Abstract
Purpose:
To evaluate the incidence, outcomes, and risk factors for hemorrhagic complications in eyes with polypoidal choroidal vasculopathy (PCV) following photodynamic therapy (PDT).
Methods:
Medical records of 94 eyes of 86 consecutive patients with PCV who underwent PDT between January 2007 and December 2014 were retrospectively reviewed. The diagnosis of PCV was based on clinical features and indocyanine green angiography. Eyes were treated with PDT monotherapy or a combination of PDT plus anti-vascular endothelial growth factor. PDT was performed at (standard [SFPDT] or reduced fluence RFPDT).
Results:
Ninety-four eyes had 119 PDT treatment sessions (mean: 1.3 sessions). Mean presenting vision was 0.46 ± 0.44 logarithm of the minimum angle of resolution (logMAR). Following PDT, ten eyes (11%) of nine patients had hemorrhagic complications such as subretinal hemorrhage (SRH; n = 5), subretinal pigment epithelium (RPE) hemorrhage (n = 1), breakthrough vitreous hemorrhage (BVH; n = 3), and SRH with sub-RPE hemorrhage and BVH (n = 1). Median interval to hemorrhage following PDT was 2 months. Age (P = 0.842), duration of symptoms (P = 0.352), number of laser spots (P = 0.219), and laser spot size (LSS) (P = 0.096) were not significantly associated with increased risk of hemorrhagic complications. Female gender was associated with reduced risk of hemorrhage (P = 0.045). SFPDT was significantly associated with increased risk of hemorrhage (P = 0.026). The probability of developing hemorrhagic complications in SFPDT group was 0.24 compared to 0.07 in RFPDT group (P = 0.039). Multivariate logistic regression analysis showed SFPDT as the only significant risk factor for hemorrhage following PDT (odds ratio 5.3, 95% confidence interval 1.1–24.8, P = 0.03). Mean final vision was 0.61 ± 0.53 logMAR at mean follow-up of 33 months (median = 22 months; range = 2–157 months).
Conclusion:
Age, LSS, number of laser spots, preexisting hemorrhages, or use of anticoagulants were not associated with increased risk of hemorrhagic complications. SFPDT was significantly associated with increased risk of hemorrhagic complications in such eyes.
Key words: Complications, hemorrhage, photodynamic therapy, polypoidal choroidal vasculopathy, treatment
Polypoidal choroidal vasculopathy (PCV) was first described by Yannuzzi et al. in 1982 as a choroidal vasculopathy that led to hemorrhagic and exudative macular degeneration.[1] Similar retinal changes were observed by Kleiner et al. who named it posterior uveal bleeding syndrome.[2] Clinically, PCV lesions usually appear as orange-red, nodule-like structures beneath the RPE associated with serous pigment epithelial detachments (PEDs), overlying neurosensory detachment, subretinal hemorrhage (SRH), sub-retinal fibrinous material, hard exudates, and drusen. On indocyanine green angiography (ICGA), PCV is characterized by polypoidal lesions with or without branching vascular networks (BVNs) of choroidal origin.[3,4] The role and efficacy of photodynamic therapy (PDT) in PCV has been well documented.[4,5,6,7,8,9,10,11,12,13,14,15] PDT results in complete regression of polyps in 71%–95% cases and leads to stable or improved vision in up to 95% cases.[5,11] However, PDT has also been associated with hemorrhagic complications in 2.2%–31% eyes. PubMed search using the keywords (polypoidal choroidal vasculopathy), (photodynamic therapy), and (hemorrhage) revealed 15 relevant reports.[4,5,10,11,12,13,14,16,17,18,19,20,21,22,23] All the published studies have reported results from East Asian countries. There is a clear lack of evidence on this aspect from India, which has an ethnically distinct population. Hereby, we report the incidence, risk factors, and outcomes of hemorrhagic complications following PDT for PCV in our study of 94 eyes of 86 Indian subjects treated over 7 years.
Methods
This was a retrospective, interventional case series. Medical records of 94 eyes of 86 consecutive patients with PCV who underwent PDT between January 2007 and December 2014 were analyzed. Institutional review board approval was sought for this study. All patients consented with a written informed consent form. The study adhered to the tenets of the Declaration of Helsinki. All patients underwent comprehensive ophthalmologic examination, including determination of best-corrected visual acuity (BCVA), intraocular pressure measurement (Goldmann applanation tonometer), indirect ophthalmoscopy, slit-lamp biomicroscopy, fluorescein angiography, ICGA (Carl Zeiss Meditec, Dublin, CA, USA), and optical coherence tomography (OCT; Cirrus OCT, Meditec, Dublin, CA, USA). Patient's data were reviewed for presenting clinical findings, ICGA features, OCT features, management, complications, outcomes, and follow-up. The diagnosis of PCV was based on clinical features and confirmed with ICGA. History of systemic diseases such as hypertension, diabetes mellitus and ischemic heart disease and history of treatment with oral anticoagulants was noted. Eyes were treated with PDT monotherapy or combination PDT plus anti-vascular endothelial growth factor (VEGF). PDT was performed at standard (SFPDT) (light dose 50 J/cm2; dose rate 600 mW/cm2) or reduced-fluence (RFPDT) (light dose 25 J/cm2; dose rate 300 mW/cm2) using a 689 nm diode laser (Opal, Lumenis, USA) for 83 s, 5 min after completion of injection of verteporfin. The greatest linear dimension (GLD) encompassed all lesion components such as polyps, BVN, and choroidal neovascularization. The laser spot size (LSS) was 1 mm larger than GLD. SFPDT or RFPDT was determined by the treating ophthalmologist [Fig. 1]. Patients undergoing combination treatment had anti-VEGF injection 2 days after PDT. Patients were followed up with at regular intervals. Re-treatment criteria included drop in vision, new or persistent visual symptoms, or signs of persistent disease activity on OCT/ICGA (e.g., intraretinal or subretinal fluid, bleeding, exudation, or leakage). Hemorrhagic complications were defined as fresh subretinal and/or sub-RPE and/or vitreous hemorrhage or an increase in the extent of preexisting subretinal and/or sub-RPE and/or vitreous hemorrhage seen within 3 months of receiving PDT. Data analysis was done by dividing eyes among groups based on PDT fluence (standard-fluence PDT [SFPDT; n = 21] or reduced-fluence PDT [RFPDT; n = 73]) and hemorrhagic complications (eyes with post-PDT hemorrhage [n = 10] or without post-PDT hemorrhage [n = 84]). Differences were considered statistically significant at P < 0.05. Independent sample t-test, Pearson's Chi-square test, and two-proportion Z-test were used to calculate P values. Univariate and multiple analyses were done by logistic regression for factors showing significant association with the occurrence of hemorrhage following PDT. Change in BCVA was determined to be significant when the change of visual acuity in logarithm of the minimum angle of resolution (logMAR) was ≥0.3.
Figure 1.
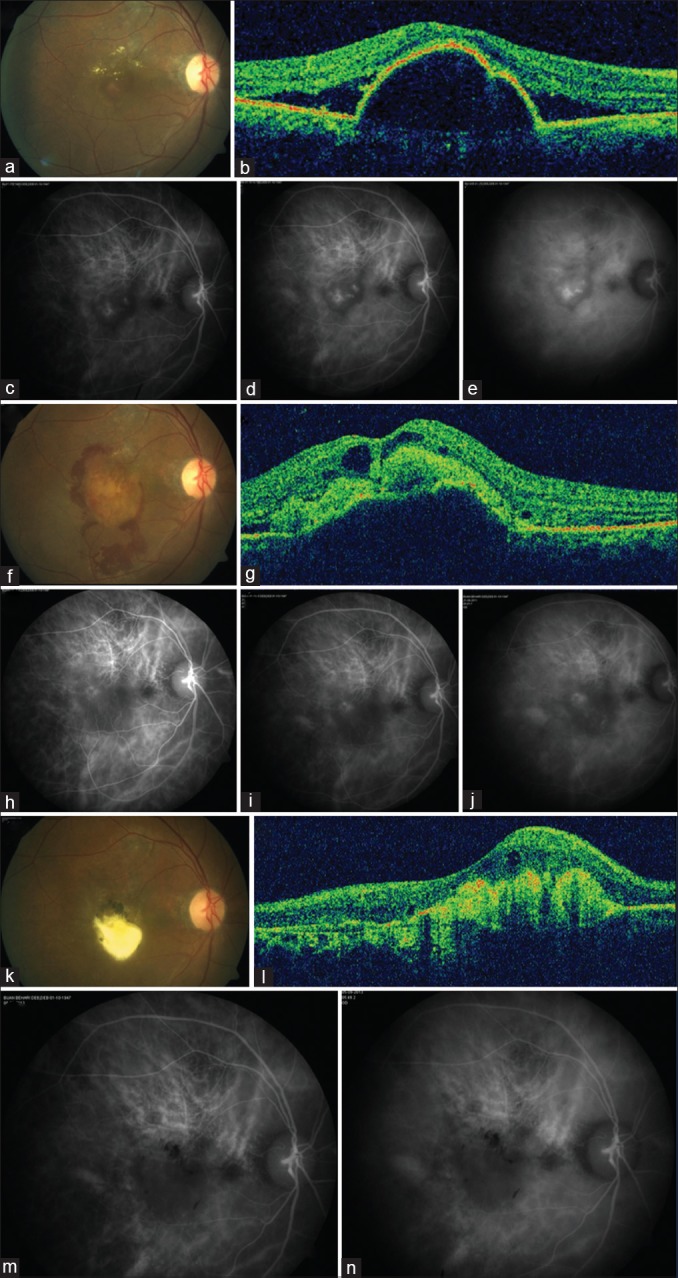
At baseline, color fundus photograph of 63-year-old male reveals serosanguineous detachment of the posterior pole (a), with a peaked pigment epithelium detachment on optical coherence tomography (b), and indocyanine green angiography revealing clusters of polyps (c-e). Best-corrected visual acuity was 0.5 logarithm of the minimum angle of resolution. The patient underwent reduced-fluence photodynamic therapy. Five weeks later, the patient presented with hemorrhage (f), optical coherence tomography revealed hemorrhagic pigment epithelium detachment and cystoid retinal changes (g), and regressing polyps on indocyanine green angiography (h-j). Best-corrected visual acuity was 0.5 logarithm of the minimum angle of resolution. Two months later, subretinal fluid resolved with subfoveal aggregation of exudates (k and l), and lack of polypoidal activity on indocyanine green angiography (m and n). Best-corrected visual acuity was 1.7 logarithm of the minimum angle of resolution. The patient was periodically observed without the need for further treatment and remained stable up to the last follow-up at 53 months. Best-corrected visual acuity was 1.3 logarithm of the minimum angle of resolution
Results
Ninety-four eyes of 86 patients were included in this study. The baseline features are summarised in Table 1. Ninety-four eyes had 119 PDT treatment sessions (mean: 1.3 sessions). Eleven patients (11.7%) underwent SFPDT-combination therapy, ten (10.6%) underwent SFPDT-monotherapy, 60 (63.8%) underwent RFPDT-combination therapy, and 13 (13.8%) underwent RFPDT-monotherapy. Following PDT, ten eyes (11%) of nine patients (seven males and two females) had hemorrhagic complications such as SRH (n = 5), sub-RPE hemorrhage (n = 1), breakthrough vitreous hemorrhage (BVH; n = 3), and SRH with sub-RPE hemorrhage and BVH (n = 1). One patient had bilateral hemorrhagic complications; BVH in one eye following SFPDT and SRH in the other following RFPDT. Individual details for the ten eyes that had hemorrhagic complications are listed in Table 2.
Table 1.
Hemorrhagic complications in eyes with polypoidal choroidal vasculopathy following photodynamic therapy: Individual details of 10 eyes with hemorrhagic complications
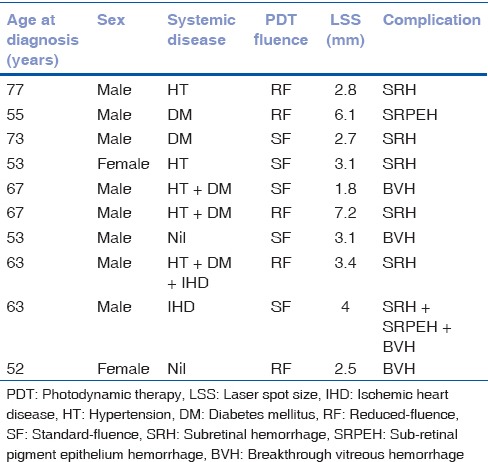
Table 2.
Hemorrhagic complications in eyes with polypoidal choroidal vasculopathy following photodynamic therapy: Baseline features
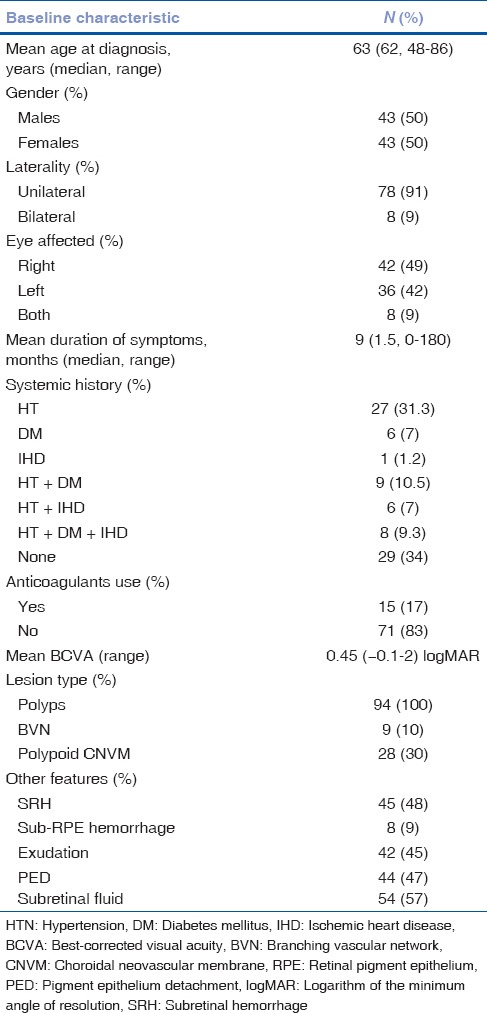
Overall, 71 of 94 eyes received anti-VEGF injections in combination with PDT (bevacizumab n = 28, ranibizumab n = 42, aflibercept n = 1). Mean interval to hemorrhage following PDT was 2 months (median 2 months, range 1–3 months). Mean presenting vision was 0.46 ± 0.44 logMAR. Mean final vision was 0.61 ± 0.53 logMAR. Mean follow-up was 33 months (median = 22 months; range = 2–157 months).
The SFPDT group (n = 21) underwent 29 PDT sessions (mean: 1.4 sessions) and RFPDT group (n = 73) underwent 80 PDT sessions (mean: 1.2 sessions). Mean LSS was 2.9 ± 1.2 mm in SFPDT group and 3 ± 1.3 mm in RFPDT group. Both groups were statistically comparable in terms of mean age (P = 0.490), gender (P = 0.804), duration of symptoms (P = 0.337), number of sessions (P = 0.338), number of laser-spots (P = 0.325), and LSS (P = 0.820). Five of 21 eyes (24%) in SFPDT group and five of 73 eyes (7%) in RFPDT group developed hemorrhagic complications (P = 0.026). The probability of developing hemorrhagic complications in SFPDT group was 0.24 compared to 0.07 in RFPDT group (relative risk = 3.3, P = 0.039). Mean presenting vision was 0.67 ± 0.51 logMAR in SFPDT group and 0.40 ± 0.39 logMAR in RFPDT group. Mean final BCVA was 0.75 ± 0.56 logMAR in SFPDT group and 0.57 ± 0.52 logMAR in RFPDT group (P = 0.167).
In eyes with hemorrhagic complications, mean presenting vision was 0.42 ± 0.61 logMAR and 0.46 ± 0.41 logMAR in eyes without hemorrhagic complications (P = 0.776). Mean LSS was 3.6 ± 1.8 mm in eyes with hemorrhagic complications and 2.9 ± 1.2 mm in eyes without hemorrhagic complications (P = 0.096). Age (P = 0.842), duration of symptoms (P = 0.352), number of laser spots (P = 0.219), and LSS (P = 0.096) were not significantly associated with increased risk of hemorrhagic complications. SFPDT was significantly associated with the occurrence of hemorrhage (P = 0.026). Female gender was associated with reduced incidence of hemorrhage (P = 0.045). Two eyes underwent pars plana vitrectomy for vitreous hemorrhage. Mean final BCVA was 0.8 ± 0.61 logMAR in eyes with hemorrhagic complications and 0.6 ± 0.52 logMAR in eyes without hemorrhagic complications (P = 0.239). Eight of 70 (11%) eyes in combination therapy group had hemorrhagic complications and two of 24 (8%) eyes in PDT monotherapy group had hemorrhagic complications (P = 0.674). Of ten eyes with hemorrhagic complications, six (60%) had preexisting hemorrhages, while four (40%) eyes had fresh bleeding. Of 45 eyes with preexisting hemorrhages at presentation, six eyes (13%) had increased hemorrhages following PDT in the form of SRH (n = 3), SRH with subretinal pigment epithelium (RPE) and BVH (n = 1), and BVH (n = 2). Of 49 eyes without preexisting hemorrhages, four eyes (8%) had fresh hemorrhages following PDT in the form of SRH (n = 2), sub-RPE hemorrhage (n = 1), and BVH (n = 1). The difference in the incidence of hemorrhagic complications between the two groups was not statistically significant (P = 0.417). Of 57 patients with systemic diseases (hypertension, diabetes mellitus, and/or ischemic heart disease), seven (12%) developed hemorrhagic complications. Of 29 patients with no systemic diseases, two (7%) patients developed hemorrhagic complications. However, the difference in the incidence among two groups was not statistically significant (P = 0.441). Of 15 patients who were on concurrent treatment with anticoagulants, two (13%) developed hemorrhagic complications (two eyes, one in SFPDT-combination group, and one in RFPDT-monotherapy group). Of 71 patients who were not on concurrent treatment with anticoagulants, seven (10%) patients developed hemorrhagic complications (eight eyes, three in SFPDT-combination group, one in SFPDT-monotherapy group, three in RFPDT-combination group, and one in RFPDT-monotherapy group). However, the difference in the incidence of hemorrhagic complications among two groups was not statistically significant (P = 0.689).
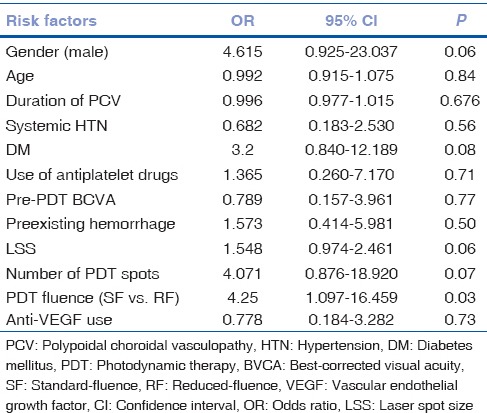
Multivariate logistic regression analysis for potential risk factors associated with hemorrhage following PDT showed the use of standard fluence as the only significant risk factor (odds ratio 5.3, 95% confidence interval 1.1–24.8, P = 0.03; [Table 3]).
Table 3.
Univariate logistic regression analysis of potential risk factors associated with hemorrhagic complications following photodynamic therapy
Discussion
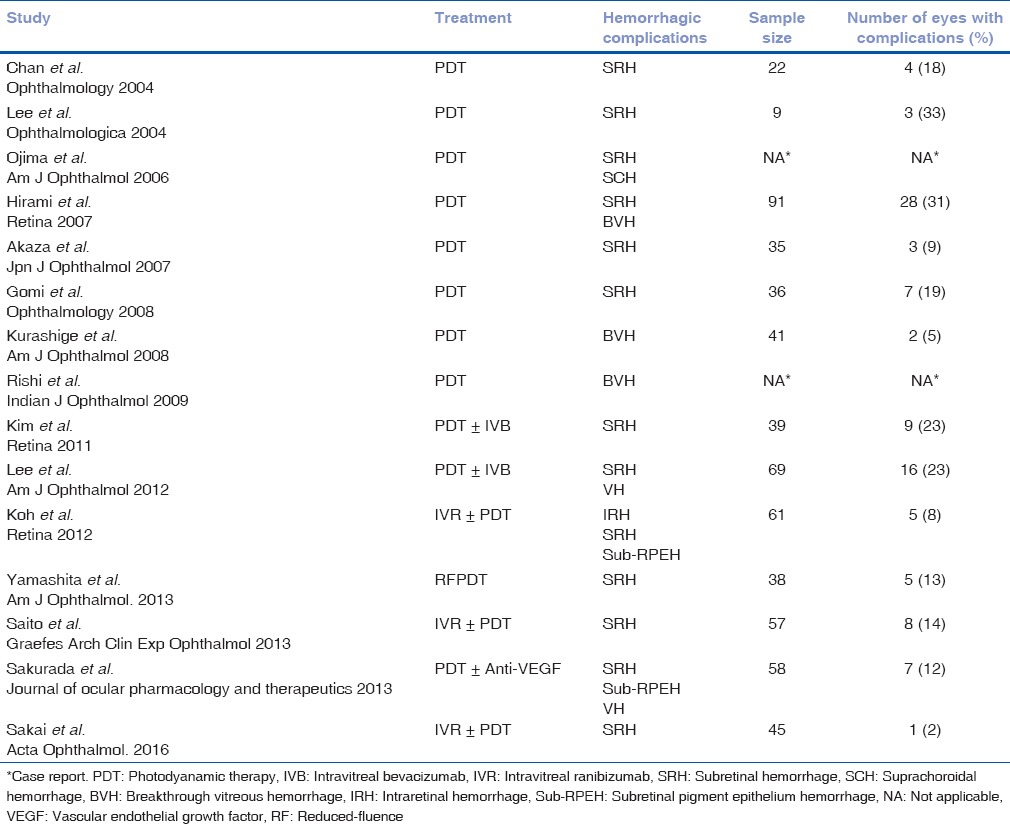
PDT is currently the most effective means of obliterating polypoidal lesions in PCV, though BVNs remain resistant to occlusion and can lead to recurrence of PCV even after treatment. PDT produces a vaso-occlusive effect on choriocapillaris when applied using the standard protocol.[24] Several studies have reported an increase in SRHs and/or BVH after treatment with PDT [Table 4].[4,5,10,11,12,13,14,16,17,18,19,20,21,22,23] Incidentally, all these studies included patients of East Asian ethnicity. In our series, among Indian subjects, we found only 11% eyes developing these complications, which is much lower than the percentage reported in the largest case series by far, in which Hirami et al. reported hemorrhagic complications in 31% (28 of 91) eyes.[4]
Table 4.
Hemorrhagic complications in eyes with polypoidal choroidal vasculopathy following photodynamic therapy: Literature review
The pathogenesis of these hemorrhagic complications is uncertain. One theory suggests that unbalanced blood pooling, owing to the immediate obliteration of both abnormal and physiological choroidal blood vessels following PDT, with an increase in blood flow in the region of fragile vessels, might be the cause of bleeding. Massive bleeding can occur subsequent to occlusion of only efferent vessels or reperfusion of only afferent vessels following PDT.[4,13,16] Another theory implicates the reactive up-regulation of VEGF after PDT.[25] An increase in the expression of VEGF in RPE cells immediately after PDT, ascribed to nonperfusion of physiologic choroid and inflammatory responses around the RPE, has been demonstrated in eyes with normal or diseased choroidal structures.[25,26,27,28] Intravitreal injection of an anti-VEGF agent could, therefore, block the adverse effects induced by the increased VEGF expression. Hence, combining PDT with an anti-VEGF agent can potentially create a synergistic effect.[24] However, Lee et al. noted a significant decrease in VEGF concentrations 1 week after treatment with PDT.[29] A recent meta-analysis suggested that combination of PDT and anti-VEGF therapy results in better long-term visual outcomes and lower incidence rates of retinal hemorrhage than PDT monotherapy.[30] However, our study did not show any significant difference in the incidence of hemorrhagic complications among the two groups (P = 0.674).
Yamashita et al. demonstrated that severe retinal hemorrhage can be prevented by employing RFPDT instead of SFPDT.[20] We also found that the percentage of eyes with hemorrhagic complications was lower in RFPDT group (five of 73 eyes, 7%) as compared to SFPDT group (five of 21 eyes, 24%), and the difference was statistically significant (P = 0.026). Larger laser irradiation spot size has also been associated with increased risk of vitreous hemorrhage after PDT.[4] In our study, though the mean LSS was larger (3.6 ± 1.8 mm) in eyes which developed hemorrhagic complications as compared to those that did not (2.9 ± 1.2), the difference was not statistically significant (P = 0.096). Eyes with hemorrhagic complications had significant visual deterioration from baseline (0.42 ± 0.61 logMAR versus 0.8 ± 0.61 logMAR). However, the mean final BCVA was not significantly different in eyes with or without complications (0.8 ± 0.61 versus 0.6 ± 0.52 logMAR, P = 0.239). Pertaining to the role of anticoagulant therapy (P = 0.689) or systemic diseases (P = 0.441) in the development of hemorrhage in eyes with PCV following PDT, our results are in keeping with those of Hirami et al., who found no causal association.[4] A potential drawback in our study is that it lacks an analysis of PED height which may be an important consideration for hemorrhage in a patient with PCV.
Conclusion
PDT can cause hemorrhagic complications in eyes with PCV. Age, duration of symptoms, number of laser spots, LSS, preexisting hemorrhages, use of anticoagulants, or concomitant hypertension, diabetes mellitus, and/or ischemic heart disease are not associated with increased risk of hemorrhagic complications. SFPDT is associated with increased risk of hemorrhagic complications while females appear to be at a lower risk for developing these complications. Visual outcome after PDT in eyes with PCV does not appear to be affected by the presence or absence of hemorrhagic complications.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Yannuzzi LA, Sorenson J, Spaide RF, Lipson B. Idiopathic polypoidal choroidal vasculopathy (IPCV) Retina. 1990;10:1–8. [PubMed] [Google Scholar]
- 2.Kleiner RC, Brucker AJ, Johnston RL. The posterior uveal bleeding syndrome. Retina. 1990;10:9–17. [PubMed] [Google Scholar]
- 3.Honda S, Matsumiya W, Negi A. Polypoidal choroidal vasculopathy: Clinical features and genetic predisposition. Ophthalmologica. 2014;231:59–74. doi: 10.1159/000355488. [DOI] [PubMed] [Google Scholar]
- 4.Hirami Y, Tsujikawa A, Otani A, Yodoi Y, Aikawa H, Mandai M, et al. Hemorrhagic complications after photodynamic therapy for polypoidal choroidal vasculopathy. Retina. 2007;27:335–41. doi: 10.1097/01.iae.0000233647.78726.46. [DOI] [PubMed] [Google Scholar]
- 5.Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, et al. EVEREST study: Efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012;32:1453–64. doi: 10.1097/IAE.0b013e31824f91e8. [DOI] [PubMed] [Google Scholar]
- 6.Quaranta M, Mauget-Faÿsse M, Coscas G. Exudative idiopathic polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Am J Ophthalmol. 2002;134:277–80. doi: 10.1016/s0002-9394(02)01516-7. [DOI] [PubMed] [Google Scholar]
- 7.Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, Slakter J, et al. Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. Retina. 2002;22:529–35. doi: 10.1097/00006982-200210000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Silva RM, Figueira J, Cachulo ML, Duarte L, Faria de Abreu JR, Cunha-Vaz JG. Polypoidal choroidal vasculopathy and photodynamic therapy with verteporfin. Graefes Arch Clin Exp Ophthalmol. 2005;243:973–9. doi: 10.1007/s00417-005-1139-4. [DOI] [PubMed] [Google Scholar]
- 9.Mauget-Faÿsse M, Quaranta-El Maftouhi M, De La Marnièrre E, Leys A. Photodynamic therapy with verteporfin in the treatment of exudative idiopathic polypoidal choroidal vasculopathy. Eur J Ophthalmol. 2006;16:695–704. doi: 10.1177/112067210601600506. [DOI] [PubMed] [Google Scholar]
- 10.Akaza E, Mori R, Yuzawa M. Long-term results of photodynamic therapy of polypoidal choroidal vasculopathy. Retina. 2008;28:717–22. doi: 10.1097/IAE.0b013e31816577cb. [DOI] [PubMed] [Google Scholar]
- 11.Chan WM, Lam DS, Lai TY, Liu DT, Li KK, Yao Y, et al. Photodynamic therapy with verteporfin for symptomatic polypoidal choroidal vasculopathy: One-year results of a prospective case series. Ophthalmology. 2004;111:1576–84. doi: 10.1016/j.ophtha.2003.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Lee SC, Seong YS, Kim SS, Koh HJ, Kwon OW. Photodynamic therapy with verteporfin for polypoidal choroidal vasculopathy of the macula. Ophthalmologica. 2004;218:193–201. doi: 10.1159/000076844. [DOI] [PubMed] [Google Scholar]
- 13.Gomi F, Ohji M, Sayanagi K, Sawa M, Sakaguchi H, Oshima Y, et al. One-year outcomes of photodynamic therapy in age-related macular degeneration and polypoidal choroidal vasculopathy in Japanese patients. Ophthalmology. 2008;115:141–6. doi: 10.1016/j.ophtha.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Kurashige Y, Otani A, Sasahara M, Yodoi Y, Tamura H, Tsujikawa A, et al. Two-year results of photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2008;146:513–9. doi: 10.1016/j.ajo.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 15.Kang HM, Koh HJ. Two-year outcome after combination therapy for polypoidal choroidal vasculopathy: Comparison with photodynamic monotherapy and anti-vascular endothelial growth factor monotherapy. Int J Ophthalmol. 2014;231:86–93. doi: 10.1159/000354546. [DOI] [PubMed] [Google Scholar]
- 16.Ojima Y, Tsujikawa A, Otani A, Hirami Y, Aikawa H, Yoshimura N. Recurrent bleeding after photodynamic therapy in polypoidal choroidal vasculopathy. Am J Ophthalmol. 2006;141:958–60. doi: 10.1016/j.ajo.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Rishi P, Kadekar A, Rishi E. Breakthrough vitreous hemorrhage after ICGA guided PDT for PCV. Indian J Ophthalmol. 2009;57:160–1. doi: 10.4103/0301-4738.45514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SJ, Yu HG. Efficacy of combined photodynamic therapy and intravitreal bevacizumab injection versus photodynamic therapy alone in polypoidal choroidal vasculopathy. Retina. 2011;31:1827–34. doi: 10.1097/IAE.0b013e318214d01e. [DOI] [PubMed] [Google Scholar]
- 19.Lee YA, Yang CH, Yang CM, Ho TC, Lin CP, Huang JS, et al. Photodynamic therapy with or without intravitreal bevacizumab for polypoidal choroidal vasculopathy: Two years of follow-up. Am J Ophthalmol. 2012;154:872–80.e2. doi: 10.1016/j.ajo.2012.03.051. [DOI] [PubMed] [Google Scholar]
- 20.Yamashita A, Shiraga F, Shiragami C, Shirakata Y, Fujiwara A. Two-year results of reduced-fluence photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol. 2013;155:96–102.e1. doi: 10.1016/j.ajo.2012.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Saito M, Iida T, Kano M, Itagaki K. Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 2013;251:2099–110. doi: 10.1007/s00417-013-2323-6. [DOI] [PubMed] [Google Scholar]
- 22.Sakai T, Okano K, Kohno H, Tsuneoka H. Three-year visual outcomes of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy. Acta Ophthalmol. 2016;94:e765–71. doi: 10.1111/aos.13130. [DOI] [PubMed] [Google Scholar]
- 23.Sakurada Y, Iijima H. Two-year results of photodynamic therapy with or without intravitreal ranibizumab for polypoidal choroidal vasculopathy. J Ocul Pharmacol Ther. 2013;29:832–6. doi: 10.1089/jop.2013.0044. [DOI] [PubMed] [Google Scholar]
- 24.Nowak-Sliwinska P, van den Bergh H, Sickenberg M, Koh AH. Photodynamic therapy for polypoidal choroidal vasculopathy. Prog Retin Eye Res. 2013;37:182–99. doi: 10.1016/j.preteyeres.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt-Erfurth U, Schlötzer-Schrehard U, Cursiefen C, Michels S, Beckendorf A, Naumann GO. Influence of photodynamic therapy on expression of vascular endothelial growth factor (VEGF), VEGF receptor 3, and pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003;44:4473–80. doi: 10.1167/iovs.02-1115. [DOI] [PubMed] [Google Scholar]
- 26.Tatar O, Shinoda K, Adam A, Eckert T, Eckardt C, Lucke K, et al. Effect of verteporfin photodynamic therapy on endostatin and angiogenesis in human choroidal neovascular membranes. Br J Ophthalmol. 2007;91:166–73. doi: 10.1136/bjo.2006.105288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatar O, Kaiserling E, Adam A, Gelisken F, Shinoda K, Völker M, et al. Consequences of verteporfin photodynamic therapy on choroidal neovascular membranes. Arch Ophthalmol. 2006;124:815–23. doi: 10.1001/archopht.124.6.815. [DOI] [PubMed] [Google Scholar]
- 28.Tatar O, Adam A, Shinoda K, Stalmans P, Eckardt C, Lüke M, et al. Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am J Ophthalmol. 2006;142:95–104. doi: 10.1016/j.ajo.2006.01.085. [DOI] [PubMed] [Google Scholar]
- 29.Lee MY, Lee WK, Baek J, Kwon OW, Lee JH. Photodynamic therapy versus combination therapy in polypoidal choroidal vasculopathy: Changes of aqueous vascular endothelial growth factor. Am J Ophthalmol. 2013;156:343–8. doi: 10.1016/j.ajo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Wang W, He M, Zhang X. Combined intravitreal anti-VEGF and photodynamic therapy versus photodynamic monotherapy for polypoidal choroidal vasculopathy: A systematic review and meta-analysis of comparative studies. PLoS One. 2014;9:e110667. doi: 10.1371/journal.pone.0110667. [DOI] [PMC free article] [PubMed] [Google Scholar]


