Abstract
Calculations indicate that selectively heating the extracellular media induces membrane temperature gradients that combine with electric fields and a temperature-induced reduction in the electropermeabilization threshold to potentially facilitate exogenous molecular delivery. Experiments by a wide-field, pulsed femtosecond laser with peak power density far below typical single cell optical delivery systems confirmed this hypothesis. Operating this laser in continuous wave mode at the same average power permeabilized many fewer cells, suggesting that bulk heating alone is insufficient and temperature gradients are crucial for permeabilization. This work suggests promising opportunities for a high throughput, low cost, contactless method for laser mediated exogenous molecule delivery without the complex optics of typical single cell optoinjection, for potential integration into microscope imaging and microfluidic systems.
Keywords: Laser, Optoporation, Thermal gradients, Electropermeabilization, Transfection
Highlights
-
•
Femtosecond lasers can transfect a single cell through optoporation.
-
•
Multiple cells are transfected with much lower power densities at 1550 nm.
-
•
Calculations show that temperature gradients contribute to the mechanism.
-
•
These mechanisms resemble those also involved in microwave biological interactions.
1. Introduction
By introducing exogenous DNA into living cells while maintaining viability, gene therapy may potentially treat numerous diseases and conditions [1], [2], [3], [4]. An ideal delivery method would be inexpensive and efficient (high delivery efficiency with high viability) with high throughput. Early gene therapy frequently used viral vectors for delivery [5]; however, side effects, such as inflammation and leukemia, prompted nonviral delivery method development [6]. Alternatives include chemical approaches, such as lipofection [7], or physical approaches, such as electric fields [8], ultrasound [9], nanoparticles [10], or lasers [11].
Electromagnetic techniques are the most common physical approach. Pulsed electric fields (PEFs) of appropriate duration and intensity electropermeabilize the membrane in a process called electroporation [12] with the membrane either resealing, as in gene therapy [13], or failing to reseal, as in cancer treatment [14] and sterilization [15]. Electroporation pulses are typically microseconds to milliseconds in duration with field strengths of approximately hundreds of volts per centimeter. PEFs of similar energy, but shorter duration (10–300 ns) and higher field strength (30–300 kV/cm) [16], may induce intracellular effects, such as apoptosis [17], changes in calcium dynamics [18], and mitochondria permeabilization [19], because the pulse duration is shorter than the charging time of the cell and on the order of the charging time of the smaller organelles. These nanosecond PEFs (nsPEFs) also create membrane pores smaller than those induced by traditional electroporation [20]. Low (~kHz–MHz) and high (~MHz) frequency AC fields similarly target external and internal membranes, respectively [21]. Lasers enable non-contact treatment and seamless integration with microscopic imaging and microfluidic systems [22]. Optical transfection has successfully delivered multiple substances, including ions, small interfering RNAs (siRNAs), and plasmids, using an automated, high-throughput process [23].
1.1. Laser mediated exogenous molecule delivery – overview
Laser-based gene delivery typically uses tightly focused beams, making it mostly a single cell permeabilization technique (optoinjection). While successful, the mechanism remains incompletely understood. Analogous to PEFs, laser pulse duration impacts the light-cell interaction. Continuous wave (CW) lasers generally favor delivery by plasma membrane heating [24]. Nanosecond lasers create shockwaves spanning multiple cell widths [24], [25], [26] while additionally inducing heat and thermoelastic stress [27]. Femtosecond lasers create free electrons at the cell surface to trigger a low density plasma that permeabilizes a single cell [23].
Laser wavelength also influences the permeabilization mechanism. The energy required for optical breakdown at femtosecond duration increases with wavelength [28]. Also, the volume of laser absorption depends upon the laser spot size (or illumination area) and absorption coefficient, which varies with wavelength [29], [30], [31], [32], [33], [34] and medium temperature [35]. Table 1 summarizes absorption coefficients for water [29] and lipids [34], [36], [37]. At 532 nm, the absorption coefficient of lipids (~0.01 cm−1) is much higher than for water (~4.47×10−4 cm−1), making water much more transparent to the beam than lipids. Thus, the resulting laser exposure predominantly interacts with the plasma membrane at the membrane/buffer interface, making heating and thermoelastic stress dominant mechanisms [24]. At longer wavelengths, laser radiation increasingly interacts with the surrounding medium. At 800 nm, the most successful wavelength for single cell optoporation [24], the absorption coefficient for water (0.02 cm−1) is five times larger than for lipids (0.004 cm−1), indicating that the laser preferentially heats the surrounding buffer to induce plasma cloud formation at the plasma membrane [23] and a membrane temperature gradient (∇T) analogous to that calculated for electric fields [38]. The ∇T may additionally induce membrane voltages due to the thermoelectric effect that could contribute to permeabilization [38]. At 1550 nm, the absorption coefficient of water (10.5 cm−1) exceeds that of lipids (0.2 cm−1) by approximately a factor of fifty, further increasing ∇T. Similarly, the absorption coefficient of water (32 cm−1) exceeds that of lipids (1.625 cm−1) at 2080 nm by approximately a factor of twenty, suggesting that ∇T may contribute to membrane permeabilization, although the mechanism remains unknown [24]. The first experimental effects of ∇T in biological samples were observed previously during microwave exposures. Greater absorption of microwave energy in the extracellular fluid [39] creates ∇T that may induce various physiological responses, without bulk heating [40], [41], such as membrane permeabilization [40], or that are irreproducible with convection heating alone [42].
Table 1.
Absorption coefficients for water and lipids at relevant laser wavelengths and the ratio (Water/Lipid) between them.
| Laser wavelength (nm) |
||||
|---|---|---|---|---|
| 532 | 800 | 1550 | 2080 | |
| Absorption Coefficient (cm−1) | ||||
| Water | 0.000447 | 0.02 | 10.5 | 32 |
| Lipid | 0.01002 | 0.004 | 0.1996 | 1.625 |
| Water/Lipid | 0.045 | 5 | 52.6 | 19.7 |
Most lasers used for transfection have peak power levels of ~10 kW, pulse durations of 17–150 fs, and wavelengths of 800–1000 nm to permeabilize a single cell [43] through low density plasma formation. Reduced cell viability [44] motivated research into femtosecond pulses of longer wavelength [45]. A 170 fs, 120 mW, 1554 nm laser transiently induced propidium iodide (PI) uptake with reduced temperature rise, shock waves, and cavitation bubbles compared to 800 nm lasers [45]. However, this study used relatively narrow focus (~2 μm) with high peak power densities at the optical breakdown level to perforate a single cell at the time [45].
This paper uses a simple analytic model to quantify and assess the potential impact of ∇T on experimental results showing that a 1550 nm, 100 fs laser with wide-field illumination in both pulse and CW modes will permeabilize cells with peak power densities three orders of magnitude below the typical optoporation threshold [45]. Comparing permeabilization levels and ∇T between pulse and CW modes will further show that ∇T may drive permeabilization and that bulk temperature rise alone is insufficient. The approach outlined here promises to be inexpensive with a higher throughput than conventional optoporation.
2. Materials and methods
Fig. 1 shows the experimental setup. The laser wavelength was ~1550 nm, average power Wavg=120 mW, pulse duration τp~100 fs, repetition rate νrep=50 MHz (or 20 ns between pulses), peak power Wpeak~24 kW (Wpeak=Wavg/[τpνrep]), and spot diameter of ~50 μm. The peak energy of the pulse (Epeak=τpWpeak) is 2.4×10−9 J. Despite similar Wpeak (~10 kW) and τp (17–150 fs) to standard optoinjection [43], the power density here is three orders of magnitude lower due to the wider illumination area (~2500 μm2 compared to 4 μm2). The resulting peak energy and average power densities are 9.6×10−4 J/cm3 and 4.8×104 W/cm3, respectively, assuming an exposure volume equal to the product of the illumination area and the absorption coefficient. In terms of area, peak energy and average power densities are 9.6×10−5 J/cm2 and 4.8×103 W/cm2, respectively. The typical laser spot measures approximately 50 μm, illuminating approximately 10–20 cells simultaneously with the exact number of cells a function of initial confluence density (approximately 80%) and the geometrical distribution of cells in the treated area.
Fig. 1.
Experimental setup for laser treatment of cells. The mechanical shutter controlling exposure time is not shown for clarity. The cells adhere to the bottom of the dish.
We followed the cell preparation method presented in more detail elsewhere [46]. We cultured adherent Chinese Hamster Ovarian cells (CHO, American Type Culture Collection (ATCC)) in F12K media supplemented with 10% FBS according to the ATCC protocol. The cells were used at early passages, typically between passage four and ten. We used a Countess® Automated Cell Counter (Invitrogen) for cell counts. We seeded the cells at approximately 50% confluency with between 7×104 and 1×105 cells/well in 24 well-plate dishes. We verified cell morphology and viability twenty-four hours after seeding and observed that the cell confluency exceeded 80%. Fresh media was added to the cells to obtain a final volume of 1 ml for cells seeded in 24 well plates. The cells were incubated with propidium iodide (PI, Sigma) at 1 μg/ml for 5 min prior to laser exposure. We generated a negative control consisting of cells incubated with PI but not exposed to the laser. We also used a positive control consisting of fixed cells that were permeabilized with 4% paraformaldehyde (PFA) and 0.1% Triton X-10. Further assessments of positive (PI uptake as expected) and negative controls (no PI uptake as expected) are discussed elsewhere [46]. The electrical conductivity of the solution with PI was 5 S/m.
We assessed PI uptake ten to fifteen minutes after laser illumination by using a Nikon Eclipse DIC phase microscope with 20× and 60× Plan Fluor objective lenses and the built-in Nikon software for image acquisition. The bright-field and cy3 images were overlaid and stitched in ImageJ software by an automated macro.
3. Results and discussion
3.1. Experimental observation of propidium iodide delivery
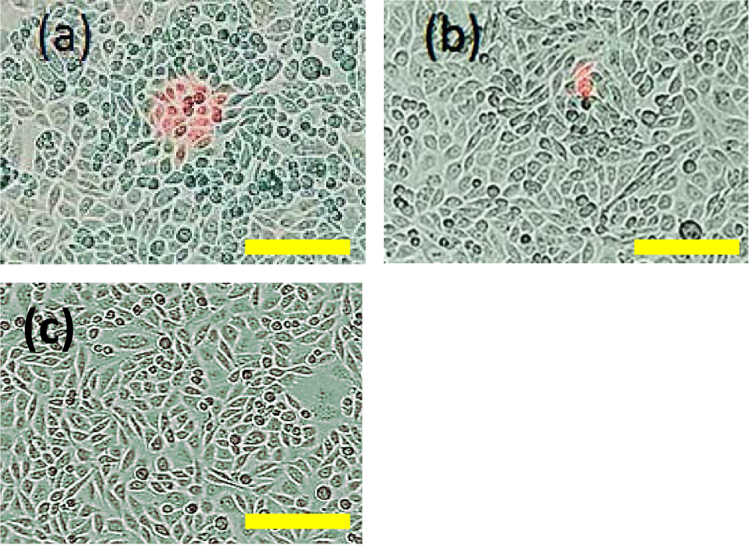
We assessed plasma membrane permeabilization following laser exposure by observing PI uptake. Fig. 2a shows that a five minute laser exposure permeabilized approximately 16 CHO cells, without changing cell viability. Fig. 2c shows the lack of PI uptake for cells in a sham experiment not treated with the laser. The delivery mechanism differs from typical optoinjection since the peak power density of 9.6×108 W/cm2 is well below the optical breakdown threshold of ~1012 W/cm2 [27], [45]. We successfully performed seven experiments with approximately 80% or higher efficiency of PI delivery to CHO cells using laser illumination with no changes in cell viability or morphology, as demonstrated by observation of morphology of cells post laser treatment and by calcein viability assays [46].
Fig. 2.
(a) CHO cells following a five minute treatment with a 1550 nm, 100 fs laser with 120 mW average power and 50 MHz repetition rate with the bar in (a) indicating the laser spot size. Permeabilized cells are stained red by propidium iodide (PI). (b) Illumination with the same laser in CW mode induced much less PI uptake. Calcein assays and morphological observations confirmed that cell viability was unchanged. (c) CHO cells not exposed to laser demonstrating the absence of membrane permeabilization by the lack of PI uptake. The typical laser spot size of 50 μm is shown by the bar above.
3.2. Calculation of laser induced temperature gradients
One potential mechanism involved in laser permeabilization and contributory to electroporation is ∇T generation [38]. PEFs induced ∇T depends upon pulse duration, repetition rate, and thermal diffusion time, τdiff [38]. Electric and laser pulses differ in their typical pulse durations and repetition rates. PEFs usually have repetition rates from 1–10 Hz and durations from microseconds to milliseconds for electroporation or 10–300 ns for nsPEFs [12], [16], [20]. Lasers for transfection typically have pulse durations from femtoseconds to nanoseconds and repetition rates as high as MHz, making the time between pulses on the order of microseconds or shorter compared to the typically much longer τdiff of 700 μs for a cell [38]. Because the absorption coefficient is proportional to the static conductivity from Drude's Law [47], we assume that the ratio between the absorption coefficients of the intracellular and extracellular fluids will be the same as between their conductivities (1.5 and 5 S/m, respectively). Since the plasma membrane has negligible impact on ∇T [38], the cell size becomes the relevant length scale for thermal diffusion, allowing us to write τdiff=ρcvR2/λ, where R is the cell radius (10 μm), ρ is the mass density of the suspension, cv is the specific heat capacity of the extracellular fluid, and λ is the thermal conductivity of the cell [38]. The reduced thermal dissipation between laser pulses will promote higher ∇T across the plasma membrane than for PEFs [38]. Laser interactions with the lipids and water directly at the membrane will be secondary because they act on the smaller length scale of the membrane, which has a much shorter τdiff. Because ∇T may impact permeabilization and molecular delivery in numerous ways, we first predict its magnitude.
We previously modeled the temperature change across a spherical cell and the resulting ∇T across the membrane for a Gaussian shaped electromagnetic pulse assuming that the temperature variation across the membrane was driven by the difference in heating between the intracellular and extracellular media [38]. For a simple first order approximation with an analytic solution, we assumed negligible cytoplasm heating, which permitted us to write the heat conduction for the extracellular fluid as [38]
| (1) |
where ΔT=T(r,t)–T0 is the temperature increase within the cell, T0 is the initial temperature of the cytoplasm, σe is the electrical conductivity of the extracellular fluid, and E is the electric field. Solving Eq. (1) analytically yields the maximum ∇T following multiple pulses, ∇T|max, for t>τdiff, τrep<τdiff, and τp<τrep, where τrep is the time between pulses, as [38]
| (2) |
where ΔTp is the temperature rise due to a single pulse.
We incorporate the laser parameters into Eq. (2) by considering two separate perspectives of laser application. A laser pulse will have a given energy density per unit volume and a given power density per unit volume. We will theoretically consider the impact of independently changing each of these on the electroporation threshold. The first condition we consider involves applying a fixed pulse energy density per unit volume, Ep=ΔTpρcv, where ρ and cv are the density and specific heat of the targeted material, respectively. The second scenario uses a fixed average power density per unit volume, 〈w〉=ΔTpρcv/τrep. Table 2 shows typical parameters for commercial 1550 nm lasers and the resulting ∇T|max. The 1 ps, 40 MHz, 1.2 W laser yields the highest ∇T (1.6×105 K/m).
Table 2.
Parameters for the 1550 nm lasers used here (first row) and others at 1550 nm that are commercially available. Here, τp=pulse duration, νrep=repetition rate, Wavg=average power, Wpeak=peak power, Ep=peak energy density, and ∇T|max=maximum calculated temperature gradient.
| τp (ps) | vrep (MHz) | Wavg (W) | Wpeak (kW) | Ep (J/cm3) | ∇T|max (K/m) |
|---|---|---|---|---|---|
| 0.1 | 50 | 0.12 | 24 | 9.6×10−4 | 2.7×106 |
| 10 | 40 | 1.5 | 3.75 | 1.5×10−2 | 1.3×107 |
| 1 | 40 | 1.2 | 30 | 1.2×10−2 | 1.6×107 |
| 0.5 | 40 | 0.5 | 25 | 5.0×10−3 | 8.0×106 |
Temperature gradients may generate plasma membrane electric fields that could contribute to or induce electropermeabilization [38]. The induced electric field, Ei, was estimated by Ei=kdT/dr, where r is the radial component and k ≈ 0.01 V/K is a conversion constant for water that only provides an initial estimate since it does not account for actual membrane structure [48]. While PEF induced ∇T generated insufficient electric fields for membrane permeabilization [38] assuming that the concomitant bulk temperature increase does not reduce electropermeabilization threshold [49], this estimate does permit assessing the laser parameters required to achieve a desired ∇T. Thus, we will assess ∇T|max>∇Tep, where ∇Tep is the minimum ∇T that may induce electropermeabilization assuming no temperature-induced change in the electropermeabilization threshold. Assuming a voltage between 0.1 and 1 V is necessary to electroporate a 10 nm membrane and the thermoelectric conversion factor from voltage to temperature is approximately 100 K/V, 109 K/m<∇Tep<1010 K/m [38]. Rearranging Eq. (2) and substituting the expressions for Ep and 〈w〉 for fixed energy density and fixed power density, respectively, gives τrep to achieve ∇Tep as
| (3) |
for fixed energy density or
| (4) |
for fixed power density, where τ*=(τdiff/τp)1/2. Note that the inequalities differ between the two requirements. Shorter τrep below the threshold in Eq. (3) will exceed the electroporation threshold for fixed energy density pulses. On the other hand, longer τrep above the threshold in Eq. (4) will exceed the electroporation threshold.
Fig. 3 shows curves satisfying ∇Tmax=∇Tep=109 K/m for constant Ep and constant 〈w〉 pulses. For a fixed energy density pulse of given τp, selecting τrep below the curve for the corresponding energy will give ∇Tmax>∇Tep. For instance, Fig. 3a shows that our laser parameters plot above the 9.6×10−4 J/cm3 curve for ∇T induced electropermeabilization by approximately three orders of magnitude, which means that the induced ∇T<∇Tep since τrep,E must fall below this curve to satisfy ∇Tep based on the inequality of Eq. (3). One can achieve ∇Tep by changing the operating parameters of the laser, such as by raising the wavelength from 1550 nm to 3000 nm. This increases the absorption coefficient of water [29] from 50 cm−1 to ~2×104 cm−1, which reduces the volume of laser irradiation absorption to 2×10−8 cm3, yielding Ep=0.32 J/cm3. A laser with Ep=0.32 J/cm3, τrep=2×10−8 s, and τp=100 fs gives ∇T~∇Tep, as demonstrated in Fig. 3a by the 0.32 J/cm3 line falling inside the data point for the laser with the given τrep and τp.
Fig. 3.
(a) Peak energy density (Ep) and (b) average power density (〈w〉) to achieve ∇Tep, the estimated temperature gradient necessary for direct electropermeabilization, as a function of pulse duration (τp) and time between pulses (τrep). Regions below (a) and above (b) the curves indicate ∇T>∇Tep, respectively. The black square represents the experimental condition used here at 1550 nm, τrep=20 ns, and tp=100 fs (Ep=9.6×10−4 J/cm3, 〈w〉=4.8x104 W/cm3).
For a laser applied with a fixed power density, the laser parameter the τrep for a given τp must fall above the curve for the corresponding energy to satisfy ∇Tmax>∇Tep. Using the same basic laser parameters with a fixed power density of 〈w〉=4.8×104 W/cm3, the square representing the laser parameters falls approximately three orders of magnitude below the corresponding 〈w〉 line, meaning that ∇Tmax<∇Tep since satisfying the inequality of Eq. (4) requires the experimental data point to be above the threshold. One may satisfy ∇Tmax=∇Tep for a laser with this τrep and τp by selecting a wavelength of 3000 nm, which increases the power density to 1.6x107 W/cm3 by reducing the absorption coefficient. Fig. 3b shows that the laser parameters then intersect the curve represented by this power density, indicating that it satisfies ∇Tmax<∇Tep. While our experimental conditions are approximately three orders of magnitude below ∇Tep, other factors may impact this threshold, which we will explore next.
3.3. Variation in temperature gradient electroporation threshold
The magnitude of ∇Tep can vary by one to two orders of magnitude because the membrane voltage for electroporation can vary by approximately an order of magnitude and several of the other parameters, such as membrane thickness, density, and thermal conductivity, may also vary. As a simple example of the influence of these variations, we consider the impact of increasing and decreasing ∇Tep by one order of magnitude from 109 K/m (from 108 to 1010 K/m) for Ep=9.6×10−4 J/cm3 and 〈w〉=4.8×104 W/cm3. Fig. 4 shows the impact of this variation in ∇Tep on the resulting thresholds as a function of τrep and τp. The changes in the threshold curves roughly scale with the changes in ∇Tep. In other words, increasing ∇Tep by an order of magnitude raises the curve by approximately an order of magnitude in τrep and decreasing it by an order of magnitude lowers the curve by approximately an order of magnitude. This provides some insight into the impact of variations on ∇Tep and what laser parameters are required to satisfy the potential range of for ∇T induced electropermeabilization.
Fig. 4.
The impact of uncertainties in the plasma membrane temperature gradient electroporation threshold ranging from 108 K/m to 1010 K/m for laser pulses of constant (a) Peak energy density (Ep) and (b) average power density (〈w〉). The black square represents the experimental condition used here at 1550 nm, τrep=20 ns, and tp=100 fs (Ep=9.6×10−4 J/cm3, 〈w〉=4.8x104 W/cm3). The experimental conditions have temperature gradients below the threshold in all cases, indicating the inability to induce electroporation through temperature gradients alone.
3.4. Bulk temperature effects on membrane permeabilization
In addition to temperature gradient effects, bulk heating may also impact membrane permeabilization and cellular behavior. Bulk heating of the cell and the laser illumination area may reduce ∇Tep [49]. For the full illumination area, we attain a thermal steady state given by Wavg/V=λΔT/Λ2, where Wavg is the average power of the laser (120 mW), V is the volume of treatment (R=50 μm, L=0.1 cm at 1550 nm), λ=0.6 W/m/K, and Λ is the heat transfer length, given by Λ−2=2.4052/R2+π2/L2. Using R⪡L and rearranging gives ΔT=10 K. Molecular dynamics (MD) simulations show that raising the temperature a few degrees kelvin reduces the electroporation threshold [49]; therefore, laser treatment reduces ∇Tep. While complicated because temperature impacts both membrane molecular properties and electroporation dynamics [50], [51], we can estimate the pore formation per unit area of the membrane by
| (5) |
where E is energy, K is Boltzmann's constant, Ea is pore formation energy, and r is pore radius [50]. Higher |S| implies closer proximity to the electroporation threshold. We assess the impact of a small temperature increase, dT, by calculating C=S(r,T+dT)/S(r,T), which gives the change of the pore formation per unit area due to dT . Assuming T̅=dT/T⪡1, we obtain T̅2–T̅+ln(C)/E˜=0, with E˜=Ea/(kT). For fixed C, increasing T̅ reduces E˜, facilitating pore formation. Alternatively, achieving the experimentally observed dT=10 K for E˜=45 [50] and T=300 K requires C=4.26.
MD simulations for assessing electroporation are often challenging because the pulses are long (~μs to ms) compared to computational capabilities (~ns). The laser pulses are subpicosecond, making them amenable to MD. MD simulations could elucidate the coupling of ∇T and electric fields on membrane permeabilization [52]. Specifically, the laser wave’s electric field will induce a membrane voltage in addition to that induced by the ∇T. Equating the laser (Wavgτp/τrep) and electromagnetic energies (ε|EL|2/2, where ε is the buffer permittivity and EL is the peak direct laser-induced electric field) yields EL=0.33 kV/cm with ε=80ε0. While much lower than the electric fields necessary to induce membrane effects for subnanosecond PEFs [53], laser exposure is more nuanced since the reduced time between laser pulses induces a greater temperature increase that may additionally reduce the electropermeabilization threshold.
3.5. Effect of CW laser heating on PI uptake and temperature gradients
One final point concerns the synergistic combination of the laser bulk temperature increase and temperature gradients. To assess the impact of bulk heating compared to temperature gradients, we also conducted experiments with the same 120 mW average power laser at 1550 nm in continuous wave (CW) mode. CW only increases ∇T up to t=τdiff, after which the cytoplasm and buffer heat together. Using CW reduces ∇T (∇T|CW=Wavgτdiff/(VρcpR)=8x105 K/m compared to 2.7×106 K/m for pulsed operation) and EL (7.5×10−4 kV/cm) by almost a factor of three compared to pulsed operation, while inducing a similar bulk temperature increase. Lower EL reduces electropermeabilization, while lower ∇T decreased membrane voltages and thermodiffusion through membrane pores. Overall, CW laser illumination induced negligible permeabilization and PI delivery, as shown in Fig. 2b. Thus, while combining ∇T, bulk temperature increase, and electric fields likely permeabilizes membranes, the same bulk temperature increase with reduced ∇T is insufficient. The synergistic combination of these effects clearly requires further investigation.
4. Conclusion
In summary, a wide-field femtosecond laser with peak power density several orders of magnitude below the optical breakdown threshold [27], [45] permeabilizes multiple cells without impacting viability. While most experiments assessed CHO cells and PI uptake, we successfully delivered other molecules to other cells, including phalloidin to CHO and NIH3T3 cells [46], or PI to rat mesenchymal cells (unpublished work). Calculations show that ∇T, bulk temperature increase, and direct laser-induced electric fields may contribute to this phenomenon; however, the reduced PI uptake in CW mode compared to pulsed mode indicates that ∇T must play a critical role. Future experiments could focus on developing interferometry systems to measure these rapid membrane temperature gradients. The importance of ∇T for laser-induced results resembles that for microwave-induced phenomena [39], [40], [41], [42]. Potential mechanisms may include electropermeabilization due to a ∇T induced membrane voltage [38], direct electropermeabilization by the laser’s electric field, a temperature-induced reduction of the electropermeabilization threshold, or some synergistic combination of these factors. Much as electric fields induce electrophoresis in addition to electroporation, ∇T may also induce thermodiffusion, in which molecules move through pores along the ∇T across the membrane [54], [55], [56]. Thermal diffusion of electrolytes, nonelectrolytes, and macromolecules arise for temperature gradients ~100–1000 K/m [56], which are three to five orders of magnitude lower than the calculated ∇T induced by the lasers described in Table 1, suggesting a potentially important contributory mechanism for molecular transport once membrane pores are formed, which requires future study. This is similar to the combination of electroporation and either electrophoresis (during the electric pulse) or diffusion (after the electric pulse), which require pore formation for ion transport [57].
We have further demonstrated that laser wavelength determines whether the surrounding buffer or the membrane will be preferentially heated based on the absorption coefficient. The wavelength of 1550 nm permeabilized cells within the 50×50 μm2 illumination area. We anticipate that the much larger absorption coefficient at 3000 nm will generate greater ∇T that could enhance biological effects. Lasers with the same peak power, frequency, and pulse widths at the 532 or 800 nm wavelengths common for optical transfection create dramatically lower ∇T due to the much smaller difference in absorption coefficient between lipids and the surrounding buffer. Much as tuning pulse durations and rise- and fall-times can control the targeting of PEF effects [16], appropriately selecting the average power, wavelength, repetition rate, illumination area, and pulse duration enables targeting laser effects to either one or multiple cells. This may yield a powerful tool for an inexpensive (the fiber laser used in these tests is relatively low cost, <$10 k), high throughput, easy to use (no complicated optics for focusing the laser beam to a narrow spot and aligning the beam to the cell of interest) laser mediated exogenous molecule delivery.
Contributor Information
Allen L. Garner, Email: algarner@purdue.edu.
V. Bogdan Neculaes, Email: neculaes@research.ge.com.
References
- 1.Glat M.J., Offen D. Cell and gene therapy in Alzheimer's disease. Stem Cells Dev. 2013;22:1490–1496. doi: 10.1089/scd.2012.0633. [DOI] [PubMed] [Google Scholar]
- 2.Bӓck S., Perӓnen J., Galli E., Pulkkila P., Lonka-Nevalaita L., Tamminen T., Voutilainen M.H., Raasmaja A., Saarma M., Mӓnnistӧ P.T., Tuominen R.K. Gene therapy with AAV2-CDNF provides functional benefits in a rat model of Parkinson's disease. Brain Behav. 2013;3:75–88. doi: 10.1002/brb3.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tilemann L., Ishikawa K., Weber T., Hajjar R.J. Gene therapy for heart failure. Circ. Res. 2012;110:777–793. doi: 10.1161/CIRCRESAHA.111.252981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson L.A., Morgan R.A., Dudley M.E. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood. 2009;114:535–546. doi: 10.1182/blood-2009-03-211714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaese R.M., Culver K.W., Miller A.D. T lymphocyte-directed gene therapy for ADA SCID: initial trial results after 4 years. Science. 1995;270:475–480. doi: 10.1126/science.270.5235.475. [DOI] [PubMed] [Google Scholar]
- 6.Niidome T., Huang L. Gene therapy progress and prospects: nonviral vectors. Gene Ther. 2002;9:1647–1652. doi: 10.1038/sj.gt.3301923. [DOI] [PubMed] [Google Scholar]
- 7.Felgner P.L., Gadek T.R., Holm M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. U. S. A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gehl J. Electroporation: Theory and methods, perspectives of drug delivery, gene therapy and research. Acta. Physiol. Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 9.Mitragotri S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev.: Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 10.Sandhu K.K., McIntosh C.M., Simard J.M. Gold nanoparticle-mediated transfection of mammalian cells. Bioconjug. Chem. 2002;13:3–6. doi: 10.1021/bc015545c. [DOI] [PubMed] [Google Scholar]
- 11.Tirlapur U.K., Konig K. Cell biology: targeted transfection by femtosecond laser. Nature. 2002;418:290–291. doi: 10.1038/418290a. [DOI] [PubMed] [Google Scholar]
- 12.Weaver J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000;28:24–33. [Google Scholar]
- 13.Satkauskas S., Ruzgys P., Venslauskas M.S. Towards the mechanisms for efficient gene transfer into cells and tissues by means of cell electroporation. Expert Opin. Biol. Ther. 2012;12:275–286. doi: 10.1517/14712598.2012.654775. [DOI] [PubMed] [Google Scholar]
- 14.Golberg A., Yarmush M.L. Nonthermal irreversible electroporation: Fundamentals, applications, and challenges. IEEE Trans. Biomed. Eng. 2013;60:707–714. doi: 10.1109/TBME.2013.2238672. [DOI] [PubMed] [Google Scholar]
- 15.Žgalin M.K., Hodžic D., Reberŝek M., Kanduŝer M. Combination of microsecond and nanosecond pulsed electric field treatments for inactivation of Escherichia coli in water samples. J. Membr. Biol. 2012;245:643–650. doi: 10.1007/s00232-012-9481-z. [DOI] [PubMed] [Google Scholar]
- 16.Schoenbach K.H., Joshi R.P., Kolb J.F. Ultrashort electrical pulses open a new gateway into biological cells. Proc. IEEE. 2004;92:1122–1137. [Google Scholar]
- 17.Beebe S.J., Fox P.M., Rec L.J. Nanosecond pulsed electric field (nsPEF) effects on cells and tissues: apoptosis induction and tumor growth inhibition. IEEE Trans. Plasma Sci. 2002;30:286–292. [Google Scholar]
- 18.Scarlett S.S., White J.A., Blackmore P.F. Regulation of intracellular calcium concentration by nanosecond pulsed electric fields. Biochim. Biophys. Acta – Biomembr. 2009;1788:1168–1175. doi: 10.1016/j.bbamem.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Napotnik T.B., Wu Y.-H., Gundersen M.A., Miklavcic D., Vernier P.T. Nanosecond electric pulses cause mitochondrial membrane permeabilization in Jurkat cells. Bioelectromagnetics. 2012;33:257–264. doi: 10.1002/bem.20707. [DOI] [PubMed] [Google Scholar]
- 20.Pakhomov A.G., Kolb J.F., White J.A., Joshi R.P., Xiao S., Schoenbach K.H. Long-lasting plasma membrane permeabilization in mammalian cells by nanosecond pulsed electric field (nsPEF) Bioelectromagnetics. 2007;28:655–663. doi: 10.1002/bem.20354. [DOI] [PubMed] [Google Scholar]
- 21.Katsuki S., Nomura N., Koga H., Akiyama H., Uchida I., Abe S. Biological effects of narrow band pulsed electric fields. IEEE Trans. Dielectr. Electr. Insul. 2003;14:663–668. [Google Scholar]
- 22.Antkowiak M., Torres-Mapa M.L., Gunn-Moore F., Dholakia K. Application of dynamic diffractive optics for enhanced femtosecond laser based cell transfection. J. Biophotonics. 2010;3:696–705. doi: 10.1002/jbio.201000052. [DOI] [PubMed] [Google Scholar]
- 23.Clark I.B., Hanania E.G., Stevens J., Gallina M., Fieck A., Brandes R., Palsson B.O., Koller M.R. Optoinjection for efficient targeted delivery of a broad range of compounds and macromolecules into diverse cell types. J. Biomed. Opt. 2006;11:014034. doi: 10.1117/1.2168148. [DOI] [PubMed] [Google Scholar]
- 24.Stevenson D.J., Gunn-Moore F.J., Campbell P., Dholakia K. Single cell optical transfection. J. R. Soc. Interface. 2010;7:863–871. doi: 10.1098/rsif.2009.0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Venugopalan V., Guerra A., III, Nahen K., Vogel A. Role of laser-induced plasma formation in pulsed cellular microsurgery and micromanipulation. Phys. Rev. Lett. 2002;88:078103. doi: 10.1103/PhysRevLett.88.078103. [DOI] [PubMed] [Google Scholar]
- 26.Soughayer J.S., Krasieva T., Jacobson S.C., Ramsey J.M., Tromberg B.J., Allbritton N.L. Characterization of cellular optoporation with distance. Anal. Chem. 2000;72:1342–1347. doi: 10.1021/ac990982u. [DOI] [PubMed] [Google Scholar]
- 27.Vogel A., Noack J., Hüttman G., Paltauf G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B. 2005;81:1015–1047. [Google Scholar]
- 28.Vogel A., Noack J. Numerical simulations of optical breakdown for cellular surgery at nanosecond to femtosecond time scales. Proc. SPIE. 2001;4260:83. [Google Scholar]
- 29.〈http://www1.lsbu.ac.uk/water/water_vibrational_spectrum.html〉 (accessed 23.08.15).
- 30.Warren S.G. Optical constants of ice from the ultraviolet to the microwave. Appl. Opt. 1984;23:1206–1225. doi: 10.1364/ao.23.001206. [DOI] [PubMed] [Google Scholar]
- 31.Quickenden T.I., Irvin J.A. The ultraviolet absorption spectrum of liquid water. J. Chem. Phys. 1980;72:4416–4426. [Google Scholar]
- 32.H. Buiteveld, J.M.H. Hakvoort, M. Donze, Optical properties of pure water, in: J.S. Jaffe (ed.) SPIE Proceedings on Ocean Optics XII, 2258, 1994, pp. 174–183
- 33.Hale G.M., Querry M.R. Optical constants of water in the 200-nm to 200-μm wavelength region. Appl. Opt. 1973;12:555–563. doi: 10.1364/AO.12.000555. [DOI] [PubMed] [Google Scholar]
- 34.R.L.P. van Veen, H.J.C.M. Sterenborg, A. Pifferi, A. Torricelli, R. Cubeddu, Biomedical Topical Meeting.Meeting, Determination of VIS- NIR absorption coefficients of mammalian fat, with time- and spatially resolved diffuse reflectance and transmission spectroscopy, 2004, Optical Society of America, Miami, FL [DOI] [PubMed]
- 35.Jansen E.D., van Leeuwen T.G., Motamedi M., Borst C., Welch A.J. Temperature dependence of the absorption coefficient of water for midinfrared laser radiation. Lasers Surg. Med. 1994;14:258–268. doi: 10.1002/lsm.1900140308. [DOI] [PubMed] [Google Scholar]
- 36.〈http://www.npsg.uwaterloo.ca/data/skin/lipid_absorption.txt〉 (accessed 23.08.15).
- 37.G.B. Altshuler, R.R. Anderson, D. Manstein, Method and Apparatus for the Selective Targeting of Lipid-rich Tissues, U. S. Patent No. 7,060,061 B2 (13.06.06).
- 38.Garner A.L., Deminsky M., Neculaes V.B., Chashihin V., Knizhnik A., Potapkin B. Cell membrane thermal gradients induced by electromagnetic fields. J. Appl. Phys. 2013;113:214701. [Google Scholar]
- 39.Saffer J.D., Profenno L.A. Microwave-specific heating affects gene expression. Bioelectromagnetics. 1992;13:75–78. doi: 10.1002/bem.2250130109. [DOI] [PubMed] [Google Scholar]
- 40.Doran T.J., Lu P.J., Vanier G.S. Microwave irradiation enhances gene and oligonucleotide delivery and induces effective exon skipping in myoblasts. Gene Ther. 2009;16:119–126. doi: 10.1038/gt.2008.144. [DOI] [PubMed] [Google Scholar]
- 41.Galvin M.J., Hall C.A., McRee D.I. Microwave radiation effects on cardiac muscle cells in vitro. Radiat. Res. 1981;86:358–367. [PubMed] [Google Scholar]
- 42.Friend A.W., Gartner S.L., Foster K.R., Howe H. The effects of high power microwave pulses on red blood cells and the relationship to transmembrane thermal gradients. IEEE Trans. Microw. Theory Technol. 1981;29:1271–1277. [Google Scholar]
- 43.Rudhall A.P., Antkowiak M., Tsampoula X. Exploring the ultrashort pulse laser parameter space for membrane permeabilisation in mammalian cells. Sci. Rep. 2012;2:858. doi: 10.1038/srep00858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson D., Agate B., Tsampoula X. Femtosecond optical transfection of cells: viability and efficiency. Opt. Express. 2006;14:7125–7133. doi: 10.1364/oe.14.007125. [DOI] [PubMed] [Google Scholar]
- 45.He H., Kong S.-K., Lee R. K-Y. Targeted photoporation and transfection in human HepG2 cells by a fiber femtosecond laser at 1554 nm. OpticsOpt. Lett. 2008;33:2961–2963. doi: 10.1364/ol.33.002961. [DOI] [PubMed] [Google Scholar]
- 46.V.B. Neculaes, K.R. Conway, A.L. Garner, E.R. Loghin, S. Yazdanfar, D.V. Dylov, B.M. Davis, C. Joo, Optical Based Delivery of Exogenous Molecules to Cells,” U. S. Patent No. 8,778,682 (issued 15 July 2014).
- 47.C. Ndebeka-Bandou, F. Carosella, R. Ferreira, et al., Free carrier absorption and inter-subband transitions in imperfect heterostructures, Semicond. Sci. Technol. 29 (2014) 023001.
- 48.Bresme F., Lervik A., Bedeaux D., Kjelstrup S. Water polarization under thermal gradients. Phys. Rev. Lett. 2008;101:020602. doi: 10.1103/PhysRevLett.101.020602. [DOI] [PubMed] [Google Scholar]
- 49.Song J., Joshi R.P., Schoenbach K.H. Synergistic effects of local temperature enhancements on cellular responses in the context of high-intensity, ultrashort electric pulses. Med. Biol. Eng. Comput. 2011;49:713–718. doi: 10.1007/s11517-011-0745-z. [DOI] [PubMed] [Google Scholar]
- 50.Garner A.L., Neculaes V.B. Extending membrane pore lifetime with AC fields: a modeling study. J. Appl. Phys. 2012;112:014701. [Google Scholar]
- 51.Joshi R.P., Hu Q., Schoenbach K.H., Hjalmarson H.P. Improved energy model for membrane electroporation in biological cells subjected to electrical pulses. Phys. Rev. E. 2002;65:041920. doi: 10.1103/PhysRevE.65.041920. [DOI] [PubMed] [Google Scholar]
- 52.M. Deminsky, B. Potapkin, 21st International Symposium on Plasma Chemistry, Cairns, Australia, 4-9 August, 2013
- 53.Schoenbach K.H., Xiao S., Joshi R.P. The effect of intense subnanosecond electrical pulses on biological cells. IEEE Trans. Plasma Sci. 2008;36:414–422. [Google Scholar]
- 54.Duhr S., Braun D. Why molecules move along a temperature gradient. Proc. Natl. Acad. Sci. U. S. A. 2006;103:19678–19682. doi: 10.1073/pnas.0603873103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reineck P., Wienken C.J., Braun D. Thermophoresis of single stranded DNA. Electrophoresis. 2010;31:279–286. doi: 10.1002/elps.200900505. [DOI] [PubMed] [Google Scholar]
- 56.Bonner F.J., Sundelöf L.-O. Thermal diffusion as a mechanism for biological transport. Z. Naturforschung. 1984;39:656–661. doi: 10.1515/znc-1984-0623. [DOI] [PubMed] [Google Scholar]
- 57.A.L. Garner, J.J. Maciejewski, A. Vadlamani, R.J. Byer, Electric pulse shape impact on biological effects: a modeling study, in: Proceedings of the 2015 Annual Report Conference on Electrical Insulation and Dielectric Phenomena, Ann Arbor, MI, 2015, pp. 632–635