Abstract
One of the pathways involved in the pathogenesis of diabetic complications is the formation of excessive levels of advanced glycation end (AGE) products. Nɛ-carboxymethyllysine (CML) is one of the best-characterized AGEs. Because little is known about the effects of AGEs on pancreatic beta cells, we investigated the effect of CML on human pancreatic cells and determined the activity and gene expression of glutathione system components. CML at a concentration of 0.5 mM induced cell death in human pancreatic beta cells, which was accompanied by increased intracellular oxidative stress. No changes in the gene expression of the receptor for AGEs (RAGE) were found, although an increase in the level of a target cytokine of RAGE after CML exposure was observed. Additionally we found that CML lowered the levels of GSH and affected the activity and expression of other components of the glutathione system. These changes indicate that the cells are even more vulnerable for oxidative stress after exposure to CML. Since beta cells are low in antioxidant enzymes and repair for oxidized DNA, CML, but most likely also other AGEs, accelerates beta cell dysfunction and increases beta cell death during chronic hyperglycemia.
Keywords: Glutathione, Beta cells, N(epsilon)-carboxymethyllysine, Advanced glycation end product, Oxidative stress
1. Introduction
Hyperglycemia, which occurs during type 2 diabetes, is associated with oxidative stress [1]. Formation of advanced glycation end products (AGEs) is one of the mechanisms that results in the increased formation of oxygen radicals. AGEs constitute a heterogeneous group of macromolecules formed by the non-enzymatic glycation of proteins, lipids and nucleic acids. AGEs can be ingested with food and are also formed in small amounts endogenously in the body as a consequence of normal metabolism [2]. During prolonged hyperglycemia AGEs can contribute to diabetic complications by the formation of crosslinks in the basal membrane and accumulation of glycated proteins which alters cellular structure and protein functions. Furthermore, interaction with the receptor for AGE (RAGE) leads to the expression of pro-inflammatory genes like interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) [3], [4]. Nɛ-carboxymethyllysine (CML) is one of the best-characterized AGEs. Elevated levels of serum CML have been associated with arterial stiffness and pose a higher risk of cardiovascular and all-cause mortality [5], [6], [7].
Pancreatic beta cells appear to be particularly vulnerable for oxidative stress. Expression and activity of the key antioxidant enzymes superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPx) is low in beta cells compared to other cell types [8]. Moreover, beta cells were found incapable to adapt their antioxidant enzyme activity in response to oxidative stress [9]. In addition, it was shown that pancreatic islets possess low repair machinery for oxidized DNA [10]. Although a lot of research has focused on the amount of antioxidant enzymes in pancreatic islets and the effect of overexpression of GPx, little is known about the levels and role of other components of the glutathione system in the beta cell.
Glutathione, a tripeptide (γ-glutamylcysteinylglycine), is the major free thiol in most living cells and it is involved in many biological processes. Within cells, GSH is found in both the reduced sulfhydryl form (GSH) and the glutathione disulfide oxidized form (GSSG). Under normal conditions, more than 90% of the glutathione pool is present in the reduced form. The balance between GSH and GSSG is tightly regulated in the cell, as a decrease in GSH can put the cell at risk for oxidative damage. An increased GSSG to GSH ratio is therefore often considered as an indicator of oxidative stress [11].
Glutathione has a diversity of crucial physiological roles, but it principally serves as an endogenous antioxidant. It functions as a cofactor for GPx, the major defense mechanism against potential toxic hydrogen peroxide and other peroxides [12]. During the detoxification process of peroxides, GSSG is formed. GSH is regenerated from GSSG by the NADPH-dependent enzyme glutathione reductase (GR) [13]. Additionally, glutathione S-transferase (GST) uses GSH as a substrate to form conjugates with electrophiles, resulting in more water soluble metabolites which are more readily excreted. Glutaredoxin (Grx) utilizes the reducing power of glutathione to catalyze disulfide reductions in the presence of NADPH and GR. Grx is involved in regulation of various cellular functions, including electron transport and protein folding [14].
Because little is known about the effects of AGEs on pancreatic beta cells, we investigated the effect of CML on pancreatic cell viability and determined the activity and expression of components belonging to the glutathione system.
2. Materials and methods
2.1. Chemicals
All chemicals were purchased from Sigma–Aldrich (Steinheim, Germany) unless stated otherwise. CML was obtained from SyMO-Chem BV (Eindhoven, Netherlands). Roswell Park Memorial Institute (RPMI) 1640 medium, Hank's Balanced Salt Solution (HBSS), trypsin-EDTA (1x), non-heat inactivated fetal calf serum (FCS), and l-glutamine were obtained from Gibco (Breda, The Netherlands).
2.2. Cell culture
The human pancreatic beta cell line 1.1E7 [15] was obtained from HPA Culture Collections. Cells were cultured in RPMI 1640 medium with 10% non-heat inactivated FCS and 2 mM l-glutamine. Cells were maintained in T75 flasks at 37 °C in a 5% CO2 atmosphere.
2.3. MTT assay
Cells were seeded at a density of 5000 cells per well in a 96-well plate and after overnight attaching, medium was removed and cells were washed with HBSS. CML was added to the plate in different concentrations (0–1 mM). Subsequently, cells were incubated for 24 h. After treatment, supernatant was removed and cells were washed with PBS. Next, 100 μl of MTT solution (0.5 mg/ml in culture medium) was added and cells were incubated for 1 h at 37 °C. After incubation, the plate was washed with PBS and the formazan crystals were dissolved in 200 μl DMSO. Cells were incubated for 30 min after which the absorbance at 540 nm was measured spectrophotometrically using a microplate reader. Relative viability is expressed as a percentage relative to untreated cells.
2.4. Measurement of intracellular oxidative stress
The production of intracellular reactive oxygen species was measured using 2,7-dichlorofluorescein diacetate (DCFH-DA) as described previously [16], [17]. Cells were seeded at a density of 5000 cells per well in a 96 well plate and after overnight attaching, medium was removed and cells were washed with HBSS. Cells were then incubated with 0.5 mM CML in the presence of 10 μM DCFH-DA. After 24 h, cells were washed with PBS to remove any DCFH-DA that was not taken up by the cells. Fluorescence (excitation 485 nm; emission 535 nm) was measured with the use of a microplate reader.
2.5. Gene expression analysis
RNA was isolated from Qiazol suspended cells according to the manufacturer's protocol and quantified spectrophotometrically. Reverse transcription reaction was performed using 500 ng of RNA, which was reverse-transcribed into cDNA using iScript™ cDNA synthesis kit (Biorad, Veenendaal, The Netherlands). Next, real time PCR was performed with a BioRad MyiQ iCycler Single Color RT-PCR detection system using Sensimix™Plus SYBR and Fluorescein (Quantace-Bioline, Alphen a/d Rijn, The Netherlands), 5 μl diluted (10×) cDNA, and 0.3 μM primers in a total volume of 25 μl. PCR was conducted as follows: denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 45 s. After PCR, a melt curve (60–95 °C) was produced for product identification and purity. β-actin was included as internal control. Primer sequences are shown in Table 1. Data were analyzed using the MyIQ software system (BioRad) and were expressed as relative gene expression (fold change) using the 2ΔΔCt method.
Table 1.
Sequences of the primers used in gene expression analysis.
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| Beta-actin (β-actin) | CCTGGCACCCAGCACAAT | GCCGATCCACACGGAGTACT |
| Receptor for AGE (RAGE) | GGCCAGGGCTAGAGTTCC | GCTGTCAGCATCAGCATCAT |
| Gamma-glutamylcystein synthetase (γ-GCS) (catalytic subunit) | GCACATCTACCACGCCGTC | CCACCTCATCGCCCCAC |
| Glutathione reductase | CAAGCTGGGTGGCACTTG | TTGGAAAGCCATAATCAGCA |
| Glutathione S-transferase pi (GSTP1) | GCTCTATGGGAAGGACCAG | CTCAAAAGGCTTCAGTTGC |
| Glutaredoxin-2 | CCGTCGCTAAATTCTCCAAA | TGGCACTCGCTGGAATC |
2.6. Cytokine release
1.1E7 cells were incubated with 0.5 mM CML for 24 h. After incubation, culture medium was collected. Cytokines released in the supernatant of the cells were measured using the Bio-plex pro assay according to manufacturer's instructions. This assay uses antibodies coupled to magnetic beads which react with 50 μl supernatant. After a series of washes to remove unbound protein, acytokine-specific biotinylated detection antibody was added to the reaction. After 30 min incubation and several washes, a streptavidin-phycoerythrin (streptavidin-PE) reporter complex was added to bind biotinylated detection antibodies. The plate was then read using the Luminex system and data was analyzed using the Bio-Plex Manager software™.
2.7. GSH/GSSG levels
1.1E7 cells were incubated with 0.5 mM CML for 24 h. After incubation, cells were washed with PBS, harvested with trypsin-EDTA and centrifuged (1000 × g, 5 min, 4 °C). Next, cells were washed with ice-cold PBS and centrifuged again. Cell pellets were then resuspended in ice-cold extraction buffer (0.1% Triton X-100 and 1.3% SSA in a 0.1 M potassium phosphate buffer with 5 mM EDTA, pH 7.5) and sonicated in icy water for 10 min. The extracts were used for determination of intracellular GSH and GSSG content using an enzymatic recycle method described by Rahman et al. [18].
2.8. Glutathione reductase activity
1.1E7 cells were incubated with 0.5 mM CML for 24 h. After incubation, cells were washed with HBSS, harvested with trypsin-EDTA and centrifuged (1000 × g, 5 min, 4 °C). Cell pellets were then resuspended in 145 mM sodium phosphate buffer pH 7.4 containing 1 mM EDTA. Next, cells were sonicated in icy water for 10 min and centrifuged (15 min, 10,000 × g, 4 °C). Final reaction mixture (1 ml) contained 0.06 mM NADPH (in 1% Na2CO3) and 50 μl sample in buffer. The reaction was started by the addition of 0.225 mM GSSG (in 0.01 M NaOH). The consumption of NADPH was followed by the decrease in absorbance at 340 nm for 3 min at 37 °C. Activity was corrected for protein content of the samples and expressed in nmol/mg protein per minute.
2.9. Glutathione transferase activity
1.1E7 cells were incubated with 0.5 mM CML for 24 h. After incubation, cells were washed with HBSS, harvested with trypsin-EDTA and centrifuged (1000 × g, 5 min, 4 °C). Cell pellets were then resuspended in 100 mM potassium phosphate buffer pH 6.5 containing 6.3 mM EDTA. Next, cells were sonicated in icy water for 10 min and centrifuged (15 min, 10.000 × g, 4 °C). Final reaction mixture (1 ml) contained 1 mM GSH and 50 μl sample in buffer. The reaction was started by the addition of 1 mM CDNB (in ethanol). The production of GS-dinitrobenzene was followed by the increase in absorbance at 340 nm for 3 min at 37 °C. With each run a spontaneous reaction was included that contained buffer instead of a sample. Activity was corrected for spontaneous reaction and for protein content of the samples and expressed in μmol/mg protein per minute.
2.10. Glutaredoxin activity
1.1E7 cells were incubated with 0.5 mM CML for 24 h. After incubation, cells were washed with HBSS, harvested with trypsin-EDTA and centrifuged (1000 × g, 5 min, 4 °C). Cell pellets were then resuspended in 100 mM potassium phosphate buffer pH 7.0 containing 1 mM EDTA. Next, cells were sonicated in icy water for 10 min and centrifuged (15 min, 10,000 × g, 4 °C). Final reaction mixture (1 ml) contained 0.5 mM GSH, 0.2 mM NADPH, 1 U glutathione reductase and 50 μl sample in buffer. The reaction was started by the addition of 0.35 mM 2-hydroxyethyl disulfide (HED). The decrease in absorbance at 340 nm, which accompanies the oxidation of NADPH, was monitored for 3 min at 37 °C. Activity was corrected for protein content of the samples and expressed in μmol/mg protein per minute.
2.11. Protein determination
Protein concentrations were determined spectrophotometrically using the DC protein assay kit (Biorad, Veenendaal, The Netherlands) according to manufacturer's protocol.
2.12. Statistical analysis
The effect of CML incubation was tested using Student's t-test for independent samples or the Mann–Whitney U test when not normally distributed. P-Values <0.05 were considered statistically significant and P-values <0.1 were considered statistical trends. We also include statistical trends because for bioactive molecules like GSH, even a small percentage change in the amount can be of biological relevance. Statistical analyses were analyzed with SPSS for Windows (version 20.0; SPSS Inc., Chicago, IL, USA).
3. Results
3.1. CML exposure causes a decrease in viability in beta cells
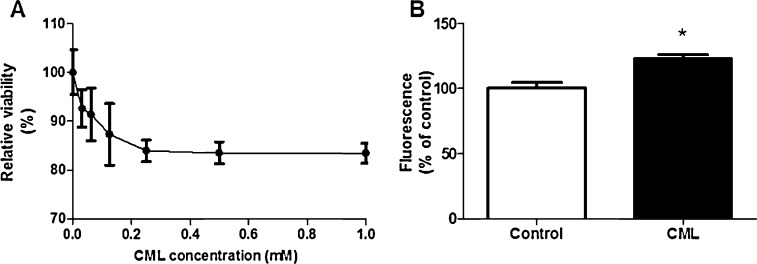
The effect of CML exposure on 1.1E7 cell viability was determined by MTT assay (Fig. 1A). At concentrations up to 0.125 mM a dose-dependent decrease in viability of 100–87% was observed, albeit with a high degree of variation between the different experiments. Above 0.125 mM the additional decrease in viability was only 4%. CML concentrations higher than 0.25 mM show a lower degree of variation between the experiments and concentrations higher than 0.5 mM did not show an additional decrease in viability, therefore a CML concentration of 0.5 mM was chosen as the exposure condition.
Fig. 1.
The effect of exposure to different concentrations Nɛ-carboxymethyllysine (CML) (0–1 mM) for 24 h on cell viability of human beta cells (A). Incubation with 0.5 mM CML for 24 h increases intracellular oxidative stress (B). Data are expressed as mean ± standard error of the mean (SEM) of three (MTT) or four (DCFH) independent experiments. *p < 0.05 compared to non-exposed cells.
3.2. CML exposure causes an increase in intracellular oxidative stress
To determine intracellular levels of reactive oxygen species we used the fluorogenic dye DCFH-DA. After diffusion into the cell, DCFH-DA is enzymatically hydrolyzed by esterases to the non-fluorescent compound DCFH. When ROS are present, DCFH can be oxidized to the highly fluorescent compound DCF. After 24 h exposure to CML we found a 23% increase in DCF fluorescence (Fig. 1B). This indicates that CML causes a significant increase in intracellular oxidative stress in the beta cell.
3.3. CML exposure increases the levels of MCP-1 in the culture medium
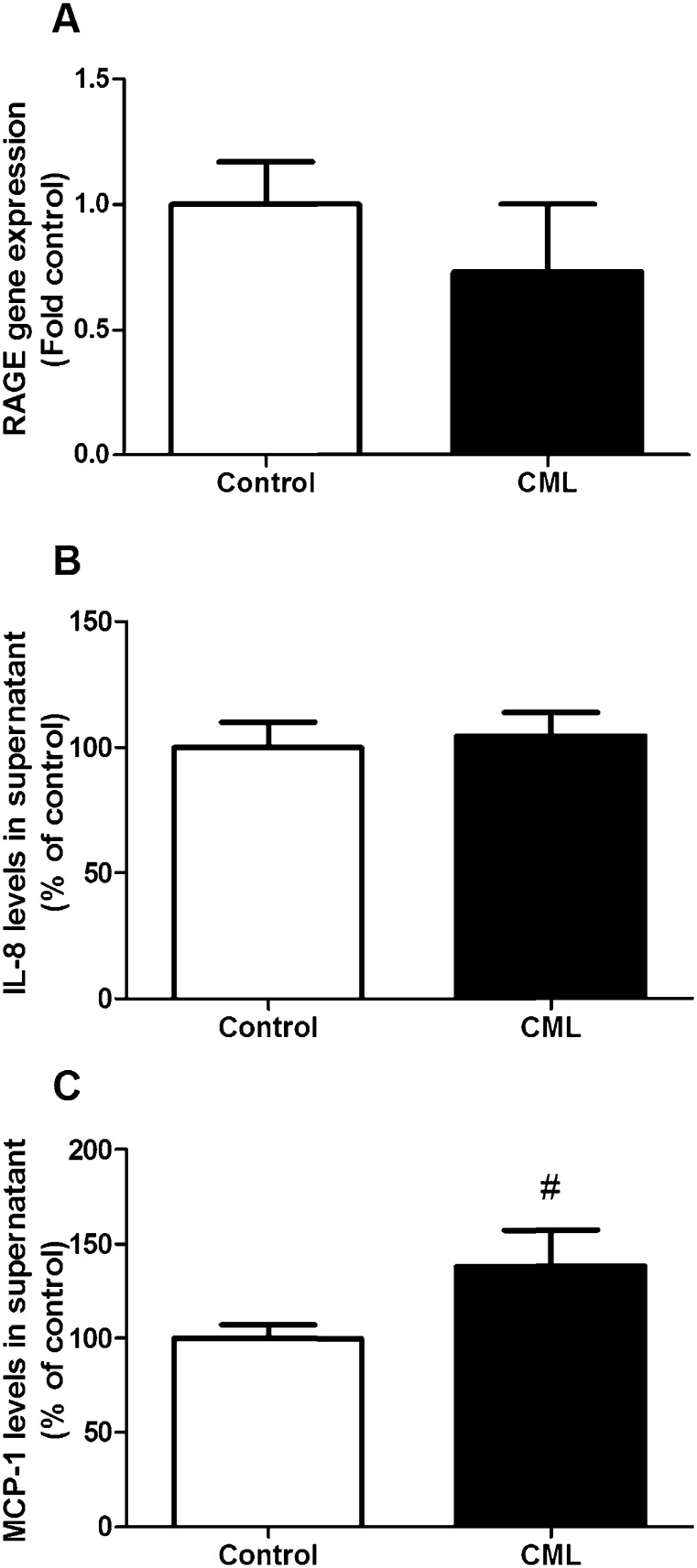
Because AGEs bind to RAGE, we measured the gene expression of this receptor in the beta cells. We did not observe an effect on gene expression after exposure to CML (Fig. 2A). Since RAGE activation is associated with an increase in pro-inflammatory genes, the levels of IL-8 and MCP-1, cytokines which are known to be upregulated by RAGE were investigated in the supernatant of cells exposed to CML [19], [20], [21]. No effects on the levels of IL-8 were observed (Fig. 2B). MCP-1 levels were increased by almost 40% (Fig. 2C). Other RAGE associated cytokines were also measured with the Luminex system, but these data are not included because the concentrations were below detection limit.
Fig. 2.
The effect of exposure to 0.5 mM CML for 24 h on gene expression of RAGE (A) and the levels of two cytokines which are elevated by RAGE signaling, IL-8 (B) and MCP-1 (C). Data are expressed as mean ± standard error of the mean (SEM) of three (RAGE expression) or four (IL-8 and MCP-1) independent experiments. #p < 0.1 compared to non-exposed cells.
3.4. Effect on components of the glutathione system
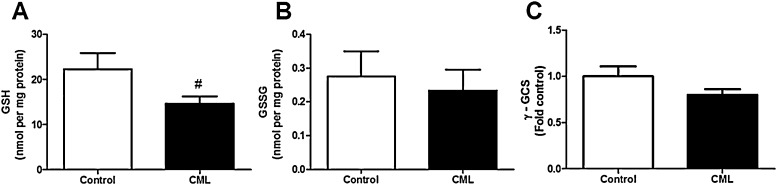
We determined the activity and gene expression of several components of the glutathione system. We observed a trend to a lower GSH concentration of the cells after CML exposure (Fig. 3A). The GSSG concentration did not change, but was very low and below the detection limit in some samples (Fig. 3B). The expression of the enzyme gamma-glutamylcystein synthetase (γ-GCS), involved in the biosynthesis of GSH, was not affected by exposure to CML (Fig. 3C).
Fig. 3.
The effect of exposure to 0.5 mM CML for 24 h on GSH content (A), GSSG content (B) and gene expression of gamma-glutamylcystein synthetase (γ-GCS) (C). Data are expressed as mean ± standard error of the mean (SEM) of three independent experiments. #p < 0.1 compared to non-exposed cells.
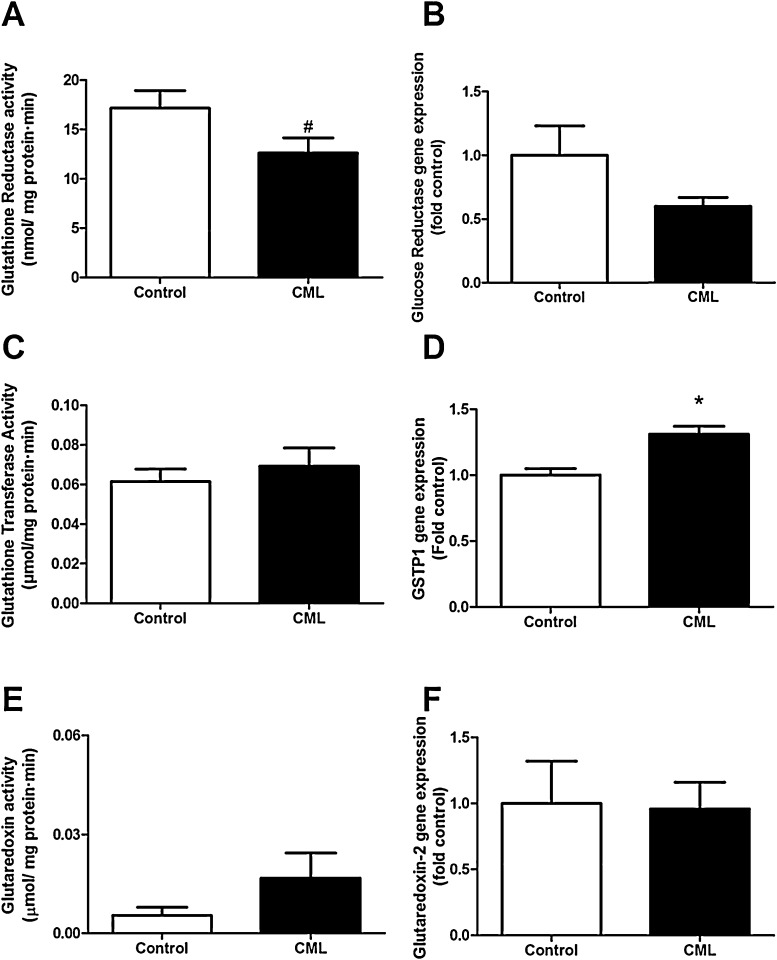
A trend toward decreased activity of GR after CML exposure was detected, which was not accompanied by a change in gene expression of this enzyme (Fig. 4A and B). We also measured GST activity, which did not show any change after CML exposure (Fig. 4C). Because GST is a large family of genes, the expression of one specific class was determined. Glutathione S-transferase pi (GSTP1) was chosen because its overexpression has been linked to the prevention of oxidative stress [22], [23]. We found an upregulation in the expression of GSTP1 when cells were exposed to CML for 24 h (Fig. 4D). We did not find any significant changes in glutaredoxin activity or gene expression (Fig. 4E and F).
Fig. 4.
The effect of exposure to 0.5 mM CML for 24 h on activity (A) and gene expression (B) of glutathione reductase; activity of glutathione transferase (C) expression of GSTP1 (D); activity of glutaredoxin (E) and expression of glutaredoxin-2 (F). Data are expressed as mean ± standard error of the mean (SEM) of three (gene expression, GR activity) or four (GST and Grx activity) independent experiments. *p < 0.05 compared to non-exposed cells; #p < 0.1 compared to non-exposed cells.
4. Discussion
AGE formation is one of the major pathways by which hyperglycemia can cause diabetic complications, therefore AGEs contribute to the pathogenesis of diabetes [24]. Beta cell dysfunction and death is involved in the progression of diabetes [25]. In this study we investigated the effect of exposure with the AGE CML on a human pancreatic beta cell line.
In this study we used a concentration of 0.5 mM CML to induce changes in glutathione components. This concentration is higher than usually found in the plasma of diabetic patients, typically reported in the nanomolar or low micromolar range [26], [27], [28]. This high concentration was chosen to determine the effects of CML in a relatively short incubation time of 24 h. Since we also use FCS in this model, it is possible that CML binds to FCS and that the actual amount of free CML reacting with the cells is much lower than 0.5 mM and might even be in the in vivo range.
Only a limited number of studies about the effect of AGE on beta cell viability and function have been published. A study in a mouse beta cell line found that exposure to AGEs increased superoxide production in the mitochondria, which led to an impairment of insulin secretion [29]. Increased oxidative stress via the mitochondria due to exposure to AGEs was also found in rat beta cells [30]. Exposure of different rodent beta cell lines to AGEs induced both proliferation and apoptosis in these cells [31]. In line with these studies, we also observed a decrease in beta cell viability after exposure to the AGE CML. This decreased viability was accompanied by an increase in oxidative stress which probably results from the interaction of CML with RAGE.
RAGE is a multiligand transmembrane receptor which belongs to the immunoglobulin gene superfamily [32]. Activation of RAGE by AGEs transduces multiple signals resulting in activation and translocation of nuclear transcription factors like NF-κB [4]. This leads to the expression of proinflammatory cytokines, including IL-8 and MCP-1 [19], [20], [21]. It has been shown that CML adducts are signal-transducing ligands for RAGE, both in vitro and in vivo [33]. However, another study found that CML-modified proteins were unable to bind to RAGE and activate proinflammatory signaling [34]. No changes in the gene expression of RAGE after exposure to CML were found, but this may be due to the relatively short incubation time of 24 h. However, increased concentrations of the proinflammatory cytokine MCP-1 were detected, which could be caused by RAGE signaling, as MCP-1 is known to be regulated by RAGE. MCP-1 is involved in the pathogenesis of diabetic nephropathy [35] and is also implicated in the destruction of beta cells in type 1 diabetes [36]. The rise in MCP-1 levels could explain the observed increase in intracellular oxidative stress in these cells since MCP-1 has been associated with the induction of oxidative stress in previous studies. MCP-1 enhanced ROS generation in monocytes from unstable angina patients [37]. Additionally, MCP-1-deficiency impaired ROS generation and attenuated oxidative stress in an ovariectomy rodent model (as a model for menopause) [38].
Previous research has shown that AGEs can increase GSSG levels in human neuroblastoma cells [39]. Also in vivo an association between AGEs and a decreased glutathione redox ratio in patients undergoing continuous ambulatory peritoneal dialysis was found [40]. We found a trend toward a decrease in GSH content in beta cells exposed to CML. The amount of GSSG was already very low and no further change could be detected after CML exposure. A decrease in GSH content has been associated with diabetes in previous studies, e.g. levels of GSH were lower in erythrocytes of type 2 diabetes patients [41]. This decrease was associated with a lower activity of the enzyme γ-GCS which is involved in the biosynthesis of GSH [41]. We did not find a change in gene expression of γ-GCS after exposing the cells to CML.
Replenishment of the GSH pool by GR is dependent on the GSSG pool and the availability of NADPH. Since we do not see changes in the GSSG concentration, the amount of available NADPH limits the glutathione reductase activity after CML exposure.
CML increased the expression of GST in both cell culture and animal models [42]. However, they also found increased GSH concentrations with a higher expression of GST. This seemed to be associated with activation of the transcription factor AP-1 by RAGE, which in turn might be involved in the induction of GST and γ-GCS [42]. We did find an increase in GSTP1 expression, however we did not find an increase in expression of RAGE and γ-GCS, which could explain why we did not find an increase in GSH.
It is known that expression of Grx is high in beta cells and that Grx might play a regulatory role in insulin exocytosis [43]. Glutaredoxin-1 expression has been linked to diabetic retinopathy, by inducing NF-κB translocation and expression of intercellular adhesion molecule-1 (ICAM-1) in rat retinal Müller cells [44]. A recent study in patients with abnormal glucose levels found a higher Grx activity in plasma and serum of these patients compared to healthy subjects [45]. We did not find any significant changes after 24 h exposure to CML in activity levels of Grx or expression of glutaredoxin-2.
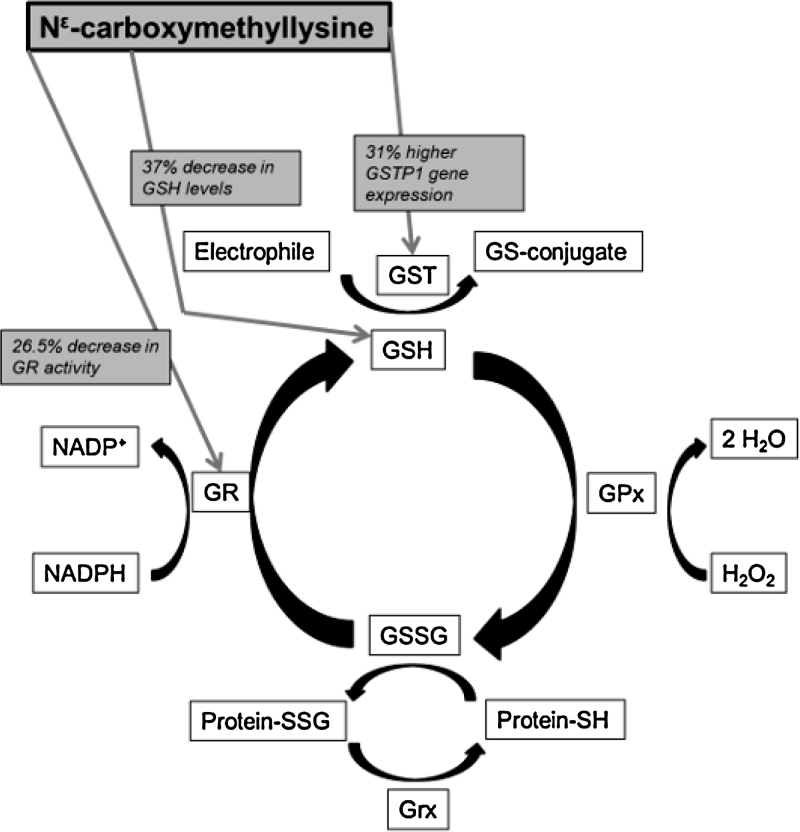
In conclusion, we found that CML was able to induce cell death in human pancreatic beta cells, which was accompanied by an increase in intracellular oxidative stress. We did not find changes in the expression of RAGE, but we found an increase in the level of a target cytokine of RAGE after CML exposure. Additionally we found that CML exposure lowered the levels of GSH. Also other components of the glutathione system were affected, we found a decrease in glutathione reductase activity and an increase in the expression of GSTP1 (Fig. 5). These changes in GSH levels and activities of components of the glutathione system indicate that the cells are even more vulnerable for oxidative stress after exposure to CML. Since beta cells are low in antioxidant enzymes and repair for oxidized DNA, it might be that AGEs like CML can accelerate beta cell dysfunction and beta cell death during hyperglycemia.
Fig. 5.
Overview of the glutathione system and the effects of 24 h incubation with 0.5 mM Nɛ-carboxymethyllysine (CML) on this system in pancreatic beta cells. GSH, glutathione (reduced form); GSSG, glutathione (oxidized form); GR, glutathione reductase; GPx, glutathione peroxidase; GST, glutathione-S-transferase; NADP, nicotinamide adenine dinucleotide phosphate (oxidized form); NADPH, nicotinamide adenine dinucleotide phosphate (reduced form); H2O2, hydrogen peroxide; H2O, water; SSG, glutathione adduct to protein; SH, thiol group; Grx, glutaredoxin.
Conflict of interest
The authors declare that there is no conflict of interests regarding the publication of this article.
Transparency document
Footnotes
Available online 27 June 2014
References
- 1.West I.C. Radicals and oxidative stress in diabetes. Diab. Med. 2000;17:171–180. doi: 10.1046/j.1464-5491.2000.00259.x. [DOI] [PubMed] [Google Scholar]
- 2.Semba R.D., Nicklett E.J., Ferrucci L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. Ser. A: Biol. Sci. Med. Sci. 2010;65A:963–975. doi: 10.1093/gerona/glq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 4.Goldin A., Beckman J.A., Schmidt A.M., Creager M.A. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 5.Semba R.D., Bandinelli S., Sun K., Guralnik J.M., Ferrucci L. Plasma carboxymethyl-lysine, an advanced glycation end product, and all-cause and cardiovascular disease mortality in older community-dwelling adults. J. Am. Geriatr. Soc. 2009;57:1874–1880. doi: 10.1111/j.1532-5415.2009.02438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Semba R.D., Ferrucci L., Sun K., Beck J., Dalal M., Varadhan R., Walston J., Guralnik J.M., Fried L.P. Advanced glycation end products and their circulating receptors predict cardiovascular disease mortality in older community-dwelling women. Aging Clin. Exp. Res. 2009;21:182–190. doi: 10.1007/bf03325227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semba R.D., Najjar S.S., Sun K., Lakatta E.G., Ferrucci L. Serum carboxymethyl-lysine, an advanced glycation end product, is associated with increased aortic pulse wave velocity in adults. Am. J. Hypertens. 2009;22:74–79. doi: 10.1038/ajh.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenzen S., Drinkgern J., Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic. Biol. Med. 1996;20:463–466. doi: 10.1016/0891-5849(96)02051-5. [DOI] [PubMed] [Google Scholar]
- 9.Tiedge M., Lortz S., Drinkgern J., Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes. 1997;46:1733–1742. doi: 10.2337/diab.46.11.1733. [DOI] [PubMed] [Google Scholar]
- 10.Modak M.A., Parab P.B., Ghaskadbi S.S. Pancreatic islets are very poor in rectifying oxidative DNA damage. Pancreas. 2009;38:23–29. doi: 10.1097/MPA.0b013e318181da4e. [DOI] [PubMed] [Google Scholar]
- 11.Townsend D.M., Tew K.D., Tapiero H. The importance of glutathione in human disease. Biomed. Pharmacother. 2003;57:145–155. doi: 10.1016/s0753-3322(03)00043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes J.D., McLellan L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999;31:273–300. doi: 10.1080/10715769900300851. [DOI] [PubMed] [Google Scholar]
- 13.Pompella A., Visvikis A., Paolicchi A., Tata V.D., Casini A.F. The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 2003;66:1499–1503. doi: 10.1016/s0006-2952(03)00504-5. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes A.P., Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid. Redox Signal. 2004;6:63–74. doi: 10.1089/152308604771978354. [DOI] [PubMed] [Google Scholar]
- 15.McCluskey J.T., Hamid M., Guo-Parke H., McClenaghan N.H., Gomis R., Flatt P.R. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J. Biol. Chem. 2011;286:21982–21992. doi: 10.1074/jbc.M111.226795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruijters E.J.B., Weseler A.R., Kicken C., Haenen G.R.M.M., Bast A. The flavanol (−)-epicatechin and its metabolites protect against oxidative stress in primary endothelial cells via a direct antioxidant effect. Eur. J. Pharmacol. 2013;715:147–153. doi: 10.1016/j.ejphar.2013.05.029. [DOI] [PubMed] [Google Scholar]
- 17.van de Wier B., Balk J.M., Haenen G.R.M.M., Giamouridis D., Bakker J.A., Bast B.C., den Hartog G.J.M., Koek G.H., Bast A. Elevated citrate levels in non-alcoholic fatty liver disease: the potential of citrate to promote radical production. FEBS Lett. 2013;587:2461–2466. doi: 10.1016/j.febslet.2013.06.019. [DOI] [PubMed] [Google Scholar]
- 18.Rahman I., Kode A., Biswas S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 19.Gu L., Hagiwara S., Fan Q., Tanimoto M., Kobata M., Yamashita M., Nishitani T., Gohda T., Ni Z., Qian J., Horikoshi S., Tomino Y. Role of receptor for advanced glycation end-products and signalling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytes. Nephrol. Dial. Transplant. 2006;21:299–313. doi: 10.1093/ndt/gfi210. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura K., Yamagishi S., Adachi H., Kurita-Nakamura Y., Matsui T., Yoshida T., Imaizumi T. Serum levels of sRAGE, the soluble form of receptor for advanced glycation end products, are associated with inflammatory markers in patients with type 2 diabetes. Mol. Med. 2007;13:185–189. doi: 10.2119/2006-00090.Nakamura. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahali S., Raviprakash N., Raghavendra P.B., Manna S.K. Advanced glycation end products (AGEs) induce apoptosis via a novel pathway: involvement of Ca2+ mediated by interleukin-8 protein. J. Biol. Chem. 2011;286:34903–34913. doi: 10.1074/jbc.M111.279190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J., Tan P.H., Tan B.K., Bay B.H. GST-pi expression correlates with oxidative stress and apoptosis in breast cancer. Oncol. Rep. 2004;12:921–925. [PubMed] [Google Scholar]
- 23.Goto S., Kawakatsu M., Izumi S.-I., Urata Y., Kageyama K., Ihara Y., Koji T., Kondo T. Glutathione S-transferase π localizes in mitochondria and protects against oxidative stress. Free Radic. Biol. Med. 2009;46:1392–1403. doi: 10.1016/j.freeradbiomed.2009.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- 25.Ashcroft F.M., Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lieuw-A-Fa M.L.M., van Hinsbergh V.W.M., Teerlink T., Barto R., Twisk J., Stehouwer C.D.A., Schalkwijk C.G. Increased levels of Nɛ-(carboxymethyl)lysine and Nɛ-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: correlation with markers of endothelial dysfunction. Nephrol. Dial. Transplant. 2004;19:631–636. doi: 10.1093/ndt/gfg619. [DOI] [PubMed] [Google Scholar]
- 27.Nin J.W., Jorsal A., Ferreira I., Schalkwijk C.G., Prins M.H., Parving H.-H., Tarnow L., Rossing P., Stehouwer C.D. Higher plasma levels of advanced glycation end products are associated with incident cardiovascular disease and all-cause mortality in type 1 diabetes: a 12-year follow-up study. Diabetes Care. 2011;34:442–447. doi: 10.2337/dc10-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanssen N.M.J., Engelen L., Ferreira I., Scheijen J.L.J.M., Huijberts M.S., van Greevenbroek M.M.J., van der Kallen C.J.H., Dekker J.M., Nijpels G., Stehouwer C.D.A., Schalkwijk C.G. Plasma levels of advanced glycation endproducts Nɛ-(carboxymethyl)lysine, Nɛ-(carboxyethyl)lysine, and pentosidine are not independently associated with cardiovascular disease in individuals with or without type 2 diabetes: the Hoorn and CODAM studies. J. Clin. Endocrinol. Metab. 2013;98:E1369–E1373. doi: 10.1210/jc.2013-1068. [DOI] [PubMed] [Google Scholar]
- 29.Coughlan M.T., Yap F.Y., Tong D.C., Andrikopoulos S., Gasser A., Thallas-Bonke V., Webster D.E., Miyazaki J., Kay T.W., Slattery R.M., Kaye D.M., Drew B.G., Kingwell B.A., Fourlanos S., Groop P.H., Harrison L.C., Knip M., Forbes J.M. Advanced glycation end products are direct modulators of beta-cell function. Diabetes. 2011;60:2523–2532. doi: 10.2337/db10-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin N., Zhang H., Su Q. Advanced glycation end-products induce injury to pancreatic beta cells through oxidative stress. Diabetes Metab. 2012;38:250–257. doi: 10.1016/j.diabet.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 31.Lim M., Park L., Shin G., Hong H., Kang I., Park Y. Induction of apoptosis of β cells of the pancreas by advanced glycation end-products, important mediators of chronic complications of diabetes mellitus. Ann. N. Y. Acad. Sci. 2008;1150:311–315. doi: 10.1196/annals.1447.011. [DOI] [PubMed] [Google Scholar]
- 32.Neeper M., Schmidt A.M., Brett J., Yan S.D., Wang F., Pan Y.C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J. Biol. Chem. 1992;267:14998–15004. [PubMed] [Google Scholar]
- 33.Kislinger T., Fu C., Huber B., Qu W., Taguchi A., Du Yan S., Hofmann M., Yan S.F., Pischetsrieder M., Stern D., Schmidt A.M. N ɛ-(carboxymethyl)lysine adducts of proteins are ligands for receptor for advanced glycation end products that activate cell signaling pathways and modulate gene expression. J. Biol. Chem. 1999;274:31740–31749. doi: 10.1074/jbc.274.44.31740. [DOI] [PubMed] [Google Scholar]
- 34.Buetler T.M., Leclerc E., Baumeyer A., Latado H., Newell J., Adolfsson O., Parisod V., Richoz J., Maurer S., Foata F., Piguet D., Junod S., Heizmann C.W., Delatour T. Nɛ-carboxymethyllysine-modified proteins are unable to bind to RAGE and activate an inflammatory response. Mol. Nutr. Food Res. 2008;52:370–378. doi: 10.1002/mnfr.200700101. [DOI] [PubMed] [Google Scholar]
- 35.Wada T., Yokoyama H., Matsushima K., Kobayashi K.i. Monocyte chemoattractant protein-1: does it play a role in diabetic nephropathy? Nephrol. Dial. Transplant. 2003;18:457–459. doi: 10.1093/ndt/18.3.457. [DOI] [PubMed] [Google Scholar]
- 36.Kutlu B., Darville M.I., Cardozo A.K., Eizirik D.L. Molecular regulation of monocyte chemoattractant protein-1 expression in pancreatic β-cells. Diabetes. 2003;52:348–355. doi: 10.2337/diabetes.52.2.348. [DOI] [PubMed] [Google Scholar]
- 37.Aukrust P., Berge R.K., Ueland T., Aaser E., Damås J.K., Wikeby L., Brunsvig A., Müller F., Forfang K., Frøland S.S., Gullestad L. Interaction between chemokines and oxidative stress: possible pathogenic role in acute coronary syndromes. J. Am. Coll. Cardiol. 2001;37:485–491. doi: 10.1016/s0735-1097(00)01110-4. [DOI] [PubMed] [Google Scholar]
- 38.Kim W.-K., Choi E.-K., Sul O.-J., Park Y.-K., Kim E.-S., Yu R., Suh J.-H., Choi H.-S. Monocyte chemoattractant protein-1 deficiency attenuates oxidative stress and protects against ovariectomy-induced chronic inflammation in mice. PLOS ONE. 2013;8:e72108. doi: 10.1371/journal.pone.0072108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deuther-Conrad W., Loske C., Schinzel R., Dringen R., Riederer P., Münch G. Advanced glycation endproducts change glutathione redox status in SH-SY5Y human neuroblastoma cells by a hydrogen peroxide dependent mechanism. Neurosci. Lett. 2001;312:29–32. doi: 10.1016/s0304-3940(01)02174-7. [DOI] [PubMed] [Google Scholar]
- 40.Şahin E., Göçmen A.Y., Koçak H., Tuncer M., Gümüşlü S. The association of advanced glycation end-products with glutathione status. Ann. Clin. Biochem. 2008;45:369–374. doi: 10.1258/acb.2007.007186. [DOI] [PubMed] [Google Scholar]
- 41.Murakami K., Kondo T., Ohtsuka Y., Fujiwara Y., Shimada M., Kawakami Y. Impairment of glutathione metabolism in erythrocytes from patients with diabetes mellitus. Metabolism. 1989;38:753–758. doi: 10.1016/0026-0495(89)90061-9. [DOI] [PubMed] [Google Scholar]
- 42.Faist V., Hofmann T., Zill H., Baynes J.W., Thorpe S.R., Sebekova K., Schinzel R., Heidland A., Wenzel E., Erbersdobler H.F. Effects of dietary Nɛ-carboxymethyllysine on expression of the biotransformation enzyme, glutathione-S-transferase, in the rat. Int. Congr. Ser. 2002;1245:313–320. [Google Scholar]
- 43.Ivarsson R., Quintens R., Dejonghe S., Tsukamoto K., in’t Veld P., Renström E., Schuit F.C. Redox control of exocytosis: regulatory role of NADPH, thioredoxin, and glutaredoxin. Diabetes. 2005;54:2132–2142. doi: 10.2337/diabetes.54.7.2132. [DOI] [PubMed] [Google Scholar]
- 44.Shelton M.D., Kern T.S., Mieyal J.J. Glutaredoxin regulates nuclear factor κ-B and intercellular adhesion molecule in Müller cells: model of diabetic retinopathy. J. Biol. Chem. 2007;282:12467–12474. doi: 10.1074/jbc.M610863200. [DOI] [PubMed] [Google Scholar]
- 45.Du Y., Zhang H., Montano S., Hegestam J., Ekberg N., Holmgren A., Brismar K., Ungerstedt J. Plasma glutaredoxin activity in healthy subjects and patients with abnormal glucose levels or overt type 2 diabetes. Acta Diabetol. 2014;51(2):225–232. doi: 10.1007/s00592-013-0498-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.