Highlights
-
•
Streptozotocin induced diabetes altered fasting blood glucose, glycated hemoglobin, body weight, serum lipid profiles, hepatic lipogenic enzymes and antioxidant activities of the rats.
-
•
Livingstone potato modulated the fasting blood glucose, glycated hemoglobin, lipid profiles, hepatic lipogenic enzymes and antioxidant status of the diabetic rats.
-
•
Treatment of diabetics with livingstone potato may lead to increased utilization of circulating glucose by the liver.
Keywords: Diabetic complications, Livingstone potato, Streptozotocin, Rats, Incorporated feeds
Abbreviations: HbA1c, glycated hemoglobin; IDH, isocitrate dehydrogenase; ME, malic enzyme; AI, atherogenic index; CRI, coronary risk index; HDL, high density lipoprotein; LDL, low density lipoprotein; VLDL, very low density lipoprotein; GAE, gallic acid equivalence; NADPH, nicotinamide adenine dinucleotide phosphate reduced; NADP+, nicotinamide adenine dinucleotide phosphate oxidized; NADH, nicotinamide adenine dinucleotide reduced; NAD+, nicotinamide adenine dinucleotide oxidized
Chemical compounds studied in this article: Streptozotocin (PubChem CID-29327), Isocitric acid (PubChem CID-5318532), Triethanolamine (PubChem CID-7618), Gallic Acid (PubChem CID-370), DPPH (PubChem CID-2735032), Quercetin (PubChem CID-5280343), NADPH (PubChem CID-12598259), GlyGly (PubChem CID-11161), Glucose (PubChem CID-79025), Triethanolamine (PubChem CID-7618), NADP (PubChem CID-5886)
Abstract
The effect of livingstone potato (Plectranthus esculenthus N.E.Br) on serum glucose, glycated hemoglobin (HbA1C), serum triglyceride, total cholesterol, high density lipoprotein (HDL), low density lipoprotein (LDL), very low density lipoprotein (VLDL), hepatic malic enzyme (ME), isocitrate dehydrogenase (IDH) and catalase activities of Streptozotocin induced diabetic rats were investigated using standard techniques. The atherogenic index (AI) and coronary risk index (CRI) of the rats were calculated as the ratios of LDL to HDL and total cholesterol to HDL, respectively. The serum glucose of the non-diabetic, diabetic control and diabetic rats given livingstone potato incorporated feeds (test feed) were 92.58 ± 3.97, 352.30 ± 4.88 and 165.50 ± 7.88 mg/dl, respectively. Intake of the test feed by the diabetic rats of group 3, resulted in significant (P < 0.05) decrease of their serum glucose, HbA1c, triglyceride, cholesterol, LDL, VLDL, AI and CRI but significant increase (P < 0.05) of hepatic levels of ME, IDH, catalase and serum HDL compared with the diabetic control rats that had significant alteration of these parameters (P < 0.05) compared with the non-diabetic rats. The feed intakes of the non-diabetic, diabetic control and diabetic rats given the test feed were 133.34 ± 1.32, 137.84 ± 5.77 and 146.38 ± 4.33 g/rat/week by the last week of experimentation. The diabetic control rats recorded significant loss of weight (P < 0.05) compared with the non diabetic rats despite increased feed intake. Chemical analysis of the standard and test feeds showed that the standard rat feed contained 15.00 ± 0.78% protein, 7.24 ± 1.20% fat, 31.55 ± 2.62% carbohydrates, energy value of 290.65 ± 4.77 kcal/100 g, 10% crude fiber and 0.12 ± 0.04 mg Gallic Acid Equivalent while the test feed contained 40.10 ± 0.16% carbohydrates, 17.22 ± 0.40% protein, 22.16 ± 0.34% fat, energy value of 428.70 ± 2.12 kcal/100 g, 8.51 ± 0.16% crude fiber, 1.3 ± 0.2 mg Gallic Acid Equivalent/g of sample and strong antioxidant activity comparable to standard quercetin. The study shows the potentials of livingstone potato in the management of diabetes and hyperlipidemia.
1. Introduction
The alteration of lipid metabolism is one of the major causes of complications arising from diabetes mellitus as it leads to increased risk of cardiovascular diseases [1]. The reversal of diabetic dyslipidaemia is thus a major strategy in diabetes treatment [2], [3].
In many countries of the world, much attention has been paid to find novel types of natural anti-diabetic drugs from various medicinal plants. These medicinal plants play important roles in the lives of rural people, particularly those in the remote parts of developing countries that have limited access to adequate health facilities. In addition, the effectiveness, limited side effects and relatively low costs of these medicinal plants make them to be widely prescribed even when their biologically active compounds are unknown [4].
Livingstone potato (Plectranthus esculenthus N.E.Br) which is known by its local name in Nigeria as rizga, is one of the widely cultivated minor root crops in the middle belt regions especially Kaduna and Plateau States of Nigeria for its finger like tubers [5]. The plant is also commonly found in Southern Africa, Malawi, Zimbabwe, Congo, Zambia and Asia [5].
Livingstone potato is used in ethnopharmacology in Africa in the treatment of digestive problems [5], stomach ache [6], pains [7] and cancer.
In our previous study [8], we described for the first time, the anti-diabetic potentials of this plant in streptozotocin induced diabetic rat models. However, there is no experimental evidence presently available in the literature with regard to its effect on serum glucose, glycated hemoglobin and lipid profiles of diabetic animals. Moreover, despite the usage of new diagnostic devices, strict glycemic targets, better treatment guidelines and increased awareness of the disease, baseline glycosylated hemoglobin has continued to remain relatively high in subjects diagnosed and treated for type 2 diabetes.
Since the alteration of lipid metabolism is one of the pathological basis for the development of diabetic complications and being that glycated hemoglobin is regarded as the best marker for glycemic control, this study was initiated to investigate the effect of livingstone potato on serum glucose, glycated hemoglobin and lipid metabolism of streptozotocin induced diabetes in rats.
2. Materials and methods
2.1. Plant materials
The fresh samples of livingstone potato (Plectranthus esculenta) were obtained at harvest from National Root Crops Research Institute (NRCRI), Umudike, Nigeria. They were identified by NRCRI, Umudike that has livingstone potato as a National Mandate as well as by a Taxonomist in Michael Okpara University of Agriculture, Umudike, Nigeria and deposited in their herbarium for authentication.
2.2. Chemicals
Streptozotocin (STZ), dl-isocitric acid, β-nicotinamide adenine dinucleotide phosphate-sodium salt, triethanolamine, hydrochloride Gly–Gly, free base, l(−)malic acid, free acid, gallic acid, DPPH and standard quercetin used were products of Sigma and Aldrich Chemical Company, United Kingdom. The kits used for lipid profile assays were obtained from Randox Laboratories Limited (UK).
2.3. Processing of the plant materials
The samples were properly washed, chopped and oven dried at 60 °C for 48 h. The dry samples were then processed to flour and incorporated into the standard rat feeds at 19.55% incorporation.
3. Animal experiments
3.1. Selection of animals
Thirty male albino rats of the wistar strain (140–208 g) obtained from the animal house of the Department of Biochemistry, University of Nigeria, Nsukka, Enugu State, Nigeria, were used for the study. The animals were kept in metabolic cages in the animal house of the Department of Biochemistry, Michael Okpara University of Agriculture, Umudike, Nigeria. The animals were acclimatized for two weeks to their diets prior to the commencement of the experiment and were maintained under a constant 12-h light and dark cycle and a room temperature of 27–30 °C. The National Institutes of Health Principles of Laboratory Animal Care [9] were observed.
3.2. Induction of diabetes
After two weeks of acclimatization, freshly prepared solution of streptozotocin (0.1 g dissolved in 5 ml of freshly prepared sodium citrate buffer 0.1 M, pH 4.5) was injected intraperitoneally to 24 of the rats at a dosage of 65 mg/kg body weight at fasting state while the remaining 6 rats were taken as the non-diabetic groups. Blood was collected from the tail vein and the blood glucose concentration was analyzed prior to the commencement of the dietary feeding using a blood glucose meter (Double G glucometer, USA). The STZ-treated rats with fasting blood glucose levels >200 mg/dl after twelve days of induction of STZ and evidence of glycosuria, were considered to be diabetic and used for the study.
3.3. Experimental procedure
The STZ treated brats with stable diabetic condition were then divided into 2 subgroups (groups 2 and 3) comprising of six animals per group while the non-diabetic group formed the first group as follows:
Group 1. Normal rats fed standard rat feeds (Non-diabetic control).
Group 2. Diabetic control rats which also received standard rat feeds.
Group 3. Diabetic rats fed livingstone potato incorporated feeds (test feed).
Their diets and water were both administered ad libitum for 28 days, after which the rats were stunned by blow, sacrificed and blood was drawn from their heart using 10 ml syringes and poured into heparin tubes for HbA1C assays while the rest of the blood samples were collected in non-anti-coagulant tubes for serum assay of lipid profile and glucose.
3.4. Biochemical analysis
The liver was removed, washed with ice-cold physiological saline immediately and stored at −20 °C until analyzed. Ten percent homogenate (w/v) of the liver was prepared in 150 mM KCl using a homogenizer at 4 °C [10] and centrifuged at 1000 × g for 5 min to remove cell debris. The supernatant was further centrifuged at 14,000 × g for 30 min at 4 °C and the supernatant was employed as the enzyme source for the assay of the malic enzyme and isocitrate dehydrogenase activities of the rats [11]. The specific activities of NADP linked malic enzyme (E.C. 1.1.1.40) and isocitrate dehydrogenase (EC 1.1.1.42) were determined using the methods of Geer et al. [12] and Bergmeyer [13]. Catalase was analyzed in the liver homogenates using the method of Sinha [14] and results were expressed as μmoles of H2O2 consumed/min/mg of wet liver tissue. The HbA1C assay was carried out using the Biosysytems (Spain) diagnostic kit method as described by Karl et al. [15] and the principle was based on the quantification of the HbA1C by a turbidimetric inhibition immunoassay after preparation of the hemolysate using tetradecyltrimethylammonium bromide as the detergent. The serum total triglycerides, cholesterol, glucose and HDL-cholesterol concentrations were analyzed using the Randox assay diagnostic kits as described by Tietz [16] and NCEP [17]. The serum total proteins were determined using Biosystems diagnostic kits as previously described [18].
The LDL-cholesterol and VLDL levels were calculated using the method of Friedewald [19]. The atherogenic index (AI) and coronary risk index (CRI) of the rats were calculated using the formula shown below as described by Omonkhua et al. [20]:
Atherogenic index (AI) = LDL-cholesterol/HDL-cholesterol; coronary risk index (CRI) = total cholesterol/HDL-cholesterol.
3.5. Preparation of the extract of the feeds for total phenolic assay
Six grams each of the flours of the test and standard rat feeds were soaked in 60 ml of water and left overnight. The mixtures were filtered (Whatman no. 1) and centrifuged at 3000 × g for 10 min for the assay of the phenolic contents of the flour samples.
3.6. Chemical analysis of the flours
The total phenolic contents of the extracts of the flour samples were determined using the Folin–Ciocalteu method as described by Singleton et al. [21]. Gallic acid was used as the standard and results were expressed in mg Gallic acid equivalents/100 g. The proximate and crude fiber contents of the flours of the test and standard rat feeds were determined using the methods of AOAC [22].
3.7. Rapid thin layer chromatrography (TLC) free radical scavenging screening
The TLC screening of the antioxidant activity of the methanolic extract of the test feed was determined using the DPPH method as proposed by Mensor et al. [23] with minor modifications. With the aid of a capillary tube, stock solutions (100 mg/ml) (instead of 1 mg/ml) of the extract were spotted on a silica gel thin layer chromatographic (TLC) plate and developed with a solvent system of ethanol: methanol (90:10). After development, the chromatograms were dried and sprayed with a 0.3 mM solution of the stable DPPH free radical. The plates were visualized for the presence of yellow spots and the degree of activity was determined qualitatively from observation of the yellow colour intensity. Yellow spot formed (within 30 min of spraying) against a purple background was taken as a positive result. Quercetin was used as the positive control for this assay.
3.8. Statistical analysis
Data was subjected to analysis using the statistical package for social sciences (SPSS), version 15.0. Results were presented as the means ± standard deviations of three determinations. One way analysis of variance (ANOVA) was used for comparison of the means. Differences between means were considered to be significant at P < 0.05 using the new Duncan multiple range test.
4. Results
The dose of the livingstone potato used in this study that produced the desired anti-diabetic action was 195.5 g/kg.
The serum glucose levels of the non-diabetic, diabetic control and diabetic rats given livingstone potato incorporated feeds were: 92.58 ± 3.97, 352.30 ± 4.88 and 165.50 ± 7.88 mg/dl, respectively (Fig. 1). Intake of the livingstone potato incorporated feed by the diabetic rats of group 3, resulted in significant reduction (P < 0.05) of their hyperglycemia compared with the diabetic control rats (Fig. 1).
Fig. 1.
Blood glucose level of rats. Values are the means + SD of three determinations. a–c Means with different superscripts differ significantly (P < 0.05) in their glucose levels.
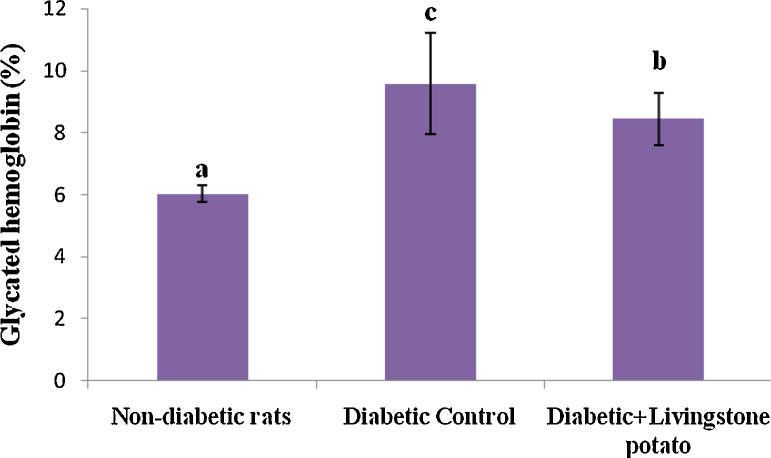
The percentage of glycated hemoglobin (HbA1c) of the rats, as shown in Fig. 2, decreased in the following order: Diabetic control rats (9.57 ± 1.63%) > Diabetic rats given livingstone potato incorporated feed (8.44 ± 0.85%) > Non-diabetic group (6.03 ± 0.28%). The diabetic rats fed livingstone potato incorporated feed had significant reduction (P < 0.05) of their HbA1C levels compared with the diabetic control rats (Fig. 2).
Fig. 2.
Glycated hemoglobin status of rats. Values are the means ± SD of three determinations. a–c Means with different superscripts differ significantly (P < 0.05) in the glycated hemoglobin contents. Biosystems reference values (%) 4–6.5 (non-diabetic); 6.0–7.0 (goal); 7.0–8.0 (good control); >8.0 (action suggested).
The diabetic control rats had significant increases (P < 0.05) of their serum cholesterol, triglycerides, LDL and VLDL levels but significant decreases of their serum HDL levels compared with the non diabetic rats (Table 1).
Table 1.
Serum lipid profile (mg/dl) of rats.
| Groups | Chol | Tri | VLDL | HDL | LDL |
|---|---|---|---|---|---|
| Group 1 | 89.15 ± 13.76a | 112.09 ± 23.33a | 22.42 ± 4.66a | 37.34 ± 6.10b | 29.39 ± 6.77a |
| Group 2 | 167.95 ± 17.95b | 183.23 ± 21.58b | 36.65 ± 4.32b | 22.01 ± 1.30a | 109.30 ± 12.72c |
| Group 3 | 96.70 ± .26a | 127.23 ± 14.62a | 25.45 ± 2.92a | 33.50 ± 3.61b | 37.76 ± 1.27b |
Values are means ± SD. a–c Means with different superscripts along each column are significantly different (P < 0.05). Chol—cholesterol; Tri—triglyceride.
The diabetic rats fed the livingstone potato incorporated feed, recorded significant reduction (P < 0.05) of their serum total cholesterol, triglycerides, VLDL and LDL but significant increase of their HDL levels compared with the diabetic control rats (Table 1).
The diabetic control rats had significant increases (P < 0.05) of their atherogenic and coronary risk indices compared with the non diabetic rats (Table 2).
Table 2.
Atherogenic index and coronary risk index of rats (units).
| Groups | Atherogenic index | Coronary risk index |
|---|---|---|
| Group 1 | 0.78 ± 0.06a | 2.38 ± 0.13a |
| Group 2 | 4.95 ± 0.29c | 7.61 ± 0.36b |
| Group 3 | 1.14 ± 0.16b | 2.90 ± 0.15a |
Values are means ± SD. a–c Means with different superscripts along each column are significantly different (P < 0.05). Units-ratio.
However, the diabetic rats given livingstone potato incorporated feed, recorded significant reduction (P < 0.05) of their atherogenic and coronary risk indices compared with the diabetic control rats (Table 2).
The hepatic malic enzyme (ME) activities of the non-diabetic, diabetic control and diabetic rats given livingstone potato incorporated feeds were 2.48 ± 0.4 units, 1.48 ± 0.16 units and 2.10 ± 0.08 units, respectively, (Fig. 3) while the hepatic isocitrate dehydrogenase (IDH) activities of the non-diabetic, diabetic control and diabetic rats given livingstone potato incorporated feeds were 2.80 ± 0.43 units, 1.62 ± 0.29 units and 2.73 ± 0.53 units, respectively (Fig. 3).
Fig. 3.
Lipogenic enzyme activities of rats. Values are the means ± SD of three determinations. a–c Means with different superscripts for each parameter are significantly different (P < 0.05): units definition: malic enzyme—μmol of pyruvate liberated/min/g protein; isocitrate dehydrogenase—μmol of α-KGT liberated/min/g protein.
There were significant reduction (P < 0.05) of the hepatic malic enzyme and isocitrate dehydrogenase activities of the diabetic control rats compared with the non-diabetic rats (Fig. 3).
However, there were significant increases (P < 0.05) of the hepatic malic enzyme and isocitrate dehydrogenase activities of the diabetic rats given livingstone potato incorporated feeds compared with the diabetic control rats (Fig. 3).
The diabetic control rats had significant reduction (P < 0.05) of their hepatic catalase activities compared with the non-diabetic rats (Table 3).
Table 3.
Hepatic catalase activity of rats.
| Groups | Catalase (μmol of H2O2 consumed/min/mg of wet liver tissue) |
|---|---|
| Non-diabetic | 82.40 ± 3.52c |
| Diabetic control | 40.75 ± 4.77a |
| Diabetic + livingstonepotato | 57.20 ± 3.97b |
a–c Values are the means ± SD of triplicate experiments. Means with different superscripts are significantly different (P < 0.05).
Intake of the livingstone potato incorporated feed by the diabetic rats of group 3 resulted in significant elevation (P < 0.05) of their hepatic catalase activity compared with the diabetic control rats (Table 3).
The body weights of the diabetic control rats were significantly reduced (P < 0.05) compared with the non-diabetic rats while the body weights of the diabetic rats given the livingstone potato incorporated feed were significantly increased (P < 0.05) compared with the diabetic control rats (Table 4).
Table 4.
Body weight of rats (g).
| Groups | Initial weight | Final weight |
|---|---|---|
| Group 1 | 185.50 ± 4.95ab | 233.00 ± 4.24c |
| Group 2 | 156.50 ± 16.26b | 102.50 ± 7.78a |
| Group 3 | 207.00 ± 1.41b | 179.00 ± 1.41b |
a–c Means with different superscripts along each column are significantly different (P < 0.05).
The feed intake of the diabetic control rats was not significantly different (P > 0.05) from that of the non-diabetic rats by the last week of experimentation while the feed intake of the diabetic rats given the livingstone potato incorporated feed was significantly higher (P < 0.05) than that of the diabetic control rats by the last week of experimentation (Table 5).
Table 5.
Feed intakes per rat (g/week).
| Groups | Week 1 | Week 2 | Week 3 | Week 4 |
|---|---|---|---|---|
| Group 1 | 118.22 ± 7.43a | 127.05 ± 8.97b | 122.00 ± 12.37b | 133.34 ± 1.32a |
| Group 2 | 117.07 ± 5.88a | 121.95 ± 9.11b | 119.60 ± 3.99a | 137.84 ± 5.77a |
| Group 3 | 119.56 ± 2.54a | 106.29 ± 5.75a | 140.24 ± 0.88c | 146.38 ± 4.33b |
Values are means ± SD. a–c Means with different superscripts along each column are significantly different (P < 0.05).
Proximate analysis of the livingstone potato incorporated and standard feeds showed that the standard rat feed contained 15.00 ± 0.78% protein, 7.24 ± 1.20% fat, 31.55 ± 2.62% carbohydrates with an energy value of 290.65 ± 4.77 kcal/100 g while the livingstone potato incorporated feed contained 40.10 ± 0.16% carbohydrates, 17.22 ± 0.40% protein, 22.16 ± 0.34% fat with an energy value of 428.70 ± 2.12 kcal/100 g.
Phytochemical and crude fiber analysis of the livingstone potato incorporated and standard rat feeds revealed that the livingstone potato incorporated feed contained 1.3 ± 0.2 mg gallic acid equivalent/g of sample and 8.51 ± 0.16% crude fiber while the standard rat feed contained 0.12 ± 0.04 mg gallic acid equivalent and 10% crude fiber (Fig. 4).
Fig. 4.
Phenolic and crude fiber composition of standard and livingstone potato incorporated feed. Values are the means ± SD of three determinations.
Antioxidant screening of the methanolic/ethanolic extract of the livingstone potato incorporated feed using rapid thin layer chromatographic assay indicated that it possessed strong antioxidant activity comparable to standard quercetin (Table 6).
Table 6.
Free radical scavenging activities of the methanolic/ethanolic extracts of livingstone potato incorporated feed using Rapid DPPH TLC screening.
| Plants | Antioxidant activity | Intensity of spot |
|---|---|---|
| Livingstone potato | Strong | +++ |
| Quercetin | Strong | +++ |
The degree of antioxidant activity, determined qualitatively from the observation of the yellow colour intensity: strong (+++).
5. Discussion
The STZ rat model of diabetes is one of the most commonly used models of human disease [24] because it mimics many of the complications of human diabetes.
The reduction of the serum glucose of the diabetic rats fed the test plant to the extent that was observed in this study is an indication of the ameliorating potentials of livingstone potato on hyperglycemia.
Glycated hemoglobin (HbA1C) is a product of the irreversible condensation of glucose with the N-terminal residue of the β-chain of hemoglobin A. The level of glycated hemoglobin is a useful and reliable tool for the assessment of glycemic control in diabetics as recommended by the International Diabetes Federation [25] as it reflects the average blood glucose concentration over the preceding 6–8 weeks [26], [27], [28], equivalent to the life time of the erythrocytes.
Several authors have reported the ameliorating actions of different anti-diabetic plants on the HbA1C status of streptozotocin diabetic rat models in short term studies [29], [30], [31].
Although the duration of this study was short, the significant reduction of the HbA1C levels of the diabetic rats fed the test feed underscores the importance of livingstone potato in the dietary management of diabetes and its complications since reduction of glycated hemoglobin is known to decrease diabetic complications [32].
Hypercholesterolemia and hyper-triglyceridemia are recognized complications of diabetes mellitus [33], [34] resulting from alterations in lipid metabolism characterized by elevated levels of cholesterol, triglycerides, VLDL and LDL and this could account for the elevated levels of cholesterol, triglycerides, VLDL and LDL in the diabetic untreated rats.
Studies have shown that atherogenic index is a good predictor of cardiovascular disease risk as well as efficient monitor of the effectiveness of lipid-lowering therapies since the LDL/HDL ratio has been considered more prognostic than LDL or HDL alone [34].
The significant reduction of the serum total cholesterol, VLDL, triglycerides, LDL-cholesterol, atherogenic and coronary risk indices together with the concomitant significant increase in the HDL levels of the diabetic rats fed the test feed, indicate the anti-hyperlipidemic potentials of livingstone potato.
The triglyceride lowering property of livingstone potato as observed in this study could indirectly have contributed to its anti-hyperglycemic activity through the mechanism of the glucose-fatty acid cycle [35] which reports that increased supply of triglycerides could constitute a source of increased free fatty acid availability and oxidation that can impair insulin action, glucose metabolism and utilization, leading to the development of hyperglycemia. Therefore, reduction of triglycerides after feeding of livingstone potato incorporated feed would also facilitate glucose oxidation, utilization and subsequently reduction of hyperglycemia.
Malic enzyme catalyzes the oxidative decarboxylation of cytoplasmic malate [10], [26]. The reaction helps to transfer cytoplasmic oxaloacetate to the mitochondrion which is important in the TCA cycle for the formation of citrate with acetyl-CoA for generating malate which can feed the cytosolic gluconeogenic pathway [10], [36].
Isocitrate dehydrogenase catalyzes the oxidative decarboxylation of isocitrate to α ketoglutarate and requires either NAD+ or NADP+, producing NADH and NADPH, respectively [37]. It is another key regulatory enzyme in lipogenesis, which supplies the NADH from the extra mitochondrial space for the generation of NADPH through the activity of the malic enzyme.
During diabetes, lipogenesis is decreased while lipolysis is increased in the hepatic tissue which results from the underutilization of glucose resulting in increased lipolysis [38] and this explains the reduced hepatic levels of the lipogenic enzymes-ME and IDH in the diabetic control rats. Similar kinds of results were reported [26], [39]. This decrease to the extent that was observed in this study could lead to decreased generation of NADPH needed for the regeneration of glutathione and other reducing agents that are needed for the maintenance of tissue integrity and oxidative balance.
However, the increased hepatic ME and IDH activities of the diabetic rats fed livingstone potato incorporated feed indicates better utilization of glucose or energy yielding intermediates by the TCA cycle [38].
Results obtained in this study indicate that treatment of diabetics with livingstone potato increases the utilization of circulating glucose by the liver.
The loss of weight by the diabetic control rats despite increased feed intake is an indication of loss of tissue proteins that contribute to body weight while the increase in weight by the diabetic rats fed the livingstone potato incorporated feed is simple due to treatment of hyperglycemia.
The recommended daily allowance for fat in diabetic patients is 15–20% of the total calorie [40]. Crude fat provided about 22.16% of the total calories of the livingstone potato incorporated feed and 7.24% of the total calories of the standard rat feed. The implication is that while the fat content of the standard rat feed failed to meet the daily requirement, the fat content of the livingstone potato incorporated feed did.
Crude protein provided about 15% of the total calories of the standard rat feed and about 17.22% of the livingstone potato incorporated feed.
The recommended daily allowance for proteins in diabetic patients is 15–20% of the total calories [40]. Thus, the range of proteins in both the standard rat feed and the livingstone potato incorporated feed did meet with this requirement.
It is worth noting that despite containing large amount of proteins, consumption of the standard rat feeds by the diabetic control rats did not translate into weight gain which is a confirmation that the loss of weight by the diabetic control rats despite eating highly proteinous diet was as a result of uncontrolled hyperglycemia while the gain in weight after feeding of livingstone potato incorporated feed resulted from glycemic control [41].
Total carbohydrates provided approximately 40.1% of the total calories for the livingstone potato incorporated feed and 31.55% of the total calories for the standard rat feed. Values obtained for both feed samples were lower than the recommended daily allowance for carbohydrates (50–70% of total calories) in diabetics [40].
Energy value of a food measures its value to the body as a fuel. The test feed was observed to contain higher energy value than the standard rat feed.
Phenolics have received considerable attention because of their potential antioxidant activity and effects on carbohydrate metabolism involving the inhibition of α-glucosidase and α-amylase, the key enzymes responsible for digestion of dietary carbohydrates to glucose [42].
Another important action of polyphenols on cardiovascular system in diabetes is the regulation of lipid and lipoprotein metabolism with resultant improvement of dyslipidemia [43].
The higher phenolic content of the livingstone potato incorporated feed compared with the standard rat feed, could be one explanation for the higher anti-diabetic and anti-hyperlipidemic actions demonstrated by livingstone potato compared with the standard rat feed in this study.
Oxidative stress has recently been implicated in pancreatic β-cell dysfunction. Several reaction mechanisms are thought to be involved in the genesis of oxidative stress in both diabetic patients and diabetic animals and they include: glucose auto-oxidation, protein glycation, formation of advanced glycation products and the polyol pathway. The production of reactive oxygen species during these processes leads to tissue damage [44].
The decrease in the hepatic catalase activity of the diabetic control rats to the extent that was observed in this study could precipitate oxidative stress to the liver while the increased hepatic catalase activity of the diabetic rats fed the livingstone potato incorporated feed shows that intake of livingstone potato by diabetics could protect the liver from oxidative damage.
The strong antioxidant activity of the methanolic/ethanolic extract of the livingstone potato incorporated feed could be the explanation for the modulation of the hepatic antioxidant status of the diabetic rats fed the livingstone potato incorporated feed. Furthermore, this strong antioxidant activity could be attributed to the phenolic content of the livingstone potato incorporated feed.
Dietary fiber decreases the absorption of cholesterol from the gut in addition to delaying the digestion and conversion of starch to simple sugars, an important factor in the management of diabetes. Dietary fibred also functions in the protection against cardiovascular disease, colorectal cancer and obesity [45].
However, it was observed that the crude fiber contents of the standard rat feeds were higher than that of the livingstone potato incorporated feed.
It is therefore plausible to suggest that the hypoglycemic and anti-hyperlipidemic actions demonstrated by livingstone potato in this study could be directly related to its phenolic constituents.
6. Conclusion
Results obtained in this study, indicate the anti-hyperlipidemic potentials of livingstone potato. The significant reduction of the HbA1C levels of the diabetic rats fed the livingstone potato incorporated feeds underscores the importance of livingstone potato in the dietary management of diabetes mellitus and its complications.
Conflicts of interest
We declare none.
Transparency document
Acknowledgement
The authors wish to thank the management of National Root Crops Research Institute, Umudike, Nigeria for sponsoring this study.
Footnotes
Available online 29 August 2014
References
- 1.Susanti E., Donosepoetro M., Patellong I., Arif M. Differences between several atherogenic parameters in patients with controlled and uncontrolled type 2 diabetes mellitus. Med. J. Indones. 2010;19(2):103–108. [Google Scholar]
- 2.Aloulou A., Khaled H., Dhouha E., Madiha B.A., Khaoula H., Bassem J., Fatma A., Abdelfattah E., Emna A. Hypoglycemic and anti-lipidemic properties of Kombucha tea in alloxan-induced diabetic rats. BMC Complement. Altern. Med. 2012;12:63. doi: 10.1186/1472-6882-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Okpe O., Ibrahim S., Njoku G.C., Ndidi U.S., Atabo S. Pancreatic islet regeneration and some liver biochemical parameters of leaf extracts of Vitex doniana in normal and streptozotocin-induced diabetic albino rats. Asian Pac. J. Trop. Biomed. 2014;4(2):124–130. doi: 10.1016/S2221-1691(14)60220-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schippers R.R. Natural Resources Institute. ACP-EU Technical Centre of Agriculture and Rural Co-operation; Chatham, UK: 2000. African Indigenous Vegetables. An Overview of the Cultivation Species. [Google Scholar]
- 5.Burkhill H.M. The useful plants of West Tropical Africa. Concepts Biochem. 1995;3:857. [Google Scholar]
- 6.Parkia M., Cooke J.A. The ethnobotany of the Midzichenda tribes of the coastal forest areas in Kenya. 2. Medicinal plant uses. S. Afr. J. Bot. 2003;69:382–395. [Google Scholar]
- 7.Lukhoba C.W., Monique S.J., Simmonds B., Alan J.P. Plectranthus: a review of ethnobotanical uses. J. Ethnopharmacol. 2006;103:1–24. doi: 10.1016/j.jep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Eleazu C.O., Eleazu K.C., Chukwuma S.C., Ironkwe C., Iroaganachi M.A., Emelike C.U. Effect of livingstonepotato (Plectranthus esculenthus N.E.Br) on diabetes and its complications in Streptozotocin induced diabetes in rats. DMJ Diab. Metab. J. 2014;38(5) doi: 10.4093/dmj.2014.38.5.366. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Research Council (NRC) National Institute of Health; Bethesda, MD, USA: 1985. Guide for the Care and Use of Laboratory Animals, Publication 8523. [Google Scholar]
- 10.Singh S.N., Praveen V., Shoba S., Radhey S., Kumria M., Ranganathan S., Sridharan K. Effect of an anti-diabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J. Ethnopharmacol. 2001;76:269–277. doi: 10.1016/s0378-8741(01)00254-9. [DOI] [PubMed] [Google Scholar]
- 11.Jin H L, Eun S Y, Jeen W. Alcohol-induced liver injury Implications for cytotoxicity and isocitrate dehydrogenase by peroxynitrite: inactivation of NADP+-dependent enzyme catalysis and regulation. J. Biol. Chem. 2003 doi: 10.1074/jbc.M302332200. [DOI] [PubMed] [Google Scholar]
- 12.Geer B.W., Krochko D., Oliver M.J., Walker V.K., Williamson J.H. Comp. Biochem. Physiol. 1980;65B:25–34. [Google Scholar]
- 13.Bergmeyer H.U. Methods Enzym. Anal. 1974;2:624–627. [Google Scholar]
- 14.Sinha K.A. Colorimetric assay of catalase. Anal Biochem. 1972;47:389. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 15.Karl J. Development and standardization of a new immunoturbidimetric HbA1c assay. Klin Lab. 1993;39:991–996. [Google Scholar]
- 16.Tietz N.W. third ed. WB Saunders Company; Philadelphia, PA: 1995. Clinical Guide to Laboratory Tests; pp. 518–519. [Google Scholar]
- 17.NCEP . Vol. 285. JAMA Publication; United States: 2001. pp. 2486–2497. (Third Report of the National Cholesterol Education Programme Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel 111)). 19. [DOI] [PubMed] [Google Scholar]
- 18.Friedman R.B., Young D.S., editors. fourth ed. Vol. 1 and 2. AACC Press; Washington, DC: 2001. (Effects of Disease on Clinical Laboratory Tests). softcover. ISBN 1-890883-45-X. [Google Scholar]
- 19.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–505. [PubMed] [Google Scholar]
- 20.Omonkhua A., Onoagbe I., Ajileye A., Aladegboye L., Adetoboye A. Long term anti-diabetic, anti-hyperlipidaemic and anti-atherogenic effects of Carica papaya leaves in Streptozotocin diabetic rats. Eur. J. Med. Plants. 2013;3(4):508–519. [Google Scholar]
- 21.Singleton V.L., Orthofer R., Lamnela-Ravenlos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 22.AOAC . 15th ed. Association of Official Analytical Chemists; Arlington, VA: 1990. Official Methods of Analysis. [Google Scholar]
- 23.Mensor L.L., Fabio S.M., Gildor G.L., Alexander S.R., Tereza C.D., Cintia S.C., Suzane G.L. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical methods. Phytother. Res. 2001;15:127–130. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 24.Ugarte M., Brown M., Hollywood K.A., Cooper G.J., Bishop P.N., Dunn W.B. Metabolomic analysis of rat serum in streptozotocin-induced diabetes and after treatment with oral triethylenetetramine (TETA) Genome Med. 2012;4(4):35. doi: 10.1186/gm334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Consensus Committee Consensus statement on the worldwide standardization of the hemoglobin A1C measurement: the American Diabetes Association, European Association for the Study of Diabetes, International Federation of Clinical Chemistry and Laboratory Medicine, and the International Diabetes Federation. Diab. Care. 2007;30(9):2399–2400. doi: 10.2337/dc07-9925. [DOI] [PubMed] [Google Scholar]
- 26.Haseena B.H., Kaladevi S.V., Varun V., Shanthi Pu Sachdanandam P. Ameliorating effect of Semecarpus anacardium on TCA cycle enzymes in high fat diet STZ induced Type 2 Diabetic rat model. J. Pharm. Res. 2011;4(12):4577–4580. [Google Scholar]
- 27.Ishrat K., Jaweed S.A., Bardapurkar J.S., Patil V.P. Study of magnesium, glycosylated hemoglobin and lipid profile in diabetic retinopathy. Indian J. Clin. Biochem. 2004;19(2):124–127. doi: 10.1007/BF02894270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cosson E., Chiheb S., Cussac-Pillegand C., Banu I., Hamo-Tchatchouang E., Nguyen M., Aout M., Charnaux N., Valensi P. Haemoglobin glycation may partly explain the discordance between HbA1c measurement and oral glucose tolerance test to diagnose dysglycaemia in overweight/obese subjects. Diab. Metab. 2013;39:118–125. doi: 10.1016/j.diabet.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Kumar V., Danish A., Firoz A., Mohammed A., Mohd M. Enhanced glycemic control, pancreas protective, antioxidant and hepatoprotective effects by umbelliferon-α-d-glucopyranosyl-(2I → 1II)-α-d-glucopyranoside in streptozotocin induced diabetic rats. SpringerPlus. 2013;2:639. doi: 10.1186/2193-1801-2-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uma B., Ansari N.M. Anti-hyperglycemic activity of aqueous extract of Embelia ribes Bum in streptozotocin induced diabetic rats. Indian J. Exp. Biol. 2008;46(1):607–613. [PubMed] [Google Scholar]
- 31.Sharma M., Siddique M., Akhter M., Shamim S., Shukla G., Pillai K. Evaluation of anti-diabetic and antioxidant effects of Seabuckthorn (Hippophae rhamnoides L.) in streptozotocin-nicotinamide induced diabetic rats. Open Conf. Proc. J. 2011;2:53–58. [Google Scholar]
- 32.Umesh C.S., Yadav K., Moorthy K., Najma Z.B. Effects of sodium-orthovanadate and Trigonella Foenum-Graecum seeds on hepatic and renal lipogenic enzymes and lipid profile during alloxan diabetes. J. Biosci. 2004;29(1):12. doi: 10.1007/BF02702565. [DOI] [PubMed] [Google Scholar]
- 33.Sharma S.R., Dwivedi S.K., Swarup D. Hypoglycemic and hypolipidaemic effects of Cinnamomum tamala Nees leaves. Indian J. Exp. Biol. 1996;34:372–374. [Google Scholar]
- 34.Onunogbo C., Ohaeri O.C., Eleazu C.O., Eleazu K.C. Chemical composition of mistletoe extract (Loranthus micranthus) and its effect on the protein, lipid metabolism and the antioxidant status of alloxan induced diabetic rats. J. Med. Res. 2012;1(4):057–063. [Google Scholar]
- 35.Randle P.J., Priestman D.A., Mistry S.C. Glucose-fatty acid interactions and the regulation of glucose disposal. J. Cell. Biochem. 1994;55(Suppl):1–11. doi: 10.1002/jcb.240550002. [DOI] [PubMed] [Google Scholar]
- 36.Bukato G., Kochan Z., Swierczynski J. Purification and properties of cytosolic and mitochondrial malic enzyme isolated from human brain. Int. J. Biochem. Cell Biol. 1995;27:47–54. doi: 10.1016/1357-2725(94)00057-3. [DOI] [PubMed] [Google Scholar]
- 37.Cherbavaz D.B., Lee M.E., Stroud R.M., Koshland D.E. Active site water molecules revealed in the 2.1 a resolution structure of a site-directed mutant of isocitrate dehydrogenase. J. Mol. Biol. 2000;295:377–385. doi: 10.1006/jmbi.1999.3195. [DOI] [PubMed] [Google Scholar]
- 38.Gomathi N., Andmalarvili T. Effect of Hibiscus rosasinensis on carbohydrate metabolizing enzymes in monosodium glutamate induced obesity in female rats. J. Cell Tissue Res. 2009;9:1969–1974. [Google Scholar]
- 39.Shanmugam K., Mallikarjuna K., Nishanth K., Li-Chen L., Shiung C., Chia-Hua K., Sathyavelu R. Nephro-protective effects of a ginger extract on cytosolic and mitochondrial enzymes against streptozotocin (STZ)-induced diabetic complications in rats. Chin. J. Physiol. 2011;54(2):79–86. doi: 10.4077/cjp.2011.amm006. [DOI] [PubMed] [Google Scholar]
- 40.Vasundev V. second ed. Jaypee Brothers Medical Publishers Pvt. Ltd; India: 2006. Fundamentals of Biochemistry. Textbook of Biochemistry; pp. 110–214. [Google Scholar]
- 41.Dolly J., Prashant K., Rai A.K., Shikha M., Geeta W. Effect of Moringa oleifera Lam. leaves aqueous therapy on hyperglycemic rats. J. Ethnopharmacol. 2009;123:392–396. doi: 10.1016/j.jep.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 42.Iwai K. Anti-diabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-A(y) mice. Plant Foods Hum. Nutr. 2008;63:163–169. doi: 10.1007/s11130-008-0098-4. [DOI] [PubMed] [Google Scholar]
- 43.Zahra B., Parvin M., Fereidoun A. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. J. Diab. Metab. Disord. 2013;12(43) doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eleazu C.O., Eleazu K.C., Chkwuma S.C., Udeme N. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J. Diab. Metab. Disord. 2013;12:60. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Monago C., Uwakwe A. Proximate composition and in vitro anti-sickling property of Nigeria Cyperus esculentus (tiger nut sedge) Trees Life J. 2009;4(2):1–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.