Abstract
Considerations on antioxidants derived from plants have continuously increased during this decade because of their beneficial effects on human health. In the present study we investigated the free radical scavenging properties of extracts from Piper guineense (P. guineense) and their inhibitory potentials against oxidative mediated ion toxicity. The free radical quenching properties of the extracts against [1,1-diphenyl-2-picrylhydrazyl (DPPH•), 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid) (ABTS•), hydroxyl radical (HO•), nitric oxide (NO•)] radical and their antioxidant potentials by FRAP and phosphomolybdenum were determined as well as their protective properties on liver enzymes. The phenolic profile was also investigated by HPLC. The results obtained, revealed that the extracts significantly inhibited the DPPH, NO, HO and ABTS radicals in a concentration depending manner. They also showed a significant ferrous ion chelating ability through FRAP and phosphomolybdenum antioxidant potential. Their polyphenol contents varied depending on the type of extracts and the solvent used. The hydroethanolic extracts (FFH) and the ethanolic extracts (FFE) of P. guineense leaves showed the higher level of phenolic compounds respectively of 21.62 ± 0.06 mg caffeic acid/g dried extract (CAE/g DE) and 19.01 ± 0.03 CAE/g DE. The HPLC phenolic compounds profile revealed a higher quantity of Eugenol, quercetin, rutin and catechin in the stem than in the leaves. The presence of these molecules could be responsible of the protective potentials of P. guineense extracts against lipid peroxidation and SOD, catalase and peroxidase.
In conclusion, P. guineense extracts demonstrated significant antioxidant property and may be used as a prospective protector against metal related toxicity.
Abbreviations: MDA, malonaldialdehyde; DPPH, 2,2-diphenyl-1-picrylhydrazyl 1,1-diphenyl-2-picrylhydrazyl radical; FRAP, ferric reducing ability of plasma; Vit C, vitamine C; ABTS, 2,2-azinobis(3-ethylbenzthiazoline)-6-sulphonic acid; BHT, butylated hydroxytoluene; RNS, reactive nitrogen species; ROS, reactive oxygen species; FRAP, ferric reducing antioxidant power; MDA, malondialdehyde; TBA, thiobarbituric acid; H2O2, hydrogen peroxide
Chemical compounds studied in this article: Eugenol (PubChem CID: 3314); 1,1-Diphenyl-2-picrylhydrazine (PubChem CID: 74358); 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulphonic acid (PubChem CID: 77519615); Ascorbic acid (PubChem CID: 54670067); Eugenol (PubChem CID: 3314); Tyrosol (PubChem CID: 10393); Quercetin (PubChem CID: 5280343); Rutin (PubChem CID: 5280805); Apigenin (PubChem CID: 5280443); Caffeic acid (PubChem CID: 689043); P-coumaric acid (PubChem CID: 637542); Syringic acid (PubChem CID: 10742); Theobromine (PubChem CID: 5429)
Keywords: P. guineense, Ion toxicity, Antioxidant, HPLC, Lipid peroxidation, Eugenol, O-coumaric acid
1. Introduction
The human body is continuously producing free radicals which, depending on their intracellular concentration and the equilibrium between them and the endogenous antioxidant species, can lead to oxidative stress. Several factors are known to be linked to the impairment of the body's potential in stabilizing free radicals. Among them, factors such as drugs, immune responses to viruses, deficiency of natural antioxidants, pollution, ultraviolet rays and tobacco could be listed [1], [2].
The body has the ability to scavenge free radicals, but if there is disequilibrium between the level of free radicals and the capacity of the body to neutralize them, oxidative stress occurs as a result [1]. The development of oxidative stress depends on the concentration reactive oxygen species (ROS) in the cell. These species have a physiological function in cell processes including the cell proliferation, the cell cycle and death as well as signal transduction [3]. Nevertheless, their high reactivity has led to the damaging effects of ROS on vital tissues [3], [4]. These destructive activities on the cells and subsequent damage of vital cell components have been associated with several severe health disorders such as endothelial dysfunctioning, angiogenesis, vascular diseases and cancer [2], [5].
Indeed, recent research have pointed out that ROS-generating enzymes such as Nox proteins are considered to be oncogenic proteins, and can cause mitochondrial dysfunction which is associated with the genesis of tumor and the physiological states involved in neo-vascularization, tumor development and progression (angiogenesis) [2]. ROS production has been demonstrated to increase the level of vascular endothelial growth factor (VEGF), which is highly unregulated in most human cancers, and which has recently emerged as the crucial rate-limiting protein in the regulation of angiogenesis in many human tumors [6], [7]. The reduction or elimination of ROS requires the action of antioxidants [5]. Antioxidants could be defined as the cellular defense mechanism against the damaging effects of high concentrations of reactive species.
Several studies have demonstrated the beneficial effects of antioxidant compounds in cancer treatment [8]. Recent researches have therefore focused on the protective role, as the preventive agent of antioxidant compounds against angiogenic aided cancer development, and support the hypothesis that antioxidant supplementation during chemotherapy has the potential to reduce dose-limiting toxicities in patients with cancer through free radical scavenging pathways or activities [2]. A growing number of research data from complementary (epidemiological and laboratory) investigations have proved that some edible plants as a whole or their documented ingredients with antioxidant potentials possess considerable protective effects on human tumor genesis [8]. Comparable evidence has also been identified to demonstrate the chemopreventive properties of phytochemicals and compounds from diets of plant origin with free-radical chelating properties on ulcers, diabetes, memory and cognitive functions, Alzheimer disease, sexual and spermatogenesis dysfunctions, atherosclerosis and neuro-vascular disorders and several other human illnesses [9], [10], [11], [12], [13], [14]. Moreover, some medicinal herbs have been demonstrated to possess both preventive and/or healing effects. Spices are well-known as sources of natural compounds that can prevent oxidative stress and thus have a significant role in chemoprevention against degenerative illnesses [10]. The therapeutic properties of spices are primarily attributed to the presence of a large range of phytochemical molecules. In fact, phytochemicals present in spices are very diversed and possess anticarcinogenic and other beneficial properties, they are therefore referred to as chemopreventers [15]. One of the predominant mechanisms of their protective action is due to their antioxidant activity and the capacity to scavenge free radicals. Among the most investigated chemopreventers are some vitamins, polyphenols and pigments of plant such as carotenoids, chlorophylls, flavonoids, and betalains [15], [16]. This medicinal potential may also be influenced by other organic and inorganic compounds such as coumarins, phenolic acids and antioxidant micronutrients (Cu, Mn, Zn) [15], [17], [18].
Piper guineense (P. guineense), commonly named African black pepper or hot leave is broadly consumed as spice in various parts of Africa such as Cameroon, Nigeria and Ghana because of its nutritional and therapeutic properties [19]. Piper guineensis is a plant of the family of Piperaceae. In African folk herbal medicine, its seeds are used for various purposes. For example, in certain regions of Nigeria, the seeds are consumed by women after child birth, to improve uterine contraction for the expulsion of the placenta and other residues from the womb, as an adjuvant in the management of rheumatic pains, as an antiasthmatic and also as weight regulator [19]. The seeds and leaf extracts have been demonstrated to possess a depolarizing neuromuscular potential in concentration depending manner. The antiparasitic, antimicrobial and antifungal properties of the leaves and seeds of P. guineense have also been reported [19], [20]. P. guineense has been used by traditional medicinal practitioners for the treatment of respiratory diseases and correction of female infertility problems [19]. Its use also in traditional medicine for male sexual weakness treatment and to prevent abortion has also been investigated [21], [22]. Previous phytochemical researches on P. guineense extracts revealed the existence of numerous compounds such as alkaloids, sterols, lignans and amides [22]. Pharmacological and physiological studies of P. guineense extracts revealed insecticidal properties. It has been shown that, eight days treatment of uncastrated mature male rats with variable doses of the alcoholic dry fruit extract of P. guineense significantly increased penis erection index, mount frequency, intromission frequency, ejaculation frequency, body weight and seminal vesicle secretion [23]. From the previous data outlined above, it is obvious that research focused on other parts of P. guineense such as its stem and leaves are scarce and its potential in preventing metal mediated oxidative damage has not been described. Therefore in the present study, we have attempted to demonstrate the free radical scavenging potential of the leaves and stem of P. guineense, to describe its antioxidant potential and the mechanism involved in this activity, to investigate its potential as a chemopreventer against metal induced damage on liver enzymes and to identify the more active extract on these activities.
2. Materials and methods
2.1. Plant material
The leaves and stem of P. guineense were collected at the Kala Mountain in the center region of Cameroon. They were identified by Mr NANA, a botanist of the National Herbarium of Cameroon, in comparison to the voucher specimens (43129-HNC).
2.2. Plant and animal material
2.2.1. Preparation of plant extracts
The collected leaves and stem barks were dried at the ambient temperature, crushed and sifted. Powders were then macerated at the ratio of 1:10 (w/v) for 48 h in ethanol for the ethanolic extract and in a mixture of water/ethanol (30/70; pH = 3) for the hydro-ethanolic extract. The mixtures were then filtered using Buchner funnel and Whatman No. 1 filter paper. This process was repeated once on the residue after 24 h. The supernatant was concentrated using a rotavaporator and the extract was dried in an oven at 55 °C for two days. Each crude extract obtained was labeled using the following codes: FFE for the leaves ethanolic extract of P. guineense; FTE: P. guineense (stem) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; FTH: P. guineense (stem) hydroethanolic extract. The different samples were then kept at 4 °C. Prior to experimentation, the solutions of the four plant extracts were dissolved using different dilutions of ethanol (25, 50, 75, 150, 300 μg/mL) of each.
2.2.2. Animals
Male albino Wistar rats weighing 200–250 g were used in this study. The rats were maintained at room temperature under lab conditions and were fed with standard diet and water ad libitum. Livers were collected after dissection of the rats under mild ether anesthesia on overnight fasted rats. This study was carried out with approval from the animal Ethics Committee of university of Yaoundé I.
2.3. Determination of free radical scavenging potential of the samples
2.3.1. Scavenging activity of DPPH radical
The DPPH assay measures the free radical scavenging capacity of the extracts as previously described by Blois [24]. Three milliliters of each of the diluted extracts were put in a test tube and 1 mL of methanol solution of DPPH (0.1 mM) was added. The mixture was kept in the dark at room temperature for 30 min and absorbance was measured at 517 nm against a blank in a spectrophotometer. The same procedure was used for vitamin C that was used as standard. The following equation was used to determine the percentage of the radical scavenging activity of each extract:
where Ao is the absorbance of the blank and As is the absorbance of the sample.
2.3.2. Scavenging effect of the ABTS+ radical
The ABTS assay was based on a previously described method of Re et al. [25] with slight modifications. ABTS radical cation (ABTS+) was produced by the reaction of a 7 mM ABTS solution with potassium persulphate (2.45 mM). The ABTS+ solution was diluted with ethanol to an absorbance of 0.70 ± 0.05 at 734 nm. The mixture was stored in the dark at room temperature for 12 h before used. After addition of 25 μL of sample extract or vitamin C used as standard to 2 mL of diluted ABTS+ solution, absorbance was measured at 734 nm after exactly 6 min. The decrease in absorbance was used for calculating the scavenging effect values. The following equation was used to determine the percentage of the radical scavenging activity of each extract:
where Ao is the absorbance of the blank and As is the absorbance of the sample.
2.3.3. Nitric oxide scavenging activity
Nitric oxide scavenging activity was determined according to previous authors [26]. The reaction mixture contained 2 mL of sodium nitroprusside (10 mM) in 0.5 mL phosphate buffer (0.5 M; pH 7.4). Various concentrations (25, 50, 75, 150, 300 μg/mL) of the extracts (0.5 mL) were added in a final volume of 3 mL. After incubation for 60 min at 37 °C, Griess reagent [α-napthyl-ethylenediamine (0.1%) and sulphanilic acid (1%) in H3PO4 (5%)] was added. The pink chromophore generated during diazotization of nitrite ions with sulphanilamide and subsequent coupling with α-napthyl-ethylenediamine was measured in a spectrophotometer at 540 nm. Ascorbic acid was used as a positive control. The scavenging ability (%) of the nitric oxide was calculated using the formula:
where Ao is the absorbance of the blank and As is the absorbance of the sample.
2.3.4. Hydroxyl radical scavenging activity
The scavenging activity of the extracts on hydroxyl radical was measured according to a previously described method [27]. To 1.5 mL of each diluted extract, 60 μL of FeCl3 (1 mM), 90 μL of 1,10-phenanthroline (1 mM), 2.4 mL of phosphate buffer (0.2 M; pH 7.8) and 150 μL of H2O2 (0.17 M) were added respectively. The mixture was then homogenized using a vortex and incubated at room temperature for 5 min. The absorbance was read at 560 nm against the blank. The percentage of the radical scavenging activity of each extract was calculated from the equation below:
where Ao is the absorbance of the blank and As is the absorbance of the sample.
2.4. Determination of the total antioxidant potential of the different samples
2.4.1. Total antioxidant activity by ferric reducing antioxidant power assay (FRAP)
The FRAP was determined using a previously described method [28] with slight modifications. The fresh FRAP reagent consisted of 500 mL of acetate buffer (300 mM; pH 3.6), 50 mL of 2,4,6-tri (2-pyridyl)-s-triazin (TPTZ) (10 mM), and 50 mL of FeCl3·6H2O (50 mM). The colorimetric measurement was carried out at 593 nm and the measurement was monitored up to 12 min on 75 μL of each extract and 2 mL of FRAP reagent. Vitamin C was used to draw a standard curve and the butylated hydroxy toluene (BHT) was used for the comparison with the different samples. The results were expressed as mg equivalent vitamin C/g of dried extract (mg equiv. VitC/g DE).
2.4.2. Phosphomolybdenum antioxidative power (PAP)
The total antioxidant activity of the extracts was evaluated by green phosphomolybdenum complex [29]. An aliquot of 10 μL of the extract solution was mixed with 1 mL of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) in a micro centrifuge tube. The tubes were incubated in a dry thermal bath at 95 °C for 90 min. After cooling, the absorbance of the mixture was measured at 695 nm against a blank. Vitamin C was used as reference to draw the standard curve and BHT was used for the comparison. The reducing capacities of the analyzed extracts were expressed as mg of ascorbic acid equivalents/g of dried extract (mg equiv. AS/g DE).
2.4.3. Reducing power assay
The reducing power of the extracts was determined by a method described by Oyaizu [30]. Different concentrations of the extracts in 1 mL of distilled water were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferrocyanide (1%). The mixtures were incubated at 50 °C for 20 min. Aliquots 2.5 mL of trichloroacetic acid (10%) were added to the mixture and centrifuged at 3000 rpm for 10 min. The supernatant of the solution (2.5 mL) was mixed with 2.5 mL of distilled water and 0.5 mL of FeCl3 (0.1%). The absorbance was measured at 700 nm.
2.5. Determination of the phenolic content of the extracts
2.5.1. Total phenol determination
The total phenol content was determined by the Folin–Ciocalteu method [31]. The reaction mixture contained 200 μL of extract, 800 μL of freshly prepared diluted Folin–Ciocalteu reagent and 2 ml of sodium carbonate (7.5%). The final mixture was diluted to 7 mL with deionized water and kept in the dark at ambient conditions for 2 h to complete the reaction. The absorbance was measured at 765 nm. Caffeic acid was used as standard and the results were expressed as mg caffeic acid/g dried extract (mg CA/g DE).
2.5.2. Determination of total flavonoid content
Total flavonoid content was determined using aluminum chloride (AlCl3) according to a known method [32] using quercetin as a standard. A volume of 0.1 mL of extract was added to 0.3 mL distilled water followed by 0.03 mL of NaNO2 (5%). After 5 min at 25 °C, 0.03 mL of AlCl3 (10%) was added. After a further 5 min, the reaction mixture was mixed with 0.2 mL of 1 mM NaOH and finally, the reaction mixture was diluted to 1 mL with distilled water and the absorbance was measured at 510 nm. The results were expressed as quercetin equivalent mg/g of dried extract (QE/g dried ext).
2.5.3. Determination of total flavonols
Total flavonols in the plant extracts were estimated using a known method [33] with some modifications. To 2.0 mL of sample, 2.0 mL of 2% ethanolic solution of AlCl3 and 3.0 mL (50 g/L) sodium acetate solutions were added. After 2.5 h of incubation at 20 °C, the absorbance was read at 440 nm. The results were expressed as quercetin equivalent (mg/g) dried extract (QE/g dried ext).
2.5.4. Quantification of phenolic compounds by HPLC
High performance liquid chromatography (HPLC) with UV detection is frequently used for the separation and detection of phenolic compounds in hydroalchoolic extracts. Samples were dissolved in pure water following the ratio (0.3 g/10 mL) and centrifuged at 4706 rpm for 10 min. The supernatant was filtered through a cellulose acetate membrane filter (0.20 μm or 0.45 μm, Schleicher & Schuell) and used for analysis. The analysis was performed on an Agilent Technologies 1200 HPLC system fitted with a SUPELCOSIL LC-18 column (length 250 mm, diameter 4.6 mm, packaging size 5 mm). The column temperature was set at 20 °C. The mobile phase consisted of a mixture of an aqueous solution of acetic acid (0.5%) by volume (“A”) and acetic nitrile (“B”). Elution was performed by using 100% of A for the first 2 min of the run, 40% of A and 60% of B from 2 to 60 min. The flow rate was set equal to 1 mL/min and the injection volume was 25 μL. Polyphenols were detected by a UV detector (280 nm). The retention times of the identified polyphenolic compounds of interest were measured by a single standard solution at a concentration of 100 mg/L.
2.6. Protective properties of the plant against oxidative damage
2.6.1. Preparation of liver homogenate
The liver was isolated from 3 normal albino Wistar rats. The organs were weighed and 10% (w/v) homogenate was prepared in phosphate buffer (0.1 M, pH 7.4 having 0.15 M KCl) using the homogenizer at 4 °C [34]. The homogenate was centrifuged at 3000 rpm for 15 min and the clear cell-free supernatant obtained was used for the study.
2.6.2. Preparation of the pro-oxidative solution
The oxidant solution was prepared, directly before its use by adding a solution of ferric chloride 100 mM to H2O2 0.50% prepared in phosphate buffer (0.1 M, pH 7.4). This solution was used for the investigation of the protective assays on liver enzymes.
2.6.3. Total protein content
The total protein content of the liver mixture was measured according to the protein kit supplier method (Human Kit-Hu102536, Boehringer Ingelheim, Germany). This result was used to express the activities of the different enzymes per gram of organs.
2.6.4. In vitro lipid peroxidation assay
Lipid peroxidation assay was performed by a formerly described protocol [35]. 0.58 mL of phosphate buffer (0.1 M; PH 7.4), 200 μL of sample, 200 μL of liver homogenate and 20 μL of ferric chloride (100 mM) were combined to form a mixture which was placed in a shaking water bath for 1 h at 37 °C. The reaction was completed by adding 1 mL of TCA (10%) and 1 mL of TBA (0.67%) to all the tubes and these were placed in a boiling water bath for 20 min. After this, the test tubes were shifted to crushed ice bath and were centrifuged at 3000 rpm for 10 min. The absorbance of the supernatant was measured at 535 nm and evaluated as nM of MDA tissue using the molar extinction coefficient of 1.56 × 105/M cm.
2.6.5. Determination of peroxidase activity
Peroxidase activity was determined by the peroxidase kit method (CAS Number 7722-84-1, Sigma–Aldrich) supplier with modifications. A solution containing the mixture of 1 mL of the oxidant solution (FeCl3, 100 mM) and the extract or vitamin C (standard) for a final concentration of 100 μg/mL was incubated for 1 h in a water bath at 37 °C. An aliquot of PBS (0.1 mL), hydrogen peroxide (50 μL), and pyrogallol solution (110 μL) were added to 625 μL of distilled water that was earlier poured into an Eppendorf tube. The plant extract (75 μL) from the mixture was thereafter added. For the blank, the control oxidant solution and vitamin C used as the standard constituted the mixture. The same reagents were used except that, the extract was replaced by distilled water (75 μL). This composition was homogenized and incubated for at least 10 min. The solution containing 100 mM, pH 6.0 PBS (40 μL) and 0.002% (v/v) of diluted liver homogenate (40 μL) was added to the blank and test mixtures respectively. These were homogenized and the increase in absorbance at 420 nm was measured after every 10 s for 3 min using a spectrophotometer (BioMate 3S UV-Visible, Thermo Scientific™ Manufacturer, Wohlen, Switzerland). One unit of peroxidase was defined as the change in absorbance/seconds/mg of protein at 420 nm using molar extinction coefficient of 12/M cm.
2.6.6. Determination of catalase activity
Prior to the test, a solution containing a mixture of 1 mL total volume of the oxidant solution and extract or vitamin C (standard) for a final concentration of 100 μg/mL was incubated for 1 h in a water bath at 37 °C. The catalase activity of the liver homogenate was assayed as previously described by Sinha [36] with modifications. An aliquot of hydrogen peroxide (0.8 mL) was introduced into an Eppendorf tube. Phosphate buffer (1.0 mL), extracted sample/vitamin C/oxidant solution (75 μL) and (0.002%, v/v) diluted homogenate (125 μL) were added. The reaction mixture (0.5 mL) was introduced into 1.0 mL of dichromate reagent (5%) and the mixture shaken vigorously. The mixture was heated in a Clifton water bath for 10 min, and allowed to cool at room temperature. The absorbance at 570 nm was taken using the spectrophotometer (BioMate 3S UV-Visible, Thermo Scientific™ Manufacturer, Wohlen, Switzerland). The absorbance obtained was extrapolated from the following standard curve y = 0.0028x + 0.0132. The catalase activity was thereafter expressed as Unit/min/mg of protein (UI/mg Prot.):
where df is the dilution factor and Abs is the absorbance
2.6.7. Superoxide dismutase (SOD) activity
The measurement of total SOD activity was performed according to the method of Misra and Fridovich with some slight modifications [37]. The principle of this method is based on the inhibition of epinephrine autoxidation. Distilled water (0.2 mL) and 2.5 mL of sodium carbonate buffer (0.05 M and pH 10.2) were added into 0.3 mL of epinephrine buffer to initiate the reaction. The absorbance at 480 nm was read for 150 s with 30 s intervals against a blank made up of 2.5 mL buffer, 0.3 mL epinephrine and 0.2 mL distilled water. The following equation allowed the calculation of the SOD activity:
where df is the dilution factor. The SOD activity was there after expressed as Unit/min/mg of protein (UI/mg Prot.).
2.7. Statistical analysis
The results were presented as mean ± SD of triplicate assays. Analyses of data were conducted using one-way ANOVA (analysis of variance) followed by Kruskal–Wallis test and Dunnett's multiple test (SPSS program version 18.0 for Windows, IBM Corporation, New York, NY, USA). The Log probit was used to determine the IC50 using the software XLstat version 7 (Addinsoft, New York, NY, USA) were used to achieve the Pearson correlation analysis (PCA). The differences were considered as significant at P < 0.05.
3. Results and discussion
Plant-derived antioxidants, especially, the phenolic compounds have benefited increased considerations because of their prospective health benefits [38]. Indeed several studies have demonstrated that foods from plant origin having antioxidants are beneficial to health since it down-regulate many degenerative processes and can efficiently lower the rate of cancer and other diseases [11], [12], [38], [39]. The recovery of antioxidant substances from plant materials is usually realized through different extraction techniques taking into account their chemistry and distribution in the plant matrix. In the present study two solvents were used for the extraction of the leaves and stem of P. guineense. The ethanol used for extraction gave a yield of about 11% and 7.46% respectively for the leaves and stem extracts while the hydro-ethanol solvent extraction produced 12.66% and 18.92%. Polar solvents are frequently used for the recovery of phenolic compounds from a plant matrix. According to this result the hydro-ethanol solvent was more active; corroborating previous studies which stated that the amount of active compounds in extracts is related to the polarity of the solvent and the hydro-alcoholic solvents have been demonstrated to increase the yields [40]. Several methods have been described in order to assess the antioxidant and the free radical quenching properties of plant materials among them, the DPPH scavenging method which has provided information on a great deal of plants and is widely used. DPPH• is a stable and organic nitrogen radical bearing no similarity to the highly reactive and transient peroxyl radicals involved in various oxidative reactions in vivo [4].
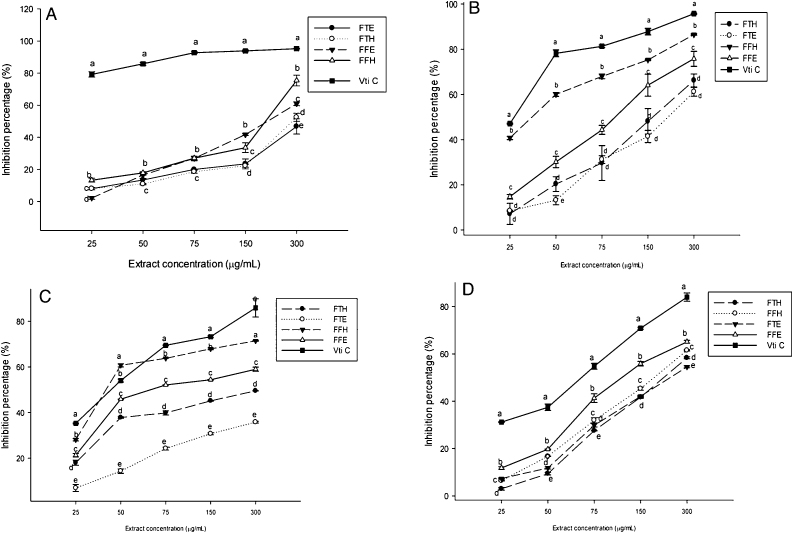
The results of DPPH scavenging potential of the plant extracts are presented in Fig. 1A. It can be seen on this figure that all the tested samples demonstrated a scavenging potential of the DPPH radical. The powerful DPPH scavenging capability demonstrated by these extracts suggests that they have a large amount of ‘reductones’ [41]. Among the tested extracts, FFH exhibited a higher (p < 0.05) inhibitory potential at the concentration of 25 μg/mL (13.31 ± 1.23%) and 300 μg/mL (75.43 ± 3.33%) demonstrating an excellent scavenging activity of DPPH radical. This result corroborates previous studies which stated that plants polyphenols can act as primary antioxidant which, effectiveness is measured by its ability to inactivate free radicals before they attack biological macromolecules [42]. Hydroxyl radical scavenging is a central antioxidant activity due to the great reactivity of hydroxyl radicals which enables it to react with a great variety of molecules found in living cells such as carbohydrates, amino acids, lipids and nucleotides [43]. Fig. 1B presents the hydroxyl radical (OH) scavenging potential of the different plant extracts. From this figure, it can be seen that the different extracts of P. guineense scavenged the hydroxyl radical in a concentration depending manner. Among the tested extracts, FFH showed a significantly (p < 0.05) higher inhibitory potential compared to the other extracts at all the concentrations varying from 40.76 ± 0.74% at the concentration of 25 μg/mL to 86.52 ± 0.28% at the concentration of 300 μg/mL. The nitric oxide (NO) radical scavenging property of the samples tested is presented in Fig. 1C. This figure shows that the extracts inhibited the NO radical in a concentration dependent manner. After vitamin C, the FFH significantly showed (p < 0.05) higher inhibitory effects among all the tested extracts. ABTS, a protonated radical, has specific absorbance maxima at 734 nm which declines with the scavenging of the proton radicals [44], [45]. The ABTS scavenging potential of the extract samples is displayed in Fig. 1D. It is found from this figure that, all the samples tested inhibited the ABTS radical. FFH showed the highest (p < 0.05) potential. The difference in the ABTS scavenging potentials of the extracts could be related to the type of phenolic compounds that have been extracted by the type of solvent. In fact the activities of phenolic acids and their esters depend on the number of free hydroxyl groups present in the molecule and which would be strengthened by steric hindrance [46]. Since, the reducing power of a compound serves as a significant indicator of its antioxidant activity [44], [47], P. guineense extracts were assayed for the reducing power activity.
Fig. 1.
Scavenging potential of the different plant extracts: (A) DPPH•, (B) OH•, (C) NO• and (D) ABTS•. Values are expressed as mean ± SD of three replicates. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; VIT C: vitamin C.
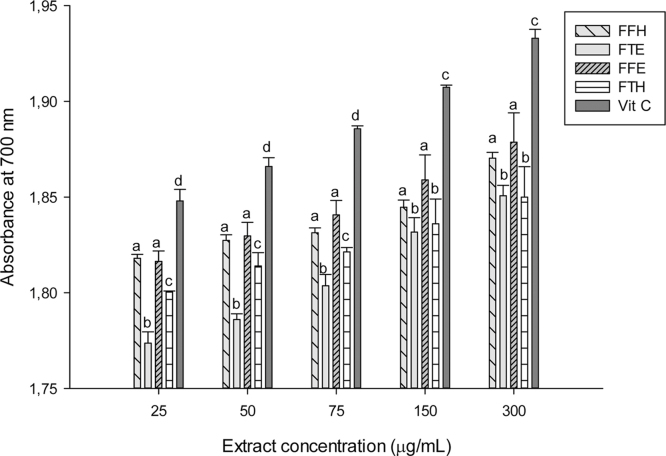
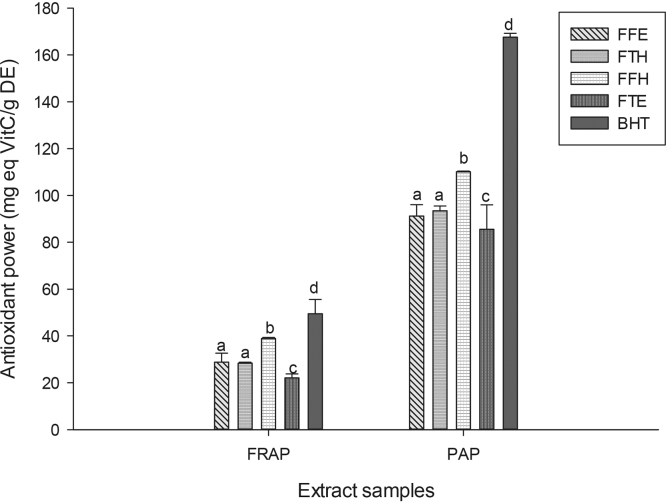
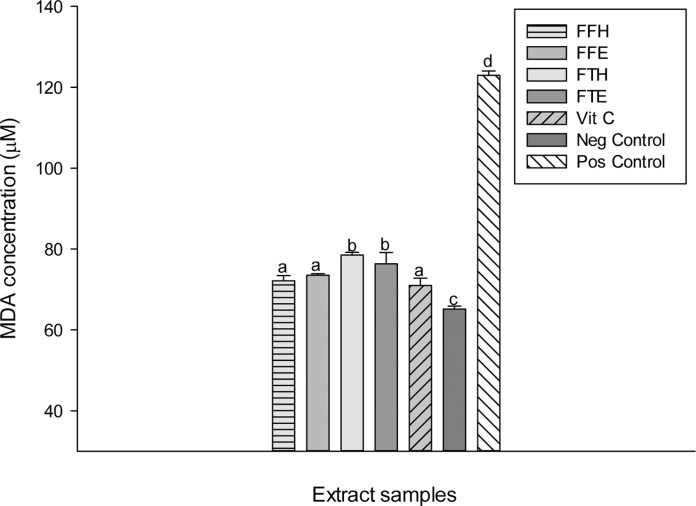
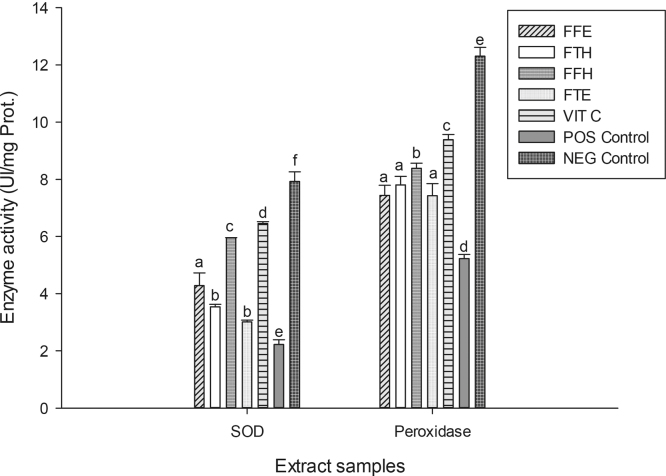
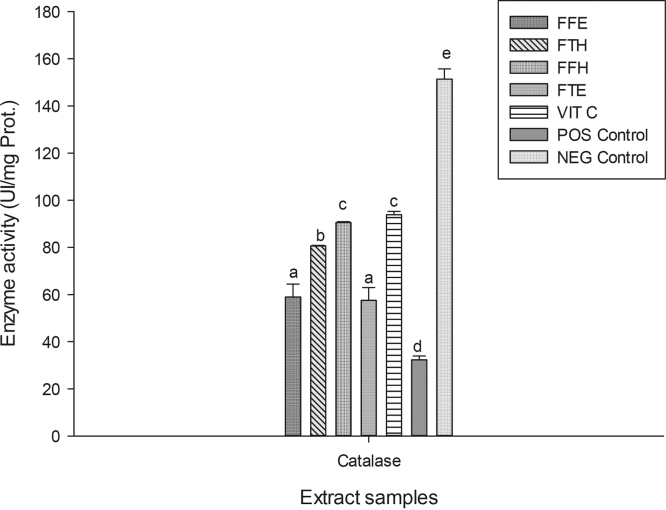
The reducing power potentials of the sample extracts are presented in Fig. 2. The result suggests that these samples have in vitro ferric reducing potentials which increased with concentration. We observed in this study an increase in absorbance with the reducing activity as shown in previous studies [44], [48]. As an alternative antioxidant property, some phenolic compounds with hydroxyl groups can conjugate transition metals, preventing metal-induced free radical formation [46]. The FRAP assay is based on the reduction of a colorless ferric complex 2,4,6-tripyridyl-s-triazine complex (Fe3+-tripyridyltriazine) to a blue-colored ferrous form (Fe2+-tripyridyltriazine) by the action of electron-donating antioxidants [49]. The different samples tested exhibited the FRAP antioxidant activity (Fig. 3). The sample FFH had the highest potential (p < 0.05) compared to the other extracts with a value of 30.53 ± 0.14 mg equiv. VitC/g DE followed by FFE (28.37 ± 0.41 mg equiv. VitC/g DE) which have similar activity with FTH. This result corroborates previous works which supported that phenolic compounds can inhibit metal-induced oxygen radical formation either by coordination with Fe2+ and enhancing autoxidation of Fe2+, or by the formation of inactive complex with Fe2+ having a relatively weaker interaction [50], [51]. In the phosphomolybdenum assay, which is a quantitative method to evaluate the antioxidant capacity by measuring its ability to quench free radicals by hydrogen donation, all the extracts exhibited different phosphomolybdenum scavenging activities (Fig. 3). The FFH extract was the most powerful antioxidant with an antioxidant value of 30.63 ± 0.14 mg equiv. VitC/g DE. This result confirms that P. guineense extracts could also act as secondary antioxidant and therefore could prevent the formation of free radicals. Plant phenolic compounds are responsible for their biological properties. These phenolic groups include a variety of compounds that could be classified as phenolic acids, flavonoids, tannins and the less common stilbenes and lignans [46]. Table 1 displays the values of the total phenol, flavonoid and flavonol content and the 50% inhibitory concentrations of the scavenging activities of P. guineense extracts. This result reveals that the levels of phenols and flavonoids contents were higher in FFH and FFE while the higher level of flavonols was noted in the FTH extract. Except for the ABTS radical, the FFH extract demonstrated the lowest IC50 regarding all the radicals tested indicating that this extract exhibited a significant scavenging property. Recent studies have shown that metals such as iron exhibited the ability to produce reactive oxygen species, resulting in lipid peroxidation. Evidence indicates that lipid peroxidation requires both Fe (III) and Fe (II), probably as a dioxygen-iron complex [52]. Iron is capable of catalyzing redox reactions between oxygen and biological macromolecules that would not occur if catalytically active iron is not present. The chelated iron acts as a catalyst for the Fenton's reaction, facilitating the conversion of superoxide anion and hydrogen peroxide to hydroxyl radical, a species often thought to initiate lipid peroxidation [52]. Inhibitory effect of the P. guineense extracts on the ion mediated toxicity was assessed by lipid peroxidation assay (Fig. 4). In biological systems the degradation of metabolites end products such as MDA are produced as a result of peroxidized polyunsaturated fatty acids of the cell membrane [53]. From our results it is obvious that ion mediated oxidation significantly increased the amount of MDA which is expressed by the increase in concentration of MDA in the positive control (oxidant) compared (p < 0.05) to the normal control (negative). The extracts from P. guineense inhibited lipid peroxidation through the reduction of MDA production. This property depended on the part of the plant extract and the solvent used for extraction. However, among these extracts, FTH showed the highest inhibitory effects. According to the results above (FRAP, and free radical scavenging assays), we can suggest that P. guineense acts as a primary or chain-breaking antioxidants which retards lipid oxidation by interfering with chain propagation, initiation, and reaction by accepting free radicals and forming stable free radicals that do not further promote initiation or propagation reactions [54]. SOD is an enzyme that catalyzes the dismutation of superoxide radicals. The SOD activity is proportional to the antioxidant potential of the samples. The effect of the different extracts from P. guineense demonstrated high capacities to inhibit free radicals leading to an increase in enzymes activity rates (Fig. 5). The negative control (without pro-oxidant and extract) showed a significantly highest enzyme activity (8.11 ± 0.07 UI/mg Prot.). Vitamin C (6.45 ± 0.06 UI/mg Prot.) used as standard showed a significant (p < 0.05) increase in SOD activity compared to the oxidant (positive control) (2.95 ± 0.33 UI/mg Prot.). The different extracts also showed increased activity, FFE (5.95 ± 0.06 UI/mg Prot.), FFH (4.03 ± 0.21 UI/mg Prot.). This increase in the activity of SOD in the presence of the extracts may be linked to the fact that plant polyphenols can reduce the concentration of this enzyme's substrate (superoxide) in the medium. In fact, ROS can be inactivated through both chemical and physical quenching pathways of compounds such as polyphenols [55]. Peroxidase and catalase are enzymes which catalyze the reduction of hydrogen peroxide (H2O2) and protect tissues from highly reactive hydroxyl radicals. The in vitro investigation of the activities of these enzymes can provide great information on the antioxidant properties of the plant extracts against H2O2 and ion related oxidative damage. The effect of the extracts of P. guineense on the activity of peroxidase is depicted in Fig. 5. The peroxidase activities of these extracts were also higher compared to those of the positive control (5.22 ± 0.17 UI/mg Prot.) with FFH (8.38 ± 0.10 UI/mg Prot.) showing the highest activity. Fig. 6 shows an increased level of catalase activity in the presence of FFH (90.57 ± 0.42 UI/mg Prot.) and FFE (80.64 ± 0.41 UI/mg Prot.) compared to the other extracts. Previous studies revealed that, active metal ions such as Fe2+ interact with H2O2 through Fenton chemistry to form hydroxyl radicals (•OH), which is the most ROS known, being able to initiate free radical chain reactions by abstracting hydrogen from almost any molecule [46].
Fig. 2.
Reductive activity of the different plant extracts. Values are expressed as mean ± SD of three replicates. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; VIT C: vitamin C.
Fig. 3.
FRAP and phosphomolybdenum (PAP) antioxidant activities of the different plant extracts. Values are expressed as mean ± SD of three replicates. Values are expressed as mean ± SD of three replicates. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; BHT: butylated hydroxyl toluene.
Table 1.
Phenolic composition and 50% inhibitory concentration (IC50) of extracts.
| Samples | Phenolic composition |
IC50 (μg/mL) |
|||||
|---|---|---|---|---|---|---|---|
| Total phenols (CAE/g dried extract) | Flavonoids (QE/g dried extract) | Flavonols (QE/g dried extract) | DPPH• | OH• | NO• | ABTS• | |
| FFE | 19.01 ± 0.03 b | 4.44 ± 0.20 a | 2.41 ± 0.26b | 134.26 ± 0.98a | 82.94 ± 2.11 a | 126.51 ± 2.21 a | 222.81 ± 3.32 a |
| FFH | 21.62 ± 0.06 c | 8.64 ± 0.17 b | 6.81 ± 0.35a | 133.95 ± 1.07 a | 53.56 ± 0.03 b | 83.39 ± 0.10 b | 258.01 ± 7.28 b |
| FTE | 9.10 ± 0.05 d | 3.29 ± 0.14 c | 1.52 ± 0.28c | 383.79 ± 6.10 b | 211.37 ± 4.00 c | 538.83 ± 5.43 c | 391.20 ± 3.54 c |
| FTH | 12.86 ± 0.00 a | 4.35 ± 0.21a | 6.67 ± 0.60a | 313.53 ± 2.77 c | 173.79 ± 3.05 d | 433.17 ± 6.90 d | 394.76 ± 7.00 c |
| Vit C | 12.49 ± 0.01 d | 27.81 ± 0.10 e | 55.84 ± 1.11 e | 70.85 ± 0.03 d | |||
Values are expressed as mean ± SD of three replicates. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; VIT C: vitamin C; CAE: caffeic acid equivalent; QE: quercitine equivalent.
Fig. 4.
Protective properties of plant extracts against lipid peroxidation. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; VIT C: vitamin C; Pos Control: oxidant (positive) control; Neg Control: normal (negative) control.
Fig. 5.
Protective properties of plant extracts: SOD and peroxidase activities. Values are expressed as mean ± SD of three replicates. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; VIT C: vitamin C; Pos Control: oxidant (positive) control; Neg Control: normal (negative) control.
Fig. 6.
Protective properties of plant extracts: catalase activity. Values are expressed as mean ± SD of three replicates. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; VIT C: vitamin C; Pos Control: oxidant (positive) control; Neg Control: normal (negative) control.
3.1. Correlation and principal component analysis (PCA)
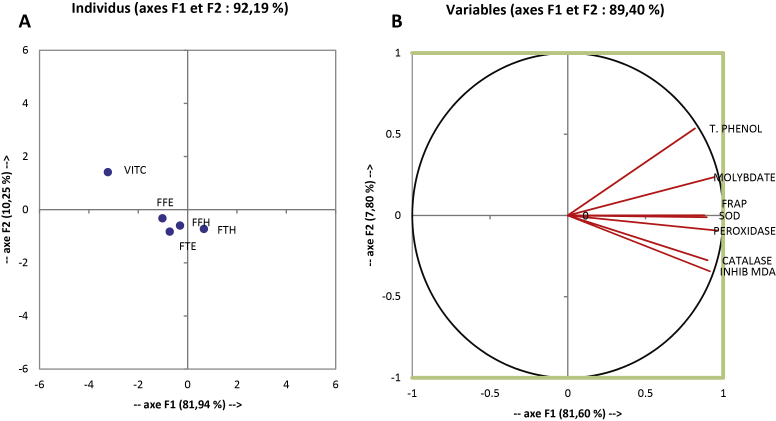
The correlation, principal component analysis of the antioxidant tests and phenolic content of BEH and BEE are represented in Fig. 7A and B and Table 2. The samples and the antioxidant tests have been projected in a single system; the PCA analysis explains 98.10% of the variation which exists in this system with a contribution of 95.91% for the axis F1 and 2.20% for the axis F2. Fig. 7a and b shows that the total phenolic content is highly positively correlated with the antioxidant test. As such, the results showed significant positive correlations between the phenolic content and the values of reducing power, FRAP, phosphomolybdenum assays (Table 3). Our results corroborate with studies which reported positive correlations between phenolic compounds from plants extracts and their antioxidant activities, supporting the relevance of phenolic compounds as oxidative stress inhibitor [56]. In the same way, the positive significant (p < 0.05) correlations values of flavonol and flavonoid with phosphomolybdenum test and FRAP values were found to be 0.908 and 0.971; 0.971 and 0.908 respectively, indicating high correlations with these assay results.
Fig. 7.
Principal component analysis results on F1XF2 axis of antioxidant and scavenging activities of the extracts tested. In the same concentration the values affected with different letters (a–f) are significantly different at p < 0.05. Values are expressed as mean ± SD of three replicates. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; MOLYBDAT: phosphomolybdenum test; Flavonol: flavonol assay; Polyphenol: polyphenol assay; Flavonoid: flavonoid assay; NO: NO radical scavenging test; ABTS: ABTS radical scavenging test; DPPH: DPPH radical scavenging test; OH: OH radical scavenging test; RED ACT: reductive activity test.
Table 2.
Correlation between antioxidant assays and polyphenols determination tests.
| DPPH | OH | NO | ABTS | RED ACT | FRAP | Molybdate | T. phenol | Flavonoid | Flavonol | |
|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | 1 | |||||||||
| OH | 0.822* | 1 | ||||||||
| NO | 0.414 | 0.673* | 1 | |||||||
| ABTS | 0.820* | 0.861* | 0.789* | 1 | ||||||
| RED ACT | 0.707* | 0.686* | 0.743* | 0.932* | 1 | |||||
| FRAP | 0.677* | 0.247 | 0.199 | 0.539* | 0.615* | 1 | ||||
| Molybdate | 0.891* | 0.673* | 0.533 | 0.859* | 0.816* | 0.829* | 1 | |||
| T. phenol | 0.914* | 0.823* | 0.692* | 0.923* | 0.828* | 0.696* | 0.953* | 1 | ||
| Flavonoid | 0.949* | 0.741* | 0.502 | 0.831* | 0.735* | 0.789* | 0.971* | 0.970* | 1 | |
| Flavonol | 0.787* | 0.535 | 0.500 | 0.681* | 0.618* | 0.827* | 0.908* | 0.895* | 0.931* | 1 |
Molybdat: phosphomolybdenum test; flavonols: flavonol assay; polyphenol: polyphenol assay; flavonoids: flavonoid assay; NO: NO radical scavenging test; ABTS: ABTS radical scavenging test; DPPH: DPPH radical scavenging test; OH: OH radical scavenging test; RED ACT: reductive activity test.
Significant values p = 0.050 (bilateral test).
Table 3.
Correlation analysis results of P. guineense extracts demonstrated by Pearson's coefficients for in vitro antioxidant assays on rat liver enzymes.
| Assays | Peroxidase | Catalase | SOD | INHIB MDA | FRAP | Molybdate | T. phenol |
|---|---|---|---|---|---|---|---|
| Peroxidase | 1 | ||||||
| Catalase | 0.927* | 1 | |||||
| SOD | 0.797 | 0.833* | 1 | ||||
| INHIB MDA | 0.905* | 0.875* | 0.739 | 1 | |||
| FRAP | 0.828* | 0.666 | 0.713 | 0.862* | 1 | ||
| Molybdate | 0.885* | 0.721 | 0.761 | 0.815* | 0.924* | 1 | |
| T. phenol | 0.761 | 0.651 | 0.720 | 0.556 | 0.640 | 0.866* | 1 |
SOD: SOD activity test; catalase: catalase activity test; peroxidase: peroxidase activity test; flavonols: flavonol assay; polyphenol: polyphenol assay; flavonoids: flavonoid assay; FRAP: FRAP antioxidant test; MDA: MDA assay; INHIB MDA: MDA inhibition percentage.
Significant values p = 0.050 (bilateral test).
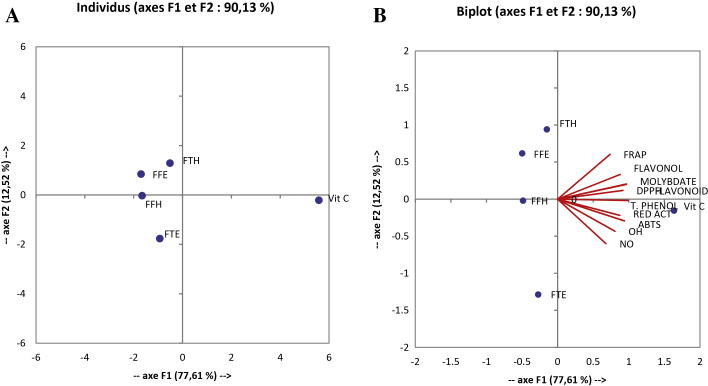
The correlation analyses of the inhibitory effects on ion mediated oxidative damages on liver enzymes in vitro by the different extracts were also conducted (Fig. 8A and B and Table 4). From these results we can suggest that the increase in antioxidant potential is related to the decrease in the inhibition of the antioxidant activity of enzymes in vitro. A positive and non-significant correlation was found between total phenol content and lipid oxidation inhibition and other enzymes involved in oxidative stress. However a significant and positive correlation was noted between the inhibition of lipid peroxidation and antioxidant capacity of extracts through FRAP and PAP activities indicating that increase of antioxidant capacity of extracts significantly reduced lipid peroxidation.
Fig. 8.
Principal component analysis results on F1XF2 axis of in vitro protective activity of P. guineense on rat liver enzymes for the tested extracts. Values are expressed as mean ± SD of three replicates. FTE: P. guineense (stem) ethanolic extract; FTH: P. guineense (stem) hydroethanolic extract; FFE: P. guineense (leaves) ethanolic extract; FFH: P. guineense (leaves) hydroethanolic extract; molybdate: phosphomolybdenum (PAP); SOD: SOD activity test; catalase: catalase activity test; peroxidase: peroxidase activity test; flavonols: flavonol assay; polyphenol: polyphenol assay; flavonoids: flavonoid assay; FRAP: FRAP antioxidant test; INHIB MDA: MDA inhibition percentage.
Table 4.
Representation of the amounts of phenolic compounds in the different plant parts.
| Phenol standards | Standard retention time |
P. guineense (stem) |
P. guineense (leaves) |
||
|---|---|---|---|---|---|
| Characteristics | T.R. (min) | A (mUA) | Conc (mg/g DW) | A (mUA) | Conc (mg/g DW) |
| 3,4-OH benzoic acid | 19.10 ± 00 | 10.12 ± 00 | 0.40 ± 00 | 48.48 ± 00 | 1.92 ± 00 |
| Apigenin | 33.49 ± 00 | 39.46 ± 00 | 0.01 ± 00 | 125.90 ± 00 | 0.03 ± 00 |
| Caffeic acid | 25.67 ± 00 | 77.25 ± 00 | 1.45 ± 00 | 132.95 ± 00 | 2.49 ± 00 |
| Catechin | 23.48 ± 00 | 62.71 ± 00 | 4.56 ± 00 | 79.81 ± 00 | 5.80 ± 00 |
| Eugenol | 29.43 ± 00 | 110.23 ± 00 | 31.87 ± 00 | 124.61 ± 00 | 36.02 ± 00 |
| Gallic acid | 14.38 ± 00 | 0 | 0 | 26.73 ± 00 | 0.68 ± 00 |
| O-coumaric acid | 25.11 ± 00 | 847.86 ± 00 | 26.17 ± 00 | 133.33 ± 00 | 4.11 ± 00 |
| OH-tyrosol | 21.91 ± 00 | 37.61 ± 00 | 3.47 ± 00 | 50.74 ± 00 | 4.68 ± 00 |
| P-coumaric acid | 30.52 ± 00 | 26.06 ± 00 | 0.51 ± 00 | 113.60 ± 00 | 2.22 ± 00 |
| Quercetin | 42.19 ± 00 | 30.14 ± 00 | 2.83 ± 00 | 90.40 ± 00 | 8.49 ± 00 |
| Rutin | 29.45 ± 00 | 55.2 ± 00 | 4.65 ± 00 | 79.2 ± 00 | 6.66 ± 00 |
| Syringic acid | 25.55 ± 00 | 88.42 ± 00 | 2.19 ± 00 | 75.9 ± 00 | 1.88 ± 00 |
| Theobromine | 17.35 ± 00 | 30.62 ± 00 | 1.03 ± 00 | 64.84 ± 00 | 2.17 ± 00 |
| Tyrosol | 21.77 ± 00 | 37.31 ± 00 | 2.17 ± 00 | 25.55 ± 00 | 1.49 ± 00 |
| Vanillic acid | 25.27 ± 00 | 48.6 ± 00 | 1.55 ± 00 | 88.34 ± 00 | 2.82 ± 00 |
Conc: concentration; DW: dried weight; T.R.: retention time; A: area.
To overcome the misunderstandings regarding the choice of the most effective antioxidative extract in vitro, in inhibiting lipid peroxidation and ions related damages, as well as to report the most reliable antioxidant activity of P. guineense extracts based on a statistical approach, the principal component analysis (PCA) was applied to the antioxidant assay data. Data obtained on the extracts were analyzed using the Varimax method for two factor loadings to see the correlations between assays that accounted for the total covariance of the plant extracts (Erkan et al., 2011). Considering the different contributions of the each single extract to F1, it can be said that the overall antioxidant activity of the extracts of P. guineense is ranked in the order FFH > FFE > FTH > TFE, based on the ABTS scavenging, phosphomolybdenum test, flavonoid, flavonol and total phenol content assay values, having much higher contributions to F1 than NO assay.
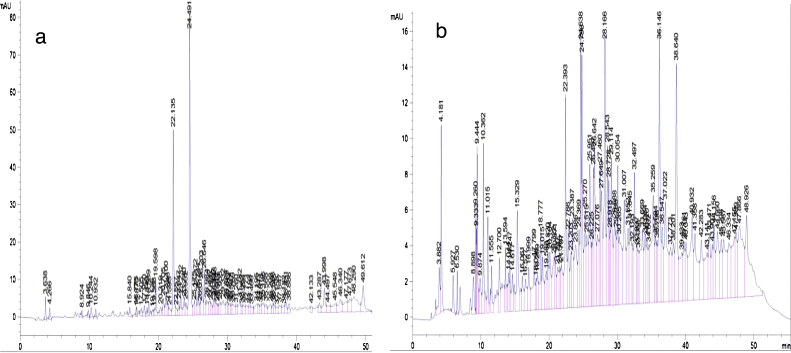
In this study, we determined the phenolic composition of leaves and stem extracts of P. guineense and the HPLC chromatograms are represented in Fig. 9 while the phenolic composition is shown in Table 4. The results from these figures revealed two majors peaks for the stem extracts while eight major picks were identified for the leaves extracts (Fig. 9A and B). The two major compounds of the stem extracts of P. guineense include Eugenol and O-coumaric acid with concentrations of 31.87 ± 00 mg/g DW and 26.17 ± 00 mg/g DW respectively. Two polyphenols (catechin and rutin) identified in the stem extracts have moderate concentrations (higher than 4 mg/g DW). The leaves appeared to be richer in phenolic compounds than stem. However only Eugenol was discovered to have the highest concentration (36.02 ± 00 mg/g DW). Contrary to the stem, five other polyphenols including quercetin, rutin, OH-tyrosol, O-coumaric acid and catechin exhibited moderate concentrations (Table 4). These results confirm our previous results obtained for total phenol content (Table 1) which show that the leaves extracts have highest total phenol content. Total phenol profile reflects the overall phenolic content of a plant or food sample. There are unidentified peaks that were not quantified in the chromatograms therefore this total phenol may not in a straight way correlate with the total amount of individual phenolic compounds detected in each samples. These results correlate those obtained in the PCA correlation where the extracts FFH and FFE were classified as the most powerful antioxidant. Nevertheless it should be noted that the flavonoid and flavonol contents of FTH are higher compared to those of FEE. This situation could more be related to the choice of the solvent than the unidentified picks of our chromatogram since the hydro-ethanolic solvent may be more efficient to extract phenolic compound that the ethanol solvent alone.
Fig. 9.
HPLC chromatograms of phenolic extracts from P. guineense recorded at 280 nm: (A) Stem and (B) leaves (TR: 19.10: 3,4-OH-benzoic acid; 33.49: Apigenin; 25.67: Caffeic acid; 23.48: catechine; 29.43: Eugenol; 14.38; gallic acid; 25.11: O-coumaric; 21.91: OH-tyrosol; 30.52: P-coumaric acid. 42.19: quercetin; 29.45: rutin; 25.55: Syringic acid; 17.35: Theobromine; 21.77: Tyrosol and 25.27: Vanillic acid).
4. Conclusion
In conclusion, the different extracts from P. guineense exhibited high free radical chelating properties and antioxidant potential. They also exhibited a high protective potential against few liver markers involved on oxidative stress, and therefore may act as inhibitor of ions mediated oxidative damages as primary antioxidant. The hydro-ethanolic extracts of P. guineense leaves (FFH) displayed the most efficient activities due to the presence of quality and higher quantity of phenols content identified in these extracts. Nevertheless, additional studies should be conducted to isolate and characterize the bioactive compounds responsible for this activity and also determine its in vivo protective effects.
Conflict interests
The authors declare that there are no conflict interests
Authors’ contributions
Moukette MB conducted the study; Eustache assisted in conducting the assays; Biapa NPC conducted the statistical analysis, Njimou conducted the HPLC, Pieme CA designed the research co-directed the research work with Ngogang YJ and provided reagents. All the authors read and approved the final manuscript. Our study received no fund from any agency.
Transparency document
Acknowledgements
We are grateful to Mr Nana from the National Herbarium for helping in collecting and identifying the plant material. We are equally very thankful to Dr. Lekeufack Martin of the University of Dschang-Cameroon, for proofreading this manuscript.
Contributor Information
Bruno Moukette Moukette, Email: mouket2006@yahoo.fr.
Pieme Constant Anatole, Email: apieme@yahoo.fr.
Cabral Prosper Nya Biapa, Email: brbiapa@yahoo.fr.
Jacques Romain Njimou, Email: jnjimou@yahoo.fr.
Jeanne Yonkeu Ngogang, Email: jngogang@yahoo.fr.
References
- 1.Ahmed M., Khan M., Khan M., Muhammad M., Khan M., Khan R. Role of medicinal plants in oxidative stress and Cancer. Sci. Rep. 2013;2:641. [Google Scholar]
- 2.Sosa V., Moliné T., Somoza R., Paciucci R., Kondoh H., LLeonart M. Oxidative stress and cancer: an overview. Ageing Res. Rev. 2012 doi: 10.1016/j.arr.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 3.García-Nebot M., Recio I., Hernández-Ledesma B. Antioxidant activity and protective effects of peptide lunasin against oxidative stress in intestinal Caco-2 cells. Food Chem. Toxicol. 2014;65:155–161. doi: 10.1016/j.fct.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Biapa N., Oben J., Ngogang J. Scavenging radical kinetic and antianaemic screening properties of some medicinal plants used in Cameroon. Int. J. Appl. Res. Nat. Prod. 2011;4:29–35. [Google Scholar]
- 5.Nanfack P., Biapa N., Pieme C., Moor V.A., Moukette B., Ngogang Y. The in vitro antisickling and antioxidant effects of aqueous extracts Zanthoxyllum heitzii on sickle cell disorder. BMC Complement. Altern. Med. 2013;13:162. doi: 10.1186/1472-6882-13-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di-Domenico F., Foppoli C., Coccia R., Perluigi M. Antioxidants in cervical cancer: chemopreventive and chemotherapeutic effects of polyphenols. Biochim. Biophys. Acta. 2012;1822:737–747. doi: 10.1016/j.bbadis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis. Annu. Rev. Med. 2006;57:1–18. doi: 10.1146/annurev.med.57.121304.131306. [DOI] [PubMed] [Google Scholar]
- 8.Choumessi A., Danel M., Chassaing S., Truchet I., Penlap V., Pieme A., Asonganyi T., Ducommun B., Valette A. Characterization of the antiproliferative activity of Xylopia aethiopica. Cell Division. 2012;7:8. doi: 10.1186/1747-1028-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Assam J., Dzoyem J., Pieme C., Penlap V. In vitro antibacterial activity and acute toxicity studies of aqueous-methanol extract of Sida rhombifolia Linn. (Malvaceae) BMC Complement. Altern. Med. 2010;10:1–7. doi: 10.1186/1472-6882-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atawodi S. Antioxidant potential of African medicinal plants. Afr. J. Biotechnol. 2005;4:128–133. [Google Scholar]
- 11.Dimo T., Rakotonirina A., Tan P., Dongo E., Dongmo A., Kamtchouing P., Azay J., Abegaz B., Cros G., Ngadjui T. Antihypertensive effects of Dorstenia psilurus extract in fructose-fed hyperinsulinemic, hypertensive rats. Phytomedicine. 2001;8:101–106. doi: 10.1078/0944-7113-00014. [DOI] [PubMed] [Google Scholar]
- 12.Dongmo F., Oben E., Momo N., Mandob E. Phytochemical constituents and antioxidant potential of some Cameroonian medicinal plants. Pharmacologyonline. 2007;2:436–452. [Google Scholar]
- 13.Dzeufiet P., Tédong L., Asongalem A., Dimo T., Sokeng S., Kamtchouing P. Hypoglycaemic and antidiabetic effect of root extracts of Ceiba pentandra in normal and diabetic rats. Afr. J. Trad. Complement. Altern. Med. 2006;3:129–136. [PMC free article] [PubMed] [Google Scholar]
- 14.Kuete V., Krusche B., Youns M., Voukeng I., Fankam A., Tankeo S.S.L., Efferth T. Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 2011;134:803–812. doi: 10.1016/j.jep.2011.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Rosa L.D., Alvarez-Parilla E., Gonzalez-Aguilar G. Blackwell Publishing; Ames, Iowa, USA: 2010. Fruit and Vegetables: Phytochemicals: Chemistry, Nutritional value and Stability; pp. 50014–58300. [Google Scholar]
- 16.Rice-Evans C., Miller N., Paganga G. Structure–antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996;20:933–956. doi: 10.1016/0891-5849(95)02227-9. [DOI] [PubMed] [Google Scholar]
- 17.Djacbou D., Pieme C., Biapa P., Beng B.P. Comparison of in vitro antioxidant properties of extracts from three plants used for medical purpose in Cameroon: Acalypha racemosa, Garcinia lucida and Hymenocardia lyrata. Asian Pac. J. Trop. Biomed. 2014;4:745–752. [Google Scholar]
- 18.Woguem V., Fogang H., Maggi F., Tapondjou L., Womeni H., Quassinti L., Bramucci M., Vitali L., Petrelli D., Lupid G., Papa F., Vittori S., Barboni L. Volatile oil from striped African pepper (Xylopia parviflora, Annonaceae) possesses notable chemopreventive, anti-inflammatory and antimicrobial potential. Food Chem. 2014;149:183–189. doi: 10.1016/j.foodchem.2013.10.093. [DOI] [PubMed] [Google Scholar]
- 19.Ekanem Q., Udoh V., Oku E. Effects of ethanol extract of Piper guineense seeds (Schum. and Thonn) on the conception of mice (Mus Musculus) Afr. J. Pharm. Pharmacol. 2010;4:362–367. [Google Scholar]
- 20.Udoh F., Theodore Y., Braide V. Effects of extract of seed and leaf of Piper guineense on skeletal muscle activity in rat and frog. Phytother. Res. 1999:110. doi: 10.1002/(SICI)1099-1573(199903)13:2<106::AID-PTR362>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Mbongue F., Kamtchouing P., Essame O., Yewah P., Dimo T., Lontsi D. Effect of the aqueous extract of dry fruits of Piper guineense on the reproductive function of adult male rats. Indian Pharmacol. Rev. 2005;7:30–32. [Google Scholar]
- 22.Noumi E., Amvam W., Lontsi D. Aphrodisiac plants used in Cameroon. Fitoterapia. 1998;69:5–34. [Google Scholar]
- 23.Miegueu P., Mbongue F., Assongalem A., Emmanuel A., Dzeufiet D., Dimo T., Kamtchouing P. Anabolic activity of alcoholic extract of Piper guineense in immature castrated rat. Pharmacologyonline. 2007;1:99–107. [Google Scholar]
- 24.Blois M. Antioxidant determinations by the use of a stable free radical. Nature. 1958:181. [Google Scholar]
- 25.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/s0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 26.Sreejayan N., Rao M. Nitric oxide scavenging activity by curcuminoids. J. Pharm. Pharmacol. 1997;49:105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu W., Zhao Y., Shu B. The radical scavenging activities of radix puerariae isoflavonoids: a chemiluminescence study. Food Chem. 2004;86:525–529. [Google Scholar]
- 28.Benzie F., Strain J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 29.Prieto P., Pineda M., Aguilar M. Spectophotometric quantitative of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999;269:337–341. doi: 10.1006/abio.1999.4019. [DOI] [PubMed] [Google Scholar]
- 30.Oyaizu M. Studies on product of browning reaction prepared from glucose amine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- 31.Singleton V., Rossi J. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965;16:144–158. [Google Scholar]
- 32.Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- 33.Kumaran A., Karunakaran R. In vitro antioxidant activities of methanol extracts of Phyllanthus species from India. Lebens-Wiss Technol. 2007;40:344–352. [Google Scholar]
- 34.Kumar S., Mishra A., Pandey A. Antioxidant mediated protective effect of Parthenium hysterophorus against oxidative damage using in vitro models. BMC Complement. Altern. Med. 2013;13:120. doi: 10.1186/1472-6882-13-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta V., Sharma M. Protective effect of Cinnamomum tejpata on lipid peroxide formation in isolated rat liver homogenate. Curr. Res. J. Biol. Sci. 2010;2:246–249. [Google Scholar]
- 36.Sinha A. Colorimetric assay of catalase. Anal. Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 37.Misra H., Fridovich I. Estimation of superoxide dismutase. J. Biochem. 1972;247:3170–3178. [PubMed] [Google Scholar]
- 38.Carocho M., Ferreira I. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013;51:15–25. doi: 10.1016/j.fct.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Pieme C., Ngoupayo J., Nkoulou C., Moukette B., Nono B., Moor V., Minkande J., Ngogang J. Syzyguim guineense extracts show antioxidant activities and beneficial activities on oxidative stress induced by ferric chloride in the liver homogenate. Antioxidants. 2014;3:1–18. doi: 10.3390/antiox3030618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Awika J. Anthocyanins from black sorghum and their antioxidant properties. Food Chem. 2005;90 293-231. [Google Scholar]
- 41.Lone I., Kaur G., Athar M., Alam S. Protective effect of Rumex patientia (English Spinach) roots on ferric nitrilotriacetate (Fe-NTA) induced hepatic oxidative stress and tumor promotion response. Food Chem. Toxicol. 2007;45:1821–1829. doi: 10.1016/j.fct.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 42.Halliwell B. Antioxidants and human disease: a general introduction. Nutr. Rev. 1997;55:44–49. doi: 10.1111/j.1753-4887.1997.tb06100.x. [DOI] [PubMed] [Google Scholar]
- 43.Halliwell B. Free radicals, antioxidants and human disease: curiosity, cause or consequences? Lancet. 1994;344:721–724. doi: 10.1016/s0140-6736(94)92211-x. [DOI] [PubMed] [Google Scholar]
- 44.Adedapo A., Jimoh F., Afolayan A., Masika P. Antioxidant activities and phenolic contents of the methanol extracts of the stems of Acokanthera oppositifolia and Adenia gummifera. BMC Complement. Altern. Med. 2008;8:54. doi: 10.1186/1472-6882-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pieme C., Penlap V., Etoa F. Screening of plants for antimicrobial and antitumor activity. Werstern African Network of Natural Products Research Scientist (WANNPRES) Afr. J. Trad. Complement. Altern. Med. 2005;2:117–205. [Google Scholar]
- 46.Dai J., Mumper J. Plant phenolics: extraction, analysis and their antioxidant and anticancer properties. Molecules. 2010;15:7313–7352. doi: 10.3390/molecules15107313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pieme C., Penlap V., Nkegoum B., Taziebou I., Ngogang J. In vivo antioxidant and potential antitumor activity extract of Leea guineensis royen ex. l. (leeaceae) on carcinomatous cells. Pharmacologyonline. 2008;1:538–547. [Google Scholar]
- 48.Moukette B., Pieme C., Biapa P., Njimou J., Moor V., Stoller M., Bravi M., Ngogang J. Phenolic content of Hypodaphnis zenkeri and its antioxidant effects against Fenton reactions’ mediated oxidative injuries on liver homogenate. Antioxidants. 2014;3:866–889. doi: 10.3390/antiox3040866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Agbor G., Oben J., Ngogang J., Xinxing C., Vinson J. Antioxidant capacity of some herbs/spices from Cameroon: a comparative study of two methods. J. Agric. Food Chem. 2005;53:6819–6824. doi: 10.1021/jf050445c. [DOI] [PubMed] [Google Scholar]
- 50.Bors W., Michel C. Chemistry of the antioxidant effect of polyphenols. Ann. N. Y. Acad. Sci. 2002;957:57–69. doi: 10.1111/j.1749-6632.2002.tb02905.x. [DOI] [PubMed] [Google Scholar]
- 51.Lia L., Chou S., Chao W. Studies on the antioxidative activities of Hsian-Tsao (Mesona procumbens Hesml) leaf gum. J. Agric. Food Chem. 2001;49:963–968. doi: 10.1021/jf001146k. [DOI] [PubMed] [Google Scholar]
- 52.Stohs S., Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radic. Biol. Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- 53.Rahimi R., Nikfar S., Larijani B., Abdollahi M. A review on the antioxidants in the management of diabetes and its complications. Biomed. Pharmacother. 2005;59:365–373. doi: 10.1016/j.biopha.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Coupland J., Clements D.M. Lipid oxidation in food emulsions. Trends Food Sci. Technol. 1996;7:83–91. [Google Scholar]
- 55.Decker E., Akoh C., Min D. Marcel Dekker; New York: 2002. Antioxidant Mechanisms. [Google Scholar]
- 56.Abrahim N., Kanthimathi M., Abdul-Aziz A. Piper betle shows antioxidant activities, inhibits MCF-7 cell proliferation and increases activities of catalase and superoxide dismutase. BMC Complement. Altern. Med. 2012;12:220. doi: 10.1186/1472-6882-12-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.