Abstract
These are many volatile organic compounds (VOCs) that are synthesized, produced from petroleum or derived from natural compounds, mostly plants. Fragrant and volatile organic compounds from plants have been used as food additives, medicines and aromatherapy. Several clinical and pathological studies have shown that chronic abuse of VOCs, mainly toluene, causes several neuropsychiatric disorders. Little is known about the mechanisms of neurotoxicity of the solvents. n-Octanal, nonanal, and 2-ethyl-1-hexanol, which are used catalyzers or intermediates of chemical reactions, are released into the environment. Essential oils have the functions of self-defense, sterilization, and antibiosis in plants. When volatile organic compounds enter the body, there is the possibility that they will pass through the blood–brain barrier (BBB) and affect the central nervous system (CNS). However, the direct effects of volatile organic compounds on neural function and their toxicities are still unclear. We compared the toxicities of n-octanal, nonanal and 2-ethyl-1-hexanol with those of five naturally derived fragrant organic compounds (FOCs), linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol and n-phenethyl alcohol. MTT assay of human neuroblastoma SK-N-SH cells showed that the IC50 values of linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol and phenethyl alcohol were 1.33, 2.3, >5, >5, and 2.39 mM, respectively, and the IC50 values of toluene, n-octanal, nonanal and 2-ethyl-1-hexanol were 850, 37.2, 8.31 and 15.1 μM, respectively. FOCs showed lower toxicities than those of VOCs. These results indicate that FOCs are safer than other compounds.
Keywords: Neurotoxicology, Volatile organic compounds, Cell culture, Fragrant compounds
1. Introduction
There are now many synthetic and petroleum-derived volatile organic compounds (VOCs). VOCs are known to show the neurotoxicity with long-term exposure [1], [2]. For example, toluene in glues, paints and cleaning solvents is known to cause sick building syndromes and to show high toxicity in the nervous system. Several clinical and pathological studies have shown that chronic abuse of VOCs, mainly toluene, causes several neuropsychiatric disorders including distractibility, hallucinations, loss of impulse control, dementia and respiratory effects [3], [4], [5], [6]. n-Octanal, nonanal and 2-ethyl-1-hexanol are including in various chemicals and pollutants in river, ambient air or aircraft [7], [8], [9]. However, little is known about their effects on neural cells. Some studies have suggested that the neurotoxicity of some solvents is due to oxidative stress [10], [11], [12], [13], [14], [15]. Neural toxicity of n-octanal, nonanal and 2-ethyl-1-hexanol has not been examined in detail. Some VOCs are highly lipophilic, leading to their storage in cerebral tissues. If the neuronal toxicity and safety of VOCs could be accurately evaluated, the compounds could be used industrial chemistry without any concerns. We compared the neural toxicities of these VOCs with that of toluene.
Essential oils extracted from various parts of plants contain 200–300 aromatic chemical substances [16], [17]. Essential oils have the functions of self-defense, sterilization, and incentive antibacterial insect in plant. Human beings have used these compounds as perfume and aromatherapy for a long time. When aromatic compounds enter the body, there is the possibility that they pass through blood–brain barrier (BBB) and affect the central nervous system (CNS) [18], [19], [20]. We compared the toxicities of n-octanal, nonanal and 2-ethyl-1-hexanol with five naturally derived fragrant organic compounds (FOCs), linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol and n-phenethyl alcohol. FOCs showed lower toxicity than those of VOCs. Linalool, one of the primary components of lavender oil, is known to decrease anxiety and have a anti-nociceptive effects [19]. Green odor, a mixture of cis-3-hexen-1-ol and trans-2-hexanal has been shown to attenuate stress response and anxiety to psychological stress in rodents [21]. Isoamyl alcohol is a fragrance ingredient used in decorative cosmetics, fine fragrances, shampoos, toilet soaps, and other toiletries as well as in non-cosmetic products such as household cleaners and detergents. Its use worldwide is in the range of 0.1–1.0 metric tons/annum [22]. Phenethyl alcohol was found to be major constituent of rose absolute and was reported to have antibacterial activity [23], [24]. n-Propyl alcohol is contained in fruits and fermented products and is used as a food additive. Many volatile organic compounds are used in daily life, and it is therefore important to elucidate to the toxicities of FOCs and VOCs on human neural cells. We examined the effects VOCs and FOCs on human neuroblastoma SK-N-SH cells and primary cultured rat neurons.
2. Materials and methods
2.1. Chemicals
Toluene, linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol, and phenethyl alcohol were obtained from Wako Pure Chemical Industries (Japan). n-Octanal, nonanal, and 2-ethyl-1-hexanol were obtained from Tokyo Chemical Industry Co., Ltd. (Japan). These substances were dissolved in dimethyl sulfoxide (DMSO) and sterilized by filtering through a 0.22 μm membrane just before the experiment. Rotenone 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), glutamine, ethylenediaminetetraacetic acid (EDTA), and trypsin were purchased from Sigma–Aldrich (St. Louis, MO). Dulbecco's modified Eagle's medium (DMEM) and high-glucose DMEM (HG-DMEM) were purchased from GIBCO. Fetal calf serum (FCS) was purchased from SAFC Biosciences SIGMA.
2.2. Cell culture
SK-N-SH neuroblastoma cells were purchased from Institute of Physical and Chemical Research (Japan) (IPCR, RIKEN). Cells were grown in DMEM medium supplemented with 2 mM glutamine and 10% heat-inactivated FCS. Cells were maintained at 37 °C under 5% CO2/95% humidity, and the medium was changed three times a week.
2.3. MTT assay
Cell viability was analyzed using the conventional MTT reduction assay [25]. Briefly, cells were seeded in 96-multiwell plates at 3 × 103 cells/well and incubated for 24 h. After pre-incubation, toluene (10–5000 μM), n-octanal (1–000 μM), nonanal (0.1–100 μM), 2-ethyl-1-hexanol, linalool (0.1–3.0 mM), cis-3-hexen-1-ol (0.1–10 mM), isoamyl alcohol (1–30 mM), n-propyl alcohol (1–30 mM), and phenethyl alcohol (1–30 mM), were added. Twenty-four hours later, cells were incubated with MTT (5 mg/ml) at 37°C for 4 h. After incubation, the medium was removed, and the cells were dissolved in an acid dissolving solution in which 5% sodium dodecyl sulfate (SDS) in 0.01 M HCl and 0.02 M HCl-isopropanol were mixed in equal amounts. Absorbance of the formazan reduction product was measured at 570 nm in a plate reader.
2.4. Primary neuronal cultures
All experimental and surgical procedures were approved by the Institutional Animal Care and Use Committee at Kinki University. Time-mated pregnant rats (Crlj:Wistar) were purchased from Charles River Laboratories International Inc. Neurons were prepared from brain cortices of E17/18 rats embryos. The tissue was dissected in Hanks’ balanced salt solution (HBSS; 0.137 M NaCl, 5.4 mM KCl, 0.25 mM Na2HPO4, 0.1% glucose, 0.44 mM KH2PO4, 1.3 mM CaCl2, 1.0 mM MgSO4, 4.2 mM NaHCO3) and incubated with 2.5 mg/ml trypsin in the presence of 1 mg/ml DNase I (SIGMA) for 30 min at 37 °C. Subsequently, the cells were triturated in a plating medium containing HG-DMEM/10% FCS using 1 ml and 200 ml pipette chips. To remove the untriturated cells, the cells were passed through a cell strainer (mesh sizes: 70 mm) and centrifuged at 1000 rpm for 2 min. Cells (1 × 106 cell/ml) were plated in wells of a 4-well culture plate coated with poly d-lysine (Nunc, Thermo Fisher Scientific Inc., MA). After 3 days, 1 μM cytosine arabinoside C (AraC) was added to the culture to inhibit glial cell proliferation in the neuronal layer. The medium was changed every 3 days for 3 weeks.
2.5. Staining of live and dead cells
The Live-Dead Cell Staining kit (BioVision Inc., Milpitas, CA) provides ready-to-use reagents for convenient discrimination between live and dead cells. The kit utilizes Live-Dye™, a cell-permeable green fluorescent dye (Ex/Em = 488/518 nm), to stain live cells. Dead cells can be easily stained by propidium iodide (PI), a cell non-permeable red fluorescent dye (Ex/Em = 488/615 nm). Stained live and dead cells can be visualized by a fluorescence microscope (Olympus, Japan) using a band-pass filter (for detection of FITC and rhodamine). The amounts of living cells and dead cells were evaluated by live and dead cell staining. Based on MTT assay data, primary neuronal cultures were incubated with 0.1 and 0.5 mM of n-octanal, nonanal, and 2-ethyl-1-hexanol. And the cultures were incubated with linalool (0.1, 0.5, 1.0, and 2.5 mM), cis-3-hexen-1-ol (1, 2.5, and 5.0 mM), isoamyl alcohol (1, 2.5, and 5.0 mM), n-propyl alcohol (1, 2.5, and 5.0 mM), and phenethyl alcohol (1, 2.5, and 5.0 mM) for 24 h, and stained with the staining solution. The areas of living cells and dead cells were measured using cellSens digital imaging software (Olympus, Japan)
3. Results
3.1. Characteristics of FOCs and VOCs
Basic chemical structures of FOCs and VOCs used in the experiments are shown in Fig. 1. Linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol, phenethyl alcohol and 2-ethyl-1-hexanol have alcohol residues. Octanal and nonanal are aldehydes. Toluene is a hydrocarbon and does not have aldehyde and alcohol residues. The partition coefficient between octanol and water (log Pow) was used as a measure for lipophilicity of the compounds (Table 1). Toluene, octanal, nonanal and 2-ethyl-1-hexanol showed relatively high values of log Pow. Linalool also showed a relatively high log Pow. Other FOCs show the lower values of log Pow than those of VOCs.
Fig. 1.
Structures of VOCs and FOCs.
Table 1.
Names, chemical classes, and log Pow values of compounds.
| Compound | Molecular formula | Chemical group | log Powa | LD50 rat (mg/kg)b (intraperitoneal) |
|---|---|---|---|---|
| Toluene | C7H8 | 2.73 | 1332 | |
| n-Octanal | C8H16O | Aldehyde | 2.78 | |
| Nonanal | C9H18O | Aldehyde | 3.27 | |
| 2-Ethyl-1-hexanol | C8H18O | Alcohol | 2.73 | 500 |
| Linalool | C10H18O | Alcohol | 2.97 | 307 |
| cis-3-Hexen-1-ol | C6H12O | Alcohol | 1.61 | 600 |
| Isoamyl alcohol | C5H12O | Alcohol | 1.42 | 813 |
| n-Propyl alcohol | C3H8O | Alcohol | 0.25 | 164 |
| Phenethyl alcohol | C8H10O | Alcohol | 1.36 |
Log Pow is the partition coefficient between octanol and water.
Hazardous Substances Data Bank (HSDB).
3.2. Exposure of SK-N-SH cells to FOCs and VOCs causes truncated life span
With the addition of different concentrations of linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol, n-phenethyl alcohol n-octanal, nonanal and 2-ethyl-1-hexanol to the culture of SK-N-SH cells, all cell viabilities decreased in a concentration-dependent manner and the IC50 values were calculated as shown in Fig. 2. The IC50 values are summarized in Table 2, Table 3. The IC50 values of linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol and phenethyl alcohol were 1.33, 2.3, >5, >5, and 2.39 mM, respectively, and the IC50 values of toluene, n-octanal, nonanal and 2-ethyl-1-hexanol were 850, 37.2, 8.31 and 15.1 μM, respectively. n-Octanal, nonanal, and 2-ethyl-1-hexanol showed higher toxicity than that of toluene, which is known to have severe neuronal toxicity. FOCs contained abundantly in essential oils show 10–100 times lower sensitivity than that of VOCs.
Fig. 2.
Inhibitory dose–response curves for VOCs and FOCs in the human neuroblastoma cell line SK-N-SH. Various concentrations of VOCs (A: toluene, B: n-octanal, C: nonanal, D: 2-ethyl-1-hexanol) and FOCs (E: linalool, F: cis-3-hexen-1-ol, G: n-phenethyl alcohol) were applied, and death of SK-N-SH cells was determined by the MTT assay. Each value is the mean + S.E. of cell viability obtained for three experiments. Theoretical curves were drawn according to the equation V = Vmax/(1 + IC50/A)n. V, A, and n represent cell viability, concentrations of VOCs and FOCs, and Hill coefficients, respectively.
Table 2.
Summary of IC50 values and Hill coefficients of VOCs.
| VOCs | IC50 (μM) | Hill coefficient |
|---|---|---|
| Toluene | 850 | 2.2 |
| n-Octanal | 37.2 | 2 |
| Nonanal | 8.31 | 1.6 |
| 2-Ethyl-1-hexanol | 15.1 | 1.7 |
Table 3.
Summary of IC50 values and Hill coefficients of FOCs.
| FOCs | IC50 (mM) | Hill coefficient |
|---|---|---|
| Linalool | 1.33 | 4.2 |
| cis-Hexen-1-ol | 2.3 | 0.97 |
| Isoamyl alcohol | >5 | |
| n-Propyl alcohol | >5 | |
| Phenethyl alcohol | 2.39 | 3.41 |
3.3. Exposure of rat primary cortical neurons cells to FOCs and VOCs causes truncated life span
Living cells and dead rat primary cultured neurons were determined by Live and Dead Staining. With the addition of different concentrations of VOCs and FOCs, all cell viabilities decreased in a concentration-dependent manner. The dead cell area and living cell are clearly distinguishable. Immunohistochemical staining with an antibody against neuronal marker MAP-2 indicated that neuronal cells were concentrated in the dead cell area (data not shown).
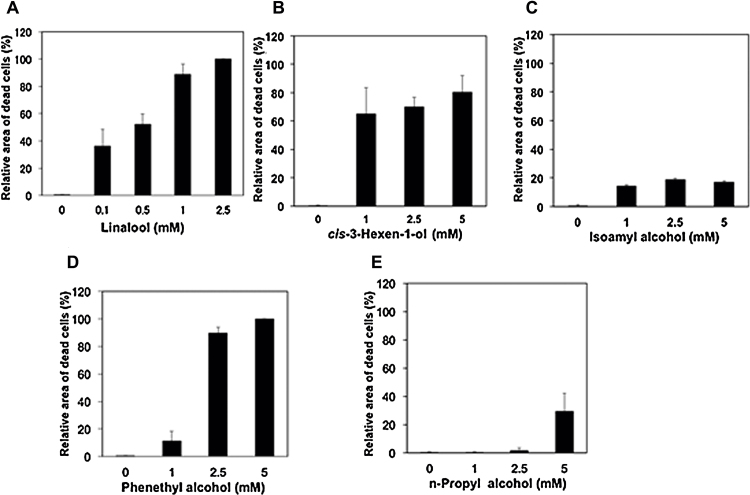
As shown in Fig. 3, almost all of the primary cultured neurons died when exposed to 0.5 mM of octanal, nonanal, or 2-ethyl-1-hexanol. The toxicity of toluene has been reported to cause the death of all primary cultured cells at concentration of 2 mM [26]. At 1 mM of isoamyl alcohol, phenethyl alcohol and n-propyl alcohol, almost all of the cells in culture dishes survived. However, at the same concentration of linalool or cis-3-hexen-1-ol, almost all of the cells in culture dishes died as shown in Fig. 4, Fig. 5. Isoamyl alcohol and n-propyl alcohol show lower toxicities than those of linalool, cis-3-hexen-1-ol and phenethyl alcohol, as was observed for SK-N-SH cells. In the experiment using primary cultured neurons, toxicities of FOCs were lower than that of VOCs, as was observed for SK-N-SK cells.
Fig. 3.
Effects of VOCs on primary rat cortical neurons. 0.1 mM and 0.5 mM of VOCs (n-octanal, nonanal, and 2-ethyl-1-hexanol) applied the primary rat cortical neurons. After 24 h of incubation with volatile compounds, the cells were stained by using Live and Dead Staining kit (A) and the dead cell area was determined. The areas of dead cells that had been exposed to 0.1 mM and 0.5 mM of VOCs (control, n-octanal, nonanal, 2-ethyl-1-hexanol) were measured and are summarized in (B) and (C), respectively.
Fig. 4.
Effects of FOCs on primary rat cortical neurons. Various amounts of FOCs (A: linalool, B: cis-3-hexen-1-ol, C: isoamyl alcohol, C: n-propyl alcohol, D: n-phenethyl alcohol) applied the primary rat cortical neurons. After 24 h of incubation with volatile compounds, the cells were stained by using Live and Dead Staining kit.
Fig. 5.
Summary of effects of FOCs on primary rat cortical neurons. Various amounts of FOCs applied the primary rat cortical neurons. After 24 h of incubation with volatile compounds, the cells were stained by using Live and Dead Staining kit and the dead cell area was determined. The areas of dead cells that had been exposed to linalool, cis-3-hexen-1-ol, isoamyl alcohol, n-propyl alcohol, n-phenethyl alcohol were measured and are summarized in (A), (B), (C), (D) and (E), respectively.
3.4. Relationship between log Pow and IC50
The partition coefficient between octanol and water (Pow) was used as a measure for the lipophilicity of the solvents (Fig. 6). Correlation coefficient between IC50 and log Pow was calculated. As log Pow increased, the IC50 decreased for the solvents except toluene and linalool. When plotted with a logarithmic scale on the ordinate axis, there was a linear relationship between IC50 values of the VOCs and FOCs and log Pow values except for toluene and linalool.
Fig. 6.
Correlation between log Pow values and IC50 values. A spreading diagram based on log Pow and IC50 of FOCs and VOCs is formed. Filled circles denote FOCs and filled diamonds denote VOCs. Correlation coefficient is shown in the diagram.
4. Discussion
Chemical compounds from plants have been used as food additives, medicines and aromatherapy. Naturally derived biogenic volatile compounds are emitted constitutively, and emission can be observed throughout the life cycle of the plant or, more often, at specific developmental stages. Synthesis of other compounds is induced after wounding and herbivore feeding or after environmental stresses. About 1700 substances have been found to be emitted from plants [27]. However, their toxicity against neurons is not clear. In this study, we compared the neuronal toxicities of synthetic volatile organic compounds and naturally derived volatile organic compounds used as fragrances and flavors.
Many synthetic and petroleum-derived VOCs are currently being used. Toluene, benzene and o-xylene are potentially harmful substances commonly found in occupational and non-occupational environments [28]. Acute exposure to toluene leads to central nervous system disorders [29]. Sarma et al. [30] reported that toluene, benzene and o-xylene induced apoptosis in vitro in the human HL-60 promyelocytic luekemia cell line, human erythromyeloblastoid leukemia K562 cell line, and human leukemic monocyte lymphoma U937 and the values of IC50 were 2.74, 4.4 and 3.79 mM, 8.75, 12.73 and 9.43 mM, and 0.84, 1.17 and 1.35 mM, respectively. They also reported that pretreatment with an ROS scavenger before exposure to these compounds decreased apoptosis. Toluene (IC50: 0.85 mM) showed higher toxicity in SK-N-SH cells than in leukemia cell lines, indicating that SK-N-SH cells are more sensitive than leukemia cell lines and that toluene toxicity has tissue specificity. In this paper, we described severe effects of other compounds on neural cells. Log Pows of n-octanal, nonanal, and 2-ethyl-1-hexanol were almost the same or higher than that toluene as shown in Table 1. n-Octanal, nonanal, and 2-ethyl-1-hexanol showed higher toxicities than that of toluene in SK-N-SH cells. These compounds used as the catalyzers or intermediates of chemical reactions are released into the environment. Hexanal, heptanal, octanal, decanal, and 6-metyl-5-hepten-2-one produced by ozone have been detected in an aircraft cabin [9]. The basal toxicities of these compounds have already been determined as shown in Table 1. We reported here for the first time that the neural toxicity of these compounds is higher than that of toluene. There are many other chemical compounds for which the neurotoxicities are not known. Further studies on the neurotoxicities of chemical volatile compounds are needed. FOCs showed much lower neural toxicities than VOCs. Dreiem et al. [10] reported that aromatic solvents with C > 7, and aliphatic and alicyclic compound C > 7 induced the production ROS in rat cerebellar granule cells in vitro. Linalool (C10) and phenethyl alcohol (C8) showed lower toxicities than those VOCs despite contain the higher numbers of carbons. Further detailed studies on ROS production needed. Even FOCs have neural toxicity, and FOCs must therefore be used carefully. Further studies of FOCs with flavors are needed. It is necessary to confirm the neurotoxicities of more volatile compounds in vitro and in vivo.
Conflict of interest
None declared.
Acknowledgments
This work was supported by MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2009–2014 (Japan). The authors wish to thank Ms. Keiko Bessho for assistance in writing this article.
References
- 1.Cranmer, Goldberg, editors. Human aspects of solvent neurobehavioral effectsNeurotoxicology. 1986;7(4):43–56. [PubMed] [Google Scholar]
- 2.Arlien-Søborg P., Hansen L., Ladefoged O., Simonsen L. Report on a conference on organic solvents and the nervous system. Neurotoxicol. Teratol. 1992;14(1):81–82. doi: 10.1016/0892-0362(92)90032-6. [DOI] [PubMed] [Google Scholar]
- 3.Pascual R., Bustamante C. Structural neuroplasticity induced by melatonin in entorhinal neurons of rats exposed to toluene inhalation. Acta Neurobiol. Exp. 2011;71(4):541–547. doi: 10.55782/ane-2011-1870. [DOI] [PubMed] [Google Scholar]
- 4.Chouanière D., Wild P., Fontana J.M., Héry M., Fournier M., Baudin V. Neurobehavioral disturbances arising from occupational toluene exposure. Am. J. Ind. Med. 2002;41(2):77–88. doi: 10.1002/ajim.10030. [DOI] [PubMed] [Google Scholar]
- 5.Filley C.M., Halliday W., Kleinschmidt-DeMasters B.K. The effects of toluene on the central nervous system. J. Neuropathol. Exp. Neurol. 2004;63(1):1–12. doi: 10.1093/jnen/63.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Rumchev K., Spickett J., Bulsara M., Phillips M., Stick S. Association of domestic exposure to volatile organic compounds with asthma in young children. Thorax. 2004;59(9):746–751. doi: 10.1136/thx.2003.013680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutta C., Som D., Chatterjee A., Mukherjee A.K., Jana T.K., Sen S. Mixing ratios of carbonyls and BTEX in ambient air of Kolkata, India and their associated health risk. Environ. Monit. Assess. 2009;148(1–4):97–107. doi: 10.1007/s10661-007-0142-0. [DOI] [PubMed] [Google Scholar]
- 8.Dąbrowska A., Nawrocki J. Aldehyde concentrations in wet deposition and river waters. Sci. Total Environ. 2013;452–453:1–9. doi: 10.1016/j.scitotenv.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Weisel C., Weschler C.J., Mohan K., Vallarino J., Spengler J.D. Ozone and ozone byproducts in the cabins of commercial aircraft. Environ. Sci. Technol. 2013;47(9):4711–4717. doi: 10.1021/es3046795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreiem A., Myhre O., Fonnum F. Relationship between lipophilicity of C6–10 hydrocarbon solvents and their ROS-inducing potency in rat cerebellar granule cells. Neurotoxicology. 2002;23(6):701–709. doi: 10.1016/S0161-813X(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 11.Mattia C.J., Ali S.F., Bondy S.C. Toluene-induced oxidative stress in several brain regions and other organs. Mol. Chem. Neuropathol. 1993;18(3):313–328. doi: 10.1007/BF03160122. [DOI] [PubMed] [Google Scholar]
- 12.Beckley J.T., Woodward J.J. The abused inhalant toluene differentially modulates excitatory and inhibitory synaptic transmission in deep-layer neurons of the medial prefrontal cortex. Neuropsychopharmacology. 2011;36(7):1531–1542. doi: 10.1038/npp.2011.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pascual R., Aedo L., Meneses J.C., Vergara D., Reyes A., Bustamante C. Solvent inhalation (toluene and n-hexane) during the brain growth spurt impairs the maturation of frontal, parietal and occipital cerebrocortical neurons in rats. Int. J. Dev. Neurosci. 2010;28(6):491–495. doi: 10.1016/j.ijdevneu.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Dreiem A., Ring A., Fonnum F. Organic solvent-induced cell death in rat cerebellar granule cells: structure dependence of c10 hydrocarbons and relationship to reactive oxygen species formation. Neurotoxicology. 2005;26(3):321–330. doi: 10.1016/j.neuro.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 15.Chalansonnet M., Carabin N., Boucard S., Cosnier F., Nunge H., Gagnaire F. Study of the potential oxidative stress induced by six solvents in the rat brain. Neurotoxicology. 2013;35:71–83. doi: 10.1016/j.neuro.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 16.Berger R.G. Flavours and fragrances chemistry. Bioprocess. Sustain. 2007:219–239. [Google Scholar]
- 17.Burt S. Essential oils: their antibacterial properties and potential applications in food – a review. Int. J. Food Microbiol. 2004;94(3):223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 18.Linck V.M., da Silva A.L., Figueiró M., Caramão E.B., Moreno P.R., Elisabetsky E. Effects of inhaled linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine. 2010;17(8–9):679–683. doi: 10.1016/j.phymed.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Woronuk G., Demissie Z., Rheault M., Mahmoud S. Biosynthesis and therapeutic properties of lavandula essential oil constituents. Planta Med. 2011;77(1):7–15. doi: 10.1055/s-0030-1250136. [DOI] [PubMed] [Google Scholar]
- 20.Sakurada T., Mizoguchi H., Kuwahata H., Katsuyama S., Komatsu T., Morrone L.A. Intraplantar injection of bergamot essential oil induces peripheral antinociception mediated by opioid mechanism. Pharmacol. Biochem. Behav. 2011;97(3):436–443. doi: 10.1016/j.pbb.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Nikaido Y., Miyata S., Nakashima T. Mixture of cis-3-hexenol and trans-2-hexenal attenuates behavioral and stress responses induced by 2,5-dihydro-2,4,5-trimethylthiazoline and electric footshock stress in rats. Physiol. Behav. 2011;103(5):547–556. doi: 10.1016/j.physbeh.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 22.McGinty D., Lapczynski A., Scognamiglio J., Letizia C.S., Api A.M. Fragrance materials review on isoamyl alcohol. Food Chem. Toxicol. 2010;48(Suppl. 4):S102–S109. doi: 10.1016/j.fct.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 23.Ulusoy S., Boşgelmez-Tinaz G., Seçilmiş-Canbay H. Tocopherol, carotene, phenolic contents and antibacterial properties of rose essential oil, hydrosol and absolute. Curr. Microbiol. 2009;59(5):554–558. doi: 10.1007/s00284-009-9475-y. [DOI] [PubMed] [Google Scholar]
- 24.Fraud S., Rees E.L., Mahenthiralingam E., Russell A.D., Maillard J.Y. Aromatic alcohols and their effect on Gram-negative bacteria, cocci and mycobacteria. J. Antimicrob. Chemother. 2003;51(6):1435–1436. doi: 10.1093/jac/dkg246. [DOI] [PubMed] [Google Scholar]
- 25.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 26.Ohtani K., Shiraishi K., Sugiyama K., Yamada Y. Evalution of the toxicity of chemicals on neural cells. Res. Rep. Eng. Kinki Univ. 2011;45:1–5. [Google Scholar]
- 27.Pichersky E., Dudareva N. The chemical diversity of floral scent. Biol. Floral Scent. 2006:27–52. [Google Scholar]
- 28.Reisch M.S. Top 50 chemicals production stagnated last year. Chem. Eng. News. 1992;70(15):16–20. [Google Scholar]
- 29.Foo S.C., Jeyaratnam J., Koh D. Chronic neurobehavioural effects of toluene. Br. J. Ind. Med. 1990;47(7):480–484. doi: 10.1136/oem.47.7.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarma S.N., Kim Y.J., Song M., Ryu J.C. Induction of apoptosis in human leukemia cells through the production of reactive oxygen species and activation of HMOX1 and Noxa by benzene, toluene, and o-xylene. Toxicology. 2011;280(3):109–117. doi: 10.1016/j.tox.2010.11.017. [DOI] [PubMed] [Google Scholar]