Highlights
-
•
Lead acetate reduced the sperm cell concentration, increased sperm abnormalities and decreased the superoxide dismutase (SOD) and catalase activities in male rats.
-
•
Addition of cinnamon to lead acetate enhanced the viability of the spermatozoa, preserved the sperm cell concentration and improved the level of SOD and catalase activities.
-
•
The expression of androgen receptor was reduced in testis of lead treated rats associated with increased the level of caspase-3 expression.
-
•
Cinnamon exhibited protective effect on reproductive system by inhibiting lead acetate induced oxidative stress and excessive cell apoptosis.
Keywords: Rats, Lead acetate, Cinnamon, Reproductive toxicity, Caspase-3, Testis
Abstract
The aim of this study was to investigate the protective effects of cinnamon on lead acetate induced reproductive toxicities in rats. Thirty-two male rats were randomly divided into 4 groups, 8 rats in each. Control rats received distilled water, while treated rats received lead acetate (30 mg/kg), cinnamon (250 mg/kg) and lead acetate and cinnamon (30 mg/kg and 250 mg/kg) for 60 days by gavage tube. In cinnamon treated rats, the relative weights of testes, epididymis, seminal and prostate glands were significantly (P < 0.05) increased compared with that in lead acetate treated rats. Sperm cell concentration and viability were significantly (P < 0.05) reduced, while sperm abnormalities were significantly (P < 0.05) increased in lead treated rats. The superoxide dismutase (SOD) and catalase activities were significantly reduced (P < 0.001) in lead acetate treated rats compared to the other groups, while the addition of cinnamon to lead acetate improved the level of SOD compared to the lead treated group. There was a marked reduction (P < 0.001) in the expression of androgen receptor and significant (P < 0.001) increase in the level of caspase-3 protein expression in the testis of lead treated rats. In conclusion, cinnamon exhibited protective effect on reproductive system by inhibiting lead acetate induced oxidative stress and excessive cell apoptosis.
1. Introduction
The diverse deleterious health effects upon exposure to heavy metals in the environment are a matter of serious concern and a global issue. Lead is the most abundant toxic metal in the environment [1]. Lead occurs naturally in the environment. However, most of the high levels found throughout the environment come from human activities. Environmental levels of lead have increased more than 1000-fold over the past three centuries as a result of human activity. The greatest increase occurred between the years 1950 and 2000, and reflected increasing worldwide use of leaded gasoline [2].
Lead does not have any detectable beneficial biological role, however on the contrary its detrimental effect on physiological, biochemical and behavioral dysfunctions have been documented in animals and humans by several investigators [3], [4]. Lead is a male reproductive toxicant [5]. Toxicity is manifested in male reproductive function by deposition of lead in testes, epididymis, vas deferens, seminal vesicle and seminal ejaculate. Lead has an adverse effect on sperm count, sperm motility and retarded the activity of spermatozoa [6]. The effect of lead on testis is still a matter of controversy where exposure to low dose of lead was found to arrest spermatogenesis [7] or to have no effect [8].
The mechanism of lead-induced oxidative stress involves an imbalance between generation and removal of ROS (reactive oxygen species) in tissues and cellular components causing damage to membranes, DNA and proteins [1]. Lead is reported to cause oxidative stress by generating the release of reactive oxygen species (ROS) such as superoxide radicals, hydrogen peroxide and hydroxyl radicals and lipid peroxides [9]. Lead acetate enhances lipid peroxidation and nitric oxide production in both serum and testes with concomitant reduction in antioxidant enzymes as catalase and superoxide dismutase [10].
The androgen receptor (AR) plays a key role in androgen action. In the male reproductive system, the testis and epididymis are major targets of androgen action, and androgen is critical for maintenance of spermatogenesis and secretory function in epididymal epithelial cells [11]. Caspases are a family of genes important for maintaining homeostasis through regulating cell death and inflammation [12].
There has been increased interest among phytotherapy researchers to use medicinal plants with antioxidant activity for protection against heavy metal toxicity [9]. Cinnamon (Cinnamomum zeylanicum), a medicinal plant belongs to Luaraceae family. This plant has many therapeutic effects. One of its most important effects is its impact on the increase of sexual ability [26]. Limited data are available on the protective effect of this substance against the toxicity of heavy metals on male reproduction. Administration of cinnamon extract before exposure to lead could reduce many of its side effects. Therefore, the present study was carried out to investigate the protective role of cinnamon extract against the effect of lead acetate on testicular functions, superoxide dismutase, expression of androgen receptor and casapase-3 in adult male albino rats.
2. Materials and methods
2.1. Preparation of materials
Lead acetate trihydrate was obtained from Oxford Lab. Co., India (CAS: 6080-56-4). Lead acetate was dissolved in distilled water at concentration of 30 mg/kg body weight of 1% solution and administrated to rats by gavage tube. For preparation of cinnamon extract, values of 10 g cinnamon was weighed and added to 100 ml of boiling distilled water. Then the solution was cleared with filter paper and was ready for administration by gavage tube. The dose of cinnamon was 250 mg/kg body weight.
2.2. Animals and housing
A total number of 32 adult male albino rats were used in the present study and their weight ranged between 130 and 150 g. Animals were raised at Faculty of Veterinary Medicine, Suez Canal University, Egypt. They were maintained in stainless steel cages with wood shavings. Food and water were supplied ad libitum. Rats were housed at a controlled temperature of 26 ± 1 °C, 60% humidity and under a 12 h light: 12 h dark schedule. The animals were divided into 4 groups. The first one (n = 8) were used as control and received only distilled water. The second one (n = 8) were administrated lead acetate at concentration of 30 mg/kg body weight of 1% solution by gavage tube. The third one (n = 8) were administrated cinnamon extract (250 mg/kg body weight) by gavage tube. The fourth one (n = 8) were administrated lead acetate at concentration of 30 mg/kg body weight of 1% solution and cinnamon extract (250 mg/kg body weight) by gavage tube for 60 days.
2.3. Organ relative weights
At the end of the study period, rats were euthanized and organs were dissected. Testes, tail of the epididymis, seminal and prostate glands are removed and weighed. The organ relative weights (organ weight/body weight × 100) were measured for each rat in treated and control groups.
2.4. Sperm concentration and morphology assay
The content of epididymis was obtained by cutting of the cuda epididymis using surgical blades then squeezed in a sterile clean watch glass. This content was diluted 5 times with 2.9% sodium citrate dihydrate solution and thoroughly mixed to estimate the sperm concentration [13]. One drop of the suspension was smeared on a glass slide and stained by Eosin Nigrosin stain to determine the viability and sperm abnormalities using the criteria of Okamura et al. [14].
2.5. Testicular superoxide dismutase (SOD) and catalase assay
Specimens from testis were collected from all experimental and control groups. The tissues were homogenized in 50 mM potassium phosphate (pH 7.4). The samples were centrifuged at 4000 rpm for 15 min, at 4 °C and the supernatants were stored at −80 °C until analysis. SOD (Biodiagnostic, Egypt) was done according to Nishikimi et al. [15] at absorbance 560 nm over 5 min. The method based on the ability of the enzyme to inhibit the phenazine methosulphate–mediated reduction of nitro blue tetrazolium dye. Catalase (Biodiagnostic, Egypt) was carried out according to Aebi [16] at absorbance of 510. The method based on the reaction of catalase with a known quantity of H2O2. The reaction was stopped after one min., with catalase inhibitor.
2.6. Histopathology
Specimens from testis were collected from all experimental and control groups and fixed in 10% neutral buffered formalin, dehydrated in ascending concentrations of ethyl alcohol (70–100%) and then prepared using standard procedures for Hematoxylin and Eosin stain as described by Bancroft et al. [17].
2.7. Immunohistochemistry of androgen receptor and caspase-3
The paraffin embedded testis were cut into 5 μm sections and mounted on positively charged slides for both androgen receptors and caspase-3 immunohistochemistry. Sections were dewaxed, rehydrated and autoclaved at 120 °C for 10 min in 10 Mm citrate buffer (pH 6). After washing with PBS, endogenous peroxidase was blocked using 0.3% H2O2 in methanol for 15 min. Slides were washed in PBS again and blocking was performed by adding blocking buffer and incubated for 30 min at room temperature. Primary monoclonal and polyclonal antibodies for androgen receptors (Cat. No. MA1-150, Thermo Fisher Scientific Co., USA) and caspase-3 (Cat. No. PAI-29157, Thermo Fisher Scientific Co., USA), respectively were added after dilution by PBS (2 μg/ml and 1:1000, respectively) and incubated for 30 min. The slides were washed three times for 3 min each with PBS. Biotinylated polyvalent secondary antibody (Cat. No. 32230, Thermo Scientific Co., UK) was applied to tissue sections and co-incubated for 30 min. The slides were washed three times for 3 min each with wash buffer. The reaction was visualized by adding Metal Enhanced DAB Substrate Working Solution to the tissue and incubated 10 min. The slides were washed two times for 3 min each with wash buffer. Counterstaining was performed by adding adequate amount of hematoxylin stain to the slide to cover the entire tissue surface. For quantitative analysis, the intensity of immunoreactive parts was used as a criterion of cellular activity after subtracting background noise. Measurement was done using an image analyzer (Image J program). From each slide of both experimental groups, 9 fields were randomly selected. The total field and immunohistochemial (IHC) stained areas were calculated and the percentage of IHC stained area calculated as follow: %IHC stained area = IHC stained area/Total area × 100.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism (Version 5.01, GraphPad Software, San Diego, USA). Data are presented as means with their standard error. Normality and homogeneity of the data were confirmed before ANOVA, differences among the experimental groups were assessed by one-way ANOVA.
3. Results
3.1. Organ relative weights
In cinnamon treated rats, the relative weights of testes, tail of the epididymis, seminal and prostate glands were significantly (P < 0.05) increased compared with that in lead acetate treated rats. Moreover, the relative weights of testes of cinnamon treated rats was significantly (P < 0.05) increased compared with that in control rats. The relative weight of all organs was not significantly differing than that of control rats when the cinnamon was administrated with lead acetate in rats (Table 1).
Table 1.
Effect of oral administration of cinnamon extract for 60 days on the weight of sexual organs of lead-treated male albino rats.
| Groups and treatment | Relative weight (g) of sexual organs (Mean ± SE) |
|||
|---|---|---|---|---|
| Testes | Seminal vesicles | Prostate glands | Tail of epididymis | |
| Control | 2.5 ± 0.4a | 0.4 ± 0.02a | 0.2 ± 0.01a | 0.5 ± 0.03a |
| Lead acetate (30 mg/kg bw) | 2.1 ± 0.1a | 0.2 ± 0.01b | 0.1 ± 0.004b | 0.3 ± 0.01b |
| Cinnamon extract (250 mg/kg bw) | 2.9 ± 0.1b | 0.4 ± 0.02a | 0.2 ± 0.004a | 0.5 ± 0.02a |
| Lead and cinnamon extract | 2.4 ± 0.1a | 0.04 ± 0.02a | 0.2 ± 0.01a | 0.4 ± 0.02a |
a and b indicated significant change from normal control and lead-treated groups respectively, at P < 0.05.
3.2. Seminal picture
In rats treated with lead acetate, the sperm cell concentration and viability were significantly (P < 0.05) reduced compared with that in other groups. Sperm abnormalities were significantly (P < 0.05) increased in lead acetate treated rats. In cinnamon treated rats, the seminal picture was improved and the percentage of sperm abnormalities was remarkably reduced without reaching a significant level. Addition of cinnamon to lead acetate in rats enhanced the viability of the spermatozoa and kept the sperm cell concentration at normal levels (Table 2).
Table 2.
Effect of oral administration of cinnamon extract for 60 days on semen picture of lead treated male albino rats.
| Groups and treatment | Sperm cell characteristics (Mean ± SE) |
||
|---|---|---|---|
| Sperm cell conc. (×106) | Viability (%) | Abnormalities (%) | |
| Control | 23.6 ± 0.8a | 78.9 ± 2.2a | 15.8 ± 0.6a |
| Lead acetate (30 mg/kg bw) | 14.5 ± 0.4b | 49.6 ± 0.5b | 32.6 ± 1.4b |
| Cinnamon extract (250 mg/kg) | 22.8 ± 0.6a | 82.9 ± 1.5a | 13.1 ± 0.7a |
| Lead and cinnamon extract | 17.9 ± 0.7a | 59.1 ± 1.8a | 28.7 ± 0.6a |
a and b indicated significant change from normal control and lead treated groups respectively, at P < 0.05.
3.3. SOD and catalase activities
SOD and catalase activities were significantly reduced (P < 0.001) in lead acetate treated rats compared to the other groups, while the addition of cinnamon to lead acetate improved the level of SOD compared to the lead treated group (Table 3).
Table 3.
Effect of oral administration of lead acetate on superoxide dismutase (SOD), catalase, androgen receptor and caspase-3 protein expression for 60 days in male albino rats.
| Groups and treatment |
||||
|---|---|---|---|---|
| Control | Cinnamon | Lead | Lead and cinnamon | |
| SOD (μ/g) | 886.3 ± 32.4a | 1000.0 ± 33.5a | 456.9 ± 25.4b | 619.1 ± 37.6c |
| Catalase (μ/g) | 0.24 ± 0.01a | 0.26 ± 0.01a | 0.15 ± 0.01b | 0.19 ± 0.01c |
| Androgen receptor | 111.2 ± 1.6a | 114.5 ± 2.1a | 85.6 ± 1.2b | 97.8 ± 2.1c |
| Caspase-3 | 43.6 ± 1.2a | 41.1 ± 0.8a | 75.4 ± 3.1b | 60.9 ± 2.5c |
a and b indicated significant change, at P < 0.0001; a and c indicated significant change, at P < 0.0001; b and c indicated significant change, at P < 0.0001 except for SOD and catalase activities at P < 0.001 and P < 0.05, respectively.
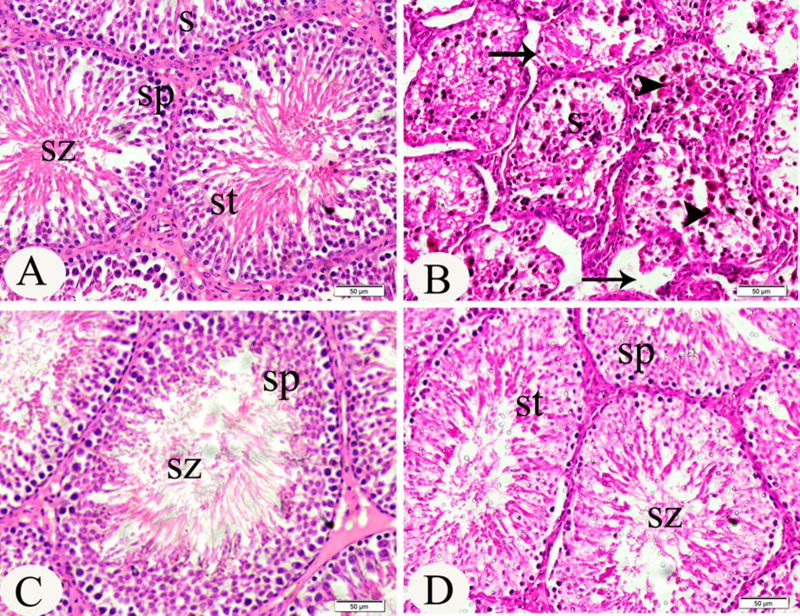
3.4. Histopatholoy of testes
Testis of control rats as well as testis of rats treated with cinnamon showed normal histological structure of active mature functioning seminiferous tubules associated with complete spermatogenic series (Fig. 1A and C). On the other hand, testis of lead treated rats showed marked degeneration of most seminiferous tubules with absence of spermatogenic series in tubular lumen and congestion in testis blood vessels (Fig. 1B). Interestingly, the testis of lead treated rat given cinnamon extract showed normal histological structure of most seminiferous tubules (Fig. 1D).
Fig. 1.
(A) Testis of control rats showing normal histological structure of active mature functioning seminiferous tubules (S) associated with complete spermatogenic series. The peripheral layer of cells is composed of spermatocytes (SP) followed by a zone of spermatids (ST) and finally spermatozoa (SZ) about to be released into the lumen (H&E). (B) Testis of lead treated rats, showing marked degeneration (d) of most seminiferous tubules with absence of spermatogenic series in tubular lumen. The arrow indicates mild disintegration of the seminiferous tubules with loss of spermatids and spermatozoa, marked degeneration (arrow) of most spermatocytes, spermatids and congestion in the testis blood vessels (H&E). (C) Testis of rats given cinnamon extract at 250 mg/kg bw for 60 days, showing normal histological structure of most seminiferous tubules (H&E). (D) Testis of lead treated rats given cinnamon extract at 250 mg/kg bw for 60 days, showing normal histological structure of most seminiferous tubules (H&E).
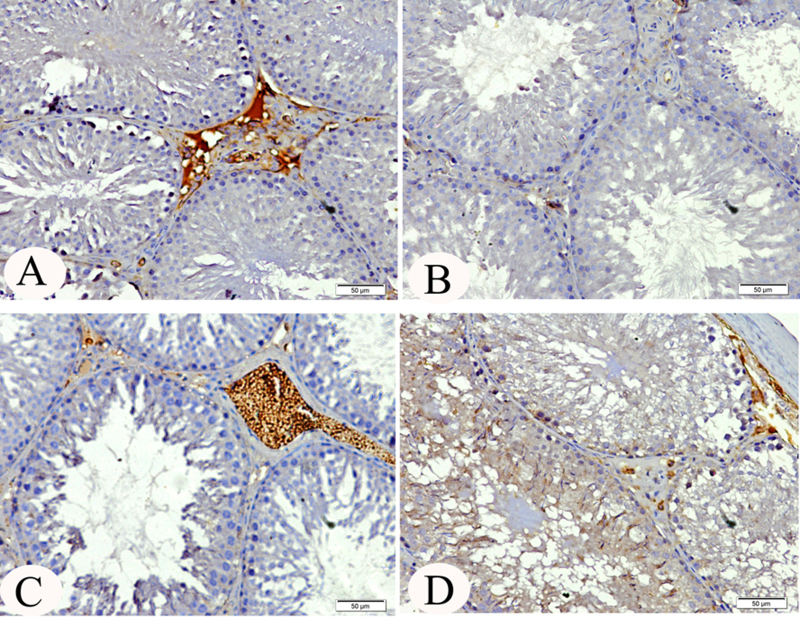
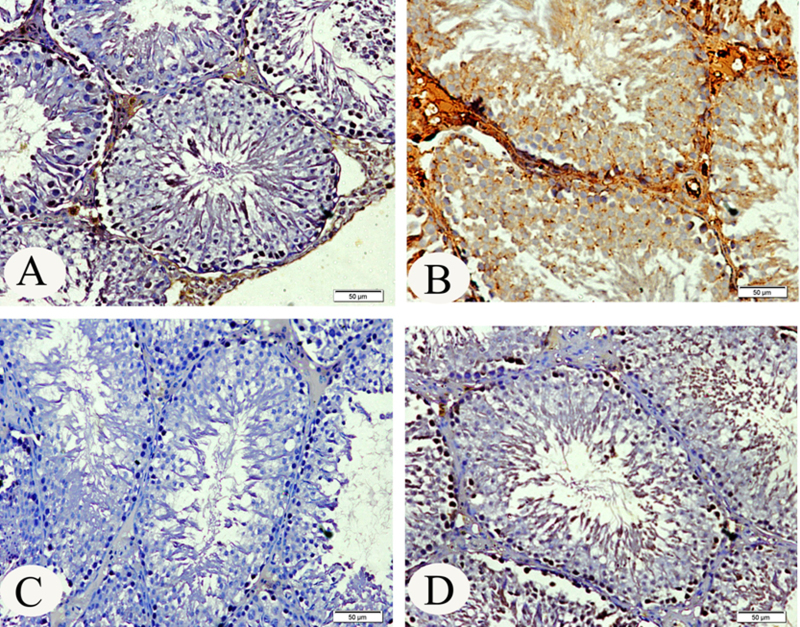
3.5. Immunohistochemistry of androgen receptor and caspase-3
There was a marked reduction (P < 0.001) in the expression of androgen receptor in the testis of lead treated rats compared to all groups (Fig. 2). The testis of cinnamon treated rats showed similar androgen receptor expression like that in the testis of control rats (Table 3). Moreover, the level of caspase-3 protein expression was significantly (P < 0.001) increased in lead treated rats compared to the expression in other groups (Table 3). The intensity of activated caspase-3 immunostaining (deep brown) is pre-dominant on spermatogonia and seminiferous tubules of lead treated rats (Fig. 3B).
Fig. 2.
(A) Testis of control rats with androgen receptor nuclear staining in the interstitial cells and lack of immunoreaction in seminiferous tubules. (B) Testis of lead treated rats, showing no immunoexpression of androgen receptor. (C) Testis of rats given cinnamon extract, showing staining in the interstitial cells. (D) Testis of lead treated rats given cinnamon extract, showing moderate androgen receptor nuclear immunoexpression in the interstitial cells.
Fig. 3.
Immunohistochemical staining of activated caspase-3 in rat testis. (A) Testis of control rats. (B) Testis of lead treated rats. The intensity of activated caspase-3 immunostaining (deep brown) is pre-dominant on spermatogonia and seminiferous tubules of lead treated group. (C) Testis of rats given cinnamon extract. (D) Testis of lead treated rats given cinnamon extract. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
4. Discussion
The present study showed that lead acetate causes a significant decrease in the male reproductive organs, testicular functions and significant alterations in the histological patterns in the testis. Our result agreed with [18] who found that the index weight of the testis, epididymis and accessory sex glands was significantly decreased in rats treated with lead compared to the control group. Several sperm parameters were severely affected following lead treatment. Epididymal sperm motility and viability decreased on lead exposure inraperitoneally [19].
The result of the present study clearly showed that the levels of SOD and catalase are remarkably decreased in rats treated with lead acetate. Lead acetate is known to cause free radical damage in tissues by two mechanisms: Increased generation of ROS, including hydroperoxides, singlet oxygen and hydrogen peroxides, and by causing direct depletion of antioxidant reserves [20], [21]. The observed decrease in circulating antioxidants and decrease in serum total antioxidants confirm the lead acetate-induced depletion of antioxidants [22]. All antioxidant enzymes including SOD and catalase decreased significantly in mitochondrial and post-mitochondrial fraction of testis of lead and cadmium treated rats [19]. There is a significant decrease in the activity levels of antioxidant enzymes superoxide dismutase and catalase in the testes of lead exposed rats [23]. In the present study, when the cinnamon and lead acetate was administrated to rats, the level of SOD was increased compared to its level in rats treated only with lead. The activities of liver SOD and catalase was significantly reduced in the carbon tetrachloride intoxicated group, while it was significantly elevated in the groups pretreated with either water or ethanol extracts of cinnamon [24].
Generally the effects of cinnamon have not yet been fully identified on reproductive system. This study concentrated on the effect of cinnamon extract on several reproductive parameters after lead exposure and its ability to correct the adverse effect of lead on seminal picture and testicular structure in rats. The improvement of reproductive parameters after cinnamon administration should be explained. One of the possible explanations is that concentration of LH, FSH and testosterone hormones have been increased significantly after cinnamon administration [25]. This effect could be due to the presence of compounds in cinnamon which affect the hypothalamus-pituitary axis and has thus increased concentrations of these hormones. The researches done by Shagauo and Davidson [26] also showed that cinnamon is capable of releasing LH hormone by affecting hypothalamus axis and increasing the secretion rate of GnRH hormone. Also, they proposed that GnRH cause proliferation of sex cells by increasing the Leydig cell activities in adult rats. In another explanation, Parivzi and Ellendorff [27] showed that cinnamaldehyde extracted from cinnamon increase norepinephrine and this hormone can increase the release of nitric oxide. Cinnamaldehyde release cAMP with connecting calcium in cell membrane and cause increase in norepinephrine secretion. Norepinephrine increase LH secretion with activation of nitric oxide. Nitric oxide affects hypothalamus axis and release gonadotropin hormone (GnRH). Gonadotorpin hormones increase secretion of other hormones such as LH and FSH of pituitary gland. LH hormone affects Leydig cells and this cells release testosterone hormone. Testosterone is the most important hormone in sex cells proliferation [27], [28].
In the present work, the testis of lead treated rats showed marked degeneration of most seminiferous tubules with absence of spermatogenic series in tubular lumen and congestion in testis blood vessels. Interestingly, the testis of lead treated rat given cinnamon extract showed normal histological structure of most seminiferous tubules. Similar changes accompanied by the accumulation of immature cells within the tubular lumen were also observed in rats under the influence of lead acetate [29]. More conspicuous degenerative changes in testicular tissues and an increase in sperm head abnormalities were observed in mice exposed to lead acetate [30].
In the present study there was a marked reduction in the expression of androgen receptor in the testis of lead treated rats. In a similar study, Paul et al. [31] found less intense immunostaining for androgen receptor in interstitial and peritubular cells of ram foeti testes from ewes reared on sewage treated pastures during pregnancy.
To date, little information exists on the effect of lead acetate on caspase-3 expression as well as the effect of cinnamon in correction of the lead toxicity. In the present study, lead induced significant increase in caspase-3 expression indicating that lead provokes apoptosis in the rat testis. Interestingly, the addition of cinnamon improved the condition by lowering the caspase-3 expressions. Apoptosis is a physiological process of selected cell deletion. As an antagonist of cell proliferation, apoptosis contributes to keeping the cell number in testicular tissue and helps to remove superfluous and damaged cells, but excessive apoptosis could cause destruction of male reproductive function [32]. The levels of caspase-3 were distinctly increased in mice treated with lead acetate Wang et al. [33]. In a similar study in China to explore the effects of expressions of caspase-3 in mice testes at different concentrations and time of lead acetate, it increased the expressions of caspase-3, which induces apoptosis of germ cells [34]. The occurrence of testicular cell apoptosis and the expression of caspase-3 in the adult male mice following lead administration were investigated. Compared with the control group, the protein levels of caspase-3 was significantly higher in experimental groups. The degree of differences was correlated with the time of lead exposure. The testicular apoptotic cell death is highly associated with lead loading and changes in caspase-3 expression may play an important role in this process [35].
5. Conclusion
It can be concluded that cinnamon may improve the reproductive parameters in male rats after lead acetate exposure. This is might be due to improve the superoxide dismutase level, androgen receptor expression and decrease caspase-3 expression in testis of male rats. However, further research is required to throw some more lights on the subject.
Conflict of interests
All authors state that there is no conflict of interest with the work done in this study.
Transparency document
Footnotes
Available online 16 October 2014
References
- 1.Patra R.C., Rautray A.K., Swarup D. Oxidative stress in lead and cadmium toxicity and its amelioration. Vet. Med. Inter. 2011:1–9. doi: 10.4061/2011/457327. (Article ID 457327) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agency for Toxic Substances and Disease Registry (ATSDR) U.S. Department of Health and Human Services, Public Health Service; Atlanta, GA: 2007. Toxicological Profile for Lead. [Google Scholar]
- 3.Goyer R.A., Cherian M.G. Ascorbic acid and EDTA treatment of lead toxicity in rats. Life Sci. 1979;24:433–438. doi: 10.1016/0024-3205(79)90215-7. [DOI] [PubMed] [Google Scholar]
- 4.Ruff H.A., Markowitz M.E., Bijur P.E., Rosen J.F. Relationships among blood lead levels, iron deficiency, and cognitive development in two-year-old children. Environ. Health Perspect. 1996;104:180–185. doi: 10.1289/ehp.96104180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winder C. Reproductive and chromosomal effect of occupational exposure to lead on the male. Reprod. Toxicol. 1989;3:221–233. doi: 10.1016/0890-6238(89)90016-6. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury A.R. Recent advances in heavy metals induced effect on male reproductive function – A retrospective. Al Ameen J. Med. Sci. 2009;2:37–42. [Google Scholar]
- 7.Ghelberg N.W., Bordas E. Lead-induced experimental lesions of the testis and their treatment. J. Appl. Toxicol. 1981;1:284–286. doi: 10.1002/jat.2550010509. [DOI] [PubMed] [Google Scholar]
- 8.Pinon Laraillade G., Thoreux Manlay A., Coffigny H., Monchaux G., Masse R., Soufir J.C. Effect of ingestion and inhalation of lead on the reproductive system and fertility of adult male rats and their progeny. Hum. Exp. Toxicol. 1993;12:165–172. doi: 10.1177/096032719301200213. [DOI] [PubMed] [Google Scholar]
- 9.El-Nekeety A.A., El-Kady A.A., Soliman M.S., Hassan N.S., Abdel-Wahhab M.A. Protective effect of Aquilegia vulgaris (L.) against lead acetate-induced oxidative stress in rats. Food Chem. Toxicol. 2009;47:2209–2215. doi: 10.1016/j.fct.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Abdel Moniem A.E., Dkhil M.A., Al-Quraishy S. Protective role of flaxseed oil against lead acetate induced oxidative stress in testes of adult rats. Afr. J. Biotechnol. 2010;9:7216–7223. [Google Scholar]
- 11.Sharpe R.M. Regulation of spermatogenesis. In: Knobil E., Neill J.D., editors. The Physiology of Reproduction. 2nd edn. Raven Press; New York: 1994. pp. 1363–1434. [Google Scholar]
- 12.Mcllwain D.R., Berger T., Mak T.W. Caspase function in cell death and disease. Cold Spring Harb. Perspect. Biol. 2013;5:a008656. doi: 10.1101/cshperspect.a008656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bearden H.J., Fuquay J.W. Reston Publishing Co., Inc.; Reston, Virginia: 1980. Applied Animal Reproduction; pp. 157–165. [Google Scholar]
- 14.Okamura A., Kamijima M., Shibata E., Ohtani K., Takagi K., Ueyama J., Watanabe Y., Omura M., Wang H., Ichihara G., Kondo T., Nakajima T. A comprehensive evaluation of the testicular toxicity of dichlorvos in Wistar rats. Toxicology. 2005;213:129–137. doi: 10.1016/j.tox.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Nishikimi M., Appaji N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46:849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Bancroft J.D., Stevens A., Turner D.R. 4th edn. Livingstone; New York, Churchill: 1996. Theory and Practice of Histological Techniques. [Google Scholar]
- 18.Hamadouche N.A., Sadi N., Kharoubi O., Slimani M., Aoues A. The protective effect of vitamin E against genotoxicity of lead acetate intraperitoneal administration in male rat. Arch. Biol. Sci. Belgrade. 2013;65:1435–1445. [Google Scholar]
- 19.Pandya C., Pillai P., Nampoothiri L.P., Bhatt N., Gupta S., Gupta S. Effect of lead and cadmium co-exposure on testicular steroid metabolism and antioxidant system of adult male rats. Andrologia. 2012;44:813–822. doi: 10.1111/j.1439-0272.2010.01137.x. [DOI] [PubMed] [Google Scholar]
- 20.Ercal N., Gurer-Orhan H., Aykin-Burns N. Toxic metals and oxidative stress part I. Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 21.Upasani C.D., Khera A., Balaraman R. Effect of lead with vitamins E, C, or spirulina on malondialdehyde: conjugated dienes and hydroperoxides in rats. Indian J. Exp. Biol. 2001;39:70–74. [PubMed] [Google Scholar]
- 22.Jackie T., Haleagrahara N., Chakravarthi S. Antioxidant effects of etlingera elatior flower extract against lead acetate – induced perturbations in free radical scavenging enzymes and lipid peroxidation in rats. BMC Res. Notes. 2011;4:67. doi: 10.1186/1756-0500-4-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anjum M.R., Reddy S.P. Effect of perinatal exposure to lead acetate on testicular lipid peroxidation in adult rats. Int. J. Pharm. Bio. Sci. 2013;4:893–898. [Google Scholar]
- 24.Moselhy S.S., Ali H.K. Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biol. Res. 2009;42:93–98. [PubMed] [Google Scholar]
- 25.Jahromi V.H., Parivar K., Forozanfar M. The Effect of cinnamon extract on spermatogenesis hormonal axis of pituitary gonad in mice. Iran. J. Appl. Anim. Sci. 2011;1:99–103. [Google Scholar]
- 26.Shagauo R.B., Davidson A.M. The effect of Cinnamomum zeylanicum on histological structure of testis in rats. Endocrinology. 2006;63:241–252. [Google Scholar]
- 27.Parivzi N., Ellendorff F. Further evidence on dual effects of norepinphrine on LH secretion. Neuro. Endocrinol. 1982;35:48–55. doi: 10.1159/000123354. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Tsukanmamoto T. Effects of nitric oxide stimulation on the brain. Drugs Today. 2000;36:38. doi: 10.1358/dot.2000.36.2-3.568781. [DOI] [PubMed] [Google Scholar]
- 29.Batra N., Nehru B., Bansal M.P. Influence of lead and zinc on rat male reproduction at biochemical and histopathological levels. J. Appl. Toxicol. 2001;21:507–512. doi: 10.1002/jat.796. [DOI] [PubMed] [Google Scholar]
- 30.Gautam A.K., Agarwal K., Shah B.A., Kumar S., Saiyed H.N. Lead induced sperm toxicity in mouse and MPG treatment. J. Environ. Biol. 2001;22:287–291. [PubMed] [Google Scholar]
- 31.Paul C., Rhind S.M., Kyle C.E., Scott H., McKinnell C., Sharpe R.M. Cellular and hormonal disruption of fetal testis development in Sheep reared on pasture treated with sewage sludge. Environ. Health Perspect. 2005;113:1580–1587. doi: 10.1289/ehp.8028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.John H. The relevance of spontaneous and chemically induced alterations in testicular germ cell apoptosis to toxicology. Toxicol. Lett. 2000;112–113:79–86. doi: 10.1016/s0378-4274(99)00253-2. [DOI] [PubMed] [Google Scholar]
- 33.Wang C., Zhang Y., Liang J., Shan G., Wang Y., Shi Q. Impacts of ascorbic acid and thiamine supplementation at different concentrations on lead toxicity in testis. Clin. Chim. Acta. 2006;370:82–88. doi: 10.1016/j.cca.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 34.Yan Z., Chunhong W.G., Duanlian Z. Effects of lead on expressions of TGFB1 and Caspase-3 in mice testes. Chin. J. Public Health. 2006;22:869–870. [Google Scholar]
- 35.Jin L., Feng C. Effects of lead on testicular cells apoptosis and expression of caspase-3, Bc-2 and Bax genes in mouse. J. Anhui Normal Univ. 2011;34:559–564. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.