Abstract
Food preservatives play important role in today's food supplies that are used to prolong the self-life of products by protecting them from deterioration caused by micro-organisms. In this study, investigations were carried out to study the impacts of food preservatives like butylated hydroxytoluene, butylated hydroxyanisole, sorbic acid, propyl gallate and sodium nitrate. The effects of these preservatives at concentration of 1000 ppm, 1500 ppm, 2000 ppm, 2500 ppm for 4 h, 8 h and 16 h of exposure period were studied on the root tips of Allium cepa. Cytological studies revealed statistically significant (p < 0.05) inhibition in mitotic index with an increase in concentration of the food preservatives when compared with the control. Most frequent cytological abnormalities observed were bridges, multipolarity, C-mitosis, stickiness and cell death. The total percentages of abnormalities were also increased with increasing concentration and time duration. The abnormalities (%) in root system caused by used preservatives were recorded as butylated hydroxytoluene < butylated hydroxyanisole < sodium nitrate < sorbic acid < propyl gallate.
Abbreviations: BHT, butylated hydroxyltoluene; BHA, butylated hydroxyanisol; SN, sodium nitrate; SA, sorbic acid; PG, propyl gallate; MI, mitotic index; CA, chromosome aberration; B, bridge; MP, multipolarity; CB, chromosomal break; BN, binucleated
Chemical compounds studied in this article: Butylated hydroxyltoluene (PubChem CID: 31404), Butylated hydroxyanisol (PubChem CID: 517036), Sodium nitrate (PubChem CID: 24268), Sorbic acid (PubChem CID: 643460), Propyl gallate (PubChem CID: 4947)
Keywords: Genotoxicity, Food preservatives, Mitotic index, Chromosomal abnormalities, Allium cepa
1. Introduction
Food preservation is one of the oldest technology used by human being. This is because increasing population of the world, food preservatives have been added to food to extend a food's freshness or self-life and prevents it from oxidizing. Sugars and salts are frequently used as preservatives. Chemical preservatives are also being used in most of food industries and are generally foolproof for preservation purposes. However, it has been proved that certain food additives, especially antimicrobial agents are genotoxic in different test systems [34], [37]. Some food preservatives are well known agent to reduce mitotic index and induce various type of chromosomal abnormalities with increasing concentrations and periods of treatments [49].
In this experiments we have selected five food preservatives, popularly used in India and USA, that are butylated hydroxyanisol (BHA), butylated hydroxytoluene (BHT), sodium nitrate (SN), propyl gallate (PG), and sorbic acid (SA). BHA and BHT are act as an antioxidant [27] and most commonly used in cereals, chewing gum, potato chips, and vegetable oils. BHA is a mixture of the isomers 3-tert-butyl-4-hydroxyanisole and 2-tert-butyl-4-hydroxyanisole. It is also used in food packaging, cosmetics, rubber and petroleum products [25]. When its higher dose administered to the Japanese house musk shrew, which have no fore-stomach, all animals died from gastrointestinal haemorrhage, on the other hand low and mild-dose groups of animals showed a 50–60% incidence of adenomatous hyperplasia of the lung [1]. IARC [22] has been reported that higher dose of BHA is possible carcinogen to humans. The food grade BHT chemically known as 3,5-di-tert-butyle-4-hydroxytoluene. Experimental studies on Bacillus subtilis and Salmonella typhimurium indicates that BHT is not harmful and did not damage DNA and not act as a mutagenic substance [29], [3], [4]. However at higher dose it was carcinogenic in mouse [7]. Leslie et al. [31] have reported that both BHA and BHT are cytotoxic and produce injury to myocardial cultured cells, and at higher dose cause cell lysis after long exposure. PG generally used in combination with BHA and BHT. It is found in meat products, chicken soup base, and chewing gum. Chemically it is propyl ester of the naturally occurring gallic acid, 3,4,5-trihydroxybenzoic acid. JECFA [25] proved it as an antioxidant and useful to preserve and stabilize the freshness of nutritional value and medicinal preparations. FAO/WHO expert committee on food additives evaluated that acceptable daily intakes (ADIs) of PG should 0.2 mg/kg body weight. PG showed toxic result when exposure from 2000 to 3800 mg/kg body weight in mice, rats, hamsters, and rabbits [51]. Sorbic acid (SA) is the trans–trans form of hexa2,4-dienoic acid a straight chain unsaturated fatty acid with molecular weight of 112.13. SA (E200) is a potent antimicrobial agent basically against moulds and yeast and now also works as growth inhibitor of bacteria. It is most commonly used in pastries, cheese, sour soup tins, fruits, beverages, sausages, sweets and ground beef [28], [35]. According to JECFA [24] sorbic acid is not harmful at higher concentration and their ADIs has been fixed at 25 mg/kg body weight, whereas sorbic acid along with its potassium salt indicated as genotoxic agent and have induced chromosome aberration (CA) and sister chromatid exchange (SCE) [20]. SN is a preservatives, colouring, and flavouring commonly used in bacon, luncheon meats, hot dog and corned beef. Nitrate (NO3−) is white crystalline powder and also naturally occurring compounds present in various form in the environment. It has been also proved that nitrates present in fruits and vegetables [30], [42]. WHO recommended that the daily intake (ADI) for nitrate should be 3.67 mg/kg body weight and for nitrite it must not be greater than 0.13 mg/kg body weight (WHO, 1973) because higher concentration of nitrates has caused cancer in adults and increased the possibility of brain tumour, leukaemia, and nasopharyngeal [50], [8]. Experiments showed that when the food preservatives were given to organisms in excessive amount, they caused toxicity. Usually nitrate–nitrite containing foods may react with endogen amines, forming carcinogenic and mutagenic compounds, i.e. nitrosoamines [10]. Higher level of nitrate in drinking water has caused chromosomal damage in children [36], [39].
Although food preservatives are part of our foodstuffs and we do not have enough information about their harmful effects. Therefore in this study, we examined the genotoxic effects of the BHA, BHT, PG, SA, and SN in the mitotic cells of Allium cepa because A. cepa has assayed to be best model plant for standard use in environmental monitoring and cytological analysis [33], [32], [12]. According to previous studies A. cepa, has been considered as one of the best established test systems, routinely being used to evaluate the genotoxicity potential of environmental chemicals due to its sensitivity and good correlation with mammalian test system [18], [13], [40]. Several pesticidal and insecticidal chemicals which have been demonstrated to induce genotoxicity in A. cepa root meristem cells are also found to produce similar effects in human lymphocytes [19]. Chauhan et al. [5] emphasized the sensitivity of Allium test system and validated the use this test as alternative to mammalian test system for monitoring the genotoxicity potential.
2. Materials and methods
The root-tip of A. cepa (2n = 16) were used as the test material and selected food preservatives were butylated hydroxyanisole (BHA), butylated hydroxytoluene (BHT), propyl gallate (PG), sorbic acid (SA), and sodium nitrate (SN) as the test substances. Chemicals procured from Sigma–Aldrich, as follows: BHA chemical formula C11H16O2, molecular weight 180.24 g/mol, BHT chemical formula C15H24O, molecular weight 220.35 g/mol, PG chemical formula C10H12O5, molecular weight 212.20 g/mol, SA chemical formula C6H8O2, molecular weight 112.1265 g/mol, SN chemical formula NaNO3, molecular weight 84.9947 g/mol.
Small bulbs (1.5–2.0 cm in diameter) of the common onion, A. cepa (2n = 16), were procured from local market. Prior to initiating the test, the outer scales of the bulbs and the dry bottom plate were removed without destroying the root primordia. Bulbs of A. cepa were placed in sand and allowed to germinate at room temperature (25 ± 2 °C). When the newly emerged roots were 1–2 cm in length, the bulb were removed from the sand washed and placed on a blotting paper to remove excess water and used for the treatment. Roots of A. cepa were treated with a series of concentrations, i.e. 1000, 1500, 2000, 2500 ppm (w/v, preservatives/distilled water), for 4, 8, 16 h. The control bulbs were grown in distilled water. After treatment, root tips were cut and washed and treated with 0.05% colchicines for 3 h. Root tips were washed and fixed in a mixture of ethanol and glacial acetic acid (3:1) for 24 h, washed thrice with distilled water, and then dyed with 2% aceto-carmine. Squashes were prepared as suggested by Sharma and [45] to determine the mitotic index and the presence of chromosomal aberrations. Three replicates were performed for each treatment and scoring was done from the three roots of each replicate. A minimum of 500 well spread metaphase cells were scored for each concentration. The MI (mitotic index) was calculated for each treatment as number of dividing cells per approximately 500 cells [14], [15]. The percentage of aberrant cells was calculated on the number of aberrant cells per dividing cells scored at each concentration for each preservatives. The cytological abnormalities were scored in the mitotic cells and the results are shown in the tables and figures. Necrotic cells were identified by light microscopy using morphological characteristics of the nucleus which exhibits a pale cytoplasm or loss of cytoplasm, and a damaged/irregular nuclear membrane with a partially intact nuclear structure. The most frequent abnormalities are shown in photomicrographs.
2.1. Statistical analysis
One-way ANOVA and multivariate analysis of the data was carried out with the SPSS computer programme. Data were expressed as mean ± standard error of mean (SEM).
3. Results
The effects of preservatives on MI and the frequency of mitotic phases are given in Table 1, Table 2, Table 3, Table 4, Table 5. Used preservatives in this experiment were found effective for significantly decrease in the overall MI in the treatment groups compared with control for all dose concentrations and treatment periods. It was observed in BHT treated roots that decrease in MI was dose dependent at 4 h of exposure as compared to control and reduction in MI was highly significant with increased exposure time. However dose concentration of 1500, 2000 and 2500 ppm at exposure of 8–16 h showed MI was insignificant with each other (Table 1). Dose concentration of BHA up to 2000–2500 ppm for the exposure period of 4–16 h showed insignificant MI but other used concentrations were found significant for MI (Table 2). In case of SN, 4 h exposure showed the reduction in MI was maintained only at concentration of 2500 ppm and reduction in MI was found parallel to control for other dose concentrations. At 8 h exposure, a significant decrease of MI observed with control but treated groups were insignificant with each other. While 16 h treatment with dose concentration 1000 ppm was observed insignificant as compared to control but other concentrations were significantly differ with control group (Table 3). Sorbic acid treatment elucidated that significant decrease of MI at 2500 ppm and maximum reduction was observed at 16 h exposure. Rest of the SA concentrations, i.e. 1000, 1500 and 2000 ppm were insignificant with each other (Table 4). Propyl gallate (PG) was found highly effective and toxic at higher concentration (2500 ppm) in comparison to other preservatives where inhibition of cell division was found at 16 h exposure. However reduction in MI was observed insignificant at 4 h and 16 h of exposure time for 2000 and 2500 ppm dose concentrations, and at 8 h of treatment all concentrations were insignificant with each other except control group (Table 5).
Table 1.
Cytogenetic analysis of A. cepa root tips exposed to different concentrations of butylated hydrotoluene for different periods.
| Time of treatment | Conc. (ppm) | Total cells | Total mitosis | MI (mean ± S.E.a) | B | Stickiness | CB and laggard | BN | Lobulated nuclei | c-Mitosis | % of abnormalities | Total abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | Control | 509.33 ± 1.86 | 319.67 ± 1.45 | 62.76 ± 2.53b | 0.33 | – | – | – | – | – | 0.10 | 0.33 ± 0.33a |
| 1000 | 505.33 ± 2.33 | 303.00 ± 3.61 | 59.95 ± 1.71ab | 4 | 1 | – | – | – | 1 | 1.98 | 6.00 ± 0.58b | |
| 1500 | 515.00 ± 1.15 | 313.33 ± 2.73 | 60.83 ± 2.62ab | 5.33 | 3 | – | – | – | 1.67 | 3.19 | 10.00 ± 0.58c | |
| 2000 | 513.00 ± 3.79 | 297.67 ± 1.45 | 58.01 ± 2.29ab | 3.33 | 2.67 | – | – | – | 1.67 | 2.58 | 7.67 ± 0.33b | |
| 2500 | 518.67 ± 1.86 | 279.00 ± 2.08 | 53.79 ± 1.50a | 5 | 3 | – | – | – | 3.33 | 4.06 | 11.33 ± 0.88c | |
| 8 h | Control | 518.33 ± 4.06 | 327.67 ± 1.45 | 63.23 ± 1.66b | – | 0.33 | – | – | – | – | 0.10 | 0.33 ± 0.33a |
| 1000 | 512.33 ± 2.33 | 310.67 ± 2.33 | 60.65 ± 2.34b | 3 | – | – | – | – | 4 | 2.25 | 7.00 ± 3.21b | |
| 1500 | 521.67 ± 4.10 | 282.33 ± 1.45 | 54.14 ± 1.70a | 4.67 | 1 | – | – | – | 4 | 3.43 | 9.67 ± 2.40b | |
| 2000 | 520.67 ± 1.86 | 270.00 ± 1.15 | 51.87 ± 1.51a | 6.33 | 3.67 | – | – | – | 5.67 | 5.80 | 15.67 ± 0.33c | |
| 2500 | 504.00 ± 1.73 | 252.33 ± 1.45 | 50.08 ± 1.60a | 9.67 | 3 | 1.33 | 1.00 | 2.00 | 3 | 7.53 | 19.00 ± 1.15c | |
| 16 h | Control | 512.67 ± 1.45 | 324.00 ± 7.02 | 63.21 ± 2.54d | 1 | – | – | – | 0.31 | 1.00 ± 0.00a | ||
| 1000 | 506.33 ± 2.03 | 278.00 ± 1.53 | 54.92 ± 2.22c | 3 | 2 | 0.67 | 1.67 | 3.00 | 1.67 | 3.72 | 11.00 ± 0.00b | |
| 1500 | 513.33 ± 2.91 | 262.33 ± 3.71 | 51.12 ± 1.54bc | 11 | 2.33 | 2 | 2.00 | 3.33 | 2.67 | 8.12 | 21.33 ± 0.88c | |
| 2000 | 519.33 ± 1.20 | 240.67 ± 6.36 | 46.34 ± 1.80ab | 7 | 4 | 1.33 | 2.33 | 4.33 | 3.33 | 8.16 | 20.00 ± 1.00c | |
| 2500 | 507.33 ± 1.76 | 221.67 ± 6.01 | 43.70 ± 1.81a | 12.33 | 5.67 | 2.33 | 2.67 | 4.00 | 4.33 | 12.93 | 28.67 ± .88d | |
MI – mitotic index; B – bridges; MP – multipolarity; CB – chromosomal break; BN – binucleated.
Means with the same letters do not significantly differ at 0.05 level (Duncon's test).
Table 2.
Cytogenetic analysis of A. cepa root tips exposed to different concentrations of butylated hydroxyanisol for different periods.
| Time of treatment | Conc. (ppm) | Total cells | Total mitosis | MI (mean ± S.E.a) | B | MP | Stickiness | CB and laggards | BN | Lobulated nuclei | c-Mitosis | % of abnormalities | Total abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | Control | 529.33 ± 1.76 | 284.33 ± 2.19 | 53.72 ± 1.11d | 0.33 | – | – | – | – | – | – | 0.12 | 0.33 ± 0.33a |
| 1000 | 514.67 ± 1.45 | 247.00 ± 3.79 | 48.00 ± 1.43c | 1.33 | 1.67 | 1.67 | 0.33 | 1.67 | 1.33 | 1.00 | 3.64 | 9.00 ± 0.58b | |
| 1500 | 535.00 ± 1.15 | 242.67 ± 2.73 | 45.36 ± 1.01b | 3.33 | 2 | 2.00 | 0.67 | 2.00 | 1.67 | 0.33 | 4.94 | 12.00 ± 1.53c | |
| 2000 | 533.67 ± 2.73 | 219.33 ± 2.33 | 41.11 ± 1.14a | 2 | 3.67 | 2.67 | 1.00 | 2.00 | 2.00 | 1.67 | 6.84 | 15.00 ± 0.58d | |
| 2500 | 519.67 ± 5.04 | 213.33 ± 2.03 | 41.05 ± 1.42a | 4 | 4 | 3.00 | 2.00 | 1.33 | 2.00 | 2.00 | 8.59 | 18.33 ± 0.67e | |
| 8 h | Control | 523.33 ± 1.67 | 274.00 ± 4.58 | 52.36 ± 1.54d | 0.33 | – | – | – | 0.33 | – | – | 0.24 | 0.67 ± 0.33a |
| 1000 | 507.33 ± 1.76 | 244.33 ± 2.33 | 48.16 ± 1.55cd | 3 | 4 | 2.00 | – | 0.33 | 0.67 | 1.67 | 4.78 | 11.33 ± 0.88b | |
| 1500 | 524.67 ± 1.86 | 234.67 ± 2.60 | 44.72 ± 1.48c | 4 | 5 | 3.00 | 0.67 | 1.00 | 0.33 | 2.33 | 5.68 | 16.33 ± 1.20bc | |
| 2000 | 532.00 ± 1.53 | 212.67 ± 2.73 | 39.97 ± 1.16a | 5.33 | 5.67 | 2.33 | 1.00 | 2.67 | 1.33 | 1.33 | 9.24 | 19.67 ± 1.45cd | |
| 2500 | 534.33 ± 1.45 | 200.33 ± 0.88 | 37.50 ± 1.02a | 7.33 | 7 | 2.33 | 1.67 | 2.33 | 1.67 | 1.67 | 11.98 | 24.00 ± 3.06d | |
| 16 h | Control | 518.00 ± 3.46 | 267.67 ± 4.81 | 51.66 ± 1.13c | 0.33 | 0.33 | – | – | – | – | 0.25 | 0.67 ± 0.33a | |
| 1000 | 536.00 ± 1.73 | 233.33 ± 2.60 | 43.48 ± 1.11b | 4.33 | 5 | 2.00 | 2.00 | 1.33 | 2.00 | 0.67 | 7.44 | 16.67 ± 0.67b | |
| 1500 | 524.67 ± 1.45 | 213.67 ± 1.86 | 40.72 ± 1.00b | 6.33 | 7 | 2.33 | 1.67 | 1.33 | 3.00 | 2.00 | 11.07 | 23.67 ± 0.88c | |
| 2000 | 525.33 ± 2.91 | 194.67 ± 1.45 | 37.06 ± 1.07a | 6.33 | 7 | 2.67 | 2.00 | 1.33 | 1.67 | 1.33 | 11.47 | 22.33 ± 1.20c | |
| 2500 | 533.33 ± 2.40 | 181.67 ± 6.01 | 34.07 ± 1.26a | 7.67 | 10.33 | 3.67 | 1.67 | 1.00 | 1.33 | 2.67 | 15.60 | 28.67 ± 0.33d | |
MI – mitotic index; B – bridges; MP – multipolarity; CB – chromosomal break.
Means with the same letters do not significantly differ at 0.05 level (Duncon's test).
Table 3.
Cytogenetic analysis of A. cepa root tips exposed to different concentrations of sodium nitrate for different periods.
| Time of treatment | Conc. (ppm) | Total cells | Total mitosis | MI (mean ± S.E.a) | B | MP | Stickiness | c-Mitosis | Binucleated | % of abnormalities | Total abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | Control | 518.67 ± 1.33 | 179.00 ± 1.15 | 34.52 ± 1.35a | – | – | – | – | – | 0.18 | 0.33 ± 0.33a |
| 1000 | 513.33 ± 5.49 | 175.67 ± 2.33 | 34.25 ± 1.16a | 1.33 | 1.33 | – | – | – | 1.51 | 2.67 ± 0.33ab | |
| 1500 | 511.67 ± 7.06 | 169.67 ± 1.20 | 33.18 ± 0.90a | 2 | 1.67 | 1 | 0.33 | – | 2.95 | 5.00 ± 0.00b | |
| 2000 | 510.33 ± 5.17 | 168.33 ± 0.33 | 32.99 ± 1.09a | 4.33 | 3.67 | 3.33 | 1.33 | – | 7.52 | 12.67 ± 1.20c | |
| 2500 | 528.67 ± 1.45 | 166.00 ± 2.65 | 31.39 ± 1.17a | 4.33 | 4.5 | 1.67 | 2.33 | 0.33 | 7.93 | 16.00 ± 1.15d | |
| 8 h | Control | 519.00 ± 4.93 | 188.00 ± 1.53 | 36.25 ± 1.18b | – | – | – | – | – | 0.00 | 0.00 ± 0.00a |
| 1000 | 544.67 ± 3.48 | 173.00 ± 6.43 | 31.75 ± 1.36a | 3 | 3.33 | 2 | 1.67 | 0.67 | 6.17 | 10.67 ± 2.33b | |
| 1500 | 518.00 ± 2.65 | 164.33 ± 8.95 | 31.71 ± 1.57a | 5.67 | 4 | 5 | 2 | – | 10.14 | 16.67 ± 0.67c | |
| 2000 | 513.00 ± 2.65 | 159.00 ± 2.00 | 30.98 ± 0.99a | 10.33 | 6 | 2.33 | – | 0.33 | 11.94 | 19.00 ± 0.58c | |
| 2500 | 525.33 ± 1.76 | 155.00 ± 2.65 | 29.51 ± 1.09a | 9.67 | – | 5.33 | 2.33 | 0.33 | 11.39 | 18.00 ± 0.58c | |
| 16 h | Control | 522.67 ± 4.26 | 188.00 ± 3.61 | 35.99 ± 1.32b | – | – | 0.33 | – | – | 0.18 | 0.33 ± 0.33a |
| 1000 | 504.33 ± 2.03 | 175.67 ± 4.81 | 34.82 ± 1.41b | 1 | 17.67 | 4.67 | 2.67 | 0.67 | 15.18 | 26.67 ± 0.88b | |
| 1500 | 514.67 ± 4.67 | 148.33 ± 4.63 | 28.81 ± 1.20a | 16 | 5 | – | 4.33 | 0.33 | 17.30 | 25.67 ± 1.20b | |
| 2000 | 521.00 ± 6.66 | 150.67 ± 4.41 | 28.95 ± 1.21a | 16.67 | 8.67 | 5 | 2 | 1 | 22.13 | 33.33 ± 1.67c | |
| 2500 | 520.33 ± 3.53 | 135.33 ± 3.84 | 26.02 ± 1.09a | 19.67 | 13 | 6 | 3 | 2 | 32.27 | 43.67 ± 1.76d | |
MI – mitotic index; B – bridges; MP – multipolarity.
Means with the same letters do not significantly differ at 0.05 level (Duncon's test).
Table 4.
Cytogenetic analysis of A. cepa root tips exposed to different concentrations of sorbic acid for different periods.
| Time of treatment | Conc. (ppm) | Total cells | Total cells | MI (mean ± S.E.a) | B | MP | Stickiness | CB and laggards | c-Mitosis | % of abnormalities | Total abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | Control | 561.33 ± 6.69 | 247.67 ± 8.25 | 44.15 ± 1.80b | .33 | – | – | – | – | 0.13 | 0.33 ± 0.33a |
| 1000 | 546.67 ± 8.29 | 230.67 ± 2.60 | 42.21 ± 1.56ab | – | 1.33 | 2.33 | – | 1 | 2.02 | 4.67 ± 1.20b | |
| 1500 | 558.33 ± 5.78 | 220.67 ± 5.78 | 39.55 ± 1.44ab | 4 | 1 | 2.67 | 1.67 | – | 4.23 | 9.00 ± 1.00c | |
| 2000 | 531.00 ± 4.73 | 209.67 ± 2.96 | 39.50 ± 1.51a | 5.33 | 3 | 1 | 1.00 | 0.67 | 5.25 | 12.67 ± 0.88d | |
| 2500 | 546.00 ± 5.57 | 211.00 ± 2.52 | 38.67 ± 1.32a | 7 | 1.33 | 3 | 1.67 | 0.33 | 6.32 | 10.67 ± 0.88cd | |
| 8 h | Control | 529.67 ± 24.31 | 242.33 ± 2.19 | 45.92 ± 1.86a | – | – | 0.67 | – | – | 0.28 | 0.67 ± 0.33a |
| 1000 | 519.67 ± 2.03 | 210.33 ± 4.98 | 40.49 ± 1.55a | 2.67 | 3.33 | 3 | 2.33 | 0.67 | 5.71 | 12.00 ± 1.00b | |
| 1500 | 520.00 ± 3.79 | 199.00 ± 2.65 | 38.28 ± 1.22a | 6.67 | 3.67 | – | 2.67 | 1 | 7.04 | 13.67 ± 1.45bc | |
| 2000 | 519.67 ± 13.32 | 197.00 ± 7.57. | 38.05 ± 2.16a | 3 | 5.67 | 5 | 3.00 | 2.67 | 9.82 | 16.67 ± 1.20cd | |
| 2500 | 513.67 ± 3.28 | 178.67 ± 4.81 | 34.80 ± 1.50a | 8.67 | 5 | 0.33 | 3.33 | 2.67 | 11.19 | 20.00 ± 1.73d | |
| 16 h | Control | 540.67 ± 8.09 | 234.33 ± 4.33 | 43.38 ± 1.45c | .33 | – | – | – | .33 | 0.29 | 0.67 ± 0.33a |
| 1000 | 538.67 ± 4.33 | 133.67 ± 11.61 | 24.81 ± 2.11b | 6.33 | 6 | 2.33 | – | 1 | 11.72 | 15.67 ± 0.33b | |
| 1500 | 515.00 ± 2.52 | 119.33 ± 2.33 | 23.17 ± 1.01ab | 10 | 5.33 | 3.67 | 2.33 | 1.33 | 18.99 | 22.67 ± 1.45c | |
| 2000 | 510.00 ± 4.58 | 112.67 ± 2.73 | 22.11 ± 1.29ab | 18 | 7 | 3.33 | 2.67 | 3.33 | 30.47 | 36.00 ± 2.65d | |
| 2500 | 532.00 ± 3.79 | 103.33 ± 7.51 | 19.43 ± 1.55a | 20.33 | 15.33 | 7.33 | 1.33 | 1.33 | 44.18 | 46.33 ± 2.96e | |
MI – mitotic index; B – bridges; MP – multipolarity; CB – chromosomal break.
Means with the same letters do not significantly differ at 0.05 level (Duncon's test).
Table 5.
Cytogenetic analysis of A. cepa root tips exposed with different concentration of propyl gallate for different periods.
| Time of treatment | Conc. (ppm) | Total cell | Total mitosis | MI (mean ± S.E.a) | B | MP | Stickiness | CB and laggards | c-Mitosis | Necrotic cells | % of abnormalities | Total abnormalities |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 h | Control | 519.67 ± 1.45 | 232.00 ± 3.61 | 44.64 ± 1.75c | 0.33 | – | – | – | – | – | 0.14 | 0.33 ± 0.33a |
| 1000 | 515.33 ± 1.45 | 222.00 ± 4.58 | 43.07 ± 1.87bc | 4.00 | 2.67 | 3.33 | 1.67 | – | – | 5.26 | 9.67 ± 0.67b | |
| 1500 | 510.67 ± 1.20 | 194.67 ± 3.28 | 38.12 ± 1.69ab | 2.33 | 1.00 | 3.33 | 1.33 | 0.33 | 0.33 | 4.44 | 8.67 ± 0.88b | |
| 2000 | 531.67 ± 3.18 | 185.00 ± 2.52 | 34.78 ± 1.33a | 1.67 | 2.67 | 3.67 | 3.00 | 1.00 | – | 6.49 | 12.00 ± 0.00c | |
| 2500 | 520.33 ± 4.84 | 181.67 ± 8.01 | 34.89 ± 1.56a | 5.67 | 3.00 | 5.00 | 2.67 | 1.00 | 0.33 | 9.73 | 17.33 ± 0.88d | |
| 8 h | Control | 522.67 ± 1.76 | 222.67 ± 3.93 | 42.59 ± 1.70b | – | 0.33 | 0.33 | – | – | – | 0.30 | 0.67 ± 0.67a |
| 1000 | 532.00 ± 1.53 | 193.00 ± 3.06 | 36.29 ± 1.72a | 4.33 | 2.67 | 5.00 | 1.00 | 1.00 | 0.67 | 7.60 | 14.67 ± 0.67b | |
| 1500 | 512.00 ± 1.73 | 181.00 ± 1.15 | 35.36 ± 1.47a | 4.67 | 5.67 | 1.00 | 2.00 | – | – | 7.37 | 13.33 ± 0.33b | |
| 2000 | 516.33 ± 3.18 | 171.67 ± 2.03 | 33.23 ± 1.33a | 6.33 | 3.67 | – | 3.00 | 0.33 | 1.00 | 8.35 | 14.00 ± 0.00b | |
| 2500 | 504.00 ± 1.53 | 164.33 ± 3.84 | 32.60 ± 1.42a | 12.67 | 3.33 | 1.00 | 3.00 | 0.67 | 1.00 | 13.19 | 22.33 ± 0.88c | |
| 16 h | Control | 535.33 ± 1.86 | 210.00 ± 4.73 | 39.22 ± 1.30d | – | 0.33 | – | – | – | – | 0.16 | 0.33 ± 0.33a |
| 1000 | 548.33 ± 1.76 | 109.00 ± 4.36 | 19.88 ± 0.96c | 3.33 | 3.00 | 1.33 | 4.00 | 1.00 | 0.67 | 12.23 | 13.33 ± 0.88b | |
| 1500 | 519.00 ± 1.73 | 77.67 ± 8.45 | 14.97 ± 0.59b | 5.00 | 2.33 | 3.00 | 3.33 | – | 4.00 | 22.74 | 16.00 ± 1.15b | |
| 2000 | 510.67 ± 1.45 | 40.67 ± 6.57 | 7.97 ± 0.37a | 2.00 | 5.33 | 3.67 | 1.67 | 6.67 | 6.00 | 62.31 | 28.00 ± 1.00d | |
| 2500 | 525.33 ± 1.76 | 36.67 ± 4.41 | 6.98 ± 0.86a | 2.00 | 3.33 | 3.00 | 4.00 | 7.33 | 10.33 | 82.72 | 30.00 ± 3.46d | |
MI – mitotic index; B – bridges; MP – multipolarity; CB – chromosomal break.
Means with the same letters do not significantly differ at 0.05 level (Duncon's test).
The food preservatives BHT, BHA, SA, PG, SN caused change in the frequencies of different cell stages and also their treatments induced a wide range of mitotic abnormalities as compared to the control in the root tip of A. cepa. Statistically significant correlation between concentration and chromosomal aberration, necrotic cells and binucleated cells in which propyl gallate was more prevalent. The highest percentage of abnormalities and total number of abnormalities was 12.93%, 28.67 (Table 1); 15.60%, 28.67 (Table 2); 32.27%, 43.67 (Table 3); 44.18%, 46.33 (Table 4); and 82.72%, 30.00 (Table 5) respectively. The types and percentage of these abnormalities are given above and abnormalities can be observed in Fig. 1. Several chromosomal abnormalities at metaphase and anaphase recorded were bridges, chromosomal break, lobulated nucleus, binucleated cell, laggard multipolarity, stickiness, C-mitosis, necrotic cell were observed in which bridges and multipolarity were more frequent.
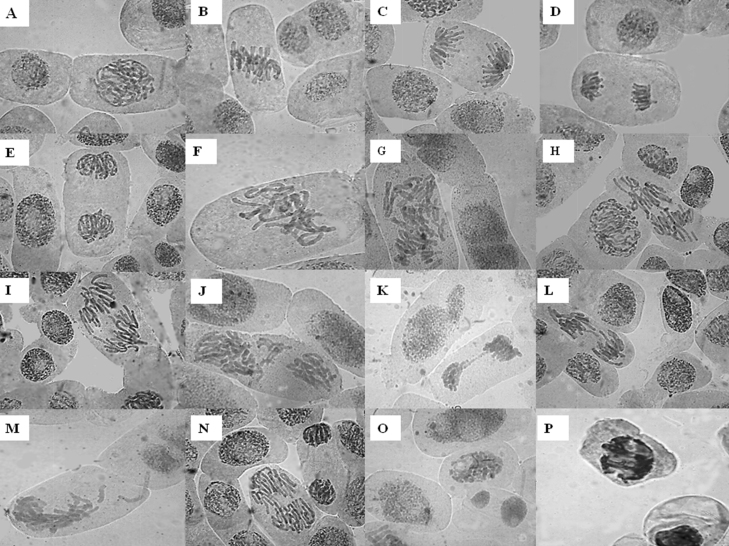
Fig. 1.
Chromosomal aberrations observed in root tip cells of Allium cepa: A – normal prophase; B – normal metaphase; C – normal anaphase; D – normal late anaphase; E – normal telophase; F – sticky metaphase; G – c-metaphase; H – abnormal anaphase with break; I – multipolar anaphase; J – abnormal anaphase with laggard; K – chromosomal bridge and lobulated nuclei; L – sticky anaphase with laggard; M – abnormal anaphase with laggard; N – multipolar anaphase with laggard; O – binucleated cell; P – necrotic cell.
Analysis of two-way ANOVA showed the effect of preservatives on mitotic index and abnormalities (Table 6), where the reduction of MI was least significant as compared to other preservatives in case of sodium nitrate, whereas chromosomal abnormalities were highly significantly increased corresponding to the time of exposure. On the other hand, all the preservatives were most effective and showed significant decrease of the MI corresponding to the concentration in comparison to control. The result of combined effect of time and concentration showed that BHT, BHA and SN were insignificant for MI in comparison to rest of the preservatives used.
Table 6.
Two-way ANOVA showing the relationship between (mitotic index and abnormalities) and (time and concentration) of the used preservatives respectively.
| Effect of treatment on MI and Ab | Time | Concentration | Time × Concentration |
|---|---|---|---|
| BHT (MI) | 16.39*** | 22.13*** | 1.83NS |
| BHA (MI) | 16.92*** | 69.55*** | 0.79NS |
| SN (MI) | 4.65* | 13.28*** | 2.25NS |
| SA (MI) | 124.40*** | 33.13*** | 6.41*** |
| PG (MI) | 339.42*** | 77.38*** | 14.61*** |
| BHT (Ab) | 76.86*** | 108.87*** | 9.20*** |
| BHA (Ab) | 51.78*** | 173.25*** | 3.90** |
| SN (Ab) | 384.83*** | 243.04*** | 31.81*** |
| SA (Ab) | 185.98*** | 145.27*** | 25.99*** |
| PG (Ab) | 63.27*** | 171.78*** | 11.59*** |
p < 0.05 significant level.
p < 0.01 significant level.
p < 0.001 significant level.
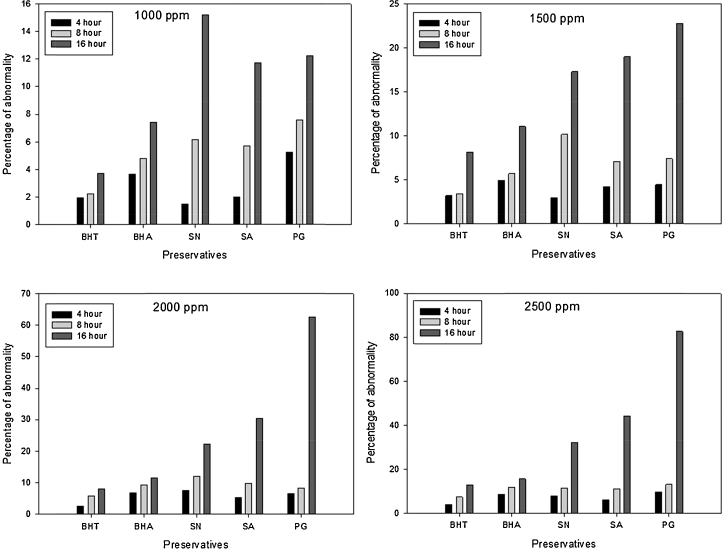
The most interesting investigation in all results was the significant increase in chromosomal abnormalities percentage, among all the five food preservatives percentage of abnormalities was found least in BHT while highest in propyl gallate (Fig. 2), which is considerably important for the use of food preservatives.
Fig. 2.
Graphical representation of percentage of abnormality of five food preservatives at different concentrations and different time periods.
4. Discussion
The impact of food preservatives BHA, BHT, SA, PG, and SN has been investigated in meristematic cells of A. cepa and significant decrease in mitotic index, increase in abnormality and other cellular activities have been noticed varying to corresponding concentration and time periods. Decrease in mitotic index might be due to inhibition of DNA synthesis [43], [47] or a blocking in the G2-phase of the cell cycle, preventing the cell from entering mitosis [52]. According to [9], [23] inhibition of DNA synthesis due to decrease in ATP level and pressure from the functioning of energy producing centre. Earlier reports revealed that food preservatives and several other chemicals have been reported as inhibitor of MI [16], [41]. Türkoğlu [49] also showed the exposure of root tips of A. cepa to high concentration of five food preservatives led to inhibition of MI and DNA synthesis. C-mitosis indicated that the inhibition of spindle formation similar to the effect of colchicine [2]. Presence of C-mitosis is commonly associated with the spindle poisons [44]. It is indicative of weak toxic effect which may be reversible indicating of risk of aneuploidy. Anaphase bridges are possibly formed by breakage and fusion of chromosomes and chromatids. Such chromosomal bridges were reported to be induced by other food preservatives like sodium benzoate and sodium sulphite in V. faba [38]. Induction of multipolarity may be caused by the disturbance of spindle formation, which is present in all the treatment group except BHT. Multipolarity also shows subsequent failure of anaphase separation or perhaps unequal translocation, shows weak toxicity. In our experiment presence of stickiness was high in all treatments especially at higher concentration indicate an effect on proteins of chromosomes and reflects a toxic effect, usually an irreversible type probably leading to cell death which has been evidenced in treatment with propyl gallate only. Although in case of other chemicals it can be regarded as physiological effect. Another aberration induced by propyl gallate was the necrotic cells at high concentration and long exposure this means that this chemical proved to be both genotoxic and clastogenic and also involve in the inhibitory action on DNA and RNA synthesis. Laggards were observed in root cells after treatment with SA, PG SA, BHT and BHA but not in SN even at higher concentration. Lagging chromosomes appears in response to failure of chromosome to move to the either of the poles. Nitrate is relatively nontoxic, but approximately 5% of all ingested nitrate is converted to the more toxic nitrite [48], [46]. Chromosome breakage is also known as clastogens observed in all treatment groups and their action on chromosome is generally regarded to involve an action on DNA [17], [6]. Binucleated cells and lobulated nucleus were observed only in case of BHT and BHA. The presence of lobulated nuclei can indicate a cell death process. Fernandes et al. [11] found that formation of nuclear buds may be because of the elimination of exceeding genetic material derived from the polyploidization process.
Comparing the results of Table 1, Table 2, Table 3, Table 4, Table 5 total abnormalities and percentage of abnormality were high at higher concentration and longer time periods which indicates that higher dose of preservatives are genotoxic to the living cells. The result also shows that least percentage of abnormalities is present in BHT and highest in propyl gallate. The order of increasing abnormalities percentage is BHT < BHA < SN < SA < PG. BHT and BHA show less abnormalities because these chemicals have antioxidative properties, Kahl and Kappus [27] also concluded that these chemicals are harmless even at higher concentration up to 3000 ppm [53], [21] but here level of abnormality of BHA and BHT show that it might be genotoxic at this concentration. BHA also known to inhibit mitotic index also reported by Jos et al. [26].
5. Conclusion
From the present investigation it appears that BHT, BHA, SA, PG, and SN, which is frequently used in packaged food have genotoxic effects on the chromosomes in a reliable plant assay, then it might be harmful to the other organisms especially to human being. In view of above, it is necessary to be aware about the level of chemicals at the time of using.
Acknowledgments
We would like to thank Head, Department of Botany, Banaras Hindu University for providing necessary laboratory facilities. The authors are also thankful to University Grant Commission, New Delhi, India for providing financial assistance (Project no: Ref. no. BOT/2011-12/UGC/BKR/P-01/659/appoint./01).
Footnotes
Available online 11 June 2014
Contributor Information
Himadri Pandey, Email: pandey.him204@gmail.com.
Vikas Kumar, Email: vikky11muir@gmail.com.
B.K. Roy, Email: bijoykrishnabotany12@gmail.com.
References
- 1.Amo H., Kubota H., Lu J., Matsuyama M. Adenomatous hyperplasia and adenomas in the lung induced by chronic feeding of butylated hydroxyanisole of Japanese house musk shrew (Suncus murinus) Carcinogenesis. 1990;11:151–154. doi: 10.1093/carcin/11.1.151. [DOI] [PubMed] [Google Scholar]
- 2.Badr A. Chromotoxic activities of two herbicides in A. cepa. Cytologia. 1983;48:451–457. [Google Scholar]
- 3.Ben-Hur E., Green M., Prager A., Rosenthal I., Riklis E. Differential protective effects of antioxidants against cell killing and mutagenesis of Salmonella typhimurium by gradiation. J. Radiat. Res. 1981;22:250–257. doi: 10.1269/jrr.22.250. [DOI] [PubMed] [Google Scholar]
- 4.Brusick D. Genotoxicity of phenotic antioxidants. Toxicol. Ind. Health. 1993;9:223–230. doi: 10.1177/0748233793009001-216. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan L.K.S., Saxena P.N., Gupta S.K. Cytogenetic effects of cypermethrin and fenvalerate on the root meristem cells of Allium cepa. Environ. Exp. Bot. 1999;42:181–189. [Google Scholar]
- 6.Chauhan L.K.S., Sundararaman V. Effects of substituted ureas on plant cells I. Cytological effects of isopruturon on the root meristem cells of A. cepa. Cytologia. 1990;55:91–98. [Google Scholar]
- 7.Clapp N.K., Tyndall R.I., Cumming R.B., Otten J.A. Effects of butylated hydroxytoluene alone or with diethylnitrosamine in mice. Food Cosmet. Toxicol. 1974;12:367–371. doi: 10.1016/0015-6264(74)90009-1. [DOI] [PubMed] [Google Scholar]
- 8.Dusdieker L.B. Nitrate in baby foods, adding to the nitrate mosaic. Arch. Pediatr. Adolesc. Med. 1994;148(5):490–494. doi: 10.1001/archpedi.1994.02170050048009. [DOI] [PubMed] [Google Scholar]
- 9.Epel D. The effects of carbon monoxide inhibition of ATP level and the date of mitosis in sea urchin egg. J. Cell Biol. 1963;17:315–319. doi: 10.1083/jcb.17.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ertuğrul N. Food additives regulations and health problems about upper limit of some food additives. Istanbul University; 1998. M.S. Thesis. [Google Scholar]
- 11.Fernandes T.C.C., Mazzeo D.E.C., Marin-Morales M.A. Mechanism of micronuclei formation in polyploidizated cells of Allium cepa exposed to trifluralin herbicide. Pest. Biochem. Physiol. 2007;88:252–259. [Google Scholar]
- 12.Fiskesjo G. Some results with Allium tests with organic mercury halogenides. Hereditas. 1969;62:314–322. doi: 10.1111/j.1601-5223.1969.tb02241.x. [DOI] [PubMed] [Google Scholar]
- 13.Fiskesjo G. The Allium test as a standard in environmental monitoring. Hereditas. 1985;102:99–112. doi: 10.1111/j.1601-5223.1985.tb00471.x. [DOI] [PubMed] [Google Scholar]
- 14.Fiskesjo G. Allium test on river water from Braan and Saxan before and after closure of a chemical factory. Ambio. 1985;14:99–103. [Google Scholar]
- 15.Fiskesjo G. Allium test for screening chemicals; evaluation of cytologic parameters. In: Wang W., Gorsuch J.W., Hughes J.S., editors. Plants for Environmental Studies. CRC Lewis Publishers; Boca Raton, NY: 1997. pp. 308–333. [Google Scholar]
- 16.Gömürgen A.N. Cytological effect of the potassium metabisulphite and potassium nitrate food preservative on root tips of Allium cepa L. Cytologia. 2005;70:119–128. [Google Scholar]
- 17.Grant W.F. Chromosome aberrations in plant as monitoring system. Environ. Health Perspect. 1978;27:37–43. doi: 10.1289/ehp.782737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant W.F. The present status of higher plant bioassays for the detection of environmental mutagens. Mutat. Res. 1994;310:175–185. doi: 10.1016/0027-5107(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 19.Grover I.S., Dhingra A.K., Adhikari N., Ladhar S.S. Genotoxicity of pesticides and plant systems. Prog. Clin. Biol. Res. 1990;340E:91–106. [PubMed] [Google Scholar]
- 20.Hasegawa M.M., Nishi Y., Ohkawa Y., Inui N. Effects of sorbic acid and its salts on chromosome aberrations, sister chromatid exchanges and gene mutations in cultured Chinese hamster cells. Food. Chem. Toxicol. 1984;22:501–507. doi: 10.1016/0278-6915(84)90219-9. [DOI] [PubMed] [Google Scholar]
- 21.Hirose M., Takesada Y., Tanaka H., Tamano S., Kato T., Shirai T. Carcinogenicity of antioxidants BHA, caffeic acid, sesamol, 4-methoxyphenol and catechol at low doses, either alone or in combination, and modulation of their effects in a rat medium-term multiorgan carcinogenesis model. Carcinogenesis. 1997;19:207–212. doi: 10.1093/carcin/19.1.207. [DOI] [PubMed] [Google Scholar]
- 22.IARC . International Agency for Research on Cancer; Lyon: 1987. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. Supplement 7; p. 59. [PubMed] [Google Scholar]
- 23.Jain A.K., Andsorbhoy R.K. Cytogenetical studies on the effects of some chlorinated pesticide III. Concluding remarks. Cytologia. 1988;53:427–436. [Google Scholar]
- 24.JECFA . FAO Nutrition Meeting Report Ser. No. 53, WHO Technical Report Ser. No. 539; Geneva: 1973. Seventeenth Report of the Joint FAO/WHO Expert Committee on Food Additives: Toxicological Evaluation of Certain Food Additives with a Review of General Principles and of Specifications. [PubMed] [Google Scholar]
- 25.JECFA . WHO Food Additives Series, No. 35. Joint FAO/WHO Expert Committee on Food Additives; Geneva: 1996. Toxicological Evaluation of Certain Food Additives and Contaminants in Food; pp. 3–86. [Google Scholar]
- 26.Jos A., Repetto G., Ríos J.C., del Peso A., Salguero M., Hazen M.J., Molero M.L., Fernández-Freire P., Pérez-Martín J.M., Labrador V., Cameán A. Ecotoxicological evaluation of the additive butylated hydroxyanisole using a battery with six different model systems and eighteen endpoint. Aquat. Toxicol. 2005;71:183–192. doi: 10.1016/j.aquatox.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Kahl R., Kappus H. Toxicity of synthetic antioxidants BHT and BHA in comparison with natural antioxidants vitamin E. Z. Lebensm. Unters. Forsh. 1993;196:329–338. doi: 10.1007/BF01197931. [DOI] [PubMed] [Google Scholar]
- 28.Karasz A.B., Maxstadt J.J., Reher J., Decocco F. Rapid screening procedure for the determination of preservatives in ground beef: sulfites, benzoates, sorbates, and ascorbates. J. Assoc. Off. Anal. Chem. 1976;59:766–769. [PubMed] [Google Scholar]
- 29.Kinae N., Hashizume T., Makita T., Tomita I., Kimura I., Kanamori H. Studies on the toxicity of pulp and paper mill effluents I. Mutagenicity of the sediment samples derived from Kraft paper mills. Water Res. 1981;15:17–24. [Google Scholar]
- 30.Laitinen S. Calculated dietary intakes of nitrate and nitrite by young Finns. Food Addit. Contam. 1993;10(4):469–477. doi: 10.1080/02652039309374170. [DOI] [PubMed] [Google Scholar]
- 31.Leslie S.W., God S.C., Acosta D. Cytotoxicity of butylated hydroxytoluene and butylated hydroxyanisole in cultured heart cells. Toxicology. 1978;10:281–289. doi: 10.1016/0300-483x(78)90078-1. [DOI] [PubMed] [Google Scholar]
- 32.Levan A., Ostergren G. The mechanism of c-mitotic action. Observations of the naphtalene series. Hereditas. 1943;29:381–443. [Google Scholar]
- 33.Levan A. The effect of colchicine on root mitoses in Allium. Hereditas. 1938;24:471–486. [Google Scholar]
- 34.Luca D., Luca V., Cotor F., Raileanu L. In vivo and in vitro cytogenetic damage induced by sodium nitrite. Mutat. Res. 1987;189:333–339. doi: 10.1016/0165-1218(87)90065-6. [DOI] [PubMed] [Google Scholar]
- 35.Luck E. Food applications of sorbic acid and its salts. Food Addit. Contam. 1990;7:711–715. doi: 10.1080/02652039009373936. [DOI] [PubMed] [Google Scholar]
- 36.McCredie M. Antenatal risk factors for malignant brain tumours in New South Wales children. Int. J. Cancer. 1994;56(1):6–10. doi: 10.1002/ijc.2910560103. [DOI] [PubMed] [Google Scholar]
- 37.Mukherjee A., Giri A.K.G., Talukder G., Sharma A. Sister chromatid exchanges and micronuclei formations induced by sorbic acid and sorbic acid-nitrite in vivo in mice. Toxicol. Lett. 1988;42:47–53. doi: 10.1016/0378-4274(88)90101-4. [DOI] [PubMed] [Google Scholar]
- 38.Njagi M., Gopalan H.N. Cytogene effects of food preservatives-sodium benzoate and sodium sulphite on Vicia faba root meristems. Mutat. Res. 1982;102:213–219. doi: 10.1016/0165-1218(82)90131-8. [DOI] [PubMed] [Google Scholar]
- 39.Preston-Martin S. N-Nitroso compounds and childhood brain tumors: a case–control study. Cancer Res. 1982;42(12):5240–5245. [PubMed] [Google Scholar]
- 40.Rank J., Nielsen M.H. Allium cepa anaphase–telophase root tip chromosome aberration assay on N-methyl-N-nitro urea, maleic hydrazide, sodium azide and ethylmethane sulphonate. Mutat. Res. 1997;390:121–127. doi: 10.1016/s0165-1218(97)00008-6. [DOI] [PubMed] [Google Scholar]
- 41.Rencüzoğullari E., Kayraldız A., İla H.B., Çakmak T., Topaktaş M. The cytogenetical effects of sodium metabisulfite, a food preservative in root tip cells of Allium cepa L. Turk J. Biol. 2001;25:361–370. [Google Scholar]
- 42.Sanchez-Echaniz J. Methemoglobinemia and consumption of vegetables in infants. Pediatrics. 2001;107(5):1024–1028. doi: 10.1542/peds.107.5.1024. [DOI] [PubMed] [Google Scholar]
- 43.Schneiderman M.H., Dewey W.C., Highfield D.P. Inhibition of DNA synthesis in synchronized Chinese hamster cell treated in G1 with cycloheximide. Exp. Cell Res. 1971;67:147–155. doi: 10.1016/0014-4827(71)90630-6. [DOI] [PubMed] [Google Scholar]
- 44.Shahin S.A., El-Amoodi K.H.H. Induction of numerical chromosomal aberrations during DNA synthesis using the fungicides nimrod and rubigan-4 in root tips of Vicia faba L. Mutat. Res. 1991;261:169–176. doi: 10.1016/0165-1218(91)90064-s. [DOI] [PubMed] [Google Scholar]
- 45.Sharma A.K. 3rd ed. Butterworth; London: 1980. Chromosome Technique Theory and Practice. [Google Scholar]
- 46.Spiegelhalder B., Eisenbrand G., Preussmann R. Influence of dietary nitrate on nitrite content of the human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 47.Sudhakar R., Gowda N., Venu G. Mitotic abnormalities induced by silk dyeing industry effluents in the cells of Allium cepa. Cytologia. 2001;66:235–239. [Google Scholar]
- 48.Tenovuo J. The biochemistry of nitrates, nitrites, nitrosamines and other potential carcinogens in human saliva. Oral Pathol. 1986;15(6):303–307. doi: 10.1111/j.1600-0714.1986.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 49.Türkoğlu Ş. Genotoxicity of five food preservatives tested on root tips of Allium cepa L. Mutat. Res. 2007;626:4–14. doi: 10.1016/j.mrgentox.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 50.U.S. Environmental Protection Agency . 1991. Integrated Risk Information System (IRIS): Nitrate.http://www.epa.gov/iris/subst/0076.htm [Google Scholar]
- 51.Van der Heijden C.A., Janssen P.J., Strip J.J. Toxicology of gallates: a review and evaluation. Food Chem. Toxicol. 1986;24:1067–1070. doi: 10.1016/0278-6915(86)90290-5. [DOI] [PubMed] [Google Scholar]
- 52.Van’t Hof J. The action of IAA and kinetin on the mitotic cycle of proliferative and stationary phase excised root meristem. Exp. Cell Res. 1968;51:167. doi: 10.1016/0014-4827(68)90167-5. [DOI] [PubMed] [Google Scholar]
- 53.Whysner J., Wang C., Zang E., Latropoulos M.J., Williams G.M. Dose–response promotion by butylated hydroxyanisole in chemically initiated tumors of the rodent forestomach. Food Chem. Toxicol. 1994;32:215–222. doi: 10.1016/0278-6915(94)90193-7. [DOI] [PubMed] [Google Scholar]