only truly curative therapy, although issues of recurrence and development of metastases remain. In case of unresectable tumor, treatment is limited, as HCC does not respond to chemotherapy and the liver does not tolerate high doses of radiotherapy [6].
HCC carries a high mortality rate and patients with chronic liver diseases usually take a long time before HCC occurs. Therefore, early diagnosis of HCC in precancerous lesions may improve the outcome of treatment, and it is necessary to encourage basic research to better understand the pathogenesis of this disease. Many experimental animal models of hepatocarcinogenesis have been described over the last decades. The most widely accepted, proposed by Farber et al. [7], combines chemical induction by diethylnitrosamine (DEN) with partial hepatectomy. Since then, DEN has been used to initiate the liver cancer either alone or in combination with other carcinogens [8], [9], [10], [11]. However, fewer studies have characterized in detail the temporal evolution of oxidative stress and cell damage implicated in hepatocarcinogenesis. Understanding changes from pre-neoplastic to carcinoma lesions in oxidative stress, inflammation and liver fibrosis could be important to improve the knowledge on the transition of chronic inflammatory liver diseases to HCC. In the current study, we used a multistage model of chronic and intermittent exposure to DEN without partial hepatectomy to get insight into changes in markers of cell damage during progression of the disease. Two different protocols of drug exposure (designed to induce advanced HCC and precancerous lesions) allowed us to study effects of time on tumor onset, liver pathology, blood chemistry, and markers of oxidative stress and cell damage in the liver.
2. Materials and methods
2.1. Animals and procedures
Male Wistar rats weighing 145–150 g were used for this study and were obtained from the Central Animal Laboratory of the Federal University of Pelotas, Rio Grande do Sul (Brazil). The rats were caged at 24 °C, under a 12-h light–dark cycle and with free access to food and water until the time of the experiments at the Animal Experimentation Division of Hospital de Clínicas de Porto Alegre (Brazil). All experiments were performed in accordance with the Guiding Principles for Research Involving Animals (NAS) under protocol number 120355.
The animals were divided into three groups: CO: control, precancerous lesions (PL) and advanced HCC. Animals in the PL group were given diethylnitrosamine (DEN, Sigma Aldrich, St. Louis, MO) at a dose of 100 mg/kg body weight i.p. once a week every 6 weeks up to 28 weeks. Animals in the advanced HCC group received DEN at a dose of 50 mg/kg body weight i.p. twice a week for the first three weeks and once a week from weeks 4 to 6 and 11 to 13 up to 19 weeks. A single dose of 2-acetylaminofluorene (2-AAF, 100 mg/kg, Sigma–Aldrich, St. Louis, MO) was administered in week 4 to both DEN groups.
Following a 12-h fast, the animals were anesthetized with ketamine hydrochloride (Ketalar®, 100 mg/kg – PubChem CID: 15851) and xylazine (50 mg/kg – PubChem CID: 5707) and subjected to blood collection for measurement of biochemical parameters.
Samples of livers for histology, biochemical and molecular analyzes were taken from the same lobe (right medial lobe). The collected sample was withdrawn from the area where the nodules were visible. The animals were killed at the end of the experiment by exsanguination under deep anesthesia, as described in the American Veterinary Medical Association (AVMA) Guidelines on Euthanasia [12].
2.2. Biochemical analysis
Serum levels of alanine aminotransferase (ALT) (U/L), aspartate aminotransferase (AST) (U/L) were determined by kinetic UV test. Gamma-glutamyl transferase (gamma-GT) (U/L), and alkaline phosphatase (AP) (U/L) were quantified by colorimetric kinetic test. They were measured using routine laboratory methods of the Hospital de Clínicas de Porto Alegre by enzymatic method (automated – Siemens Advia 1800 Chemistry system).
2.3. Histology
For histological examination, a specimen of liver was trimmed and fixed by immersion in 10% buffered formalin for 24 h. The blocks were dehydrated in a graded ethanol series and embedded in paraffin wax. Serial 3-μm sections were stained with hematoxylin and eosin and picrosirius red.
The percentage of fibrosis (%) in the liver tissue was determined by morphometric measurements. Ten images from each slide were captured from randomly selected high-power fields (200× magnification) containing the conjunctive tissue area positive. Morphometric assessment of the percentage of the ratios of conjunctive tissue relative to whole liver were performed using the Adobe Photoshop CS5 Extended 10.0 (Adobe Systems, San Jose, CA), according to the protocol described by Souza et al. [13].
2.4. Lipid peroxidation and cytosolic superoxide dismutase (SOD)
The livers were excised, weighed, and immediately frozen at −70 °C. Frozen tissue from each rat was homogenized in ice-cold phosphate buffer (KCl 140 mM, phosphate 20 mM, pH 7.4) and centrifuged at 3000 rpm for 10 min. Protein concentration in the liver homogenates was determined using a bovine albumin solution [14]. Lipid peroxidation was determined by measuring the concentration of TBARS (nmol/mg protein) [15]. Spectrophotometric absorbance was determined in the supernatant as 535 nm. Cytosolic SOD (EC 1.15.1.1) was assayed as described by Misra and Fridovich [16].
2.5. Western blot
Western blot analysis was performed on cytosolic extracts prepared by liver tissue homogenization in 140 mM NaCl, 15 mM EDTA (PubChem CID: 6049), 20 mM glycerol (10%), and a protease inhibitor cocktail [17]. The mixture was incubated on ice for 30 min and centrifuged for 30 min at 12,000 g and 4 °C. The supernatant fraction was collected and stored at −80 °C in aliquots until use. Protein concentration was measured by the Bradford assay [14]. Samples containing 50–100 μg of protein were separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis (9–12% acrylamide) and transferred to polyvinylidene fluoride (PVDF) membranes [18], [19]. The membranes were then blocked with 5% nonfat dry milk in Tris-buffered saline containing 0.05% Tween 20 (TTBS) for 1 h at room temperature and probed overnight at 4 °C with polyclonal anti-TGF-1β (SC31609/25 kDa), anti-eNOS (SC8311/140 kDa), anti-iNOS (SC7271/130 kDa), anti-NQO1 (SC376023/32 kDa), anti-Keap1 (SC 33569/69 kDa), and anti-Nrf2 (SC30915/57 kDa) antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:200–1:1000 dilution with TTBS in 5% nonfat dry milk, and anti-HSP70 (H5147/73 and 72 kDa) (Sigma–Aldrich, St. Louis, MO, USA) antibody at 1:5000 dilution with TTBS in 5% nonfat dry milk, and anti-GAPDH (G9545/37 kDa) antibody (Sigma–Aldrich, St. Louis, MO, USA) antibody at 1:1000 dilution with TTBS in 5% nonfat dry milk. After washing with TTBS, the membranes were incubated for 1 h at room temperature with secondary HRP-conjugated antibody (Dako, Glostrup, Denmark, 1:4000). Protein detection was performed via chemiluminescence using a commercial ECL kit (Amersham Pharmacia Biotech, Little Chalfont, Great Britain) [20]. The density of the specific bands was quantified with an L-Pix Chemi Molecular Imaging densitometer.
2.6. Statistical analysis
Means and standard deviations (SD) were calculated for all data. Significant differences between means were evaluated by one-way analysis of variance (ANOVA). In the case of significance, Tukey's test was applied. P values <0.05 were deemed significant. All analyses were carried out using SPSS 18.0.
3. Results
3.1. Body weight and blood biochemical analyses
Rats with advanced HCC showed a slower growth rate than the PL and control animals, reaching at the time of sacrifice a body weight approximately 30% lower than that of controls, with a significant increase in the hepatosomatic ratio (Table 1). Blood analyses indicated that AST, ALT, AP and GGT levels were significantly higher in the advanced HCC group compared to control rats. Enzyme levels for the PL group also differed from those in control rats, although values were lower than those in the HCC group (Table 1).
Table 1.
Effects of advanced HCC and precancerous lesions on body weight, hepatosomatic ratio, serum levels of AST, ALT, AP and GGT, hepatic TBARS and liver SOD activity.
| Control | Advanced HCC | Precancerous lesions | |
|---|---|---|---|
| Initial weight (g) | 146 ± 6 | 147 ± 7 | 154 ± 6 |
| Final weight (g) | 486 ± 8 | 323 ± 53a | 437 ± 26b |
| HSR (%) | 3.1 ± 0.2 | 12.4 ± 6.6a | 3.5 ± 0.4b |
| AST (U/L) | 102 ± 10 | 238 ± 70a | 160.5 ± 15a,b |
| ALT (U/L) | 49 ± 4 | 160 ± 27a | 71 ± 7a,b |
| AP (U/L) | 71 ± 12 | 249 ± 84a | 141 ± 57a,b |
| GGT (U/L) | 2.2 ± 0.9 | 113.8 ± 6.6a | 10.2 ± 1.7a,b |
| TBARS (nmol/mg prot) | 0.11 ± 0.02 | 0.16 ± 0.03a | 0.20 ± 0.02a |
| SOD (U SOD) | 12.7 ± 0.4 | 8.7 ± 1.8a | 15.9 ± 2.0a,b |
Values are expressed as means ± SD for 8–10 rats. HSR, hepatosomatic ratio; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; GGT, gamma-glutamyl transferase.
p < .05 vs. control.
p < .05 vs. advanced HCC.
3.2. Histology and morphometric study
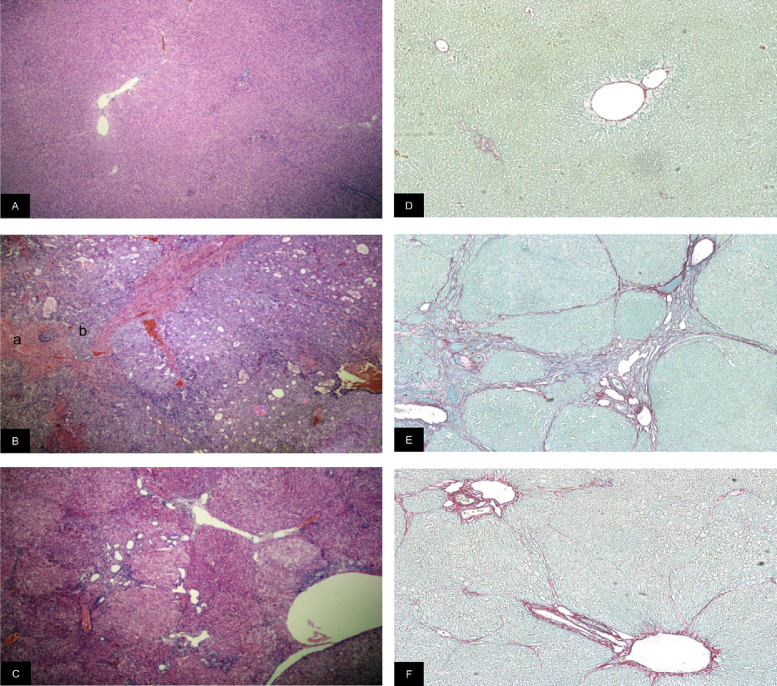
The liver histology of animals in the advanced HCC group was characterized by chronic damage and areas of cellular atypia such as large nucleoli, increased nucleus to cytoplasm ratio and increased mitotic index at 19 weeks. The signs observed included lymphocytic infiltration, cells with enlarged nuclei, extremely atypical hepatocytes. Loss of normal hepatic parenchyma was present, with a pseudo-acinar and trabecular growth pattern. Moderate and large nodules were present (20% and 80% of rats, respectively) [21]. Animals in the group with precancerous lesions had developed early-stage HCC or preneoplastic lesions at 28 weeks. The liver histology in this group was consistent with multiple nodules of regeneration (small nodules in 100% of animals) and preneoplastic foci (Fig. 1). Distorted lobular architecture was also observed, with increased mitotic index and hepatocellular damage such fibrosis and cirrhosis. The cytologic criteria included nuclear and cytoplasmic changes, multinucleation, centrally located nuclei, prominent nucleoli and increased cell density [22].
Fig. 1.
Photomicrographs of liver sections. Left panels: tissue samples counter stained with hematoxylin-eosin. (A) Control: normal hepatic parenchyma. (B) Advanced HCC: non-cancerous cells (a) and atypical hepatocytes (b). Abnormal hepatic parenchyma was present, with a pseudo-acinar and trabecular growth pattern. (C) Precancerous lesions: low-grade dysplastic nodules (DN) and collagen deposition (black arrows) (Original magnification 40×). Right panels: tissue samples counter stained with picrosirius red. (D) Control: normal hepatic parenchyma. (E) Advanced HCC: intense deposits of fibrosis. (F) Precancerous lesions: moderated deposits of fibrosis (Original magnification 100×).
The percentage of fibrosis in the liver tissue was determined by morphometric measurement of picrosirius red-stained samples. Data obtained indicate that the extent of fibrotic tissue increased slightly in rats with precancerous lesions and augmented markedly in animals with advanced HCC (control: 1.7 ± 0.1; precancerous lesions: 3.8 ± 1.5; advanced HCC: 12.3 ± 2.9; p < .05).
3.3. Markers of lipid peroxidation and SOD activity
Determination of lipid peroxidation in liver tissue was performed by the TBARS method, which showed a significant increase of malondialdehyde formation in both groups of DEN-treated rats. TBARS increased by 81% in the PL group when compared to control animals, while rats with advanced HCC had values approximately 25% lower than that of PL group. Liver activity of the antioxidant enzyme SOD was significantly increased in PL rats (+13%) and reduced in the advanced HCC group (−32%) when compared to control animals (Table 1).
3.4. TGF-1β, Keap-1, Nrf2, NQO1, eNOS, iNOS and HSP70 expression
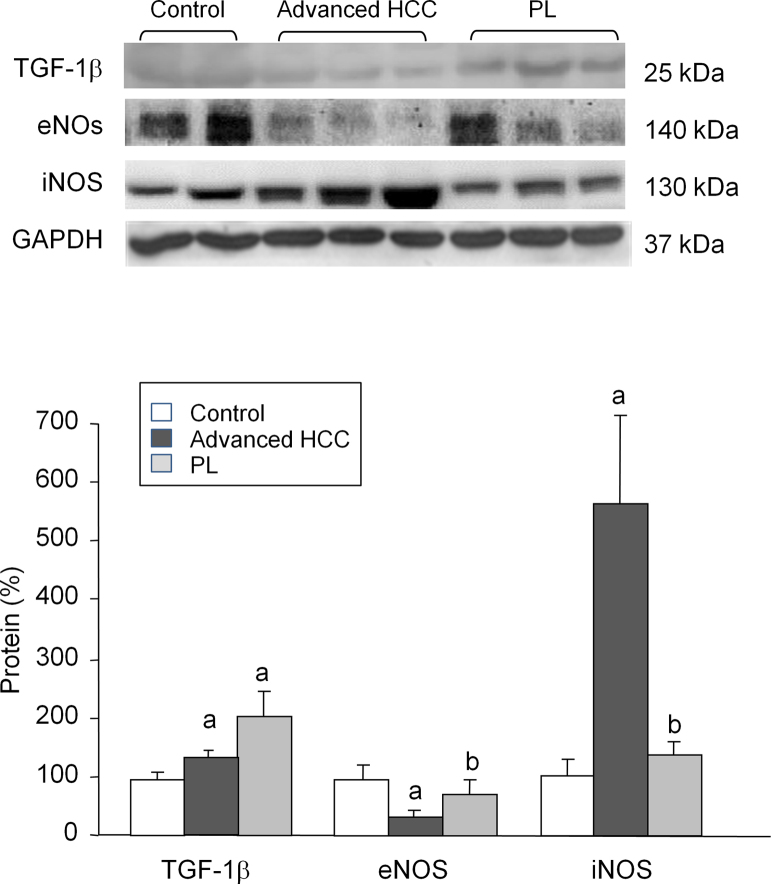
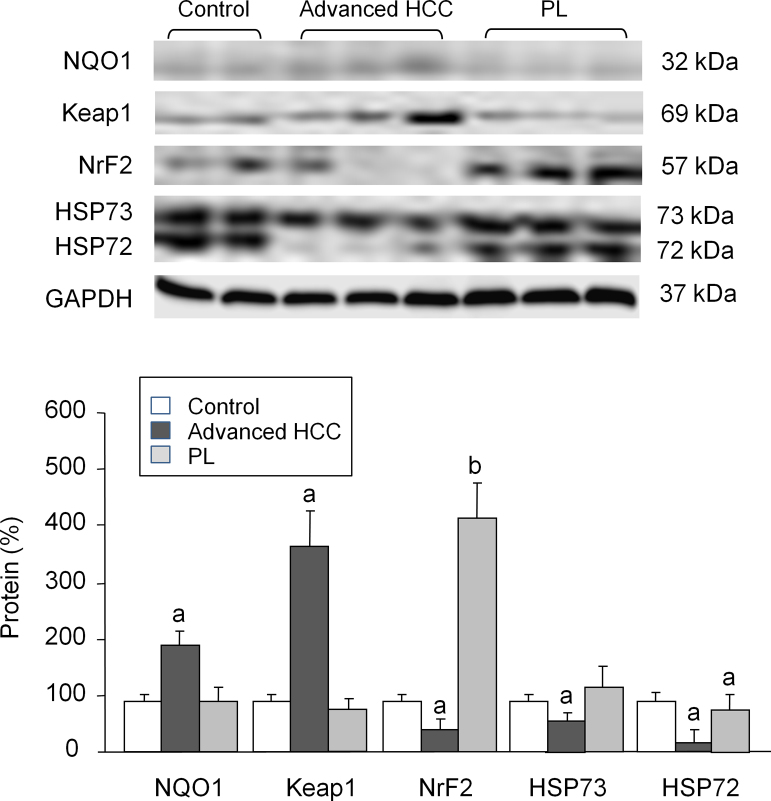
To evaluate the effects of early and advanced HCC on development of fibrosis, the expression of TGF-1β was quantified by measurement of protein expression. Both PL and advanced HCC animals exhibited a significant induction of TGF-1β, which reached a higher extent in the first group (+98%) (Fig. 2). Concerning markers of inflammation, eNOS expression was reduced (−60%), whereas iNOS expression increased strongly in animals with advanced HCC (Fig. 2). Protein markers related to oxidative stress were also evaluated. The advanced HCC group exhibited a significant induction of NQO1 protein as compared with the control group (+82%). Rats in the PL group overexpressed nuclear factor Nrf2 (+260%), while in the advanced HCC group Nrf-2 expression was reduced (−56%) and Keap-1 was markedly overexpressed (+308%). Expression of the main isoforms of the HSP family (constitutive HSP 73 and stress-inducible HSP72) decreased significantly in animals with advanced HCC (−32% and −74%, respectively) (Fig. 3).
Fig. 2.
Western blot analysis of TGF-1β, eNOS, and iNOS. Protein from liver extracts was separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis followed by immunoblotting. (A) Representative images. (B) Densitometric quantification. Values are expressed as means ± SD (n = 5). ap < .05 vs. control, bp < .05 vs. advanced HCC.
Fig. 3.
Western blot analysis of NQO1, Keap1, Nrf2, HP73 and HP72. Protein from liver extracts was separated by sodium dodecylsulfate-polyacrylamide gel electrophoresis followed by immunoblotting. (A) Representative images. (B) Densitometric quantification. Values are expressed as means ± SD (n = 5). ap < .05 vs. control, bp < .05 vs. advanced HCC.
4. Discussion
This study provides evidence of the activation/inhibition of different proteins involved in oxidative stress and cell damage in a multistage animal model of hepatic carcinogenesis. Blood chemistry, liver histology, markers of oxidative stress and expression of different proteins related to HCC pathogenic mechanisms were measured in rats with early/precancerous lesions (PL) or late-stage HCC reached through different protocols of DEN administration.
DEN is a potent hepatocarcinogenic agent [23], which is hydrolyzed to nitrosamine, generating an electrophilic radical. The rats that were exposed most frequently to low doses of DEN had developed advanced HCC at 19 weeks, whereas animals exposed to high doses on fewer occasions developed preneoplastic lesions or early HCC by 28 weeks. In addition, we used 2-AAF on week 4 to inhibit the proliferation of normal hepatocytes [8], [24]. With this design, HCC was already established by weeks 17–19 in the advanced HCC group, suggesting that the therapeutic window to address inflammation occurs on week 4 or 5, whereas cirrhosis is established by weeks 10–12.
Aminotransferase levels were significantly increased in the advanced HCC group. By week 19, there was an elevation of both ALT and AST, indicative of liver injury and hepatocellular damage [25], reaching values similar to those previously reported in other models of progressive cirrhosis induced in rat by repeated injections of DEN [9], [26]. Furthermore, AP was elevated concomitantly with a significant increase in the GGT level, which indicates the presence of obstructive and cholestatic disease. After analysis of the dimensions of the masses found, we believe that tumor nodules caused compression of the hepatic ducts. Both ALP and GGT have been confirmed as useful factor for confirmation of stages in HCC [10], and it is known that elevated GGT associates with increased cancer risk [27], [28], seeming to be involved in the activation of pro-oncogenes or the inactivation of tumor-suppressor genes [29].
The effects of HCC stages on development of fibrosis were evaluated by quantifying TGF-1β expression and percentage of fibrosis (%). TGF-1β was significantly increased in all rats with precancerous lesions, while the intense deposits of fibrosis was more prominent in animals with advanced HCC. This result may suggest that the TGF-1β is first activated in the early stage of HCC. Due to this activation, stellate cells (HSC) respond with intense deposits of fibrosis observed in the late stage of HCC. A strong association exists between fibrosis and HCC, because TGF-1β is an important peptide mediator of hepatic stellate cells (HSC), which activate and stimulate matrix synthesis, leading to progressive liver failure [30]. A wealth of evidence suggests the existence of reciprocal signaling and positive feedback loop between precancerous hepatocytes and stellate cells. This cycle enhances the growth of hepatocytes and HSC activation, which promotes carcinogenesis by altering the stromal environment and promoting angiogenesis. Furthermore, the accumulation of extracellular matrix would lead to increased proliferation and decreased apoptosis, favoring carcinogenesis [31]. TGF-1β signaling in carcinogenesis is complex; in early-stage HCC, it acts as a tumor suppressor, but in the late phase it plays a role as a tumor promoter [61].
We also studied the behavior of the inflammatory mediator nitric oxide (NO), evaluating eNOS and iNOS expression in cytoplasmic extract of livers with advanced HCC and precancerous lesions. iNOS expression was increased in advanced HCC, whereas eNOS expression decreased significantly. Hanahan and Weinberg [32], [33] have proposed six biological hallmarks necessary for tumor development, Overexpression of iNOS acts on three of these six markers. This occurs when overexpressed iNOS interacts on two important molecular pathways, IKK/NF-kappaB and RAS/ERK. Activation of these pathways triggers the transcription of genes that control cell growth, angiogenesis, and inhibition of cell death [34], [35]. Regarding the role eNOS in carcinoma, Decker et al. [36] demonstrated that eNOS overexpression was associated with fewer and smaller tumor lesions as well as increased animal survival. However, eNOS−/− knockout animals developed larger tumors and had worse survival. This vascular dysfunction in chronic liver disease is an important sign that precedes carcinoma [36].
After determination of proteins classically involved in chronic liver diseases, we assessed oxidative stress, by measuring the cytosolic concentration of TBARS and quantifying SOD activity. TBARS was already increased in the PL groups compared to controls. DEN is hydrolyzed in nitrosamine, generating the ethyl radical, responsible for an increase in intensification of oxidative stress. Many studies have linked oxidative stress to pathogenesis and disease prognosis [37], [38], [39]. One of the key factors in carcinogenesis is an imbalance of the redox state, favoring the formation of several toxic products such as malondialdehyde and 4-hydroxynonenal, which can attack lipids, proteins and DNA, leading to carcinogenicity and mutagenicity [40], [41]. In this study, SOD activity was reduced in advanced HCC, whereas increased in early HCC, signaling the presence of the superoxide anion. Similar results, showing increases in oxidative stress and reduction in SOD levels in animals with HCC have been previously reported, indicating that the decrease of SOD activity intensifies with the disease progression [8], [42].
In addition to SOD activity, NQO1 expression was also determined. While SOD activity was significantly reduced in animals with advanced HCC, NQO1 protein expression increased significantly. Most solid tumors express high levels of NQO1 [43], and biochemical studies have shown that NQO1 is induced by numerous chemicals, including polycyclic aromatic hydrocarbons and azo dyes. Two regulatory elements responsible for the NQO1 gene are the antioxidant response element (ARE) and the xenobiotic response element (XRE) [44]. According to Venugopal and Jaiswal [45] an increase in NQO1 expression occurs in response to the generation of ROS caused by inflammation or xenobiotic exposure. Conversely, precancerous lesions showed augmented SOD activity with no increase in NOQ1 protein expression. These findings suggest that NQO1 acts directly as a superoxide anion scavenger, although less efficiently than SOD [46]. Its lack results in additional loss of protection against oxidative stress, exposing the cell to the aggressions of ROS. Tissues with high SOD levels and low NQO1 expression may have decreased clearance of superoxide anion, generating other reactive species and worsening liver injury [47].
In this study, Keap1/Nrf2 were assessed in animals with PL and advanced HCC. There is doubt as to whether Nrf2 is a tumor suppressor or oncogenic [48]. Under basal conditions, Nrf2 is sequestered in the cytoplasm by Keap1, but induction of oxidative stress is able to dissociate Nrf2 from Keap1, leading to its translocation to the nucleus and subsequent increase on antioxidant genes expression [49]. We observed that animals in late-stage (advanced) HCC showed Keap1 overexpression and Nrf2 downregulation compared to animals in the PL group. It is known that the Nrf2 system could be induced by chemical carcinogens [50]. Activation of this factor facilitates cytoprotection and contributes to the proliferation and survival of tumor cells, whereas its inhibition results in degradation [51], [52], allowing an increase in ROS attacks to the cell. The role of Nrf2 is dependent on the stage of carcinogenesis. In the inflammatory phase, with precancerous lesions, increased activation of Nrf2 aims to reduce oxidative stress, thus contributing to tumor suppression [53]. Meanwhile, maintaining Nrf2 activation during the tumorigenesis stage may facilitate the transformation of dysplastic nodules into malignant cancer cells and make them resistant to treatment [53], [54]. During the development of carcinoma, an increase in Nrf2 protein is associated with poor prognosis [48]. In our work, Nrf2 and Keap1 changes observed in both PL and HCC groups were in parallel with the changes on SOD activity, contributing to liver injury during hepatocarcinogenesis.
Another interesting finding from our investigation was the significant reduction in the expression of HSP70 in liver tissue with advanced HCC. HSP70 has strong cytoprotective effects and functions as a molecular chaperone in protein folding, transport, and degradation [55]. HSP70 downregulation is associated with carcinogenesis of the oral epithelium, and is a marker of HCC [56]. HSP70 downregulation also correlates with poor prognosis in breast cancer [57], endometrial cancer [58], and pancreatic cancer [56]. Rohde et al. [59] reported that HSP70 is not a condition for the growth of tumor cells, but plays an important role in maintaining the deregulated tumor cell cycle. Chuma et al. [60] evaluated the expression of HSP70 in liver tissue with and without cancer, and identified HSP70 as a molecular marker of HCC progression.
In conclusion, we have shown a multistage induction of HCC in rats through chronic and intermittent exposure to carcinogenic agents. Changes in SOD and NrF2 and TGF-1β stand out as markers of oxidative stress and cell damage in early HCC. TGF-1β stimulates extracellular matrix formation and contributes to cell proliferation, SOD and Nrf2 overexpression act as cytoprotectives factors in precancerous lesions. In advanced HCC, however, there is a decreased expression of HSP70, and an increase in the expression of NQO1 and iNOS, that interact with important genes controlling cell growth, angiogenesis and apoptosis. These results confirm that oxidative stress and fibrosis plays an important role in liver carcinogenesis, suggesting that a multi-step process involving different molecular mechanisms could be implicated in the progression of chronic inflammatory liver diseases to HCC. Factors involved in oxidative stress and fibrosis can constitute not only potential biomarkers but also therapeutical targets for treatment of HCC.
Conflict of interest
The authors of this article declare that they have no conflicts of interest.
Transparency document
Acknowledgements
This study was supported by grants from the Brazilian agencies Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundo de Incentivo à Pesquisa e Eventos (FIPE)/Hospital de Clínicas de Porto Alegre (HCPA), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and Laboratório Experimental de Hepatologia e Gastroenterologia (HCPA/UFRGS).
References
- 1.Ferlay J., Shin H.R., Bray F., Forman D., Mathers C., Parkin D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Cervello M., Montalto G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006;12:5113–5121. doi: 10.3748/wjg.v12.i32.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bruix J., Boix L., Sala M., Llovet J.M. Focus on hepatocellular carcinoma. Cancer Cell. 2004;5:215–219. doi: 10.1016/s1535-6108(04)00058-3. [DOI] [PubMed] [Google Scholar]
- 4.Halegoua-De Marzio D., Hann H.W. Then and now: the progress in hepatitis B treatment over the past 20 years. World J. Gastroenterol. 2014;20(2):401–413. doi: 10.3748/wjg.v20.i2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forner A., Llovet J.M., Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 7.Farber E., Solt D., Cameron R., Laishes B., Ogawa K., Medline A. Newer insights into the pathogenesis of liver cancer. Am. J. Pathol. 1977;89:477–482. [PMC free article] [PubMed] [Google Scholar]
- 8.Malik S., Bhatnagar S., Chaudhary N., Katare D.P., Jain S.K. DEN+2-AAF-induced multistep hepatotumorigenesis in Wistar rats: supportive evidence and insights. Protoplasma. 2013;250:175–183. doi: 10.1007/s00709-012-0392-8. [DOI] [PubMed] [Google Scholar]
- 9.Nagahara T., Okano J., Fujise Y., Abe R., Murawaki Y. Preventive effect of JTE-522, a selective cyclooxygenase-2 inhibitor, on DEN-induced hepatocarcinogenesis in rats. Biomed. Pharmacother. 2010;64:319–326. doi: 10.1016/j.biopha.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 10.Park D.H., Shin J.W., Park S.K., Seo J.N., Li L., Jang J.J., Lee M.J. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. Toxicol. Lett. 2009;191:321–326. doi: 10.1016/j.toxlet.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 11.Yoshino H., Futakuchi M., Cho Y.M., Ogawa K., Takeshita F., Imai N., Tamano S., Shirai T. Modification of an in vivo lung metastasis model of hepatocellular carcinoma by low dose N-nitrosomorpholine and diethylnitrosamine. Clin. Exp. Metastasis. 2005;22:441–447. doi: 10.1007/s10585-005-2807-9. [DOI] [PubMed] [Google Scholar]
- 12.AVMA . 2013. AVMA Guidelines for the Euthanasia of animals: 2013 edition; pp. 1–102. [Google Scholar]
- 13.Souza A., Meurer L., Silveira T.R., Gregorio C., Reus N., Uribe C., Matte U., Santos J.L. Angiopoietin 1 and angiopoietin 2 are associated with medial thickening of hepatic arterial branches in biliary atresia. Pediatr. Res. 2014;75(1):22–28. doi: 10.1038/pr.2013.177. [DOI] [PubMed] [Google Scholar]
- 14.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 15.Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 16.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 17.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 18.Mauriz J.L., Molpeceres V., García-Mediavilla M.V., González P., Barrio J.P., González-Gallego J. Melatonin prevents oxidative stress and changes in antioxidant enzyme expression and activity in the liver of aging rats. J. Pineal Res. 2007;42:222–230. doi: 10.1111/j.1600-079X.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 19.San-Miguel B., Alvarez M., Culebras J.M., González-Gallego J., Tuñón M.J. N-acetyl-cysteine protects liver from apoptotic death in an animal model of fulminant hepatic failure. Apoptosis. 2006;11:1945–1957. doi: 10.1007/s10495-006-0090-0. [DOI] [PubMed] [Google Scholar]
- 20.Tuñón M.J., San-Miguel B., Crespo I., Laliena A., Vallejo D., Alvarez M., Prieto J., González-Gallego J. Melatonin treatment reduces endoplasmic reticulum stress and modulates the unfolded protein response in rabbits with lethal fulminant hepatitis of viral origin. J. Pineal Res. 2013;55:221–228. doi: 10.1111/jpi.12063. [DOI] [PubMed] [Google Scholar]
- 21.Thoolen B., Kate F.J.W., Diest P.J., Malarkey D.E., Elmore S.A., Maronpot R.R. Comparative histomorphological review of rat and human hepatocellular proliferative lesions. J. Toxicol. Pathol. 2012;25:189–199. doi: 10.1293/tox.25.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thoolen B., Maronpot R.R., Harada T., Nyska A., Rousseaux C., Nolte T., Malarkey D., Kaufmann W., Küttler K., Deschl U., Nakae D., Gregson R., Vinlove M., Brix A., Singh B., Belpoggi F., Ward J.M. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol. Pathol. 2010;38:5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- 23.Williams G.M., Iatropoulos M.J., Wang C.X., Ali N., Rivenson A., Peterson L.A., Schulz C., Gebhardt R. Diethylnitrosamine exposure-responses for DNA damage, centrilobular cytotoxicity, cell proliferation and carcinogenesis in rat liver exhibit some non-linearities. Carcinogenesis. 1996;17:2253–2258. doi: 10.1093/carcin/17.10.2253. [DOI] [PubMed] [Google Scholar]
- 24.Bagnyukova T.V., Tryndyak V.P., Montgomery B., Churchwell M.I., Karpf A.R., James S.R., Muskhelishvili L., Beland F.A., Pogribny I.P. Genetic and epigenetic changes in rat preneoplastic liver tissue induced by 2-acetylaminofluorene. Carcinogenesis. 2008;29:638–646. doi: 10.1093/carcin/bgm303. [DOI] [PubMed] [Google Scholar]
- 25.Mauriz J.L., Matilla B., Culebras J.M., González P., González-Gallego J. Dietary glycine inhibits activation of nuclear factor kappa B and prevents liver injury in hemorrhagic shock in the rat. Free Radic. Biol. Med. 2001;31:1236–1244. doi: 10.1016/s0891-5849(01)00716-x. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs B.C., Hoshida Y., Fujii S., Wei L., Yamada S., Lauwers G.Y. Epidermal growth factor receptor inhibition attenuates liver fibrosis and development of hepatocellular carcinoma. Hepatology. 2014;59:1577–1590. doi: 10.1002/hep.26898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guiu B., Deschamps F., Boulin M., Boige V., Malka D., Ducreux M., Hillon P., de Baère T. Serum gamma-glutamyl-transferase independently predicts outcome after transarterial chemoembolization of hepatocellular carcinoma: external validation. Cardiovasc. Intervent. Radiol. 2012;35:1102–1108. doi: 10.1007/s00270-011-0293-9. [DOI] [PubMed] [Google Scholar]
- 28.Strasak A.M., Rapp K., Brant L.J., Hilbe W., Gregory M., Oberaigner W., Ruttmann E., Concin H., Diem G., Pfeiffer K.P., Ulmer H., Group V.a.P.S. Association of gamma-glutamyltransferase and risk of cancer incidence in men: a prospective study. Cancer Res. 2008;68(10):3970–3977. doi: 10.1158/0008-5472.CAN-07-6686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanigan M.H., Gallagher B.C., Townsend D.M., Gabarra V. Gamma-glutamyl transpeptidase accelerates tumor growth and increases the resistance of tumors to cisplatin in vivo. Carcinogenesis. 1999;20:553–559. doi: 10.1093/carcin/20.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gressner O.A., Weiskirchen R., Gressner A.M. Biomarkers of liver fibrosis: clinical translation of molecular pathogenesis or based on liver-dependent malfunction tests. Clin. Chim. Acta. 2007;381:107–113. doi: 10.1016/j.cca.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 31.Zhang D.Y., Friedman S.L. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hanahan D., Weinberg R.A. The hallmarks of cancer review. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 33.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 34.Frau M., Biasi F., Feo F., Pascale R.M. Prognostic markers and putative therapeutic targets for hepatocellular carcinoma. Mol. Aspects Med. 2010;31:179–193. doi: 10.1016/j.mam.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 35.Muntané J., la Mata M.D. Nitric oxide and cancer. World J. Hepatol. 2010;2:337–344. doi: 10.4254/wjh.v2.i9.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Decker N.K., Abdelmoneim S.S., Yaqoob U., Hendrickson H., Hormes J., Bentley M., Pitot H., Urrutia R., Gores G.J., Shah V.H. Nitric oxide regulates tumor cell cross-talk with stromal cells in the tumor microenvironment of the liver. Am. J. Pathol. 2008;173:1002–1012. doi: 10.2353/ajpath.2008.080158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vásquez-Garzón V.R., Macias-Pérez J.R., Jiménez-García M.N., Villegas V., Fattel-Fazenta S., Villa-Treviño S. The chemopreventive capacity of quercetin to induce programmed cell death in hepatocarcinogenesis. Toxicol. Pathol. 2013;41:857–865. doi: 10.1177/0192623312467522. [DOI] [PubMed] [Google Scholar]
- 38.Kretzmann N., Filmann H., Mauriz J.L., Marroni C.A., Marroni N., González-Gallego J., Tuñón M.J. Effects of glutamine on proinflamamtory gene expression and activation of nuclear factor kappa B and signal transducers and activators of transcription in TNBS-induced colitis. Inflamm. Bowel Dis. 2008;14:1504–1513. doi: 10.1002/ibd.20543. [DOI] [PubMed] [Google Scholar]
- 39.Mauriz J.L., González P., Jorquera F., Olcoz J.L., González-Gallego J. Caspase inhibition does not protect against liver damage in hemorrhagic shock. Shock. 2003;19:33–37. doi: 10.1097/00024382-200301000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 41.De Zwart L.L., Meerman J.H., Commandeur J.N., Vermeulen N.P. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic. Biol. Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]
- 42.Mauriz J.L., Gonzalez P., Duran M.C., Molpeceres V., Culebras J.M., Gonzalez-Gallego J. Cell-cycle inhibition by TNP-470 in an in vivo model of hepatocarcinoma is mediated by a p53 and p21WAF1/CIP1 mechanism. Transl. Res. 2007;149:46–53. doi: 10.1016/j.trsl.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Ross D., Beall H.D., Siegel D., Traver R.D., Gustafson D.L. Enzymology of bioreductive drug activation. Br. J. Cancer Suppl. 1996;27:S1–S8. [PMC free article] [PubMed] [Google Scholar]
- 44.Ross D., Kepa J.K., Winski S.L., Beall H.D., Anwar A., Siegel D. NAD(P)H: quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem. Biol. Int. 2000;129:77–97. doi: 10.1016/s0009-2797(00)00199-x. [DOI] [PubMed] [Google Scholar]
- 45.Venugopal R., Jaiswal A.K. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc. Natl. Acad. Sci. U. S. A. 1996;93:14960–14965. doi: 10.1073/pnas.93.25.14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siegel D., Gustafson D.L., Dehn D.L., Han J.Y., Boonchoong P., Berliner L.J., Ross D. NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol. Pharmacol. 2004;65:1238–1247. doi: 10.1124/mol.65.5.1238. [DOI] [PubMed] [Google Scholar]
- 47.Dinkova-Kostova A.T., Talalay P. NAD(P)H:quinone acceptor oxidoreductase 1 (NQO1), a multifunctional antioxidant enzyme and exceptionally versatile cytoprotector. Arch. Biochem. Biophys. 2010;501:116–123. doi: 10.1016/j.abb.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sporn M.B., Liby K.T. NRF2 and cancer: the good, the bad and the importance of context. Nat. Rev. Cancer. 2012;12:564–571. doi: 10.1038/nrc3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jeong W.S., Jun M., Kong A.N. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Signal. 2006;8:99–106. doi: 10.1089/ars.2006.8.99. [DOI] [PubMed] [Google Scholar]
- 50.Jung K.A., Kwak M.K. The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules. 2010;15:7266–7291. doi: 10.3390/molecules15107266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kansanen E., Kuosmanen S.M., Leinonen H., Levonen A.L. The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 2013;1:45–49. doi: 10.1016/j.redox.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Linehan W.M., Rouault T.A. Molecular pathways: fumarate hydratase-deficient kidney cancer--targeting the Warburg effect in cancer. Clin. Cancer Res. 2013;19:3345–3352. doi: 10.1158/1078-0432.CCR-13-0304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Satoh H., Moriguchi T., Takai J., Ebina M., Yamamoto M. Nrf2 prevents initiation but accelerates progression through the Kras signaling pathway during lung carcinogenesis. Cancer Res. 2013;73:4158–4168. doi: 10.1158/0008-5472.CAN-12-4499. [DOI] [PubMed] [Google Scholar]
- 54.Jaramillo M.C., Zhang D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013;27:2179–2191. doi: 10.1101/gad.225680.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gupta S., Deepti A., Deegan S., Lisbona F., Hetz C., Samali A. HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol. 2010;8:e1000410. doi: 10.1371/journal.pbio.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boonjaraspinyo S., Boonmars T., Kaewkes S., Laummaunwai P., Pinlaor S., Loilome W., Yongvanit P., Wu Z., Puapairoj A., Bhudhisawasdi V. Down-regulated expression of HSP70 in correlation with clinicopathology of cholangiocarcinoma. Pathol. Oncol. Res. 2012;18:227–237. doi: 10.1007/s12253-011-9432-5. [DOI] [PubMed] [Google Scholar]
- 57.Lazaris A.C.h., Chatzigianni E.B., Panoussopoulos D., Tzimas G.N., Davaris P.S., Golematis B.C.H. Proliferating cell nuclear antigen and heat shock protein 70 immunolocalization in invasive ductal breast cancer not otherwise specified. Breast Cancer Res. Treat. 1997;43:43–51. doi: 10.1023/a:1005706110275. [DOI] [PubMed] [Google Scholar]
- 58.Nanbu K., Konishi I., Komatsu T., Mandai M., Yamamoto S., Kuroda H., Koshiyama M., Mori T. Expression of heat shock proteins HSP70 and HSP90 in endometrial carcinomas. Correlation with clinicopathology, sex steroid receptor status, and p53 protein expression. Cancer. 1996;77:330–338. doi: 10.1002/(SICI)1097-0142(19960115)77:2<330::AID-CNCR16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 59.Rohde M., Daugaard M., Jensen M.H., Helin K., Nylandsted J., Jäättelä M. Members of the heat-shock protein 70 family promote cancer cell growth by distinct mechanisms. Genes Dev. 2005;19:570–582. doi: 10.1101/gad.305405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chuma M., Sakamoto M., Yamazaki K., Ohta T., Ohki M., Asaka M., Hirohashi S. Expression profiling in multistage hepatocarcinogenesis: identification of HSP70 as a molecular marker of early hepatocellular carcinoma. Hepatology. 2003;37:198–207. doi: 10.1053/jhep.2003.50022. [DOI] [PubMed] [Google Scholar]
- 61.Sakamoto M., Effendi K., Yohei M. Molecular diagnosis of multistage hepatocarcinogesis. Jpn J Clin Oncol. 2010;40(9):891–896. doi: 10.1093/jjco/hyq099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.