Highlights
-
•
Krill oil is a safe dietary source of omega-3 fatty acids.
-
•
The no adverse effect level (NOAL) of krill oil was 5%.
-
•
Krill oil showed no mutagenic activity in bacteria.
Abbreviations: ANOVA, analysis of variance; DHA, docosahexaenoic acid; EPA, eicosapentaenoic acid; FOB, functional observation battery; KO, krill oil; NOAEL, no observed adverse effect level; SO, soya bean oil
Keywords: Krill oil, Omega-3 fatty acids, Phospholipids, Eicosapentaenoic acid, Docosahexaenoic acid, Astaxanthin
Abstract
The safety of krill oil was assessed in a subchronic toxicity study and in a genotoxicity test. In a 13-week study, rats were fed krill oil or control diets. There were no differences noted in body weight, food consumption or in the functional observation battery parameters in either gender. Differences in both haematology and clinical chemistry values were noted in the krill oil-treated groups. However these findings were of no toxicological significance. Significant decreases in absolute and covariant heart weight in some krill oil-treated animals were noted although no corresponding histological changes were observed. In addition, periportal microvesicular hepatocyte vacuolation was noted histologically in males fed 5% krill oil. This finding was not associated with other indications of hepatic dysfunction. Given that the effects of the 13-week toxicity study were non-toxic in nature, the no observed adverse effect level (NOAEL) for the conditions of this study was considered to be 5% krill oil. The genotoxicity experiments documented no mutagenicity of krill oil in bacteria.
1. Introduction
The beneficial effects of long-chain omega-3 polyunsaturated fatty acids (PUFAs), eicosapentaenoic acid (EPA, 20:5) and docosahexaenoic acid (DHA, 22:6), which are present in fatty fish and omega-3 supplements like fish oil, have been well documented. Omega-3 fatty acids have been demonstrated to reduce plasma lipids [1], reduce blood pressure [2], prevent arrhythmias [3] and reduce platelet aggregation [4]. In addition to the positive effects of omega-3 fatty acids demonstrated for cardiovascular disease [5], studies also suggest that these long-chain fatty acids are important for immune function, bone-turnover, insulin sensitivity and cognitive function, amongst many other physiological functions and their general importance for body composition homeostasis [6], [7], [8].
Hence the European Food Safety Authority (EFSA) allows for health claims for 250 mg/day EPA + DHA to contribute on normal function of the heart, for either 2 g/day DHA or 2 g/day EPA + DHA on maintenance of normal blood triglyceride levels and for 3 g EPA + DHA on maintenance of normal blood pressure. Moreover, EFSA has established health claims on food containing at least 82.5 mg choline per 100 g. The choline nutrition claim covers maintenance of normal liver function, lipid metabolism and homocysteine metabolism.
The short-chain omega-3 fatty acid α-linolenic acid (ALA) is produced by plants, but in humans the enzymatic conversion efficiency to the longer chain PUFAs, EPA and DHA, is rather low [9]. Phytoplankton has the ability to convert ALA to EPA and DHA and by feeding on phytoplankton these PUFAs are enriched over the aquatic food chain. Therefore omega-3 PUFAs are found in high quantities in e.g. krill, fish and marine mammals.
Krill are shrimp-like crustaceans mostly found in the Arctic and Antarctic oceans [10]. They are abundant and underutilised in the food industry and are characterised by large energy stores. Most omega-3 supplements sold in today's market contain EPA and DHA in the form of triglycerides (fish oil) or as ethyl-esters. However, in nature, omega-3 fatty acids are also present as fatty acyl-chains in phospholipids. In krill oil produced from Antarctic krill (Euphausia superba) a high proportion of the omega-3 fatty acids are present in this molecular form [11]. More specifically, the phospholipids mainly consist of phosphatidylcholines, making krill an excellent natural source of omega-3 containing phosphatidylcholine [12]. Several animal studies have demonstrated that phospholipids themselves, and in particular phosphatidylcholine [13], [14], [15], have beneficial effects on the hepatic lipid metabolism and might have a potential to affect cardiovascular disease (reviewed in [16]). It was also shown that the tissue uptake of omega-3 PUFAs is improved, when they are provided in the form of phospholipids instead of triglycerides [11].
In addition to the high presence of phospholipids, krill also contains the red antioxidant molecule astaxanthin [17]. The presence of astaxanthin in krill oil contributes to the stability of the omega-3 PUFAs in krill oil [18], [19], [20], [21], and gives it the characteristic dark red colour.
To the best of our knowledge, there is no publication describing the safety of krill oil in genotoxicity studies. Therefore, a 13-week study in Wistar rats assessing subchronic toxicity and an Ames test to investigate potential mutagenic activity of krill oil was performed.
2. Materials and methods
2.1. Test materials
Superba™ krill oil was supplied by Aker BioMarine Antarctic AS, Oslo, Norway. The raw materials were assayed (Nofima Ingredients, Bergen, Norway) for elemental composition, chemical characteristics, and fatty acid composition and met previously set specifications (Table 1).
Table 1.
Fatty acid composition of krill oil mixed into the diets fed to Wistar rats for 13 weeks.
| Fatty acid | Common name | Krill oila |
|---|---|---|
| 14:0 | Myristic acid | 6.4 |
| 16:0 | Palmitic acid | 13.6 |
| 18:0 | Stearic acid | 0.6 |
| 20:0 | Eicosanoic acid | nd |
| 22:0 | Docosanoic acid | nd |
| 16:1 n-7 | Palmitoleic acid | 2.5 |
| 18:1 (n-9) + (n-7) + (n-5) | Elaidic acid | 10.6 |
| 20:1 (n-9) + (n-7) | Eicosenoic acid | 0.5 |
| 22:1 (n-11) + (n-9) + (n-7) | 11-docosenoic acid | 0.2 |
| 24:1 n-9 | Nervonic acid | nd |
| 16:2 n-4 | Hexadecadienoic acid | 0.3 |
| 16:3 n-4 | Hexadecatrienoic acid | 0.4 |
| 18:2 n-6 | Linoleic acid | 1.3 |
| 18:3 n-6 | γ-Linoleic acid | 0.1 |
| 20:2 n-6 | Eicosadienoic acid | 0.1 |
| 20:3 n-6 | Dihomo-γ-linolenic acid | nd |
| 20:4 n-6 | Arachidonic acid | 0.3 |
| 22:4 n-6 | Adrenic acid | nd |
| 18:3 n-3 | α-Linolenic acid | 1.2 |
| 18:4 n-3 | Stearidonic acid | 2.9 |
| 20:3 n-3 | Eicosatrienoic acid | 0.1 |
| 20:4 n-3 | Eicosatetraenoic acid | 0.4 |
| 20:5 n-3 | Eicosapentaenoic acid (EPA) | 12.8 |
| 21:5 n-3 | Heneicosapentenoic acid | 0.3 |
| 22:5 n-3 | Docosapentaenoic acid | 0.4 |
| 22:6 n-3 | Docosahexaenoic acid n-3 (DHA) | 7.4 |
g/100 g oil. nd = not detected. Lower detection limit for fatty acid measurements was 0.1 g/100 g.
2.2. Subchronic toxicity study
The design and conduct of the subchronic toxicity study was performed based on the regulatory guidelines OECD No.408, OPPTS 870.3100 and US FDA Redbook.
2.2.1. Animals
The study was performed at Charles River, Tranent, Edinburgh, UK. Forty male and forty female Han Wistar rats were received from Charles River UK Limited and were acclimatised for a period of 14 days. At study start, the animals were approximately 7 weeks old with male animals in the weight range of 179–229 g and females of 109–162 g. Animals were allocated to cages on racks separated by sex and treatment group. All animals were housed in the same room, but the control animals were placed on a separate rack. Two to three animals were housed per cage in suspended polycarbonate cages. For environmental enrichment, each cage of rats was provided wooden chew sticks (Tapvei Estonia OÜ, Harjumma, Estonia). Rats were provided food and water (domestic mains water) ad libitum during this period. During the study the animals were kept at 19–23 °C, 40–70% humidity and a fixed light cycle (light hours were 0700–1900 h). Studies were conducted in accordance with the OECD Principles of Good Laboratory Practice as incorporated into the United Kingdom Statutory Instrument for GLP, and as accepted by regulatory authorities throughout the European Community, United States (FDA and EPA) and Japan (MHLW, MAFF and METI).
2.2.2. Animal treatment
Four groups of 10 male and 10 female Han Wistar rats were fed diets supplemented with a total of 8% oil. Diets were incorporated with 8% soya bean oil (8% SO), 1.7 (1.7% KO, 6.3% SO), 3.3 (3.3% KO, 4.7% SO) and 5% krill oil (5% KO, 3% SO) for a period of 13 weeks. The high dose of krill oil corresponded to 2.5 g to 5 g/kg of body weight. After conversion to human equivalent doses (HED), the studied dose range provides a 24- to 48-fold safety margin with the recommended supplement level of 1 g/day. The diets are based on the standard RM1 diet and were prepared by Special Diet Services (Witham, UK) according to their in-house standard operating procedures. The exact description of the RM1 diet is given at: http://www.sdsdiets.com/pdfs/RM1P-E-FG.pdf. Since the RM1 diet contained 3% soya bean oil, and 5% krill oil was added on top in the high dose group, the fat content of the control group had to be adjusted by the addition of 5% soya bean oil. This assured similar calorie content in all 4 diets.
The prepared diets were stored frozen at −20 °C, thawed, and given to the animals on a daily discard and top-up routine. The diets were verified for stability and homogeneity by Nofima AS (Bergen, Norway). After inclusion of krill oil into the final diet form, the recovery of EPA and DHA was: EPA: 98.2, 97.5 and 96.1%; DHA: 97.1, 96.5 and 95.5%, in the 1.7, 3.3 and 5% krill oil diets, respectively. The homogeneity was verified by measuring EPA and DHA in 3 samples of each of the 3 krill oil diets. The standard deviation of EPA and DHA measured in the 3 krill oil diets was always less than 3.6%. All measurements were performed at the beginning of the study.
2.2.3. Animal observations
Well-being of the animals was monitored daily and once each week, all animals received a detailed clinical examination. Moreover, the animals’ eyes were examined before, during and at the end of the experiment.
Additionally, specific clinical observations were recorded each day throughout the treatment period to examine for reaction to treatment. Body weights were recorded once during pre-trial, then weekly throughout the treatment. The quantity of food consumed by each cage of animals was measured and recorded daily throughout pre-trial and treatment period and are given as g/animal/day for all weeks. Water consumption was qualitatively evaluated by visual inspection on a weekly basis.
At the end of treatment period (week 12/13), detailed neurotoxicological observations were made of all animals, including parameters of a Functional Observation Battery (FOB). Most of the assessments were based on scaled observations of the animals’ behaviour/status and included home cage and open field evaluations. In addition, ease of removal from cage, body temperature, condition of the eyes, condition of the coat, presence of salivation and overall ease of handling were recorded. Open field observations in a standardised arena (2 min observation period) included latency, level of mobility, rearing, grooming, urination/defecation, arousal, posture, tremor/convulsions, vocalisation, piloerection, palpebral closure, gait abnormalities, stereotypy and/or unusual behaviours. Additional functional tests were performed at an approximately standardised time of day (reaction to sound and reaction to touching the rump with a blunt probe). Quantitative measurements including grip strength (using a method derived from Meyer et al. [22]), pain perception (using a method derived from D’Amour and Smith [30]), landing foot splay and motor activity.
2.2.4. Haematology, coagulation and clinical chemistry
Blood samples for haematology, coagulation and clinical chemistry were obtained from all surviving animals via the orbital sinus under isoflurane anaesthesia on the morning of necropsy. The animals were not deprived of food overnight prior to sampling. Approximately 0.5 mL of whole blood was transferred into tubes containing EDTA for measurement of haematology parameters using the ADVIA 120 automated haematology analyser (Bayer, Munich, Germany). Haemoglobin, red blood cell count, haematocrit, white blood cell count, mean cell volume, mean cell haemoglobin concentration, platelet count, reticulocytes, neutrophils, lymphocytes, monocytes, eosinophils, basophils and large unclassified cells were all quantified. In trisodium citrate-treated blood (with a blood to citrate ratio of 9:1), prothrombin time and activated partial thromboplastin time were measured with an ACL Advance coagulation analyser (Diamond Diagnostics, MA, USA). A blood film smear was made from all EDTA haematology samples and stained for possible examination.
Blood collected for clinical chemistry was taken into lithium heparin tubes, centrifuged and analysed with a Roche P module clinical chemistry analyser using a Roche test kit (Roche, Basel, Switzerland) for urea, glucose, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, total protein, albumin, cholesterol, total bilirubin, calcium and phosphate. Sodium and potassium was analysed using Roche P module clinical chemistry analyser using indirect Ion Selective Electrode. Globulin was calculated by subtraction of the albumin concentration from the total protein concentration; albumin:globulin ratio was calculated by (albumin)/(total protein − albumin).
2.2.5. Urinalysis
Urine samples were collected over a 4-h period from all animals during week 13. The animals were housed individually in metabolic cages and were deprived of food and water. For fresh urine, the following parameters were evaluated: volume (weighing of urine sample), specific gravity (manual assessment using a refractometer), colour, pH, protein, glucose, ketones, urobilinogen, bilirubin, pigments (Aution JET 9UB test strips measured using an Aution Jet AJ4270 analyser, Menarini Diagnostics, Florence, Italy) and microscopy of the spun deposits (epithelial cells, crystals, white blood cells, red blood cells, organisms, casts, other abnormalities).
2.2.6. Necropsy and histopathology
After 13 weeks of treatment all animals were sacrificed in a random order by exsanguination after anaesthesia (carbon dioxide). The animals were non-fasted to minimise stress due to food deprivation. Terminal body weight of each animal was recorded, followed by severance of major blood vessels. All animals in the 4 different groups were subjected to a detailed necropsy examination. In addition, more than 40 different tissues from the control group and animals given 5% krill oil were subject to a more comprehensive histopathological examination.
A complete external and internal examination, which included body orifices, respiratory tract and cranial, thoracic and abdominal cavities, was performed. Representative tissues were taken from all animals and fixed in 10% neutral buffered formalin or Davidson's fluid (only eyes, optic nerve and testis): abnormal tissue, adrenal glands, aortic arch, blood smear, brain, eyes, epididymis, gastro-intestinal tract, harderian gland, heart, implant, kidney and ureter, liver, lung, mesenteric lymph node, nasal cavity, oesophagus, optic nerve, ovaries, pancreas, pituitary, prostate, rib, salivary glands, sciatic nerve, seminal vesicles, spinal cord, skin and mammary gland, spleen, sternum, submandibular lymph node, testis, thigh muscle, thyroid with parathyroid, tongue, trachea, urinary bladder, thymus, uterus and vagina. Sections were cut 4–6 μm thick, and stained with haematoxylin and eosin (H&E) (unless otherwise stated) and evaluated by a pathologist.
The following organs were weighed: adrenal glands, brain, epididymides, heart, kidneys, liver, lung, ovaries, pituitary gland, prostate, spleen, testes, thymus and thyroid.
2.2.7. Data analysis
Unless otherwise stated, all statistical tests were two-sided and performed at the 5% significance level using in-house software and performed as described below. Pairwise comparisons were performed between the different doses of krill oil and the control group for males and females separately. Quantitative data, body weight, food consumption, haematology, coagulation, clinical chemistry and selected urinalysis, motor activity and quantitative FOB measurements were analysed for homogeneity of variance using the ‘F-Max’ test. If the group variances appeared homogeneous, a parametric ANOVA was used and pairwise comparisons made using Fisher's F protected LSD method via Student's t-test i.e. pairwise comparisons were made only if the overall F-test was significant. If the variances were heterogeneous, log or square root transformations were used in an attempt to stabilise the variances. If the variances remained heterogeneous, then a Kruskal–Wallis non-parametric ANOVA was used and pairwise comparisons made using chi squared protection (via z tests, the non-parametric equivalent of Student's t-test). Organ weights were analysed using ANOVA as above and by analysis of covariance (ANCOVA) using terminal kill body weight as covariate. In addition, organ weights as a percentage of terminal body weight were analysed using ANOVA as above. Histological incidence data were analysed using Fisher's Exact Probability Test.
2.3. Genotoxicity studies
Studies were conducted in accordance with the OECD Principles of Good Laboratory Practice as incorporated into the United Kingdom Statutory Instrument for GLP.
2.3.1. Dose-finding toxicity test for Ames assay
First, a toxicity test was performed as an initial dose-finding test to establish suitable exposure levels for the Ames test. The Salmonella typhimurium strain TA100 was used to test toxicity of different doses of krill oil (up to 5000 μg per plate) in the presence and absence of metabolic activation (S9 mix). The S9-based metabolic activation system is described in more detail by [23]. The S9 enzymes used in the experiment (supernatant post-mitochondrial fraction obtained after centrifugation at 9000 g) were prepared in-house from the livers of Aroclor 1254-treated adult, male Fischer rats, as described by Ames et al. [24]. The method used for the toxicity test was similar to the ‘Direct plate incorporation method’ used for the Ames test described below.
2.3.2. Ames assay
Krill oil was assayed for mutagenic activity using the Ames test [24], a well-established assay for reverse mutations in amino acid-requiring strains of S. typhimurium (his−) and Escherichia coli (trp−). Four strains of S. typhimurium, TA1535, TA100, TA1537 and TA98 (obtained in 1976 from Professor B.N. Ames, Department of Biochemistry, University of California, USA) and one strain of E. coli, WP2uvrA (obtained in 1976 from the National Collection of Industrial Bacteria, Aberdeen, Scotland) were used.
Two mutation experiments were performed using the five bacteria strains. The first experiment was performed by the ‘Direct plate incorporation method’ (2 mL top agar, 0.5 mL S9 mix or 0.05 M phosphate buffer (pH 7.4), 0.1 mL bacterial culture and 0.05 mL test item or ethanol are thoroughly mixed and immediately poured onto minimal medium plates) while the second was performed by the ‘Pre-incubation method’ (S9 or 0.05 M phosphate buffer, bacteria and test item or control material (as before) are placed in a shaking incubator 37 °C for 20 min, then 2 mL of soft agar added and mixed and poured onto minimal medium plates). Both tests were performed in triplicate plates in the presence and absence of S9 mix (S9 mixed 1:9 with the co-factors required for mixed-function oxidase activity: NADP, G-6-P, MgCl2 and KCl). Just before use, the test substance was dissolved and diluted in ethanol. The same concentration of ethanol was also added to the control groups. Six concentrations of krill oil were tested: 17, 50, 167, 500, 1667 and 5000 μg per plate, and positive control chemicals were included in each experiment (2-aminoanthracene, sodium azide, 9-aminoacridine, 2-nitrofluorene and N-ethyl-N-nitro-N-nitrosoguanadine). The highest concentration represents the predetermined maximum, as recommended by relevant guidelines (OECD guideline 471, the European Commission Annex V Text Method B13 and B14, ICH Guidelines CPMP/ICH/141/95 and CPMP/ICH/174/95 and USA EPA 712-C-98-247).
Following incubation at 37 °C for 2–3 days, the number of his+ and trp+ revertants were counted using an electronic image capture system (Perceptive Instruments’ Sorcerer Colony Counter). Plates were also examined microscopically for precipitates and for micro-colony growth.
3. Results
3.1. Subchronic toxicity study
Mortality. There was one male animal belonging to the control group sacrificed prematurely during the study. This animal was euthanised on Day 81 of the study having previously displayed clinical observations including a subcutaneous mass on left ventral abdomen, abnormal respiration, weight loss and abnormally pale faeces. Histologically, a mammary adenoma was observed, which accounted for the subcutaneous mass observed at necropsy.
Clinical observations. There were no notable clinical signs that could be attributable to treatment. All animals however, were noted to have abnormal coloured faeces (pale and/or yellow) evident throughout treatment. This was considered to be a result of the diet and not to be of toxicological significance.
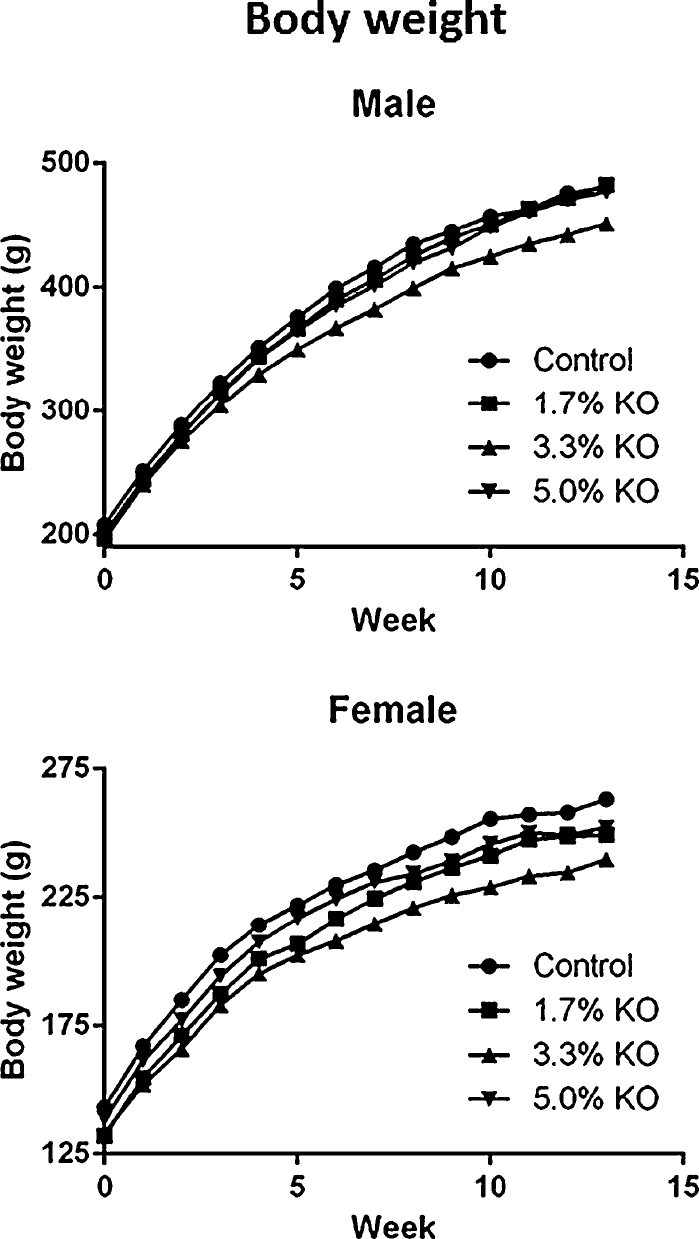
Body weight. Group mean body weights at all dose levels and in both sexes throughout treatment, were statistically similar in comparison to control (Fig. 1).
Fig. 1.
Group mean body weights of male and female Han Wistar rats fed diets containing krill oil (KO) for 13 weeks. No significant changes were observed between control and krill oil groups (p < 0.05).
Food consumption. The food consumption in control and krill oil groups was measured weekly and is given as g/animal/day (Table 2). Throughout treatment, only one isolated incidence of statistical significance was noted against the control group in males receiving the 3.3% krill oil diet during week 7.
Table 2.
Feeding (% krill oil in feed).
| Week |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −1 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
| Males | ||||||||||||||
| Control | 18.4 ± 0.4 | 20.4 ± 0.7 | 21.2 ± 0.4 | 21.3 ± 1.1 | 21.5 ± 1.0 | 22.6 ± 0.8 | 22.7 ± 0.5 | 22.3 ± 0.9 | 22.4 ± 0.8 | 22.2 ± 0.4 | 21.7 ± 1.0 | 20.8 ± 2.0 | 20.8 ± 2.2 | 19.8 ± 1.1 |
| 1.7%KO | 18.1 ± 0.6 | 21.6 ± 0.5 | 21.6 ± 0.7 | 22.6 ± 0.9 | 22.7 ± 1.0 | 23.1 ± 1.1 | 22.9 ± 1.3 | 22.3 ± 0.6 | 22.1 ± 1.0 | 22.9 ± 0.6 | 21.4 ± 0.3 | 21.4 ± 0.5 | 21.7 ± 0.9 | 21.4 ± 1.1 |
| 3.3%KO | 18.2 ± 0.5 | 20.0 ± 1.0 | 20.6 ± 1.3 | 20.1 ± 1.7 | 20.8 ± 0.9 | 20.4 ± 0.9 | 20.3 ± 0.8 | 19.8b ± 1.0 | 20.6 ± 1.4 | 21.0 ± 1.8 | 19.7 ± 1.5 | 19.7 ± 1.5 | 18.8 ± 1.2 | 19.3 ± 1.0 |
| 5%KO | 18.1 ± 1.2 | 20.7 ± 1.3 | 20.9 ± 1.2 | 21.5 ± 1.4 | 22.6 ± 1.6 | 22.3 ± 1.8 | 22.1 ± 1.7 | 21.5 ± 1.5 | 21.8 ± 1.2 | 21.2 ± 1.4 | 21.0 ± 1.6 | 22.1 ± 2.5 | 20.9 ± 1.7 | 20.9 ± 2.5 |
| Females | ||||||||||||||
| Control | 13.5 ± 0.6 | 14.9 ± 0.4 | 15.3 ± 0.8 | 15.5 ± 0.9 | 16.4 ± 1.3 | 15.9 ± 1.4 | 15.2 ± 1.8 | 15.1 ± 1.7 | 15.0 ± 1.5 | 15.5 ± 2.0 | 15.4 ± 2.1 | 14.1 ± 1.4 | 13.7 ± 0.8 | 13.6 ± 0.7 |
| 1.7%KO | 12.3 ± 0.7 | 14.7 ± 0.7 | 15.3 ± 1.0 | 16.3 ± 1.9 | 15.7 ± 1.0 | 15.3 ± 1.4 | 16.5 ± 2.6 | 16.7 ± 3.3 | 14.5 ± 0.3 | 15.9 ± 1.4 | 15.8 ± 2.6 | 14.7 ± 0.4 | 14.8 ± 1.9 | 13.5 ± 1.1 |
| 3.3%KO | 12.9 ± 1.0 | 13.9 ± 1.4 | 14.3 ± 1.2 | 14.9 ± 1.0 | 15.2 ± 1.0 | 14.5 ± 0.7 | 14.2 ± 0.7 | 14.4 ± 0.4 | 14.1 ± 0.7 | 14.6 ± 0.7 | 13.9 ± 0.8 | 14.7 ± 1.3 | 12.9 ± 0.5 | 13.6 ± 0.6 |
| 5%KO | 13.0 ± 0.7 | 14.8 ± 1.0 | 15.3 ± 1.1 | 15.7 ± 1.3 | 16.4 ± 1.0 | 16.0 ± 1.2 | 15.7 ± 1.7 | 15.1 ± 1.4 | 14.3 ± 1.3 | 14.9 ± 1.1 | 15.3 ± 1.7 | 15.0 ± 1.3 | 13.9 ± 0.7 | 13.2 ± 0.7 |
KO: krill oil. The values given are calculated by dividing the weekly food consumption of animals per cage by the number of animals in each cage (animals housed 2 or 3 per cage). All values are means of 4 cages ± SD.
Significantly different from control:
= p < 0.01
Achieved dosage. The overall mean achieved dosages were 0, 1044, 1929, and 2999 mg krill oil/kg body weight/day in males and 0, 1256, 2346, and 3532 mg krill oil/kg body weight/day for females corresponding to dietary inclusion levels of 0, 1.7, 3.3 and 5% (Table 3).
Table 3.
Krill oil intake by dietary administration (mg/kg body weight/day).
| 1.7% KO | 3.3% KO | 5% KO | |
|---|---|---|---|
| Males | 1044 ± 272 | 1929 ± 491 | 2999 ± 752 |
| Females | 1256 ± 253 | 2346 ± 424 | 3532 ± 710 |
KO: krill oil. The data represent average krill oil intake throughout the 13-week study period and was calculated by using weekly data of body weights and food consumption.
Water consumption. Visual inspection of water bottles revealed no intergroup differences throughout the treatment period.
Ophthalmoscopy. There were no findings that were considered to be due to treatment with krill oil.
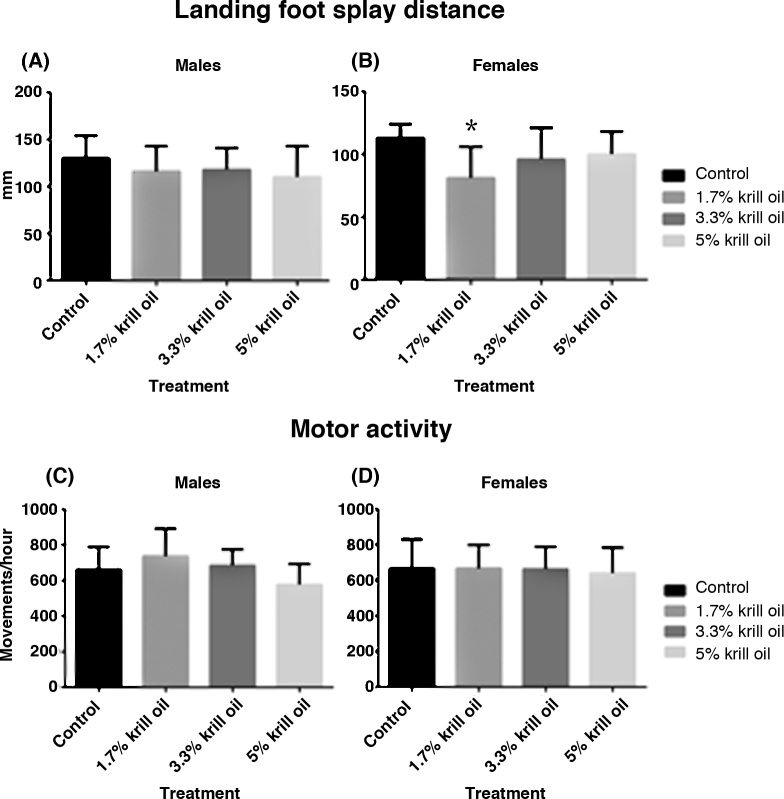
Functional observation battery parameters. There were no differences in the functional observation battery parameters that were considered to be due to the consumption of diets containing krill oil. A reduced distance between landing foot splay was noted to be significantly lower in females fed diet containing 1.7% krill oil compared to control (Fig. 2B). Due to the very small magnitude of the difference and the absence of any differences in the higher dose level or in the male groups (Fig. 2A) this was considered not to be related to treatment. There were no differences in overall motor activity that were attributed to the consumption of diets containing krill oil (Fig. 2C and D). However, when individual 5-min intervals were compared to the control group, then activity was noted to be significantly lower in males fed 5% krill oil at the 36–40 min interval of the 60-min period (data not shown). However, due to the absence of any significant changes in the other 11 intervals for the males and no significant changes in any intervals for females, in addition to the lack of any in-life clinical observations suggesting subdued behaviour and/or lethargy etc., the toxicological significance of these findings remain unclear.
Fig. 2.
Functional observation parameters include the distance between landing foot splay in male (A) and female (B) animals, as well as motor activity measurements in male (C) and (D) rats. All data are mean ± SD (n = 10). (*) Significantly different from control group (p < 0.05).
Haematology and coagulation. There were no differences in coagulation parameters that were considered to be due to the consumption of diets containing krill oil (Table 4). However, while male mean cell haemoglobin values were not changed in the krill oil groups compared to the control group, this parameter was significantly increased in the female 1.7% krill oil group. Furthermore, in females given 1.7% and 5% krill oil, there was also a significant increase in mean cell volume, whereas male values remained unchanged in all groups. No other haematology parameters were affected.
Table 4.
Haematology and coagulation.
| Control | 1.7% KO | 3.3% KO | 5% KO | |
|---|---|---|---|---|
| Males | ||||
| No. of animals | 6 | 10 | 7 | 10 |
| Haemoglobin (g/dL) | 15.7 ± 0.8 | 15.5 ± 0.6 | 15.2 ± 0.5 | 15.3 ± 0.7 |
| Red blood cells (×1012/L) | 8.55 ± 0.70 | 8.40 ± 0.37 | 8.20 ± 0.28 | 8.57 ± 0.46 |
| Haematocrit (L/L) | 0.43 ± 0.02 | 0.43 ± 0.02 | 0.42 ± 0.01 | 0.43 ± 0.02 |
| Mean cell haemoglobin (pg) | 18.5 ± 1.5 | 18.4 ± 0.7 | 18.6 ± 0.7 | 17.8 ± 0.8 |
| Mean cell volume (fL) | 50.6 ± 3.2 | 50.9 ± 1.9 | 51.1 ± 1.4 | 50.0 ± 1.5 |
| Mean cell haemoblobin concentration (g/dL) | 36.5 ± 0.9 | 36.2 ± 1.0 | 36.4 ± 0.5 | 35.7 ± 0.9 |
| Reticulocytes (%) | 1.8 ± 0.3 | 2.1 ± 0.3 | 2.0 ± 0.2 | 1.9 ± 0.2 |
| White blood cells (×109/L) | 6.41 ± 1.27 | 6.75 ± 1.56 | 5.87 ± 1.21 | 6.01 ± 1.38 |
| Neutrophils (×109/L) | 1.12 ± 0.37 | 1.17 ± 0.41 | 0.84 ± 0.17 | 0.85 ± 0.31 |
| Lymphocytes (×109/L) | 5.00 ± 1.04 | 5.24 ± 1.43 | 4.77 ± 1.04 | 4.88 ± 1.11 |
| Monocytes (×109/L) | 0.10 ± 0.02 | 0.13 ± 0.06 | 0.10 ± 0.03 | 0.11 ± 0.04 |
| Eosinophils (×109/L) | 0.14 ± 0.05 | 0.14 ± 0.03 | 0.11 ± 0.04 | 0.12 ± 0.03 |
| Basosophils (×109/L) | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.00 |
| Large unclassified cells (×109/L) | 0.04 ± 0.01 | 0.05 ± 0.03 | 0.05 ± 0.02 | 0.03 ± 0.02 |
| Platelets (×109/L) | 747 ± 119 | 672 ± 57 | 699 ± 66 | 674 ± 110 |
| Prothrombin time (s) | 15 ± 1 (8) | 15 ± 1 | 15 ± 1 (10) | 15 ± 1 (9) |
| Activated partial thromboplastin time (s) | 20 ± 2 (8) | 22 ± 2 | 22 ± 3 (9) | 22 ± 2 (8) |
| Females | ||||
| No. of animals | 9 | 9 | 8 | 8 |
| Haemoglobin (g/dL) | 15.0 ± 0.6 | 14.9 ± 0.4 | 14.6 ± 0.6 | 15.0 ± 0.4 |
| Red blood cells (×1012/L) | 8.06 ± 0.45 | 7.65 ± 0.31 | 7.87 ± 0.46 | 7.84 ± 0.26 |
| Haematocritt (L/L) | 0.42 ± 0.02 | 0.41 ± 0.02 | 0.40 ± 0.02 | 0.42 ± 0.01 |
| Mean cell haemoglobin (pg) | 18.6 ± 0.8 | 19.4 ± 0.7a | 18.5 ± 0.6 | 19.2 ± 0.9 |
| Mean cell volume (fL) | 51.5 ± 1.8 | 53.1 ± 1.8a | 51.3 ± 1.1 | 53.3 ± 1a |
| Mean cell haemoblobin concentration (g/dL) | 36.1 ± 0.9 | 36.6 ± 0.7 | 36.1 ± 0.8 | 36 ± 1 |
| Reticulocytes (%) | 2.6 ± 0.5 | 2.2 ± 0.3 | 2.6 ± 0.3 | 2.6 ± 0.7 |
| White blood cells (×109/L) | 4.54 ± 0.56 | 4.68 ± 0.95 | 4.93 ± 1.21 | 4.75 ± 1.25 |
| Neutrophils (×109/L) | 0.62 ± 0.2 | 0.66 ± 0.11 | 0.71 ± 0.25 | 0.75 ± 0.46 |
| Lymphocytes (×109/L) | 3.71 ± 0.65 | 3.77 ± 0.83 | 3.99 ± 1.05 | 3.78 ± 0.86 |
| Monocytes (×109/L) | 0.08 ± 0.02 | 0.10 ± 0.05 | 0.10 ± 0.05 | 0.10 ± 0.04 |
| Eosinophils (×109/L) | 0.09 ± 0.03 | 0.10 ± 0.02 | 0.07 ± 0.02 | 0.10 ± 0.05 |
| Basophils (×109/L) | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| Large unclassified cells (×109/L) | 0.03 ± 0.02 | 0.03 ± 0.02 | 0.04 ± 0.02 | 0.02 ± 0.01 |
| Platelets (×109/L) | 692 ± 108 | 749 ± 100 | 679 ± 100 | 663 ± 166 |
| Prothrombin time (s) | 15 ± 1 | 14 ± 1 (10) | 15 ± 1 | 15 ± 1 |
| Activated partial thromboplastin time (s) | 21 ± 2 (8) | 21 ± 2 (10) | 23 ± 3 | 21 ± 3 (7) |
KO: krill oil. All values are means ± SD for 6–10 animals, with the exceptions described above in parenthesis.
Significantly different from control:
p < 0.05.
Clinical chemistry. Significantly increased plasma triglyceride levels were observed in female rats receiving 1.7% krill oil (Table 5). Increases in blood total protein in animals treated with krill oil, achieving statistical significance against control, were noted in both sexes. Globulin levels were also statistically significantly increased in both males and females fed diets containing 5% krill oil. This change was reflected in the albumin–globulin ratio which was reduced, achieving statistical significance in males only. Other minor differences achieving statistical significance in clinical chemistry parameters were noted in both males treated at 3.3% krill oil (decreased urea volume and cholesterol) and 5% (increased total protein and globulin and decreased globulin/albumin). In females, significant changes were seen in all krill oil groups: 1.7% (increased triglycerides, total protein and albumin), 3.3% (increased lactate dehydrogenase) and 5% (increased lactate dehydrogenase, total protein and globulin). As the differences had no supporting histological evidence, and due to the small magnitude of the difference and lack of any dose response, their toxicological significance was considered to be minimal.
Table 5.
Clinical chemistry at termination.
| Control | 1.7% KO | 3.3% KO | 5% KO | |
|---|---|---|---|---|
| Males | ||||
| No. of animals | 8 | 10 | 10 | 10 |
| Alkaline phosphatase (iu/L) | 97 ± 17 | 109 ± 40 | 92 ± 17 | 114 ± 16 |
| Alanine aminotransferase (iu/L) | 23 ± 5 | 25 ± 4 | 25 ± 6 | 24 ± 4 |
| Aspartate aminotransferase (iu/L) | 60 ± 8 | 63 ± 13 | 59 ± 7 | 55 ± 8 |
| Lactate dehydrogenase (iu/L) | 102 ± 27 | 126 ± 152 | 97 ± 34 | 117 ± 37 |
| Urea (mmol/L) | 5.7 ± 0.3 | 5.8 ± 0.5 | 5.2 ± 0.3a | 6.1 ± 0.5 |
| Glucose (mmol/L) | 11.94 ± 1.57 | 10.81 ± 1.25 | 11.21 ± 1.08 | 11.34 ± 2.10 |
| Total bilirubin (μmol/L) | 1.7 ± 0.0 | 1.7 ± 0.0 | 1.7 ± 0.0 | 1.7 ± 0.0 |
| Cholesterol (mmol/L) | 1.9 ± 0.2 | 1.7 ± 0.3 | 1.5 ± 0.1b | 2.1 ± 1.8 |
| Triglycerides (mmol/L) | 2.53 ± 0.60 | 2.31 ± 1.00 | 1.9 ± 0.32 | 2.51 ± 1.87 |
| Total protein (g/L) | 66 ± 2 | 67 ± 3 | 68 ± 2 | 69 ± 3a |
| Albumin (g/L) | 42 ± 1 | 43 ± 2 | 43 ± 2 | 41 ± 4 |
| Globulin (g/L) | 24 ± 2 | 25 ± 2 | 25 ± 2 | 29 ± 3c |
| Albumin:globulin ratio | 1.7 ± 0.1 | 1.7 ± 0.1 | 1.7 ± 0.2 | 1.5 ± 0.2b |
| Sodium (mmol/L) | 142 ± 3 | 142 ± 2 | 141 ± 1 | 143 ± 2 |
| Potassium (mmol/L) | 4.2 ± 0.2 | 4.1 ± 0.3 | 4.1 ± 0.2 | 4.2 ± 0.2 |
| Phosphate (mmol/L) | 1.24 ± 0.27 | 1.32 ± 0.19 | 1.33 ± 0.09 | 1.35 ± 0.15 |
| Calcium (mmol/L) | 2.68 ± 0.04 | 2.68 ± 0.07 | 2.66 ± 0.03 | 2.69 ± 0.05 |
| Females | ||||
| No. of animals | 10 | 10 | 10 | 10 |
| Alkaline phosphatase (iu/L) | 111 ± 76 | 58 ± 23 | 63 ± 32 | 56 ± 20 |
| Alanine aminotransferase (iu/L) | 19 ± 4 | 19 ± 2 | 25 ± 13 | 23 ± 4 |
| Aspartate aminotransferase (iu/L) | 60 ± 8 | 55 ± 6 | 57 ± 7 | 59 ± 9 |
| Lactate dehydrogenase (iu/L) | 73 ± 10 | 80 ± 22 | 105 ± 33a | 162 ± 122c |
| Urea (mmol/L) | 7 ± 0.9 | 6.4 ± 1.0 | 6.3 ± 0.7 | 6.5 ± 0.6 |
| Glucose (mmol/L) | 10.75 ± 1.48 | 10.06 ± 0.84 | 9.93 ± 1.32 | 9.91 ± 1.23 |
| Total bilirubin (μmol/L) | 1.9 ± 0.5 | 1.7 ± 0.0 | 1.7 ± 0.0 | 1.7 ± 0.0 |
| Cholesterol (mmol/L) | 1.2 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.3 | 1.3 ± 0.4 |
| Triglycerides (mmol/L) | 1.39 ± 0.78 | 2.58 ± 1.65a | 1.76 ± 0.87 | 1.71 ± 1.56 |
| Total protein (g/L) | 69 ± 3 | 73 ± 4b | 71 ± 3 | 73 ± 3b |
| Albumin (g/L) | 49 ± 3 | 53 ± 3b | 52 ± 3 | 51 ± 3 |
| Globulin (g/L) | 19 ± 2 | 20 ± 2 | 20 ± 1 | 22 ± 2c |
| Albumin:globulin ratio | 2.6 ± 0.3 | 2.7 ± 0.3 | 2.6 ± 0.2 | 2.3 ± 0.3 |
| Sodium (mmol/L) | 141 ± 1 | 142 ± 2 | 141 ± 1 | 142 ± 2 |
| Potassium (mmol/L) | 3.6 ± 0.2 | 3.8 ± 0.2 | 3.6 ± 0.2 | 3.8 ± 0.3 |
| Phosphate (mmol/L) | 1.12 ± 0.24 | 1.01 ± 0.15 | 1.11 ± 0.12 | 1.22 ± 0.12 |
| Calcium (mmol/L) | 2.68 ± 0.04 | 2.73 ± 0.08 | 2.70 ± 0.08 | 2.68 ± 0.07 |
KO: krill oil. All values are means ± SD for 8–10 animals.
Significantly different from control:
p < 0.05,
p < 0.01,
p < 0.001
Urinalysis. There were no differences in urinalysis parameters that were considered to be due to the consumption of diets containing krill oil (Table 6). An isolated incidence of statistical significance was noted in urinary pH in males treated with diets containing 5% krill oil in comparison to control. As this was an isolated incidence and did not show any dose response, the difference was not considered to be of toxicological significance. Due to the small volume of several female samples, an accurate comparison could not be made against the control groups, although no numerical differences were noted.
Table 6.
Urinalysis at week 13.
| Control | 1.7% KO | 3.3% KO | 5% KO | |
|---|---|---|---|---|
| Males | ||||
| Urine specific gravity | 1.055 ± 0.012 | 1.047 ± 0.015 | 1.057 ± 0.012 | 1.053 ± 0.013 |
| Urine volume (mL) | 0.7 ± 0.5 | 0.6 ± 0.5 | 0.3 ± 0.2 | 0.7 ± 0.6 |
| pH | 8.0 ± 1.1 | 7.8 ± 1.0 | 7.4 ± 0.9 | 6.8 ± 0.3b |
| Females | ||||
| Urine specific gravity | 1.058 ± (−) | 1.043 ± 0.020 | 1.072 ± (−) | 1.044 ± (−) |
| Urine volume (mL) | 0.1 ± 0.0 | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.2 ± 0.1 |
| pH | 6.6 ± 0.5 | 7.1 ± 0.5 | 6.5 ± 0.0 | 6.7 ± 0.4 |
KO: krill oil. All values are means ± SD. The number of samples in males was: urine specific gravity: n = 6–9; urine volume: n = 9–10; pH: n = 9–10. (−): Due to the small number of several female samples (urine specific gravity: n = 1–3; urine volume: n = 5–10; pH: n = 2–5), an accurate comparison could not be made against the control groups, although no numerical differences were noted.
Significantly different from control:
p < 0.01.
Organ weight. Organ weights are presented as percentage of terminal body weight (Table 7) and as absolute weights (Table 8). The only significant finding was a decreased absolute heart weight in male (3.3% krill oil) and female (all krill oil groups), compared to control animals. However, after adjustment for body weight, no significant changes in organs weights were observed.
Table 7.
Relative organ weights (% of body weight).
| Control | 1.7% KO | 3.3% KO | 5% KO | |
|---|---|---|---|---|
| Males | ||||
| No. of animals | 9 | 10 | 10 | 10 |
| Adrenals | 0.013 ± 0.002 | 0.014 ± 0.001 | 0.014 ± 0.001 (8) | 0.012 ± 0.002 |
| Brain | 0.43 ± 0.04 | 0.42 ± 0.03 | 0.46 ± 0.04 | 0.42 ± 0.03 |
| Epididymides | 0.29 ± 0.05 | 0.28 ± 0.04 | 0.29 ± 0.06 | 0.27 ± 0.04 (9) |
| Heart | 0.29 ± 0.02 | 0.26 ± 0.02 | 0.26 ± 0.02 | 0.27 ± 0.02 |
| Kidneys | 0.61 ± 0.02 | 0.61 ± 0.09 | 0.62 ± 0.04 | 0.62 ± 0.11 |
| Liver | 3.3 ± 0.2 | 3.4 ± 0.4 | 3.5 ± 0.2 | 3.7 ± 0.3 |
| Lung | 0.38 ± 0.04 | 0.39 ± 0.04 | 0.4 ± 0.07 | 0.42 ± 0.08 |
| Pituitary | 0.0018 ± 0.0003 | 0.0018 ± 0.0003 (7) | 0.0022 ± 0.0006 | 0.0020 ± 0.0002 |
| Prostate | 0.10 ± 0.02 | 0.11 ± 0.03 | 0.11 ± 0.02 | 0.10 ± 0.02 |
| Spleen | 0.15 ± 0.02 | 0.15 ± 0.01 | 0.15 ± 0.02 | 0.15 ± 0.01 |
| Testes | 0.76 ± 0.09 | 0.72 ± 0.10 | 0.75 ± 0.15 | 0.70 ± 0.06 (9) |
| Thymus | 0.11 ± 0.02 | 0.11 ± 0.01 | 0.10 ± 0.02 | 0.10 ± 0.02 |
| Thyroid | 0.0057 ± 0.0012 | 0.0058 ± 0.0008 | 0.0528 ± 0.0013 (9) | 0.0067 ± 0.0017 |
| Females | ||||
| No. of animals | 10 | 10 | 10 | 10 |
| Adrenals | 0.029 ± 0.007 | 0.029 ± 0.005 | 0.029 ± 0.005 | 0.029 ± 0.005 (9) |
| Brain | 0.70 ± 0.09 | 0.72 ± 0.06 | 0.75 ± 0.04 | 0.75 ± 0.06 |
| Heart | 0.34 ± 0.04 | 0.32 ± 0.01 | 0.35 ± 0.03 | 0.32 ± 0.03 |
| Kidneys | 0.66 ± 0.08 | 0.65 ± 0.05 | 0.67 ± 0.07 | 0.67 ± 0.03 |
| Liver | 3.2 ± 0.3 | 3.4 ± 0.3 | 3.5 ± 0.4 | 3.6 ± 0.4 |
| Lung | 0.54 ± 0.09 | 0.52 ± 0.07 | 0.54 ± 0.08 | 0.53 ± 0.07 |
| Ovaries | 0.042 ± 0.008 | 0.040 ± 0.005 | 0.041 ± 0.001 | 0.044 ± 0.012 |
| Pituitary | 0.0045 ± 0.0007 | 0.0045 ± 0.0007 | 0.0050 ± 0.0010 | 0.0050 ± 0.0008 (9) |
| Spleen | 0.21 ± 0.02 | 0.23 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.03 |
| Thymus | 0.17 ± 0.04 | 0.15 ± 0.03 | 0.15 ± 0.03 | 0.14 ± 0.03 |
| Thyroid | 0.0075 ± 0.0016 | 0.0093 ± 0.0015 | 0.0078 ± 0.0016 | 0.0080 ± 0.0012 (9) |
| Uterus | 0.25 ± 0.16 | 0.26 ± 0.09 | 0.26 ± 0.11 | 0.25 ± 0.07 |
KO: krill oil. No significant changes were observed between control and krill oil groups (p < 0.05). All values are means ± SD for 9–10 animals, with the exceptions described above in parenthesis. Paired organs were weighed together.
Table 8.
Absolute organ weights (g).
| Control | 1.7% KO | 3.3% KO | 5% KO | |
|---|---|---|---|---|
| Males | ||||
| No. of animals | 9 | 10 | 10 | 10 |
| Final body weight | 481 ± 51 | 479 ± 38 | 450 ± 51 | 475 ± 46 |
| Adrenals | 0.062 ± 0.007 | 0.065 ± 0.005 | 0.060 ± 0.004 (8) | 0.059 ± 0.011 |
| Brain | 2.05 ± 0.05 | 2.02 ± 0.06 | 2.03 ± 0.08 | 2.00 ± 0.11 |
| Epididymides | 1.36 ± 0.17 | 1.33 ± 0.19 | 1.31 ± 0.17 | 1.30 ± 0.15 (9) |
| Heart | 1.36 ± 0.13 | 1.26 ± 0.12 | 1.18 ± 0.14b | 1.26 ± 0.11 |
| Kidneys | 2.95 ± 0.34 | 2.89 ± 0.36 | 2.78 ± 0.32 | 2.90 ± 0.33 |
| Liver | 15.96 ± 1.95 | 16.12 ± 2.38 | 15.85 ± 2.23 | 17.52 ± 1.96 |
| Lung | 1.82 ± 0.17 | 1.87 ± 0.30 | 1.81 ± 0.24 | 2.01 ± 0.38 |
| Pituitary | 0.009 ± 0.001 | 0.009 ± 0.001 (7) | 0.010 ± 0.003 | 0.009 ± 0.001 |
| Prostate | 0.49 ± 0.1 | 0.55 ± 0.14 | 0.50 ± 0.11 | 0.49 ± 0.08 |
| Spleen | 0.69 ± 0.05 | 0.73 ± 0.06 | 0.69 ± 0.09 | 0.71 ± 0.06 |
| Testes | 3.62 ± 0.27 | 3.44 ± 0.43 | 3.34 ± 0.52 | 3.36 ± 0.18 (9) |
| Thymus | 0.54 ± 0.09 | 0.54 ± 0.09 | 0.47 ± 0.08 | 0.47 ± 0.09 |
| Thyroid | 0.027 ± 0.005 | 0.028 ± 0.004 | 0.024 ± 0.006 (9) | 0.032 ± 0.009 |
| Females | ||||
| No. of animals | 10 | 10 | 10 | 10 |
| Final body weight | 263 ± 38 | 249 ± 17 | 240 ± 9 | 251 ± 25 |
| Adrenals | 0.076 ± 0.016 | 0.072 ± 0.012 | 0.070 ± 0.012 | 0.074 ± 0.009 (9) |
| Brain | 1.82 ± 0.08 | 1.77 ± 0.04 | 1.80 ± 0.06 | 1.86 ± 0.08 |
| Heart | 0.90 ± 0.12 | 0.79 ± 0.05b | 0.83 ± 0.06a | 0.80 ± 0.06b |
| Kidneys | 1.71 ± 0.2 | 1.61 ± 0.13 | 1.60 ± 0.16 | 1.68 ± 0.15 |
| Liver | 8.43 ± 1.01 | 8.44 ± 0.85 | 8.46 ± 1.14 | 9.05 ± 1.04 |
| Lung | 1.40 ± 0.24 | 1.30 ± 0.18 | 1.29 ± 0.17 | 1.34 ± 0.24 |
| Ovaries | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.03 |
| Pituitary | 0.012 ± 0.002 | 0.011 ± 0.002 | 0.012 ± 0.003 | 0.012 ± 0.002 (9) |
| Spleen | 0.55 ± 0.08 | 0.57 ± 0.07 | 0.57 ± 0.05 | 0.59 ± 0.08 |
| Thymus | 0.44 ± 0.12 | 0.38 ± 0.09 | 0.36 ± 0.09 | 0.35 ± 0.05 |
| Thyroid | 0.020 ± 0.005 | 0.023 ± 0.005 | 0.019 ± 0.004 | 0.020 ± 0.003 (9) |
| Uterus | 0.65 ± 0.44 | 0.63 ± 0.20 | 0.63 ± 0.25 | 0.63 ± 0.21 |
KO: krill oil. All values are means ± SD for 9–10 animals, with the exceptions detailed above in parenthesis. Paired organs were weighed together.
Significantly different from control:
p < 0.05,
p < 0.01.
Macroscopic findings. Prominent lobulation of the liver was observed in 6/10, 7/10 and 7/10 males receiving 1.7, 3.3 and 5% krill oil respectively, and in 1/10 females receiving 1.7% krill oil. Hepatic lobulation in a normal liver is only seen or defined on microscopic evaluation. However, if there is a microscopic finding which follows a pattern (portal, periportal, centrilobular, etc.) the lobular appearance of the liver becomes prominent and can be seen macroscopically.
Microscopic findings. Periportal microvesicular hepatocyte vacuolation (minimal to mild) was observed in 5/10 males which received 5% krill oil (Table 9). This correlated with the findings of prominent liver lobulation observed at necropsy. In males which received 5% krill oil, the incidence of this finding was statistically significant when compared with controls (p < 0.05). The hepatocyte vacuolation was not associated with hepatocellular necrosis or inflammation, and there were no clinical pathology findings suggesting liver impairment, therefore, this finding was considered adaptive and non-adverse. There was no hepatocyte vacuolation observed in animals receiving 1.7% or 3.3% krill oil.
Table 9.
Histological findings.
| Males |
Females |
|||
|---|---|---|---|---|
| Control | 5% KO | Control | 5% KO | |
| Liver | (10) | (10) | (10) | (10) |
| Microvesicular, periportal hepatocyte vacuolation | ||||
| Minimal | 0 | 4 | 0 | 0 |
| Mild | 0 | 1 | 0 | 0 |
| Total incidence | 0 | 5* | 0 | 0 |
KO: krill oil. There was no hepatocyte vacuolation observed in animals receiving 1.7% or 3.3% krill oil.
Significantly different from control (p < 0.05).
3.2. Genotoxicity study
Dose-finding toxicity test for Ames assay. With krill oil doses ranging from 17 to 5000 μg per plate, no increased bacterial numbers (S. typhimurium strain TA100 ± S9 mix) of revertant colonies over those occurring spontaneously could be observed (Table 10). Thus, no toxicity of krill oil to bacteria became evident. Some precipitation was observed at the two highest krill oil concentrations tested.
Table 10.
Dose-finding toxicity test.
| Krill oil dose per plate (μg) | Mean revertants per plate |
|
|---|---|---|
| Without metabolic activation (−S9 mix) | With metabolic activation (+S9 mix) | |
| 0 | 65 | 68 |
| 17 | 51 | 77 |
| 50 | 82 | 75 |
| 167 | 50 | 90 |
| 500 | 66 | 54 |
| 1667 | 55p | 90p |
| 5000 | 60p | 75p |
p = Precipitate.
Strain used: S. typhimurium TA100.
Ames assay. The Ames assay was performed on 5 different bacterial strains with either vehicle or 6 different krill oil doses (up to 5000 μg per plate). The assay revealed that the average numbers of his+ and trp+ revertant colonies were not increased using either the ‘Direct plate incorporation method’ (Table 11) or the ‘Pre-incubation method’ (Table 12). Therefore, there was no evidence of mutagenic activity of krill oil to any of the strains tested.
Table 11.
First mutation assay (direct plate incorporation method).
| Treatment (μg/plate) | Revertants per plate |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA1535 |
TA1537 |
TA98 |
TA100 |
WP2uvrA |
||||||
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| 0 | 17.0 ± 6.9 | 20.3 ± 6.8 | 9.0 ± 3.6 | 13.3 ± 2.3 | 35.0 ± 3.0 | 51.0 ± 6.1 | 92.0 ± 6.9 | 110.3 ± 21.5 | 6.3 ± 2.5 | 6.0 ± 5.6 |
| 17 | 19.0 ± 10.6 | 18.3 ± 7.4 | 12.3 ± 2.5 | 16.3 ± 5.1 | 34.0 ± 1.7 | 54.7 ± 8.7 | 102.0 ± 5.7 | 108.3 ± 2.9 | 6.7 ± 5.0 | 5.7 ± 1.2 |
| 50 | 17.3 ± 4.5 | 21.0 ± 4.2 | 11.7 ± 5.0 | 14.0 ± 3.6 | 26.3 ± 9.7 | 46.7 ± 3.1 | 104.0 ± 17.0 | 110.0 ± 8.7 | 8.0 ± 2.6 | 8.3 ± 1.2 |
| 167 | 11.0 ± 5.6 | 24.3 ± 3.8 | 14.7 ± 3.2 | 16.3 ± 3.5 | 37.3 ± 10.3 | 50.7 ± 6.5 | 97.7 ± 3.5 | 118.3 ± 13.3 | 9.3 ± 5.5 | 6.7 ± 2.1 |
| 500 | 18.0 ± 7.9 | 20.3 ± 3.1 | 11.3 ± 4.0 | 14.3 ± 3.2 | 31.7 ± 3.5 | 48.5 ± 12.0 | 97.3 ± 9.6 | 103.3 ± 9.6 | 9.0 ± 2.6 | 2.7 ± 2.1 |
| 1667 | 13.3 ± 3.5p | 20.3 ± 3.8p | 13.7 ± 8.3p | 14.7 ± 2.3p | 35.0 ± 8.7p | 50.7 ± 4.5p | 102.7 ± 12.2p | 118.7 ± 10.8p | 5.0 ± 4.6p | 5.3 ± 1.2p |
| 5000 | 12.7 ± 3.8p | 21.0 ± 7.2p | 12.3 ± 0.6p | 13.3 ± 3.2p | 35.0 ± 13.5p | 59.7 ± 7.8p | 77.0 ± 32.9p | 115.7 ± 1.5p | 4.7 ± 2.5p | 12.7 ± 0.6p |
| Pos. control | 474.3 ± 4.0 | 477.0 ± 38.6 | 8695.7 ± 582.8 | 416.3 ± 40.5 | 838.0 ± 34.6 | 544.3 ± 27.4 | 1129.7 ± 38.9 | 841.0 ± 10.1 | 96.3 ± 18.6 | 448.7 ± 41.8 |
p Precipitate.
All colony numbers of revertants are means of 3 plates ± SD.
Positive controls for each strain were as follows: Strains with S9 activation: 2 μg/plate 2-aminoanthracene (TA1535 and TA1537), 0.5 μg/plate (TA98 and TA100) and 20 μg/plate (WP2uvrA). Strains without S9 activation: 1 μg/plate sodium azide (TA1535 and TA100), 2 μg/plate N-ethyl-N-nitro-N-nitrosoguanidine (WP2uvrA), 1 μg/plate 2-nitrofluorene (TA98) and 80 μg/plate 9-aminoacridine (TA1537).
Table 12.
Second mutation assay (pre-incubation method) TDENDOFDOCTD.
| Treatment | Revertants per plate (μg/plate) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA1535 |
TA1537 |
TA98 |
TA100 |
WP2uvrA |
||||||
| −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | −S9 | +S9 | |
| 0 | 11.0 ± 2.0 | 17.0 ± 3.6 | 11.0 ± 2.0 | 9.3 ± 2.5 | 27.7 ± 4.7 | 39.3 ± 9.8 | 87.3 ± 17.2 | 98.7 ± 0.6 | 2.3 ± 1.5 | 8.0 ± 5.3 |
| 17 | 14.3 ± 4.6 | 14.0 ± 2.6 | 8.7 ± 2.9 | 16.0 ± 1.0 | 30.3 ± 2.1 | 41.0 ± 6.1 | 92.0 ± 6.2 | 99.0 ± 7.0 | 5.0 ± 1.0 | 5.7 ± 0.6 |
| 50 | 17.0 ± 1.0 | 15.7 ± 2.3 | 9.7 ± 2.5 | 12.7 ± 2.5 | 34.7 ± 3.8 | 42.0 ± 14.7 | 91.7 ± 7.2 | 98.7 ± 11.1 | 2.7 ± 2.1 | 7.0 ± 2.0 |
| 167 | 9.0 ± 3.0 | 17.3 ± 3.2 | 11.7 ± 3.8 | 12.7 ± 3.1 | 31.0 ± 1.7 | 46.0 ± 8.5 | 95.3 ± 22.4 | 98.7 ± 12.1 | 5.7 ± 1.2 | 7.0 ± 3.0 |
| 500 | 7.7 ± 2.3 | 17.0 ± 5.3 | 11.7 ± 1.5 | 16.3 ± 5.5 | 34.3 ± 7.2 | 43.7 ± 11.6 | 89.7 ± 5.5 | 94.0 ± 8.7 | 1.3 ± 1.2 | 6.0 ± 3.6 |
| 1667 | 12.3 ± 3.2 | 22.3 ± 7.5 | 9.0 ± 0.0 | 14.7 ± 3.2 | 33.0 ± 7.2 | 51.7 ± 2.1 | 93.0 ± 6.1 | 108.3 ± 18.5 | 4.0 ± 3.0 | 11.3 ± 3.2 |
| 5000 | 10.3 ± 3.1p | 13.0 ± 1.7p | 13.7 ± 5.8p | 15.7 ± 2.3p | 31.0 ± 1.7p | 51.0 ± 6.1p | 89.3 ± 4.6p | 85.3 ± 11.0p | 7.0 ± 1.7p | 9.0 ± 2.0p |
| Pos. control | 528.0 ± 5.6 | 299.3 ± 28.6 | 5014.3 ± 432.3 | 204.7 ± 17.0 | 622.0 ± 55.0 | 302.0 ± 22.6 | 1260.3 ± 25.7 | 872.3 ± 64.7 | 157.7 ± 22.0 | 515.3 ± 39.7 |
p Precipitate.
All colony numbers of revertants are means of 3 plates ± SD.
Positive controls for each strain were as follows: Strains with S9 activation: 2 μg/plate 2-aminoanthracene (TA1535 and TA1537), 0.5 μg/plate (TA98 and TA100) and 20 μg/plate (WP2uvrA). Strains without S9 activation: 1 μg/plate sodium azide (TA1535 and TA100), 2 μg/plate N-ethyl-N-nitro-N-nitrosoguanidine (WP2uvrA), 1 μg/plate 2-nitrofluorene (TA98) and 80 μg/plate 9-aminoacridine (TA1537).
Result Evaluation: For S. typhimurium strains TA1535, TA1537, and TA98 and for E. coli WP2uvrA, at least a doubling of the mean concurrent vehicle control value was required before mutagenic activity was suspected (in addition, an absolute mean plate count of at least 20 colonies per plate was required). For S. typhimurium strain TA100, a 1.5-fold increase over the control value was considered indicative of a mutagenic effect.
4. Discussion
The results of the 13-week toxicity study in rats demonstrate a lack of toxicologically significant adverse effects following oral administration of krill oil at doses up to 5% inclusion levels in the diet. Daily administration of krill oil at inclusion levels up to 5% for at least 13 weeks resulted in in-life clinical observations which included abnormally coloured faeces (pale and/or yellow). This was considered a result of the manufactured diet which itself ranged in colour from pale white through yellow to red (due to the astaxanthin content in krill oil). There were no deaths noted over the duration of treatment with krill oil. And no differences were noted in body weight or food consumption. Additionally there were no differences noted in the functional observation parameters in either sex which could be attributed to treatment with krill oil.
Several differences in both haematology and clinical chemistry values were noted in the krill oil-treated groups when compared to control; however the findings were considered to be of no toxicological significance since the changes were small, were not dose-dependent, were not observed in both sexes, and were not related to histopathological changes.
Periportal microvesicular hepatocyte vacuolation was present only in animals fed the highest krill oil dose of 5%. The microvesicular vacuolation might have been caused by accumulation of triglycerides (hepatic lipidosis) due to the high dose of lipids given [25]. This is commonly found to be a compensatory, transient response that has been observed previously [26]. Additionally, hepatic lipidosis can occur as the result of excessive delivery of free fatty acids either from the gut or from adipose tissue [27]. However, there was no microvesicular vacuolation with lower doses of krill oil supplementation, and in none of the groups were other indications of hepatic dysfunction observed. Moreover, krill oil at a level of 2.5% in the diet has been shown to be effective in decreasing liver fat in two different obesity models, where the animals had abnormally high hepatic lipid levels [28], [29].
There was no significant change in plasma triglycerides levels in animals fed diets containing krill oil, except for the females receiving the lowest dose of krill oil (1.7%). This observation could be explained by variability due to the non-fasted state of the animals. Given that the effects of the 13-week toxicity study were not adverse in nature, the no-observed-adverse-effect-level (NOAEL) for the conditions of this study was considered to be 5% krill oil (equating to 2999 mg krill oil/kg body weight/day for males and 3532 mg krill oil/kg body weight/day for females). The safety of krill oil was further supported in a mutagenicity test, which showed that this substance was not mutagenic when tested in ethanol up to and beyond the limit of its solubility (predetermined maximum of 5000 μg) in the Ames Test system with S. typhimurium TA1535, TA1537, TA98 and TA100 and E. coli strain WP2uvrA. The results in the current paper are based on a subacute toxicity study in rats. Because krill oil is recommended for lifetime consumption, future experiments should also address the toxicological potential of krill oil in a chronic long-term study. Moreover, a teratogenicity study as well as a micronucleus test have been performed in a separate study without showing any negative effects of krill oil, thereby further confirming the safety of krill oil consumption (data to be published).
5. Conclusions
In summary, the dietary administration of krill oil at inclusion levels up to 5% to rats for at least 13 weeks demonstrated no adverse toxicological in-life, haematology and/or blood chemistry effects. Findings were restricted to periportal microvesicular hepatocyte vacuolation in male animal fed diets containing 5% krill oil with no correlated increase in organ weight.
Conflict of interest
Kjetil Berge and Lena Burri are employees of Aker BioMarine Antarctic AS.
Transparency document
Acknowledgements
This work was funded by Aker BioMarine Antarctic AS, Oslo, Norway and by Norwegian Research Council grant nr. 199360. Many thanks go to Laura Stibich for carefully editing the manuscript.
Footnotes
Available online 28 September 2014
Contributor Information
Bruce Robertson, Email: bruce.robertson@crl.com.
Lena Burri, Email: lena.burri@akerbiomarine.com.
Kjetil Berge, Email: kjetil.berge@akerbiomarine.com.
References
- 1.Eslick G.D., Howe P.R., Smith C., Priest R., Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta-analysis. Int. J. Cardiol. 2009;136:4–16. doi: 10.1016/j.ijcard.2008.03.092. [DOI] [PubMed] [Google Scholar]
- 2.Miller P.E., Van Elswyk M., Alexander D.D. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am. J. Hypertens. 2014;27:885–896. doi: 10.1093/ajh/hpu024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Caterina R., Madonna R., Zucchi R., La Rovere M.T. Antiarrhythmic effects of omega-3 fatty acids: from epidemiology to bedside. Am. Heart J. 2003;146:420–430. doi: 10.1016/S0002-8703(03)00327-2. [DOI] [PubMed] [Google Scholar]
- 4.Knapp H.R. Dietary fatty acids in human thrombosis and hemostasis. Am. J. Clin. Nutr. 1997;65:1687S–1698S. doi: 10.1093/ajcn/65.5.1687S. [DOI] [PubMed] [Google Scholar]
- 5.Harris W.S., Dayspring T.D., Moran T.J. Omega-3 fatty acids and cardiovascular disease: new developments and applications. Postgrad. Med. 2013;125:100–113. doi: 10.3810/pgm.2013.11.2717. [DOI] [PubMed] [Google Scholar]
- 6.Calder P.C. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 7.Morris M.C., Evans D.A., Bienias J.L., Tangney C.C., Bennett D.A., Wilson R.S., Aggarwal N., Schneider J. Consumption of fish and n-3 fatty acids and risk of incident Alzheimer disease. Arch. Neurol. 2003;60:940–946. doi: 10.1001/archneur.60.7.940. [DOI] [PubMed] [Google Scholar]
- 8.Morris M.C., Evans D.A., Tangney C.C., Bienias J.L., Wilson R.S. Fish consumption and cognitive decline with age in a large community study. Arch. Neurol. 2005;62:1849–1853. doi: 10.1001/archneur.62.12.noc50161. [DOI] [PubMed] [Google Scholar]
- 9.Arterburn L.M., Hall E.B., Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am. J. Clin. Nutr. 2006;83:1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 10.Hamner W.M., Hamner P.P., Strand S.W., Gilmer R.W. Behavior of Antarctic krill, Euphausia superba: chemoreception, feeding, schooling, and molting. Science. 1983;220:433–435. doi: 10.1126/science.220.4595.433. [DOI] [PubMed] [Google Scholar]
- 11.Burri L., Hoem N., Banni S., Berge K. Review. Marine omega-3 phospholipids: metabolism and biological activities. Int. J. Mol. Sci. 2012;13:15401–15419. doi: 10.3390/ijms131115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winther B., Hoem N., Berge K., Reubsaet L. Elucidation of phosphatidylcholine composition in krill oil extracted from Euphausia superba. Lipids. 2011;46:25–36. doi: 10.1007/s11745-010-3472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buang Y., Wang Y.M., Cha J.Y., Nagao K., Yanagita T. Dietary phosphatidylcholine alleviates fatty liver induced by orotic acid. Nutrition. 2005;21:867–873. doi: 10.1016/j.nut.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 14.Hung M.C., Shibasaki K., Yoshida R., Sato M., Imaizumi K. Learning behaviour and cerebral protein kinase C, antioxidant status, lipid composition in senescence-accelerated mouse: influence of a phosphatidylcholine-vitamin B12 diet. Br. J. Nutr. 2001;86:163–171. doi: 10.1079/bjn2001391. [DOI] [PubMed] [Google Scholar]
- 15.Schneider H., Braun A., Fullekrug J., Stremmel W., Ehehalt R. Lipid based therapy for ulcerative colitis-modulation of intestinal mucus membrane phospholipids as a tool to influence inflammation. Int. J. Mol. Sci. 2010;11:4149–4164. doi: 10.3390/ijms11104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn J.S., Wat E., Kamili A., Tandy S. Dietary phospholipids, hepatic lipid metabolism and cardiovascular disease. Curr. Opin. Lipidol. 2008;19:257–262. doi: 10.1097/MOL.0b013e3282ffaf96. [DOI] [PubMed] [Google Scholar]
- 17.Takaichi S., Matsui K., Nakamura M., Muramatsu M., Hanada S. Fatty acids of astaxanthin esters in krill determined by mild mass spectrometry. Comp. Biochem. Physiol. B: Biochem. Mol. Biol. 2003;136:317–322. doi: 10.1016/s1096-4959(03)00209-4. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi K., Kakizono T., Nishio N., Nagai S., Kurimura Y., Tsuji Y. Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl. Microbiol. Biotechnol. 1997;48:351–356. [Google Scholar]
- 19.Naguib Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000;48:1150–1154. doi: 10.1021/jf991106k. [DOI] [PubMed] [Google Scholar]
- 20.Nakagawa K., Kiko T., Miyazawa T., Carpentero Burdeos G., Kimura F., Satoh A., Miyazawa T. Antioxidant effect of astaxanthin on phospholipid peroxidation in human erythrocytes. Br. J. Nutr. 2011;105:1563–1571. doi: 10.1017/S0007114510005398. [DOI] [PubMed] [Google Scholar]
- 21.Terao J. Antioxidant activity of beta-carotene-related carotenoids in solution. Lipids. 1989;24:659–661. doi: 10.1007/BF02535085. [DOI] [PubMed] [Google Scholar]
- 22.Meyer O.A., Tilson H.A., Byrd W.C., Riley M.T. A method for the routine assessment of fore- and hindlimb grip strength of rats and mice. Neurobehav. Toxicol. 1979;1:233–236. [Google Scholar]
- 23.McGregor D.B., Edwards I., Riach C.G., Cattanach P., Martin R., Mitchell A., Caspary W.J. Studies of an S9-based metabolic activation system used in the mouse lymphoma L5178Y cell mutation assay. Mutagenesis. 1988;3:485–490. doi: 10.1093/mutage/3.6.485. [DOI] [PubMed] [Google Scholar]
- 24.Ames B.N., McCann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat. Res. 1975;31:347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 25.Gopinath C., Prentice D.E., Lewis D.J. MTP Press Limited; Lancaster: 1987. Atlas of Experimental Toxicological Pathology. [Google Scholar]
- 26.Ferramosca A., Conte A., Damiano F., Siculella L., Zara V. Differential effects of high-carbohydrate and high-fat diets on hepatic lipogenesis in rats. Eur. J. Nutr. 2014;53:1103–1114. doi: 10.1007/s00394-013-0613-8. [DOI] [PubMed] [Google Scholar]
- 27.Krugner-Higby L., Caldwell S., Coyle K., Bush E., Atkinson R., Joers V. The effects of diet composition on body fat and hepatic steatosis in an animal (Peromyscus californicus) model of the metabolic syndrome. Comp. Med. 2011;61:31–38. [PMC free article] [PubMed] [Google Scholar]
- 28.Batetta B., Griinari M., Carta G., Murru E., Ligresti A., Cordeddu L., Giordano E., Sanna F., Bisogno T., Uda S., Collu M., Bruheim I., Di Marzo V., Banni S. Endocannabinoids may mediate the ability of (n-3) fatty acids to reduce ectopic fat and inflammatory mediators in obese Zucker rats. J. Nutr. 2009;139:1495–1501. doi: 10.3945/jn.109.104844. [DOI] [PubMed] [Google Scholar]
- 29.Tandy S., Chung R.W., Wat E., Kamili A., Berge K., Griinari M., Cohn J.S. Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J. Agric. Food Chem. 2009;57:9339–9345. doi: 10.1021/jf9016042. [DOI] [PubMed] [Google Scholar]
- 30.D’Amour F.E., Smith D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941;72:74–79. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.