Abstract
Erectile dysfunction (ED) affected the lives of more than 300 million men worldwide. Erectile dysfunction drugs (EDD), known as phosphodiesterase inhibitors (PDEIs), have been used for treatment of ED. It has been shown that oxidative stress plays an important role in the progression of erectile dysfunction. Oxidative stress can be alleviated or decreased by antioxidant enzymes. Therefore, the present study aims at investigating the changes in the activity of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione reductase as well as protein expression of glutathione peroxidase and glutathione S-transferase after treatment of male rats with a daily dose of sildenafil (1.48 mg/kg), tadalafil (0.285 mg/kg) and vardenafil (0.285 mg/kg) for three weeks. In addition, levels of reduced glutathione and malondialdyhyde (MDA) were assayed. The present study showed that sildenafil, vardenafil, and tadalafil treatments significantly decreased the levels of glutathione, MDA and the activity of glutathione reductase. In addition, vardenafil and sildenafil increased the activity of superoxide dismutase and catalase. Interestingly, western immunoblotting data showed that vardenafil induced the activity of glutathione peroxidase (GPX) and its protein expression, whereas tadalafil and sildenafil inhibited such enzyme activity and its protein expression. In addition, the protein expression of GST π isozyme was markedly reduced after treatment of rats with sildenafil. It is concluded that ED drugs induced the activities of both SOD and catalase which consequently decreased MDA level. Therefore, decrement in MDA levels could increase nitric oxide–cGMP level which in turn promotes the erection mechanism.
Abbreviations: ED, erectile dysfunction; EDD, erectile dysfunction drugs; PDEIs, phosphodiesterase inhibitors; PDE-5, phosphodiesterase type-5; MDA, malondialdyhyde; GST, glutathione S-transferase; SOD, superoxide dismutase; CAT, catalase; ROS, reactive oxygen species; GPx, glutathione peroxidase; GSH, glutathione; GR, glutathione reductase
Keywords: Oxidative stress, Glutathione, Glutathione peroxidase, Glutathione reductase, Catalase
1. Introduction
Erectile dysfunction (ED) is the inability to maintain penile erection for satisfactory sexual performance. Phosphodiesterase type-5 (PDE-5) is present in most vascular beds as well as cardiac myocytes [43], [13], [25]. PDE-5 inhibitors have been developed for treatment of erectile dysfunction through diminishing the degradation of cGMP [8], [38], [3]. PDE-5 inhibitors are sildenafil citrate (Viagra), tadalafil (Cialis) and vardenafil (Levitra). All over the world, these drugs have been used extensively in treatment of ED. It has been found that vardenafil increased intracavernosal pressure more quickly and to a greater extent than sildenafil in a rabbit model [41], [40]. In addition, vardenafil is more potent and selective biochemically toward ED than sildenafil. Tadalafil is effective for up to 36 h in men and has a long-acting inhibition on PDE-5 [16], [29].
There is a growing interest among researchers regarding the role of oxidative stress and reactive oxygen species (ROS) in the pathophysiological mechanism of ED [48], [15]. Oxidative stress occurs when there is an imbalance between prooxidants and the ability of the antioxidants to scavenge excess reactive oxygen species [53], [56]. However, its role in ED has not been investigated comprehensively. Previous study showed a significant association between the production of reactive oxygen species (ROS) and erectile dysfunction especially in diabetic animal models [27], [10]. It has been found that combined treatment of diabetic ED with PDE5 inhibitors and antioxidant agents are more effective than PDE5 inhibitors alone. Treatment of diabetic patients with ED with l-carnitine (an antioxidant) and sildenafil was found to reduce monocyte oxidative activity and the amount of endothelial dysfunction markers [35]. In addition, it has been found that Vitamin E enhanced the therapeutic effect of the PDE5 inhibitor supporting the potential use of oxygen free radical scavengers in salvaging erectile function in diabetic patients [14]. Moreover, combined treatment of diabetic patients with antioxidants plus sildenafil was found to improve the action of sildenafil which may be due to neutralization of reactive oxygen species [55]. In addition, Incubation of endothelial cells with sildenafil was found to attenuate the oxidant burden [33]. Moreover, vardenafil treatment was found to reduce DNA breakage and oxidative stress as well as increased the cGMP levels in the aortic wall [30].
Superoxide dismutase (SOD), an antioxidant enzyme, converts superoxide anion (O•−2) to hydrogen peroxide (H2O2) and molecular oxygen (O2) [26]. SOD is a promising therapeutic target for ED [15]. Also, sildenafil was found to reduce superoxide formation and increased levels of cGMP, cAMP and glutathione in corpus cavernosum of rabbits and in hypertensive rats [49]. In addition, chronic treatment of rats with sildenafil was found to restore the elevated biological markers of oxidative stress and cyclooxygenase derived vasoconstrictors to their normal levels [6]. ROS, formed during regular oxygen molecule metabolism primarily in the vascular endothelium, include H2O2 and peroxynitrite (OONO2). Increment in ROS level was found to inhibit SOD activity which consequently reduces the bioavailable NO concentration through induction of peroxynitrite level [21].
Recently, it has been found that oxidative stress plays an important role in ED which can be alleviated by antioxidant enzymes [32]. Therefore, the present study aimed at investigating the alterations in activities of superoxide dismutase and catalase as antioxidant enzymes as well as protein expression of glutathione peroxidase and glutathione S-transferase after treatment of rats with sildenafil, vardenafil and/or tadalafil.
2. Materials and methods
2.1. Chemicals
Sildenafil, tadalafil, vardenafil pills manufactured by Pfizer Pharmaceutical Company, Lilly Corporation, Schering-Plough Corporation, USA, respectively, were obtained from local drug stores at Egypt. Sulfosalcylic acid, bis-(3-caboxy-4-nitrophenyl)-disulfide, 1-chloro-2, 4-dinitrobenzene and all other chemicals were purchased from Sigma Chemical Co., St. Louis, MO, USA. Monoclonal antibodies of glutathione S-transferase π and glutathione peroxidase were obtained from ABCAM, UK.
2.2. Animals
Fifty male Sprague–Dawely rats (weighing 100–120 gm) were obtained from the animal house of Faculty of Medicine, Alexandria University, Alexandria, Egypt. The rats were housed in standard cages where food and water were provided ad libitum. After a period of acclimation, animals were divided into four groups. The first group (11 rats) was used as control (C) and received ddH2O as vehicle. Thirteen rats in the second, third, and fourth group received an oral daily dose of sildenafil (1.48 mg/kg), tadalafil (0.285 mg/kg) and vardenafil (0.285 mg/kg) respectively, for three weeks. These doses have been chosen according to the manufacturers of these drugs. At the end of the experimental period, rats were anesthetized with diethyl ether and sacrificed by cervical decapitation. The thoracic cavity of rats was opened for whole body and liver tissues were removed. Also, fasting blood samples were collected in heparinized tubes and plasma samples were obtained after centrifugation at 4000 rpm for 20 min and stored at −80 °C until use.
2.3. Enzyme assessments
At the designated time point, the thoracic cavity of rats was opened for whole body. Liver tissues were vigorously washed in an iced solution of 0.25 M sucrose, which contained 0.001 M EDTA, to avoid contamination from erythrocyte-containing enzymes. Liver tissues were homogenized in 3 vol (w/v) 0.1 M phosphate buffer, (pH 7.4) and centrifuged at 12000 × g for 20 min at 4 °C. Reduced glutathione level was estimated in the supernatant of liver tissue homogenate using sulfosalcylic acid for protein precipitation and bis-(3-caboxy-4-nitrophenyl)-disulfide for color development [34]. Glutathione reductase activity was assayed by monitoring the oxidation of NADPH at 340 nm using the method of James et al. [24]. A unit of enzyme activity represents 1 nmol of NADPH oxidized/min/mg protein. GST activity was determined according to the method of Lee et al. [31]. The conjugate of GSH with l-chloro-2, 4-dinitrobenzene (CDNB) was measured at 340 nm using a double beam spectrophotometer. A unit of enzyme activity is defined as the amount of enzyme that catalyses the formation of 1 mmol of CDNB conjugate/mg protein/min under the assay conditions. Calculations were made using a molar extinction coefficient of 9.6 mM−1 cm−1. The hepatic lipid peroxidation end product, malondialdehyde (MDA), was measured as thiobarbituric acid reactive substance (TBARS), according to the method of Tappel and Zalkin [52]. The color intensity of the reactants (MDA) was measured at 532 nm. An extinction coefficient of 156,000 M−1 cm−1 was used for the calculation.
2.4. Glutathione peroxidase
Glutathione peroxidase enzyme activity (GPx; EC. 1.11.1.9) was assayed according to the method of Chiu et al. [12]. The reaction mixture (1 mL) containing 0.05 mL of the enzyme source, 0.05 M Tris–HCl buffer (pH 7.6), 1.5 mM GSH, and cumene hydroperoxide was incubated for 5 min at 37 °C. In another tube, the control sample was prepared without cumene hydroperoxides and incubated for 5 min at 37 °C. To both control and test samples, 1.0 mL of TCA (15%) were added, while 0.1 mL cumene hydroperoxide was added to the control only. Both tubes were incubated for 10 min at 37 °C and centrifuged at 3000 rpm for 20 min. Tris–HCL buffer (pH 8.9) and 1.5 mM DTNB were added to 1 mL supernatant for both sample and control. The optical density of the yellow color obtained was measured at 412 nm within 5 min. Result was expressed as U/gm tissue.
2.5. Superoxide dismutase activity
SOD activity was measured according to the method of Sun et al. [50]. Estimation of SOD activity was based on the generation of superoxide radicals produced by xanthine and xanthine oxidase, which react with nitroblue tetrazolium (NTB) to form formazan dye. Generated formazan dye was then measured spectrophotometrically at 560 nm. The degree of inhibition of this reaction was expressed as mM/min/ml plasma.
2.6. Catalase assay
Catalase (CAT) activity was determined by measuring the decrease in the absorbance of H2O2 solution decomposed by the enzyme [4]. The quantity of H2O2 decomposed over specified time was calculated using the molar absorbance coefficient. Absorbance was measured at wavelength 240 nm and catalase activity was expressed in IU/L plasma.
2.7. Western immunoblotting technique
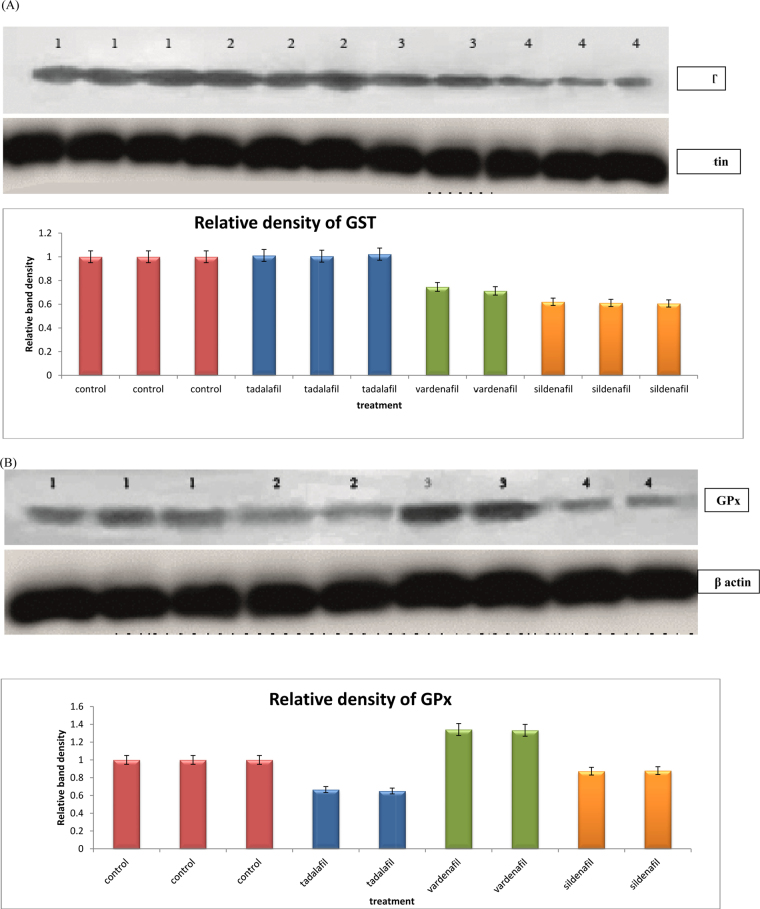
From pooled sample of each treatment, 20 μg of S-9 liver supernatant proteins were prepared and subjected to 10% SDS-polyacrylamide gel electrophoresis. Proteins were transblotted to Hybond-C nitrocellulose membranes (Amersham, UK). The membranes were stained by Ponceau to verify the transfer of comparable amounts of cellular protein. Nonspecific binding sites of the membranes were blocked by 5% Bovine Serum Albumin (BSA) for 1 h at 37 °C. The blots were then incubated with specific anti- rabbit primary antibodies against GST π isoenzyme and Gpx3, at a dilution of 1:1000 incubated overnight at room temperature, followed by a 2-h incubation period with anti- rabbit peroxidase-labelled secondary antibody at a dilution of 1:15000 at room temperature. Both GST π and glutathione peroxidase isozymes were visualized after binding with their specific monoclonal antibodies. Chemiluminescence signals were detected according to the manufacturer’s instructions (Abcam, UK). The band intensity was measured using quantity one software program.
2.8. Histopathological examination of liver tissues
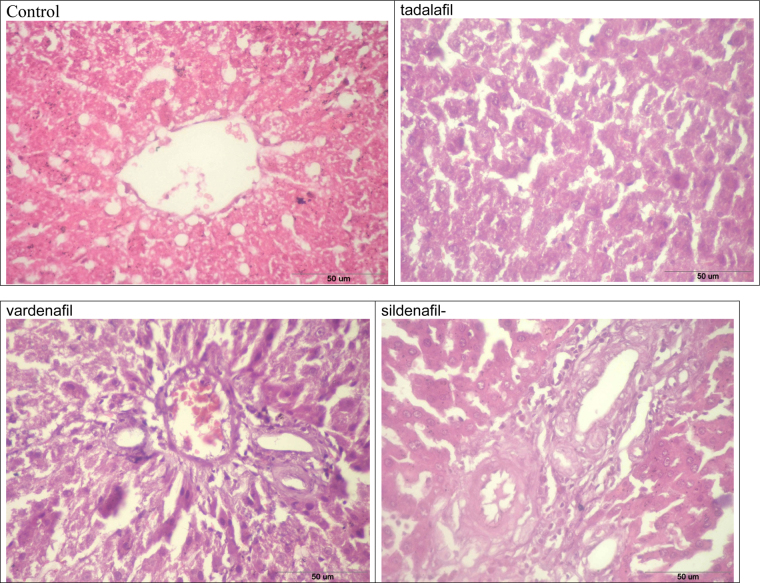
The liver tissues were fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer for 24 h. Blocks of liver tissue were taken from the median lobe and prepared for embedding in glycol methacrylate (Historesin, Leica) using standard techniques. Subsequently, sections of 5 μm thicknesses were obtained and stained with Eosin and Hematoxylin dyes for microscopic assessment. The histological sections were examined under a light microscope using a 121 intersection grid placed at the ocular lens, coupled to a 100× objective. The hepatic parenchyma was classified as one of the following: hepatocyte cytoplasm or degenerative hepatocytes, central vein, portal space, and inflammatory infiltrate.
2.9. Statistical analysis
Means and standard errors of each treatment were calculated and compared by a Student’s t-test. The level of all significances for all experiments was set at P < 0.05.
3. Results
The present study showed that MDA levels were significantly decreased by 28, 36 and 23% after treatment of rats with tadalafil, vardenafil, and sildenafil, respectively (Table 1). On the other hand, vardenafil and sildenafil significantly increased the activity of superoxide dismutase (SOD) by 35, 17% and catalase by 56, 32%, respectively (Table 1). Tadalafil treatment did not change SOD activity and increased catalase by 47%. The activity of GR and GSH levels were markedly decreased after treatment of rats with tadalafil, vardenafil, and sildenafil by 22, 51, 38 and 23, 51, and 36%, respectively (Table 1). There are different isozymes of GST and their total activities did not change after treatment of rats with either tadalafil or sildenafil, whereas vardenafil induced such activity by 25% (Table 1). GST π is one of these isozymes and its protein expression was potentially decreased after treatment of rats with either vardenafil or sildenafil treatments, whereas tadalafil did not change such expression (Fig. 1A). The activity of glutathione peroxidase (GPX) and its protein expression were inhibited after treatment of rats with either tadalafil or sildenafil whereas vardenafil induced such activity and expression (Table 1 and Fig. 1B). The histopathological examination showed that sildenafil citrate and vardenafil caused inflammation and fibrosis in tissues of rat livers (Fig. 2D and C), whereas tadalafil did not cause any changes (Fig. 2B).
Table 1.
Changes in the activity of antioxidant enzymes and free radical levels after treatment of rats with a daily dose of tadalafil, vardenafil and/or sildenafil for consecutive three weeks.
| Enzymes | Control | Tadalafil | Vardenafil | Sildenafil |
|---|---|---|---|---|
| GST (Unit /mg protein) | 0.79 ± 0.015 | 0.79 ± 0.02 | 0.60 ± 0.017 | 0.74 ± 0.013 |
| (NS) | (−25%, P < 0.05)** | (NS) | ||
| Glutathione reductase (nM oxidized NADPH/mg protein/min) | 95.6 ± 2.67 | 75.0 ± 3.36 | 46.0 ± 2.1 | 59.7 ± 1.57 |
| (−22%, P < 0.001)* | (−51%, P < 0.001)* | (−38%, P < 0.001)* | ||
| Glutathione peroxidase (IU/g tissue) |
34.2 ± 2.2 | 21.9 ± 3.2 | 42.7 ± 3.0 | 24.6 ± 3.1 |
| (−36%, P < 0.05)** | (+25%, P < 0.05)** | (−28%, P < 0.05)** | ||
| Glutathione (μM GSH/g liver) | 2.1 ± 0.13 | 1.65 ± 0.09 | 1.03 ± 0.04 | 1.36 ± 0.02 |
| (−23%, P < 0.001)* | (−51%, P < 0.001)* | (−36%, P < 0.001)* | ||
| TBARS (μM/g liver) | 16.1 ± 0.48 | 11.6 ± 0.29 | 10.3 ± 0.38 | 12.36 ± 0.11 |
| (−28%, P < 0.001)* | (−36%, P < 0.001)* | (−23%, P < 0.001)* | ||
| Catalase activity (IUnit/L) |
168.9 ± 0.8 | 247.0 ± 3.4 | 269.1 ± 4.73 | 223.0 ± 0.9 |
| (+47%, P < 0.001)* | (+56%, P < 0.001)* | (+32%, P < 0.001)* | ||
| SOD activity (mM/min/mL) | 2.5 ± 0.039 | 2.7 ± 0.20 | 3.5 ± 0.12 | 2.9 ± 0.09 |
| (NS) | (+35%, P < 0.001)* | (+17%, P < 0.05)** |
Values are expressed as Mean ± SEM.
NS: values are not significant statistically compared to the control group where P > 0.05.
Values are significantly different compared to control group where P < 0.05.
Values are significantly different compared to control group where P < 0.001.
Fig. 1.
Western immunoblotting analysis showed the influence of tadalafil, vardenafil, and sildenafil on the protein expression of (A) Glutathione S-transferase π isozyme, (B) Glutathione peroxidase (GPx3 isozyme). In both figures A and B, Lanes 1–4 represented the pooled proteins of matched control, tadalafil, vardenafil, and sildenafil-treated rats, respectively. The band intensity was measured using Quantity one software program.
Fig. 2.
Histopathological examination showed moderate inflammation and marked fibrosis in the liver tissues of male rats after treatment with vardenafil and sildenafil with no changes in the liver of tadalafil-treated rats.
4. Discussion
The role of oxidative stress and reactive oxygen species in the pathophysiological mechanisms of some diseases have been extensively studied [1], [23]. Quenching of free radicals by EDD could sustain the bioavailability of NO for vasodilatation which might be a new possible mechanism of actions of ED medications since most cases of erectile dysfunction (ED) are associated with oxidative stress [57], [46], [39]. Induction of SOD and CAT activities might be a defence mechanism to protect the cell against oxidative insult resulted from high levels of free radicals [37], [9], [7]. Previous study showed that sildenafil reduced malondialdyhyde (MDA) levels and increased the activity of antioxidant enzymes in rats [5]. In agreement with this finding, MDA levels were significantly decreased after treatment of rats with tadalafil, vardenafil, and sildenafil. Decrement in MDA levels might be due to induction of superoxide dismutase and catalase activities since tadalafil, vardenafil and sildenafil significantly increased the activities of these enzymes. In agreement with our finding, sildenafil depresses hydrogen peroxide generation by inducing the activity of superoxide dismutase (SOD)-mimetic by preventing reactive oxygen species (ROS) generation [22], [18]. Also, it has been found that sildenafil improved the antioxidants concentrations and reduced oxidative stress in patients [17]. Moreover, it has been found that tadalafil was more effective than sildenafil because it increased SOD activity in rats [44], [36] and decreased MDA levels in their cavernous tissues [11]. In addition, it has been found that tadalafil is not only a PDE5 inhibitor, but also act as an effective antioxidant agent for the penis and also inhibits the production of lipid peroxidation via inhibition of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity which in turn reduces superoxide anion formation [44], [28].
Glutathione reductase and glutathione play an important role in scavenging of free radicals [2], [42], [47]. The reduced form of glutathione (GSH) is the most important cellular antioxidant and is also an essential cofactor for nitric oxide (NO) synthase that synthesizes NO from l-arginine [19]. Therefore, GSH depletion could lead to reduction of NO synthesis, and consequently impairs vasodilation in the corpora cavernosa [51]. In the present study, the activity of GR and GSH levels were markedly decreased in liver tissues after treatment of rats with sildenafil, vardenafil and tadalafil. In accordance with the present study, it has been found that sildenafil reduced GSH levels in both prostate and brain tissues of rat [54], [20] and in red blood cell of human [51]. It has been proposed that the total GSH level is partially dependent on the reduction of the oxidized form of glutathione (GSSG) to the reduced form (GSH) by glutathione reductase [45]. It seems that there are different sources of GSH pool such as GSH synthase and γ-gluatamyltranspeptidase because the activity of glutathione reductase is markedly inhibited after the treatment of rats with ED drugs.
It seems from the present study and others that changes in glutathione peroxidase activity is dependent on animal species since sildenafil did not change glutathione peroxidase activity in human erythrocyte [37]. However, glutathione peroxidase (GPX) activity and its protein expression were inhibited after treatment of rats with either tadalafil or sildenafil whereas vardenafil induced such activity and expression of its protein. Therefore, inhibition of glutathione peroxidase, glutathione reductase activities, and depletion of glutathione levels may enhance liver toxicity. Supporting this finding, the histopathological examination showed that sildenafil citrate caused inflammation and fibrosis in rat liver tissues, whereas tadalafil did not cause any changes. Therefore, inhibition of GST π expression after treatment of rats with either sildenafil or vardenafil might be due to inflammation and fibrosis caused by these drugs. This inhibition might potentiate liver toxicity and probably other organs since GST π isozyme plays a significant role in the protection of liver against toxicity induced by a wide variety of toxic compounds [47].
It is concluded that ED drugs decreased free radical levels and increased the antioxidant capacity by increasing SOD and CAT activities, which can attenuate the oxidative stress resulted from many endogenous sources. This attenuation might be a new possible mechanism that can be added to EDDs beside their action as PDEIs.
Acknowledgment
Authors are grateful to STDF, Cairo, Egypt, for their financial support of MSc. thesis of Basant M. Salama, Department of Biotechnology, IGSR, Alexandria University, Egypt.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.06.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Agarwal A., Nandipati K.C., Sharma R.K., Zippe C.D., Raina R. Role of oxidative stress in the pathophysiological mechanism of erectile dysfunction. J. Androl. 2006;27:335–347. doi: 10.2164/jandrol.05136. [DOI] [PubMed] [Google Scholar]
- 2.Arrick B.A., Nathan C.F. Glutathione metabolism as a determinant of therapeutic efficiency: a review. Cancer Res. 1984;44:4224–4228. [PubMed] [Google Scholar]
- 3.Attinà T.M., Drummond I.D., Malatino L.S., Maxwell S.R., Webb D.J. Phosphodiesterase type 5 inhibition improves arterial stiffness after exercise but not exercise capacity in hypertensive men. Am. J. Hypertens. 2013;26:342–350. doi: 10.1093/ajh/hps057. [DOI] [PubMed] [Google Scholar]
- 4.Beers R.F., Sizer I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952;1:133–140. [PubMed] [Google Scholar]
- 5.Beheshtian A., Salmasi A.H., Payabvash S., Kiumehr S., Ghazinezami B., Rahimpour S., Tavangar S.M., Dehpour A.R. Protective effets of sildenafil administration on testicular torsion/detorsion damage in rats. World J. Urol. 2008;26:197–202. doi: 10.1007/s00345-008-0243-6. [DOI] [PubMed] [Google Scholar]
- 6.Behr-Roussel D., Oudot A., Compagnie S., Gorny D., Le Coz O., Bernabe J., Wayman C., Alexandre L., Giuliano F. Impact of a long-term sildenafil treatment on pressor response in conscious rats with hypertriglyceridemia. Am. J. Hypertens. 2008;21:1258–1263. doi: 10.1038/ajh.2008.273. [DOI] [PubMed] [Google Scholar]
- 7.Bivalacqua T.J., Musicki B., Hsu L.L., Berkowitz D.E., Champion H.C., Burnett A.L. Sildenafil citrate-restored eNOS and PDE5 regulation in sickle cell mouse penis prevents priapism via control of oxidative/nitrosative stress. Plos One. 2013;8:e68028. doi: 10.1371/journal.pone.0068028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boolell M., Allen M.J., Ballard S.A., Gepi-Attee S., Muirhead G.J., Naylor A.M., Osterloh I.H., Gingell C. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int. J. Impot. Res. 1996;8:47–52. [PubMed] [Google Scholar]
- 9.Cadirci E., Halici Z., Odabasoglu F., Albayrak A., Karakus E., Unal D., Atalay F., Ferah I., Unal B. Sildenafil treatment attenuates lung and kidney injury due to overproduction of oxidant activity in a rat model of sepsis: a biochemical and histopathological study. Clin. Exp. Immunol. 2011;166:374–384. doi: 10.1111/j.1365-2249.2011.04483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castela A., Gomes P., Domingues V.F., Paíga P., Costa R., Vendeira P., Costa C. Role of oxidative stress-induced systemic and cavernosal molecular alterations in the progression of diabetic erectile dysfunction. J. Diabetes. 2014;(June 9) doi: 10.1111/1753-0407.12181. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Chen Y., Li X.X., Lin H.C., Qiu X.F., Gao J., Dai Y.T., Wang R. The effects of long-term administration of tadalafil on STZ-induced diabetic rats with erectile dysfunction via a local antioxidative mechanism. Asian J. Androl. 2012;14:616–620. doi: 10.1038/aja.2012.22. 8207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiu D.T., Stults F.H., Tappel A.L. Purification and properties of rat lung soluble glutathione peroxidase. Biochim. Biophys. Acta. 1976;445:558–566. doi: 10.1016/0005-2744(76)90110-8. [DOI] [PubMed] [Google Scholar]
- 13.Das A., Xi L., Kukreja R.C. Phosphodiesterase-5 inhibitor, sildenafil preconditions adult cardiac myocytes against necrosis and apoptosis: essential role of NO signaling. J. Biol. Chem. 2005;280:12944–12955. doi: 10.1074/jbc.M404706200. [DOI] [PubMed] [Google Scholar]
- 14.De Young L., Yu D., Bateman R.M., Brock G.B. Oxidative stress and antioxidant therapy: their impact in diabetes-associated erectile dysfunction. J. Androl. 2004;25:830–836. doi: 10.1002/j.1939-4640.2004.tb02862.x. [DOI] [PubMed] [Google Scholar]
- 15.Deng W., Bivalacqua T.J., Champion H.C., Hellstrom W.J., Murthy S.N., Kadowitz P.J. Superoxide dismutase a target for gene therapeutic approach to reduce oxidative stress in erectile dysfunction. Methods Mol. Biol. 2010;610:213–227. doi: 10.1007/978-1-60327-029-8_13. [DOI] [PubMed] [Google Scholar]
- 16.Eardley I., Cartledge J. Tadalafil (Cialis) for men with erectile dysfunction. Int. J. Clin. Pract. 2002;56:300–304. [PubMed] [Google Scholar]
- 17.El-Far M., El-Motwally A.E.G., Hashem I.A., Bakry N. Biochemical role of intravaginal sildenafil citrate as a novel antiabortive agent in unexplained recurrent spontaneous miscarriage: first clinical study of four case reports from Egypt. Clin. Chem. Lab. Med. 2009;47:1433–1438. doi: 10.1515/CCLM.2009.311. [DOI] [PubMed] [Google Scholar]
- 18.Fernandes M.A., Marques R.J., Vicente J.A., Santos M.S., Monteiro P., Moreno A.J., Custódio J.B. Sildenafil citrate concentrations not affecting oxidative phosphorylation depress H2O2 generation by rat heart mitochondria. Mol. Cell. Biochem. 2008;309:77–85. doi: 10.1007/s11010-007-9645-9. [DOI] [PubMed] [Google Scholar]
- 19.Ghigo D., Alessio P., Foco A., Bussolino F., Costamagna C., Heller R., Garbarino G., Pescarmona G.P., Bosia A. Nitric oxide synthesis is impaired in glutathione-depleted human umbilical vein endothelial cells. Am. J. Physiol. 1993;265:C728–C732. doi: 10.1152/ajpcell.1993.265.3.C728. [DOI] [PubMed] [Google Scholar]
- 20.Guzmán D.C., Olguín H.J., Brizuela N.O., García E.H., Mejía G.B., Jacobo A.J., Abarca L.S., Betancourt E.T. Effect of prostaglandin E1 (PGE1) and sildenafil on serotonin metabolism and some oxidative damage markers in rat prostate gland and brain. Andrologia. 2011;43:266–272. doi: 10.1111/j.1439-0272.2010.01067.x. [DOI] [PubMed] [Google Scholar]
- 21.Hirata H., Kawamoto K., Kikuno N., Kawakami T., Kawakami K., Saini S., Yamamura S., Dahiya R. Restoring erectile function by antioxidant therapy in diabetic rats. J. Urol. 2009;182:2518–2522. doi: 10.1016/j.juro.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 22.Hotston M., Jeremy J.Y., Bloor J., Greaves N.S., Persad R., Angelini G., Shukla N. Homocysteine and copper interact to promote type 5 phosphodiesterase expression in rabbit cavernosal smooth muscle cells. Asian J. Androl. 2008;10:905–913. doi: 10.1111/j.1745-7262.2008.00380.x. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh H.J., Liu C.A., Huang B., Tseng A.H., Wang D.L. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J. Biomed. Sci. 2014;21:3–7. doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.James N.S., Gay R.J., Hilf R. Influence of estrogen on glutathione levels and glutathione-metabolizing enzymes in uteri and R3230AC mammary tumors of rats. Biochim. Biophys. Acta. 1980;630:485–496. doi: 10.1016/0304-4165(80)90003-3. [DOI] [PubMed] [Google Scholar]
- 25.Kass D.A. Cardiac role of cyclic-GMP hydrolyzing phosphodiesterase type 5: from experimental models to clinical trials. Curr. Heart Fail. Rep. 2012;9:192–199. doi: 10.1007/s11897-012-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelso G.F., Maroz A., Cochemé H.M., Logan A., Prime T.A., Peskin A.V., Winterbourn C.C., James A.M., Ross M.F., Brooker S. A mitochondria-targeted macrocyclic Mn(II) superoxide dismutase mimetic. Chem. Biol. 2012;19:1237–1246. doi: 10.1016/j.chembiol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Kim J.H., Kim H., Kim Y.H., Chung W.S., Suh J.K., Kim S.J. Antioxidant effect of captopril and enalapril on reactive oxygen species-induced endothelial dysfunction in the rabbit abdominal aorta. Korean J. Thorac. Cardiovasc. Surg. 2013;46:14–21. doi: 10.5090/kjtcs.2013.46.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohda Y., Gemba M. Modulation by cyclic AMP and phorbol myristate acetate of cephaloridine-induced injury in rat renal cortical slices. Jpn. J. Pharmacol. 2001;85:54–59. doi: 10.1254/jjp.85.54. [DOI] [PubMed] [Google Scholar]
- 29.Koka S., Das A., Salloum F.N., Kukreja R.C. Phosphodiesterase-5 inhibitor tadalafil, attenuates oxidative stress and protects against myocardial ischemia/reperfusion injury in type 2 diabetic mice. Free Radic. Biol. Med. 2013;60:80–88. doi: 10.1016/j.freeradbiomed.2013.01.031. [DOI] [PubMed] [Google Scholar]
- 30.Korkmaz S., Radovits T., Barnucz E., Neugebauer P., Arif R., Hirschberg K., Loganathan S., Seidel B., Karck M., Szabó G. Dose-dependent effects of a selective phosphodiesterase-5-inhibitor on endothelial dysfunction induced by peroxynitrite in rat aorta. Eur. J. Pharmacol. 2009;615:155–162. doi: 10.1016/j.ejphar.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 31.Lee C.Y., Johnson L., Cox R.H., McKinney J.D., Lee S.M. Mouse liver glutathione S-transferases. Biochemical and immunological characterization. J. Biol. Chem. 1981;256:8110–8116. [PubMed] [Google Scholar]
- 32.Lushchak V.I. Glutathione homeostasis and functions: potential targets for medical interventions. J. Amino Acids. 2012;2012:736837. doi: 10.1155/2012/736837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Milara J., Juan G., Ortiz J.L., Guijarro R., Losada M., Serrano A., Morcillo E.J., Cortijo J. Cigarette smoke-induced pulmonary endothelial dysfunction is partially suppressed by sildenafil. Eur. J. Pharm. Sci. 2010;39:363–372. doi: 10.1016/j.ejps.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell J.R., Jollow D.J., Potter W.Z., Gillette J.R., Brodie B.B. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J. Pharmacol. Exp. Ther. 1973;187:211–217. [PubMed] [Google Scholar]
- 35.Morano S., Mandosi E., Fallarino M., Gatti A., Tiberti C., Sensi M., Gandini L., Buchetti B., Lenti L., Jannini E.A. Antioxidant treatment associated with sildenafil reduces monocyte activation and markers of endothelial damage in patients with diabetic erectile dysfunction: a double-blind, placebo-controlled study. Eur. Urol. 2007;52:1768–1774. doi: 10.1016/j.eururo.2007.04.042. [DOI] [PubMed] [Google Scholar]
- 36.Ozdegirmenci O., Kucukozkan T., Akdag E., Topal T., Haberal A., Kayir H., Oter S., Akyol M., Uzbay T. Effects of sildenafil and tadalafil on ischemia/reperfusion injury in fetal rat brain. J. Matern. Fetal. Neonatal. Med. 2011;24:317–323. doi: 10.3109/14767058.2010.492061. [DOI] [PubMed] [Google Scholar]
- 37.Perk H., Armagan A., Nazıroğlu M., Soyupek S., Hoscan M.B., Sütcü R., Ozorak A., Delibas N. Sildenafil citrate as a phosphodiesterase inhibitor has an antioxidant effect in the blood of men. J. Clin. Pharm. Ther. 2008;33:635–640. doi: 10.1111/j.1365-2710.2008.00962.x. [DOI] [PubMed] [Google Scholar]
- 38.Porst H., Rosen R., Padma-Nathan H., Goldstein I., Giuliano F., Ulbrich E., Bandel T. The efficacy and tolerability of vardenafil, a new, oral, selective phosphodiesterase type 5 inhibitor, in patients with erectile dysfunction: the first at-home clinical trial. Int. J. Impot. Res. 2001;13:192–199. doi: 10.1038/sj.ijir.3900713. [DOI] [PubMed] [Google Scholar]
- 39.Pravenec M., Kozich V., Krijt J., Sokolová J., Zídek V., Landa V., Simáková M., Mlejnek P., Silhavy J., Oliyarnyk O. Folate deficiency is associated with oxidative stress, increased blood pressure, and insulin resistance in spontaneously hypertensive rats. Am. J. Hypertens. 2013;26:135–140. doi: 10.1093/ajh/hps015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roustit M., Blaise S., Allanore Y., Carpentier P.H., Caglayan E., Cracowski J.L. Phosphodiesterase-5 inhibitors for the treatment of secondary Raynaud’s phenomenon: systematic review and meta-analysis of randomized trials. Ann. Rheum. Dis. 2013;72:1696–1699. doi: 10.1136/annrheumdis-2012-202836. [DOI] [PubMed] [Google Scholar]
- 41.Saenz D.T.I., Angulo J., Cuevas P., Fernández A., Moncada I., Allona A., Lledó E., Körschen H.G., Niewöhner U., Haning H. The phosphodiesterase inhibitory selectivity and the in vitro and in vivo potency of the new PDE-5 inhibitor vardenafil. Int. J. Impot. Res. 2001;13:282–290. doi: 10.1038/sj.ijir.3900726. [DOI] [PubMed] [Google Scholar]
- 42.Seelig G.F., Meister A. Glutathione biosynthesis; 7-glutamylcysteine synthetase from rat kidney. Methods Enzymol. 1985;113:379–390. doi: 10.1016/s0076-6879(85)13050-8. [DOI] [PubMed] [Google Scholar]
- 43.Senzaki H., Smith C.J., Juang G.J., Isoda T., Mayer S.P., Ohler A., Paolocci N., Tomaselli G.F., Hare J.M., Kass D.A. Cardiac phosphodiesterase 5 (cGMP specific) modulates beta-adrenergic signaling in vivo and is down-regulated in heart failure. FASEB J. 2001;15:1718–1726. doi: 10.1096/fj.00-0538com. [DOI] [PubMed] [Google Scholar]
- 44.Serarslan Y., Yönden Z., Ozgiray E., Oktar S., Güven E.O., Söğüt S., Yilmaz N., Yurtseven T. Protective effects of tadalafil on experimental spinal cord injury in rats. J. Clin. Neurosci. 2010;17:349–352. doi: 10.1016/j.jocn.2009.03.036. [DOI] [PubMed] [Google Scholar]
- 45.Sheweita S.A., Mostafa M.H. N-nitrosamines and their effects on the level of glutathione, glutathione reductase and glutathione S-transferase activities in the liver of male mice. Cancer Lett. 1996;99(1 (Jan. 19)):29–34. doi: 10.1016/0304-3835(95)04034-x. [DOI] [PubMed] [Google Scholar]
- 46.Sheweita S.A., Sheikh B.Y. Can dietary antioxidants reduce the incidence of brain tumors? Curr. Drug. Metabol. 2011;12:587–593. doi: 10.2174/138920011795713733. [DOI] [PubMed] [Google Scholar]
- 47.Sheweita S.A., Tilmisany A.K. Cancer and phase II drug-metabolizing enzymes. Curr. Drug. Metabol. 2003;4:45–58. doi: 10.2174/1389200033336919. [DOI] [PubMed] [Google Scholar]
- 48.Sheweita S.A., Tilmisany A.M., Al-Sawaf H. Mechanisms of male infertility: role of antioxidants. Curr. Drug. Metabol. 2005;6:495–501. doi: 10.2174/138920005774330594. [DOI] [PubMed] [Google Scholar]
- 49.Shukla N., Rossoni G., Hotston M., Sparatore A., Del Soldato P., Tazzari V., Persad R., Angelini G.D., Jeremy J.Y. Effect of hydrogen sulphide-donating sildenafil (ACS6) on erectile function and oxidative stress in rabbit isolated corpus cavernosum and in hypertensive rats. BJU Int. 2009;103:1522–1529. doi: 10.1111/j.1464-410X.2009.08415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun Y., Oberley L.W., Li Y. A simple method for clinical assay of superoxide dismutase. Clin. Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 51.Tagliabue M., Pinach S., Di Bisceglie C., Brocato L., Cassader M., Bertagna A., Manieri C., Pescarmona G.P. Glutathione levels in patients with erectile dysfunction, with or without diabetes mellitus. Int. J. Androl. 2005;28:156–162. doi: 10.1111/j.1365-2605.2005.00528.x. [DOI] [PubMed] [Google Scholar]
- 52.Tappel A.L., Zalkin H. Lipide peroxidation in isolated mitochondria. Arch. Biochem. Biophys. 1959;80:326–332. [Google Scholar]
- 53.Terai T., Nagano T. Site-specific oxidative stress induction. Chem. Biol. 2007;14:877–878. doi: 10.1016/j.chembiol.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 54.Thakur T., Sharma S., Kumar K., Deshmukh R., Sharma P.L. Neuroprotective role of PDE4 and PDE5 inhibitors in 3-nitropropionic acid induced behavioral and biochemical toxicities in rats. Eur. J. Pharmacol. 2013;714:515–521. doi: 10.1016/j.ejphar.2013.06.035. [DOI] [PubMed] [Google Scholar]
- 55.Vicari E., La Vignera S., Condorelli R., Calogero A.E. Endothelial antioxidant administration ameliorates the erectile response to PDE5 regardless of the extension of the atherosclerotic process. J. Sex. Med. 2010;7:1247–1253. doi: 10.1111/j.1743-6109.2009.01420.x. [DOI] [PubMed] [Google Scholar]
- 56.Zabłocka A., Janusz M. The two faces of reactive oxygen species. Postepy Hig. Med. Dosw. 2008;62:118–124. [PubMed] [Google Scholar]
- 57.Zhang Q., Radisavljevic Z.M., Siroky M.B., Azadzoi K.M. Dietary antioidants improve arteriogenic erectile dysfunction. Int. J. Androl. 2011;34:225–235. doi: 10.1111/j.1365-2605.2010.01083.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.