Highlights
-
•
Arsenic, cadmium and lead levels in tobacco and smoke from a worldwide sample of 568 cigarettes are presented and discussed.
-
•
Enhanced retention of cadmium, but not lead, was observed in cigarettes with carbon filters compared to the other cigarettes.
-
•
Differences in speciation between cadmium, arsenic and lead were related to their distribution in ash, butt and smoke streams.
-
•
The transient formation of organometallic derivatives could adequately explain the observed cadmium selective filtration.
Keywords: Cadmium, Lead, Metal, Tobacco, Cigarette, Filtration, Activated carbon, Smoke chemistry, Transfer
Abstract
Arsenic, cadmium and lead levels in tobacco filler and cigarette smoke were determined in a 568-sample worldwide survey. Median tobacco levels for arsenic, cadmium and lead were 237, 769 and 397 ng/g respectively, comparable to those previously reported albeit somewhat lower for lead and cadmium. Median mainstream smoke yields for arsenic, cadmium and lead were <3.75, 18.2, and <12.8 ng/cig. under ISO, and <8.71, 75.1 and <45.7 ng/cig. under Health Canada Intense (HCI) smoking regime respectively. In the case of cigarettes with activated carbon, a selective retention of cadmium but not lead or arsenic was observed. This effect was more pronounced under ISO than under HCI smoking regimes. Cadmium selective retention by activated carbon was confirmed by testing specially designed prototype cigarettes and the causes for this selective filtration were investigated. The differences between cadmium, arsenic and lead in terms of their speciation in tobaccos and in cigarette smoke could be related to their distribution in the ash, butt, mainstream (in gas-phase and particulate-phase) and sidestream smoke of a smoked cigarette. The possible formation of organometallic cadmium derivatives in the smoke gas-phase is discussed, the presence of which could adequately explain the observed cadmium selective filtration.
1. Introduction
The health effects of environmental or workplace exposure to heavy metals and arsenic have been the subject of extensive research [1], [2]. Cadmium, in particular, has been linked with overall cancer mortality [3] and, more specifically, with cancers of the lung, pancreas, breast, prostate, endometrium and urinary bladder [4]. It has also been linked with non-cancer morbidity, kidneys and bones being major target organs [5], [6], [7], [8].
Heavy metals have been reported to be associated with the toxicity of tobacco products and tobacco smoke [9], [10] and a number of elements have been identified as contributors to this toxicity. Canadian regulations require that levels of cadmium, lead, arsenic, nickel, chromium, selenium and mercury be reported in tobacco, mainstream and sidestream smoke [11]. Among these elements, arsenic and cadmium appear in the abbreviated list of harmful and potentially harmful constituents whose level in tobacco should be reported according to a guidance document issued by the U.S. Food and Drug Administration (FDA) [12]. In particular, cadmium was listed by the International Agency for Research on Cancer as a Group 1 human carcinogen [4]. It was also selected as a priority toxicant by the World Health Organization for smoke delivery reporting [13] and recommended for regulatory policy in a subsequent report [14]. Cadmium has been included in different prioritization lists of smoke constituents based on risk assessments [15], [16], [17].
In the absence of specific occupational exposure, the main sources of cadmium uptake are food and tobacco smoke. The body burden of cadmium was assessed as being approximately two-fold higher in smokers than in non-smokers [18], [7], [19]. The impact of smoking on the lead body burden is observed through a sequestration in bones [20], [21], [22], but not in blood [23], [24], while no effect from smoking could be observed in the case of arsenic [25], or mercury [26], [27]. Surveys also showed that smoking is not an important source for nickel [28]. Finally, the smoke delivery levels of nickel, chromium and selenium are in most cases below the quantification limits of the protocols commonly used for their determination [29]. Conversely, sizeable amounts of cadmium, lead and arsenic can be found in tobacco smoke [30]. In the light of these observations, the present study focuses on cadmium (Cd), lead (Pb) and arsenic (As).
The cigarette delivery of elements to mainstream smoke can be addressed as a combination of two factors, the amount of these elements present in tobacco and their transfer rate, which is specific to element speciation and is impacted by cigarette design. The transfer of elements during smoking has been the subject of a number of studies over decades. Nevertheless, despite this wealth of information, it is difficult to obtain a clear model of elements transfer to smoke (sidestream or mainstream), or their retention (in ash or butt). Even for the specific subject of the phase-distribution for each element in the smoke aerosol, there is a lack of agreement. This point is central to a discussion on transfer since a compound must be at least partly present in the gas-phase to be selectively removed from mainstream smoke by adsorbents. The uncertainty that prevails about the elements transfer or speciation is likely due to the complexity of the quantification of elements yields at trace levels, despite dramatic improvements in instrumentation and analytical methods over the years. Sample contamination is a constant problem. The small size of the data sets taken into account in many studies is an additional cause for discrepancies among authors’ assessments.
Based on data from three worldwide market surveys of commercial cigarettes performed between 2008 and 2012, which included the determination of tobacco and mainstream smoke levels of As, Cd and Pb, we investigated the transfer of each of these elements from tobacco to mainstream smoke generated under both International Organization for Standardization (ISO) and Health Canada Intense (HCI) machine-smoking regimes. Of particular interest is the fact that market surveys data can very effectively evidence selective removal of an element by activated carbon through a comparison of its filtration to that of nicotine. Results, including data from specially designed prototypes, are discussed and the conclusions strengthened by a review of the relevant literature on elements specific filtration.
2. Materials and methods
2.1. Market surveys brands sampling
In order to best observe the impact of cigarette design and tobacco blend, brands were selected to cover as many cigarette design specificities as possible, rather than sampling based on local market share. 568 samples of commercial brands from 27 different manufacturers were bought in 2008 (205 samples), 2009 (63 samples) and 2012 (300 samples) at the point of sale in 23 countries. Because in some cases a same brand could be analyzed in different market surveys, 489 brands were actually investigated by the 568 samples. With the exception of one papirosi cigarette, all were conventional cigarettes, excluding e.g., bidis and herbal products. Different blend types were included in the sampled set, with a large proportion of American and Virginia blends. The dimension of sampled cigarettes covered the whole available range, with diameters between 5.2 mm (superslim) and 8.0 mm (magnum), and rod lengths between 70 mm and 100 mm. Among the sampled brands, filter designs included single and multiple-plug configurations with up to 4 plugs. In some brands, filters contained activated carbon, present either in the tow or in a cavity between two plugs. Some non-filter brands were also sampled. The numbers of samples selected per country are presented in Table 1, including information regarding their filter design.
Table 1.
Origin and design of the market brands; number of samples analyzed.
| Country | Filter |
Number of samples | |||||
|---|---|---|---|---|---|---|---|
| Activated carbon |
No carbon |
No filter |
|||||
| ISO | HCI | ISO | HCI | ISO | HCI | ||
| Argentina | 3 | 3 | |||||
| Australia | 22 | 32 | 32 | ||||
| Belgium | 8 | 1 | 9 | ||||
| Brazil | 8 | 12 | 12 | ||||
| China | 1 | 1 | 9 | 9 | 10 | ||
| Denmark | 4 | 4 | 4 | ||||
| France | 16 | 26 | 2 | 2 | 28 | ||
| Germany | 13 | 17 | 1 | 1 | 18 | ||
| Great Britain | 17 | 30 | 30 | ||||
| Greece | 2 | 12 | 14 | ||||
| Hungary | 10 | 10 | 20 | ||||
| Italy | 2 | 3 | 17 | 34 | 37 | ||
| Japan | 30 | 61 | 10 | 32 | 1 | 94 | |
| Korea | 10 | 22 | 2 | 8 | 30 | ||
| Lithuania | 2 | 8 | 10 | ||||
| Mexico | 14 | 18 | 1 | 1 | 19 | ||
| Romania | 10 | 21 | 31 | ||||
| Russia | 20 | 28 | 53 | 78 | 2 | 2 | 108 |
| Spain | 1 | 17 | 18 | ||||
| Switzerland | 3 | 7 | 10 | ||||
| Taiwan | 8 | 7 | 15 | ||||
| Turkey | 5 | 6 | 5 | 7 | 13 | ||
| USA | 3 | 3 | 3 | ||||
| Total number | 68 | 157 | 193 | 403 | 6 | 8 | 568 |
2.2. Prototype cigarettes investigating a high activated carbon loading
Prototype cigarettes were manufactured to study the impact of adsorbents on cadmium, arsenic and lead filtration. The control cigarette (without activated carbon) was designed to mimic a commercial king-size American blend with a 27-mm cellulose acetate plug, a ventilation set at 35% and a resistance to draw of 100 mm H2O. The cigarette had a 7.5-mg tar delivery under ISO machine-smoking regime. The test prototype differed only from the control in the filter design. The test prototype filter was a 27-mm composite filter, consisting of a 7-mm plug of cellulose acetate at the mouth end abutted to a 20-mm Dalmatian plug into which 80 mg of activated carbon was embedded. The prototype cigarette was designed to same resistance to draw as the control. The test had a 7.2-mg tar delivery under ISO machine-smoking regime.
2.3. Smoke and tobacco analyses
The analyses of the different components in both tobacco filler and smoke were conducted under contract to Philip Morris International by Labstat International ULC (Kitchener, Ont., Canada), an ISO 17025 accredited laboratory, and were performed according to the official Health Canada methods [31]. Alkaloids in tobacco fillers were analyzed by gas chromatography according to method T-301 [32]; three replicates per sample were conducted. Cadmium, lead and arsenic were analyzed in tobacco fillers according to method T-306 [33]. Three replicates per sample were conducted. After conditioning according to ISO [34], cigarettes were smoked under both ISO [35] and HCI [36] machine-smoking regimes. Tar, nicotine and CO in mainstream smoke were analyzed according to method T-115 [36]. Eight replicates per sample were performed. Cadmium, lead and arsenic were analyzed in mainstream smoke according to method T-109 [37], with a rotary smoking machine equipped with an electrostatic precipitator. Three replicates per sample were conducted in the case of the market surveys, and 4 per sample in the case of the assessment of dedicated prototypes. The mainstream smoke yields of samples bought in 2012 were from a set that had only been analyzed using the HCI machine-smoking regime. A 3R4F Kentucky reference cigarette was used as a monitor in all analyses and all determinations were within internally specified target values. Specifically, cadmium, lead and arsenic smoke deliveries for this cigarette under ISO smoking regime were 34.6 ± 3.2, 12.3 ± 1.1 and 3.05 ± 0.35 ng/cigarette respectively, in line with a recently organized ring trial results [38].
2.4. Data treatment
In one Korean brand, nicotine was reported as below limit of quantification (LOQ) in analyses under the ISO machine-smoking regime. This sample was removed from the data set since the assessment of nicotine transfer was part of the data analysis. Only 267 data points were thus available for the smoke yields obtained under the ISO machine-smoking regime, while 567 data points were considered for the analysis of smoke yields obtained under the HCI machine-smoking regime. Data below the limits of quantification were reported as <LOQ. In the statistical treatment of the data distribution, the number of data points below the LOQ are given. As a conservative estimate, these samples are attributed the LOQ value in the calculation of medians and quartiles.
3. Results
3.1. Elements and nicotine levels in cigarette fillers
Tobacco levels of arsenic, cadmium and lead were measured in the blend of each sample. All measured values were above LOQ. Descriptive statistics for the results are presented in Table 2, together with a range of typical mean values reported in previously published surveys. Nicotine levels were also measured since nicotine transfer was required for the assessment of the elements transfer.
Table 2.
Tobacco filler analyses, all 568 samples.
| Analyte (unit) | Mean | Minimum | 1st quartile | Median | 3rd quartile | Maximum | Typical published mean values |
|---|---|---|---|---|---|---|---|
| Nicotine (mg/g) | 16.8 | 6.35 | 15.6 | 16.9 | 18.2 | 25.5 | – |
| Arsenic (ng/g) | 256 | 60.9 | 198 | 237 | 282 | 2065 | 90–780a |
| Cadmium (ng/g) | 898 | 187 | 645 | 769 | 998 | 4672 | 650–3630b |
| Lead (ng/g) | 466 | 183 | 336 | 397 | 487 | 4634 | 440–12,160c |
Limit of quantification for the elements: As, 60 ng/g; Cd, 144 ng/g; Pb, 126 ng/g.
All levels calculated on a dry-weight basis.
3.2. Smoke analyses from market samples
Smoke yields of arsenic, cadmium, lead and nicotine were measured for each sample under HCI machine-smoking regime. In addition, the yields under ISO machine smoking regime were also obtained from a subset of the samples (267 retained for the study). Unlike the filler levels, these smoke yields were below the analytical limit of quantification for many samples, especially when the samples were smoked under ISO. The numbers of samples with levels determined below the limit of quantification are highlighted alongside the descriptive statistics in Table 3 (ISO yields) and Table 4 (HCI yields). Because samples with yields below LOQ were attributed the LOQ value in the calculation of medians and quartiles, some of the statistical data in Table 3, Table 4 are reported as <LOQ, which then provides an upper limit estimate.
Table 3.
Distribution of the results of mainstream smoke analyses under ISO, 267 samples.
| Analyte (unit) | Number of samples below LOQ | Mean | Minimum | 1st quartile | Median | 3rd quartile | Maximum | Typical published mean values |
|---|---|---|---|---|---|---|---|---|
| Nicotine (mg/cig.) | 0 | 0.621 | 0.072 | 0.486 | 0.635 | 0.787 | 1.40 | – |
| Arsenic (ng/cig.) | 228 | <3.81 | <3.75 | <3.75 | <3.75 | <3.75 | 43.8 | 2.8–5.5a |
| Cadmium (ng/cig.) | 23 | 25.9 | <1.59 | 8.48 | 18.2 | 27.8 | 299 | 1.6–260b |
| Lead (ng/cig.) | 147 | <19.4 | <12.8 | <12.8 | <12.8 | 18.2 | 221 | 2.0–980c |
Limit of quantification for the elements: As, 3.75 ng/cig.; Cd, 1.59 ng/cig.; Pb, 12.8 ng/cig.
[30], [40], [61], [62]. In a literature survey published in 1997 [65], values up to 1400 ng/cig. were reported.
Table 4.
Distribution of the results for mainstream smoke analyses under HCI, all 567 samples.
| Analyte (unit) | Number of samples below LOQ | Mean | Minimum | 1st quartile | Median | 3rd quartile | Maximum | Typical published mean values |
|---|---|---|---|---|---|---|---|---|
| Nicotine (mg/cig.) | 0 | 1.68 | 0.71 | 1.43 | 1.67 | 1.92 | 3.13 | – |
| Arsenic (ng/cig.) | 400 | <8.71 | <7.5 | <7.5 | <7.5 | 8.29 | 81.9 | 7.5–14.5 a |
| Cadmium (ng/cig.) | 0 | 75.1 | 14.1 | 44.6 | 62.9 | 87.6 | 508 | 43.5–197.1b |
| Lead (ng/cig.) | 94 | <45.7 | <25.7 | 31.2 | 39.7 | 50.2 | 379 | 25.7–93.2c |
3.2.1. ISO mainstream smoke yields (267 samples)
Descriptive statistics for the results of all smoke analytical determinations for nicotine and all selected elements performed under the ISO machine-smoking regime are given in Table 3, together with a range of typical mean values reported in previous published surveys. The limits of quantification for each element and number of samples with yields below these limits are also presented.
3.2.2. HCI mainstream smoke yields (567 samples)
Descriptive statistics for the results of all smoke analytical determinations for nicotine and all selected elements performed under the HCI machine-smoking regime are given in Table 4, together with a range of typical mean values reported in previous published surveys. The limits of quantification for each element and number of brands with yields below these limits are also provided.
3.2.3. Smoke yields normalized to nicotine
The distribution of smoke yields for the elements after normalization with corresponding nicotine yields was addressed, since data normalization has been recommended when dealing with data sets derived from brands with diverse design features, particularly with reference to regulation [39]. Only normalized data for cadmium are reported in Table 5. The large number of values below LOQ for lead and arsenic makes any estimate for the distribution of their normalized yields meaningless. For comparison, mean values for nicotine-normalized cadmium yields of samples available in the published literature are also reported in Table 5.
Table 5.
Distribution of nicotine-normalized mainstream smoke yields for cadmium in all measured samples, expressed as ng/mg nicotine, smoked under ISO and HCI machine-smoking regimes.
| Smoking regime | Mean | Minimum | 1st quartile | Median | 3rd quartile | Maximum | Published mean values |
|---|---|---|---|---|---|---|---|
| ISO | 35.0 | 5.38 | 16.3 | 28.1 | 38.7 | 243 | 57.7; 58.6a |
| HCI | 44.0 | 9.86 | 28.5 | 37.8 | 51.1 | 216 | 55.5; 71.4a |
Under ISO, 23 samples have cadmium yields below LOQ; in this case samples were attributed the LOQ (1.59 ng/cig.) divided by the nicotine yield. No cadmium yield was found below LOQ under HCI.
3.3. Elements transfer to mainstream smoke; results from market surveys
In general, the transfer rates of elements may be influenced by a broad range of cigarette design parameters, as recently reported [40]. In the present case, we performed an analysis of variance (ANOVA) on the transfer rates of cadmium and lead (insufficient amount of data for arsenic) under ISO and HCI smoking regimes as response variables, taking the presence of activated carbon in the filter, measured filter ventilation, filter length, and cigarette diameter as independent design features. The results for lead transfer rates show a strong residual contribution of more than 85% to the total variance under the HCI smoking regime, and significant apparent contributions to the total variance from filter ventilation (ISO smoking regime) or cigarette diameter (HCI smoking regime). In the case of cadmium transfer, under both ISO and HCI smoking regimes more than 30% of the total variance can be attributed to the presence of activated carbon in the filter, and 30–40% is a residual contribution.
If one assumes that lead and cadmium reside in the particulate phase, the contribution of the cigarette design features to the variance of yields should cancel out if we instead take the ratio of their transfer rates to nicotine transfer rates as response variables, since nicotine is entirely present in the smoke particle-phase throughout its transfer across the unburnt tobacco and the filter [41], [42], [43]. Indeed, the same ANOVA performed on metals transfer rates normalized to nicotine transfer rates showed that for lead 84% and 96% of the total variance is contained in the residuals under ISO and HCI smoking regimes respectively. For cadmium, however, there remains a large contribution from the presence of activated carbon in the filter, which accounts for about 50% of the total variance under both ISO and HCI smoking regimes. The residual contribution is at a level of 40–50%. This last result suggests that a specific filtration of cadmium by activated carbon may be taking place, which would imply that cadmium is partly present in the gas-phase.
These results can also be investigated as graphs, showing in abscissa the mainstream smoke nicotine yield divided by the nicotine level in the tobacco filler, and in ordinate the equivalent for each selected element, both in percent. The amount of burnt tobacco during smoking cancels out in such a ratio. This made it possible to study the elements transfer to mainstream smoke across the diverse set of surveyed samples. A smoke component that would be totally in the particulate-phase is expected to experience a transfer that would remain in a constant ratio to the nicotine transfer. Taking into account the experimental variability, the expected plot from market map data would then show a cloud close to a line going through the origin. Conversely, in case a retention process takes place on top of TPM filtration, the corresponding data points will show up below the other points. This approach can thus provide a sensitive indicator for the existence and extent of any selective retention that would be in addition to particle-phase removal by filtration. A semi-quantitative assessment was obtained by performing a linear regression forced through the origin.
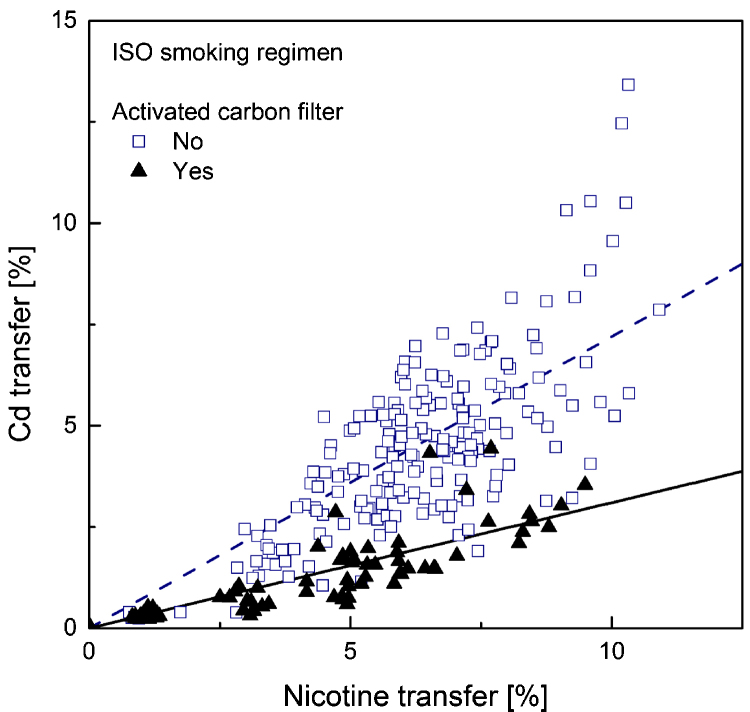
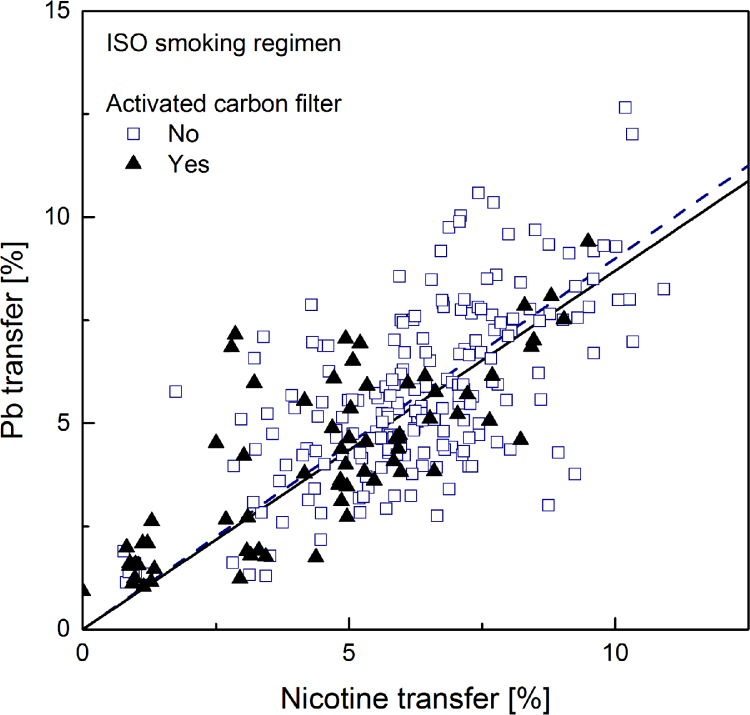
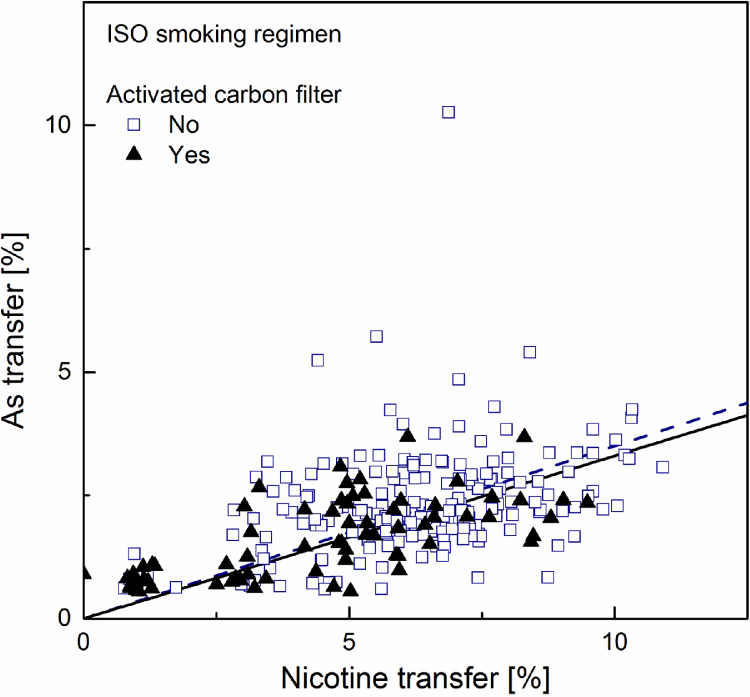
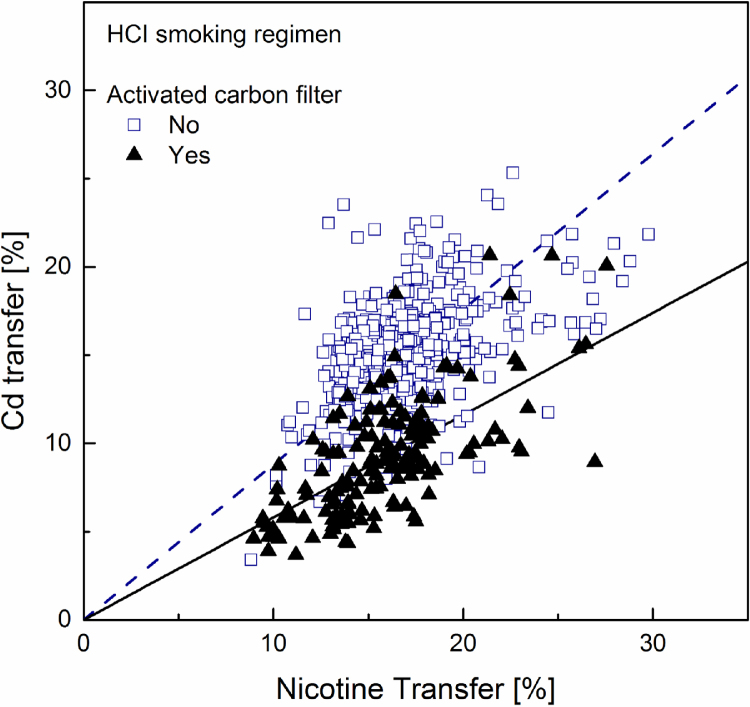
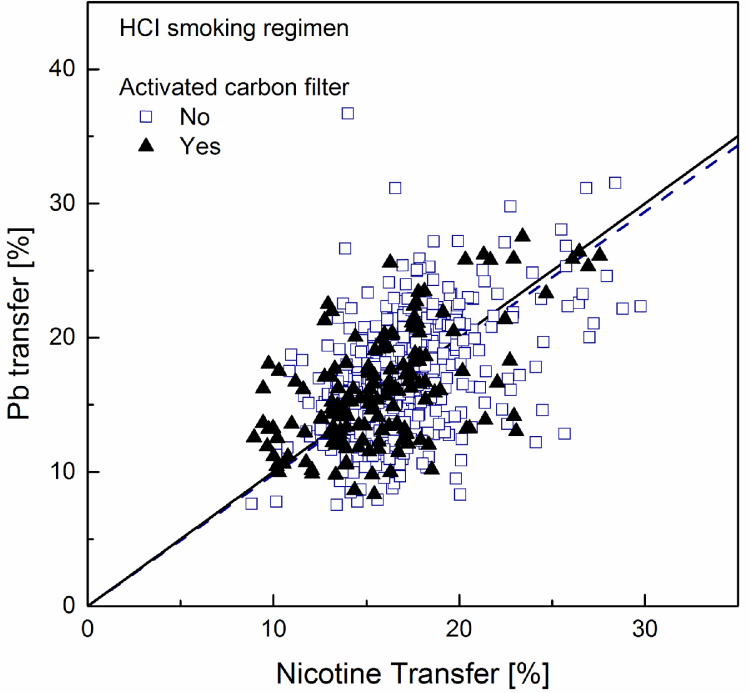
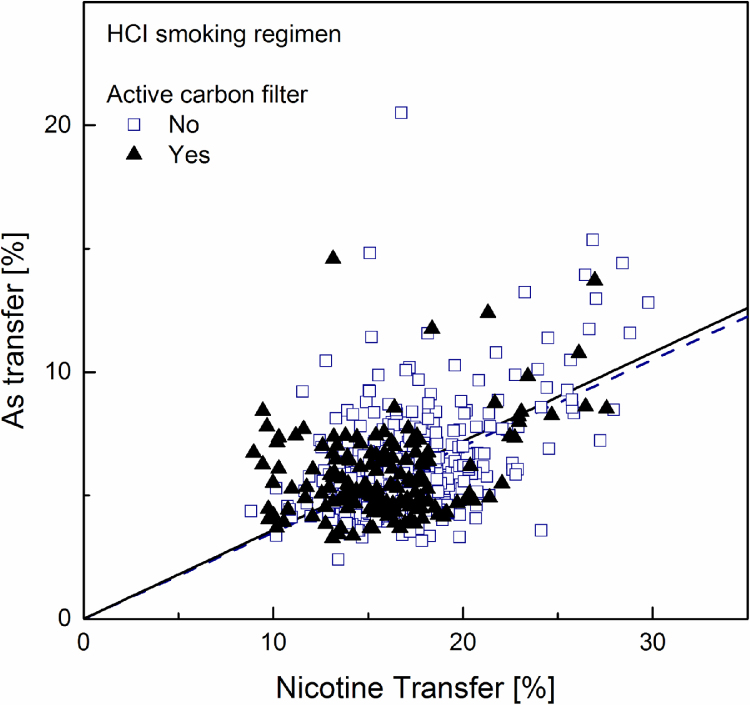
Fig. 1, Fig. 2, Fig. 3 show the patterns obtained from the data sets for Cd, Pb and As respectively when smoke is generated under the ISO machine-smoking regime. Fig. 4, Fig. 5, Fig. 6 show the patterns obtained from the data sets for Cd, Pb and As respectively when smoke is generated under the HCI machine-smoking regime. It should be noted that, with a nicotine transfer of about 20% and 47% under ISO and HCI machine-smoking regimes respectively, the data point corresponding to the non-filter papirossi cigarette could not be made visible in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7. The data were nevertheless included in all calculations. The linear regressions (forcing the intercept to zero) calculated for both activated carbon-filtered and non-carbon-filtered cigarettes and visualized in Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, are presented in Table 6 together with the corresponding standard error. In this calculation, the LOQ was entered in place of the smoke element level whenever the analytical determination was below this value. Clearly this provides an upper estimate for the linear regressions that are forced through zero. In the case of arsenic this certainly brings an issue. As an alternative, one might consider for instance removing all data below LOQ from the data set, or input the LOD or a percentage of the LOQ in place of the LOQ. Any of these choices remains arbitrary and would not alter the bases of the conclusions. To gauge the uncertainty brought by the limitations of the analytical determinations, the results of the calculations obtained by removing all data below LOQ from the data sets are also given in Table 6.
Fig. 1.
Linear regression plot (forced through zero) for cadmium transfer rate to mainstream smoke versus that for nicotine, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon.
Fig. 2.
Linear regression plot (forced through zero) for lead transfer rate to mainstream smoke versus that for nicotine, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon.
Fig. 3.
Linear regression plot (forced through zero) for arsenic transfer rate to mainstream smoke versus that for nicotine, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon.
Fig. 4.
Linear regression plot (forced through zero) for cadmium transfer rate to mainstream smoke versus that for nicotine, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon.
Fig. 5.
Linear regression plot (forced through zero) for lead transfer rate to mainstream smoke versus that for nicotine, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon.
Fig. 6.
Linear regression plot (forced through zero) for arsenic transfer rate to mainstream smoke versus that for nicotine, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon.
Fig. 7.
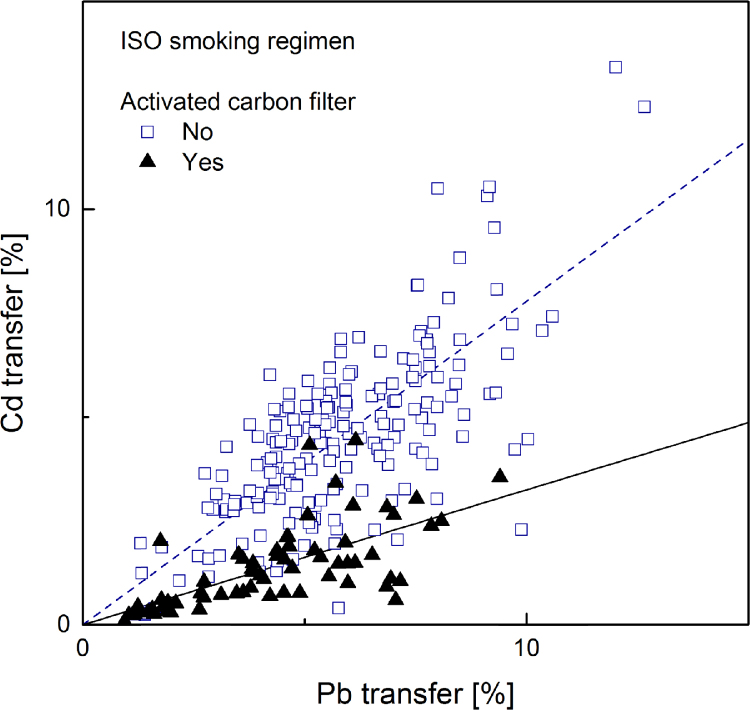
Linear regression plot (forced through zero) for cadmium transfer rate to mainstream smoke versus that of lead, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon. Smoke generated under ISO machine smoking regime (267 samples).
Table 6.
Slopes from plots of elements transfer against nicotine transfer ± standard error samples with and without activated carbon smoked under 2 machine-smoking regimes.
| Analyte | Data < LOQ input as LOQ: | ISO smoking regime |
HCI smoking regime |
||
|---|---|---|---|---|---|
| No carbon | Activated carbon | No carbon | Activated carbon | ||
| Cadmium | Included | 0.72 ± 0.02 | 0.31 ± 0.01 | 0.88 ± 0.01 | 0.58 ± 0.01 |
| Not included | 0.73 ± 0.02 | 0.31 ± 0.02 | 0.88 ± 0.01 | 0.58 ± 0.01 | |
| Lead | Included | 0.90 ± 0.02 | 0.87 ± 0.03 | 0.98 ± 0.01 | 1.00 ± 0.02 |
| Not included | 0.86 ± 0.03 | 0.81 ± 0.03 | Unchanged | Unchanged | |
| Arsenic | Included | 0.35 ± 0.01 | 0.33 ± 0.02 | 0.35 ± 0.01 | 0.36 ± 0.01 |
| Not included | 0.29 ± 0.01 | 0.23 ± 0.01 | 0.54 ± 0.03 | 0.54 ± 0.03 | |
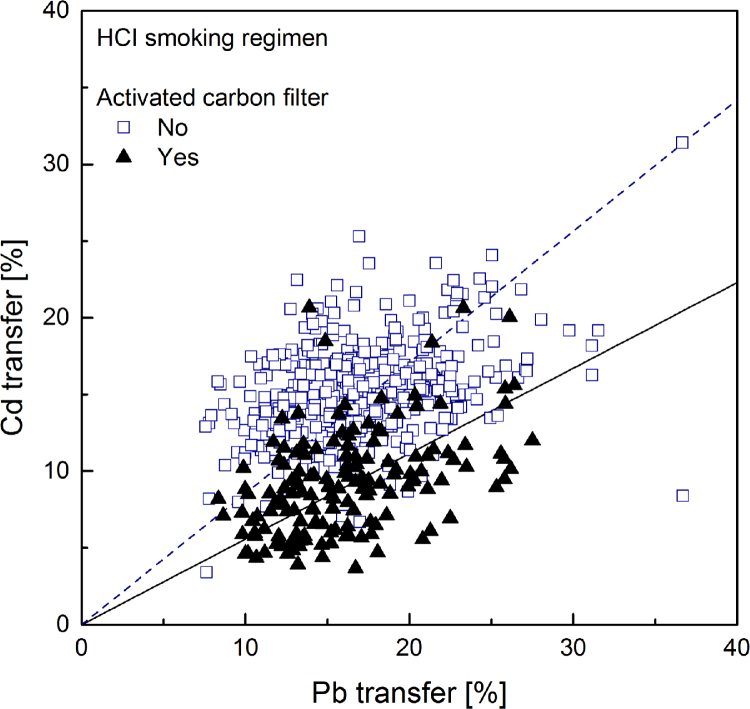
Cadmium transfer was plotted against lead transfer for all samples in order to more accurately estimate their relative importance. The plots from smoke data obtained under ISO and HCI machine-smoking regimes are given in Fig. 7, Fig. 8 respectively.
Fig. 8.
Linear regression plot (forced through zero) for cadmium transfer rate to mainstream smoke versus that of lead, computed separately for samples with (solid regression line) and without (dotted regression line) activated carbon. Smoke generated under HCI machine smoking regime (567 samples).
Linear regressions forcing the intercept to zero were calculated from plots of cadmium transfer as a function of lead transfer shown in Fig. 7, Fig. 8. Separate regressions were made for samples with activated carbon in the filter and samples without any activated carbon. The calculated slopes and the associated standard error are reported in Table 7.
Table 7.
Slopes from plots of cadmium transfer against lead transfer (Fig. 7, Fig. 18) comparing samples with and without activated carbon smoked under 2 machine-smoking regime.
| ISO smoking regime | HCI smoking regime | |
|---|---|---|
| No carbon in filter | 0.78 ± 0.02 | 0.85 ± 0.01 |
| Activated carbon filter | 0.32 ± 0.02 | 0.56 ± 0.01 |
Results expressed as slope ± standard error.
3.4. Results from dedicated prototype cigarettes
To further ensure that the observed selective filtration of cadmium can really be attributed to the presence of activated carbon in the market survey samples, we prepared a specific set of prototypes, as described in Section 2, differing only in the presence or not of activated carbon in the filter. These prototypes were smoked under HCI machine-smoking regime and the 3 selected elements Cd, Pb, and As were measured. The means and standard error of the mainstream smoke yield of each element are presented in Table 8, expressed both on a per-cigarette basis and normalized to the nicotine yields.
Table 8.
Elements smoke yields under HCI machine-smoking regime, expressed per cigarette and per nicotine yield, for prototypes with and without activated carbon in the filter.
| Analyte | HCI yields per cigarettea (ng/cigarette) |
HCI yields per mg nicotine (ng/mg nicotine) |
||
|---|---|---|---|---|
| No carbon in filter | 80-mg activated carbon in filter | No carbon in filter | 80-mg activated carbon in filter | |
| Cadmium | 74 ± 4 | 28 ± 1 | 43 | 16 |
| Lead | 18.8 ± 0.4 | 19.4 ± 0.4 | 11.0 | 11.3 |
| Arsenic | 3.3 ± 0.2 | 3.6 ± 0.1 | 1.9 | 2.1 |
Results expressed as mean ± standard error.
4. Discussion
The results obtained for cadmium, arsenic and lead in mainstream smoke demonstrate that a selective retention of cadmium is occurring in activated carbon filters, while it is not the case for arsenic and lead. This phenomenon is possible only if cadmium is present to some degree in the gas-phase of mainstream smoke [44], [45]. After reviewing the results obtained for cigarette fillers and mainstream smoke, the discussion focuses on possible explanations for the differences in filtration observed in the case of cadmium.
4.1. Cadmium, lead and arsenic levels in cigarette fillers
The observed cadmium levels obtained from a large set of worldwide commercial samples are consistent with previously reported distributions for smaller, single-country data sets [9], [46], [47], [48], [49], [50], as well as with the levels reported for 755 samples of Burley, flue-cured and oriental tobacco leaves collected in 13 countries during the period 2001–2003 [51].
The distribution of lead levels measured from 568 samples in the present study is consistent with the distributions of smaller datasets recently reported for single countries [9], [46], [47], [48], [49], [50], [52], [53], [54], [55], [56], [57], but substantially lower than the distributions given in older reports [58], [59]; this is essentially linked to the appearance of unleaded gasoline in the eighties.
The distribution of arsenic levels is in line with the results obtained from smaller datasets of commercial products from single countries [48], [50], [55], and the mean of 400 ng/g obtained from 1431 samples of Burley, flue-cured and oriental tobacco leaves collected in 20 countries during the period 2002–2004 [60].
4.2. Cadmium, lead and arsenic levels in cigarette mainstream smoke
The observed cadmium levels in mainstream smoke generated under ISO and HCI machine-smoking regime are consistent with data obtained for smaller datasets [30], [46], [48], [61], [62], [63], [64]; the present data are much narrower in range than the historical results provided in an early review [65]. The ranges and median values for cadmium smoke yields expressed per mg nicotine are slightly higher under the more intense smoking regime. This is in line with the results reported for Canadian [48] or American brands [9] as well as in worldwide surveys [61]; it shows that cadmium yield increases more than nicotine yield when the intensity of the smoking regime is increased.
The lead levels in mainstream smoke generated under ISO and HCI machine-smoking regimes are consistent with results obtained from smaller datasets [30], [46], [48], [61], [62], [64], [66] and narrower than the range of historical results provided in an early review [65]. If adjusted for nicotine yields, the range and median values for lead yields are very similar under both regimes, suggesting that, in contrast to cadmium yields, lead yields behave in the same way as nicotine when machine-smoking conditions are changed.
The arsenic levels in mainstream smoke (for the samples above LOQ) are slightly higher than previously reported for UK brands [62], or international brands from Philip Morris [61], while their distribution is clearly narrower than the span of historical data gathered in an earlier review in which levels up to 1400 ng/cigarette had been reported [65].
Even after nicotine normalization, the range of elements yields was wide, which was a consequence of the spread of the elements levels in tobacco.
4.3. Evidence for cadmium specific filtration from mainstream smoke
4.3.1. Market surveys data
Of particular interest in the market surveys data is the fact that, at equal nicotine transfer, the cadmium transfer in a sample containing activated carbon in the filter is much smaller than that of a sample without carbon in the filter. No such trend could be observed in the data regarding either lead or arsenic. This is readily apparent by visually comparing Fig. 1, Fig. 4 – showing cadmium transfer against nicotine transfer, to the similar plots obtained for lead and arsenic, or by directly comparing cadmium and lead transfers among all samples in Fig. 7, Fig. 8.
This effect can be quantified using the slopes of the regression lines reported in Table 6. Considering the ratio of slopes as the ratio of averaged yields of samples with equal nicotine transfer, the results correspond to cadmium yields reductions in the presence of activated carbon amounting to 57% in smoke generated under the ISO machine-smoking regime and 34% under the HCI machine-smoking regime. Direct comparison of cadmium and lead transfers (Table 7) shows that for smoke generated under the ISO regime cadmium transfer is about 22% lower than that of lead. It is 15% lower under the HCI regime; the more intense puffing and the suppression of the filter ventilation weaken the efficiency of cadmium filtration. As expected, in the presence of activated carbon this difference is substantially increased. The present results are in agreement with those reported in the survey of the Japanese market [63].
4.3.2. High carbon level prototypes data
The mainstream smoke yields of cadmium, lead and arsenic from cigarettes with a very high load of activated carbon (80 mg) in the filter were compared to those of the matched control. Smoking was done under the HCI regime, which is less favorable to selective filtration by adsorbents than the ISO regime [67], [68]. Results clearly show a selective retention of cadmium in the activated carbon-containing prototype, cadmium yield being reduced by 62%. Adjusting for nicotine yields provides exactly the same result. As observed in the market survey, lead and arsenic yields were essentially unchanged (3% and 10% higher for the activated carbon-containing prototype respectively). These results are in agreement with the reported mainstream smoke yields of a series of products from a market test addressing extreme levels of activated carbon [69]. Cadmium yields were uniformly reduced by about 69% in cigarettes containing 60, 80 and even 120 mg of activated carbon, while yields of lead and arsenic were unchanged. The fact that cadmium removal is the same at all filter loadings suggests that all available gas-phase cadmium was already retained by the filter with a 60-mg carbon load.
4.4. Issues regarding the different filtration of cadmium and lead
There is strong evidence showing that cadmium can be partially removed from cigarette mainstream smoke by activated carbon, and is therefore present in notable amounts in the smoke gas-phase. There is no indication that this could be true for lead or arsenic. Although this is the first time that such an observation has been made using a very large and diverse set of cigarettes, different studies have already identified the fact that cadmium retention in the butt was higher than that for nicotine [47], [70], [71], [72], and the effect of activated carbon was mentioned in a survey of the Japanese market [63].
Of issue is the fact that published literature regarding the presence of metals in gas-phase smoke is not consistent with either the observed selective adsorption of up to 70% of cadmium or the total absence thereof in the case of lead. Indeed, values between <1% and up to 28% were reported for cadmium in the gas-phase, while substantially larger values, between 18% and 71%, were reported for lead [70], [72], [73], [74], [75], [76], [77]. This cannot be attributable to mere sampling issues, since observations of higher proportions of lead in the gas-phase compared to cadmium were derived in most cases from analysis of the same sample.
The goal of the following investigation was therefore to clarify the physical chemistry behind the transfer and retention of cadmium, and compare it to that of lead. There is a wealth of information that can be used. The heating undergone by metals during cigarette smoldering and puffing is fairly well known in terms of time and temperature. Detailed information is also available regarding elements speciation in the course of thermal processes (biomass combustion or gasification, refuse incineration, or smelting for extractive metallurgy) and the impact of speciation on their volatility is known. Such studies can provide insights on metals volatility and reactivity under the conditions of smoke generation. Conclusions could therefore be postulated regarding elements speciation that would match, when a cigarette is smoked, their distribution among ash, sidestream and mainstream smoke, and account for the observed filter retention.
4.5. Elements distribution among smoke streams, ash and butt
A number of studies reported the levels of elements in the tobacco filler of a set of cigarettes, together with smoke yields [46], [72], [75], [77], [78], [79], [80], [81], [82]. In some studies the results were supplemented with information on the elements levels in ashes or butt after smoking. All studies were performed under the ISO machine-smoking regime. The data were scattered, reflecting differences in the cigarettes design and very different study protocols or methods [83], [84]. The following conclusions can nevertheless be drawn.
Cadmium transfer from tobacco to sidestream smoke is well documented, and ranges between 40% and 55%. It is collected with the particulate matter [79]. Lead transfer to sidestream smoke is less precisely established, but indications are that it could be much lower than that for cadmium. Lower values were found whenever sidestream smoke yield was directly measured rather than calculated by difference. Transfers as low as 2–5% were then observed [81], [79], the latter team having used a standard sampling method [85]. Ash retention is moderate for cadmium (about 20–30%) but higher for lead and arsenic (at least 50%, up to 75%). Cadmium transfer to ISO mainstream smoke is about 3–10% for a filter cigarette, up to 22% for a non-filter cigarette. From the regressions of market data obtained in the present study, cadmium transfer is only 72% of that for nicotine, i.e., about 20% lower than that for lead. This means for lead a transfer in the range of 3–12% for a filter cigarette, similar to what is cited in recent reviews [9], [84]. Of the cadmium that exits a cigarette filter devoid of adsorbing material, two thirds can be removed by activated carbon, while this is not observed for lead or arsenic. When the amount of activated carbon is increased the amount of retained cadmium reaches a plateau at ca. 70%. This suggests that in mainstream smoke some of the cadmium species are partially present in the gas-phase.
4.6. Inferred elements speciation in high-temperature processes
From the information available from studies of other thermal processes, inferences can be made on the elements speciation during their volatilization from tobacco through a thermal process and their transport within a multi-phase system. The following discussion covers the high temperature behavior of elements, their ensuing reactivity at elevated temperature, and the potential transfer of the airborne elements, both to sidestream and mainstream smoke, including deposition and filtration.
4.6.1. Elements volatilization
Speciation in tobacco: Elements speciation has an impact on thermal volatilization, therefore speciation of the investigated elements in tobacco is an important factor. Cadmium is efficiently taken up by tobacco from the soil and transported systemically throughout the whole plant, either bound (e.g., to glutathione) or chelated (e.g., to peptides) [86]. Cadmium is primarily present as a part of organic molecules and can therefore be released at a low temperature [87]. Conversely, lead was reported to be present as PbS, PbOH or PbCO3 [88], or bound to inorganic material with P, Mn, Si or Al [89], forms from which lead is difficult to volatilize [90]. In tobacco, arsenic is mainly present as inorganic matter, partly identified as arsenites As(III)O33− and arsenates As(V)O43−. The As(V) species are often predominant and are the least volatile [91], [92], [93], [94].
Volatilization: Downstream of the combustion zone the atmosphere is very hot (ca. 900 °C) and reducing (essentially devoid of oxygen and rich in H2 or CH4). Under such conditions cadmium can be released in the gas phase as Cd(0) [95]. Cd(0) is thermodynamically preferred [96] but e.g., CdCO3 decomposition would directly yield CdO [90]. Cadmium release from biomass is very effective, higher than 90% above 450 °C [97]. Some lead volatilization from biomass is observed above 500 °C, but the interaction with the matrix in which lead is embedded is a limiting factor [87]. Only about 85% of the lead present in wood could be volatilized by pyrolysis at 850 °C, essentially as Pb and PbO [98], [87]. In cigarette smoke generation, such interactions would cause most of the lead to remain in the ash. Arsenic is released as As(III) in a reducing atmosphere, mostly As2O3 [99]. As(III) derivatives can be released from biological material above 600 °C [100], but arsenic is highly prone to interactions with other elements that cause it to remain in the ash, in particular with sulfur [95] and calcium – e.g., from CaCO3 present in the cigarette paper [99], [101], [90] that would yield calcium arsenate [102]. A high retention of arsenic in the ash is therefore expected. In ash As(III) is further oxidized to As(V) [92].
4.6.2. Elements high-temperature reactions
The elements’ gas-phase reactivity is critical, since elements’ speciation has a large impact on their volatility. The major inorganic elements in tobacco that could react are potassium (ca. 4%), calcium (ca. 2–3%), chlorine (0.5–2%) magnesium (ca. 0.6%), sulfur (0.2–0.6%), phosphorous (ca. 0.4%) and sodium (ca. 0.1%) [103], [104], [105], [106], [107].
Cadmium in fresh smoke collected at the filter exit has been shown to be in the Cd(II) oxidation state [108]. This implies that cadmium, emitted as Cd(0) as detailed above, undergoes oxidative reactions. This may be from reaction with oxygen diffusing from the outside air, forming CdO. Reaction with sulfur is less likely, as biomass sulfates only release sulfur above 850 °C [109]. Furthermore, sulfide formation is hindered by calcium (present in high amount in tobacco and paper) [101]. Both CdO and CdS being non-volatile, they will be either in the ash or in the smoke particle-phase. From thermodynamics, chlorides are favored over sulfides above 300 °C for both cadmium and lead [96]. They were found to be the preferred species up to at least 600 °C [110], [111]. The reaction of lead with chloride, even from lead oxide, is spontaneous at elevated temperature [111], and no PbCl4 is formed [109]. CdCl2 and PbCl2 are the most volatile documented species for lead and cadmium. Indeed, chloride formation is used to increase the volatilization of both cadmium and lead in high temperature treatments [95]. Chloride formation is expected to take place in cigarettes. Large amounts of melted KCl crystals were found in a cigarette extinguished during a puff both in front of the char line [103] and in the ash [112], demonstrating chloride availability. Chlorine content of straw (0.5–2%) is very similar to that of tobacco and large amounts of CdCl2 are found in fly ash from straw combustion [113]. In theory, a reaction with chlorine is also possible for arsenic. If released as the volatile species As2O3, arsenic can react with chlorine to yield AsCl3, a volatile compound [95]. In both As2O3 and AsCl3 arsenic is in the As(III) oxidation state, the speciation shown as mostly prevalent in fresh cigarette smoke [92], [93].
4.6.3. Elements transfer under puffing conditions
As vapors move away from the burning coal their temperature drops very fast, causing most elemental species to nucleate or deposit. Elements can deposit on aerosol particles, remaining airborne. If they deposit on tobacco, they may be mobilized in a consecutive puff. The temperature at which lead and cadmium will deposit depends on their speciation. In biomass fluidized bed gasification, cadmium in the exit gas is still found mostly in the gas-phase at 380 °C but lead condenses to the particle-phase as soon as the temperature drops to below 500 °C [97]. This is, however, in the absence of chlorine. Pure CdCl2 starts vaporizing above 400 °C [111]. Chlorides are the most volatile documented species for lead and cadmium, being liberated at 600 °C from most matrices [114]. In a study performed under reduced pressure on pure PbCl2 and CdCl2, nanometer scale nucleation was observed below 150 °C [115]. Indeed, PbCl2 and CdCl2 were shown to be removed by filtration from an aerosol at 120 °C [114]. In cigarette mainstream smoke, they are therefore part of the TPM when they reach the filter.
4.6.4. Elements transfer under smoldering conditions
Since according to [115] CdCl2 could be sublimed in substantial amounts at 400 °C, this cadmium species should readily transfer to sidestream smoke since gases escape from a smoldering cigarette at about 350 °C [116]. As CdCl2 condenses out of the gas-phase below 150 °C, it should be a particle-phase compound immediately after leaving the cigarette. The same conclusions should apply to PbCl2 except that the lead species may be liberated at higher temperature with a lower yield. These conclusions are fully consistent with observations made from sidestream smoke sampling when using the fishtail method [117]. Only 2 and 4% of the sidestream smoke yield of lead and cadmium were respectively found deposited on the collection flask, showing their presence in the particle-phase. Conversely, 28% of the arsenic was retained on the walls, showing a presence of volatile arsenic, likely in the form As2O3 [79].
4.7. Conclusions from a consolidation of published information
The amount retained in ash can be understood from a review of available literature. Cadmium is always reported as being more volatile than lead during thermal treatments. In tobacco it is primarily present bound to organic material and is therefore mobilized at relatively low temperature. Lead and arsenic are present in a large part as inorganic, non-volatile compounds and can readily form such compounds upon tobacco combustion, notably by reaction with calcium. This explains the observed differences in the amounts found in ashes (Cd 20–30%, Pb and As 50–70%). The transfer to sidestream smoke can also be understood from published information. The fact that approximately 40–55% of cadmium present in a cigarette is exhausted to sidestream smoke and collected with the particulate matter is consistent with the formation of CdCl2, where cadmium is in the Cd(II) oxidation state, as expected from speciation studies [108]. Lead can also be chlorinated, but a much lower transfer is observed. This is likely because less lead is volatilized, although the extent of the difference in sidestream transfer between lead and cadmium could be associated with the presence of a volatile cadmium derivative. In mainstream smoke, both lead and cadmium are expected to be present as oxides or chlorides, all derivatives in the particulate matter at filter level. Overall, the transfer of lead and cadmium to mainstream smoke should not be very different. The fact that cadmium is selectively retained by activated carbon in a cigarette filter, while lead is not, shows that some reactions remain unaccounted for and suggests that a large part of the cadmium (and not lead) is present as a gas-phase species, even at temperatures approaching ambient. This species is unlikely to be CdCl2, first because the same retention would be observed with lead (PdCl2 and CdCl2 share similar physical properties) [109], but also because both metal di-chlorides have been shown to be only present in the particulate matter below 150 °C [115]. PbCl4, absent from high temperature chlorine reaction products, is not expected to be found in smoke [109].
4.8. Alternative mechanism for cadmium transfer to cigarette smoke
A remaining possibility is the reaction of cadmium with radicals. Primary radicals, mostly carbon-centered such as alkyl radicals, are formed by tobacco decomposition in the hot zone. These very reactive species can further react to yield secondary radicals, some carbon-centered like acyl or alkylamino radicals, but most oxygen-centered [118]. Primary radicals do not react in totality and, in fact, both methyl and ethyl radicals were observed as important radical species in mainstream smoke at filter exit. The yield of carbon-centered radicals from the reference cigarette 2R4F smoked with the ventilation blocked was estimated at 265 nmole/cig. [119].
Gas-phase reaction of cadmium with short hydrocarbon radicals can yield organometallic derivatives. Indeed a well-studied and documented example is the reaction with methyl radicals. Cd(0) and CH3˙ readily react in the gas-phase to yield dimethylcadmium Cd(CH3)2 at or below 300 °C [120], [121]. Unlike CdCl2 or CdS, Cd(CH3)2 is a volatile compound (bp 105.5 °C), which readily reacts with water to yield cadmium hydroxide but does not oxidize spontaneously in air. In fact, there is a gradation in stability among the Group 12 methyl derivatives, with Cd(CH3)2 ranking in an intermediate position between dimethylmercury, quite stable and dimethylzinc, very reactive toward oxygen and water [122]. Indicative of its stability, Cd(CH3)2 toxicity could be assessed, including through animal inhalation studies, and a maximum 8-h work-place exposure has been set at 1 μg/m3 [123].
While CH3• is the most abundant alkyl radical generated in the high temperature zone, homologue radicals with higher carbon content are also present that could react in the same way. In fact many other radicals present in smoke could be expected to react with Cd(0) but very little information is available on such reactions. Thus, the following discussion is focused on Cd(CH3)2, since its reactivity is well documented and it is epitomical when discussing the consequences of the transitory formation of a volatile and reactive cadmium derivative. It should however be understood that Cd(CH3)2 may not be the main cadmium volatile intermediate that is actually formed in smoke.
4.9. Expected outcome of cadmium radical reactions
4.9.1. Transfer to mainstream smoke
Cd(CH3)2 could certainly move to the filter during a puff, and exit the cigarette with mainstream smoke. Because of its reactivity, Cd(CH3)2 will deposit onto the unburnt tobacco downstream with a high efficiency; yet, elements captured on the unburnt tobacco during a puff can be mobilized in subsequent puffs, so that this capture is not incompatible with the observed cadmium transfer to mainstream smoke (only 5–10%). The consequence of this high capture is a yield per puff that increases with puff number, which has indeed been observed [78]. Moreover, in such a case it is expected that a higher smoke flow rate through the tobacco rod would decrease the retention of gas-phase cadmium since it is diffusion-controlled. This was also observed. Compared to the ISO yields, cadmium yield was found to be more increased under HCI than nicotine was, whereas lead yield remains to a constant ratio to nicotine (Table 6, Table 8). Specifically, a high and flow rate-sensitive capture of cadmium by the tobacco filler was evidenced by studies where the deposited cadmium was separately assessed in the unburnt tobacco and in the filter plug after machine-smoking the cigarettes using both ISO conditions and undefined “heavy” puffing conditions [82].
4.9.2. Transfer to sidestream smoke
The fact that elements captured on the unburnt tobacco during a puff can be mobilized by subsequent heating also increases the possibility of transfer to sidestream smoke. Hot gases can diffuse out of a smoldering cigarette as sidestream emission, the temperature of this gas stream is about 350 °C [116]. Cadmium can diffuse out as CdCl2, which would be gaseous. This is above the decomposition temperature of Cd(CH3)2, which is below 300 °C, however, and Cd(CH3)2 is unlikely to be present in sidestream smoke.
4.9.3. Mainstream smoke selective filtration
The main consequence of the formation of Cd(CH3)2 is of course the fact that it would be efficiently adsorbed on a carbon bed in the filter. Any Cd(CH3)2 retained on an adsorbent, or a Cambridge filter, should be readily hydrolyzed by the water present on the surface. This yields the hydroxide, a Cd(II) species that is not volatile. The fact that adsorbed cadmium cannot be re-emitted may in part explain why cadmium selective filtration remains effective under the HCI machine-smoking regime, in contrast with other volatile compounds for which adsorption processes are strongly hindered under these conditions.
In fact Cd(CH3)2 reactivity with water contributes to making its presence in smoke plausible. Since most Cd(CH3)2 would be trapped by a Cambridge filter, it is counted as particle-phase material, explaining how a low and variable gas-phase percentage could be observed and reported in the literature, while in fact up to 60% of the cadmium can actually be retained by an activated carbon.
4.9.4. Difference between cadmium and lead
No report of the formation of a lead derivative through gas-phase reaction of Pb(0) with free radicals could be found in the literature. Even if some derivatives could be made, it is likely that, unlike Cd(0), Pb(0) would condense out of the gas-phase before the temperature would be low enough for an organo-compound to be stable. These observations may explain why lead is not selectively retained by activated carbon in the filter.
5. Conclusions
The study of arsenic, cadmium and lead levels in tobacco and smoke was performed on a set of surveys gathering a large number of samples (568) with a large diversity of origins, tobacco blend types and cigarette designs. This ensured that the observations accurately reflected the trends and correlations that prevailed among the samples while allowing a greater degree of precision than previously obtained by using smaller data sets.
For comparative purposes, the elements transfer in each sample was estimated from the ratio of their mainstream smoke yield to the elements level in the tobacco rod. Cadmium transfer was clearly lower in the cigarettes with an activated carbon filter compared to the other samples with the same nicotine transfer. This was not observed in the case of lead or arsenic. The effect was also observed under the more intense HCI regime despite being less pronounced than under the ISO regime. Test cigarettes with an 80-mg carbon load smoked under HCI machine-smoking regime showed a 62% retention of cadmium, while arsenic and lead yields were unchanged.
The distribution of the elements levels in the tobacco of the sampled cigarettes was rather wide, but the levels are close to most of the recent results reported either for specific countries or for international datasets. They are lower than those reported in older datasets, possibly due to a reduced contamination from the environment and changes in agronomic practices. The observed variability of the elements smoke yields normalized to nicotine remains quite large in this study. It is essentially due to the variability of the tobacco content of the elements, with the exception of the reduced cadmium yields observed in the cigarettes containing activated carbon in their filter.
From the large body of literature on heavy metals levels and yields, it appears that the specificity of cadmium can be traced to its volatility, such that the amount sequestered in the ash is no more than 20–30% while volatile cadmium chloride can readily transfer to the sidestream smoke, where about 45% of the cadmium originally present in the tobacco is found. Conversely, 50–75% of lead and arsenic are retained in the ash and the lower volatility of lead results in a lower yield of chloride conversion. Estimates for the levels of lead in sidestream smoke are much less precise than those for cadmium; they are also lower, in some studies accounting for only a few percent of the tobacco content. The reason for the increased removal of cadmium from mainstream smoke when activated carbon is present in the filter is yet to be proven, but a potential explanation is the formation of cadmium organometallic derivatives from free-radical reactions in the smoke gas-phase at intermediate temperature (300 °C and below). Dimethylcadmium, in particular, can be formed under these conditions. Such compounds are not stable in the presence of water, but their transitional occurrence during the smoke transfer through the cigarette could explain the strong experimental evidence made regarding metals selective filtration that is otherwise difficult to reconcile with published data on cadmium transfer and phase distribution in smoke.
Transparency document
References
- 1.Järup L. Hazards of heavy metal contamination. Br. Med. Bull. 2003;68:167–182. doi: 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- 2.Sharma R.K., Agrawal M. Biological effects of heavy metals: an overview. J. Environ. Biol. 2005;26:301–313. [PubMed] [Google Scholar]
- 3.Adams S.V., Passarelli M.N., Newcomb P.A. Cadmium exposure and cancer mortality in the Third National Health and Nutrition Examination Survey cohort. Occup. Environ. Med. 2012;69:153–156. doi: 10.1136/oemed-2011-100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.IARC . Lyon; France: 2012. Cadmium and Cadmium Compounds, A Review of Human Carcinogens: Arsenic, Metals, Fibres, and Dusts; pp. 121–145. [Google Scholar]
- 5.Bhattacharyya M.H. Cadmium osteotoxicity in experimental animals: mechanisms and relationship to human exposures. Toxicol. Appl. Pharmacol. 2009;238:258–265. doi: 10.1016/j.taap.2009.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Järup L., Akesson A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009;238:201–208. doi: 10.1016/j.taap.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 7.Satarug S., Garrett S.H., Sens M.A., Sens D.A. Cadmium, environmental exposure, and health outcomes. Environ. Health Perspect. 2010;118:182–190. doi: 10.1289/ehp.0901234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thévenod F., Lee W.K. Toxicology of cadmium and its damage to mammalian organs. Met. Ions Life Sci. 2013;11:415–490. doi: 10.1007/978-94-007-5179-8_14. [DOI] [PubMed] [Google Scholar]
- 9.Caruso R.V., O’Connor R.J., Stephens W.E., Cummings K.M., Fong G.T. Toxic metal concentrations in cigarettes obtained from U.S. smokers in 2009: results from the International Tobacco Control (ITC) United States survey cohort. Int. J. Environ. Res. Publ. Health. 2014;11:202–217. doi: 10.3390/ijerph110100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pappas R.S. Toxic elements in tobacco and in cigarette smoke: inflammation and sensitization. Metallomics. 2011;3:1181–1198. doi: 10.1039/c1mt00066g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Health-Canada . 2000. Canadian Ministry of Justice: Tobacco Reporting Regulations, SOR/2000-273. Registration 2000-06-26. Part 3: Emissions from Designated Tobacco Products. [Google Scholar]
- 12.U.S. Department of Health and Human Services. Food and Drug Administration . 2012. Harmful and Potentially Harmful Constituents in Tobacco Products and Tobacco Smoke; Established List. Federal Register 77, 20034-20037. [Google Scholar]
- 13.WHO, Study-Group . 2008. Mandated Lowering of Toxicants in Cigarette Smoke: Tobacco-specific Nitrosamines and Selected Other Constituents, The Scientific Basis of Tobacco Product Regulation. WHO Technical Report Series 951; pp. 45–124. [Google Scholar]
- 14.WHO . 2012. Toxic elements in tobacco and in cigarette smoke, The Scientific Basis of Tobacco Product Regulation Fourth Report of a WHO Study Group; WHO Technical Report Series 967, Annex 1. [PubMed] [Google Scholar]
- 15.Cunningham F.H., Fiebelkorn S., Meredith C. A novel application of the Margin of Exposure approach: segregation of tobacco smoke toxicants. Food Chem. Toxicol. 2011;49:2921–2933. doi: 10.1016/j.fct.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 16.Pankow J.F., Watanabe K.H., Toccalino P.L., Luo W., Austin D.F. Calculated cancer risks for conventional and “Potentially Reduced Exposure Product” cigarettes. Cancer Epidemiol.: Biomark. Prev. 2007;16:584–592. doi: 10.1158/1055-9965.EPI-06-0762. [DOI] [PubMed] [Google Scholar]
- 17.Xie J., Marano K.M., Wilson C.L., Liu H., Gan H., Xie F., Naufal Z.S. A probabilistic risk assessment approach used to prioritize chemical constituents in mainstream smoke of cigarettes sold in China. Regul. Toxicol. Pharmacol. 2011;62:355–362. doi: 10.1016/j.yrtph.2011.10.017. [DOI] [PubMed] [Google Scholar]
- 18.Marano K.M., Naufal Z.S., Kathman S.J., Bodnar J.A., Borgerding M.F., Garner C.D., Wilson C.L. Cadmium exposure and tobacco consumption: biomarkers and risk assessment. Regul. Toxicol. Pharmacol. 2012;64:243–252. doi: 10.1016/j.yrtph.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Tellez-Plaza M., Navas-Acien A., Caldwell K.L., Menke A., Muntner P., Guallar E. Reduction in cadmium exposure in the United States population, 1988–2008: the contribution of declining smoking rates. Environ. Health Perspect. 2011;120:204–209. doi: 10.1289/ehp.1104020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brito J.A.A., Costa I.M., e Silva A.M., Marques J.M.S., Zagalo C.M., Cavaleiro I.I.B., Fernandes T.A.P., Gonçalves L.L. Changes in bone Pb accumulation: cause and effect of altered bone turnover. Bone. 2014;64:228–234. doi: 10.1016/j.bone.2014.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Farias P., Echavarria M., Hernandez-Avila M., Villanueva C., Amarasiriwardena C., Hernandez L., Aro A., Hu H. Bone, blood and semen lead in men with environmental and moderate occupational exposure. Int. J. Environ. Health Res. 2005;15:21–31. doi: 10.1080/09603120400018782. [DOI] [PubMed] [Google Scholar]
- 22.Theppeang K., Glass T.A., Bandeen-Roche K., Todd A.C., Rohde C.A., Links J.M., Schwartz B.S. Associations of bone mineral density and lead levels in blood, tibia, and patella in urban-dwelling women. Environ. Health Perspect. 2008;116:784–790. doi: 10.1289/ehp.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barregard L., Fabricius-Lagging E., Lundh T., Mölne J., Wallin M., Olausson M., Modigh C., Sallsten G. Cadmium, mercury, and lead in kidney cortex of living kidney donors: impact of different exposure sources. Environ. Res. 2010;110:47–54. doi: 10.1016/j.envres.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 24.Forte G., Madeddu R., Tolu P., Asara Y., Marchal J.A., Bocca B. Reference intervals for blood Cd and Pb in the general population of Sardinia (Italy) Int. J. Hyg. Environ. Health. 2011;214:102–109. doi: 10.1016/j.ijheh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 25.Marano K.M., Naufal Z.S., Kathman S.J., Bodnar J.A., Borgerding M.F., Wilson C.L. Arsenic exposure and tobacco consumption: biomarkers and risk assessment. Regul. Toxicol. Pharmacol. 2012;64:225–232. doi: 10.1016/j.yrtph.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Batáriová A., Spěváčková V., Beneš B., Čejchanová M., Šmíd J., Černá M. Blood and urine levels of Pb, Cd and Hg in the general population of the Czech Republic and proposed reference values. Int. J. Hyg. Environ. Health. 2006;209:359–366. doi: 10.1016/j.ijheh.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Bjermo H., Sand S., Nälsén C., Lundh T., Enghardt Barbieri H., Pearson M., Lindroos A.K., Jönsson B.A.G., Barregård L., Darnerud P.O. Lead, mercury, and cadmium in blood and their relation to diet among Swedish adults. Food Chem. Toxicol. 2013;57:161–169. doi: 10.1016/j.fct.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 28.Bernhard D., Rossmann A., Wick G. Metals in cigarette smoke. IUBMB Life. 2005;57:805–809. doi: 10.1080/15216540500459667. [DOI] [PubMed] [Google Scholar]
- 29.Counts M.E., Hsu F.S., Laffoon S.W., Dwyer R.W., Cox R.H. Mainstream smoke constituent yields and predicting relationships from a worldwide market sample of cigarette brands: ISO smoking conditions. Regul. Toxicol. Pharmacol. 2004;39:111–134. doi: 10.1016/j.yrtph.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Krivan V., Schneider G., Baumann H., Reus U. Multi-element characterization of tobacco smoke condensate. Fresen. J. Anal. Chem. 1994;348:218–225. [Google Scholar]
- 31.Health-Canada . Analytical methods to support reporting regulations under the Tobacco Act. In: Canadian-Ministry-of-Justice, editor. Tobacco Reporting Regulations. Part 3: Emissions from Designated Tobacco Products Modified: 2007-11-28, Ottawa, OT, Canada. 2007. [Google Scholar]
- 32.Health-Canada . 1999. Method T-301, Determination of Alkaloids in Whole Tobacco, SOR/2000-273. Registration 2000-06-26. Canadian Ministry of Justice: Tobacco Reporting Regulations. Part 3: Emissions from Designated Tobacco Products. Available at: http://www.hc-sc.gc.ca/hc-ps/alt_formats/hecs-sesc/pdf/tobac-tabac/legislation/reg/indust/method/_whole-entier/alkaloids-eng.pdf. [Google Scholar]
- 33.Health-Canada . 1999. Method T-306, Determination of Ni, Pb, Cd, Cr, As, Se and Hg in Whole Tobacco, SOR/2000-273. Registration 2000-06-26. Canadian Ministry of Justice: Tobacco Reporting Regulations. Part 3: Emissions from Designated Tobacco Products. Available at: http://www.hc-sc.gc.ca/hc-ps/alt_formats/hecs-sesc/pdf/tobac-tabac/legislation/reg/indust/method/_whole-entier/metal-eng.pdf. [Google Scholar]
- 34.ISO-3402 . 4th ed. International Organization for Standardization; 1999. Tobacco and Tobacco Products—Atmosphere for Conditioning and Testing. [Google Scholar]
- 35.ISO-3308 . 4th ed. International Organization for Standardization; Geneva, Switzerland: 2000. Routine analytical cigarette-smoking machine – Definitions and standard conditions. [Google Scholar]
- 36.Health-Canada . 1999. Method T-115, Determination of “tar”, nicotine and carbon monoxide in mainstream tobacco smoke, SOR/2000-273. Registration 2000-06-26. Canadian Ministry of Justice: Tobacco Reporting Regulations. Part 3: Emissions from Designated Tobacco Products. [Google Scholar]
- 37.Health-Canada . 1999. Method T-109, Determination of Ni, Pb, Cd, Cr, As and Se in Mainstream Tobacco Smoke, SOR/2000-273. Registration 2000-06-26. Canadian Ministry of Justice: Tobacco Reporting Regulations. Part 3: Emissions from Designated Tobacco Products. Available at: http://www.hc-sc.gc.ca/hc-ps/alt_formats/hecs-sesc/pdf/tobac-tabac/legislation/reg/indust/method/_main-principal/metal-eng.pdf. [Google Scholar]
- 38.Purkis S.W., Intorp M. Analysis of reference cigarette smoke yield data from 21 laboratories for 28 selected analytes as a guide of selection of new CORESTA recommended methods. Beitr. Tabakforsch. Int. 2014;26:57–73. [Google Scholar]
- 39.Burns D.M., Dybing E., Gray N., Hecht S., Anderson C., Sanner T., O’Connor R., Djordjevic M., Dresler C., Hainaut P., Jarvis M., Opperhuizen A., Straif K. Mandated lowering of toxicants in cigarette smoke: a description of the World Health Organization TobReg proposal. Tob. Control. 2008;17:132–141. doi: 10.1136/tc.2007.024158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pappas R.S., Fresquez M.R., Martone N., Watson C.H. Toxic metal concentrations in mainstream smoke from cigarettes available in the USA. J. Anal. Toxicol. 2014;38:204–211. doi: 10.1093/jat/bku013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.John E. Nicotine particle/gas phase distribution trends: denuder tube studies. In: Coresta, editor. Coresta SSPT Meeting, Graz (Austria), Paper ST 26. 2011. [Google Scholar]
- 42.Lipowicz P., Piadé J.J. Evaporation and subsequent deposition of nicotine from mainstream cigarette smoke in a denuder tube. J. Aerosol Sci. 2004;35:33–45. [Google Scholar]
- 43.Pankow J.F., Tavakoli A.D., Luo W., Isabelle L.M. Percent free base nicotine in the tobacco smoke particulate matter of selected commercial and reference cigarettes. Chem. Res. Toxicol. 2003;16:1014–1018. doi: 10.1021/tx0340596. [DOI] [PubMed] [Google Scholar]
- 44.Branton P.J., McAdam K.G., Duke M.G., Liu C., Curle M., Mola M., Proctor C.J., Bradley R.H. Use of classical adsorption theory to understand the dynamic filtration of volatile toxicants in cigarette smoke by active carbons. Adsorpt. Sci. Technol. 2011;29:117–138. [Google Scholar]
- 45.Hearn B.A., Ding Y.S., Vaughan C., Zhang L., Polzin G.M., Caudill S.P., Watson C.H., Ashley D.L. Semi-volatiles in mainstream smoke delivery from select charcoal-filtered cigarette brand variants. Tob. Control. 2010;19:223–230. doi: 10.1136/tc.2009.032680. [DOI] [PubMed] [Google Scholar]
- 46.Csalári J., Szántai K. Transfer rate of cadmium, lead, zinc and iron from the tobacco-cut of the most popular Hungarian cigarette brands to the combustion products. Acta Aliment. 2002;31:279–288. [Google Scholar]
- 47.Galażyn-Sidorczuk M., Brzóska M.M., Moniuszko-Jakoniuk J. Estimation of Polish cigarettes contamination with cadmium and lead, and exposure to these metals via smoking. Environ. Monit. Assess. 2008;137:481–493. doi: 10.1007/s10661-007-9783-2. [DOI] [PubMed] [Google Scholar]
- 48.Hammond D., O’Connor R.J. Constituents in tobacco and smoke emissions from Canadian cigarettes. Tob. Control. 2008;17:i24–i31. doi: 10.1136/tc.2008.024778. [DOI] [PubMed] [Google Scholar]
- 49.Maurí-Aucejo A.R., Campos-Candel A., Marín-Sáez R., Llobat-Estellés M. Extraction of Cd, Pb, Ni, Cu, and Zn from tobacco samples by pressurized fluid extraction and determination by atomic spectrometry. At. Spectrosc. 2008;29:217–223. [Google Scholar]
- 50.Viana G.F.d.S., Garcia K.S., Menezes-Filho J.A. Assessment of carcinogenic heavy metal levels in Brazilian cigarettes. Environ. Monit. Assess. 2011;181:255–265. doi: 10.1007/s10661-010-1827-3. [DOI] [PubMed] [Google Scholar]
- 51.Lugon-Moulin N., Martin F., Krauss M.R., Ramey P.B., Rossi L. Cadmium concentration in tobacco (Nicotiana tabacum L.) from different countries and its relationship with other elements. Chemosphere. 2006;63:1074–1086. doi: 10.1016/j.chemosphere.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Ashraf M.W. Levels of heavy metals in popular cigarette brands and exposure to these metals via smoking. Sci. World J. 2012. 2012 doi: 10.1100/2012/729430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asubiojo O.I., Adebiyi F.M., Ayenimo J.G., Olukoko O.O., Oyekunle J.A.O. Chemical analysis of tobacco cigarette for organochlorine insecticides and heavy metal composition. Toxicol. Environ. Chem. 2009;91:611–618. [Google Scholar]
- 54.Kazi T.G., Jalbani N., Arain M.B., Jamali M.K., Afridi H.I., Sarfraz R.A., Shah A.Q. Toxic metals distribution in different components of Pakistani and imported cigarettes by electrothermal atomic absorption spectrometer. J. Hazard. Mater. 2009;163:302–307. doi: 10.1016/j.jhazmat.2008.06.088. [DOI] [PubMed] [Google Scholar]
- 55.Lazarević K., Nikolić D., Stosić L., Milutinović S., Videnović J., Bogdanović D. Determination of lead and arsenic in tobacco and cigarettes: an important issue of public health. Cent. Eur. J. Publ. Health. 2012;20:62–66. doi: 10.21101/cejph.a3728. [DOI] [PubMed] [Google Scholar]
- 56.Massadeh A.M., Alali F.Q., Jaradat Q.M. Determination of cadmium and lead in different cigarette brands in Jordan. Environ. Monit. Assess. 2005;104:163–170. doi: 10.1007/s10661-005-1609-5. [DOI] [PubMed] [Google Scholar]
- 57.Stephens W.E., Calder A., Newton J. Source and health implications of high toxic metal concentrations in illicit tobacco products. Environ. Sci. Technol. 2005;39:479–488. doi: 10.1021/es049038s. [DOI] [PubMed] [Google Scholar]
- 58.Pesch H.J., Bloß S., Schubert J., Seibold H. The mercury, cadmium and lead content of cigarette tobacco: comparative analytical-statistical studies in 1987 and 1991 employing Zeeman-AAS. Fresen. J. Anal. Chem. 1992;343:152–153. [Google Scholar]
- 59.Rickert W.S., Kaiserman M.J. Levels of lead, cadmium, and mercury in Canadian cigarette tobacco as indicators of environmental change: results from a 21-year study (1968–1988) Environ. Sci. Technol. 1994;28:924–927. doi: 10.1021/es00054a025. [DOI] [PubMed] [Google Scholar]
- 60.Lugon-Moulin N., Martin F., Krauss M., Ramey P., Rossi L. Arsenic concentration in tobacco leaves: a study on three commercially important tobacco (Nicotiana tabacum L.) types. Water Air Soil Pollut. 2008;192:315–319. [Google Scholar]
- 61.Counts M.E., Morton M.J., Laffoon S.W., Cox R.H., Lipowicz P.J. Smoke composition and predicting relationships for international commercial cigarettes smoked with three machine-smoking conditions. Regul. Toxicol. Pharmacol. 2005;41:185–227. doi: 10.1016/j.yrtph.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 62.Gregg E., Hill C., Hollywood M., Kearney M., McAdam K.G., McLaughlin D., Purkis S., Williams M. The UK smoke constituents testing study. Summary of results and comparison with other studies. Beiträge Tabakforschung Int. 2004;21:117–138. [Google Scholar]
- 63.Hyodo T., Maruta Y., Itaya H., Mikita A., Kodera T., Meger M. Evaluation of functional relationships for predicting mainstream smoke constituent machine yields for conventional cigarettes from the Japanese market. Regul. Toxicol. Pharmacol. 2007;48:194–224. doi: 10.1016/j.yrtph.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Pappas R.S., Polzin G.M., Zhang L., Watson C.H., Paschal D.C., Ashley D.L. Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem. Toxicol. 2006;44:714–723. doi: 10.1016/j.fct.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Smith C.J., Livingston S.D., Doolittle D.J. An international literature survey of “IARC Group I Carcinogens” reported in mainstream cigarette smoke. Food Chem. Toxicol. 1997;35:1107–1130. doi: 10.1016/s0278-6915(97)00063-x. [DOI] [PubMed] [Google Scholar]
- 66.Smith C.J., Perfetti T.A., Rumple M.A., Rodgman A., Doolittle D.J. “IARC Group 2A Carcinogens” reported in cigarette mainstream smoke. Food Chem. Toxicol. 2000;38:371–383. doi: 10.1016/s0278-6915(99)00156-8. [DOI] [PubMed] [Google Scholar]
- 67.Piadé J.J., Wajrock S., Jaccard G., Janeke G. Formation of mainstream cigarette smoke constituents prioritized by the World Health Organization – yield patterns observed in market surveys, clustering and inverse correlations. Food Chem. Toxicol. 2013;55:329–347. doi: 10.1016/j.fct.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 68.Purkis S.W., Cahours X., Rey M., Teillet B., Troude V., Verron T. Some consequences of using cigarette machine smoking regimes with different intensities on smoke yields and their variability. Regul. Toxicol. Pharmacol. 2011;59:293–309. doi: 10.1016/j.yrtph.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 69.Podraza K.F. 2006. Marlboro UltraSmooth benchmarking data, Letter to Dr. Laugesen, Health New Zealand Ltd. Dated February 1st 2006, Bates Nb 3104262330-3104262342. Accessed October 2013 at http://legacy.library.ucsf.edu/tid/ima80g00/pdf. [Google Scholar]
- 70.Bache C.A., Lisk D.J., Shane B.S., Hoffmann D., Adams J.D. Effectiveness of cigarette filter tips for reducing cadmium in relation to other mainstream smoke constituents. Drug Chem. Toxicol. 1987;10:189–193. doi: 10.3109/01480548709042981. [DOI] [PubMed] [Google Scholar]
- 71.Scherer G., Barkemeyer H. Cadmium concentrations in tobacco and tobacco smoke. Ecotoxicol. Environ. Saf. 1983;7:71–78. doi: 10.1016/0147-6513(83)90050-7. [DOI] [PubMed] [Google Scholar]
- 72.Westcott D.T., Spincer D. The cadmium, nickel and lead content of tobacco and cigarette smoke. Beiträge Tabakforschung. 1974;7:217–221. [Google Scholar]
- 73.Gutenmann W.H., Bache C.A., Lisk D.J., Hoffmann D., Adams J.D., Elfving D.C. Cadmium and nickel in smoke of cigarettes prepared from tobacco cultured on municipal sludge-amended soil. J. Toxicol. Environ. Health. 1982;10:423–431. doi: 10.1080/15287398209530264. [DOI] [PubMed] [Google Scholar]
- 74.Kalcher K., Kern W., Pietsch R. Cadmium and lead in the smoke of a filter cigarette. Sci. Total Environ. 1993;128:21–35. doi: 10.1016/0048-9697(93)90177-8. [DOI] [PubMed] [Google Scholar]
- 75.Nitsch A., Kalcher K., Greschonig H., Pietsch R. 1991. Schwermetalle in Tabaken und in Tabakrauch ll: Spurenelemente Cadmium, Blei, Kupfer, Kobalt und Nickel in oesterreichischen Zigaretten und deren Rauchkondensaten und Rauchgasen Beiträge zur Tabakforschung International 15; pp. 19–32. [Google Scholar]
- 76.Perinelli M.A., Carugno N. Determination of trace metals in cigarette smoke by flameless atomic absorption spectrometry. Beiträge Tabakforschung. 1978;9:214–217. [Google Scholar]
- 77.Szadkowski D., Schultze H., Schaller K.-H., Lehnert G. Zur oekologischen Bedeutung des Schwermetallgehaltes von Zigaretten. Blei-, Cadmium- und Nickelanalysen des Tabaks sowie der Gas- und Partikelphase. Arch. Hyg. Bakteriol. 1969;153:1–8. [PubMed] [Google Scholar]
- 78.Chang M.J., Naworal J.D., Connell C.T. Direct introduction of cigarette smoke for puff-by-puff trace metals analysis by inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2006;21:574–581. [Google Scholar]
- 79.Chang M.J., Walker K., McDaniel R.L., Connell C.T. Impaction collection and slurry sampling for the determination of arsenic, cadmium, and lead in sidestream cigarette smoke by inductively coupled plasma-mass spectrometry. J. Environ. Monit. 2005;7:1349–1354. doi: 10.1039/b509048b. [DOI] [PubMed] [Google Scholar]
- 80.Menden E.E., Elia V.J., Michael L.W., Petering H.G. Distribution of cadmium and nickel of tobacco during cigarette smoking. Environ. Sci. Technol. 1972;6:830–832. [Google Scholar]
- 81.Suna S., Asakawa F., Jitsunari F., Manabe Y., Gotou A., Fukunaga I., Nakajima T. Assessment of cadmium and lead released from cigarette smoke. Jpn. J. Hyg. 1991;46:1014–1024. doi: 10.1265/jjh.46.1014. [DOI] [PubMed] [Google Scholar]
- 82.Wu D., Landsberger S., Larson S.M. Determination of the elemental distribution in cigarette components and smoke by instrumental neutron activation analysis. J. Radioanal. Nucl. Chem. 1997;217:77–82. [Google Scholar]
- 83.Morgan W.R., Akers C.H. Philip-Morris USA; Richmond, VA: 1982. Transfer of metals into mainstream cigarette smoke, Philip Morris R&D Report 82-282. Bates 2021565869-2021565907. Accessed September 2014 at http://legacy.library.ucsf.edu/tid/ehb68e00/pdf. [Google Scholar]
- 84.Rodgman A., Perfetti T.A. 2nd edition. CRC Press; Boca Raton, FL, USA: 2013. The Chemical Components of Tobacco and Tobacco Smoke. [Google Scholar]
- 85.ISO-20773-2 . 2nd edition. International Organization for Standardization; Geneva, Switzerland: 2013. Cigarettes – Determination of nicotine-free dry particulate matter and nicotine in sidestream smoke – method using a routine analytical linear smoking machine equipped with a fishtail chimney. [Google Scholar]
- 86.Vögeli-Lange R., Wagner G.J. Subcellular localization of cadmium and cadmium-binding peptides in tobacco leaves: implication of a transport function for cadmium-binding peptides. Plant Physiol. 1990;92:1086–1093. doi: 10.1104/pp.92.4.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Lith S.C., Jensen P.A., Frandsen F.J., Glarborg P. Release to the gas phase of inorganic elements during wood combustion. Part 2: influence of fuel composition. Energy Fuels. 2008;22:1598–1609. [Google Scholar]
- 88.Utsunomiya S., Jensen K.A., Keeler G.J., Ewing R.C. Direct identification of trace metals in fine and ultrafine particles in the detroit urban atmosphere. Environ. Sci. Technol. 2004;38:2289–2297. doi: 10.1021/es035010p. [DOI] [PubMed] [Google Scholar]
- 89.Pacyna J.M. Atmospheric emissions of arsenic, cadmium, lead and mercury from high temperature processes in power generation and industry. In: Hutchinson T.C., Meema K.M., editors. Lead, Mercury, Cadmium and Arsenic in the Environment. John Wiley & Sons Ltd.; New York: 1987. pp. 69–87. [Google Scholar]
- 90.Ménard Y., Asthana A., Patisson F., Sessiecq P., Ablitzer D. Thermodynamic study of heavy metals behaviour during municipal waste incineration. Process Saf. Environ. Prot. 2006;84:290–296. [Google Scholar]
- 91.Benning J., Liu C., McAdam K.G. 2014. Arsenic speciation in selected tobacco products, STPOST 11, Coresta Congress, Quebec (Canada) [Google Scholar]
- 92.Campbell R.C.J., Stephens W.E., Finch A.A., Geraki K. Controls on the valence species of arsenic in tobacco smoke with implications for health and regulation. Environ. Sci. Technol. 2014;48:3449–3456. doi: 10.1021/es4039243. [DOI] [PubMed] [Google Scholar]
- 93.Liu C., Wright C.G., McAdam K.G., Taebunpakul S., Heroult J. Arsenic speciation in tobacco and cigarette smoke. Beiträge Tabakforschung Int. 2012;25:375–380. [Google Scholar]
- 94.Taebunpakul S., Liu C., Wright C., McAdam K., Heroult J., Braybrook J., Goenaga-Infante H. Determination of total arsenic and arsenic speciation in tobacco products: from tobacco leaf and cigarette smoke. J. Anal. At. Spectrom. 2011;26:1633–1640. [Google Scholar]
- 95.Wu H., Glarborg P., Frandsen F.J., Dam-Johansen K., Jensen P.A., Sander B. Trace elements in co-combustion of solid recovered fuel and coal. Fuel Process. Technol. 2013;105:212–221. [Google Scholar]
- 96.Díaz-Somoano M., Martínez-Tarazona M.R. Trace element evaporation during coal gasification based on a thermodynamic equilibrium calculation approach. Fuel. 2003;82:137–145. [Google Scholar]
- 97.Šyc M., Pohořelý M., Jeremiáš M., Vosecký M., Kameníková P., Skoblia S., Svoboda K., Punčochář M. Behavior of heavy metals in steam fluidized bed gasification of contaminated biomass. Energy Fuels. 2011;25:2284–2291. [Google Scholar]
- 98.van Lith S.C., Alonso-Ramírez V., Jensen P.A., Frandsen F.J., Glarborg P. Release to the gas phase of inorganic elements during wood combustion. Part 1: development and evaluation of quantification methods. Energy Fuels. 2006;20:964–978. [Google Scholar]
- 99.Hirsch M.E., Sterling R.O., Huggins F.E., Helble J.J. Speciation of combustion-derived particulate phase arsenic. Environ. Eng. Sci. 2000;17:315–327. [Google Scholar]
- 100.Krivan V., Arpadjan S. Radiotracer study of the behaviour of arsenic in the graphite furnace. Fresen. Z. Anal. Chem. 1989;335:743–747. [Google Scholar]
- 101.Goodarzi F. Characteristics and composition of fly ash from Canadian coal-fired power plants. Fuel. 2006;85:1418–1427. [Google Scholar]
- 102.Sterling R.O., Helble J.J. Reaction of arsenic vapor species with fly ash compounds: kinetics and speciation of the reaction with calcium silicates. Chemosphere. 2003;51:1111–1119. doi: 10.1016/S0045-6535(02)00722-1. [DOI] [PubMed] [Google Scholar]
- 103.Baliga V.L., Thurston M.E., Miser D.E., Sharma R.K., Chan G., Hajaligol M.R. Physical characterization of the cigarette coal. Part II: puff burn. J. Anal. Appl. Pyrol. 2004;72:83–96. [Google Scholar]
- 104.Baskevitch N., Horler W. LTR Industries; 2001. Organic Potassium. Its Role in Controlling the Rate of Burn and tar Content of Tobacco Products, Report to Coresta. Available from http://legacy.library.ucsf.edu/tid/dew20d00;jsessionid=40C75FC25145D7BA58B78D93A3A68162 (accessed August 2014) [Google Scholar]
- 105.Campbell C.R. Flue-cured tobacco. In: Campbell C.R., editor. Reference sufficiency ranges for plant analysis in the southern region of the United States, NC Dept of Agriculture & Consumer Services. Southern Cooperative Series, Raleigh (NC). Bulletin 394. 2000. [Google Scholar]
- 106.Leffingwell J.C. Leaf chemistry – basic chemical constituents of tobacco leaf and differences among tobacco types. In: Davis D.L., Nielsen M.T., editors. Tobacco Production, Chemistry and Technology. Blackwell Sciences Ltd.; Oxford, UK: 1999. pp. 265–284. [Google Scholar]
- 107.Müller A.L.H., Müller C.C., Antes F.G., Barin J.S., Dressler V.L., Flores E.M.M., Müller E.I. Determination of bromide, chloride, and fluoride in cigarette tobacco by ion chromatography after microwave-induced combustion. Anal. Lett. 2012;45:1004–1015. [Google Scholar]
- 108.Hu J., Liu C., McAdam K. Synchrotron X-ray analysis on oxidation states of arsenic and cadmium in cut tobacco powder, cigarette ash and mainstream smoke total particulate matter. In: Coresta, editor. Coresta SSPT Meeting, Aix-en-Provence, France, SSPTPOST 19. 2009. [Google Scholar]
- 109.Vogel C., Adam C., Unger M. Heavy metal removal from sewage sludge ash analyzed by thermogravimetry. J. Therm. Anal. Calorim. 2011;103:243–248. [Google Scholar]
- 110.Lu-shi S., Abanades S., Lu J., Flamant G., Gauthier D. Volatilization of heavy metals during incineration of municipal solid wastes. J. Environ. Sci. (China) 2004;16:635–639. [PubMed] [Google Scholar]
- 111.Matsuno M., Tomoda K., Nakamura T. Volatilization mechanism of Pb from fly ash in municipal waste incinerator. Mater. Trans. 2003;44:2481–2488. [Google Scholar]
- 112.Liu C., Parry A. Potassium organic salts as burn additives in cigarettes. Beiträge Tabakforschung Int. 2003;20:341–347. [Google Scholar]
- 113.Hansen H.K., Pedersen A.J., Ottosen L.M., Villumsen A. Speciation and mobility of cadmium in straw and wood combustion fly ash. Chemosphere. 2001;45:123–128. doi: 10.1016/s0045-6535(01)00026-1. [DOI] [PubMed] [Google Scholar]
- 114.Rio S., Verwilghen C., Ramaroson J., Nzihou A., Sharrock P. Heavy metal vaporization and abatement during thermal treatment of modified wastes. J. Hazard. Mater. 2007;148:521–528. doi: 10.1016/j.jhazmat.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 115.Tsukada M., Nishikawa N., Horikawa A., Wada M., Liu Y., Kamiya H. Emission potential of condensable suspended particulate matter from flue gas of solid waste combustion. Powder Technol. 2008;180:140–144. [Google Scholar]
- 116.Baker R.R. Variation of sidestream gas formation during the smoking cycle. Beiträge Tabakforschung Int. 1982;11:181–193. [Google Scholar]
- 117.Proctor C.J., Martin C., Beven J.L., Dymond H.F. Evaluation of an apparatus designed for the collection of sidestream tobacco smoke. Analyst. 1988;113:1509–1513. doi: 10.1039/an9881301509. [DOI] [PubMed] [Google Scholar]
- 118.Wooten J.B. Gas-phase radicals in cigarette smoke: a re-evaluation of the steady-state model and the Cambridge filter pad. Mini-Rev. Org. Chem. 2011;8:412–426. [Google Scholar]
- 119.Gerardi A.R., Coleman W.M.I. New methodologies for qualitative and semi-quantitative determination of carbon-centered free radicals in cigarette smoke using liquid chromatography–tandem mass spectrometry and gas chromatography–mass selective detection. Beiträge Tabakforschung Int. 2010;24:58–71. [Google Scholar]
- 120.Czerniak M.R., Easton B.C. An investigation of the pyrolysis of dimethylcadmium and diethyltelluride by in-situ gas sampling and analysis. J. Cryst. Growth. 1984;68:128–135. [Google Scholar]
- 121.Hails J.E., Cole-Hamilton D.J., Stevenson J., Bell W., Foster D.F., Ellis D. A chemical assessment of the suitability of allyl-iso-propyltelluride as a Te precursor for metal organic vapour phase epitaxy. J. Cryst. Growth. 2001;224:21–31. [Google Scholar]
- 122.Wulfsberg G. University Science Books; Sausalito, CA, USA: 1991. Principles of Descriptive Inorganic Chemistry; Chapter 10: The Hydrides and Organometallic Derivatives of the Elements; pp. 355–356. [Google Scholar]
- 123.Spiridonova E.Y. Experimental studies of the dimethylcadmium toxic properties. Meditsina Truda I Promyshlennaya Ekol. 1991:14–17. [PubMed] [Google Scholar]
- 124.Fresquez M.R., Pappas R.S., Watson C.H. Establishment of toxic metal reference range in tobacco from US cigarettes. J. Anal. Toxicol. 2013;37:298–304. doi: 10.1093/jat/bkt021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.O’Connor R.J., Li Q., Stephens W.E., Hammond D., Elton-Marshall T., Cummings K.M., Giovino G.A., Fong G.T. Cigarettes sold in China: design, emissions and metals. Tob. Control. 2010;19:i47–i53. doi: 10.1136/tc.2009.030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Watanabe T., Kasahara M., Nakatsuka H., Ikeda M. Cadmium and lead contents of cigarettes produced in various areas of the world. Sci. Total Environ. 1987;66:29–37. doi: 10.1016/0048-9697(87)90074-x. [DOI] [PubMed] [Google Scholar]
- 127.Kalaitzoglou M., Samara C. Yields of cadmium, tar, nicotine and carbon monoxide in mainstream smoke of Greek cigarettes: a comparative study. Beiträge Tabakforschung Int. 1999;18:235–244. [Google Scholar]
- 128.King B., Borland R., Fowles J. Mainstream smoke emissions of Australian and Canadian cigarettes. Nicotine Tob. Res. 2007;9:835–844. doi: 10.1080/14622200701485109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.