Abstract
In this study 11 commercial roll-your-own (RYO) tobacco brands sold in Spain and the reference tobacco 3R4F have been smoked and several components of the mainstream tobacco smoke have been analyzed. Cigarettes were prepared using commercial tubes, and were smoked under smoking conditions based on the ISO 3308. The gaseous and condensed fractions of the smoke from RYO brands and 3R4F have been analyzed and compared. RYO tobaccos, as opposed to 3R4F, present lower amounts of condensed products in the traps than in the filters. In general, RYO tobaccos also provide lower yields of most of the compounds detected in the gas fraction. The yield of CO is between 15.4 and 20.4 mg/cigarette. In most of the cases studied, RYO tobaccos deliver higher amounts of nicotine than the 3R4F tobacco. On average, the yield of the different chemical families of compounds appearing in the particulate matter retained in the cigarette filters tends to be around three times higher than those obtained from 3R4F, whereas similar values have been obtained in the particulate matter retained in the traps located after the filters. It can be concluded that RYO tobaccos are not less hazardous than the reference tobacco, which may be contrary to popular belief.
Keywords: RYO tobacco, Tar, CO, Nicotine, Mainstream smoke
1. Introduction
The importance of and interest in the analysis of mainstream tobacco smoke has been recognized from years [39], [19], [9], [20], [40]. Such studies provide very valuable information for establishing relationships between tobacco constituents and smoke products and the formation of harmful and hazardous compounds [5], [32]. They also provide knowledge of the effect of cigarette design parameters on the smoke composition for several purposes, such as the discrimination among tobacco types [1], the smoke composition [13], or the contribution of certain pollutants from tobacco smoke to indoor air [29], for example. In this context, the need for including the analysis of reference cigarettes has been recognized [35] in the studies because it allows the replication and comparison of experiments performed in different laboratories. In our study the inclusion of a reference tobacco is especially needed because, in addition to the above reason providing a reference of the quality of our data, it allows a comparison with the results of other authors and smoking conditions.
The habits of tobacco consumption have changed in the last years, and the use of roll-your-own (RYO) cigarettes has increased noticeably, mainly due to being cheaper than buying factory-made (FM) cigarettes, but also because it is easier to control the amount of tobacco used by rolling thinner cigarettes, and the erroneous belief that they are less hazardous [44]. In 2008, the prevalence of the consumption of RYO in Spain was 8.7%, but increased 200% in the 2007–2010 period, with an increase of 60% in 2010, probably because of its lower price and the erroneous argument that it could be less toxicant because it contains fewer additives than FM cigarettes [18]. According to Rosenberry et al. [36], in 2013 the data about the prevalence of these cigarettes in several countries is as follows: United Kingdom (28.4%), Canada (17.1%), Australia (24.2%); Malaysia (17%), Thailand (58%), and U.S. (6.7%), although the current use in the United States could be greater. Young et al. [43] have published an interesting study on the prevalence and attributes of roll-your-own smokers in Australia, Canada, the United Kingdom and the United States.
The application of comprehensive tobacco control legislation, particularly large cigarette tax increases can contribute to a decrease of the prevalence of smoking and consumption of cigarettes. However, the eventual problems related to the potential use of RYO as a cheaper alternative, especially among the less favored population, have to be considered [3], [26], [6]. As Kaiserman and Rickert [24] state, cigarette manufacturers are able to adjust the deliveries of tar, nicotine, and CO by manipulating the components used in FM cigarettes (tobacco, filters and papers). However, the tar delivered from RYO cigarettes can vary by approximately 60% depending on the tube and filter combination used for wrapping the cigarettes. Therefore, smokers should be advised, that not only the yields of tar, nicotine and CO from tobacco have to be taken into account in order to make their choices of the less hazardous product. The use of slim cigarettes results in lower yields of some toxicants [37]. However, according to Darral and Figgins [14], there is a lack of information that neglects to inform the smoking public in any detail about the hazards associated with either particular brands or particular rolling techniques.
Different authors have studied the mainstream smoke from RYO cigarettes. In 1990, Appel et al. [2] reported that the emissions of benzene and benzo(α)pyrene and lead were similar for cigars, RYO cigarettes and pipe tobacco, and the lead values were below the limit of reliable quantitation in the cases studied. However, the potential exposures of benzene and benzo(α)pyrene exceeded the No Significant Risk levels. Darral and Figgins [14] point out that there are few published studies focused on the yields of smoke from hand-rolling tobaccos. These authors found that the yields of tar in the mainstream smoke from RYO cigarettes produced by 57% of the smokers were above the current maximum of 15 mg/cigarette for manufactured cigarettes. Moreover, 77% of RYO smokers produced cigarettes with a nicotine yield higher than 1.1 mg/cigarette, in comparison with 8% of manufactured cigarettes that declared nicotine yields higher than this value. These results agree with those reported by Castaño Calduch et al. [10] that analyzed up to 70% of the fine-cut tobacco market in Spain, and showed that the yields of nicotine, tar and CO were in the range of 1–1.7, 13.7–18.5 and 13.5–18.4 mg/cigarette respectively, and in many cases they were higher than the maxima legislated of 1, 10 and 10 mg/cigarette, respectively.
In a previous work [30] we analyzed the mainstream smoke from the 10 commercial top selling cigarette brands in Spain, showing that despite the gaseous hydrocarbons and that most of the compounds of the vapor phase presented similar relative proportions among brands, the relative yield of some very well known toxic compounds may vary substantially from brand to brand. Moreover, the brands with the lowest total yield for the compounds in the vapor fraction were not coincident with those showing the lowest yield in the total particulate matter retained in the traps located after the cigarette filters. Thus, the need to establish more adequate parameters to determine tobacco toxicity was pointed out because the harmfulness of any individual compound appearing in the mainstream smoke can be very different, and the relative proportion of them is not always proportional to tar, CO or nicotine, that are the parameters regulated by legislation. In another paper [31], the effect of certain zeolite-type catalysts on the mainstream smoke composition has also been studied.
The main objective of the present work is to study the composition of the gases evolved from 11 RYO tobacco brands sold in Spain, and compare them with the 3R4F reference tobacco smoked under the same conditions. This study could represent an interesting contribution in order to avoid the lack of analytical data regarding the smoke from RYO tobaccos. In addition, this study would provide the reference for further studies focused on the effect of several catalysts to reduce the toxicity of RYO tobacco smoke.
2. Materials and methods
Table 1 shows the 11 RYO tobacco brands selected for the study carried out in the present work, as well as the corresponding manufacturing tobacco companies and market share in Spain accumulated up to February 2014, http://www.cmtabacos.es/wwwcmt/paginas/ES/mercadoEstadisticas.tmpl [12]. All of them account for approximately 2/3 of the Spanish market and represent an accumulated value of sales from January and February 2014 of around 934 tons. The different brands have been labeled with letters from A to K in a different order than that shown in Table 1 for privacy reasons. The reference 3R4F tobacco from the University of Kentucky [35], [34] has also been included in Table 1.
Table 1.
Brands of RYO tobacco studied in this work.
| Brands | Tobacco company | Market share (% kg) |
|---|---|---|
| 3R4F | University of Kentucky | |
| Pueblo | Pöschl Tabak | 12.1 |
| Golden Virginia | Imperial Tobacco | 3.3 |
| Domingo Natural | Gryson NV | 4.9 |
| Malboro | Philip Morris International | 4.9 |
| Camel | JTI | 4.3 |
| Chesterfield | Philip Morris International | 7.1 |
| Winston | JTI | 8.7 |
| Ducados Rubio | Imperial Tobacco | 14.0 |
| Pall-Mall | British American Tobacco | 5.7 |
| American Spirit | Santa Fe Natural Tobacco Company | 0.9 |
| Cross Road | Scandinavian tobacco group | 0.6 |
The cigarettes for the smoking experiments were prepared by weighing the empty tube and the tube completely refilled, by hand, with the corresponding tobacco. The procedure used for filling the tubes was the same in all the cases. However, the differences in the apparent density and other physical characteristics of the tobacco strands employed in each brand made it difficult to prepare cigarettes with the same amount of tobacco and the same compacting degree. We have tried to use masses of tobacco that permit us to keep the pressure drop in the rods (at around 1.5 kPa). Thus, different amounts of tobacco per cigarette were used for each brand, and the results obtained for the composition of the mainstream smoke have been expressed in terms of mass of materials per gram of smoked tobacco. Nevertheless, the mass of tobacco per cigarette was in the range of the values employed in other works focused on the study of RYO tobacco, Kaiserman and Rickert [24] used 900 mg and similar condition conditions as used in the present work and Castaño Calduch et al. [10] used 750 mg using the ISO 15592-2 conditioning conditions.
The tubes used that include the filter plus the void paper cylinder were Mascotte Hulzen X-LONG. These tubes have no ventilation holes and were selected since they more likely correspond to the conditions of the RYO cigarettes smoked by consumers, because the paper used (that also covers the filter) has typically no ventilation holes. The 3R4F reference tobacco from the emptied 3R4F cigarettes has been used in these experiments using the same tubes as those used for the RYO tobaccos. Thus, the results obtained with the non-ventilated tubes permit us to connect the results obtained for RYO tobaccos with other data reported in the literature through the comparison of the results corresponding to 3R4F smoked under different conditions.
All the cigarettes, after preparation, were also conditioned for at least 48 h at 22 °C and a relative humidity of 60%, based on the ISO 3402 standard [22]. Although the number of puffs is different for the different tobaccos, in this work the cigarettes have been smoked until a butt length of 33.2 mm (filter length +8 mm), since is the largest of 23 mm, filter length +8 mm (33.2 mm) or paper length +3 mm (28.2 mm), according to the ISO 4387 standard [23]. The study of the cigarettes prepared by using exactly the same tubes and procedure with the RYO and 3R4F tobaccos enables us to compare the results obtained in the present work with those reported by other authors that also use the 3R4F reference (i.e., [35], [7], [33] or [40]) as well as with the results corresponding to other additive-tobacco systems that are being studied by our research team.
Cigarettes were smoked in a smoking machine under smoking conditions based on the ISO 3308 [21], in a similar way as in other works [35], [30], [31], the main difference being the absence of ventilation holes in the tubes. This condition has a great influence on the yields obtained as will be discussed later. The smoking machine employed allows five cigarettes to be smoked simultaneously and the pressure of aspiration was never higher than 1.5 kPa. Puff volume was 35 mL, taken during 2.0 s, with a puff frequency of 60 s. Each experiment was repeated twice. Typically, standard deviations lower than 20% are obtained [31]. Ten cigarettes were smoked for each experiment, and the mainstream smoke obtained was analyzed in all cases. The mainstream smoke, after passing through the cigarette filter, passed through another trap (based on the ISO 3308, we use a glass microfibre filter Wathman trap of 47 mm diameter, conditioned as the tobacco samples) in order to retain the less volatile compounds that could condense in the mouth and lungs of smokers, and was finally collected in a Tedlar bag, as described elsewhere [30], [31]. Thus, the mainstream smoke has be considered as comprising three fractions, i.e., the gaseous fraction which is that collected in the Tedlar bag, the total particulate matter (TPM) and the condensed fraction collected in the cigarette filters. Last is going to name TPM-F in order to improve the comparative process. The yields obtained for the different fractions and compounds appearing in the mainstream smoke were compared for all the studied RYO brands and, finally the comparison between the RYO tobaccos and the reference 3R4F tobacco was also carried out.
The gaseous fraction compounds were analyzed by GC/TCD in a Shimadzu GC-14 chromatograph with a CTRI column (for CO), and by GC/MS or GC/FID in an Agilent 6890N chromatograph with a GS-GASPRO column (for the other components). The particulate matter condensed in filters and traps was extracted separately with isopropanol, according to the ISO 4387, dried and analyzed by GC/MS in an Agilent 6890N chromatograph with a HP-5-MS column. In order to make sure of extracting all the matter, three successive extraction stages with fresh isopropanol were performed. Nevertheless, a single extraction stage yields most of the matter. The filters have been conditioned and 60 mL of isopropanol in three stages of 20 mL each were used. 31 and 74 compounds were analyzed respectively in the gaseous and in the TPM fractions of the mainstream smoke. The conditions for the analysis and quantification of the analytes have been described elsewhere [30], [31].
3. Results and discussion
3.1. Preliminary analysis
Table 2 shows the number of puffs, the amount of tobacco per cigarette, the amount of smoked tobacco in the smoking runs and the total particulate matter condensed in filters and traps (TPM-F and TPM), both expressed as mg/g of smoked tobacco.
Table 2.
Number of puffs, initial and final amounts of tobacco and global yields obtained for the different smoking runs. The results reported correspond to the mean values of the 10 cigarettes smoked in each experiment.
| Sample | Number of puffs | g of tobacco/cigarette | g of smoked tobacco/cigarette | TPM-F (mg/g of smoked tobacco) | TPM (mg/g of smoked tobacco) | TPM-F + TPM (mg/g of smoked tobacco) |
|---|---|---|---|---|---|---|
| 3R4F | 8 | 0.76 | 0.62 | 15.8 | 18.6 | 34.4 |
| A | 10 | 0.92 | 0.77 | 21.5 | 19.4 | 40.9 |
| B | 9 | 0.84 | 0.67 | 24.1 | 17.9 | 42.0 |
| C | 9 | 0.89 | 0.77 | 16.8 | 13.7 | 30.5 |
| D | 9.5 | 0.85 | 0.70 | 22.1 | 19.6 | 41.8 |
| E | 10 | 0.94 | 0.76 | 24.6 | 19.1 | 43.7 |
| F | 9 | 0.87 | 0.71 | 20.1 | 18.8 | 38.9 |
| G | 8.3 | 0.86 | 0.70 | 25.4 | 20.1 | 45.5 |
| H | 7.5 | 0.64 | 0.55 | 26.0 | 22.0 | 47.9 |
| I | 11 | 0.96 | 0.79 | 25.2 | 18.9 | 44.0 |
| J | 12 | 1.10 | 0.91 | 20.2 | 13.8 | 33.9 |
| K | 8.3 | 0.82 | 0.64 | 22.6 | 18.1 | 40.7 |
As can be seen in Table 1, the amount of tobacco smoked was in the range of 0.55–0.91 g/cigarette, and in most cases it was around 0.6–0.7 g/cigarette. The amount of tobacco was in the range of 1.10 and 0.64 g/cigarette. These results agree with the expected linear correlation between the amount of tobacco per cigarette and the amount of tobacco smoked per cigarette which can be clearly appreciated in Table 1. There is also a linear correlation between the number of puffs and the amount of tobacco per cigarette that indicates the need to increase the air supply for burning the same length of cigarette when the amount of tobacco in the cigarette also increases.
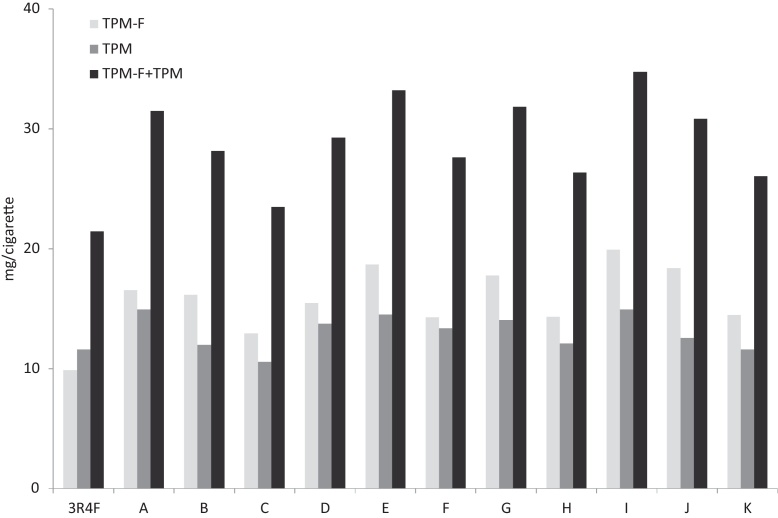
Fig. 1 shows the yield of particulate matter condensed in the filters and the traps and the sum of both for all brands analyzed. In order to facilitate the comparison with the values reported in the bibliography; the data have been expressed in terms of mg/cigarette. The represented values have been obtained by multiplying the results of Table 2, TPM mg/g of smoked tobacco, by the amount of g of tobacco smoked/cigarette corresponding to each case. Similar changes of the units will be made in the following paragraphs with the sole purpose of being able to perform comparisons with the results reported by other authors.
Fig. 1.
Yields of the total particulate matter expressed as mg/cigarette.
In general, for RYO tobacco and 3R4F, the yield of TPM is within the 18–22 mg/g smoked tobacco or 11–15 mg/cigarette ranges. Only brand C presents a slightly lower yield. Worth mentioning is the case of brand J, which is outside the mentioned range of TPM in terms of mg/g tobacco smoked but within the range observed for the other RYOs when TPM is expressed as mg/cigarette. This is, obviously, due to the fact that the brand J cigarettes had the highest amounts of tobacco per cigarette and tobacco smoked per cigarette. TPM for 3R4F is higher than TPM-F, but for RYO tobaccos the opposite trend is observed. The total amount of TPM + TPM-F for the reference tobacco is lower than for RYO tobaccos, with the only exception of brands C and J, and only when the data are expressed in terms of mg/g of tobacco smoked. The values reported by Bodnar et al. [7] for tar are around 30.6 mg/cigarette for the 3R4F reference tobacco and 34.4 mg/cigarette for the average of the 61 brands of manufactured cigarettes studied. These values are much higher than our TPM that, in accordance with the amount of tobacco per 3R4F cigarette (Table 1) would be TPM-F = 9.9 mg/cigarette and TPM = 11.6 mg/cigarette, thus demonstrating the effect of the more intense smoking regime used in that work and the absence of ventilation holes in the tubes used.
In accordance with Table 2, the highest value of TPF-F + TPM is obtained for brand H, with 47.9 mg/g smoked tobacco, but E, G and I tobaccos also yield high amounts of TPF-F + TPM. The lowest value of TPF-F + TPM is obtained for brands C and J, with values of 30.5 and 33.9 mg/g smoked tobacco. Differences between brands are not too high. 3R4F produces 34.4 mg/g smoked tobacco. This yield is in the range of the RYO brands giving the lowest yields expressed in mg/g of smoked tobacco.
The study of mainstream tobacco smoke involves the analysis of its carbon monoxide content. CO is under specific regulations in many countries due its known high toxicity, and the generation of this compound is greatly influenced by many variables including the type of tobacco [11], [15]. Table 3 shows the results obtained for the yield of CO expressed as mg/g of smoked tobacco and in mg/cigarette. As can be seen, the yields of CO are, in all cases, greater than 10 mg/cigarette, limiting value for cigarettes according to the Spanish legislation (RD 1079/2002 [17]), ranging from 15.4 mg/cigarette for brand J to 20.4 mg/cigarette for brand E. Nevertheless, it must be considered that the amount of tobacco per cigarette is higher in these tubes than in the usual ones (typically around 0.7 g/cigarette) and, additionally, the tubes have no ventilation holes. In fact, we obtained 11 mg of CO/cigarette when the reference 3R4F cigarettes were smoked in our laboratory, following the same procedure as in the present work and emptying and refilling the own 3R4F tubes (with ventilation holes), which agrees with the typical value provided by the supplier of 12 mg/cigarette. In this study this reference tobacco has a yield 17.4 mg/cigarette, thus, it could be concluded that several RYO tobaccos (i.e., those providing CO yields lower than the 3R4F reference tobacco) yield CO that could be below the authorized limit if they are smoked with ventilation holes. Kaiserman and Rickert [24] reported an average value of 17.69 mg/cigarette for RYO tobacco smoked in unperforated tubes, which is a value very similar to those obtained in the present study, where the average value is 17.7 mg/cigarette. Bodnar et al. [7] obtained 32.1 mg of CO per cigarette for the reference 3R4F tobacco smoked under the Canadian Intense regime, and a mean value of 32.3 mg CO/cigarette for the 61 commercial cigarette brands studied (including several slim cigarettes). The influence of the more intense smoking regime in addition to the lack of ventilation holes in the filters used is responsible for these high values. The amount of tobacco in that work ranged from 0.61 to 1 g/cigarette for the cigarettes with around 24–25 mm rod size.
Table 3.
Yield of CO.
| CO (mg/g of smoked tobacco) | CO (mg/cigarette) | |
|---|---|---|
| 3R4F | 27.9 | 17.4 |
| A | 25.6 | 19.6 |
| B | 27.0 | 18.0 |
| C | 19.7 | 15.1 |
| D | 23.8 | 16.5 |
| E | 26.7 | 20.4 |
| F | 27.3 | 19.3 |
| G | 27.6 | 19.4 |
| H | 32.1 | 17.7 |
| I | 22.5 | 17.8 |
| J | 16.9 | 15.4 |
| K | 24.5 | 15.7 |
Other very important compound to be considered is nicotine, because this is the compound responsible for the addictive characteristics of tobacco. Nicotine, together with CO and tar, are the three parameters under the Spanish (as many other countries) regulations (RD 1079/2002 [17]). Table 4 shows the amount of nicotine in the condensed fraction retained in the traps and the filters, expressed as mg/g of smoked tobacco, as well as the results obtained for the amount of nicotine retained in traps expressed in terms of mg/cigarette, which is the value suitable for the comparison with the values reported in the bibliography. As will be seen in the following sections, nicotine is the major individual compound appearing both in TPM and TPM-F. According to Table 4, the trend observed is now different to that described for CO, and the RYO tobaccos studied in this work yield higher amounts of nicotine than the 3R4F tobacco. When the 3R4F reference cigarettes were smoked using their own 3R4F tubes (i.e., with ventilation holes), 0.65 mg of nicotine/cigarette was obtained in the TPM. This value agrees with the data reported by the supplier (the yield of nicotine in TPM agrees with the value of 0.73 mg/cigarette reported by the supplier when taking into consideration the different conditions used and that the cigarettes have been emptied and refilled). As previously commented, the higher values obtained in the present work are a consequence of the absence of ventilation holes. As in the case of CO, the values of nicotine reported by Bodnar et al. [7] are higher than those obtained in the present work. Kaiserman and Rickert [24] obtained an average value for nicotine of 1.14 mg/cigarette when smoking in conditions very similar to those used in the present work and Castaño Calduch et al. [10] reported values between 1 and 1.17 mg/cigarette.
Table 4.
Yields of nicotine.
| Sample | Nicotine in TPM-F (mg/g smoked tobacco) | Nicotine in TPM (mg/g smoked tobacco) | Nicotine in TPM (mg/cigarette) |
|---|---|---|---|
| 3R4F | 1.09 | 1.90 | 1.19 |
| A | 2.66 | 3.60 | 2.76 |
| B | 2.12 | 2.74 | 1.83 |
| C | 1.83 | 2.41 | 1.84 |
| D | 1.98 | 2.54 | 1.77 |
| E | 1.55 | 2.35 | 1.80 |
| F | 1.44 | 2.09 | 1.48 |
| G | 1.24 | 2.35 | 1.65 |
| H | 1.41 | 2.74 | 1.51 |
| I | 2.02 | 3.15 | 2.49 |
| J | 0.96 | 2.19 | 1.99 |
| K | 1.75 | 2.84 | 1.82 |
An interesting fact is that TPM-F is higher than TPM, the amount of nicotine is higher in TPM than in TPM-F, thus indicating that this compound tends to be more concentrated in the traps. It is interesting to point out that, even though the overall yield of TPM-F was lower than TPM for the 3R4F reference, nicotine is also more concentrated in TPM than in TPM-F.
3.2. Analysis of the vapor phase
The results obtained are shown in Table 5. The total gas yield has been calculated as the sum of the yields of the different analyzed compounds and, as can be seen, ranges from 4.66 mg/g of tobacco smoked for brand K to 6.98 mg/g of tobacco smoked for brand E. 3R4F is within this range, but very close to its highest end, with a value of 6.73 mg/mg of tobacco smoked.
Table 5.
Yields of the different compounds analyzed in the gaseous fraction.
| mg/g smoked tobacco | 3R4F | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Methane | 1.92 | 1.45 | 1.36 | 1.36 | 1.18 | 1.23 | 1.57 | 1.24 | 0.1 | 1.48 | 1.79 | 1.25 |
| Ethane | 0.72 | 0.58 | 0.53 | 0.55 | 0.50 | 0.52 | 0.64 | 0.53 | 0.38 | 0.61 | 0.70 | 0.49 |
| Ethylene | 0.44 | 0.30 | 0.29 | 0.27 | 0.26 | 0.27 | 0.33 | 0.30 | 0.27 | 0.31 | 0.34 | 0.26 |
| Ethyne | 0.06 | 0.03 | 0.03 | 0.03 | 0.03 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 |
| Propane | 0.31 | 0.26 | 0.25 | 0.25 | 0.23 | 0.24 | 0.29 | 0.25 | 0.18 | 0.28 | 0.30 | 0.22 |
| Propene | 0.39 | 0.29 | 0.28 | 0.26 | 0.26 | 0.26 | 0.32 | 0.28 | 0.24 | 0.30 | 0.32 | 0.25 |
| Iso-butane | 0.05 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 |
| Chloromethane | 0.10 | 0.08 | 0.13 | 0.08 | 0.05 | 0.07 | 0.04 | 0.04 | 0.08 | 0.07 | 0.08 | 0.04 |
| Butane | 0.09 | 0.08 | 0.07 | 0.08 | 0.07 | 0.07 | 0.09 | 0.08 | 0.05 | 0.08 | 0.09 | 0.07 |
| 1-Butene | 0.09 | 0.07 | 0.06 | 0.06 | 0.06 | 0.06 | 0.08 | 0.07 | 0.06 | 0.07 | 0.08 | 0.06 |
| 1,2-Propadiene | 0.03 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 |
| 1,3-Butadiene | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 |
| Isobutene | 0.06 | 0.07 | 0.04 | 0.06 | 0.06 | 0.06 | 0.07 | 0.06 | 0.06 | 0.07 | 0.08 | 0.05 |
| cis-2-Butene | 0.08 | 0.05 | 0.01 | 0.01 | 0.05 | 0.04 | 0.06 | 0.03 | 0.04 | 0.05 | 0.06 | 0.05 |
| Pentane | 0.01 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 | 0.02 | 0.02 | 0.03 | 0.02 |
| Methanethiol | 0.05 | 0.03 | 0.04 | 0.00 | 0.02 | 0.03 | 0.04 | 0.02 | 0.03 | 0.03 | 0.02 | 0.01 |
| Hydrogen cyanide | 0.02 | 0.02 | 0.02 | 0.01 | 0.01 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.02 |
| 1-Pentene | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 |
| Furan | 0.03 | 0.03 | 0.01 | 0.02 | 0.02 | 0.02 | 0.03 | 0.03 | 0.02 | 0.03 | 0.03 | 0.02 |
| Isoprene | 0.40 | 0.48 | 0.31 | 0.20 | 0.31 | 0.26 | 0.37 | 0.40 | 0.26 | 0.32 | 0.54 | 0.37 |
| Hexane | 0.01 | 0.02 | 0.02 | 0.01 | 0.02 | 0.01 | 0.03 | 0.02 | 0.01 | 0.02 | 0.03 | 0.02 |
| 1-Hexene | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Benzene | 0.27 | 0.18 | 0.15 | 0.18 | 0.17 | 0.16 | 0.20 | 0.19 | 0.17 | 0.21 | 0.19 | 0.18 |
| Acetaldehyde | 1.01 | 1.06 | 1.15 | 1.13 | 1.08 | 2.61 | 0.74 | 1.61 | 1.32 | 1.49 | 0.87 | 0.86 |
| Acrolein | 0.05 | 0.05 | 0.06 | 0.08 | 0.05 | 0.04 | 0.05 | 0.03 | 0.05 | 0.02 | 0.03 | 0.02 |
| Propionaldehyde | 0.06 | 0.04 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 |
| Acetonitrile | 0.16 | 0.09 | 0.06 | 0.07 | 0.07 | 0.06 | 0.07 | 0.07 | 0.09 | 0.06 | 0.04 | 0.05 |
| Toluene | 0.06 | 0.04 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.03 | 0.03 |
| 2,5-Dimethylfuran | 0.01 | 0.03 | 0.02 | 0.03 | 0.02 | 0.02 | 0.03 | 0.01 | 0.01 | 0.01 | 0.04 | 0.02 |
| Crotonaldehyde | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| Isobutyraldehyde | 0.02 | 0.04 | 0.03 | 0.05 | 0.02 | 0.03 | 0.01 | 0.03 | 0.03 | 0.02 | 0.02 | 0.01 |
| Sum | 6.73 | 6.01 | 5.62 | 5.18 | 5.20 | 6.98 | 5.71 | 5.95 | 5.00 | 5.90 | 6.02 | 4.66 |
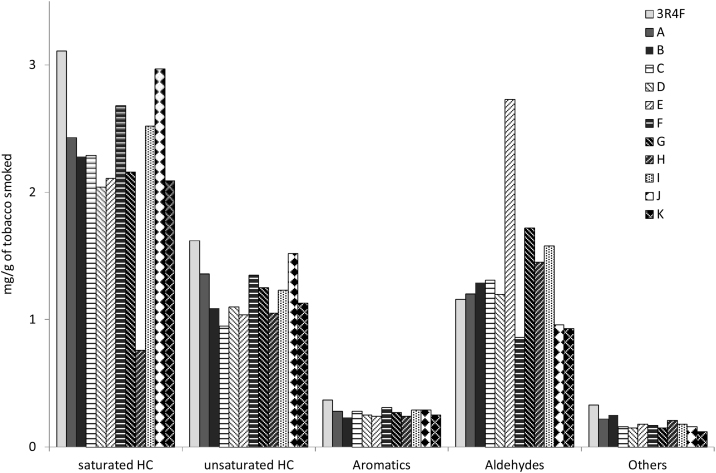
Hydrocarbons show very similar amounts in all the RYO brands, with some exceptions such as the low value of methane when smoking brand H or of cis-2-butene when brands B and C are smoked. In general, the highest values seem to be shown by brands J and F, and the lowest by D and H. No clear tendency has been observed for the oxygenated or other heteroatom-containing compounds; it is worth mentioning the comparatively high values of Acetaldehyde from brand E, Acrolein from brand C or Chloromethane from brand B. In order to make the comparison easier, the chemical compounds of Table 5 have been grouped by chemical families such as aliphatic saturated or unsaturated hydrocarbons, aromatics, aldehydes and other compounds, where nitrogenous and other heteroatom-containing compounds have been included. The corresponding yields are shown in Fig. 2. As can be seen, the major compounds are aliphatic hydrocarbons, with higher overall yields of saturated hydrocarbons than of unsaturated hydrocarbons, followed by aldehydes. Aromatics and other compounds appear to a noticeably lower extent. The already commented on noticeably high value of aldehydes in brand E and the low yield of saturated hydrocarbons of brand H could be highlighted. The maximum values for all the chemical families have been obtained in the gases evolved when the 3R4F reference was smoked, with the only exception of the yields of aldehydes, which are close to the average value obtained for RYO tobaccos if brand E is not taken into account.
Fig. 2.
Yields of the different chemical families appearing in the gaseous fraction of the mainstream smoke.
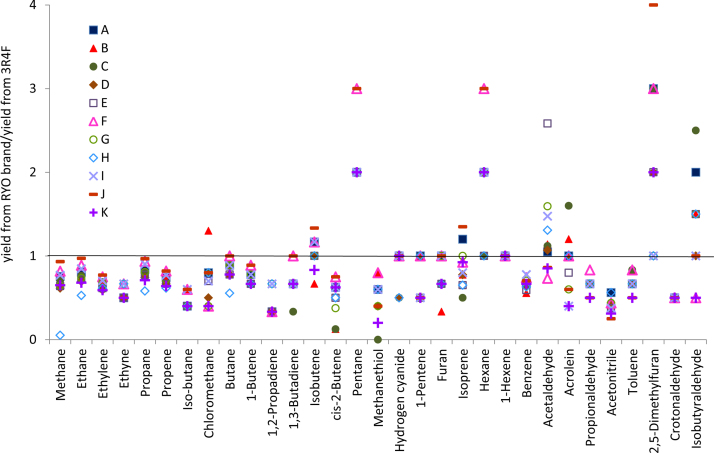
According to Table 5, and in good agreement with the comments of the previous paragraph, a higher amount of the gaseous compounds has been obtained from 3R4F than from most of the studied RYO brands. In order to facilitate the comparison between each chemical compound in RYO tobacco and the reference tobacco, normalized yields of these compounds have been obtained by dividing each particular yield by the corresponding value obtained for 3R4F. The results obtained are shown in Fig. 3. In this figure, a value of unity means that the yield obtained from the RYO brand is equal to the yield obtained from the reference tobacco. As can be seen, with the only exceptions of pentane, hexane, acetaldehyde, 2,5-dimethylfuran and isobutyraldehyde, the other 26 compounds are obtained in lower amounts when smoking the different RYO tobaccos than when smoking the 3R4F reference tobacco.
Fig. 3.
Relative yields for compounds obtained in the gaseous fraction of the mainstream smoke.
3.3. Particulate matter
Table 6, Table 7 contain, respectively, the results corresponding to the condensed materials – also so-called liquid fraction – from filters and traps. Compounds have been arranged according to their retention time. As can be seen, the amount of most compounds with low retention time is higher in the filters than in the traps, while the opposite is true for the heaviest compounds. This behavior has already been observed in another work [30], and has been related with different aspects, such as the different affinity of any particular compound for the filter and the trap, their relative concentration and the differences in their vapor pressures. According to Kalaitzoglou and Samara [25], an increase in the pressure drop during smoking may cause desorption of PAHs from the TPM which might favor their distribution in the gas phase, whereas compounds from other chemical classes may behave differently. The pressure drop through the cigarette might be related to the density and size of the filters, the ventilation rates, among other design features of the cigarettes, and also on the smokers’ habits. It may also change between puffs, affecting the way the different compounds are adsorbed or desorbed in filters and traps, making the interpretation of this phenomenon difficult.
Table 6.
Yields of the different compounds analyzed in the TPM-F fraction.
| mg/g smoked tobacco | 3R4F | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyridine, 4-methyl- | 0.007 | 0.014 | 0.013 | 0.011 | 0.017 | 0.013 | 0.012 | 0.011 | 0.017 | 0.000 | 0.007 | 0.000 |
| Pyrazine, methyl- | 0.007 | 0.009 | 0.015 | 0.010 | 0.012 | 0.010 | 0.007 | 0.009 | 0.019 | 0.000 | 0.004 | 0.000 |
| Furfural | 0.085 | 0.090 | 0.100 | 0.136 | 0.143 | 0.087 | 0.100 | 0.113 | 0.121 | 0.000 | 0.089 | 0.000 |
| 2-Pentanone, 4-hydroxy-4-methyl- | 0.010 | 0.004 | 0.007 | 0.013 | 0.005 | 0.004 | 0.000 | 0.000 | 0.003 | 0.000 | 0.002 | 0.000 |
| Ethanol, 2-(1-methylethoxy)- | 0.009 | 0.006 | 0.009 | 0.015 | 0.011 | 0.006 | 0.011 | 0.000 | 0.000 | 0.022 | 0.000 | 0.016 |
| 2-Furanmethanol | 0.010 | 0.000 | 0.025 | 0.050 | 0.050 | 0.015 | 0.026 | 0.027 | 0.000 | 0.016 | 0.000 | 0.038 |
| Pyridine, 3-methyl- | 0.017 | 0.035 | 0.027 | 0.016 | 0.026 | 0.049 | 0.040 | 0.054 | 0.047 | 0.046 | 0.019 | 0.018 |
| 2-Propanone, 1-(acetyloxy)- | 0.028 | 0.025 | 0.047 | 0.047 | 0.051 | 0.026 | 0.028 | 0.036 | 0.033 | 0.032 | 0.017 | 0.033 |
| 4-Cyclopentene-1,3-dione | 0.025 | 0.022 | 0.087 | 0.062 | 0.075 | 0.047 | 0.041 | 0.040 | 0.020 | 0.052 | 0.024 | 0.037 |
| Styrene | 0.002 | 0.004 | 0.007 | 0.005 | 0.005 | 0.007 | 0.005 | 0.005 | 0.003 | 0.012 | 0.005 | 0.010 |
| 2-Cyclopenten-1-one, 2-methyl- | 0.026 | 0.034 | 0.056 | 0.057 | 0.067 | 0.045 | 0.034 | 0.049 | 0.052 | 0.058 | 0.031 | 0.061 |
| 2-Acetylfuran | 0.015 | 0.028 | 0.033 | 0.029 | 0.036 | 0.036 | 0.031 | 0.037 | 0.039 | 0.019 | 0.020 | 0.029 |
| 2(5H)-furanone | 0.013 | 0.018 | 0.024 | 0.027 | 0.029 | 0.021 | 0.020 | 0.028 | 0.028 | 0.008 | 0.016 | 0.015 |
| Pyrazine, 2,3-dimethyl- | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.000 | 0.002 | 0.003 | 0.000 | 0.000 | 0.000 |
| 2-Hydroxycyclopent-2-en-1-one | 0.013 | 0.013 | 0.022 | 0.033 | 0.027 | 0.012 | 0.011 | 0.006 | 0.004 | 0.020 | 0.008 | 0.025 |
| Pyridine, 3,5-dimethyl- | 0.000 | 0.015 | 0.000 | 0.000 | 0.000 | 0.014 | 0.008 | 0.016 | 0.019 | 0.004 | 0.009 | 0.002 |
| 2,5-Dimethyl-2-cyclopentenone | 0.004 | 0.005 | 0.010 | 0.009 | 0.011 | 0.007 | 0.005 | 0.000 | 0.000 | 0.015 | 0.000 | 0.012 |
| 2(3H)-furanone, 5-methyl- | 0.003 | 0.005 | 0.004 | 0.006 | 0.006 | 0.000 | 0.000 | 0.006 | 0.007 | 0.003 | 0.004 | 0.005 |
| Butanoic acid, 3-methyl- | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ethanol, 2-butoxy- | 0.009 | 0.006 | 0.008 | 0.012 | 0.009 | 0.011 | 0.011 | 0.000 | 0.000 | 0.007 | 0.000 | 0.011 |
| Benzaldehyde | 0.007 | 0.008 | 0.022 | 0.030 | 0.025 | 0.008 | 0.010 | 0.007 | 0.011 | 0.031 | 0.005 | 0.028 |
| Furfural, 5-methyl- | 0.037 | 0.061 | 0.052 | 0.084 | 0.080 | 0.052 | 0.057 | 0.058 | 0.062 | 0.060 | 0.058 | 0.076 |
| Pyridine, 3-ethenyl- | 0.012 | 0.024 | 0.020 | 0.016 | 0.023 | 0.018 | 0.017 | 0.025 | 0.029 | 0.031 | 0.012 | 0.025 |
| 2(5H)-furanone, 3-methyl- | 0.009 | 0.012 | 0.014 | 0.028 | 0.018 | 0.014 | 0.012 | 0.011 | 0.013 | 0.011 | 0.007 | 0.006 |
| Phenol | 0.063 | 0.125 | 0.128 | 0.162 | 0.156 | 0.108 | 0.102 | 0.122 | 0.130 | 0.128 | 0.090 | 0.125 |
| 2-Isopropylfuran | 0.008 | 0.011 | 0.013 | 0.009 | 0.016 | 0.017 | 0.000 | 0.016 | 0.015 | 0.014 | 0.000 | 0.012 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 0.030 | 0.023 | 0.033 | 0.029 | 0.039 | 0.012 | 0.012 | 0.057 | 0.031 | 0.000 | 0.027 | 0.037 |
| Limonene | 0.005 | 0.024 | 0.068 | 0.077 | 0.085 | 0.058 | 0.048 | 0.012 | 0.011 | 0.065 | 0.023 | 0.080 |
| 2,3-Dimethyl-2-cyclopenten-1-one | 0.021 | 0.022 | 0.023 | 0.035 | 0.043 | 0.020 | 0.027 | 0.033 | 0.026 | 0.038 | 0.016 | 0.040 |
| Indeno | 0.002 | 0.010 | 0.018 | 0.016 | 0.014 | 0.009 | 0.010 | 0.011 | 0.012 | 0.032 | 0.007 | 0.024 |
| o-Cresol | 0.026 | 0.037 | 0.030 | 0.044 | 0.047 | 0.042 | 0.039 | 0.042 | 0.040 | 0.034 | 0.029 | 0.033 |
| 2-Acetylpyrrole | 0.003 | 0.014 | 0.020 | 0.021 | 0.017 | 0.021 | 0.026 | 0.014 | 0.000 | 0.006 | 0.008 | 0.014 |
| Phenol, 4-methoxy- | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ethanone, 1-phenyl- | 0.005 | 0.007 | 0.016 | 0.010 | 0.014 | 0.011 | 0.011 | 0.009 | 0.010 | 0.012 | 0.005 | 0.013 |
| p-Cresol | 0.041 | 0.094 | 0.094 | 0.111 | 0.119 | 0.074 | 0.069 | 0.083 | 0.092 | 0.092 | 0.052 | 0.101 |
| 2-Ethyl thiophene | 0.003 | 0.000 | 0.000 | 0.000 | 0.023 | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Phenol, 2-methoxy- | 0.023 | 0.036 | 0.035 | 0.039 | 0.047 | 0.032 | 0.029 | 0.036 | 0.040 | 0.039 | 0.026 | 0.043 |
| 2-Propanamine | 0.010 | 0.011 | 0.014 | 0.015 | 0.018 | 0.009 | 0.008 | 0.000 | 0.000 | 0.019 | 0.000 | 0.014 |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 0.010 | 0.024 | 0.030 | 0.031 | 0.034 | 0.026 | 0.026 | 0.025 | 0.027 | 0.021 | 0.018 | 0.023 |
| Benzene acetonitrile | 0.004 | 0.009 | 0.012 | 0.011 | 0.013 | 0.009 | 0.007 | 0.011 | 0.013 | 0.020 | 0.005 | 0.013 |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 0.012 | 0.002 | 0.022 | 0.113 | 0.031 | 0.009 | 0.020 | 0.011 | 0.010 | 0.062 | 0.020 | 0.116 |
| Phenol, 2,4-dimethyl- | 0.007 | 0.009 | 0.000 | 0.000 | 0.000 | 0.016 | 0.016 | 0.000 | 0.000 | 0.027 | 0.000 | 0.020 |
| Phenol, 4-ethyl- | 0.000 | 0.017 | 0.032 | 0.013 | 0.039 | 0.000 | 0.013 | 0.018 | 0.019 | 0.014 | 0.013 | 0.023 |
| Naphthalene | 0.006 | 0.007 | 0.025 | 0.007 | 0.007 | 0.023 | 0.006 | 0.000 | 0.038 | 0.012 | 0.005 | 0.012 |
| Ethanone, 1-(3-methylphenyl)- | 0.003 | 0.011 | 0.025 | 0.015 | 0.017 | 0.022 | 0.009 | 0.000 | 0.000 | 0.000 | 0.010 | 0.000 |
| p-Cresol 2-methoxy | 0.002 | 0.011 | 0.006 | 0.007 | 0.007 | 0.010 | 0.005 | 0.006 | 0.007 | 0.000 | 0.004 | 0.013 |
| 2,3-Dihydro-benzofuran | 0.007 | 0.023 | 0.021 | 0.023 | 0.027 | 0.019 | 0.020 | 0.019 | 0.020 | 0.026 | 0.011 | 0.010 |
| 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 0.000 | 0.000 | 0.000 | 0.061 | 0.043 | 0.000 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 | 0.049 |
| 1H-inden-1-one, 2,3-dihydro- | 0.011 | 0.018 | 0.019 | 0.025 | 0.032 | 0.015 | 0.017 | 0.014 | 0.016 | 0.019 | 0.009 | 0.020 |
| Hydroquinone | 0.020 | 0.073 | 0.064 | 0.082 | 0.060 | 0.056 | 0.032 | 0.042 | 0.040 | 0.038 | 0.027 | 0.081 |
| 1H-indole | 0.019 | 0.053 | 0.037 | 0.087 | 0.083 | 0.043 | 0.057 | 0.067 | 0.061 | 0.038 | 0.041 | 0.044 |
| 4-Vinyl-2-methoxy-phenol | 0.013 | 0.016 | 0.025 | 0.020 | 0.037 | 0.016 | 0.013 | 0.022 | 0.017 | 0.026 | 0.011 | 0.028 |
| Nicotine | 1.094 | 2.661 | 2.116 | 1.833 | 1.985 | 1.554 | 1.445 | 1.241 | 1.414 | 2.024 | 0.957 | 1.750 |
| 1H-indole, 3-methyl- | 0.008 | 0.013 | 0.019 | 0.017 | 0.016 | 0.011 | 0.012 | 0.010 | 0.020 | 0.019 | 0.010 | 0.017 |
| Myosmine | 0.010 | 0.020 | 0.027 | 0.018 | 0.026 | 0.014 | 0.008 | 0.010 | 0.019 | 0.016 | 0.004 | 0.016 |
| Phenol, 2-methoxy-4-(2-propenyl)- | 0.006 | 0.009 | 0.008 | 0.012 | 0.017 | 0.008 | 0.005 | 0.008 | 0.006 | 0.014 | 0.005 | 0.007 |
| Nicotyrine | 0.003 | 0.014 | 0.021 | 0.016 | 0.018 | 0.014 | 0.010 | 0.009 | 0.010 | 0.013 | 0.006 | 0.014 |
| Norsolanadione | 0.003 | 0.013 | 0.016 | 0.018 | 0.020 | 0.012 | 0.013 | 0.011 | 0.010 | 0.013 | 0.007 | 0.018 |
| 2,3′-Bipyridine | 0.007 | 0.024 | 0.022 | 0.024 | 0.024 | 0.012 | 0.014 | 0.015 | 0.017 | 0.011 | 0.008 | 0.011 |
| 1,4-Dihydrophenantrhene | 0.000 | 0.016 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.005 | 0.011 | 0.000 | 0.014 |
| Megastigmatrienone | 0.006 | 0.024 | 0.000 | 0.000 | 0.000 | 0.019 | 0.018 | 0.014 | 0.000 | 0.009 | 0.000 | 0.015 |
| 2,4-Dimethyl-6-(2-furyl) pyridine | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.011 | 0.009 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 |
| N-propyl-nornicotine | 0.004 | 0.013 | 0.012 | 0.009 | 0.012 | 0.006 | 0.008 | 0.007 | 0.008 | 0.010 | 0.004 | 0.007 |
| Cotinine | 0.005 | 0.015 | 0.017 | 0.013 | 0.015 | 0.011 | 0.017 | 0.030 | 0.015 | 0.011 | 0.000 | 0.024 |
| 5-Tetradecene | 0.006 | 0.009 | 0.021 | 0.013 | 0.015 | 0.009 | 0.008 | 0.007 | 0.013 | 0.013 | 0.009 | 0.015 |
| N(β)-formylnornicotine | 0.004 | 0.008 | 0.019 | 0.010 | 0.011 | 0.007 | 0.006 | 0.005 | 0.009 | 0.000 | 0.002 | 0.008 |
| Neophytadiene | 0.078 | 0.248 | 0.205 | 0.160 | 0.215 | 0.139 | 0.151 | 0.150 | 0.181 | 0.184 | 0.133 | 0.244 |
| Farnesol | 0.006 | 0.013 | 0.021 | 0.014 | 0.013 | 0.014 | 0.015 | 0.027 | 0.009 | 0.019 | 0.012 | 0.018 |
| Hexadecanoic acid, ethyl ester | 0.000 | 0.014 | 0.005 | 0.012 | 0.027 | 0.010 | 0.014 | 0.009 | 0.000 | 0.008 | 0.000 | 0.012 |
| 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl- | 0.004 | 0.014 | 0.030 | 0.017 | 0.019 | 0.009 | 0.012 | 0.011 | 0.016 | 0.013 | 0.013 | 0.022 |
| Heptacosane | 0.009 | 0.026 | 0.042 | 0.030 | 0.029 | 0.025 | 0.023 | 0.025 | 0.026 | 0.026 | 0.016 | 0.029 |
| Triacontane | 0.004 | 0.040 | 0.044 | 0.048 | 0.043 | 0.030 | 0.042 | 0.034 | 0.045 | 0.027 | 0.022 | 0.025 |
| Octadecane | 0.012 | 0.071 | 0.100 | 0.089 | 0.094 | 0.064 | 0.081 | 0.070 | 0.079 | 0.050 | 0.052 | 0.056 |
| Tocopherol | 0.010 | 0.073 | 0.089 | 0.080 | 0.067 | 0.058 | 0.063 | 0.052 | 0.076 | 0.063 | 0.071 | 0.092 |
Table 7.
Yields of the different compounds analyzed in the TPM fraction.
| mg/g smoked tobacco | 3R4F | A | B | C | D | E | F | G | H | I | J | K |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pyridine, 4-methyl- | 0.003 | 0.003 | 0.001 | 0.002 | 0.003 | 0.001 | 0.000 | 0.003 | 0.002 | 0.000 | 0.000 | 0.000 |
| Pyrazine, methyl- | 0.002 | 0.001 | 0.001 | 0.002 | 0.002 | 0.001 | 0.000 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 |
| Furfural | 0.015 | 0.007 | 0.003 | 0.006 | 0.007 | 0.003 | 0.000 | 0.000 | 0.006 | 0.000 | 0.003 | 0.000 |
| 2-Pentanone, 4-hydroxy-4-methyl- | 0.009 | 0.000 | 0.004 | 0.012 | 0.001 | 0.003 | 0.004 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| Ethanol, 2-(1-methylethoxy)- | 0.005 | 0.005 | 0.005 | 0.006 | 0.007 | 0.004 | 0.006 | 0.002 | 0.000 | 0.010 | 0.003 | 0.009 |
| 2-Furanmethanol | 0.002 | 0.000 | 0.003 | 0.005 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Pyridine, 3-methyl- | 0.004 | 0.005 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 | 0.003 | 0.003 | 0.001 | 0.001 | 0.002 |
| 2-Propanone, 1-(acetyloxy)- | 0.005 | 0.002 | 0.001 | 0.002 | 0.002 | 0.001 | 0.002 | 0.002 | 0.001 | 0.002 | 0.001 | 0.002 |
| 4-Cyclopentene-1,3-dione | 0.004 | 0.002 | 0.007 | 0.003 | 0.004 | 0.003 | 0.005 | 0.004 | 0.001 | 0.000 | 0.001 | 0.000 |
| Styrene | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.003 | 0.001 | 0.003 |
| 2-Cyclopenten-1-one, 2-methyl- | 0.009 | 0.004 | 0.004 | 0.005 | 0.007 | 0.002 | 0.005 | 0.006 | 0.005 | 0.003 | 0.002 | 0.003 |
| 2-Acetylfuran | 0.004 | 0.003 | 0.001 | 0.001 | 0.002 | 0.002 | 0.003 | 0.003 | 0.002 | 0.002 | 0.001 | 0.002 |
| 2(5H)-furanone | 0.002 | 0.002 | 0.000 | 0.001 | 0.001 | 0.002 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 |
| Pyrazine, 2,3-dimethyl- | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2-Hydroxycyclopent-2-en-1-one | 0.005 | 0.002 | 0.003 | 0.004 | 0.004 | 0.002 | 0.003 | 0.001 | 0.001 | 0.004 | 0.001 | 0.004 |
| Pyridine, 3,5-dimethyl- | 0.001 | 0.002 | 0.000 | 0.000 | 0.000 | 0.001 | 0.002 | 0.002 | 0.002 | 0.000 | 0.001 | 0.000 |
| 2,5-Dimethyl-2-cyclopentenone | 0.003 | 0.001 | 0.001 | 0.001 | 0.003 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 |
| 2(3H)-furanone, 5-methyl- | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Butanoic acid, 3-methyl- | 0.000 | 0.002 | 0.007 | 0.012 | 0.007 | 0.004 | 0.008 | 0.006 | 0.000 | 0.005 | 0.000 | 0.006 |
| Ethanol, 2-butoxy- | 0.010 | 0.000 | 0.006 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.002 | 0.000 | 0.003 |
| Benzaldehyde | 0.004 | 0.001 | 0.001 | 0.003 | 0.003 | 0.001 | 0.002 | 0.002 | 0.002 | 0.001 | 0.000 | 0.001 |
| Furfural, 5-methyl- | 0.011 | 0.006 | 0.001 | 0.002 | 0.002 | 0.003 | 0.006 | 0.004 | 0.004 | 0.005 | 0.003 | 0.007 |
| Pyridine, 3-ethenyl- | 0.002 | 0.002 | 0.000 | 0.000 | 0.002 | 0.001 | 0.002 | 0.002 | 0.002 | 0.000 | 0.000 | 0.000 |
| 2(5H)-furanone, 3-methyl- | 0.002 | 0.002 | 0.001 | 0.001 | 0.001 | 0.001 | 0.002 | 0.001 | 0.001 | 0.000 | 0.001 | 0.000 |
| Phenol | 0.025 | 0.039 | 0.018 | 0.020 | 0.019 | 0.014 | 0.016 | 0.018 | 0.014 | 0.026 | 0.014 | 0.024 |
| 2-Isopropylfuran | 0.004 | 0.004 | 0.002 | 0.002 | 0.004 | 0.004 | 0.004 | 0.004 | 0.003 | 0.003 | 0.003 | 0.001 |
| 2-Cyclopenten-1-one, 2-hydroxy-3-methyl- | 0.017 | 0.014 | 0.000 | 0.000 | 0.000 | 0.006 | 0.008 | 0.007 | 0.005 | 0.000 | 0.005 | 0.000 |
| Limonene | 0.016 | 0.011 | 0.022 | 0.021 | 0.025 | 0.009 | 0.006 | 0.007 | 0.007 | 0.026 | 0.007 | 0.023 |
| 2,3-Dimethyl-2-cyclopenten-1-one | 0.005 | 0.006 | 0.004 | 0.008 | 0.007 | 0.003 | 0.005 | 0.004 | 0.003 | 0.007 | 0.003 | 0.008 |
| Indeno | 0.002 | 0.002 | 0.002 | 0.004 | 0.004 | 0.000 | 0.001 | 0.001 | 0.001 | 0.005 | 0.000 | 0.002 |
| o-Cresol | 0.016 | 0.016 | 0.007 | 0.011 | 0.009 | 0.009 | 0.011 | 0.009 | 0.009 | 0.008 | 0.008 | 0.017 |
| 2-Acetylpyrrole | 0.000 | 0.002 | 0.003 | 0.007 | 0.005 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Phenol, 4-methoxy- | 0.000 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Ethanone, 1-phenyl- | 0.000 | 0.000 | 0.002 | 0.002 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| p-Cresol | 0.023 | 0.037 | 0.018 | 0.022 | 0.017 | 0.017 | 0.019 | 0.017 | 0.018 | 0.028 | 0.015 | 0.031 |
| 2-Ethyl tiophene | 0.005 | 0.006 | 0.000 | 0.000 | 0.000 | 0.006 | 0.004 | 0.001 | 0.002 | 0.000 | 0.006 | 0.000 |
| Phenol, 2-methoxy- | 0.010 | 0.011 | 0.005 | 0.009 | 0.006 | 0.004 | 0.006 | 0.006 | 0.006 | 0.005 | 0.004 | 0.013 |
| 2-Propanamine | 0.009 | 0.009 | 0.007 | 0.008 | 0.004 | 0.005 | 0.005 | 0.000 | 0.000 | 0.011 | 0.004 | 0.014 |
| 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | 0.012 | 0.010 | 0.000 | 0.009 | 0.000 | 0.009 | 0.013 | 0.012 | 0.009 | 0.009 | 0.006 | 0.012 |
| Benzene acetonitrile | 0.000 | 0.000 | 0.005 | 0.007 | 0.010 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one | 0.021 | 0.039 | 0.046 | 0.080 | 0.083 | 0.012 | 0.023 | 0.006 | 0.004 | 0.058 | 0.026 | 0.093 |
| Phenol, 2,4-dimethyl- | 0.005 | 0.000 | 0.014 | 0.014 | 0.014 | 0.000 | 0.008 | 0.000 | 0.000 | 0.014 | 0.000 | 0.017 |
| Phenol, 4-ethyl- | 0.005 | 0.014 | 0.016 | 0.019 | 0.017 | 0.002 | 0.008 | 0.004 | 0.006 | 0.000 | 0.003 | 0.000 |
| Naphthalene | 0.004 | 0.002 | 0.000 | 0.000 | 0.003 | 0.004 | 0.012 | 0.015 | 0.013 | 0.000 | 0.006 | 0.000 |
| Ethanone, 1-(3-methylphenyl)- | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| p-Cresol 2-methoxy | 0.002 | 0.000 | 0.000 | 0.002 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| 2,3-Dihydro-benzofuran | 0.008 | 0.018 | 0.017 | 0.015 | 0.020 | 0.012 | 0.010 | 0.011 | 0.010 | 0.017 | 0.010 | 0.021 |
| 2-Furancarboxaldehyde, 5-(hydroxymethyl)- | 0.000 | 0.000 | 0.006 | 0.044 | 0.032 | 0.000 | 0.013 | 0.000 | 0.000 | 0.005 | 0.018 | 0.033 |
| 1H-inden-1-one, 2,3-dihydro- | 0.012 | 0.012 | 0.008 | 0.009 | 0.021 | 0.002 | 0.007 | 0.004 | 0.003 | 0.000 | 0.004 | 0.000 |
| Hydroquinone | 0.031 | 0.072 | 0.033 | 0.080 | 0.053 | 0.049 | 0.091 | 0.053 | 0.027 | 0.084 | 0.040 | 0.146 |
| 1H-indole | 0.024 | 0.070 | 0.036 | 0.049 | 0.061 | 0.057 | 0.044 | 0.044 | 0.043 | 0.017 | 0.026 | 0.014 |
| 4-Vinyl-2-methoxy-phenol | 0.007 | 0.008 | 0.016 | 0.009 | 0.017 | 0.006 | 0.005 | 0.007 | 0.006 | 0.025 | 0.006 | 0.024 |
| Nicotine | 1.897 | 3.599 | 2.741 | 2.409 | 2.545 | 2.345 | 2.088 | 2.354 | 2.743 | 3.147 | 2.188 | 2.839 |
| 1H-indole, 3-methyl- | 0.005 | 0.014 | 0.010 | 0.010 | 0.010 | 0.008 | 0.006 | 0.005 | 0.010 | 0.015 | 0.007 | 0.024 |
| Myosmine | 0.013 | 0.031 | 0.024 | 0.014 | 0.016 | 0.017 | 0.013 | 0.012 | 0.016 | 0.026 | 0.004 | 0.029 |
| Phenol, 2-methoxy-4-(2-propenyl)- | 0.007 | 0.009 | 0.007 | 0.004 | 0.009 | 0.007 | 0.004 | 0.003 | 0.006 | 0.025 | 0.004 | 0.006 |
| Nicotyrine | 0.007 | 0.022 | 0.017 | 0.005 | 0.006 | 0.011 | 0.012 | 0.008 | 0.013 | 0.014 | 0.008 | 0.010 |
| Norsolanadione | 0.010 | 0.023 | 0.014 | 0.012 | 0.014 | 0.015 | 0.014 | 0.012 | 0.018 | 0.018 | 0.010 | 0.016 |
| 2,3′-Bipyridine | 0.010 | 0.022 | 0.023 | 0.018 | 0.014 | 0.014 | 0.016 | 0.015 | 0.019 | 0.019 | 0.013 | 0.018 |
| 1,4-Dihydrophenantrhene | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.018 | 0.000 | 0.018 |
| Megastigmatrienone | 0.006 | 0.026 | 0.010 | 0.010 | 0.009 | 0.007 | 0.012 | 0.009 | 0.010 | 0.015 | 0.021 | 0.024 |
| 2,4-Dimethyl-6-(2-furyl) pyridine | 0.000 | 0.010 | 0.000 | 0.000 | 0.000 | 0.017 | 0.012 | 0.009 | 0.015 | 0.000 | 0.005 | 0.000 |
| N-propyl-nornicotine | 0.007 | 0.013 | 0.012 | 0.009 | 0.010 | 0.006 | 0.013 | 0.009 | 0.009 | 0.010 | 0.008 | 0.011 |
| Cotinine | 0.017 | 0.021 | 0.022 | 0.011 | 0.018 | 0.023 | 0.026 | 0.033 | 0.030 | 0.019 | 0.013 | 0.026 |
| 5-Tetradecene | 0.008 | 0.011 | 0.018 | 0.011 | 0.013 | 0.010 | 0.010 | 0.010 | 0.010 | 0.016 | 0.008 | 0.023 |
| N(β)-formylnornicotine | 0.015 | 0.021 | 0.013 | 0.017 | 0.021 | 0.018 | 0.016 | 0.020 | 0.024 | 0.024 | 0.009 | 0.021 |
| Neophytadiene | 0.120 | 0.297 | 0.202 | 0.147 | 0.198 | 0.160 | 0.174 | 0.161 | 0.181 | 0.241 | 0.165 | 0.331 |
| Farnesol | 0.010 | 0.017 | 0.020 | 0.010 | 0.015 | 0.018 | 0.022 | 0.028 | 0.011 | 0.036 | 0.018 | 0.029 |
| Hexadecanoic acid, ethyl ester | 0.000 | 0.017 | 0.000 | 0.019 | 0.024 | 0.012 | 0.013 | 0.014 | 0.006 | 0.009 | 0.005 | 0.011 |
| 2,6,10,14,18,22-Tetracosahexaene, 2,6,10,15,19,23-hexamethyl- | 0.013 | 0.020 | 0.030 | 0.019 | 0.020 | 0.015 | 0.010 | 0.010 | 0.015 | 0.023 | 0.017 | 0.022 |
| Heptacosane | 0.021 | 0.031 | 0.041 | 0.030 | 0.030 | 0.038 | 0.030 | 0.032 | 0.024 | 0.032 | 0.023 | 0.032 |
| Triacontane | 0.013 | 0.051 | 0.060 | 0.043 | 0.040 | 0.053 | 0.053 | 0.038 | 0.045 | 0.037 | 0.032 | 0.042 |
| Octadecane | 0.032 | 0.081 | 0.122 | 0.087 | 0.084 | 0.110 | 0.109 | 0.074 | 0.083 | 0.076 | 0.065 | 0.090 |
| Tocopherol | 0.037 | 0.111 | 0.096 | 0.066 | 0.077 | 0.112 | 0.088 | 0.049 | 0.088 | 0.119 | 0.105 | 0.146 |
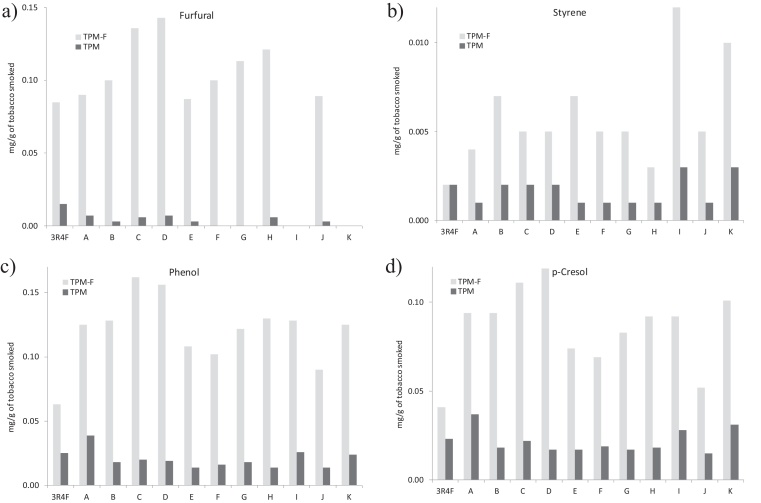
The general distribution of the lightest compounds between filters and traps agrees with that observed for the overall TPM-F + TPM yields from RYO, shown in Table 2. The distribution of the heaviest compounds however follows the same tendency as the overall TPM-F + TPM from 3R4F, and also that of Nicotine, as already commented (i.e., the behavior of nicotine resembles more that of the heaviest compounds). Fig. 4 shows the cases of furfural, styrene, phenol and p-cresol, as examples of this type of behavior, even although, in view of the respective retention time in the chromatograms, p-cresol cannot be classified within the lightest compounds of the mixture. As Fig. 4 reflects, in general, 3R4F yields lower amounts of these types of compounds in filters than RYO tobaccos, but it yields larger amounts in the traps. Brands I and K yield almost no furfural whereas they yield the largest amounts of Styrene.
Fig. 4.
Examples of some compounds showing higher yields in TPM-F than in TPM. (a) Furfural, (b) styrene, (c) phenol and (d) p-cresol.
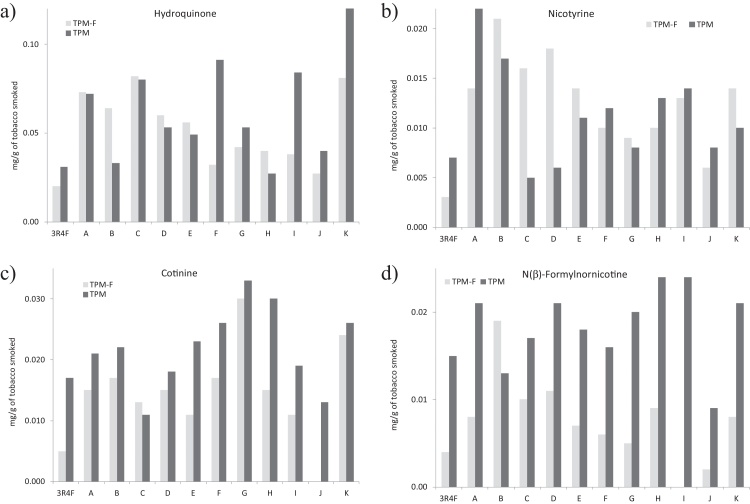
Fig. 5 shows the behavior of hydroquinone, nicotyrine, and especially cotinine and N(β)-formylnornicotine, as examples of heavier compounds being more concentrated in the traps than in the filters. Once again 3R4F is one of the tobaccos yielding the lowest amounts in the filters, whereas in the traps the yields obtained for this tobacco are closer to the average yields. According to Borgerding and Klus [8], the Hydroquinone yield is more sensitive to the tobacco blend type than other smoke constituents and, as can be seen in Table 6, Table 7 and in Fig. 5, it is delivered to a much higher extent in traps, where its value varies between 0.027 and 0.146 mg/g of smoked tobacco, for brands H and K, respectively. It is worth mentioning that the yields of the heaviest compounds obtained in TPM from the reference tobacco are usually lower than that of the rest of RYO tobacco brands.
Fig. 5.
Examples of some compounds showing higher yields in TPM than in TPM-F. (a) Hydroquinone, (b) nicotyrine, (c) cotinine and (d) N(β)-formylnornicotine.
In order to facilitate the comparison of the compounds found in the filter and in the traps among the different brands, the series of compounds listed in Table 6, Table 7 have been grouped by families, according to their chemical functionality, in the same manner as described elsewhere [31]. The families considered, arranged according to the established priority, are the following: nitrogenous, carbonylic, phenolic, epoxies, aromatics, aliphatics and polyaromatic hydrocarbons (PAHs). Nicotine belongs to the nitrogenous compounds group and its behavior has been already discussed in a previous section. The yields of Nicotine are as high as the sum of all the other compounds, thus it has not been included in its group in order to allow a clearer discussion of the behavior of the other groups. The results obtained for the yields of the different chemical families in the filters and in the traps corresponding to the 3R4F reference are shown in Table 8, Table 9. These tables also include the ratio between the yield of each family when smoking each RYO brand and the yield of the same family when smoking the 3R4F reference tobacco.
Table 8.
Yields of the different chemical family compounds analyzed in the TPM-F fraction from the 3R4F reference and ratio between the yield of each family from each RYO brand and from 3R4F.
| 3R4F, mg/g of smoked tobacco | (mg/mg of smoked RYO tobacco)/(mf/g 3R4F smoked tobacco) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | ||
| Nitrogenous | 0.124 | 2.369 | 2.365 | 2.347 | 2.655 | 2.110 | 2.070 | 2.363 | 2.461 | 1.950 | 1.110 | 1.813 |
| Carbonylics | 0.375 | 1.248 | 1.812 | 2.439 | 2.249 | 1.351 | 1.339 | 1.500 | 1.430 | 1.343 | 1.043 | 1.748 |
| Epoxies | 0.025 | 1.364 | 2.372 | 3.263 | 3.686 | 2.038 | 1.841 | 2.446 | 1.399 | 2.230 | 0.434 | 2.354 |
| Aromatics | 0.002 | 2.210 | 4.429 | 2.728 | 2.753 | 3.939 | 2.797 | 2.958 | 1.645 | 14.302 | 2.824 | 13.455 |
| Others | 0.032 | 1.538 | 3.356 | 3.733 | 4.479 | 2.809 | 3.011 | 1.233 | 0.619 | 3.614 | 1.115 | 3.960 |
| PAHs | 0.007 | 4.322 | 5.689 | 3.023 | 2.813 | 4.263 | 2.195 | 1.516 | 7.322 | 7.339 | 1.617 | 6.624 |
| Phenolics | 0.213 | 2.346 | 2.394 | 2.678 | 2.803 | 1.967 | 1.812 | 2.022 | 2.194 | 2.235 | 1.545 | 2.647 |
| Aliphatics | 0.113 | 3.646 | 3.913 | 3.160 | 3.680 | 2.469 | 2.802 | 2.666 | 3.193 | 2.780 | 2.176 | 3.457 |
Table 9.
Yields of the different chemical family compounds analyzed in the TPM fraction from the 3R4F reference and ratio between the yield of each family from each RYO brand and from 3R4F.
| 3R4F, mg/g of smoked tobacco | (mg/mg of smoked RYO tobacco)/(mf/g 3R4F smoked tobacco) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | ||
| Nitrogenous | 0.120 | 1.9799 | 1.4584 | 1.3288 | 1.5097 | 1.4107 | 1.2953 | 1.2922 | 1.4643 | 1.2933 | 0.7834 | 1.4134 |
| Carbonylics | 0.157 | 1.0254 | 0.7543 | 1.3701 | 1.3086 | 0.4902 | 0.8194 | 0.5046 | 0.4970 | 0.8316 | 0.6705 | 1.3230 |
| Epoxies | 0.014 | 1.6149 | 1.6326 | 1.5594 | 1.9604 | 1.1419 | 1.0319 | 1.0739 | 0.9650 | 1.4517 | 0.8775 | 1.5586 |
| Aromatics | 0.005 | 0.1999 | 0.2909 | 0.3507 | 0.3880 | 0.1993 | 0.2458 | 0.1963 | 0.2050 | 0.4463 | 0.1038 | 0.5097 |
| Others | 0.046 | 0.8809 | 1.2647 | 1.0772 | 1.2679 | 0.8951 | 0.9875 | 0.9588 | 0.4473 | 1.7115 | 0.7182 | 1.5188 |
| PAHs | 0.006 | 0.6924 | 0.4098 | 0.7056 | 1.2311 | 0.6751 | 2.3152 | 2.9265 | 2.4031 | 3.9875 | 1.0825 | 3.7058 |
| Phenolics | 0.168 | 1.9253 | 1.3715 | 1.5248 | 1.4370 | 1.3037 | 1.5284 | 0.9893 | 1.0763 | 1.9874 | 1.1837 | 2.5226 |
| Aliphatics | 0.207 | 2.3634 | 2.2753 | 1.6184 | 1.8566 | 1.8654 | 1.8610 | 1.5655 | 1.7247 | 2.0493 | 1.4937 | 2.6068 |
As already commented on for the individual compounds, 3R4F is the tobacco yielding the lowest amounts of the different families, as indicated by the fact that most of the ratios in Table 8 are higher than one, with the average value of the yield ratio in filters around 3. With regard to the TPM-F, tobacco J is the one showing the lowest yields among all the RYO brands, except for aromatics, and provides lower yields of epoxies than the 3R4F tobacco. Contrarily the aromatics are the compounds that this brand yields in higher amounts in relation to the 3R4F tobacco. Brand I is at the other end providing the larger amounts of aromatics and PAH. According to Table 8 it appears that RYO brands generally provide much higher yields in the filters of the group of others and PAH, in comparison with 3R4F. As can be seen in Table 9, the behavior observed for TPM is somewhat different. All brands including the 3R4F yield similar results and the average value of all ratios is around 1.3. Moreover, it can also be observed that the number of families and/or brands showing lower values than the reference tobacco (i.e., ratios lower than 1) is larger than in the case of TPM-F. According to these results, all the studied RYO brands provide noticeably lower yields of aromatics than 3R4F.
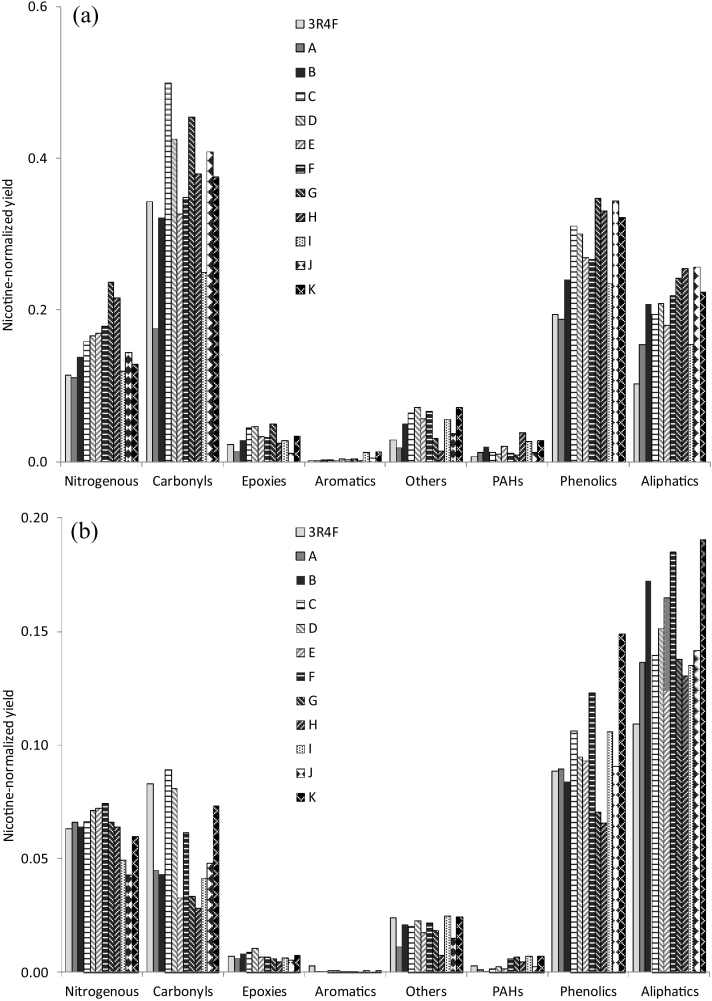
Some authors use the yields expressed as mass of component per mg of nicotine, i.e., the nicotine-normalized yields in order to compare commercial brand styles [7], because of the interest in focusing attention on nicotine, which is mainly responsible for the addictive character of smoking tobacco. Fig. 6 shows the nicotine-normalized yields obtained for the studied RYO brands and the 3R4F reference, thus allowing the comparison with respect to this reference tobacco. The first question that merits a comment is that all these ratios are lower than one, showing the already commented on fact that nicotine is the major compound appearing both in TPM and TPM-F. The data represented in Fig. 6 indicate that the different RYO brand tobaccos studied in this work generate in general higher amounts of the considered chemical families with respect to nicotine than those containing the 3R4F reference. In other words, the overall TPM of the RYO tobaccos seems to be more harmful than that of the reference tobacco per mg of nicotine inhaled. As stated previously, the nitrogenous group includes other nitrogen-containing compounds other than nicotine. Regarding TPM-F (Fig. 6a), the only cases where RYO tobaccos show lower yields than the reference tobacco are: almost all the families of brand A, carbonyls of brands B, E and I and other compounds of brand H. With respect to the TPM, the nicotine yields obtained tend to be lower than those in TPM-F. In general, they tend to be also higher for the RYO tobaccos than for the reference tobacco, but in this case there are many more cases that deviate from this behavior.
Fig. 6.
Ratio between the nicotine-normalized yields obtained in (a) the TPM-F and (b) the TPM for the different chemical families in the RYO brands studied and the 3R4F reference.
4. Conclusions
RYO tobaccos present lower amounts of condensed products in the traps than in the filters. In general, the yields of TPM are in the range 18–22 mg/g of smoked tobacco. However, the opposite effect is observed for 3R4F because the amount of TPM obtained from 3R4F is higher than TPM-F. Moreover, the total of TPM (TPM + TPM-F) for the reference tobacco is lower than for RYO tobaccos, with the only exception of brands C and J. The yields of nicotine are in the ranges of 0.96–2.66 and 1.90–3.60 mg/g of tobacco smoked in TPM-F and TPM, respectively, with brand A being the one yielding the highest value. Nicotine may tend to be more concentrated in the traps than in the filters.
The total gas yields ranged from 4.66 to 6.98 mg/g of tobacco, with the 3R4F reference being very close to the highest end. In fact, with the only exceptions of pentane, hexane, acetaldehyde, 2,5-dimethylfuran and isobutiraldehyde, most of the other 26 compounds analyzed in the vapor phase are obtained in lower amounts from the different RYO tobaccos than from 3R4F.
Regarding the heaviest compounds, whose main tendency is to appear more concentrated in TPM than in TPM-F, 3R4F is one of the tobaccos yielding the lowest amounts in the filters, whereas in the traps the yields obtained for this tobacco are closer to the average yields. On average, the yield of the different chemical families of compounds appearing in TPM-F tends to be around three times higher than those obtained from 3R4F. Among the RYO tobacco brands, J is the one showing the lowest yields except for aromatics, and brand I is at the other end providing the larger amounts of aromatics and PAH. However, all RYO brands as well as the 3R4F tobacco show very similar yields of the considered chemical families of compounds appearing in TPM, where all the studied RYO brands provide noticeably lower yields of aromatics than 3R4F.
Considering the results obtained it could be concluded that the RYO brands studied deliver more nicotine and most of the products than in the condensed fraction than the 3R4F reference tobacco. Only the gas fraction seems to be lower for these tobaccos than for the reference one, despite there being no significant differences between the CO yielded by 3R4F and the 11 RYO brands. Accordingly it can be stated that RYO tobaccos are definitively not less hazardous than the reference tobacco, which may be contrary to popular belief.
Transparency document
Acknowledgements
Financial support for this investigation has been provided by the Spanish “Secretaría de Estado de Investigación” del Ministerio de Ciencia e Innovación (CTQ2008-01023) and Generalitat Valenciana (PROMETEO/2012/015).
Footnotes
Available online 22 May 2014
Contributor Information
A. Marcilla, Email: antonio.marcilla@ua.es.
A. Gómez-Siurana, Email: amparo.gomez@ua.es.
References
- 1.Adam T., Ferge T., Mitschke S., Streibel T., Baker R.R., Zimmermann R. Discrimination of three tobacco types (Burley, Virginia and Oriental) by pyrolysis single-photon ionisation-time-of-flight mass spectrometry and advanced statistical methods. Anal. Bioanal. Chem. 2005;381:487–499. doi: 10.1007/s00216-004-2935-0. [DOI] [PubMed] [Google Scholar]
- 2.Appel R.B.R., Guirguis G., Kim I.S., Garbin O., Fraccia M., Flessel C.P., Kizer K.W., Book S.A., Warriner T.E. Benzene, benzo(α)pyrene and lead in smoke from tobacco products other than cigarettes. Am. J. Public Health. 1990;80(5):560–564. doi: 10.2105/ajph.80.5.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayo-Yusuf O.A., Olutol B.G. “Roll-your-own” cigarette smoking in South Africa between 2007 and 2010. BMC Public Health. 2013;13:597–603. doi: 10.1186/1471-2458-13-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker R.R., Bishop L.J. The pyrolysis of tobacco ingredients. J. Anal. Appl. Pyrolysis. 2004;71:223–311. [Google Scholar]
- 6.Benjakul S., Termsirikulchai L., Hsia J., Kenggapanich M., Puckcharern H., Touchchai C., Lohtongmongkol A., Andes L., Asma S. Current manufacture cigarette smoking and roll-your-own cigarette smoking in Thailand: findings from the 2009 global adult tobacco survey. BMC Public Health. 2013;13:277–283. doi: 10.1186/1471-2458-13-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bodnar J.A., Morgan W.T., Murphy P.A., Ogden M.W. Mainstream smoke chemistry analysis of samples from the 2009 US cigarette market. Regul. Toxicol. Pharmacol. 2012;64:35–42. doi: 10.1016/j.yrtph.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Borgerding M., Klus H. Analysis of complex mixtures–cigarette smoke. Exp. Toxicol. Pathol. 2005;57:43–73. doi: 10.1016/j.etp.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 9.Busch C., Streibel T., Liu C., McAdam K.G., Zimmermann R. Pyrolysis and combustion of tobacco in a cigarette smoking simulator under air and nitrogen atmosphere. Anal. Bioanal. Chem. 2012;403:419–430. doi: 10.1007/s00216-012-5879-9. [DOI] [PubMed] [Google Scholar]
- 10.Castaño Calduch T., Hernert Giménez C., Campo San Segundo M.T., Ysa Valle M., Pons Carlos-Roca A. Fine-cut tobacco: a priority for public health and consumer advocacy. Gac. Sanit. 2012;26(3):267–269. doi: 10.1016/j.gaceta.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Chen M., Xu Z., Chen G., Ge S., Yin C., Zhou Z., Sun W., Li Y., Zhong F. The generation of carbon monoxide and carbonyl compounds in reconstituted tobacco sheet. J. Therm. Anal. Calorim. 2014;115(1):961–970. [Google Scholar]
- 12.Comisionado para el Mercado de Tabacos, Ministerio de Hacienda y Administraciones Públicas, Gobierno de España. Estadísticas. Ranking por marcas y por kilos, http://www.cmtabacos.es/wwwcmt/paginas/ES/mercadoEstadisticas.tmpl (accessed 02.04.14).
- 13.Dagnon S., Stoilova A., Ivanov I., Nikolova S. The effect of cigarette design parameters on the content of phenols in mainstream tobacco smoke, Beitr. Tabakforsch. 2011;24(4):187–193. [Google Scholar]
- 14.Darral K.G., Figgins J.A. Roll-your-own smoke yields: theoretical and practical aspects. Tob. Control. 1998;7:168–175. doi: 10.1136/tc.7.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Djulančić N., Radojičić V., Srbinovska M. The influence of tobacco blend composition on carbon monoxide formation in mainstream cigarette smoke. Arh. Hig. Rada Toksikol. 2013;64:107–113. doi: 10.2478/10004-1254-64-2013-2250. [DOI] [PubMed] [Google Scholar]
- 17.Gobierno de España, REAL DECRETO 1079/2002, de 18 de octubre, por el que se regulan los contenidos máximos de nicotina, alquitrán y monóxido de carbono de los cigarrillos, el etiquetado de los productos del tabaco, así como las medidas relativas a ingredientes y denominaciones de los productos del tabaco, BOE núm. 251 de 19 de octubre de 2002.
- 18.Granda-Orive J.I., Jiménez-Ruiz C.A. Some thoughts on hand-rolled cigarette. Arch. Bronconeumol. 2011;47(9):425–426. doi: 10.1016/j.arbres.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Gregg E., Hill C., Hollywoood M., Kearney M., McAdam K., McLaughlin D., Purkis S., Willliams M. The UK Smoke constituents testing study. Summary of results and comparison with other studies. Beitr. Tabakforsch. 2004;21(2):117–138. [Google Scholar]
- 20.Hertz R., Streibel S., Liu C., McAdam K., Zimmermann R. Microprobe sampling-photo ionization-time-of-flight mass spectrometry for in situ chemical analysis of pyrolysis and combustion gases: examination of the thermo-chemical processes within a burning cigarette. Anal. Chim. Acta. 2012;714:104–113. doi: 10.1016/j.aca.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 21.ISO 3308 . 2000. Routine analytical cigarette-smoking machine – definitions and standard conditions. [Google Scholar]
- 22.ISO 3402 . 1991. Tobacco and tobacco products – atmosphere for conditioning and testing. [Google Scholar]
- 23.ISO 4387 . 2000. Cigarettes – determination of total and nicotine-free dry particulate matter using a routine analytical smoking machine. [Google Scholar]
- 24.Kaiserman M.J., Rickert W.S. Handmade cigarettes: it's de tube that counts. Am. J. Public health. 1992;82(1):107–109. doi: 10.2105/ajph.82.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalaitzoglou M., Samara C. Gas/particle partitioning and yield levels of polycyclic aromatic hydrocarbons and n-alkanes in the mainstream cigarette smoke of commercial cigarette brands. Food Chem. Toxicol. 2006;44:1432–1442. doi: 10.1016/j.fct.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 26.Leatherdale S.T., Burkhalter R. Roll-your-own tobacco use among Canadian youth: is it a bigger problem than we think? BMC Public Health. 2012;12:557–563. doi: 10.1186/1471-2458-12-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu H., Zhu L. Pollution patterns of polycyclic aromatic hydrocarbons in tobacco smoke. J. Hazard. Mater. 2007;139:193–198. doi: 10.1016/j.jhazmat.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Marcilla A., Martínez I., Berenguer D., Gómez-Siurana A., Beltrán M.I. Comparative study of the main characteristics and composition of the mainstream smoke of ten cigarette brands sold in Spain. Food Chem. Toxicol. 2012;50:1317–1333. doi: 10.1016/j.fct.2012.01.046. [DOI] [PubMed] [Google Scholar]
- 31.Marcilla A., Gómez-Siurana A., Berenguer D., Martínez-Castellanos I., Beltrán M.I. Reduction of tobacco smoke components yields by zeolites and synthesized Al-MCM-41. Microporous Mesoporous Mater. 2012;161:14–24. [Google Scholar]
- 32.Pandey S.K., Kim K.H. A review of environmental tobacco smoke and its determination in air. Trends Anal. Chem. 2010;29:804–819. [Google Scholar]
- 33.Piadé J.-J., Wajrock S., Jaccard G., Janeke G. Formation of mainstream cigarette smoke constituents prioritized by the World Health Organization – yield patterns observed in market surveys, clustering and inverse correlations. Food Chem. Toxicol. 2013;55:329–347. doi: 10.1016/j.fct.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Reference Cigarette Program – UK College of Agriculture, 3R4F Preliminary Analysis, http://www2.ca.uky.edu/refcig/ (accessed 04.04.14).
- 35.Roemer E., Schramke H., Weiler H., Buettner A., Kausche S., Weber S., Berges A., Stueber M., Muench M., Trelles-Sticken E., Pype J., Kohlgrueber K., Voelkel H., Wittke S. Mainstream smoke chemistry and in vitro and in vivo toxicity of the reference cigarettes 3R4F and 2R4F. Beitr. Tabakforsch. 2012;25(1):316–335. [Google Scholar]
- 36.Rosenberry Z.R., Strasses A.A., Canlas L.L., Potts J.L., Pickwort W.B. Make your own cigarettes: characteristics of the product and the consumer. Nicotine Tob. Res. 2013;15(8):1453–1457. doi: 10.1093/ntr/nts271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siu M., Mladjenovich N., Soo E. The analysis of mainstream smoke emissions of Canadian “super slim” cigarettes. Tob. Control. 2013;22(6):e10. doi: 10.1136/tobaccocontrol-2012-050450. [DOI] [PubMed] [Google Scholar]
- 39.Torikai K., Yoshida S., Takasaki H. Effects of temperature, atmosphere and pH on the generation of smoke compounds during tobacco pyrolysis. Food Chem. Toxicol. 2004;42:1409–1417. doi: 10.1016/j.fct.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 40.Uchiyama S., Tomizawa T., Inaba Y., Kunugita N. Simultaneous determination of volatile organic compounds and carbonyls in mainstream cigarette smoke using a sorbent cartridge followed by two-step elution. J. Chromatogr. A. 2013;1314:31–37. doi: 10.1016/j.chroma.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Young D., Borland R., Hammond D., Cummings K.M., Devlin E., Yong H.H., O’Connor R.J. Prevalence and attributes of roll-your-own smokers in the International Tobacco Control (ITC) Four Country Survey. Tob. Control. 2006;15(Suppl. III):iii76–iii82. doi: 10.1136/tc.2005.013268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Young D., Yong H.H., Borland R., Shahab L., Hammond D., Cummings K.M., Wilson N. Trends in roll-your-own smoking: findings from the ITC four-country survey (2002–2008) J. Environ. Public Health. 2012 doi: 10.1155/2012/406283. (article ID: 406283) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.