Abstract
Erythrophleum ivorense and Parquetina nigrescens are found growing in tropical regions and they are used in African traditional medicine to treat various ailments including wounds, boils and anaemic conditions. Some species of plant in the Erythrophleum genus are also known to be poisonous and toxic to several livestock. However, there is no information on the toxicity of E. ivorense and P. nigrescens. This study is to determine the cytotoxicity and subchronic toxicity properties of methanol leaf extract (EIML) and methanol stem barks extract (EIMB) of E. ivorense and methanol leaf and aerial part extract of P. nigrescens (PNML). Concentrations from 0.1 to 100 μg/mL of the extracts were used to determine the influence of the extracts on the release of lactate dehydrogenase (LDH) from HaCaT keratinocytes. The EIML and EIMB extracts showed increase in LDH released from HaCaT keratinocytes at 0.1–10 μg/mL and 1–100 μg/mL for the PNML extracts (p > 0.05). Wistar rats were orally administered with 100, 300 and 1000 mg/kg body weight of the extracts (EIML, EIMB and PNML, respectively) for 35 days. Tissues from the kidney and liver of the rats treated with lower doses (100–300 mg/kg body weight) of EIML extract showed highly vascularized kidneys with numerous glomerular tufts, healthy hepatocytes and sinusoids in liver. However, there were persistent renal tissue inflammation and glomerular degeneration in kidney, and increased inflammatory infiltrates with few vacuolations and scarrings in liver in rats treated with higher extract dose of 1000 mg/kg body weight of rat. The rats treated with EIMB extract showed persistent renal and hepatocyte inflammations with glomerular and hepatocyte necrosis at all administered doses (100, 300 and 1000 mg/kg body weight) which are indications of renal and hepatic toxicities. Though rats administered with 100 and 300 mg/kg of PNML extract showed renal haemorrhage and inflammation and hepatic inflammation, the rats administered with 1000 mg/kg body weight showed restoring glomerular tufts and improved vasculature and liver with reduced inflammatory infiltrates with healthy hepatocytes. Phytochemical screening of EIML, EIMB and PNML extracts revealed the presence of alkaloids, tannins, flavonoids, sterols, cardiac glycosides and terpenoids.
Keywords: Wistar rats, Toxicity, Lactate dehydrogenase, Cytotoxicity, HaCaT keratinocytes
1. Introduction
Traditional medicine practice is a prominent aspect of the primary healthcare systems in most developing countries. It is estimated that about 80% of the world's population especially people from developing countries depend on traditional medicines of plant origin [25]. The inclusion of plants in traditional medicines dates back to several thousands of years [1] and it is on the surge due to their great source of bioactive compounds employed in pharmaceutical intermediates and chemical agents for synthetic drugs [25], and the fact that they are cost-effective and lack the complexity of compounded pharmaceutical preparations [38], [40]. However, many herbal preparations and traditional folk medicines have not been thoroughly tested or investigated [22].
Erythrophleum ivorense (A Chev.) is a large tree found growing in tropical regions in Africa including Ghana, Cote d’Ivoire and Liberia. It is also described as the ‘ugly’ plant. It can grow up to 40 m tall, usually bole cylindrical, but it may occasionally be fluted at the base, with or without buttresses at old age. The diameter is usually 60–90 cm [8], [20].
Parquetina nigrescens (Afzel.) Bullock belongs to the family Ascelpiadaceae. It is a slender, glaborous twining shrub that grows up to tops of forest trees. The plant is present in low bushes in savannah areas, farm clearings in forests and transition forests in West African countries including Ghana and Cote d’Ivoire [20]. The leaves and roots of P. nigrescens are used as poultice to treat wounds, boils, carbuncles and worm infections in ethno-medicine [5].
Aside from the therapeutic values of some of the compounds from plants, others are also known to be toxic and harmful to humans and animals. Several Erythrophleum spp. including Erythrophleum lascianthum and Erythrophleum guineense have been studied to be toxic to livestock and humans [2]. They have been used as hunting poisons for animals and suffering or test drinks for people convicted of serious crimes in some communities [12].
The kidney and liver are organs of metabolism and excretion, respectively, of xenobiotic molecules such as saponins, alkaloids, tannins etc. [20]. These organs can suffer from diverse diseases or disorders [38]. Lactate dehydrogenase enzymes are cytoplasmic in origin in intact cells of animals. However, these enzymes may leak through phospholipid membrane channels of cytoplasm of cells and be measured extracellularly, indicative of injury or damage to the cells. Plants possess several metabolites with varied pharmacological effects. The need to evaluate the potential toxicity of these compounds and that of many therapeutic molecules has led to the development of various cytotoxicity assays [35]. The study was therefore to investigate the cytotoxicity of methanol leaf and aerial parts extract of P. nigrescens and methanol leaf and stem bark extracts of E. ivorense on HaCaT keratinocytes and in vivo toxicity effect the extracts on kidney and liver tissues of Wistar rats.
2. Materials and methods
2.1. Plant materials and chemicals
The leaves and barks of E. ivorense were collected from the Botanic Garden, University of Ghana, in November 2011 by Mr. John Yaw Amponsah. The leaves and aerial parts of P. nigrescens were collected by Mr. Eric Gyebi from Jachie in the Bosomtwi District of the Ashanti Region, Ghana, in December 2011. The plant materials were authenticated by Dr. Alex Asase, Department of Botany of University of Ghana, Legon, Ghana. Voucher specimens of the plant materials have been kept in the Ghana Herbarium, University of Ghana, Legon, Accra, Ghana. Unless stated otherwise, all the chemicals were purchased from GPR, BDH, Poole, UK.
2.2. Preparation of extracts
Fresh leaves and stem bark of E. ivorense and leaves and other aerial parts of P. nigrescens were washed with tap water to remove debris and soil particles. The plant materials were dried at room temperature (28–30 °C) for 6 days before the dried plant materials were powdered using laboratory mill machine (Type 8, Christy and Norris Limited, UK).
Eight hundred grams (800 g) of the powdered leaf, 650 g of powdered bark of E. ivorense and 700 g of powdered leaf and other aerial parts of P. nigrescens were and each soaked in 2.5 L of 70% (v/v) methanol in a stoppered container. These were shaken for about 5 min and left to extract by means of maceration (shaking the mixture intermittently) at 28 °C for 72 h [28]. The mixtures were filtered into a porcelain crucible using a fine mesh. The supernatant was concentrated below 40 °C using rotary evaporator and then lyophilized. The yields of the methanol leaf extract of E. ivorense (EIML), methanol bark extract of E. ivorense (EIMB), and methanol leaf and other aerial parts extracts of P. nigrescens (PNML) were determined.
2.3. Phytochemical analysis
The phytochemical constituents of the methanol extracts were determined using methods described by [16], [36].
2.4. HPLC profile of methanol extracts
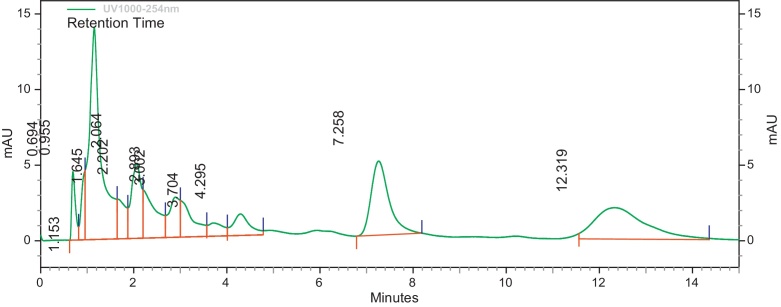
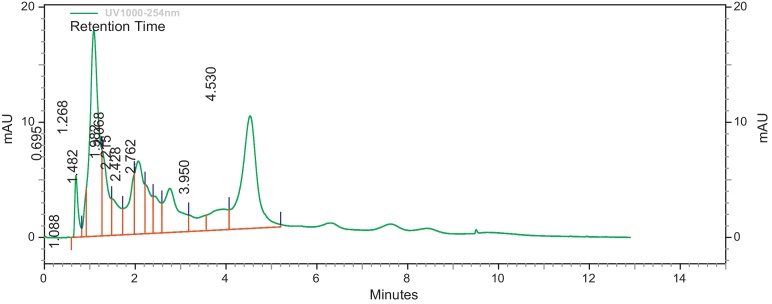
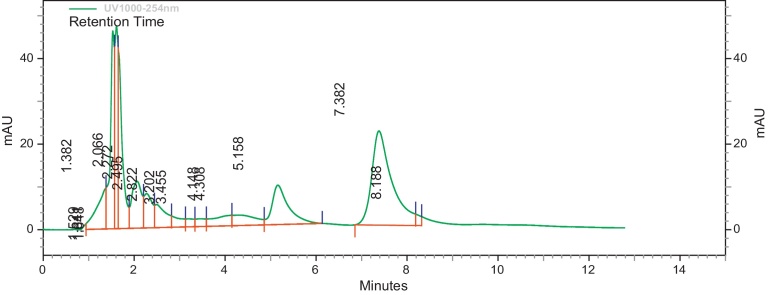
Modified methods of [11], [34] were used to determine the HPLC profile of the methanol extracts (EIML, EIMB and PNML) using a reverse phase Jupiter C18 300R column (250 mm × 4.6 mm). Concentrations of 10 mg/mL of extracts were prepared with methanol–water (3:7, v/v) which is the same as the mobile phase and a volume of 10 μL injected into the columns. The run time for the column of extracts was 10 min at 22 °C under a pump pressure of 21 MPa and flow rate of 1.0 mL/min. The resultant chromatograms were observed at a wavelength of 254 nm. The retention times and area under curve of the chromatograms were then determined.
2.5. Ethical approval for animal studies
Ethical clearance and approval for the subchronic toxicity studies in Wistar rats was given by the Ethical Committee on Animals of the Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana in accordance with the Guide for Care and Use of Laboratory Animals, NIH, Department of Health Services Publication, USA, no. 83-23, revised 1985 [15]. Approval of cytotoxicity studies was made by the local Ethical Committee of University of Muenster, Muenster, Germany (2006-177-f-S).
2.6. Handling and preparation of test laboratory animals
Fifty (50) male Wistar rats of weight ranging between 95 and 150 g were obtained from the Department of Pharmacology, Faculty of Pharmacy and Pharmaceutical Sciences, Kwame Nkrumah University of Science and Technology, Kumasi, Ghana and housed in stainless steel cages containing wood shavings for bedding. They were to acclimated holding facilities for 2 weeks before experimental procedure was started. The rats were randomly divided into 10 groups (5 rats per group), which consisted of 9 treatment groups and one untreated group (control). They were fed on normal commercial dietary pellets (GAFCO, Tema, Ghana) and given tap water ad libitum during the study. Environmental conditions were maintained at a temperature of 29 ± 2 °C and a relative humidity of 40 ± 10% with 12 h light/dark cycle.
2.7. Administration of extracts to animals
The rats were fasted for 12 h prior to administration of doses. The extracts were administered to the rats as aqueous suspensions of finely grounded powder for thirty-five (35) days via oral gavage, using a curved, ball-tipped stainless steel feeding needle connected to a syringe at the concentrations. Each group was made of five rats. Group 1 rats were treated with 100 mg/kg of methanol leaf extract of E. ivorense (EIML); Group 2 rats were treated with 300 mg/kg of EIML; Group 3 rats were treated with 1000 mg/kg of EIML; Group 4 rats were treated with 100 mg/kg of EIMB; Group 5 rats were treated with 300 mg/kg of EIMB; Group 6 rats were treated with 1000 mg/kg of EIMB; Group 7 rats were treated with 100 mg/kg of methanol leaf and other aerial part extract of P. nigrescens (PNML); Group 8 rats were treated with 300 mg/kg of PNML; Group 9 rats were treated with 1000 mg/kg of PNML and Group 10 rats were administered with distilled water.
2.8. Biochemical tests for determination of toxicity
The animals were fasted overnight prior to necropsy and blood collection. The animals were anaethesized prior to euthanization and then decapitated after neck dislocation. Blood samples were taken through the jugular veins in the animals in each group into complete blood count (CBC) bottles containing ethylenediaminetetraacetic acid (EDTA-2K). Haematological analyses which measured parameters such as red blood cell count, haemoglobin concentration, haematocrit, mean corpuscular cell volume, mean corpuscular cell haemoglobin, mean corpuscular cell haemoglobin concentration, platelet count, white blood cell count, and differential WBC count were determined using automatic haematology analyzer (Hitachi 7060, Japan).
Portions of uncoagulated blood were centrifuged at 3000 rpm for 10 min and analyzed using a 7060 autoanalyzer (Hitachi, Tokyo). Serum biochemical indicators such as glucose, total cholesterol, blood urea nitrogen (BUN), creatinine, total protein, albumin, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), total bilirubin, creatine kinase, albumin/globulin ratio, triglycerides, phosphorus, calcium and chloride were measured.
2.9. Histopathological studies
Histopathological examinations were performed on the kidney and liver of both treated and untreated Wistar rats as described by methods by Wasfi et al. [37] and Guntupalli et al. [18]. The tissues were fixed in 10% formalin. They were then dehydrated sequentially in ethanol concentrations of 50–100%. The tissues are then rinsed in xylene to remove the dehydrant (ethanol) and finally embedded in paraffin for strengthening and easy dissection. Prior to sectioning the tissues, they are ‘de-paraffinized’ by rinsing in xylene, followed by washing in decreasing concentration of ethanol (100–50%) before rehydrating the tissues with water. Tissue sections of thickness 6 μm were made and stained with haematoxylin–eosin (H–E) dye to impart contrast for photomicroscopic viewing. The tissues were then observed under a light microscope at magnification (600×).
2.10. In vitro cytotoxicity
Cytoplasmic enzyme lactate dehydrogenase (LDH) released from the cytosol of HaCaT keratinocytes when damaged or under stress was determined according to the method described by Agyare et al. [6]. Concentrations (0.10, 1.0, 10.0, 50.0 and 100.0 μg/mL) of the extracts in 100 μL HaCaT keratinocytes medium were made in 96-well microtitre plates and each well seeded with 105 keratinocyte cells and then incubated for 48 h at 35 °C with 5% of CO2. After incubation 25 μL of this supernatant was pipetted into a 96-well microtitre plate and 25 μL lysis buffer added to both the supernatant and the adherent lysed cells in the wells. This was incubated at 28 °C for 1 h and frequently agitated. Afterwards, 25 μL of substrate mix was added to both the untreated supernatant and lysed cell medium in the microtitre plate and incubated in dark at 20 °C for 30 min. The reactions were finally halted by the addition of 10 μL HCL–isopropanol solution to each well. The above procedure was repeated for untreated HaCaT keratinocytes and 10% Triton X-100 in FCS as negative and positive controls, respectively. After incubation for 4 h measurement of LDH enzyme released into the 25 μL supernatant and the absorbance were determined at 450 nm against 690 nm.
2.11. Statistical analysis
Haematological and serum biochemistry data were expressed as means ± standard error using GraphPad prism version 5.0 windows (GraphPad Software, San Diego, CA, USA). A one-way analysis of variance (ANOVA) was done for the data followed by Newman–Keuls post-test. The values of p < 0.05 were considered to be statistically significant.
3. Results
3.1. Phytochemical screening
The preliminary phytochemical screening of the methanol extracts (EIML, EIMB and PNML) revealed the presence of secondary metabolites including saponins, tannins, flavonoids and alkaloids (Table 1). The yields of EIML, EIMB and PNML extracts were 9.76, 15.54, 7.68% (w/w) related to the dried plant materials, respectively and the yield was determined by dividing the final powdered extract (g) by the total weight of the dried plant material (g) multiplied by 100%.
Table 1.
Phytochemical constituents of methanol extracts of E. ivorense and P. nigrescens.
| Phytochemical | EIML | EIMB | PNML |
|---|---|---|---|
| Saponins | + | + | + |
| Hydrolysable tannins | − | + | − |
| Condensed tannins | + | − | + |
| Alkaloids | + | + | + |
| Terpenoids | − | + | + |
| Sterols | + | − | + |
| Flavonoids | + | + | + |
| Cardiac glycosides | − | + | + |
Key: (+), presence of secondary metabolite; (−), absence of secondary metabolites.
3.2. HPLC profile
The HPLC profile of the methanol extracts was determined using different solvent systems. These HPLC profiles are used as identification for the plants. Different peaks in the chromatograms represent different compounds or constituents present in the plant extract (Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
HPLC profile of methanol leaf extract of E. ivorense at λ 254 nm.
Fig. 2.
HPLC profile of methanol bark extract of E. ivorense at λ 254 nm.
Fig. 3.
HPLC profile of methanol leaf and aerial parts extract of P. nigrescens at λ 254 nm.
3.3. Subchronic toxicity studies
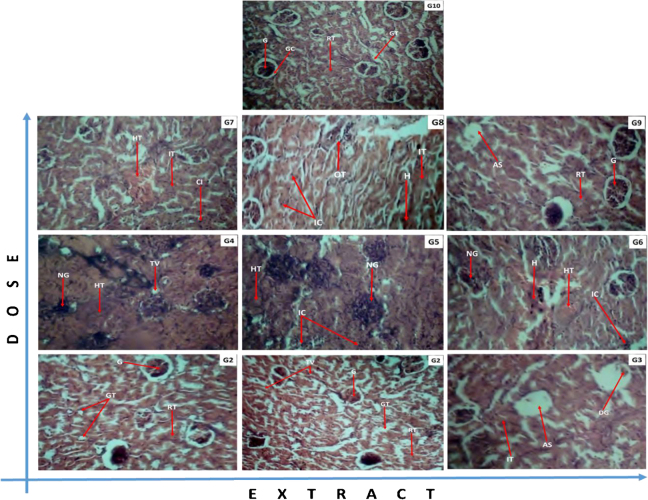
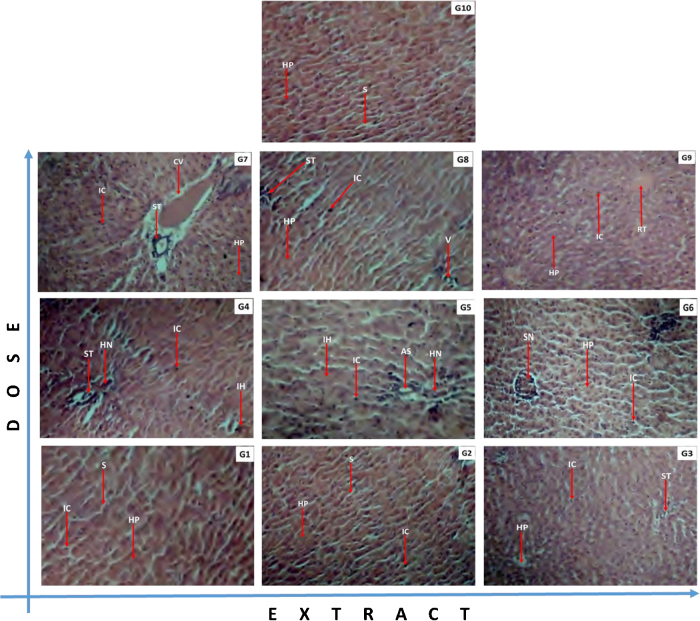
Sections of liver and kidney tissues from rats of all 10 groups were made and histopathological studies were done on them. The tissues of animals from treated group showed diverse morphological changes as compared to tissues sectioned from untreated group indicative of toxicity effects of the extracts administered. The renal tissues showed inflammation glomerular degeneration, hyalinized tissues and haemorrhage (Fig. 4). The hepatic tissues revealed scarring, inflammation, occlusion or congestive veins and necrosis (Fig. 5).
Fig. 4.
Effects of E. ivorense leaf and stem bark and P. nigrescens extracts on kidney tissue of treated and untreated Wistar rats. (G1) EIML 100 mg/kg: improved vasculature and a good number of glomerular tufts with no notable changes. (G2) EIML 300 mg/kg: highly vascularized kidney with a good number of glomerular tufts. (G3) EIML 1000 mg/kg: Persistent renal tissue inflammation and glomerular degeneration, evident of reduced immunity. (G4) EIMB 100 mg/kg: diseased kidney with profuse renal tissue inflammation, glomerular necrosis and vacuolation, evident of kidney degeneration. (G5) EIMB 300 mg/kg: profuse glomerular degeneration with persistent renal tissue inflammation, indicative of persistent poor treatment response. (G6) EIMB 1000 mg/kg: persistent renal toxicity with profuse haemorrhage, glomerular degeneration, reflective of treatment failure. (G7) PNML 100 mg/kg: persistent hyalination and haemorrhage with focal inflammatory cells infiltration indicative of persistent kidney damage due to reduced immunity. (G8) PNML 300 mg/kg: profuse haemorrhage and persistent renal tissue inflammation reflective of poor immune response. (G9) PNML 1000 mg/kg: a good number of restoring glomerular tufts with improved vasculature suggestive of recovery. (G10) Control 000 mg/kg: Normal kidney highly vascularized with a good number of glomerular tufts. Legend: RnT: renal tissue; RV: reduced vacuolation; TV: tissue vasculature; AS: apoptotic space; DG: degenerating glomeruli; G: glomeruli; GT: glomerular tubules; H: haemorrhage; HT: hyalinized tissue; IC: infiltrating cells; IT: inflamed tissue; NG: necrotic glomerulus; OT: occluding tubules.
Fig. 5.
Effects of E. ivorense leaf and stem bark and P. nigrescens extracts on liver tissue of both treated and untreated Wistar rats. (G1) EIML 100 mg/kg: healthy hepatocytes and sinusoids, indicative of effective treatment response. (G2) EIML 300 mg/kg: marginal inflammatory infiltrates with healthy hepatocytes and sinusoids, indicative of recovery. (G3) EIML 1000 mg/kg: increased inflammatory infiltrates with few vacuolations and scarrings, indicative of persistent disease. (G4) EIMB 100 mg/kg: diseased liver with profuse inflammatory cell infiltration and hepatocyte necrosis. (G5) EIMB 300 mg/kg: inflamed liver with hepatocyte necrosis, suggestive of sustained hepatotoxicity. (G6) EIMB 1000 mg/kg: sustained inflammation with satellite hepatocyte necrosis, suggestive of impending hepatotoxicity. (G7) PNML 100 mg/kg: increased inflammatory infiltrates with scarring and central venous congestion suggestive of poor immunity. (G8) PNML 300 mg/kg: persistent inflammation with few scarrings and occluding vasculature, evident of sustained disease. (G9) PNML 1000 mg/kg: reduced inflammatory infiltrates with healthy hepatocytes and tissue proliferation, indicative of liver restitution. (G10) Control 000 mg/kg: normal liver with healthy hepatocytes and sinusoids. Legend: AS: apoptotic space; CV: congested vein; HN: hepatocyte necrosis; HP: hepatocytes; IC: inflammatory cells; IH: inflamed hepatocytes; RT: regenerating tissue; S: sinusoids; SN: satellite necrosis; ST: scar tissue; V: vacuolation.
3.4. Cytotoxicity studies
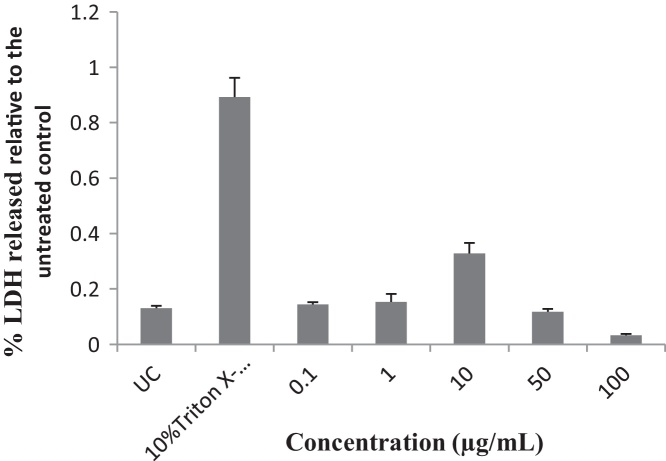
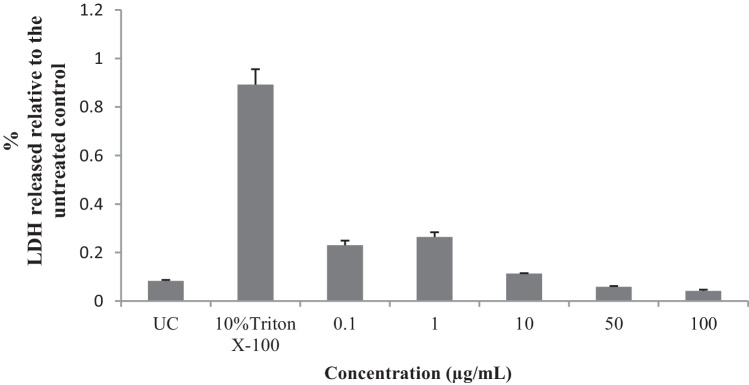
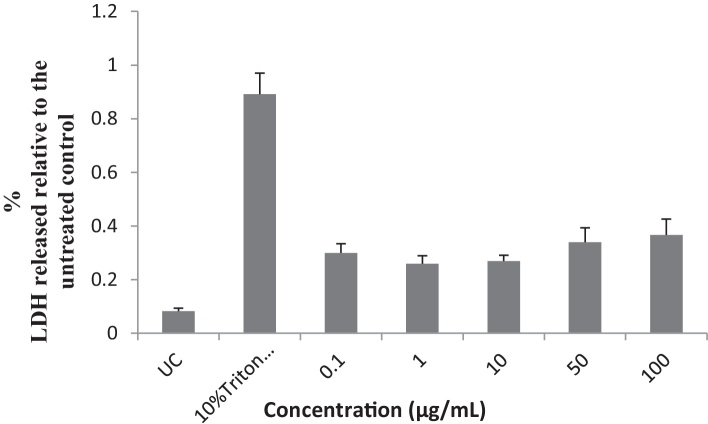
Concentrations of 0.1, 1 and 10 μg/mL of the EIML and EIMB extracts and 0.1–100 μg/mL of the PNML extracts showed increases in the amount of LDH released from the HaCaT keratinocytes as compared to the untreated cells (Fig. 6, Fig. 7, Fig. 8).
Fig. 6.
Influence of methanol leaf extract of E. ivorense on the release of LDH from HaCaT keratinocyte cells. UC, untreated cells.
Fig. 7.
Influence of methanol bark extract of E. ivorense on the release of LDH from HaCaT keratinocyte cells. UC, untreated cells.
Fig. 8.
Influence of methanol leaf and other aerial part extract of P. nigrescens on the release of LDH from HaCaT keratinocyte cells. UC, untreated cells.
3.5. Subchronic toxicity studies
Several haematological and serum biochemistry parameters were measured for possible indication or otherwise of end organ subchronic toxicity in the Wistar rats. The mean white blood cell counts measured in rats treated with the extracts were lower than the untreated rats but red blood cells, haemoglobin and haematocrits concentrations of the animals in the treated groups were higher than those of the untreated groups (Table 2). In the serum biochemistry analysis, animals treated with the methanol extracts (EIML, EIMB and PNML) showed higher concentrations of albumin, cholesterol and total bilirubin (Table 3). The values obtained for the negative controls or from the untreated animals with respect to the serum and haematological parameters investigated were consistent with reference values for rats from literature values [23], [24].
Table 2.
Effects of methanol extracts on haematological parameters of Wistar rats in subchronic toxicity studies.
| Parameters | Extracts and their concentration (mg/kg body weight) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EIML 100 | EIML 300 | EIML 1000 | EIMB 100 | EIMB 300 | EIMB 1000 | PNML 100 | PNML 300 | PNML 1000 | CONTROL | |
| WBC X 103/μL | 11.0 ± 0.50 | 11.2 ± 0.60 | 7.18 ± 0.75 | 16.2 ± 0.12 | 13.2 ± 1.00 | 12.1 ± 0.74 | 8.20 ± 0.47* | 8.16 ± 1.11* | 11.0 ± 0.25 | 12.0 ± 0.58 |
| RBC X 106/μL | 8.81 ± 0.19* | 8.43 ± 0.08* | 8.24 ± 0.12* | 8.58 ± 0.18* | 8.31 ± 0.08* | 7.74 ± 0.05 | 7.98 ± 0.12 | 8.27 ± 0.19* | 8.42 ± 0.14* | 7.25 ± 0.33 |
| HGB (g/dL) | 15.6 ± 0.27* | 15.1 ± 0.21* | 15.0 ± 0.24* | 15.5 ± 0.20* | 14.9 ± 0.12* | 14.2 ± 0.09* | 14.5 ± 0.27* | 14.9 ± 0.28* | 15.4 ± 0.31* | 13.2 ± 0.26 |
| HCT (%) | 56.3 ± 1.25* | 54.0 ± 0.54* | 54.4 ± 1.17* | 54.6 ± 0.83* | 52.6 ± 0.46* | 50.8 ± 1.25* | 50.0 ± 1.25* | 51.2 ± 1.33* | 54.2 ± 1.35* | 43.9 ± 2.15 |
| MCV (fL) | 63.9 ± 0.75 | 64.1 ± 0.55 | 66.0 ± 0.87* | 63.7 ± 0.70 | 63.3 ± 0.71 | 65.6 ± 1.64* | 62.7 ± 0.41 | 61.9 ± 0.52 | 64.3 ± 0.64 | 60.8 ± 1.27 |
| MCHC (g/dL) | 27.8 ± 0.21* | 28.0 ± 0.37* | 27.6 ± 0.37* | 28.4 ± 0.50 | 28.3 ± 0.16 | 28.1 ± 0.79 | 28.9 ± 0.69 | 29.2 ± 0.31 | 28.4 ± 0.21 | 30.0 ± 0.43 |
| PLT X 103/μL | 702 ± 56.10 | 730 ± 35.4 | 688 ± 31.8 | 839 ± 117 | 767 ± 36.7 | 647 ± 23.1 | 748 ± 32.4 | 665 ± 23.4 | 751 ± 19.2 | 596 ± 42.7 |
| LYM (%) | 78.4 ± 4.74 | 73.8 ± 1.85 | 69.3 ± 1.52 | 72.6 ± 2.60 | 69.5 ± 1.96 | 72.8 ± 7.69 | 72.8 ± 11.1 | 77.4 ± 1.78 | 74.6 ± 1.46 | 78.9 ± 1.12 |
| NEUT (%) | 21.6 ± 4.74 | 26.2 ± 1.85 | 30.7 ± 1.52* | 27.4 ± 2.60* | 30.5 ± 1.96* | 27.2 ± 7.69 | 27.3 ± 11.1* | 22.6 ± 1.78 | 25.4 ± 1.46* | 21.1 ± 1.12 |
| LYM # X 103/μL | 8.54 ± 0.48 | 8.26 ± 0.59 | 4.98 ± 0.53* | 10.2 ± 1.16 | 9.20 ± 0.94 | 8.40 ± 1.82 | 7.73 ± 1.06 | 6.32 ± 0.87 | 8.18 ± 0.35 | 9.47 ± 0.90 |
| NEUT#X 103/μL | 2.42 ± 0.58 | 2.90 ± 0.11 | 2.20 ± 0.26 | 3.97 ± 0.85 | 3.98 ± 0.17 | 2.80 ± 0.40 | 4.70 ± 3.20 | 1.84 ± 0.27 | 2.78 ± 0.14 | 2.53 ± 0.19 |
WBC – white blood cells; RBC – red blood cells; HGB – haemoglobin; HCT – haematocrit; MCV – mean corpuscular volume; MCHC – mean corpuscular haemoglobin concentration; PLT – platelets; LYM – lymphocytes; NEUT – neutrophils.
p < 0.05 (statistically significant).
Table 3.
Effects of methanol extracts on general biochemical parameters of treated and untreated Wistar rats.
| Parameters | Extracts and their concentration (mg/kg body weight) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EIML 100 | EIML 300 | EIML 1000 | EIMB 100 | EIMB 300 | EIMB 1000 | PNML 100 | PNML 300 | PNML 1000 | CONTROL | |
| Tot protein (g/L) | 92.8 ± 2.11 | 92.7 ± 1.44 | 85.1 ± 1.22 | 89.5 ± 3.31 | 82.5 ± 1.50 | 84.6 ± 3.76 | 85.7 ± 5.20 | 83.0 ± 2.50 | 85.0 ± 1.66 | 85.2 ± 3.36 |
| ALT/GPT (U/L) | 6.94 ± 1.46* | 32.6 ± 10.6* | 48.5 ± 17.2* | 34.7 ± 14.6* | 21.8 ± 6.33* | 30.5 ± 22.9* | 64.0 ± 20.3* | 47.1 ± 11.4* | 36.9 ± 9.42* | 112 ± 4.02 |
| AST/GOT (U/L) | 3.46 ± 0.61 | 3.52 ± 0.78 | 4.28 ± 1.28 | 4.13 ± 0.85 | 5.52 ± 0.53 | 4.23 ± 1.24 | 5.33 ± 0.74 | 4.26 ± 0.74 | 4.30 ± 0.86 | 4.30 ± 0.46 |
| Total bilirubin | 2.78 ± 0.11* | 2.69 ± 0.12* | 2.87 ± 0.15* | 2.66 ± 0.29* | 2.87 ± 0.20* | 2.63 ± 0.12* | 2.41 ± 0.09 | 2.36 ± 0.04 | 2.31 ± 0.11 | 1.94 ± 0.09 |
| Gamma-GT (U/L) | 4.62 ± 1.22 | 4.20 ± 0.72 | 9.22 ± 2.23 | 8.60 ± 3.86 | 7.06 ± 3.83 | 5.03 ± 1.44 | 11.1 ± 7.90 | 1.86 ± 0.56 | 4.30 ± 1.64 | 6.37 ± 2.88 |
| Creatinine (μmol/L) | 81.0 ± 4.58 | 72.5 ± 3.94 | 75.5 ± 5.46 | 73.9 ± 5.15 | 76.2 ± 1.70 | 75.8 ± 6.05 | 72.1 ± 7.73 | 71.3 ± 2.91 | 73.8 ± 1.92 | 59.7 ± 3.20 |
| Cholestrol (mmol/L) | 6.25 ± 0.13 | 6.19 ± 0.11 | 6.19 ± 0.39 | 5.97 ± 0.21 | 5.58 ± 0.16 | 5.51 ± 0.38 | 5.55 ± 0.52 | 5.72 ± 0.23 | 5.75 ± 0.11 | 5.10 ± 0.20 |
| HDL chol (mmol/L) | 1.34 ± 0.08 | 1.39 ± 0.06 | 1.40 ± 0.08 | 1.23 ± 0.10 | 1.35 ± 0.00 | 1.54 ± 0.13 | 1.27 ± 0.19 | 1.32 ± 0.04 | 1.33 ± 0.10 | 1.06 ± 0.09 |
| LDL chol (mmol/L) | 4.60 ± 0.09 | 4.50 ± 0.09 | 4.48 ± 0.26 | 4.52 ± 0.11 | 4.03 ± 0.13 | 3.70 ± 0.28 | 4.03 ± 0.31 | 4.15 ± 0.17 | 4.30 ± 0.07 | 3.58 ± 0.16 |
| Glucose (mmol/L) | 0.48 ± 0.11* | 0.33 ± 0.04* | 0.40 ± 0.07* | 0.40 ± 0.09* | 0.39 ± 0.05* | 0.25 ± 0.02* | 0.43 ± 0.11* | 0.35 ± 0.04* | 0.33 ± 0.01* | 1.17 ± 0.28 |
| Amylase (U/L) | 803 ± 55.3 | 769 ± 34.80 | 837 ± 35.50 | 807 ± 11.6 | 848 ± 35.2 | 809 ± 67.3 | 785 ± 12.0 | 751 ± 19.80 | 828 ± 7.77 | 810 ± 71.50 |
ALT/GPT – Alanine aminotransferase; AST/GOT – Aspartate aminotransferase Tot protein – total protein; Gamma GT – Gamma glutamyl transferase; HDL chol – high density lipoprotein cholesterol; LDL chol – low density lipoprotein cholesterol
p < 0.05 (statistically significant)
4. Discussion
Plants secondary metabolites are a wide range of molecules that have various pharmacological effects. These effects include therapeutic actions, defense mechanism for the plants and toxic effects on organs of animals and humans. The EIML, EIMB and PNML extracts revealed the presence of flavonoids, tannins, alkaloids and saponins (Table 1).
With respect to biochemical analysis of the blood samples of the treated animals, there were significant increases (p < 0.05) in the mean counts of red blood cells (RBC), haemoglobin (HGB) and haematocrit concentrations at all doses of administration of the extracts as compared to the untreated control group. Other studies have reported significant increases in these haematological parameters at 400, 800 and 1600 mg/kg dose levels of aqueous leaf extract of P. nigrescens [4] and at 50 and 100 mg/kg body weight of aqueous root extract of the same plant [26]. This may support the folkloric use of parts of this plant in Nigeria to treat anaemia.
The EIMB extract showed increase in WBCs and lymphocytes (p > 0.05) at dose of 100 mg/kg. The reduction in the mean counts of WBCs (p > 0.05) in rats administered with aqueous leaf extract of P. nigrescens has also been reported in previous reports [3], [26]. Owoyele et al. [27] also reported decrease in mean counts of WBCs in rats treated with lower doses of 50 and 100 mg/kg body weight of aqueous root extracts of P. nigrescens. The active principles including phenols, cardiac glycosides, terpenoids, saponins, alkaloids, tannins, and steroids may be responsible for these effects.
The various concentrations of the extracts increased the destruction of the erythrocytes or decreased its production or proliferation. Haemolytic activity provides the basic data and information on the interaction between compounds or extracts and biological agents at cellular level. Haemolytic activity of any compound or extract is an indicator of general cytotoxicity towards normal healthy cells [10]. The presence of saponins in the extracts exhibit haemolytic activity in the cells by creating changes in the erythrocyte membrane [21].
Increase in these lipid profiles have been reported for P. nigrescens aqueous root extract at 100 and 150 mg/kg body weight by other studies. Increase in lipid profile such as HDL and LDL cholesterol in the Wistar rats may be useful indicators in investigating the influence of these extracts on metabolism of lipids and how animals and humans may be prone to coronary diseases from intake of preparations from these plants [27].
The reduction in the blood glucose level (p < 0.05) from all the doses of extracts administered may justify the folkloric use of P. nigrescens as an antidiabetic agent [30]. E. ivorense should be investigated as a possible source of antidiabetic agent.
Neutrophils are the predominant granulocytes that are seen in initial stages of acute inflammation [7]. These neutrophils are packed with granules containing inflammatory factors like leukotrienes [32]. These inflammatory factors could be involved in the evidence of inflammation shown in the micrograph of tissues (Fig. 4, Fig. 5). These inflammations could cause a reduced flow of blood through kidneys [29]. The result is a surge in blood creatinine concentration (Table 3) and a decrease in the renal plasma clearance of creatinine [14]. Cheesbrough [9] showed increase in serum creatinine levels of rats treated with aqueous leaf extract of Erythrophleum africanum and the associated reduction in renal function. Since kidney and liver are organs of metabolism and excretion respectively of xenobiotic molecules such as saponins, alkaloids and tannins, the presence of these secondary metabolites in E. ivorense may be responsible for the observed hepatorenal toxicities [19].
The liver tissues of rats administered with EIML extract showed increased inflammatory infiltrates with few vacuolations and scarrings (cirrhosis), indicative of persistent damage while the EIMB extract treated rats showed liver tissue with profuse inflammatory cell infiltration and hepatocyte necrosis. The PNML extract at 1000 mg/kg body weight showed reduced inflammatory infiltrates with healthy hepatocytes and tissue proliferation, indicative of liver restitution (Fig. 5, G9).
Alanine transaminase (ALT), aspartate transaminase (AST) and γ-glutamyl (GGT) are enzymes found in the cytoplasm of cells and they are involved in amino acid metabolism but only released into systemic circulation after cells have been damaged [8], [31]. Hassan et al. [19] reported increases in serum levels of ALT and AST in treating rats with 2000–3000 mg/kg bwt of E. africanum extracts and suggested that the extracts must have affected the permeability of liver cell membranes and made them leaky, thus the leakage of ALT and AST to raise their serum levels. This was observed in the histopathology findings for the kidney and liver of animals treated with the various doses of the extracts with the exception of 100 mg/kg body weight of EIML (Fig. 4, Fig. 5) which had no effect on both organs. An increase in the level of ALT and AST in blood serum of treated rats above normal ranges in the untreated rats may explain the liver damages by the methanol extracts (EIML, EIMB and PNML).
Spier et al. [33] reported that hydrolysable tannins which are astringents bind to proteins in plasma and body organs resulting in coagulation and necrosis. Blood from all the animals treated with the extracts (EIML, EIMB and PNML) showed increase in serum total protein concentration. Hassan et al. [19] has also reported increases in the concentration of serum total protein in rats administered with extract of E. africanum. The increases in serum total protein concentration have been attributed to liver injury and hepatic toxicity [17], [13]. The methanol extracts (EIML, EIMB and PNML) may therefore be toxic to animals due to the measured increase in serum total protein concentration. The increasing doses of the extracts (EIML, EIMB and PNML) used in the study lead to increase and pronounced toxicity in both livers and kidneys of all the treated animals.
When cells are stressed, lysed or injured, they lose the integrity of their cytoplasmic membrane. Lactate dehydrogenase (LDH) enzymes which are found in their cytosol can be measured extracellularly as they leak through the disrupted cytoplasmic membrane. This study showed an increase in the amount of LDH released though not statistically significant (p > 0.05) from the keratinocyte cells treated with concentrations of 1–10 μg/mL of the EIML and EIMB extracts and 1–100 μg/mL of the PNML extracts as compared with the untreated cells (negative control). Triton X-100 is a cell lysing agent and thus HaCaT keratinocytes treated with it showed marked release of LDH enzymes, evident of pronounced cell damage but not as compared to the extract treated cells. The synergistic effects of plant metabolites may sometimes be toxic and cause damage to cells treated with them. The methanol extracts of these plant materials may thus possess compounds that may be toxic to morphology and function of cells of humans and animals. This cytotoxic property may support the overall in vivo toxicity observed in the kidney and liver sections of the Wistar rats. There was reduction in the release of LDH as the concentration of the extracts increases and this may be due the presence of bioactive agents in the extracts which in higher concentrations may serve as protective mechanism for the cells or delay or reduce apoptosis. There is a need to identify and characterize the individual compounds or agents and the subsequent protective mechanism to cell damage by higher concentrations of the extracts.
5. Conclusion
Methanol leaf and bark extracts of E. ivorense and leaf and other aerial part extract of P. nigrescens may exhibit dose and time dependent toxicity to animals and humans. The kidney and liver tissues from rats administered with the extracts showed diseased conditions including inflammation of cells, necrotic tissues and infiltration cells. The extracts exhibited cytotoxicity on HaCaT keratinocytes at low concentrations but not statistically significant.
Acknowledgements
We are grateful to Dr. Alex Asase and Mr. John Yaw Amponsah of the Department of Botany, University of Ghana, Accra and Mr. Eric Gyebi, for the identification and collection of the plant materials. We thank Mr. Thomas Ansah, Department of Pharmacology for the technical assistance in the animal studies. We thank Prof. Dr. Andreas Hensel, Institute for Pharmaceutical Biology and Phytochemistry, University of Muenster, Germany for the cytotoxicity studies. We also thank Dr. Paul Poku Sampane Ossei, a consultant pathologist and Head of Department of Pathology, Komfo Anokye Teaching Hospital, Kumasi, Ghana for the revision of the histopathological report.
Footnotes
Available online 3 July 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.06.009.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Abu-Rabia A. Urinary diseases and ethnobotany among pastoral nomads in the Middle East. J. Ethnobiol. Ethnomed. 2005;1(1):4. doi: 10.1186/1746-4269-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adeoye B.A., Oyedapo O.O. Toxicity of Erythrophleum guineense stem-bark: role of alkaloidal fractions. Afr. J. Tradit. CAM. 2004;1(1):45–54. [Google Scholar]
- 3.Agbor G.A., Odetola A.A. Haematological studies of Parquetina nigrescens on haemorrhagic anaemic rats. Afr. J. Med. Med. Sci. 2001;30(1–2):105–109. [PubMed] [Google Scholar]
- 4.Agbor G.A., Odetola A.A. Effect of Parquetina nigrescens on erythrocyte indices and serum electrolytes of rats following acute blood loss. Pak. J. Biol. Sci. 2005;8(4):527–531. [Google Scholar]
- 5.Agyare C., Asase A., Lechtenberg M., Niehues M., Deters A., Hensel A. An ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing in Bosomtwi-Atwima-Kwanwoma area, Ghana. J. Ethnopharmacol. 2009;125(3):393–403. doi: 10.1016/j.jep.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Agyare C., Lechtenberg M., Deters A., Petereit F., Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine. 2011;18(7):617–624. doi: 10.1016/j.phymed.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 7.Alberts B. Leukocyte functions and percentage breakdown. Mol. Biol. Cell. 2005;4:41–43. [Google Scholar]
- 8.Burkill H.M. 2nd ed. vol. 3. Families J–L. Royal Botanic Gardens; Kew, Richmond, United Kingdom: 1995. p. 857. (The Useful Plants of West Tropical Africa). [Google Scholar]
- 9.Cheesbrough M. 2nd ed. vol. 2. ELBS; Cambridge: 1991. pp. 508–511. (Medical Laboratory Manual for Tropical Countries). [Google Scholar]
- 10.Da Silva E., Shahgaldian P., Coleman A.W. Haemolytic properties of some water-soluble para-sulphonato-calix-[n]-arenes. Int. J. Pharm. 2004;273(1–2):57–62. doi: 10.1016/j.ijpharm.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 11.Ding Y., Wu E.Q., Liang C., Chen J., Tran M.N., Hong C.H., Jang Y., Park K.L., Bae K.H., Kim Y.H., Kang J.S. Discrimination of cinnamon bark and cinnamon twig samples sourced from various countries using HPLC-based fingerprint analysis. Food Chem. 2011;127(2):755–760. doi: 10.1016/j.foodchem.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Dongmo A.B., Kamanyi A., Anchang M.S., Chungag-Anye Nkeh B., Njamen D., Nguelefack T.B., Nole T., Wagner H. Anti-inflammatory and analgesic properties of the stem bark extracts of Erythrophleum suaveolens (Caesalpiniaceae). Guillemin and Perrottet. J. Ethnopharmacol. 2001;77(2):137–141. doi: 10.1016/s0378-8741(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 13.Emerson F.S., Sharada A.C., Devi P.U. Toxic effects of crude root extract of Plumbago rosea (Rakta chitraka) on mice and rats. J. Ethnopharmacol. 1993;38(1):79–84. doi: 10.1016/0378-8741(93)90081-f. [DOI] [PubMed] [Google Scholar]
- 14.Fox S.I. 8th ed. McGraw-Hill Publishers; Boston, MA, USA: 2003. Human Physiology; p. 551. [Google Scholar]
- 15.Garber J.C., Barbee R.W., Bielitzki J.T., Clayton L.A., Donovan J.C., Hendriksen C.F.M., Kohn D.F., Lipman N.S., Melcher J., Quimby F.W. vol. 8. The National Academic Press; Washington, DC: 2010. p. 220. (Guide for the care and use of laboratory animals). [Google Scholar]
- 16.Harborne J.B. 3rd ed. Chapman and Hall; London: 1988. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 17.Gatsing D., Aliyu R., Kuiate J.R., Garba I.H., Jaryum K.H., Tedongmo N., Tchouanguep F.M., Adoga G.I. Toxicological evaluation of the aqueous extract of Allium sativum bulbs on laboratory mice and rats. Cameroon J. Exp. Biol. 2006;1(1):39–45. [Google Scholar]
- 18.Guntupalli M.M., Chandana V.R., Pushpagadarn P., Shllwaikar A. Hepatoprotective effects of rubiadin, a major constituents of Rubia cordifolia L. J. Ethnopharmacol. 2006;103:484–490. doi: 10.1016/j.jep.2005.08.073. [DOI] [PubMed] [Google Scholar]
- 19.Hassan S.W., Ladan M.J., Dogondaji R.A., Umar R.A., Bilbis L.S., Massan L.G., Matazu I.K. Phytochemical and toxicological studies of aqueous leaves extracts of Erythrophleum africanum. Pak. J. Biol. Sci. 2007;10:3815–3821. doi: 10.3923/pjbs.2007.3815.3821. [DOI] [PubMed] [Google Scholar]
- 20.Irvine F.R. Oxford University Press; London: 1961. Woody Plants of Ghana. [Google Scholar]
- 21.Kumar G., Karthik L., Rao V.B. Haemolytic activity of Indian n=medicinal plants towards erythrocytes: an in vitro study. Elixir Appl. Bot. 2011;40:5534–5537. [Google Scholar]
- 22.Kunwar R.M., Uprety Y., Burlakoti C., Chowdhary C.L., Bussmann R.W. Indigenous use and ethnopharmacology of medicinal plants in Far-west Nepal. Ethnobot. Res. Appl. 2009;7:005–028. [Google Scholar]
- 23.Lang P.L. Charles Rivers Laboratories; MA: 1993. Hematology Parameters for the CrI: CD® BR Rat. http://www.criver.com/files/pdfs/rms/cd/rm_rm_r_hematology_parameters_crIcd_br_rat.aspx (accessed June, 2014) [Google Scholar]
- 24.Meingassner J.G., Schmook F.P. vol. 3, No. 1., Charles Rivers Laboratories; MA: 1990. (Reference Values for Cr I: CD® (SD) BR Rats). No. 1; http://legacy.library.ucsf.edu/tid/qab37e00/pdf (accessed in June, 2014) [Google Scholar]
- 25.Nath S., Choudhury M.D., Rouchoudhury S., Talukar D., Sirokin A.V., Bokova Z., Kadasi A., Marumiakova N., Kolesarova A. Restorative aspect of castor plant on mammalian physiology: a review. J. Microbiol. Biotechnol. Food Sci. 2011;1(2):236–246. [Google Scholar]
- 26.Nsiah K., Terlabi E.O., Woode E., Obiri D.D., Ansah C., Duwiejua M. Toxicological assessment of Parquetina nigrescens extracts in rats. J. Sci. Technol. (Ghana) 2006;26(3):24–31. [Google Scholar]
- 27.Owoyele B.V., Oyelowo O.T., Biliaminu S.A., Alaran O.N., Alimi S.A., Saliu R.S. Haematological and biochemical studies on Parquetina nigrescens root extract in albino rats. J. Appl. Pharm. Sci. 2011;1(10):176–179. [Google Scholar]
- 28.Parekh J., Karathia N., Chanda S. Screening of some traditionally used medicinal plants for potential antibacterial activity. Indian J. Pharm. Sci. 2006;68(6):832–834. [Google Scholar]
- 29.Rosner M.H., Okusa M.D. Acute kidney injury associated with cardiac surgery. Clin. J. Am. Soc. Nephrol. 2006;1(1):19–32. doi: 10.2215/CJN.00240605. [DOI] [PubMed] [Google Scholar]
- 30.Saba A.B., Oyagbemi A.A., Azeez O.I. Antidiabetic and haematinic effects of Parquetina nigrescens on alloxan induced type-1 diabetes and normocytic normochromic anaemia in Wistar rats. Afr. Health Sci. 2010;10(3):276–282. [PMC free article] [PubMed] [Google Scholar]
- 31.Sallie R., Tredger M.J., Williams R. Drugs and the liver: testing liver function. Biopharm. Drug Dispos. 1991;12(4):251–259. doi: 10.1002/bdd.2510120403. [DOI] [PubMed] [Google Scholar]
- 32.Shen W.C., Louie S.G. CRC Press, Francis and Taylor Publishers, E-book; Boca Raton, FL, USA: 2005. Immunology for Pharmacy Students; p. 2. [Google Scholar]
- 33.Spier S.J., Smith B.P., Seawright A.A. Oak toxicosis in cattle in Northern California: clinical and pathological findings. J. Am. Vet. Med. Assoc. 1987;191(8):959–964. [PubMed] [Google Scholar]
- 34.Srivastava A., Mishra H., Verma R.K., Gupta M.M. Chemical fingerprinting of Andrographis paniculata using HPLC, HPTLC and densitometry. Phytochem. Anal. 2004;15(5):280–285. doi: 10.1002/pca.779. [DOI] [PubMed] [Google Scholar]
- 35.Todd M.D., Lin X., Stankowski L.F., Jr., Desai M., Wolfgang G.H. Toxicity screening of a combinatorial library: correlation of cytotoxicity and gene induction to compound structure. J. Biomol. Screen. 1999;4(5):259–268. doi: 10.1177/108705719900400507. [DOI] [PubMed] [Google Scholar]
- 36.Trease G.E., Evans W.C. 16th ed. Elsevier Limited; London: 2009. A Textbook of Pharmacognosy. [Google Scholar]
- 37.Wasfi I.A., Bashir A.K., Arniri M.H., Abdalla A.A. National Committee for Clinical Laboratory Standard (Approved standard M2-A3); Villano and Pennsylvania M2-A3: 1994. Performance Standard for Antimicrobial Disk Susceptibility Tests. [Google Scholar]
- 38.Wolf P.L. Biochemical diagnosis of liver disease. Indian J. Clin. Biochem. 1999;14(1):59–90. doi: 10.1007/BF02869152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zirihi G.N., Mambu L., Guede-Guina F., Bodo B., Grellier P. In vitro antiplasmodial activity and cytotoxicity of 33 West African plants used for treatment of malaria. J. Ethnopharmacol. 2005;98(3):281–285. doi: 10.1016/j.jep.2005.01.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.