Highlights
-
•
A novel aromatic mutagen, 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one (ABAQ) formed in physiological conditions has a dysplasia-initiating activity in mouse colon, with or without inflammation.
-
•
High-grade dysplasia induced by ABAQ has high proliferative activity.
-
•
High-grade dysplasia induced by ABAQ expresses β-catenin, but does not show PDCD4 expression.
-
•
ABAQ is formed under physiological conditions in diabetic patients who have risk of cancer development in the colon.
-
•
Our findings provide a scientific basis for further research on the involvement of ABAQ in human health.
Abbreviations: ABAQ, 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one; AOM, azoxymethane; DSS, dextran sodium sulfate; HCA, heterocyclic amine; H&E, hematoxylin and eosin; i.g, intragastric; MeIQx, 2-amino-3,8-dimethylimidazo[4,5-flquinoxaline; PAH, polycyclic aromatic hydrocarbons; PhIP, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine
Keywords: Benzoazepinoqunolinone, Heterocyclic amines, Maillard reaction, Fenton reaction, High-grade dysplasia, PDCD4, Colon, Dextran sodium sulfate, Initiation, Mice
Abstract
The benzoazepinoqunolinone derivative, 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one (ABAQ), which is produced in a mixture of glucose and tryptophan incubated at 37 °C under physiological conditions in the presence or absence of hydroxyl radicals caused by the Fenton reaction, is a novel aromatic mutagen. In the current study, we determined the tumor-initiating potency of ABAQ using an inflammation-relate, two-stage mouse colon carcinogenesis model. Male Crj: CD-1 (ICR) mice were treated with the single intragastric administration (100 or 200 mg/kg body weight) of ABAQ followed by subsequent 1-week oral exposure to 2% dextran sodium sulfate (DSS) in drinking water. The ABAQ treatment alone resulted in high-grade dysplasia, which is a precursor to colorectal cancer, in the colon. Following the administration of DSS after ABAQ treatment, the incidence and frequency of high-grade dysplastic lesions increased; the values were highest in the mice treated with 200 mg/kg body weight of ABAQ followed by DSS. The lesions expressing β-catenin in their nuclei and cytoplasm exhibited high proliferation activity without the expression of programmed cell death 4. These findings indicate that ABAQ has a tumor-initiating activity in the mouse colon, with or without inflammation, although the potential pro-inflammatory effect of high doses of ABAC should be investigated.
1. Introduction
Human people are continuously exogenously exposed to a variety of chemicals that have been shown to have mutagenic or carcinogenic properties in experimental systems [1]. Cooking meat and fish at a high temperature (above 180 °C) forms mutagenic and carcinogenic heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs). HCAs are formed by the pyrolysis of creati(ni)ne with sugars with specific amino acids. Since a high temperature is needed, only fried, broiled or barbecued meat contains a significant amount of HCAs [2]. Experimental studies of HCAs began with Dr. Sugimura's discovery that cooked meat and fish contain potent mutagens [2]. Some HCAs were later shown to be complete carcinogens that induce liver, colon, mammary and prostate tumors in rodents and monkeys [2], [3]. Certain HCAs are consistently identified in well-done meat products consumed in the North American diet. Although a causal link has not been fully established, a majority of epidemiology studies have linked the consumption of well-done meat products to cancer of the colon and breast. Several HCAs thus represent an important class of carcinogens in foods and have been classified as “possibly carcinogenic to humans (Group 2B)” or “probably carcinogenic to humans (Group 2A)” by the IARC [4] and “Group 2: reasonably anticipated to be human carcinogens (R)” by the NTP [5]. Similar to most other chemical carcinogens, HCAs must be metabolized by CYP1A2 or CYP1B1 to chemically reactive electrophiles prior to reacting with DNA in order to exert their carcinogenic potency in both rats and humans [2].
Certain compounds that are mutagenic and carcinogenic in cooked foods are formed by the Maillard reaction of reducing sugars and amino acids. Indeed, 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) are mutagenic and carcinogenic HCAs formed through the Maillard reaction in meat and fish cooked at a high temperature [2]. These compounds are suggested to be formed by the reaction of creatine with Maillard reaction products from glucose and amino acids by heating at a high temperature of 128 °C [2], [3], [6]. The Maillard reaction can also occur at physiological temperatures. In a series of our study performed to clarify the formation of mutagens during the Maillard reaction in glucose and amino acids, we recently identified a novel aromatic mutagen, 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one (ABAQ, C16H11N3O2, MW = 277.28, Fig. 1a), formed in the mixture of glucose and tryptophan incubated at 37 °C and a pH of 7.4 in the presence or absence of hydroxyl radicals produced by the Fenton reaction [7]. ABAQ exhibits a strong mutagenic activity toward S. typhimurium TA98 and YG1024 with S9 mix [7]. The mutagenic potency of ABAQ is comparable to that of PhIP [7]. ABAQ also revealed mutagenicity in the liver of gpt delta transgenic mice [8].
Fig. 1.
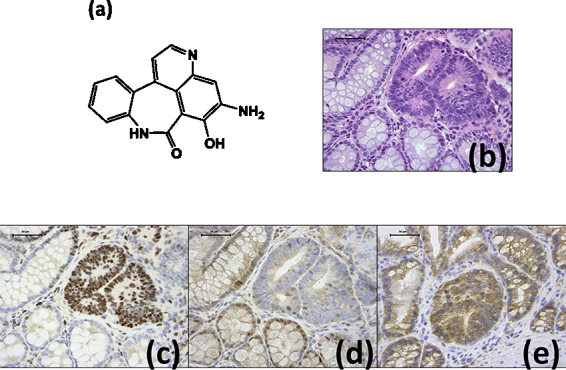
(a) Chemical structure of ABAQ (ABAQ, C16H11N3O2, MW = 277.28). (b) A high-grade colonic dysplasia on H&E-stained section from a mouse in group 2 (200 ppm ABAQ + DSS). Note: Nuclear atypia in the crypts and Paneth's granules (red in color) in the cytoplasm of some dysplastic crypt cells. H&E stain; bar, 50 μm. (c) Many nuclei in the high-grade dysplasia in a serial section from (b) are positive for MCM2. MCM2 immunohistochemistry; bar, 50 μm. (d) Most nuclei in the high-grade dysplasia in a serial section from (b) are negative for PDCD4, whereas the nuclei in the surrounding normal crypts are positive for PDCD4. PDCD4 immunohistochemistry; bar, 50 μm. (e) The cytoplasmic expression of β-catenin is present in the high-grade dysplasia lesion developed in the colon of a mouse from group 2 (200 ppm ABAQ + DSS). Some nuclei are weakly positive for β-catenin. β-Catenin immunohistochemistry; bar, 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of the article.)
In order to understand the effects of ABAQ on human health, it is important to elucidate its tumor-initiating ability in rodents. The current study thus aimed to determine whether the novel mutagen ABAQ possesses a tumor-initiation activity in the colon in in vivo experiment in mice. We used an experimental model employing a colitis-inducing agent, dextran sodium sulfate (DSS), which has a powerful tumor-promotion activity [9], since the tumor-initiation activity of PhIP [10] and aminophenylnorharman [11] can be detected within a short-term period in this model. The single administration with ABAQ in two doses via gavage resulted in development of high-grade dysplasia in the inflamed colon induced by DSS in male mice. The immunohistochemical analysis revealed the lesions with a high proliferative activity and cytoplasmic and/or nuclear expression of β-catenin to be negative for programmed cell death 4 (PDCD4), suggesting the tumor-initiation ability of ABAQ.
2. Materials and methods
2.1. Animals, chemicals and diet
Male Crlj: CD-1 (ICR) mice (Charles River Japan, Tokyo, Japan) 5 weeks of age were used. The mice were maintained at Gifu University Animal Facility according to the Institutional Animal Care Guidelines. All animals were housed in plastic cages (3 or 4 mice/cage) with free access to drinking water and a pelleted basal diet, CRF-1 (Oriental Yeast, Tokyo, Japan), under controlled conditions of humidity (50 ± 10%), light (12/12-h light/dark cycle) and temperature (23 ± 2 °C). After seven days of quarantine, they were randomized according to body weight into experimental and control groups. ABAQ was synthesized as previously described [7]; its purity was confirmed to be > 99% by HPLC. DSS with a molecular weight of 36,000–50,000 (Cat No. 160110) was purchased from MP Biochemicals, LLC (Aurora, OH, USA).
2.2. Experimental procedure for evaluating the tumor-initiating activity
The present study was approved by the Experimental Animal Research Committee of Gifu University. A total of 38 male ICR mice were divided into five experimental and solvent control groups. Groups 1 through 3 were treated with the single intragastric (i.g.) intubation of ABAQ at a dose of 100 or 200 mg/kg body weight. Starting one week after the ABAQ treatment, the animals in groups 1 (n = 15) and 2 (n = 15) were given 2% (w/v) DSS in drinking water for seven days, followed by no further treatments for 14 weeks. Groups 3 (n = 5) and 4 (n = 5) were given ABAQ (200 mg/kg body weight) alone and 2% DSS alone, respectively. Group 5 (n = 8) was given solvent (physiological saline) alone and served as an untreated control group. All animals were sacrificed via CO2 asphyxiation at week 16. The colons were flushed with saline, excised, measured for length (from the ileocecal junction to the anal verge), cut open longitudinally along the main axis and washed with saline. After carefully macroscopically inspecting the colons, the tissues were cut and fixed in 10% buffered formalin for at least 24 h. A histological examination was performed on paraffin-embedded sections following hematoxylin and eosin (H&E) staining. The presence or absence of mucosal ulceration, dysplasia and colonic neoplasms was examined according to our previous report [9]. A histopathological examination was also performed for other organs.
2.3. Immunohistochemistry of minichromosome maintenance protein 2 (MCM2), β-catenin and programmed cell death 4 (PDCD4)
We used paraffin-embedded sections of the colons of the mice in all groups for the immunohistochemical analysis. Serial histological sections (4 μm thickness) were made from each paraffin wax block. Immunohistochemical staining was performed automatically (Ventana Benchmark XTsystem; Ventana, Touchstone, Arizona, USA), according to the manufacturer's instructions. The primary antibodies were anti-MCM2 rabbit monoclonal antibody (no. 3619, anti-MCM2 (D7611)XP, 1:400 dilution; Cell Signaling Technology, Inc., Danvers, MA, USA), anti-β-catenin rabbit polyclonal antibody (#9661, 1:200 dilution; Cell Signaling Technology) and anti-PCDC4 rabbit polyclonal antibody (ab51495, 1:500 dilution; Abcam, Inc. Cambridge, MA, USA). In each case, the positive and negative controls were run concurrently. As the final step, the sections were lightly counterstained with Mayer's hematoxylin (Merck, Tokyo, Japan).
Immunoreactivity for antibodies against MCM2, PDCD4 and β-catenin was assessed in the lesions (high-grade dysplasia) that developed in groups 1 through 3 using a microscope (Olympus BX41, Olympus Optical Co., Tokyo, Japan). The intensity and localization of the immunoreactivity against the primary antibodies were determined by a pathologist (T.T.) who was unaware of the treatment group to which the slide belonged.
2.4. Statistical analysis
All measurements were compared using a one-way ANOVA with either Tukey's correction or Fisher's exact probability test (GraphPad Instat version 3.05, GraphPad Software, San Diego, CA), with a value of p < 0.05 as the criterion for significance.
3. Results
3.1. General observations
As listed in Table 1, the mean liver (p < 0.05) and relative liver weights (p < 0.01), and mean colon length (p < 0.05) in group 1 (100 mg/kg ABAQ + 2% DSS) were significantly larger than those observed in group 3 (200 mg/kg ABAQ alone). The mean liver weight and colon length of group 3 were significantly lower than that of group 5 (solvent control) (p < 0.05 for each comparison).
Table 1.
Body, liver and relative liver weights and colon lengths in all groups.
| Group no. | Treatment (no. of mice examined) | Body weight (g) | Liver weights (g) | Relative liver weight (g/100 g body weight) | Colon length (cm) |
|---|---|---|---|---|---|
| 1 | ABAQ a (100 mg/kg bw) → 2% DSS (15) | 49.9 ± 7.29 b | 2.5 ± 0.53 c | 5.0 ± 0.48 d | 16.5 ± 1.30 c |
| 2 | ABAQ (200 mg/kg bw) → 2% DSS (15) | 44.8 ± 9.46 | 2.2 ± 0.56 | 4.8 ± 0.37 c | 15.6 ± 1.31 |
| 3 | ABAQ (200 mg/kg bw) (5) | 42.0 ± 3.46 | 1.7 ± 0.23 e | 4.1 ± 0.29 | 14.3 ± 0.42 e |
| 4 | Solvent → 2% DSS (5) | 47.6 ± 1.62 | 2.1 ± 0.13 | 4.5 ± 0.35 | 15.9 ± 1.33 |
| 5 | None (8) | 51.6 ± 7.95 | 2.5 ± 0.45 | 4.8 ± 0.58 | 16.4 ± 1.21 |
ABAQ, 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one; DSS, dextran sodium sulfate.
Mean ± SD.
c,d Significantly different from group 3 (cp < 0.05 and dp < 0.01) according to a one-way ANOVA and the Tukey–Kramer Multiple Comparison test.
Significantly different from group 5 (p < 0.05).
3.2. Pathological findings of the liver and colorectum of mice treated with ABAQ and/or DSS
There were no tumors in any organs, including the colorectum, in all groups. Fatty changes were observed in the liver of two mice (13%) in group 1, but not in other groups. As indicated in Table 2, colonic dysplasia (high-grade, Fig. 1b) developed in groups 1 through 3. DSS exposure increased the multiplicity of high-grade dysplasia induced by ABAQ. There were no dysplastic lesions in the mice in groups 4 and 5.
Table 2.
Incidence and multiplicity of colonic lesions in the mice in each group.
| Group no. | Treatment (no. of mice examined) | Mucosal ulcer | High-grade dysplasia |
|---|---|---|---|
| 1 | ABAQ a (100 mg/kg bw) → 2% DSS (15) | 10/15 (67%) 1.40 ± 1.18 b | 4/15 (27%) c,d 0.53 ± 1.13 |
| 2 | ABAQ (200 mg/kg bw) → 2% DSS (15) | 11/15 (73%) 1.27 ± 1.16 | 10/15 (67%) e 1.60 ± 1.50 |
| 3 | ABAQ (200 mg/kg bw) (5) | 0/5 (0%) 0 | 1/5 (20%) 0.20 ± 0.45 |
| 4 | Solvent → 2% DSS (5) | 5/5 (100%) 1.80 ± 0.84 f | 0/5 (0%) 0 |
| 5 | None (8) | 0/8 (0%) 0 | 0/8 (0%) 0 |
ABAQ, 5-amino-6-hydroxy-8H-benzo[6,7]azepino[5,4,3-de]quinolin-7-one; DSS, dextran sodium sulfate.
Mean ± SD.
c,d,e Significantly different from group 2 (cp < 0.05), group 3 (dp < 0.05) and group 4 (ep < 0.05) according to the Fisher's exact probability test.
Significantly different from group 5 (p < 0.05) according to a one-way ANOVA and the Tukey–Kramer Multiple Comparison test.
3.3. Immunohistochemical expression of MCM2, β-catenin, and PDCD4 in the high-grade dysplasia lesions
The nuclei in the high-grade dysplasia lesions were positive for MCM2 (Fig. 1c), reflecting a high proliferation activity, and negative for PDCD4 (Fig. 1d). A cytoplasmic and nuclear expression of β-catenin was observed in the high-grade dysplasia lesions (Fig. 1e).
4. Discussion
In this study, we confirmed the tumor-initiating ability of ABAQ in an inflammation-associated, two-stage mouse carcinogenesis model. Importantly, ABAQ treatment alone produced lesions exhibiting high-grade dysplasia lesions that are preneoplastic for colorectal cancer, although the incidence and multiplicity were low and statistically insignificant from group 5 (untreated control). One week of exposure to DSS after the single i.g. administration of ABAQ increased the incidence and number of high-grade dysplasia lesions in the colorectum. The dysplasia-inducing potency was considered to be dose-dependent, although only two doses of ABAQ were applied in this study.
In this study, dosing of ABAQ (100 mg/kg) followed by DSS (group 1) increased the liver weight, while 200 mg/kg of ABAQ (group 2) did not. However, both doses of ABAQ increased the relative liver weight. Fatty degeneration observed in the liver of a few mice of group 1 may be related to these changes. As to the colon length, the treatment of ABAQ alone (group 3) shortened the colon, suggesting pro-inflammatory action of ABAQ, but the colon lengths of groups 1 and 2 were increased when given DSS. In order to proof the dose dependency and discard the possible pro-inflammatory effect at higher doses of ABAQ, which might contribute to the tumor development, it would be interesting to use three or four different doses (from 50 to 200 mg/kg, for instance). Such an experiment is planned in our laboratory.
The Maillard reaction in vivo is involved in aging [12] and a variety of chronic diseases, such as diabetes and related retinopathy and nephropathy [13]. Pyrraline is formed as an advanced glycation end product in the Maillard reaction between glucose and the ɛ-amino group of lysine under physiological conditions [14]. The serum [15] and urine [16] concentrations of pyrraline are known to be increased in diabetic patients, and pyrraline is detected in individuals with diabetic glomerulosclerosis [17]. Epidemiological [18], [19] and experimental investigations [20], [21] have indicated a positive association between diabetes and cancer development in several tissues, including the colorectum, suggesting that certain chemicals formed by the Maillard reaction in the body increase the risk of cancer in patients with diabetes. However, little is known about mutagens formed through the Maillard reaction in vivo.
In this study, we observed high-grade dysplasia in the colorectum of the mice treated with ABAQ alone and ABAQ followed by DSS exposure. Although no colorectal neoplasms developed, these findings are of importance since high-grade dysplasia is known to be a precursor lesion of inflammatory conditions in the colon, such as inflammatory bowel disease (IBD) [22]. In the fact, the high-grade dysplasia observed in this study increased the degree of proliferation, as estimated on MCM2 immunohistochemistry, and altered the expression of β-catenin in the cytoplasm and nuclei. More importantly, almost null expression of PDCD4 was observed in the high-grade dysplasia lesions. This finding is in accordance with the results showing a negative expression of PDCD4 in patients with sporadic colorectal cancer [23], [24], IBD-related colorectal cancer [22], [25] and dysplasia in IBD [22], [25].
In conclusion, the results of the current study indicate the potential tumor-initiating activity of ABAQ in the colon, with and without inflammation. Although the ABAQ level has not been determined in healthy subjects, ABAQ is a potential novel endogenous mutagen and tumor-initiating compound, as shown in this study and a previous investigation [7]. Since the mutagenic activity of ABAQ is comparable to that of PhIP [7], additional studies of the carcinogenicity of ABAQ in the colon and other tissues are required. Our findings provide a scientific basis for further research on the involvement of ABAQ in human health.
Conflict of interest
The authors declare no financial or commercial conflicts of interests.
Acknowledgments
This work was partly supported by a Grant-in-Aid for the 3rd Terms Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health, Labour and Welfare of Japan, Grants-in-Aid from Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, Grants-in-Aid for National Cancer Center Research and Development Fund, Grants-in-Aid for the U.S.-Japan Cooperative Medical Science Program, from the Ministry of Health, Labor and Welfare of Japan, and a grant from Takeda Science Foundation.
Footnotes
Available online 30 April 2014
References
- 1.Tanaka T., Shimizu M., Kohchi T., Moriawaki H. Chemical-induced carcinogenesis. J. Exp. Clin. Med. 2013;5:203–209. [Google Scholar]
- 2.Sugimura T., Wakabayashi K., Nakagama H., Nagao M. Heterocyclic amines: mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wakabayashi K., Nagao M., Esumi H., Sugimura T. Food-derived mutagens and carcinogens. Cancer Res. 1992;52:2092s–2098s. [PubMed] [Google Scholar]
- 4.IARC . IARC, WHO; Lyon: 1993. Some Naturally Occurring Substances: Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins; p. 599. [Google Scholar]
- 5.NTP NTP 12th Report on Carcinogens. Rep Carcinog. 2011;12 iii-499. [PubMed] [Google Scholar]
- 6.Jagerstad M., Olsson K., Grivas S., Negishi C., Wakabayashi K., Tsuda M., Sato S., Sugimura T. Formation of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in a model system by heating creatinine, glycine and glucose. Mutat. Res. 1984;126:239–244. doi: 10.1016/0027-5107(84)90002-2. [DOI] [PubMed] [Google Scholar]
- 7.Nishigaki R., Watanabe T., Kajimoto T., Tada A., Takamura-Enya T., Enomoto S., Nukaya H., Terao Y., Muroyama A., Ozeki M., Node M., Hasei T., Totsuka Y., Wakabayashi K. Isolation and identification of a novel aromatic amine mutagen produced by the Maillard reaction. Chem. Res. Toxicol. 2009;22:1588–1593. doi: 10.1021/tx900119j. [DOI] [PubMed] [Google Scholar]
- 8.Totsuka Y., Watanabe T., Coulibaly S., Kobayashi S., Nishizaki M., Okazaki M., Hasei T., Wakabayashi K., Nakagama H. In vivo genotoxicity of a novel heterocyclic amine, aminobenzoazepinoquinolinone-derivative (ABAQ), produced by the Maillard reaction between glucose and l-tryptophan. Mutat. Res. 2014;760:48–55. doi: 10.1016/j.mrgentox.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka T., Kohno H., Suzuki R., Yamada Y., Sugie S., Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka T., Suzuki R., Kohno H., Sugie S., Takahashi M., Wakabayashi K. Colonic adenocarcinomas rapidly induced by the combined treatment with 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and dextran sodium sulfate in male ICR mice possess beta-catenin gene mutations and increases immunoreactivity for beta-catenin, cyclooxygenase-2 and inducible nitric oxide synthase. Carcinogenesis. 2005;26:229–238. doi: 10.1093/carcin/bgh292. [DOI] [PubMed] [Google Scholar]
- 11.Kohno H., Totsuka Y., Yasui Y., Suzuki R., Sugie S., Wakabayashi K., Tanaka T. Tumor-initiating potency of a novel heterocyclic amine, aminophenylnorharman in mouse colonic carcinogenesis model. Int. J. Cancer. 2007;121:1659–1664. doi: 10.1002/ijc.22864. [DOI] [PubMed] [Google Scholar]
- 12.Aronson D. Pharmacological prevention of cardiovascular aging—targeting the Maillard reaction. Br. J. Pharmacol. 2004;142:1055–1058. doi: 10.1038/sj.bjp.0705832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCance D.R., Dyer D.G., Dunn J.A., Bailie K.E., Thorpe S.R., Baynes J.W., Lyons T.J. Maillard reaction products and their relation to complications in insulin-dependent diabetes mellitus. J. Clin. Invest. 1993;91:2470–2478. doi: 10.1172/JCI116482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith P.R., Somani H.H., Thornalley P.J., Benn J., Sonksen P.H. Evidence against the formation of 2-amino-6-(2-formyl-5-hydroxymethyl-pyrrol-1-yl)-hexanoic acid (‘pyrraline’) as an early-stage product or advanced glycation end product in non-enzymic protein glycation. Clin. Sci. (Lond.) 1993;84:87–93. doi: 10.1042/cs0840087. [DOI] [PubMed] [Google Scholar]
- 15.Odani H., Shinzato T., Matsumoto Y., Takai I., Nakai S., Miwa M., Iwayama N., Amano I., Maeda K. First evidence for accumulation of protein-bound and protein-free pyrraline in human uremic plasma by mass spectrometry. Biochem. Biophys. Res. Commun. 1996;224:237–241. doi: 10.1006/bbrc.1996.1013. [DOI] [PubMed] [Google Scholar]
- 16.Portero-Otin M., Pamplona R., Bellmunt M.J., Bergua M., Nagaraj R.H., Prat J. Urinary pyrraline as a biochemical marker of non-oxidative Maillard reactions in vivo. Life Sci. 1997;60:279–287. doi: 10.1016/s0024-3205(96)00628-5. [DOI] [PubMed] [Google Scholar]
- 17.Miyata S., Monnier V. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J. Clin. Invest. 1992;89:1102–1112. doi: 10.1172/JCI115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inoue M., Iwasaki M., Otani T., Sasazuki S., Noda M., Tsugane S. Diabetes mellitus and the risk of cancer: results from a large-scale population-based cohort study in Japan. Arch. Intern. Med. 2006;166:1871–1877. doi: 10.1001/archinte.166.17.1871. [DOI] [PubMed] [Google Scholar]
- 19.Yuhara H., Steinmaus C., Cohen S.E., Corley D.A., Tei Y., Buffler P.A. Is diabetes mellitus an independent risk factor for colon cancer and rectal cancer? Am. J. Gastroenterol. 2011;106:1911–1921. doi: 10.1038/ajg.2011.301. quiz 1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hata K., Kubota M., Shimizu M., Moriwaki H., Kuno T., Tanaka T., Hara A., Hirose Y. Monosodium glutamate-induced diabetic mice are susceptible to azoxymethane-induced colon tumorigenesis. Carcinogenesis. 2012;33:702–707. doi: 10.1093/carcin/bgr323. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M., Shirakami Y., Iwasa J., Shiraki M., Yasuda Y., Hata K., Hirose Y., Tsurumi H., Tanaka T., Moriwaki H. Supplementation with branched-chain amino acids inhibits azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Clin. Cancer Res. 2009;15:3068–3075. doi: 10.1158/1078-0432.CCR-08-2093. [DOI] [PubMed] [Google Scholar]
- 22.Tanaka T., Sugie S. Recent advances in pathobiology and histopathological diagnosis of inflammatory bowel disease. Pathol. Discov. 2013;1:1–6. [Google Scholar]
- 23.Fassan M., Pizzi M., Giacomelli L., Mescoli C., Ludwig K., Pucciarelli S., Rugge M. PDCD4 nuclear loss inversely correlates with miR-21 levels in colon carcinogenesis. Virchows Arch. 2011;458:413–419. doi: 10.1007/s00428-011-1046-5. [DOI] [PubMed] [Google Scholar]
- 24.Mudduluru G., Medved F., Grobholz R., Jost C., Gruber A., Leupold J.H., Post S., Jansen A., Colburn N.H., Allgayer H. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer. 2007;110:1697–1707. doi: 10.1002/cncr.22983. [DOI] [PubMed] [Google Scholar]
- 25.Ludwig K., Fassan M., Mescoli C., Pizzi M., Balistreri M., Albertoni L., Pucciarelli S., Scarpa M., Sturniolo G.C., Angriman I., Rugge M. PDCD4/miR-21 dysregulation in inflammatory bowel disease-associated carcinogenesis. Virchows Arch. 2013;462:57–63. doi: 10.1007/s00428-012-1345-5. [DOI] [PubMed] [Google Scholar]


