Graphical abstract
Chemical compounds studied in this article: (E)-Nerolidol (Pubchem CID: 5284507), Spathulenol (Pubchem CID: 92231), (E)-Caryophyllene (PubchemCID: 5281515), δ-Cadinene (Pubchem CID: 12306055), Viridiflorene (Pubchem CID: 10910653), ⿿-Copaene (PubchemCID:70678558), Aromadendrene (Pubchem CID: 91354), ⿿-trans-Bergamotene (Pubchem CID: 6429302)
Keywords: SMART, Essential oil, Propolis, Genotoxicity, Antifungal activity, Antibacterial activity
Highlights
-
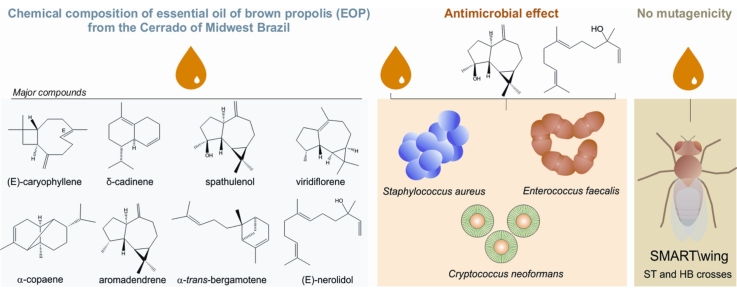
Sesquiterpenes were the prevalent class in the essential oil of propolis from the Cerrado biome in Midwest Brazil (EOP).
-
The mutagenic and antimicrobial potentials of EOP and its chemical composition were investigated for the first time.
-
EOP was not mutagenic to Drosophila melanogaster larvae, even when tested against the high-bioactivation (HB) cross.
-
The antimicrobial activities of EOP are not associated to DNA damage induction (SMART assay).
-
(E)-Nerolidol and spathulenol isolated from EOP showed antimicrobial activity.
Abstract
Biological, and particularly antimicrobial, activities have been demonstrated for the essential oil of propolis samples worlwide, yet their mutagenic effects remain unknown. To correlate antimicrobial effects with mutagenic risks, the present study evaluated the antifungal and antibacterial activities of the essential oil obtained from brown propolis collected from the Cerrado biome in Midwest Brazil (EOP), testing it against nine pathogenic microorganisms. Evaluation of mutagenic potential was based on the somatic mutation and recombination test (SMART) performed on wing cells of standard (ST) and high-bioactivation (HB) crosses of Drosophila melanogaster. EOP was extracted by hydrodistillation, and sesquiterpenes were characterized by GC⿿MS as its major constituents. The crude oil proved active against Cryptococcus neoformans and Enterococcus faecalis, as did two of its major constituents, spathulenol and (E)-nerolidol ⿿ the latter being also active against Staphylococcus aureus ⿿ isolated using chromatographic procedures. No significant increase in the number of somatic mutations was observed in the offspring of ST or HB crosses ⿿ the latter exhibiting enhanced levels of metabolizing enzymes of the cytochrome P450 type ⿿ treated with 0.05%, 0.1%, and 0.2% EOP. These findings revealed no mutagenic activity of EOP, even when tested against the HB strain, and demonstrated that its antimicrobial activities are not associated with DNA damage induction (investigated with SMART), suggesting the potential of EOP as a natural preservative.
1. Introduction
Bees have the ability to collect plant compounds that can protect the beehive against microorganisms. Propolis (bee glue) is one of the protective products resulting from this behavior [1]. Comprising fixed and volatile constituents, propolis has a highly variable chemical composition, depending on local vegetation [1], [2], [3]. This variability has encouraged the investigation of extracts and essential oils obtained from propolis samples from different regions, particularly biomes threatened with extinction, among them the Brazilian Cerrado (a tropical savanna hotspot) in Mato Grosso do Sul state, Midwest Brazil. Brazilian propolis samples have been the subject of continuous scientific research and shown to be potential providers of bioactive agents, including those with antimicrobial and (anti)genotoxic properties [4], [5], [6], [7]. Despite the relative scarcity of studies on the essential oils of propolis (EOP) from different world regions, a wider variability in chemical composition has been found in EOP than among non-volatile constituents [3]. To date, however, EOP has not been investigated for its mutagenic potential. Evaluating the genetic toxicology of natural products, such as EOP, employed in the pharmaceutical and food industries, as well as in traditional medicine, is crucial for a more balanced picture of their risks and benefits to humans and other living organisms. Also, investigating the mutagenic potential of EOP allows its mechanism of action to be elucidated, revealing possible correlations between antimicrobial activity and genotoxicity.
In addition to evaluating the genotoxic potential of the essential oil extracted from a propolis sample collected from Mato Grosso do Sul state (using the somatic mutation and recombination test⿿SMART) and its antifungal and antibacterial properties, the present study investigated the main chemical composition of this material.
SMART uses two Drosophila melanogaster marker genes for the shape of wing hairs ⿿ termed multiple wing hairs (mwh, 3-0.3) and flare (flr3, 3-38.8) ⿿ and is based on inducing genetic changes that prevent heterozygosis from occurring in larval cells heterozygous for these two recessive genes [8]. When genetic changes take place in cells undergoing mitosis, a clone of mutant cells is generated, which can be phenotypically detected as a mutant spot on the surface of adult wings. The induced lesions are detected as cell clusters (mutant clones) phenotypically expressing marker genes flr3 or mwh, responsible for changing the shape of trichomes [8]. The test identifies agents that induce point mutations, chromosomal changes, recombinations, deletions, translocations, and aneuploidies, among other phenomena. The genome sequencing of D. melanogaster revealed high evolutionary conservation, compared with the human genome, not only in terms of DNA sequences, but particularly with regard to genic functions, while having high genetic homology. Data obtained from proteome analysis are equally important, since 60% of the 289 genes implicated in human diseases have homologs in Drosophila, while 75% have similar protein sequences in both organisms [9], thus revealing a high conservation between biochemical routes and regulatory functions in both species [10]. Given these similarities, Drosophila is considered a suitable model for the study of genotoxicity and its molecular mechanisms [11].
2. Materials and methods
2.1. Essential oil extraction, GC⿿MS analysis, and isolation of (E)-nerolidol and spathulenol
Fresh brown propolis (500 g) produced by Apis mellifera L. in the Cerrado landscape in Campo Grande, Mato Grosso do Sul, was purchased from Apiário Vovô Pedro (Campo Grande, MS, Brazil) in January, 2010.
The essential oil of propolis (EOP) was obtained by hydrodistillation in a Clevenger-type apparatus, and stored at 4 °C until time of GC⿿MS and bioassays. GC⿿MS analysis was carried out on a gas chromatograph coupled to a mass spectrometer (Shimadzu QP2010 Plus) equipped with an Rtx capillary column (30 m ÿ 0.25 mm, 0.25 μm film thickness), employing helium as the carrier gas at a flow rate of 1 mL min⿿1. The injector temperature was 220 °C. The oven temperature was programmed to increase by 3 °C min⿿1 from 60 to 240 °C, and the split ratio was set at 1:10. MS was recorded in electron impact mode at 70 eV; ion source temperature was 240 °C and full-scan spectra were acquired over the range of 40⿿500 u. Calculation of the percentages of oil components was based on measurements of normalized GC peak areas in relation to the total area of all the sample constituents. Individual constituents were identified by comparison of their GC retention indices (RI, relative to the retention times of a series of C8⿿C21 n-alkanes) [12] and mass spectra with those from the MS library (NIST05/FFNSC) and also from the literature for MS/RI data [13], [14]. C8⿿C20 and C21 n-alkane standards were purchased from Sigma and Fluka, respectively.
EOP (252.8 mg) was chromatographed on a silica gel 230⿿400 mesh (Merck) column (30 cm ÿ 2.5 cm) eluted with hexane, hexane:EtOAc 2%, and hexane:EtOAc 10% to yield 157 fractions (F1⿿F157) of 10 mL each. (E)-Nerolidol (1, 6.8 mg) and spathulenol (2, 5.2 mg) were obtained from F135 and F148, respectively. 1H and 13C NMR spectra of 1 and 2 were obtained in CDCl3 (Cambridge Isotope Laboratories) on a Bruker DPX-300 spectrometer operating at 300.13 MHz (1H)/75.47 MHz (13C) equipped with a 5 mm probe. Chemical shifts are reported in ppm, using TMS as an internal standard (dδ = 0 ppm), and coupling constants (J) are expressed in hertz.
2.2. Antimicrobial assays
The antifungal and antibacterial evaluations employed the following strains from the American Type Culture Collection (ATCC, Rockville, MD, USA): Candida albicans (ATCC 90028), Candida krusei (ATCC 6258), Candida parapsilosis (ATCC 22019), Cryptococcus neoformans (ATCC 32045), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Klebsiella pneumoniae (ATCC 700603), Enterococcus faecalis (ATCC 29218), and Staphylococcus aureus (ATCC 25923). Amphotericin B and chloramphenicol (Sigma) were used as the reference antimycotic and antibacterial controls, respectively.
A broth microdilution method was employed to determine minimum inhibitory concentrations (MICs) of EOP, and compounds 1 and 2, based on Clinical and Laboratory Standards Institute guidelines for fungi and bacteria [15], [16]. The tests were performed in plates containing Mueller⿿Hinton agar and RPMI-1640 (Cultilab) supplemented with glutamine, devoid of sodium bicarbonate, and amended with phenol red and antibiotics against bacterial and fungal strains, respectively.
EOP was dissolved in 5% dimethylsulfoxide to obtain a 1000 μg/mL stock solution. The solution was poured into 96-well microplates and diluted to yield final concentrations of 1000, 500, 250, 125, 62.5, 31.25, 16.625, and 7.8125 μg/mL. Compounds 1 and 2 were solubilized to achieve a 200 μg/mL stock solution and subsequently diluted to concentrations of 200, 100, 50, 25, 12.5, 6.25, 3.12, and 1.56 μg/mL.
The strains were suspended in saline solution (0.9%) to obtain a density of 108 CFU/mL (0.5 McFarland turbidity standard). The plates were incubated at 35 °C for 24 h (bacterial strains), 35 °C for 48 h (Candida strains), and 35 °C for 72 h (C. neoformans). The control tests were performed simultaneously, using amphotericin B and chloramphenicol in 5% dimethylsulfoxide. MIC was defined as the lowest concentration of essential oil at which a microorganism does not exhibit visible growth. The tests were performed in duplicate and repeated once. To improve the accuracy of MIC determination, the broth dilution method using resazurin (Sigma) to detect microorganism viability was also employed, according to standard protocols [17], as follows: after the plates were read, 30 μL of resazurin solution in sterile distilled water (0.1 mg/mL) was added to each well and the plates incubated at 35 °C for 2 h for visual evaluation of color shift. A shift from purple to pink indicates reduction of resazurin, revealing microbial growth. MIC was defined as the lowest concentration of essential oil preventing this color shift.
2.3. Mutagenicity assays
Three mutant D. melanogaster strains, provided by Dr. Mário Antônio Spanó, of the Universidade Federal de Uberlândia, Brazil, were maintained in a light⿿dark incubator at 23 ± 1 °C and 60⿿70% relative humidity: (1) multiple wing hairs (mwh), with genetic constitution y;mwh jv; (2) flare (flr3), with genetic constitution flr3/In(3LR)TM3 ri pp sep l(3) 89Aa bx34e e BdS; and (3) ORR; flare-3, with genetic constitution ORR; flr3/In(3LR)TM3, ri pp sep l(3) 89Aa bx34e e BdS (the last of which has chromosomes 1 and 2 derived from the DDT-resistant Oregon R strain, characterized by a high level of enzymes of the cytochrome P450 (CYP450) type, conferring high sensitivity to promutagens and procarcinogens) [18].
For SMART, two crossings were performed⿿namely, (1) standard (ST), in which virgin flr3 females were selected and crossed with mwh males [8], and (2) high bioactivation (HB), from virgin ORR/flr3 females and mwh males [19].
For chronic treatment, third-instar larvae of ST and HB crosses were collected and transferred to glass vials containing 1.5 g of alternative culture medium (instant mashed potato flakes, Yoki Alimentos, Brazil) rehydrated with 5 mL of EOP (0.05%, 0.1%, or 0.2%). Both assays included a negative control (Milli-Q water with 1% Tween-40 and 3% ethanol) and a positive control, the genotoxic agent doxorubicin hydrochloride (DXR, Glenmark Farmacêutica), at 0.125 mg/mL. The experiments were carried out at 23 ± 1 °C under 60⿿70% relative humidity.
The following progenies were obtained: marker-heterozygous (MH) (mwh+/+flr3) flies, with wild wing phenotype, and balancer-heterozygous (BH) (mwh+/+TM3, BdS) flies, with serrated wing edges resulting from the balancing chromosome.
After feeding on the culture medium and completing the metamorphosis cycle, the emerging adults were collected and fixed in 70% ethanol. Male and female MH individuals were separated and their respective wings were arranged in pairs on slides, fixed in Faure's solution (30 g of gum arabic, 50 g of chloral hydrate, 100 mL of water, and 20 mL of glycerol), and their ventral and dorsal surfaces were observed under an optical microscope (400ÿ magnification).
Cells with a missing wild allele develop hairs with a mutant phenotype during the pupal stage, and can be easily distinguished from other cells [20]. Mutant spots were classified as (1) small single spots (one or two mwh or flr3 spots), (2) large single spots (three or more mwh or flr3 spots), which can result from different genetic events⿿namely, mitotic recombination, point mutation, and chromosomal aberration⿿or (3) twin spots (both mwh and flr3 genotypes), produced by mitotic recombination of the flr3 proximal marker and the centromere of chromosome 3 [8], [20].
The data collected were tabulated by type and size of mutant spots and subjected to statistical treatment as described by Frei and Würgler [21], to yield the following statistical diagnoses: positive, weakly positive, inconclusive, or negative.
The frequency of each type of mutant spot per wing in a treated series was compared with its corresponding negative control using the conditional binomial test of Kastenbaum and Bowman [22]. To assess negative results, multiplication factors (m) were introduced in the test, as follows: m = 2 for single small spots and total spots, given their high spontaneous frequency, and m = 5 for single large spots and twin spots, which rarely arise spontaneously [8], [21]. The criterion for positive diagnosis was that the frequency of mutations resulting from treatment had to be m times higher than that obtained in the negative control [23].
The frequency of clone formation per 105 cells per cell division was calculated as n/NC, where n is the total number of mutant clones, N is the total number of wings and C is the approximate number of cells per wing.
3. Results
EOP was obtained by hydrodistillation, with a yield of 0.07% (w/w). GC⿿MS (Table 1) revealed 31 compounds present at concentrations higher than 0.1%, 93.5% of which belonged to the sesquiterpene class, with a predominance of hydrocarbon sesquiterpenes. Most structural types belonged to the caryophyllane, cadinane, aromadendrane, copaane, and farnesane classes, with (E)-caryophyllene (7.85%), δ-cadinene (7.67%), spathulenol (6.65%), viridiflorene (4.52%), α-copaene (4.01%), aromadendrene (3.85%), α-trans-bergamotene (3.73%), and (E)-nerolidol (3.72%) as major constituents.
Table 1.
Chemical composition of essential oil of propolis (EOP) from the Cerrado biome in Midwest Brazil.
| Compoundsa, b | RIc | RI lit.d | Composition (%) |
|---|---|---|---|
| α-Pinene | 939 | 933 | 0.15 |
| α-Cubebene | 1351 | 1351 | 0.62 |
| α-Ylangene | 1372 | 1375 | 0.43 |
| α-Copaene | 1377 | 1376 | 4.01 |
| 7-epi-Sesquithujene | 1391 | 1391 | 0.17 |
| β-Elemene | 1393 | 1390 | 0.45 |
| α-Gurjunene | 1410 | 1409 | 1.98 |
| α-cis-Bergamotene | 1415 | 1415 | 0.11 |
| (E)-Caryophyllene | 1418 | 1418 | 7.85 |
| β-Gurjunene | 1433 | 1432 | 0.25 |
| α-trans-Bergamotene | 1436 | 1434 | 3.73 |
| Aromadendrene | 1439 | 1441 | 3.85 |
| α-Himachalene | 1449 | 1451 | 0.50 |
| α-Humulene | 1454 | 1454 | 1.24 |
| allo-Aromadendrene | 1461 | 1460 | 2.01 |
| γ-Muurolene | 1477 | 1479 | 2.49 |
| Germacrene D | 1481 | 1480 | 2.92 |
| α-Curcumene | 1482 | 1480 | 1.21 |
| β-Selinene | 1486 | 1485 | 1.74 |
| δ-Selinene | 1491 | 1492 | 0.38 |
| Viridiflorene | 1495 | 1496 | 4.52 |
| α-Muurolene | 1500 | 1499 | 1.72 |
| β-Bisabolene | 1508 | 1505 | 1.72 |
| β-Curcumene | 1511 | 1515 | 0.57 |
| γ-Cadinene | 1514 | 1513 | 1.99 |
| δ-Cadinene | 1524 | 1523 | 7.67 |
| trans-Cadina-1,4-diene | 1533 | 1534 | 0.37 |
| α-Cadinene | 1538 | 1538 | 0.27 |
| (E)-Nerolidol | 1565 | 1563 | 3.72 |
| Spathulenol | 1580 | 1578 | 6.65 |
| Allyl-3-prenylcinnamate | 2022 | 2016 | 0.70 |
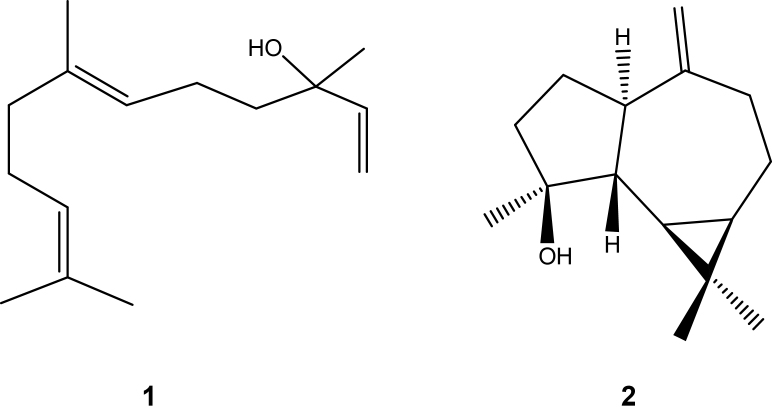
In the present study, two of the predominant constituents ⿿ the sesquiterpene alcohols (E)-nerolidol (1) and spathulenol (2) (Fig. 1) ⿿ were isolated by chromatographic procedures and identified using 1H and 13C NMR techniques and by comparing with authentic samples or published data [24]. The antimicrobial properties of these two constituents, along with those of the crude essential oil, were evaluated in vitro against four fungal and five bacterial strains, by measuring their respective MICs using the broth microdilution method (Table 2). The crude oil proved active against C. neoformans and E. faecalis (MIC = 250 and 500 μg/mL, respectively), as did compounds 1 and 2 (MIC = 200 μg/mL for both compounds and both microorganisms), while 1 also exhibited activity against S. aureus (MIC = 200 μg/mL).
Fig. 1.
Structures of sesquiterpenes (E)-nerolidol (1) and spathulenol (2) isolated from EOP.
Table 2.
Antifungal and antibacterial activities of EOP and (E)-nerolidol (1) and spathulenol (2) (MIC values in μg/mL).
| Microbial strains | EOP | 1 | 2 | Controla | Controlb |
|---|---|---|---|---|---|
| Cryptococcus neoformans | 250 | 200 | 200 | 0.25 | ⿿ |
| Candida albicans | >1000 | >200 | >200 | 0.50 | ⿿ |
| C. krusei | >1000 | >200 | >200 | 0.25 | ⿿ |
| C. parapsilosis | >1000 | >200 | >200 | 0.25 | ⿿ |
| Enterococcus faecalis | 500 | 200 | 200 | ⿿ | 0.50 |
| Staphylococcus aureus | >1000 | 200 | >200 | ⿿ | 0.50 |
| Escherichia coli | >1000 | >200 | >200 | ⿿ | 0.50 |
| Pseudomonas aeruginosa | >1000 | >200 | >200 | ⿿ | 8.00 |
| Klebsiella pneumoniae | >1000 | >200 | >200 | ⿿ | 4.00 |
Amphotericin B.
Chloramphenicol.
To examine whether the antimicrobial activity of the present EOP sample was directly associated with DNA damage, the genotoxic potential of the crude oil was evaluated by SMART, performed on wing cells of D. melanosgaster, using ST and HB crosses (the latter is characterized by high levels of CYP, rendering it highly sensitive to promutagens and procarcinogens). As shown in Table 3, the frequencies of formation of mutant spots in the offspring of ST and HB crosses treated with three EOP concentrations (0.05, 0.1, and 0.2%) in independent experiments did not differ significantly from those obtained for negative and positive controls. These results revealed that EOP was not mutagenic to somatic cells of D. melanogaster larvae at the doses tested, even after metabolization (as demonstrated for the HB cross, which has high metabolic bioactivation).
Table 3.
Frequencies of mutant spots on wings of D. melanogaster MH descendants derived from standard (ST) and high bioactivation (HB) crosses, after chronic treatment of larvae with different concentrations of EOP.
| Genotypes and concentration (%) |
Number of flies (N) | Spots per fly (number of spots) statistical diagnosisa |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Small single spots (1⿿2 cells)bm = 2 | Large single spots (>2 cells)bm = 5 |
Twin spots m = 5 | Total spots m = 2 | Spots with mwh clonesc (n) |
Frequency of clone formation per 105 cells per cell divisiond (n/NC)e |
|||||||||||
| mwh/flr3 | Fr | N | D | Fr | N | D | Fr | N | D | Fr | N | D | Cf | |||
| ST | ||||||||||||||||
| DXR | 40 | 1.95 | (39) | + | 1.50 | (30) | + | 1.35 | (27) | + | 4.80 | (96) | + | |||
| Negative control | 20 | 0.70 | (14) | 0 | (0) | 0 | (0) | 0.70 | (14) | 14 | 1.43 | |||||
| EOP (0.05) | 40 | 0.18 | (7) | ⿿ | 0 | (0) | i | 0.03 | (1) | i | 0.20 | (8) | ⿿ | 08 | 0.41 | ⿿1.02 |
| EOP (0.1) | 40 | 0.18 | (7) | ⿿ | 0.08 | (3) | i | 0 | (0) | i | 0.25 | (10) | ⿿ | 10 | 0.51 | ⿿0.92 |
| EOP (0.2) | 40 | 0.38 | (15) | ⿿ | 0 | (0) | i | 0.05 | (2) | i | 0.43 | (17) | ⿿ | 17 | 0.87 | ⿿0.56 |
| HB | ||||||||||||||||
| DXR | 40 | 0.80 | (16) | + | 0.70 | (14) | + | 0.60 | (12) | + | 2.10 | (42) | + | |||
| Negative control | 40 | 0.60 | (24) | 0.05 | (2) | 0 | (1) | 0.65 | (26) | 26 | 1.54 | |||||
| EOP (0.05) | 40 | 0.70 | (28) | ⿿ | 0.05 | (2) | i | 0 | (0) | i | 0.75 | (30) | ⿿ | 30 | 1.33 | 0.20 |
| EOP (0.1) | 40 | 0.45 | (18) | ⿿ | 0.13 | (5) | i | 0.03 | (1) | i | 0.40 | (16) | ⿿ | 15 | 1.43 | ⿿0.56 |
| EOP (0.2) | 40 | 0.68 | (27) | ⿿ | 0.13 | (5) | i | 0.05 | (2) | i | 0.85 | (34) | i | 34 | 1.54 | 0.41 |
Statistical diagnosis (D) according to Frei and Würgler [21]: +, positive; w+, weakly positive; ⿿, negative; i, inconclusive. m, multiplication factor for assessment of significantly negative results. Significance levels: α = β = 0.05 when compared with respective control. Fr: frequency. N: number of spots.
Including rare flr3 single spots.
Considering mwh clones from mwh single and twin spots.
Only single mwh spots were observed in heterozygous mwh/TM3 individuals, since TM3 balancer chromosome does not carry the mutant flr3 gene.
Induction frequencies corrected for spontaneous incidence estimated from negative controls.
C = 48 800 (approximate number of examined cells per fly).
4. Discussion
Very low percentages of volatile compounds (generally up to 1%) can be found in propolis samples worldwide [3], as occurred with the yield obtained in the present study for the essential oil of propolis (EOP) from the Cerrado biome in Midwest Brazil (0.07%).
Sesquiterpene-type compounds, characterized as the main components of EOP, were also shown to predominate in essential oil samples of propolis from Southeast Brazil investigated by Albuquerque et al. [25], and Oliveira et al. [26]. However, monoterpenes were also described as propolis volatile compounds from the country⿿s Northeast and South regions [27], [28]. This variability in composition can be explained by differences in local flora biodiversity [3].
In the present study, EOP, as well as two of its predominant constituents⿿(E)-nerolidol (1) and spathulenol (2), isolated using chromatographic procedures, showed antimicrobial properties against C. neoformans and E. faecalis while 1 was also active against S. aureus. The MIC values obtained for 1 and 2 were comparable to or lower than those for crude oil tested against the same microorganisms, suggesting that 1 and 2 are two of the principal bioactive constituents of the essential oil.
Compound 1 is known for its inhibitory activity against E. coli and S. aureus and its efficacy as a topical antifungal agent against Microsporum gypseum in a guinea pig model [29], [30]. The activity of 2 against S. aureus, S. epidermidis, and E. coli has also been reported ⿿ e.g., in a study of the essential oil of Helichrysum fulgidum [31] ⿿ corroborating the attribution to sesquiterpenes (93.5% of the compounds identified) of the antimicrobial effect observed for the present EOP sample. Although the remaining major propolis compounds identified by GC⿿MS in the present sample, namely (E)-caryophyllene, δ-cadinene, viridiflorene, α-copaene, aromadendrene, and α-trans-bergamotene, were not individually tested, δ-cadinene and caryophyllene have been associated with the antimicrobial activity of essential oils [32], [33].
Some known antimicrobial agents have been reported to promote DNA damage [34]⿿including strand breakage as a consequence of oxidative stress, either in mammalian or microbial genomes [35], [36], [37]. Although sesquiterpenes are known for their wide range of biological activities, including antimicrobial and antigenotoxic activities, literature reports the induction of DNA damage by sesquiterpene-type compounds with antimicrobial properties [38], [39]. However, the present study demonstrated that, although bearing antimicrobial activity, EOP did not show any genotoxicity at the doses tested, even after metabolization (HB cross), unlike genotoxic effects reported for some alcoholic extracts of propolis at higher concentrations [5], [40], [41]. These effects might be due to the complex chemical composition of crude propolis extracts, which are known to contain flavonoids and their glycosides, prenylated and non-prenylated cinnamic acid derivatives, non-volatile diterpenes, among other compounds [2], [42], however absent in propolis essential oils. In fact, the principal compounds identified in EOP (hydrocarbon and oxygenated sesquiterpenes) have not been reported elsewhere as inducing DNA damage. For instance, sesquiterpene trans-caryophyllene (also termed β-caryophyllene or (E)-caryophyllene) showed no mutagenicity when evaluated in bacterial reverse-mutation assays on Salmonella typhimurium his⿿ TA98 and TA100 or on E. coli strains [43], [44], and also no increased micronuclei frequency in human lymphocytes, protecting these cells against the genotoxic damage induced by ethyl methanesulfonate [45]. Copaiba oil-resin extracted from the trunk of Copaifera sp., which contains high levels of (E)-caryophyllene, in addition to other sesquiterpenes identified in EOP, produced no genotoxic effects in mice using the micronucleus and comet assays [46]. Copaene, other main component of EOP, was not genotoxic when evaluated on human lymphocytes using sister chromatid exchange and micronucleus assays [47]. At high doses, (E)-nerolidol (also termed trans-nerolidol) has been shown to induce clastogenicity in mouse cells and to be very weakly genotoxic to them, albeit not cytotoxic on comet and micronucleus assays [48]. A mixture of cis- and trans-nerolidol, however, was not mutagenic on reverse-mutation tests using S. typhimurium his⿿ TA 98 and TA 100 strains [44]. (E)-nerolidol was reported to predominate in the essential oil of Piper gaudichaudianum in a study that also demonstrated the cytotoxic effects of the crude oil and a mixture of cis- and trans-nerolidol against Saccharomyces cerevisiae, despite an absence of mutagenicity [49]. Similarly, data obtained in the present study suggest that the (E)-nerolidol concentration found in EOP did not induce mutagenicity in a D. melanogaster model.
5. Conclusions
This pioneering evaluation of the chemical composition of EOP from the Cerrado biome in Midwest Brazil, along with the investigation of its mutagenic and antimicrobial potentials, revealed that the crude oil and two of its constituents ⿿ (E)-nerolidol and spathulenol ⿿ exhibited antimicrobial properties. Also, EOP was not mutagenic to somatic cells of D. melanogaster larvae, even when tested against the HB strain, which has high metabolic bioactivation. These results indicate the antimicrobial potential of EOP and show that its antifungal and antibacterial properties are not associated with induction of DNA damage (SMART assay), thus suggesting the potential utility of employing volatile constituents of propolis from the Cerrado landscape of Midwest Brazil as natural preservatives. Further in vivo experiments are warranted to confirm the absence of potential mutagenic risks posed by EOP.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Transparency document
Acknowledgements
The authors are grateful to Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia de Mato Grosso do Sul-FUNDECT-MS (0155/10) and Conselho Nacional de Desenvolvimento Científico e Tecnológico-CNPq (478016/2010-7) for their financial support and to CNPq and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the scholarships awarded. Thanks are also due to Mario Antônio Spanó (Universidade Federal de Uberlândia, Brazil) for providing the mutant D. melanogaster lines.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.11.007
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Bankova V.S., Castro S.L., Marcucci M.C. Propolis: recent advances in chemistry and plant origin. Apidologie. 2000;31:3–15. [Google Scholar]
- 2.Pereira A.S., Seixas F.R.M.S., Aquino Neto F.R. Propolis: 100 years of research and future perspectives. Quím. Nova. 2002;25:321–326. [Google Scholar]
- 3.Bankova V., Popova M., Trusheva B. Propolis volatile compounds: chemical diversity and biological activity: a review. Chem. Central J. 2014;18:28–35. doi: 10.1186/1752-153X-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavares D.C., Lira W.M., Santini C.B., Takahashi C.S., Bastos J.K. Effects of propolis crude hydroalcoholic extract on chromosomal aberrations induced by doxorubicin in rats. Planta Med. 2007;73:1531–1536. doi: 10.1055/s-2007-993737. [DOI] [PubMed] [Google Scholar]
- 5.Pereira A.D., de Andrade S.F., de Oliveira Swerts M.S., Maistro E.L. First in vivo evaluation of the mutagenic effect of Brazilian green propolis by comet assay and micronucleus test. Food Chem. Toxicol. 2008;46:2580–2584. doi: 10.1016/j.fct.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Salomão K., Pereira P.R.S., Campos L.C., Borba C.M., Cabello P.H., Marcucci M.C., Castro S.L. Brazilian propolis: correlation between chemical composition and antimicrobial activity. Evid. Based Complement. Alternat. Med. 2008;5:317–324. doi: 10.1093/ecam/nem058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Santos G.S., Tsutsumi S., Vieira D.P., Bartolini P., Okazaki K. Effect of Brazilian propolis (AF-08) on genotoxicity, cytotoxicity and clonogenic death of Chinese hamster ovary (CHO-K1) cells irradiated with (60)Co gamma-radiation. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2014;762:17–23. doi: 10.1016/j.mrgentox.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Graf U., Würgler F.E., Katz A.J., Frei H., Juon H., Hall C.B., Kale P.G. Somatic mutation and recombination test in Drosophila melanogaster. Environ. Mutagen. 1984;6:153–188. doi: 10.1002/em.2860060206. [DOI] [PubMed] [Google Scholar]
- 9.Tinckoo S., Russell S. Drosophila melanogaster as a model system for drug discovery and pathway screening. Curr. Opin. Pharmacol. 2002;2:555–560. doi: 10.1016/s1471-4892(02)00206-0. [DOI] [PubMed] [Google Scholar]
- 10.St. John M.A.R., Xu T. Understanding human cancer in a fly? Am. J. Hum. Genet. 1997;61:1006–1010. doi: 10.1086/301619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bier E. Drosophila the golden bug, emerges as a tool for human genetics. Nat. Rev. Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- 12.Van den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas⿿liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/s0021-9673(01)80947-x. [DOI] [PubMed] [Google Scholar]
- 13.Adams R.P. 4th ed. Allured Pub. Corp; Carol Stream: 2009. Identification of essential oil components by gas chromatography/mass spectroscopy. [Google Scholar]
- 14.Fernandes-Silva C.C., Lima C.A., Negri G., Salatino M.L.F., Salatino A., Mayworm M.A.S. Composition of the volatile fraction of a sample of Brazilian green propolis and its phytotoxic activity. J. Sci. Food Agric. 2015 doi: 10.1002/jsfa.7045. [DOI] [PubMed] [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards . National Committee for Clinical Laboratory Standards; Wayne, PA: 2002. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard. [Google Scholar]
- 16.National Committee for Clinical Laboratory Standards . NCCLS; Wayne, PA: 2003. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard. [Google Scholar]
- 17.Alves E.G., Vinholis A.H.C., Casemiro L.A., Furtado N.A.J.C., Silva M.L.A., Cunha W.R., Martins C.H.G. Comparative study of screening techniques for antibacterial activity evaluation of plant crude extracts and pure compounds. Quím. Nova. 2008;31:1224–1229. [Google Scholar]
- 18.Hällström I., Blanck A. Genetic regulation of the cytochrome P-450 system in Drosophila melanogaster. I. Chromosomal determination of some cytochrome P-450-dependent reactions. Chem. Biol. Interact. 1985;56:157–171. doi: 10.1016/0009-2797(85)90003-1. [DOI] [PubMed] [Google Scholar]
- 19.Graf U., van Schaik N. Improved high bioactivation cross for the wing somatic mutation and recombination test in Drosophila melanogaster. Mutat. Res. Environ. Mutagen. Relat. Subj. 1992;271:59–67. doi: 10.1016/0165-1161(92)90032-h. [DOI] [PubMed] [Google Scholar]
- 20.Guzmán-Rincón J., Graf U. Drosophila melanogaster somatic mutation and recom bination test as a biomonitor. In: Butterworth F.M., Corkum L.D., Guzmán-Rincón J., editors. Biomonitors and Biomarkers as Indicators of Environmental Change: A Handbook. Plenum Press; New York: 1995. pp. 169–181. [Google Scholar]
- 21.Frei H., Würgler F.E. Statistical methods to decide whether mutagenicity test data from Drosophila assays indicate a positive, negative, or inconclusive result. Mutat. Res. Environ. Mutagen. Relat. Subj. 1988;203:297–308. doi: 10.1016/0165-1161(88)90019-2. [DOI] [PubMed] [Google Scholar]
- 22.Kastenbaum M.A., Bowman K.O. Tables for determining the statistical significance of mutation frequencies. Mutat. Res. Fund. Mol. Mech. Mutagen. 1970;9:527–549. doi: 10.1016/0027-5107(70)90038-2. [DOI] [PubMed] [Google Scholar]
- 23.Andrade H.H.R., Reguly M.L., Lehmann M. Drosophila Cytogenetics Protocols. Humana Press; New Jersey: 2004. Wing somatic mutation and recombination test; pp. 389–412. [DOI] [PubMed] [Google Scholar]
- 24.Miyazawa M., Nankai H., Kameoka H. Biotransformations of acyclic terpenoids, (±)-trans-nerolidol and geranylacetone, by Glomerella cingulata. J. Agric. Food Chem. 1996;44:1543–1547. [Google Scholar]
- 25.Albuquerque I.L., Alves L.A., Lemos T.L., Dorneles C.A., de Morais M.O. Constituents of the essential oil of Brazilian green propolis from Brazil. J. Essent. Oil Res. 2008;20:414–415. [Google Scholar]
- 26.Oliveira A.P., França H.S., Kuster R.M., Teixeira L.A., Rocha L.M. Chemical composition and antibacterial activity of Brazilian propolis essential oil. J. Venom. Anim. Toxins Trop. Dis. 2010;16:121–130. [Google Scholar]
- 27.Torres R.N.S., Lopes J.A.D., Moita Neto J.M., Citó A.M.G.L. Constituintes voláteis de própolis piauiense. Quím. Nova. 2008;31:479–485. [Google Scholar]
- 28.Simionatto E., Facco J.T., Morel A.F., Giacomelli S.R., Linares C.E.B. Chiral analysis of monoterpenes in volatile oils from propolis. J. Chil. Chem. Soc. 2012;57:1240–1243. [Google Scholar]
- 29.Brehm-Stecher B.F., Johnson E.A. Sensitization of Staphylococcus aureus and Escherichia coli to antibiotics by the sesquiterpenoids nerolidol, farnesol, bisabolol, and apritone. Antimicrob. Agents Chemother. 2003;47:3357–3360. doi: 10.1128/AAC.47.10.3357-3360.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S.-J., Han J.-I., Lee G.-S., Park M.-J., Choi I.-G., Na K.-J., Jeung E.-B. Antifungal effect of eugenol and nerolidol against Microsporum gypseum in a guinea pig model. Biol. Pharm. Bull. 2007;30:184–188. doi: 10.1248/bpb.30.184. [DOI] [PubMed] [Google Scholar]
- 31.Bougatsos C., Ngassapa O., Runyoro D.K., Chinou I.B. Chemical composition and in vitro antimicrobial activity of the essential oils of two Helichrysum species from Tanzania. Z. Naturforsch. C. 2004;59:368–372. doi: 10.1515/znc-2004-5-614. [DOI] [PubMed] [Google Scholar]
- 32.Pérez-López A., Cirio A.T., Rivas-Galindo V.M., Aranda R.S., de Torres N.W. Activity against Streptococcus pneumoniae of the essential oil and δ-cadinene isolated from Schinus molle fruit. J. Essent. Oil Res. 2011;23:25–28. [Google Scholar]
- 33.Xiong L., Peng C., Zhou Q.-M., Wan F., Xie X.-F., Guo L., Li X.-H., He C.-J., Dai O. Chemical composition and antibacterial activity of essential oils from different parts of Leonurus japonicus Houtt. Molecules. 2013;18:963–973. doi: 10.3390/molecules18010963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomé S., Bizarro C.R., Lehmann M., de Abreu B.R.R., de Andrade H.H.R., Cunha K.S., Dihl R.R. Recombinagenic and mutagenic activities of fluoroquinolones in Drosophila melanogaster. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2012;742:43–47. doi: 10.1016/j.mrgentox.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 35.Lamore S.D., Cabello C.M., Wondrak G.T. The topical antimicrobial zinc pyrithione is a heat shock response inducer that causes DNA damage and PARP-dependent energy crisis in human skin cells. Cell Stress Chap. 2010;15:309–322. doi: 10.1007/s12192-009-0145-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neto J.B.A., da Silva C.R., Neta M.A.S., Campos R.S., Siebra J.T., Silva R.A.C., Gaspar D.M., Magalhães H.I.F., de Moraes M.O., Lobo M.D.P., Grangeiro T.B., Carvalho T.S.C., Diogo E.B.T., da Silva Júnior E.N., Rodrigues F.A.R., Cavalcanti B.C., Júnior H.V.N. Antifungal activity of naphthoquinoidal compounds in vitro against fluconazole-resistant strains of different Candida species: a special emphasis on mechanisms of action on Candida tropicalis. PLoS One. 2014;9:e93698. doi: 10.1371/journal.pone.0093698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mandal S.M., Chakraborty A., Hossain M., Mahata D., Porto W.F., Chakraborty R., Mukhopadhyay C.K., Franco O.L., Hazra T.K., Basak A. Amphotericin and anidulafungin directly interact with DNA and induce oxidative damage in the mammalian genome. Mol. BioSyst. 2015;11:2551–2559. doi: 10.1039/c5mb00366k. [DOI] [PubMed] [Google Scholar]
- 38.Looi C.Y., Arya A., Cheah F.K., Muharram B., Leong K.H., Mohamad K., Wong W.F., Rai N., Mustafa M.R. Induction of apoptosis in human breast cancer cells via caspase pathway by vernodalin isolated from Centratherum anthelminticum (L.) seeds. PLoS One. 2013;8:e56643. doi: 10.1371/journal.pone.0056643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arghiani N., Matin M.M., Bahrami A.R., Iranshahi M., Sazgarnia A., Rassouli F.B. Investigating anticancer properties of the sesquiterpene ferutinin on colon carcinoma cells, in vitro and in vivo. Life Sci. 2014;109:87–94. doi: 10.1016/j.lfs.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Montoro A., Soriano J.M., Barquinero J.F., Almonacid M., Montoro A., Verdú G., Sahuquillo V., Villaescusa J.I., Sebastià N. Assessment in vitro of cytogenetic and genotoxic effects of propolis on human lymphocytes. Food Chem. Toxicol. 2012;50:216–221. doi: 10.1016/j.fct.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 41.Fernandes F.H., da Rosa Guterres Z., Garcez W.S., Lopes S.M., Corsino J., Garcez F.R. Assessment of the (anti) genotoxicity of brown propolis extracts from Brazilian Cerrado biome in a Drosophila melanogaster model. Food Res. Int. 2014;62:20–26. [Google Scholar]
- 42.Salatino A., Fernandes-Silva C.C., Righi A.A., Salatino M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011;28:925–936. doi: 10.1039/c0np00072h. [DOI] [PubMed] [Google Scholar]
- 43.Di Sotto A., Evandri M.G., Mazzanti G. Antimutagenic and mutagenic activities of some terpenes in the bacterial reverse mutation assay. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2008;653:130–133. doi: 10.1016/j.mrgentox.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Gonçalves O., Pereira R., Gonçalves F., Mendo S., Coimbra M.A., Rocha S.M. Evaluation of the mutagenicity of sesquiterpenic compounds and their influence on the susceptibility towards antibiotics of two clinically relevant bacterial strains. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011;723:18–25. doi: 10.1016/j.mrgentox.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 45.Di Sotto A., Mazzanti G., Carbone F., Hrelia P., Maffei F. Inhibition by β-caryophyllene of ethyl methanesulfonate-induced clastogenicity in cultured human lymphocytes. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2010;699:23–28. doi: 10.1016/j.mrgentox.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Almeida M.R., Darin J.D.C., Hernandes L.C., de Souza Ramos M.F., Antunes L.M.G., de Freitas O. Genotoxicity assessment of Copaiba oil and its fractions in Swiss mice. Genet. Mol. Biol. 2012;35:664–672. doi: 10.1590/S1415-47572012005000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Türkez H., ÿelik K., ToĿar B. Effects of copaene, a tricyclic sesquiterpene, on human lymphocytes cells in vitro. Cytotechnology. 2014;66:597–603. doi: 10.1007/s10616-013-9611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pículo F., Guiraldeli Macedo C., de Andrade S.F., Maistro E.L. In vivo genotoxicity assessment of nerolidol. J. Appl. Toxicol. 2011;31:633–639. doi: 10.1002/jat.1607. [DOI] [PubMed] [Google Scholar]
- 49.Sperotto A.R.M., Moura D.J., Péres V.F., Damasceno F.C., Caramão E.B., Henriques J.A.P., Saffi J. Cytotoxic mechanism of Piper gaudichaudianum Kunth essential oil and its major compound nerolidol. Food Chem. Toxicol. 2013;57:57–68. doi: 10.1016/j.fct.2013.03.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.