Abstract
Zinc oxide (ZnO) nanoparticles have been widely used in industry, cosmetics, and biomedicine. Recent studies suggested that these nanoparticles could have a major impact on the cardiovascular system. Endothelial progenitor cells (EPCs) contribute to postnatal endothelial repair and regeneration. The present study dissected the effects of ZnO nanoparticles on vasculogenesis using human endothelial colony forming cells (ECFCs), which participate in post-natal vasculogenesis. Two types of ZnO particles were used (nano and micro), in addition to zinc chloride solutions with zinc ion concentrations equal to those in ZnO nanoparticles. Twenty-four-hour exposure induced cytotoxicity in a dose-dependent manner and increased ECFCs apoptosis in all groups. The exposure also reduced the functional capacity of ECFCs on Matrix gel to form tubules, compared with the control cells. These effects were associated with downregulation of expression of vascular endothelial growth factor receptor, VEGFR2 and CXC chemokine receptor, CXCR4. The results suggest that ZnO nanoparticles suppress vasculogenesis from ECFCs through downregulation of the expression of receptors related to vasculogenesis. These effects are based the concentration of released Zn(II).
Abbreviations: CXCR4, CX chemokine receptor; ECFCs, endothelial colony forming cells; EPCs, endothelial progenitor cells; ICP-AES, inductively coupled plasma atomic emission spectrometry; PdI, polydispersity index; VEGFR2, vascular endothelial growth factor A receptor 2; ZnCl2, zinc chloride; ZnO, zinc oxide
Keywords: Nanoparticles, Zinc oxide, Vasculogenesis, Endothelial progenitor cells, Tube formation, VEGFR2, CXCR4
1. Introduction
The recent explosive development in the field of nanotechnology provides potentially promising applications for manufactured nanomaterials in a variety of fields. The rapidly developing field of nanotechnology potentially exposes human to engineered nanoparticles through different routes, including inhalation (respiratory tract), ingestion (gastrointestinal tract), dermal (skin), and injection (blood circulation) [1]. Therefore, the establishment of a new discipline of nanotoxicology is needed to address the new potential threats associated with the widespread use of new nanoparticles, which should provide support for any further growth of a safe and sustainable nanotechnology industry, since nanoparticles could, at least theoretically, have adverse effects on the lungs and other organs [2].
The relative surface area of particles correlates with the degree of their toxicity [3]. Furthermore, nanoparticles can generate reactive oxygen species, resulting in induction of oxidative stress and inflammation [4], [5]. Zinc oxide (ZnO) nanoparticles are among the most commonly used nanomaterials, and are widely used in paint, pharmaceutical, and cosmetics industries, in addition to biomedicine. Recent studies reported worrying harmful effects for ZnO nanoparticles; they have been demonstrated to increase the expression of adhesion molecules in vascular endothelial cells [6], [7] and to induce infiltration of macrophages and lipid accumulation in the intimal and medial areas of the arterial wall in an animal model [8]. Our group has also showed that ZnO nanoparticles increase macrophage cholesterol uptake [9]. Considered together, the new data suggest that ZnO nanoparticles could potentially increase the risk of cardiovascular diseases, including atherosclerosis.
Endothelial progenitor cells (EPCs) are one subset of progenitor cells that originate in the bone-marrow and are mobilized to the post-natal circulation. These cells can differentiate into mature endothelial cells and home to sites of tissue injury where they play an important role in facilitating vascular repair and tissue regeneration [10]. Previous studies have demonstrated a clear link between reduced numbers and dysfunctionality of circulating EPCs and increased cardiovascular risk [11]. Indeed, EPC dysfunction may not only be a marker of cardiovascular risk, but may also represent a pathophysiologic link between cardiovascular risk factors and the development of atherosclerosis [12]. Evidence based on epidemiological and toxicological studies indicates that high concentrations of particle masses <2.5 μm (PM2.5) are associated with high risk of cardiovascular events and death from cardiovascular diseases [13], [14]. The endothelium is a sensitive target of PM, and animals and humans exposed to PM show endothelial dysfunction [15] and EPC depletion [16], suggesting that PM exposure can potentially harm the endothelium by causing acute and direct loss of flow-mediated dilation and attenuation of EPC-mediated endothelial repair and regeneration [17].
ZnO nanoparticles can potentially increase the incidence of cardiovascular diseases, although the mechanism(s) behind this relation remain unclear. Our working hypothesis is that exposure to ZnO nanoparticles results in functional impairments of EPCs. The present study dissected the effects of ZnO nanoparticles on endothelial tubule formation, using an in vitro set-up of human endothelial colony forming progenitor cells (ECFCs). These cells are commonly used in vascular regenerative medicine and regarded as a promising target for antiangiogenic tumor therapy due to their robust proliferative potential and profound vessel-forming capacity [18]. The study also determined the role of Zn(II) ion on EPCs function using ZnO nano- and micro-particles and zinc chloride (ZnCl2).
2. Methods
2.1. Nanoparticle preparation and characterization
Nano-sized ZnO particles (ZnO-n1) (MKN-ZnO-020; mkNano, Mississauga, ONT, Canada) and (ZnO-n2) (351-34492; WAKO Pure Chemical Industries, Osaka, Japan) with primary diameter of 20 nm, micro-sized ZnO particles (ZnO-micro) (WAKO Pure Chemical Industries) with primary diameter of 5 μm, and zinc chloride (ZnCl2) (Sigma–Aldrich, St. Louis, MO) were used in the present study. We previously characterized TiO2 and ZnO nanoparticles from the same lot by not only dynamic light scattering (DLS), but also transmission electron microscope (TEM), and then established a suitable and reproducible protocol for the preparation of suspension of TiO2 and ZnO nanoparticles [19]. As described in this paper, nanoparticles were suspended in serum containing culture media and dispersed using a sonicator (Branson Sonifier model 450, Danbury, CT, 80% pulsed mode, 100 W, 15 min). The hydrodynamic size of the particles in media was measured four times after 1 h on standing using DLS technology with a Zetasizer Nano-S (Malvern Instruments, Worcestershire, UK). Dispersion status was described by the intensity-weighted hydrodynamic average diameter (z-average) and polydispersity index (PdI), which reflect the broadness of the size distribution (scale range from 0 to 1, with 0 being monodispersion and 1 being polydispersion).
2.2. Cell culture and cytotoxicity assay
Human circulating EPCs with in vitro clonogenic capacity, also known as endothelial colony-forming cells (ECFCs), obtained from Lonza Group (Basel, Switzerland), were cultured in endothelial growth medium (EGM-2 BulletKit, Lonza Group) at 37 °C in 5% CO2. The ECFCs were isolated from the primary cord blood mononuclear cells. Cells were passaged with trypsin-EDTA, trypsin neutralizing solution and Hanks’ Balanced Salt Solution (HBSS) (Life Technologies, Carlsbad, CA) every 2–3 days and experiments were performed using ECFCs between passages 6 and 8.
ECFCs were seeded overnight at 1.5 × 104 cells per well on 96-well plates before the experiment. Particles were dispersed in serum containing cell culture medium, the final concentration of the particles ranged from 1 to 100 μg/ml. The concentration range is corresponding with the dose used in previous studies using human aortic endothelial cells (HAECs) [6] and human umbilical vein endothelial cells (HUVECs) [9] exposed to ZnO nanoparticles. It was assumed that Zn(II) concentration in 25 mg/ml of ZnO nanoparticles was equal to that in 307 mM (41.8 mg/ml) of ZnCl2 based on the data reported in a previous paper [20], and calculated the concentration of ZnCl2 corresponding to the nanoparticles. Cytotoxicity was determined after incubation of the dispersed ZnO particles or dissolved ZnCl2 for 24 h, by MTS assay as indicated by the CellTiter 96 AQueous One Solution (Promega, Madison, WI), which is a colorimetric method for determining the number of viable cells. The serum containing cell culture medium was used during incubation with the particles. After 24-h incubation, the cells were incubated with fresh medium (phenol red-free) containing MTS reagent for 1 h before absorbance measurements at 490 nm. The effect of particles on cell proliferation was calculated as percentage of inhibition of cell growth relative to the control.
2.3. Inductively coupled plasma-atomic emission spectroscopy (ICP-AES)
The stock suspensions were diluted to a final volume of 15 ml EGM-2 complete to a final concentration of 1, 10, or 100 μg/ml ZnO particles. In the same way, 1.67, 16.7, or 167 μg/ml ZnCl2 solution was prepared. Each sample was incubated at 37 °C in 5% CO2 for 0, 1, 6, and 24 h. After centrifugation for 20 min at 10,000 × g, 10 ml of the supernatant was transferred into a test tube that contained 0.5 ml of concentrated nitric acid (HNO3; Wako Pure Chemical Industries). The solution was then subjected to zinc analysis by inductively coupled plasma atomic emission spectrometry (ICP-AES), as described previously [21]. The residual 5 ml containing the precipitate (ZnO nanoparticles that were left in dispersion medium) was acidified with 0.5 ml of concentrated nitric acid, transferred into a tube and treated with 60% perchloric acid until all contents were dissolved. The resulting solution was filled up to 50 ml with water and analyzed for zinc contents using ICPS-7500 (Shimadzu, Kyoto, Japan).
2.4. Tubule formation assay
ECFCs (5.0 × 104 cells) were placed in 24-well plates pre-coated with solidified Matrigel Matrix (BD Biosciences, San Jose, CA) and exposed to different doses (10 or 25 μg/ml) of the dispersed ZnO particles or dissolved 16.7 or 41.8 μg/ml ZnCl2 for 24 h at 37 °C. The cells were stained with CalceinAM (BD Biosciences) and tubule formation was examined under a fluorescence microscope (×40) (model FSX100, Olympus, Tokyo), as described previously [22]. Six representative fields were selected and the length or area of complete tubes formed by cells was measured by a computer digital imaging software (CellSens Software, Olympus).
2.5. Apoptosis assay
ECFCs were seeded overnight at 2.0 × 105 cells per well on 24-well plates before the experiment. After incubation with the dispersed ZnO particles or dissolved ZnCl2 for 24 h, the cells were harvested with EDTA-trypsin, washed twice with PBS, and stained with fluorescein isothiocyanate (FITC)–annexin V and propidium iodide using TACS Annexin V apoptosis detection kit (Trevigen, Gaithersburg, MD). The labeled cells were analyzed by flow cytometry (FACS Canto II, BD Biosciences). The fluorescent signal was detected through the FITC channel with at least 10,000 events acquired for each sample.
2.6. RNA isolation and analysis of RT-PCR
ECFCs (2.0 × 105 cells) were grown overnight in 12-well plates at 37 °C, and then exposed to 25 μg/ml of the dispersed ZnO particles or dissolved ZnCl2 for 1, 3, or 6 h at 37 °C. Total RNA from the cells was isolated by using ReliaPrep RNA cell miniprep system (Promega) using the protocol provided by the manufacturer. The concentration of total RNA was quantified by spectrophotometry (ND-1000; NanoDrop Technologies, Wilmington, DE). RNA was reverse transcribed to single-strand cDNA using SuperScript III First-Strand Synthesis System for RT-PCR (Life Technologies). cDNA (n = 4 in each group) was subjected to quantitative PCR analysis with FastStart Universal Probe Master Mix (Roche, Basel, Switzerland) using primers for human vascular endothelial growth factor A receptor 2, VEGFR2, CX chemokine receptor, CXCR4, and phosphatase and tensin homolog deleted on chromosome 10, PTEN, using an ABI 7000 Real-Time PCR system (Life Technologies), as described previously [23]. The gene expression level was normalized to that of β-actin in the same cDNA.
2.7. Statistical analysis
All parameters were expressed as mean ± standard deviation (SD). Statistical analyses were performed using one-way analysis of variance (ANOVA) followed by Dunnett's post hoc test. A p value less than 0.05 was considered statistically significant.
3. Results
3.1. Characterization of nanoparticles in suspension
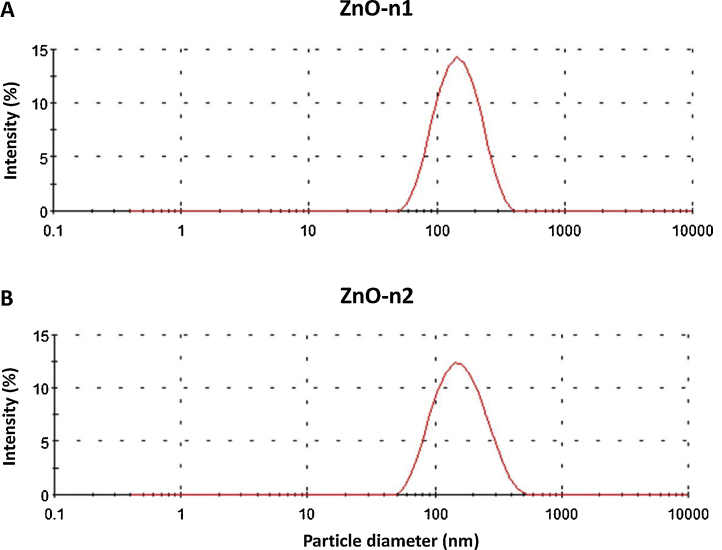
Both nano-sized ZnO particles were dispersed in the culture medium of ECFCs. The intensity-weighted hydrodynamic mean diameter of the dispersed nanoparticles was measured by DLS technology. Table 1 shows the mean hydrodynamic diameter and PdI of dispersed ZnO nanoparticles in the medium. The analysis software of DLS provided the value for the size distribution based on number and volume of particles. Based on the observed dispersion, the presence of nano-sized particles was confirmed in the medium (Fig. 1); the number of particles measuring less than 100 nm was 83.4 ± 2.3% and 78.7 ± 1.5% and the volume of particles measuring less than 100 nm was 43.2 ± 1.7% and 42.7 ± 1.7%, for ZnO-n1 and ZnO-n2 nanoparticles, respectively. Both ZnO-n1 and ZnO-n2 nanoparticles were dispersed to a similar extent.
Table 1.
Characterization of nano-sized ZnO particles.
| Particles | Primary diameter (nm) | Medium | Hydrodynamic size (nm) | PdI |
|---|---|---|---|---|
| ZnO-n1 | 20 | EGM-2 (12% FBS) | 116.7 ± 1.3 | 0.282 ± 0.006 |
| ZnO-n2 | 20 | EBM-2 (12% FBS) | 116.6 ± 0.7 | 0.171 ± 0.007 |
Data are mean ± SD of four independent experiments.
PdI: polydispersity index, EGM: endothelial growth medium, FBS: fetal bovine serum.
Fig. 1.
Histogram of particle size distribution measured by dynamic light scattering technology. (A) ZnO-n1 and (B) ZnO-n2 nanoparticles suspensions were dispersed using a sonicator at 100 W, 80% pulse mode, for 15 min.
3.2. Amount of zinc analyzed by ICP-AES
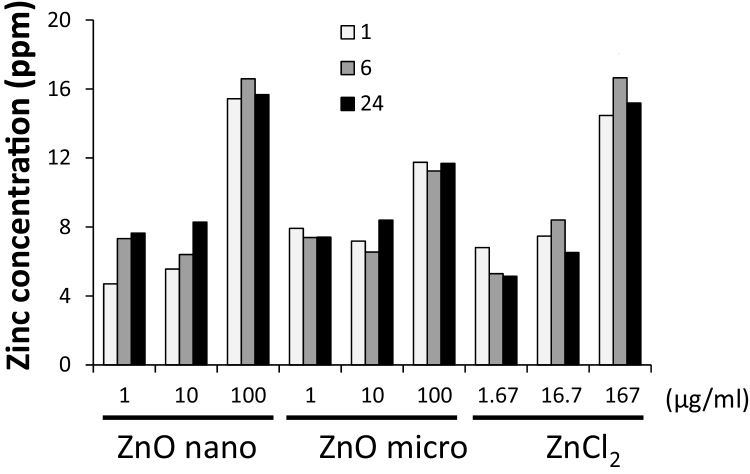
ICP-AES was used to analyze the amount of zinc in the supernatant and precipitate of the dispersed ZnO particles and dissolved ZnCl2 solution. The total amount of zinc was almost the same in the dispersed 1 or 10 μg/ml of ZnO-n1 and ZnO-micro particles and the corresponding concentration (1.67 or 16.7 μg/ml) of ZnCl2 solution (Fig. 2). The results also demonstrated that the total amount of zinc was almost the same in the solutions of dispersed 100 μg/ml of ZnO-n1 nanoparticles and the corresponding concentration of ZnCl2 (167 μg/ml). The amount of zinc was lower in the solution of 100 μg/ml of ZnO-micro particles than in the solution of dispersed 100 μg/ml of ZnO-n1 nanoparticles. However, since zinc amount was not measured as appropriate replicates in the present study, there may be no ability to comment on variability or perceived differences.
Fig. 2.
Zinc ion content in the supernatant and precipitate of solutions dispersed ZnO particles or dissolved ZnCl2. Results are ICP-AES measurements of supernatants and precipitates of 1, 10, 100 μg/ml of ZnO-n1 nanoparticles, ZnO-micro particle, and dissolved ZnCl2 at the corresponding concentrations for 1, 6, and 24 h.
3.3. Cytotoxicity
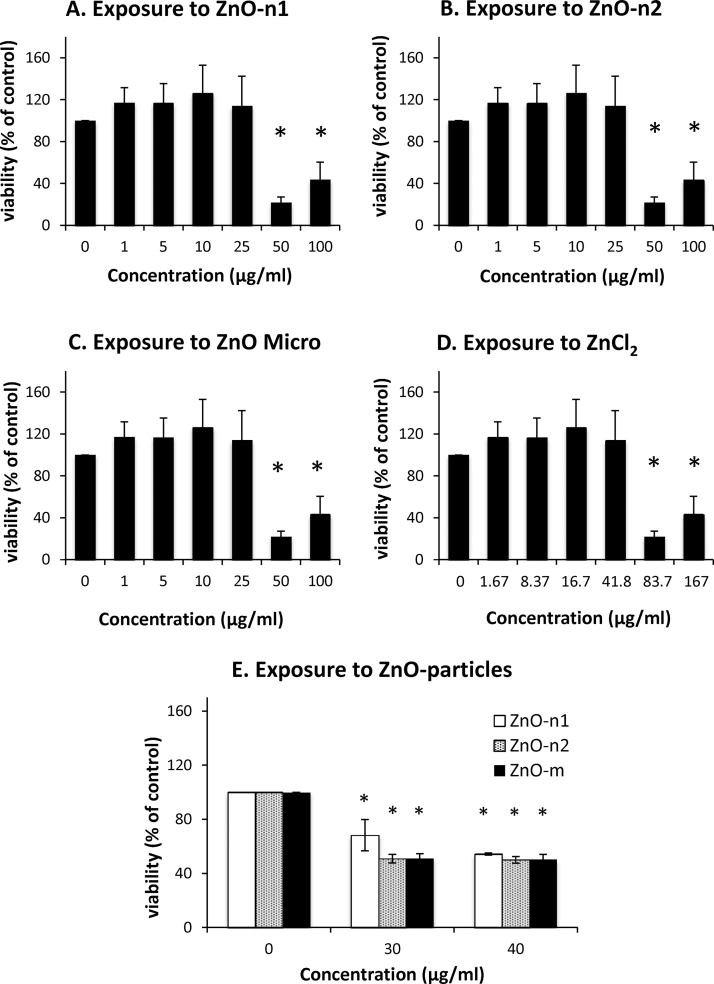
ECFCs were exposed for 24 h to the dispersed ZnO particles at a concentration ranging from 1 to 100 μg/ml and dissolved ZnCl2 at the corresponding concentrations. The MTS assay showed that incubation of ECFCs in the presence of nano- and micro-sized ZnO particles at 50–100 μg/ml significantly induced cytotoxicity (Fig. 3A–C). Cytotoxicity was also significantly induced after exposure to the corresponding concentration of 50 or 100 μg/ml (83.7 or 167 μg/ml) of ZnCl2 (Fig. 3D). The MTS assay showed that incubation of ECFCs in the presence of ZnO particles at more than 30 μg/ml significantly induced cytotoxicity (Fig. 3E). Therefore, we used particle concentrations of 10 and 25 μg/ml and the corresponding concentration (16.7 or 41.8 μg/ml) of ZnCl2 to analyze tube formation.
Fig. 3.
Effects of dispersed ZnO particles and dissolved ZnCl2 on cytotoxicity. Cytotoxicity was measured by MTS assay. ECFCs were exposed to (A) ZnO-n1 and (B) ZnO-n2 nanoparticles and (C) ZnO-micro particle at concentrations ranging from 1 to 100 μg/ml, (D) ZnCl2 at concentrations ranging from 1.67 to 167 μg/ml, and (E) ZnO particles at the concentration of 30 and 40 μg/ml for 24 h. Data are mean ± SD of six replicates. *p < 0.05 vs. control (0 μg/ml).
3.4. Effects of ZnO particles on tubule formation
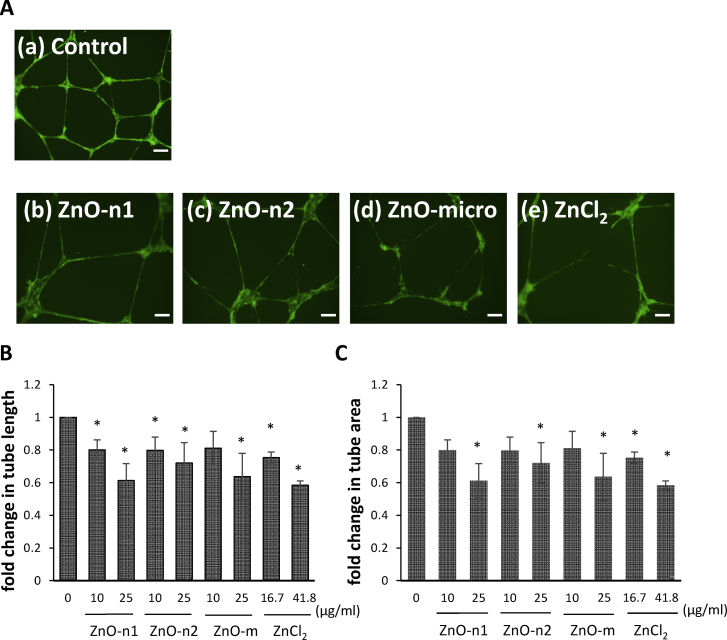
To determine the effects of dispersed ZnO particles and dissolved ZnCl2 on angiogenesis, we evaluated their effects on tubule formation. Under control conditions, ECFCs cells formed a well organized tubule network (Fig. 4A(a)). However, exposure of ECFCs to the dispersed ZnO particles and dissolved ZnCl2 resulted in marked disruption of the tubule network (Fig. 4A(b)–(e)). The tubule length was significantly lower in ECFCs exposed to 10 or 25 μg/ml of ZnO-n1 and ZnO-n2 nanoparticles and ZnO-micro particles, and the corresponding concentration (16.7 or 41.8 μg/ml) of ZnCl2 than in the control (Fig. 4B). Furthermore, the tubule area was significantly lower in ECFCs exposed to 25 μg/ml of ZnO-n1 and ZnO-n2 nanoparticles and ZnO-micro particle and the corresponding concentration (41.8 μg/ml) of ZnCl2 than in the control (Fig. 4C). The tubule area was significantly smaller even after exposure to 16.7 μg/ml of ZnCl2. Quantification of tubule formation confirmed the microscopic observation on the effects of the dispersed ZnO particles and dissolved ZnCl2 exposure on angiogenesis.
Fig. 4.
Tubule formation assay of ECFCs. (A) Representative images of tubule formation from ECFCs exposed to 25 μg/ml of ZnO particles or the corresponding concentration of ZnCl2 for 24 h. Scale bar = 200 μm. (B) Tubule length and (C) tubule area from ECFCs exposed to 10 or 25 μg/ml of the dispersed ZnO particles and corresponding concentrations of dissolved ZnCl2. Six representative fields were selected in each culture well and the length or area of complete tubes formed was measured by a computer digital imaging software. Data are mean ± SD from minimum three cell culture wells of 1–3 independent experiments. *p < 0.05 vs. control (0 μg/ml).
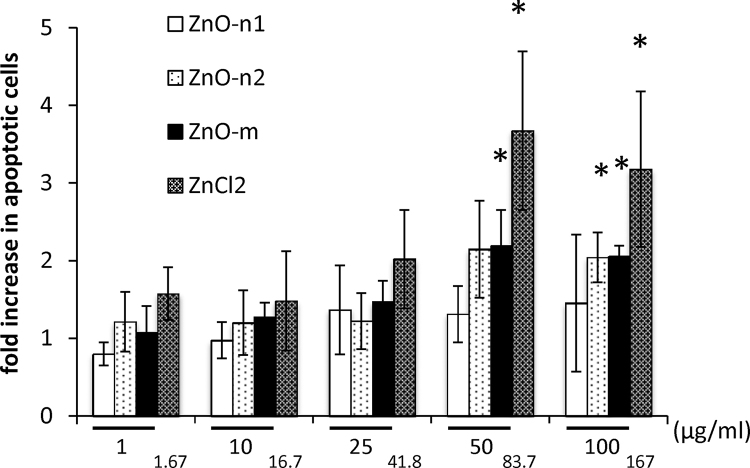
3.5. Effects of ZnO particles on apoptosis
Annexin V staining of ECFCs (annexin-V positive and propidium-iodide negative/annexin-V positive and propidium-iodide positive) demonstrated apoptosis of a proportion of cells among the adherent cells at 24 h after exposure to 50 and 100 μg/ml of ZnO-micro particles and the corresponding concentration of ZnCl2 (Fig. 5). The fold increase in this population of annexin-V positive apoptotic cells was especially significant after exposure of ECFCs to 16.7 μg/ml of ZnO-n2 nanoparticles, compared with the control (Fig. 5). The effects of ZnCl2 were much more pronounced as compared to the other groups in particular at the concentration of 50 and 100 μg/ml.
Fig. 5.
Effects of ZnO on apoptosis of ECFCs assessed by flow cytometry. ECFCs were exposed to ZnO-n1, ZnO-n2 nanoparticles and ZnO-micro particle at concentrations ranging from 1 to 100 μg/ml, or to ZnCl2 at the indicated concentrations for 24 h. Data are mean ± SD of four replicates. *p < 0.05 vs. control (0 μg/ml).
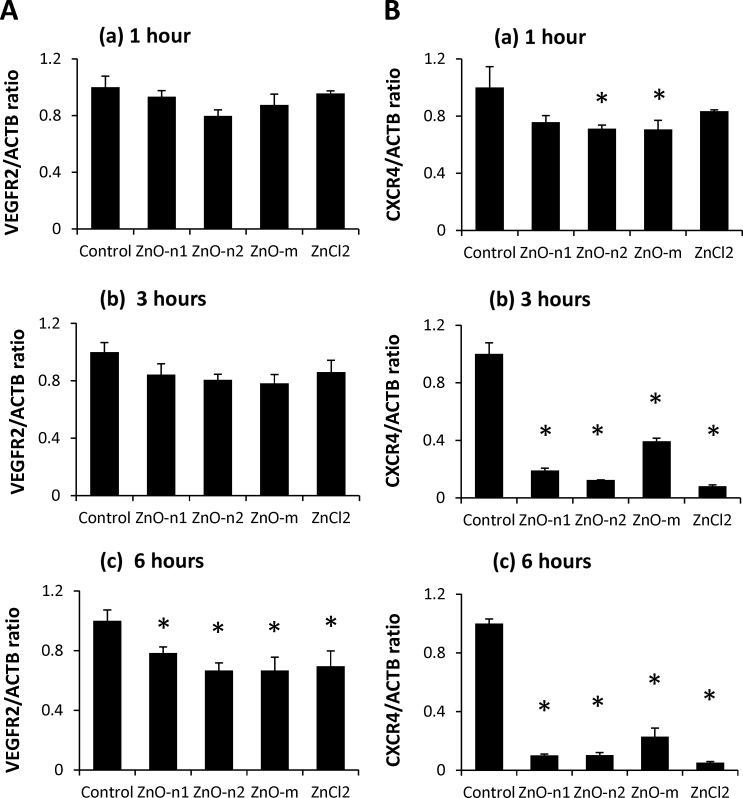
3.6. Effects of ZnO particles on VEGFR2 and CXCR4 expression
The expression level of VEGFR2 mRNA was significantly lower in ECFCs exposed to 25 μg/ml of ZnO particles and the corresponding concentration of ZnCl2 for 6 h than in the control (Fig. 6A(c)). There was no significant difference in the mRNA expression level in ECFCs exposed to the dispersed ZnO particles or dissolved ZnCl2 for 1 or 3 h only (Fig. 6A(a) and (b)). The expression level of CXCR4 mRNA was significantly lower in ECFCs exposed to 25 μg/ml of ZnO particles or the corresponding concentration of ZnCl2 for 3 or 6 h than in the control (Fig. 6B(b) and (c)). The change in CXCR4 mRNA level was observed in ECFCs exposed to ZnO-n2 and ZnO-micro particles from 1 h after exposure (Fig. 6A(a)). Exposure to ZnO particles or ZnCl2 did not affect the expression level of PTEN mRNA (data not shown).
Fig. 6.
Effects of dispersed ZnO particles or dissolved ZnCl2 on the expression levels of membrane type receptors. Expression levels of (A) VEGFR2 and (B) CXCR4 mRNA in ECFCs exposed to 25 μg/ml of dispersed ZnO particles and the corresponding concentration of dissolved ZnCl2 for (a) 1 h, (b) 3 h, or (c) 6 h. Data are mean ± SD of three or four replicates. *p < 0.05 vs. control (0 μg/ml).
4. Discussion
The present study demonstrated that in vitro exposure of ECFCs to ZnO nanoparticles attenuated tubule formation. Since exposure to ZnO micro-sized particles or dissolved ZnCl2 had similar effects, these changes are considered to be based on the concentration of the released Zn(II). We also demonstrated that in vitro exposure of ECFCs to dispersed ZnO particles and dissolved ZnCl2 suppressed the expression of the membrane type receptors involved in vasculogenesis.
Previous studies suggested that inhaled particles in the lungs may cause systemic inflammation through oxidative stress, which also mediates endothelial dysfunction [24]. Several studies demonstrated that nano-sized particles can cross the pulmonary epithelial barrier and enter the bloodstream [25], [26], although translocation of nanoparticles from the lung into the circulation was slow and very low [27]. The results of animal studies on alveolar translocation of nanoparticles suggest the existence of the same pathway in humans; however, the extent of extrapulmonary translocation is highly dependent on particle surface characteristics/chemistry, as well as particle size [28]. In addition to the lungs, several types of nanoparticles have been reported to translocate and accumulate in the bone marrow after administration to animals [29], [30]. These results suggest that nanoparticles could be taken up by bone marrow-derived mononuclear cells and provide a mechanism for the direct effects on such cells. Therefore, we focused in the present study on the direct effects of ZnO nanoparticles on cultured EPCs in order to explore the cellular mechanisms responsible for the cardiovascular effects of nanoparticles.
EPCs differentiate into mature endothelial cells and play an important role in facilitating vascular repair and tissue regeneration [10]. Previous studies demonstrated that the numbers and functionality of EPCs may predict the clinical outcome and prognosis of cardiovascular diseases [31], [32]. Previous studies showed that exposure to PM was associated with a decrease in the number of EPCs [16], [17]. Moreover, diesel exhaust particles impaired neoangiogenesis due to a reduction in the number of circulating EPCs and a significant increase in apoptosis of EPCs [33]. Chronic exposure to tobacco smoke is not only associated with low EPC levels [34] but also impaired EPC functional activities (e.g., chemotaxis, migration, and adhesion) [35]. Thus, various species and sizes of particles can impair the function of EPCs. In the present study, incubation of ECFCs in the presence of nano- and micro-sized ZnO particles at the high concentrations (50–100 μg/ml) significantly induced cytotoxicity. To confirm if the effects are specifically related to Zn(II), we need further studies exposing to other metal-containing nanoparticles at the same condition.
Metal oxide nanoparticles are newly developed materials used in a variety of applications. Nano-sized ZnO and TiO2 have been applied extensively in different fields, including industry, cosmetics, and biomedicines. It has been reported that ZnO particles induce inflammatory responses and adhesion molecules in endothelial cells in a dose-dependent manner [6], [8]. We have also shown recently that ZnO nanoparticle can potentially enhance THP-1 monocyte cell migration, adhesion of THP-1 cells to human umbilical vein endothelial cells and uptake modified LDL by THP-1 macrophages in vitro [9]. In the present study, the functional capacity of ECFCs for tubule formation on Matrix gel was reduced in cells exposed to 10 or 25 μg/ml of ZnO nanoparticles compared with the control cells. Our findings are in agreement with those of previous studies, which reported that exposure to inhaled nickel nanoparticles resulted in dysfunction of bone-marrow EPC [36].
Hammond [37] reported that workers exposed to ZnO nanoparticle at concentrations of approximately 725 mg/m3 for 3 h have experienced metal fume fever. In a previous in vitro study [38], ZnO nanoparticles were exposed to primary cultured rat alveolar epithelial cell monolayers at the concentration from 11 to 176 μg/ml, based on an assumption that ZnO deposited in the lung could be 8.8 μg/cm2 if the workers are exposed to ZnO nanoparticles at 725 mg/m3 for 24 h, with a respiratory minute volume of 20 l/min, ∼70 m2 surface area of airspaces of the lung, and 30% of inhaled ZnO deposition in the lung. Previous studies have reported that approximately 10–15% of inhaled nanoparticles translocate into the blood vessels [39]. In the present study, the functional capacity of ECFCs for tubule formation was reduced in cells exposed to ZnO nanoparticles at 10 or 25 μg/ml, which are comparable to 3.9 or 9.9 μg/cm2, respectively. Although this value is relatively high, but the results suggest that the concentrations adopted in the present study are relevant to ZnO concentrations that can suppress vasculogenesis in human EPCs.
In the present study, the functional capacity of ECFCs for tubule formation was reduced to the same extent in cells exposed to either the ZnO-micro particles or dissolved ZnCl2. These results are consistent with previous studies demonstrating that dissolved zinc ions seem to mediate the toxic effects of ZnO particles [40]. On the other hand, a recent study suggested other factors play an important role in the toxic effects of ZnO nanoparticles based on the finding of negligible toxic contribution of released Zn(II) ions in A549 cell lines [41]. Moreover, another study demonstrated that rapid, pH-dependent dissolution of ZnO nanoparticles inside the phagosomes is the main cause of ZnO nanoparticles-induced tissue injury [42] and that soluble intracellular Zn(II) ions, rather than soluble extracellular Zn(II) ions, are key components in modulating the bioactivity of ZnO nanoparticles [43]. In the present study, our results indicated that dissolved Zn(II) ions in the culture medium impaired tubule formation in EPCs exposed to ZnO nanoparticles; consistent with the previous studies that demonstrated that ZnO nanoparticles toxicity is largely due to the release of toxic Zn(II) ions which occurs predominantly in extracellular compartment in the culture medium [20]. We previously demonstrated a significant correlation between the amount of metal uptaken by HUVECs and the concentration of ZnO particles in the medium, as determined by ICP-Mass Spectrometry, and showed increase in free intracellular zinc ions in HUVECs exposed to ZnO particles by measuring a zinc-specific fluorescent (zinquin ethyl ester) [9]. We believe that intracellular Zn-content increases with increasing exposure concentration of ZnO particles in the ECFCs culture medium, although the intracellular Zn-content was not measured in the present study. To demonstrate the importance of intracellular Zn(II) ions for the bioactivity or toxic effects of ZnO nanoparticles, we need to compare the intracellular Zn concentrations between the groups.
In the present study, exposure of ECFCs to either dispersed ZnO particles or dissolved ZnCl2 resulted in a significant increase in apoptosis, compared with the control. The effects of ZnCl2 were much more pronounced as compared to the other groups in particular at concentrations of 50 and 100 μg/ml. The amount of zinc in the solution of 100 μg/ml of ZnO-micro particles was lower than those in the solution of the corresponding concentration of ZnCl2 (167 μg/ml) although the amount of zinc in the solutions of ZnO nanoparticles was almost the same as the corresponding concentration of ZnCl2. The difference in the results of the apoptosis assay may reflect the difference in the concentration of intracellular Zn(II) ions resulting from exposures to ZnO particles and ZnCl2. Apoptosis is an important process during normal development as it contributes to the maintenance of tissue homeostasis. Several studies have indicated that endothelial cell apoptosis is closely involved in vascular growth and vessel regression and limitation of angiogenesis [44]. Previous studies showed that exposure to ZnO nanoparticles induced apoptosis of macrophages [45], [46]. Although EPCs are less sensitive to apoptosis compared with mature endothelial cells [47], apoptosis is an important regulatory cellular mechanism under pathophysiological conditions involving EPCs. Since induction of apoptosis is a key element in the regulation of EPC numbers and function [48], our results suggest that it represents one of the mechanisms of the observed anti-proliferative effects of dispersed ZnO particles and dissolved ZnCl2 on ECFCs.
Vascular endothelial growth factor (VEGF) and its receptor, VEGFR2, and chemokine stromal cell-derived factor-1α (SDF-1α) and its receptor, CXCR4 signal pathway, play key roles in mobilization and development of vascular networks [49]. Indeed, increased expression of these receptors has been described to result in increased EPCs homing to the ischemic zone and that it is a suitable therapeutic target in angiogenesis after myocardial infarction [50], [51]. In the present study, exposure of ECFCs to ZnO nanoparticles significantly decreased the expression levels of VEGFR2 and CXCR4 in all groups. These results suggest that the exposure-induced reduction of functional capacity of ECFCs for tubule formation was due to the down-regulation of membrane type receptors related to vasculogenesis. This finding is in agreement with a previous study, which showed significant correlation between CXCR4 expression level and in vitro angiogenic function as well as ability to promote angiogenesis and repair of vascular injury in vivo [52].
5. Conclusion
The present study demonstrated that exposure of ECFCs to ZnO nanoparticles attenuated vasculogenesis and this effect was probably related to the concentration of released Zn(II) in the culture medium. We also demonstrated that Zn(II) released from the dispersed ZnO particles and dissolved ZnCl2 suppressed the expression of membrane type receptors related to vasculogenesis.
Conflicts of interest
The authors declare no financial conflict of interest.
Transparency document
Acknowledgments
The authors thank Chihara Higashionna, Fukumi Matsuyama, and Atsushi Suzuki for ICP-AES analysis and Kumi Nakao for the help in preparation of the manuscript. This work was supported in part by a grant from the Japan Society for the Promotion of Science (NEXT Program #LS056) and a grant-in aid for Scientific Research (#26293149).
References
- 1.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environ. Health Perspect. 2005;113:823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donaldson K., Stone V., Tran C.L., Kreyling W., Borm P.J. Nanotoxicology. Occup. Environ. Med. 2004;61:727–728. doi: 10.1136/oem.2004.013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sager T.M., Kommineni C., Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part. Fibre Toxicol. 2008;5:17. doi: 10.1186/1743-8977-5-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xia T., Kovochich M., Brant J., Hotze M., Sempf J., Oberley T., Sioutas C., Yeh J.I., Wiesner M.R., Nel A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6:1794–1807. doi: 10.1021/nl061025k. [DOI] [PubMed] [Google Scholar]
- 5.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M., Somasundaran P., Klaessig F., Castranova V., Thompson M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 6.Gojova A., Guo B., Kota R.S., Rutledge J.C., Kennedy I.M., Barakat A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: effect of particle composition. Environ. Health Perspect. 2007;115:403–409. doi: 10.1289/ehp.8497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsou T.C., Yeh S.C., Tsai F.Y., Lin H.J., Cheng T.J., Chao H.R., Tai L.A. Zinc oxide particles induce inflammatory responses in vascular endothelial cells via NF-κB signaling. J. Hazard. Mater. 2010;183:182–188. doi: 10.1016/j.jhazmat.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Li C.H., Liao P.L., Shyu M.K., Liu C.W., Kao C.C., Huang S.H., Cheng Y.W., Kang J.J. Zinc oxide nanoparticles-induced intercellular adhesion molecule 1 expression requires Rac1/Cdc42, mixed lineage kinase 3, and c-Jun N-terminal kinase activation in endothelial cells. Toxicol. Sci. 2012;126:162–172. doi: 10.1093/toxsci/kfr331. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki Y., Tada-Oikawa S., Ichihara G., Yabata M., Izuoka K., Suzuki M., Sakai K., Ichihara S. Zinc oxide nanoparticles induce migration and adhesion of monocytes to endothelial cells and accelerate foam cell formation. Toxicol. Appl. Pharmacol. 2014;278:16–25. doi: 10.1016/j.taap.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Asahara T., Masuda H., Takahashi T., Kalka C., Pastore C., Silver M., Kearne M., Magner M., Isner J.M. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ. Res. 1999;85:221–228. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- 11.Hill J.M., Zalos G., Halcox J.P., Schenke W.H., Waclawiw M.A., Quyyumi A.A., Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 12.Wassmann S., Werner N., Czech T., Nickenig G. Improvement of endothelial function by systemic transfusion of vascular progenitor cells. Circ. Res. 2006;99:e74–e83. doi: 10.1161/01.RES.0000246095.90247.d4. [DOI] [PubMed] [Google Scholar]
- 13.Miller K.A., Siscovick D.S., Sheppard L., Shepherd K., Sullivan J.H., Anderson G.L., Kaufman J.D. Long-term exposure to air pollution and incidence of cardiovascular events in women. N. Engl. J. Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 14.Mills N.L., Tornqvist H., Gonzalez M.C., Vink E., Robinson S.D., Söderberg S., Boon N.A., Donaldson K., Sandström T., Blomberg A., Newby D.E. Ischemic and thrombotic effects of dilute diesel-exhaust inhalation in men with coronary heart disease. N. Engl. J. Med. 2007;357:1075–1082. doi: 10.1056/NEJMoa066314. [DOI] [PubMed] [Google Scholar]
- 15.Brook R.D., Bard R.L., Burnett R.T., Shin H.H., Vette A., Croghan C., Phillips M., Rodes C., Thornburg J., Williams R. Differences in blood pressure and vascular responses associated with ambient fine particulate matter exposures measured at the personal versus community level. Occup. Environ. Med. 2011;68:224–230. doi: 10.1136/oem.2009.053991. [DOI] [PubMed] [Google Scholar]
- 16.O’Toole T.E., Hellmann J., Wheat L., Haberzettl P., Lee J., Conklin D.J., Bhatnagar A., Pope C.A., 3rd Episodic exposure to fine particulate air pollution decreases circulating levels of endothelial progenitor cells. Circ. Res. 2010;107:200–203. doi: 10.1161/CIRCRESAHA.110.222679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haberzettl P., Lee J., Duggineni D., McCracken J., Bolanowski D., O’Toole T.E., Bhatnagar A., Conklin D.J. Exposure to ambient air fine particulate matter prevents VEGF-induced mobilization of endothelial progenitor cells from the bone marrow. Environ. Health Perspect. 2012;120:848–856. doi: 10.1289/ehp.1104206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hofmann N.A., Reinisch A., Strunk D. Endothelial colony-forming progenitor cell isolation and expansion. Methods Mol. Biol. 2012;879:381–387. doi: 10.1007/978-1-61779-815-3_23. [DOI] [PubMed] [Google Scholar]
- 19.Wu W., Ichihara G., Suzuki Y., Izuoka K., Oikawa-Tada S., Chang J., Sakai K., Miyazawa K., Porter D., Castranova V., Kawaguchi M., Ichihara S. Dispersion method for safety research on manufactured nanomaterials. Ind. Health. 2014;52:54–65. doi: 10.2486/indhealth.2012-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buerki-Thurnherr T., Xiao L., Diener L., Arslan O., Hirsch C., Maeder-Althaus X., Grieder K., Wampfler B., Mathur S., Wick P., Krug H.F. In vitro mechanistic study towards a better understanding of ZnO nanoparticle toxicity. Nanotoxicology. 2012;7:402–416. doi: 10.3109/17435390.2012.666575. [DOI] [PubMed] [Google Scholar]
- 21.Moon C.S., Zhang Z.W., Shimbo S., Hokimoto S., Shimazaki K., Saito T., Shimizu A., Imai Y., Watanabe T., Ikeda M. A comparison of the food composition table-based estimates of dietary element intake with the values obtained by inductively coupled plasma atomic emission spectrometry: an experience in a Japanese population. J. Trace Elem. Med. Biol. 1996;10:237–244. doi: 10.1016/S0946-672X(96)80041-1. [DOI] [PubMed] [Google Scholar]
- 22.Walker V.G., Li Z., Hulderman T., Schwegler-Berry D., Kashon M.L., Simeonova P.P. Potential in vitro effects of carbon nanotubes on human aortic endothelial cells. Toxicol. Appl. Pharmacol. 2009;236:319–328. doi: 10.1016/j.taap.2009.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Ichihara S., Yamada Y., Liu F., Murohara T., Itoh K., Yamamoto M., Ichihara G. Ablation of the transcription factor Nrf2 promotes ischemia-induced neovascularization by enhancing the inflammatory response. Arterioscler. Thromb. Vasc. Biol. 2010;30:1553–1561. doi: 10.1161/ATVBAHA.110.204123. [DOI] [PubMed] [Google Scholar]
- 24.Courtois A., Andujar P., Ladeiro Y., Baudrimont I., Delannoy E., Leblais V., Begueret H., Galland M.A., Brochard P., Marano F., Marthan R., Muller B. Impairment of NO-dependent relaxation in intralobar pulmonary arteries: comparison of urban particulate matter and manufactured nanoparticles. Environ. Health Perspect. 2008;116:1294–1299. doi: 10.1289/ehp.11021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemmar A., Hoet P.H., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M.F., Vanbilloen H., Mortelmans L., Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 26.Choi H.S., Ashitate Y., Lee J.H., Kim S.H., Matsui A., Insin N., Bawendi M.G., Semmler-Behnke M., Frangioni J.V., Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat. Biotechnol. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kreyling W.G., Semmler M., Erbe F., Mayer P., Takenaka S., Schulz H., Oberdörster G., Ziesenis A. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J. Toxicol. Environ. Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 28.Kreyling W.G., Semmler-Behnke M., Seitz J., Scymczak W., Wenk A., Mayer P., Takenaka S., Oberdörster G. Size dependence of the translocation of inhaled iridium and carbon nanoparticle aggregates from the lung of rats to the blood and secondary target organs. Inhal. Toxicol. 2009;21:55–60. doi: 10.1080/08958370902942517. [DOI] [PubMed] [Google Scholar]
- 29.Bazile D.V., Ropert C., Huve P., Verrecchia T., Marlard M., Frydman A., Veillard M., Spenlehauer G. Body distribution of fully biodegradable [14C]-poly (lactic acid) nanoparticles coated with albumin after parenteral administration to rats. Biomaterials. 1992;13:1093–1102. doi: 10.1016/0142-9612(92)90142-b. [DOI] [PubMed] [Google Scholar]
- 30.Cagle D.W., Kennel S.J., Mirzadeh S., Alford J.M., Wilson L.J. In vivo studies of fullerene-based materials using endohedral metallofullerene radiotracers. Proc. Natl. Acad. Sci. U. S. A. 1999;96:5182–5187. doi: 10.1073/pnas.96.9.5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasa M., Fichtlscherer S., Aicher A., Adler K., Urbich C., Martin H., Zeiher A.M., Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 32.Werner N., Kosiol S., Schiegl T., Ahlers P., Walenta K., Link A., Böhm M., Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N. Engl. J. Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 33.Pöss J., Lorenz D., Werner C., Pavlikova V., Gensch C., Speer T., Alessandrini F., Berezowski V., Kuntz M., Mempel M., Endres M., Böhm M., Laufs U. Diesel exhaust particles impair endothelial progenitor cells, compromise endothelial integrity, reduce neoangiogenesis, and increase atherogenesis in mice. Cardiovasc. Toxicol. 2013;13:290–300. doi: 10.1007/s12012-013-9208-0. [DOI] [PubMed] [Google Scholar]
- 34.Kondo T., Hayashi M., Takeshita K., Numaguchi Y., Kobayashi K., Iino S., Inden Y., Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler. Thromb. Vasc. Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 35.Heiss C., Amabile N., Lee A.C., Real W.M., Schick S.F., Lao D., Wong M.L., Jahn S., Angeli F.S., Minasi P., Springer M.L., Hammond S.K., Glantz S.A., Grossman W., Balmes J.R., Yeghiazarians Y. Brief secondhand smoke exposure depresses endothelial progenitor cells activity and endothelial function: sustained vascular injury and blunted nitric oxide production. J. Am. Coll. Cardiol. 2008;51:1760–1771. doi: 10.1016/j.jacc.2008.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Liberda E.N., Cuevas A.K., Gillespie P.A., Grunig G., Qu Q., Chen L.C. Exposure to inhaled nickel nanoparticles causes a reduction in number and function of bone marrow endothelial progenitor cells. Inhal. Toxicol. 2010;22:95–99. doi: 10.3109/08958378.2010.515269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hammond J.W. Metal fume fever in the crushed stone industry. J. Ind. Hyg. Toxicol. 1944;26:117–119. [Google Scholar]
- 38.Kim Y.H., Fazlollahi F., Kennedy I.M., Yacobi N.R., Hamm-Alvarez S.F., Borok Z., Kim K.J., Crandall E.D. Alveolar epithelial cell injury due to zinc oxide nanoparticle exposure. Am. J. Respir. Crit. Care Med. 2010;182:1398–1409. doi: 10.1164/rccm.201002-0185OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Geiser M., Rothen-Rutishauser B., Kapp N., Schürch S., Kreyling W., Schulz H., Semmler M., Im Hof V., Heyder J., Gehr P. Ultrafine particles cross cellular membranes by nonphagocytic mechanisms in lungs and in cultured cells. Environ. Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Song W., Zhang J., Guo J., Zhang J., Ding F., Li L., Sun Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010;199:389–397. doi: 10.1016/j.toxlet.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Xu M., Li J., Hanagata N., Su H., Chen H., Fujita D. Challenge to assess the toxic contribution of metal cation released from nanomaterials for nanotoxicology – the case of ZnO nanoparticles. Nanoscale. 2013;5:4763–4769. doi: 10.1039/c3nr34251d. [DOI] [PubMed] [Google Scholar]
- 42.Cho W.S., Duffin R., Howie S.E., Scotton C.J., Wallace W.A., Macnee W., Bradley M., Megson I.L., Donaldson K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011;8:27. doi: 10.1186/1743-8977-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shen C., James S.A., de Jonge M.D., Turney T.W., Wright P.F., Feltis B.N. Relating cytotoxicity, zinc ions, and reactive oxygen in ZnO nanoparticle-exposed human immune cells. Toxicol. Sci. 2013;136:120–130. doi: 10.1093/toxsci/kft187. [DOI] [PubMed] [Google Scholar]
- 44.Muñoz-Chápuli R., Quesada A.R., Angel Medina M. Angiogenesis and signal transduction in endothelial cells. Cell. Mol. Life Sci. 2004;61:2224–2243. doi: 10.1007/s00018-004-4070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilhelmi V., Fischer U., Weighardt H., Schulze-Osthoff K., Nickel C., Stahlmecke B., Kuhlbusch T.A., Scherbart A.M., Esser C., Schins R.P., Albrecht C. Zinc oxide nanoparticles induce necrosis and apoptosis in macrophages in a p47phox- and Nrf2-independent manner. PLOS ONE. 2013;8:e65704. doi: 10.1371/journal.pone.0065704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy R., Singh S.K., Chauhan L.K., Das M., Tripathi A., Dwivedi P.D. Zinc oxide nanoparticles induce apoptosis by enhancement of autophagy via PI3K/Akt/mTOR inhibition. Toxicol. Lett. 2014;227:29–40. [Google Scholar]
- 47.He T., Peterson T.E., Holmuhamedov E.L., Terzic A., Caplice N.M., Oberley L.W., Katusic Z.S. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler. Thromb. Vasc. Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 48.Gensch C., Clever Y.P., Werner C., Hanhoun M., Böhm M., Laufs U. The PPAR-gamma agonist pioglitazone increases neoangiogenesis and prevents apoptosis of endothelial progenitor cells. Atherosclerosis. 2007;192:67–74. doi: 10.1016/j.atherosclerosis.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y.B., Liu Y.F., Lu X.T., Yan F.F., Wang B., Bai W.W., Zhao Y.X. Rehmannia glutinosa extract activates endothelial progenitor cells in a rat model of myocardial infarction through a SDF-1 α/CXCR4 cascade. PLOS ONE. 2013;8:e54303. doi: 10.1371/journal.pone.0054303. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 50.Tang Y.L., Zhu W., Cheng M., Chen L., Zhang J., Sun T., Kishore R., Phillips M.I., Losordo D.W., Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ. Res. 2009;104:1209–1216. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Frederick J.R., Fitzpatrick J.R., 3rd, McCormick R.C., Harris D.A., Kim A.Y., Muenzer J.R., Marotta N., Smith M.J., Cohen J.E., Hiesinger W., Atluri P., Woo Y.J. Stromal cell-derived factor-1alpha activation of tissue-engineered endothelial progenitor cell matrix enhances ventricular function after myocardial infarction by inducing neovasculogenesis. Circulation. 2010;122(11 Suppl.):S107–S117. doi: 10.1161/CIRCULATIONAHA.109.930404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oh B.J., Kim D.K., Kim B.J., Yoon K.S., Park S.G., Park K.S., Lee M.S., Kim K.W., Kim J.H. Differences in donor CXCR4 expression levels are correlated with functional capacity and therapeutic outcome of angiogenic treatment with endothelial colony forming cells. Biochem. Biophys. Res. Commun. 2010;398:627–633. doi: 10.1016/j.bbrc.2010.06.108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.