Abstract
Swept source optical coherence tomography-angiography (OCT-angiography) gives us a unique opportunity to study the vasculature of choroidal lesions in vivo. We describe the OCT angiographic characteristics of circumscribed choroidal hemangioma before and after laser photocoagulation. Medium-sized choroidal vessels/vessels on the tumor surface become predominantly visible after laser photocoagulation due to laser-induced damage to the overlying choriocapillaris. OCT-angiography adds a new dimension to in vivo analysis of vascular changes in choroid due to choroidal tumors and their response to therapy.
Key words: Autofluorescence, laser photocoagulation, medium-sized choroidal vessels, Sattler's layer, swept source optical coherence tomography-angiography
Choroidal hemangiomas can be circumscribed or diffuse. We aim to describe the swept source optical coherence tomography-angiography (OCT-angiography, source OCT, DRI OCT Triton, Topcon Inc.,) features of circumscribed choroidal hemangioma (CCH). OCT-angiography is a noninvasive modality to evaluate retinal and choroidal vasculature without the need for dyes, in contrast to fundus fluorescein angiography or indocyanine green angiography. We also describe the changes in choroidal vasculature overlying these tumors following laser photocoagulation as assessed by OCT-angiography.
Laser photocoagulation with confluent laser burns was done in all patients using a frequency-doubled neodymium-doped yttrium aluminum garnet (Nd-YAG) (532 nm) laser.
Case Report
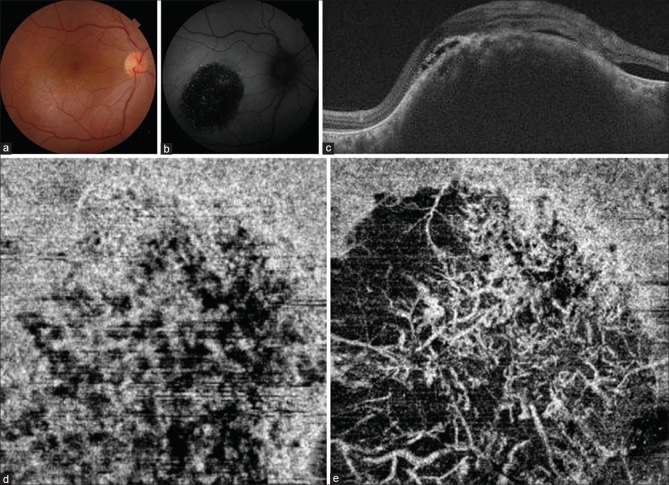
The first patient was a 55-year-old male with a visual acuity of 6/18 in the right eye. Fundus showed a deep, smooth round mass inferotemporal to the fovea with overlying patchy retinal pigment epithelial (RPE) changes [Fig. 1a]. There was no evidence of port-wine stain in the skin. Fundus autofluorescence showed a dark patch with a few speckled autofluorescent areas [Fig. 1b]. Fluorescein angiography showed the typical early hyperfluorescence. Ultrasound-B scan revealed a mass lesion in the choroid with uniform moderate-to-high internal reflectivity. OCT revealed a choroidal mound with convex inner surface elevating the overlying retina with associated subretinal fluid [Fig. 1c]. The details of the deeper layers of the choroid were not clear as the large mass obscured them. The choroidal-scleral interface under the mass could not be made out in the OCT. OCT-angiography scan taken below the level of the RPE (30-μ section) showed multiple relatively well-defined areas of reduced decorrelation signal (hypoflow) [Fig. 1d] as opposed to a diffuse hyperflow decorrelation signal seen at this level in normal eyes. The patient opted for frequency-doubled Nd-YAG (532 nm) laser as he could not afford photodynamic therapy (PDT), the standard treatment modality. However, the pathology of choroidal hemangioma lies in the deep choroid. Long wavelength lasers such as PDT (689 nm) and transpupillary thermotherapy (810nm) are preferred as such waves may penetrate the deep choroidal layers. On the contrary, a superficial effect of 532 nm laser over retinal pigment epithelium and choriocapillaris may be expected in view of its shorter wavelength. Thick hemangiomas, especially of the cavernous variety, may need repeated lasers to make the fovea free of fluid.
Figure 1.
(a) Color fundus photograph of the first patient shows an elevated reddish mass, with ill-defined margins, inferotemporal to the fovea suggesting a choroidal hemangioma. (b) Corresponding autofluorescence image reveals predominantly hypoautofluorescence with a few specks of hyperautofluorescence over the mass. (c) The swept source OCT reveals the elevated mound of the choroidal hemangioma and subretinal fluid. (d) The OCT-angiogram segmented below the level of the retinal pigment epithelium (30-μ) shows well-defined hypoflow areas within the choriocapillaris layer. (e) OCT-angiogram of the same patient through the same area 1 month after the laser shows disruption of the choriocapillaris layer and predominance of crisscrossing medium-sized vessels over the tumor
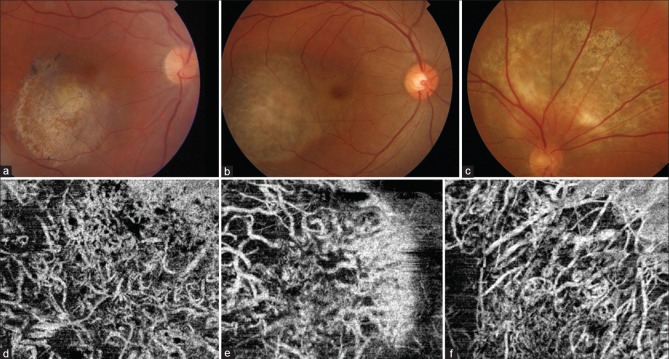
OCT-angiography images were repeated at a 1-month follow-up after the laser therapy [Fig. 1e]. The images segmented below the level of the RPE (30-μ section) now revealed a drastic reduction in signal from the choriocapillaris [Fig. 1e]. Postlaser, well-defined crisscrossing vessels similar to middle layer choroidal vessels (Sattler's layer) could be predominantly visualized over the choroidal hemangioma at this level [Fig. 1e]. We performed OCT-angiography at 1-year follow-up on three patients of choroidal hemangioma (including Case 1) treated similarly with laser photocoagulation [Table 1]. All the patients maintained visual acuity, and the fovea was free of fluid at final follow-up [Table 1]. The fundus images revealed marked loss of pigmentation with whitening of the surface of the hemangiomas [Fig. 2a–c]. OCT-angiography revealed similar crisscrossing vessels (looking even more prominent) as seen at 1-month follow-up. Despite repeated laser treatment, there was no damage/loss of these vessels [Fig. 2d–f].
Table 1.
Details of the eyes with choroidal hemangioma which underwent laser

Figure 2.
(a-c) Postlaser 1-year follow-up fundus images of the cases of choroidal hemangioma (a-case 1, b-case 2, and c-case 3). (d-f) Corresponding OCT-A images of the three patients in the figure above showing absence of choriocapillaris with persisting medium-sized crisscrossing vessels
Discussion
OCT-angiography helped us to noninvasively study the changes in choroidal vasculature after laser in choroidal hemangioma. OCT with enhanced depth imaging of choroidal hemangiomas shows expansion of the medium vessels (265%) and large vessels (576%) compared to normal population.[1] Notably, no compression of choriocapillaris may be seen.[1] On histopathology, circumscribed CCH may involve cavernous vessels (44%), capillary vessels (7%), or both (49%).[2] The OCT-angiography images of our patient taken at the choriocapillaris level before treatment are suggestive of some alteration in flow pattern at this level. The lack of normal RPE autofluorescence and mild attenuation of RPE signal on OCT-B scan may also suggest focal damage to the overlying RPE. This may be secondary to a disturbance of flow through the choriocapillaris. In a series of 34 eyes with choroidal hemangioma, the intrinsic autofluorescence of CCH was iso-autofluorescent in 58% of cases and hypoautofluorescent in 42% of cases.[3] We have also been able to document in vivo, the damage occurring to the choriocapillaris following laser photocoagulation. Obstruction of choriocapillaris within 24 hours of the laser has been documented earlier in experimental models on cats.[4] In our patient, following the loss of signal of the choriocapillaris, the medium-sized vessels over the tumor could be visualized. The 1-year follow-up scans of three of our patients showed the persistence of these vessels and the loss of flow in the choriocapillaris layer secondary to the laser [Table 1]. In a few experimental studies on animals, some restoration of the choriocapillaris has been described with time, following laser photocoagulation.[5] However, in all our cases, there was no appreciable restoration of flow in the choriocapillaris in the lasered area. Human studies on histopathological evaluation of old laser scars also show disruption of choriocapillaris and lack of recovery.[6,7] The medium-sized vessels over the tumor did not show any signs of loss/damage despite multiple sittings of laser photocoagulation. A study on en-face OCT of 22 eyes of CCH did not reveal any morphological differences between treated and untreated eyes.[8] This study also noted a multi-lobular pattern of the CCH and hyper-reflective halo around the tumor.[8]
As the choroidal hemangioma predominantly seems to be lying at a deeper level, i.e. within and below the middle vascular layer of the choroid, does the application of laser photocoagulation to it serve any purpose? The proposed mechanism of action of the laser in choroidal hemangioma may include intense photocoagulation to induce obliteration of the tumor as proposed by L'Esperance.[9] However, this may require multiple settings of the laser/xenon arc laser which was reportedly noted to have deeper penetration and improved efficacy over argon laser.[9] According to Gass, the main objective of the laser is to collapse the overlying cystic retina to the surface of the tumor to further cause absorption of the subretinal fluid.[10] Changes in outer blood-retinal barrier associated with photocoagulation may also contribute due to anatomical remodeling of RPE and functional improvement.
OCT-angiography imaging of our cases provides in vivo evidence that application of laser energy mainly destroys the choriocapillaris overlying the tumor and may not have much effect on the medium-sized vasculature of the tumor/choroid itself.
As of now, the gold standard therapy for the management of subfoveal or intraretinal fluid due to choroidal hemangioma is PDT. However, this therapy is costly, may require repeated applications, and also has the potential of causing overlying choroidal atrophy. Although 532nm laser may not reduce the size of the choroidal hemangioma, it still may be effective in making the fovea free of intraretinal or subretinal fluid in cases of large extrafoveal choroidal hemangiomas as hypothesized by Gass. This may require repetition of laser treatment up to three times a year as seen in our cases. Volumetric assessment of the choroidal hemangioma was not done in our series as the determination of the posterior extent of the lesion was difficult. However, clinically and qualitatively on OCT, we did not appreciate any change in the size of choroidal hemangioma after treatment. The goal of our treatment was to reduce the leakage from the lesion so as to make the fovea dry of fluid rather than to reduce the size of the choroidal hemangioma.
Further research and analysis of more cases of choroidal hemangiomas treated with various treatment modalities using a longer follow-up should make us wiser regarding the most appropriate form of therapy for such lesions.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Rojanaporn D, Kaliki S, Ferenczy SR, Shields CL. Enhanced depth imaging optical coherence tomography of circumscribed choroidal hemangioma in 10 consecutive cases. Middle East Afr J Ophthalmol. 2015;22:192–7. doi: 10.4103/0974-9233.150629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witschel H, Font RL. Hemangioma of the choroid. A clinicopathologic study of 71 cases and a review of the literature. Surv Ophthalmol. 1976;20:415–31. doi: 10.1016/0039-6257(76)90067-9. [DOI] [PubMed] [Google Scholar]
- 3.Ramasubramanian A, Shields CL, Harmon SA, Shields JA. Autofluorescence of choroidal hemangioma in 34 consecutive eyes. Retina. 2010;30:16–22. doi: 10.1097/IAE.0b013e3181bceedb. [DOI] [PubMed] [Google Scholar]
- 4.Perry DD, Risco JM. Choroidal microvascular repair after argon laser photocoagulation. Am J Ophthalmol. 1982;93:787–93. doi: 10.1016/0002-9394(82)90475-5. [DOI] [PubMed] [Google Scholar]
- 5.Perry DD, Reddick RL, Risco JM. Choroidal microvascular repair after argon laser photocoagulation. Ultrastructural observations. Invest Ophthalmol Vis Sci. 1984;25:1019–26. [PubMed] [Google Scholar]
- 6.Stitt AW, Gardiner TA, Archer DB. Retinal and choroidal responses to panretinal photocoagulation: An ultrastructural perspective. Graefes Arch Clin Exp Ophthalmol. 1995;233:699–705. doi: 10.1007/BF00164672. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DJ, Green WR. Argon laser panretinal photocoagulation for diabetic retinopathy. Scanning electron microscopy of human choroidal vascular casts. Arch Ophthalmol. 1987;105:239–42. doi: 10.1001/archopht.1987.01060020093036. [DOI] [PubMed] [Google Scholar]
- 8.Flores-Moreno I, Caminal JM, Arias-Barquet L, Rubio-Caso MJ, Catala-Mora J, Vidal-Martí M, et al. En face mode of swept-source optical coherence tomography in circumscribed choroidal haemangioma. Br J Ophthalmol. 2016;100:360–4. doi: 10.1136/bjophthalmol-2015-307099. [DOI] [PubMed] [Google Scholar]
- 9.Lanzetta P, Virgili G, Ferrari E, Menchini U. Diode laser photocoagulation of choroidal hemangioma. Int Ophthalmol 1995. 1996;19:239–47. doi: 10.1007/BF00132693. [DOI] [PubMed] [Google Scholar]
- 10.Gass JD. Stereoscopic Atlas of Macular Diseases: Diagnosis and Treatment. St Louis: Mosby; 1997. [Google Scholar]