Abstract
Gastrointestinal mucositis induced during cancer treatment is considered a serious dose-limiting side effect of chemotherapy and/or radiotherapy. Frequently, interruption of the cancer treatment due to this pathology leads to a reduction in cure rates, increase of treatment costs and decrease life quality of the patient. Natural products such as Bidens pilosa L. (Asteraceae), represent a potential alternative for the treatment of mucositis given its anti-inflammatory properties. In this study, B. pilosa glycolic extract was formulated (BPF) with poloxamer, a mucoadhesive copolymer, was used for treatment of 5-fluorouracil (5-FU)-induced mucositis in mice. As expected, animals only treated with 5-FU (200 mg/kg) presented marked weight loss, reduction of intestinal villi, crypts and muscular layer, which was associated with severe disruption of crypts, edema, inflammatory infiltrate and vacuolization in the intestinal tissue, as compared to the control group and healthy animals only treated with BPF. On the other hand, the treatment of intestinal mucositis-bearing mice with BPF (75, 100 or 125 mg/kg) managed to mitigate clinical and pathologic changes, noticeably at 100 mg/kg. This dose led to the restoration of intestinal proliferative activity through increasing Ki-67 levels; modulated the expression of Bax, Bcl2 and p53 apoptotic markers protecting intestinal cells from cell death. Moreover, this treatment regulated lipid peroxidation and inflammatory infiltration. No acute toxic effects were observed with this formulation. This work demonstrated that BPF was safe and effective against 5-FU-induced intestinal mucositis in mice. Additional studies are already in progress to further characterize the mechanisms involved in the protective effects of this technological formulation toward the development of a new medicine for the prevention and treatment of intestinal injury in patients undergoing chemotherapy/radiotherapy.
Keywords: 5-Fluorouracil, Bidens pilosa L. (Asteraceae), Chemotherapy, Mucoadhesive formulation, Mucositis, Intestinal injury
1. Introduction
Gastrointestinal mucositis induced during cancer treatment is considered a serious dose-limiting side effect of chemotherapy and/or radiotherapy, since this condition promotes severe ulceration and inflammation of the gastrointestinal tract, mainly in the small intestine [1], [2], [3]. Interruption of the cancer treatment due to mucositis usually leads to a reduction in cure rates, increase treatment costs, decrease quality of life and, consequently worsening prognosis of the disease [3], [4], [5].
Currently, there is no curative intervention for inflammatory mucositis induced by the cancer treatment. Palliative measures for mucositis in the clinic includes oral cryotherapy, soft laser application and systemic administration of medicines, such as glucocorticoids and growth factors [2], [6], [7], [8]. Considering the high cost of some therapeutic procedures and the low efficacy, the development of alternative treatments, such as using natural products, it has been considered [9].
Bidens pilosa L. (Asteraceae), is a plant used for nutritional and medicinal purposes, commonly found in tropical and subtropical regions such as South America [10], [11], [12]. In Brazil, it is popularly known as picão-preto [13] and all parts of the B. pilosa, including its aerial components and/or roots, are widely used [11]. It is traditionally used as a diuretic, anti-rheumatic and antidiabetic agent. However, its anti-inflammatory properties raised interest to treat inflammatory gastrointestinal diseases [10], [12], [14], [15].
Gastric antisecretory/antiulcer [16], [17], hepatoprotective [18], [19], antihyperglycemic [20], immunomodulatory, anti-inflammatory [21], [22] and antitumor [23], [24] activities of B. pilosa have been scientifically demonstrated. In addition, antibacterial [25], antihypertensive [26], chemopreventive [27], antimalarial [28], [29] and antioxidant [10], [24], [30] properties have also been reported.
Phytochemistry analysis of this plant has demonstrated a variety of compounds, which justifies its wide pharmacological properties and uses [23]. Aliphatic hydrocarbon derivatives, simple aromatic hydrocarbons, phenylpropanoid groups, sesquiterpenes, phytosterols, chalcones and terpenes have been isolated, but polyacetylene and flavonoids are considered the main metabolites in B. pilosa [11], [16], [19], [22], [26], [27], [28], [29], [30], [31].
There is no doubt about the need to develop therapeutic strategies in order to preventing and treating mucositis. In this context, the association of a new pharmacologically active material (B. pilosa extracts) with a mucoadhesive platform could further improve the management of chemotherapy-induced mucositis.
Mucoadhesion is defined as the attachment of a macromolecule to the mucus layer. The oral administration of mucoadhesive formulations may improve the efficacy of topical treatments and increase drug absorption, since mucoadhesion can prolong the residence time of the dosage form in the disease absorption site [32]. Mucoadhesive systems for oral administration have been proposed for the treatment of several disorders such as colitis and mucositis [33], [34], [35], [36]. There are few reports in the literature on the treatment of chemotherapy-induced intestinal mucositis with mucoadhesive dosage forms [33], [34]. Bioadhesion can be obtained through non-specific interactions between the mucoadhesive polymer and the mucus (mucoadhesion) or through the specific interaction of a ligant to a specific site at the cellular surface [37]. Polymers that non-specifically bind to the mucosa can provide simultaneous treatment of the mucositis wounds in the buccal, stomach and intestinal sites, which can all be affected by chemotherapy at the same time. Poloxamer 407 is a thermoreversible non-specific mucoadhesive copolymer consisting of ethylene oxide (EO) and propylene oxide (PO) blocks (EOx–POy–EOx). It also exhibits solubilization properties improving the apparent solubility of poorly water-soluble drugs, and is used in various pharmaceutical applications [38].
In this study, B. pilosa glycolic extract (BPE) was incorporated in a liquid formulation (BPF) based on poloxamer and used to prevent the toxic effects induced by chemotherapy using an in vivo model of 5-FU-induced mucositis.
2. Materials and methods
2.1. Chemicals
Ecobidens® (B. pilosa glycolic extract, BPE) and butylated hydroxytoluene were obtained from Chemyunion (Sorocaba, SP, Brazil) and Mapric (São Paulo, SP, Brazil), respectively. Polyethylene glycol l400, propylene glycol and sodium azide were acquired from Labsynth (Diadema, SP, Brazil). 5-Fluorouracil, hexadecyltrimethylammonium bromide, poloxamer 407, bovine serum albumin (BSA), n-butanol and ortho-dianisidine were purchased from Sigma–Aldrich (St. Louis, MO, USA). ImmunoCruz™ mouse ABC staining systems (sc-2017 and 2018), monoclonal mouse anti-mouse p53 (clone 3H2820) and polyclonal rabbit anti-mouse Bax (clone P-19) antibodies were acquired from Santa Cruz Biotechnology (CA, USA), whereas 3,3 diaminobenzidine (DAB), Duet strept ABC complex/HRP kit 0492 and anti-human Bcl-2 (clone 124) antibodies were obtained from Dako (Carpinteria, CA, USA). Anti-human ki-67 (clone MM1) antibodies were acquired from Novocastra (Newcastle, UK). Trichloroacetic acid, hydrogen peroxide (H2O2), tris, hydrochloricacid (HCl), methanol and xylene were obtained from Vetec (Rio de Janeiro, RJ, Brazil). Thiobarbituric acid, hematoxylin and eosin staining were acquired from Merck (Darmstadt, HE, Germany). Xylazine and ketamine were purchased from Syntec (Cotia, SP, Brazil) and König (Santana de Parnaíba, SP, Brazil), respectively.
2.2. Mucoadhesive formulation based on polaxamer containing B. pilosa (BPF)
According to the manufacturer's descriptions, B. pilosa glycolic extract (BPE) (Ecobidens®, Chemyunion, Brazil) is comprised of water (54–78%, w/w), glycerol (5–25%, w/w), B. pilosa extract (10–25%, w/w), benzyl alcohol (0.5–1%, w/w) and potassium sorbate (0.3–0.8%, w/w). BPE, which does not have mucoadhesive properties, was used as the active pharmaceutical ingredient for the preparation of the BPF, as described below.
BPF was prepared by mixing polyethylene glycol 400 and propilenglycol (1:1, v/v) in a heated reactor (65–70 °C). Then 15% (w/v) poloxamer 407 was added to the mixture under mechanical stirring until completely dispersed. After that, BPE was added to the organic phase and the pH of the final preparation was adjusted with 0.1 M citric acid (pH 6.5). BPF was then placed in an amber flask and stored under refrigeration (4° C) until use. A blank mucoadhesive formulation (control) was prepared as described above, but the BPE was replaced by a mixture of purified water and glycerin (75:25, v/v).
The total polyphenol content of the BPE was evaluated by the Folin Ciocalteau method, as described by Swain and Hillis [39] and modified by Roesler et al. [40]. Total polyphenols were 88.2 ppm, which was within the range (20–200 ppm) of the analysis certificate issued by the Ecobidens® manufacturers.
2.3. Animals
The experiments were carried out on male Swiss mice (age: 7–8 weeks; weight: 35–40 g) obtained from the Bioterium at the Federal University of Goiás. All efforts were realized to ensure the welfare of mice. In this sense, loss of body weight, food/water consumption and changes in activity and behavior of the animals were monitored daily as a clinical indication of animal suffering to determine when the animals should be humanely sacrificed in accordance with Hubrecht and Kirkwood [41]. The animals were acclimatized for a week prior to beginning the experiments and kept under constant environmental conditions with light–dark cycles and controlled temperature, while water and food were provided ad libitum. The Research Ethics Committee of the University approved the experimental protocol (UFG 036/2012). At the end of each experiment, the mice were anesthetized with xylazine (10 mg/kg) and ketamine hydrochloride (100 mg/kg) administered intraperitoneally and euthanized by cervical dislocation [41].
2.4. Experimental design
Intestinal mucositis was induced by the intraperitoneal administration of 200 mg/kg 5-FU (0.5 mL/animal) diluted in sterile water, as described by Wu et al. [42]. Mice were treated orally (gavage) with doses of BPF or mucoadhesive formulation without BPE (blank formulation, BF) (0.5 mL/animal) for 6 days, while doses of 5-FU or its vehicle were administered on the 4th to 6th days. On the 7th day, the animals were euthanized. For body weight and histomorphometry analysis, animals (5 mice per group) were distributed as follows: Group I – Control (blank formulation, BF); Group II – 75 mg/kg BPF; Group III – 100 mg/kg BPF; Group IV – 125 mg/kg BPF; Group V – BF + 5-FU; Group VI – 75 mg/kg BPF + 5-FU; Group VII – 100 mg/kg BPF + 5-FU; Group VIII – 125 mg/kg BPF + 5-FU. For the other assays, groups I, V and VII were chosen.
2.5. Mice body weight analysis
The animals were weighed daily during the induction process and treatment of intestinal mucositis. The values were expressed as the variation in body weight (%) in relation to the initial weight at the beginning of experiment [43].
2.6. Intestinal morphometry and histopathological analysis
The small intestine of each animal was dissected, washed with a phosphate buffered solution (PBS) and kept in 10% phosphate buffered formalin (pH 7.4) for histomorphometry evaluation. Intestinal macroscopic cross-sections were obtained, dehydrated in graded ethanol (70–100%), cleared in xylene and paraffin-embedded. Paraffin-embedded tissue sections of 5 μm thickness were obtained using a microtome (Leica RM 2155, Heidelberg, BW, Germany). Following, the slides were dewaxed in xylene, hydrated using graded ethanol and stained with hematoxylin and eosin (H&E). Subsequently, ten random fields of each slide were photographed using a light microscope (Axio Scope A1 Carl Zeiss, Jena, TH, Germany) at 20× objective.
During the intestinal morphometry examination, the intestinal villi, crypts and muscle layer were analyzed using AxioVision 40 software (Carl Zeiss, Jena, TH, Germany). The semi-quantitative histopathological examination was adapted from Wright et al. [44]. The severity of the damage caused by the intestinal mucositis was assessed by ten different parameters: reduction of the intestinal crypts and villi; disruptions and abscess formation in the crypts; thickening of the outer muscle layer; integrity of the epithelium and muscular layer; inflammatory cell infiltration; vacuolization and edema. Scores for each parameter ranging from 0 to 3 (0 = normal; 1 = mild; 2 = moderate; 3 = severe) were totaled to reach a histological damage index (HDI) maximum of 30.
2.7. Immunohistochemistry for detection of Bcl2, Bax, p53 and Ki-67
Paraffin-embedded intestinal sections of 3 μm thickness were obtained and mounted on silane-coated microscope slides. Each slide was deparaffinized in xylene, rehydrated in graded ethanol and washed with tris-buffered saline (TBS) (pH 7.2–7.4). Antigen retrieval followed in a citrate buffer (pH 6.0), at 95 °C for 20 min. After that, the slides were cooled to 25 °C for 10 min, washed with TBS and submitted to endogenous peroxidase blocking in H2O2 (3%) for 30 min. Subsequently, a nonspecific protein blocking was done using bovine serum albumin (BSA) (1%) at room temperature for 20 min. The slides were afterwards incubated at 4 °C overnight with one of the following primary monoclonal antibodies: anti-human Ki-67 (at 1:100), anti-human Bcl-2 (at 1:50), anti-mouse p53 (at 1:50), and anti-mouse Bax (at 1:50). After incubation, the materials were washed with TBS. Incubation with secondary antibodies followed, in accordance with the manufacturer's instructions of ImmunoCruz™ mouse ABC staining systems sc-2017 and 2018 (Santa Cruz, CA, USA) or Duet strept ABC complex/HRP kit 0492 (Dako, Carpinteria, CA, USA). Antibody binding was visualized through the incubation with 3.3 diaminobenzidine (DAB) for 7 min, followed by counterstaining with Mayer's hematoxylin. The assays were carried out in triplicate. Negative controls were obtained by omitting the primary antibody, which was replaced by 1% PBS–BSA.
The slides were analyzed and photographed using a light microscope at 20× objective. About 100 epithelial cells of the small intestine were counted in ten consecutive fields to determine the percentage of cells expressing Bcl2, Bax and p53, all antingens involved in the apoptotic process, and Ki-67, an indicator of cell proliferation.
2.8. Preparation of intestinal tissue homogenates
A small intestinal segment of each animal was removed and stored at −20 °C for subsequent analysis. After thawing the samples, 250 mg of each intestine were washed in phosphate buffer (pH 6.0) and transferred to tubes after which 5 mL of hexadecyltrimethylammonium bromide buffer (0.5%, w/v) (pH 6.0) were added to each intestinal fragment. Subsequently, the tissues were homogenized using Ultra-turrax® T 25 digital (IKA, BW, Germany) at 10,000 rpm for 5 min. Posteriorly, the samples were centrifuged at 2000 rpm, 4 °C for 15 min. The supernatants obtained were used for assays. The experiments were carried out in triplicate.
2.9. Myeloperoxidase (MPO) activity
A 10 μL volume of supernatant from each tissue was mixed with 200 μL of phosphate buffer containing H2O2 (0.1%, v/v) and ortho-dianisidine (1.3%, w/v). After 10 min incubation at room temperature (25 °C), the reaction was halted by adding 50 μL sodium azide (1.3%, w/v). The optical density reading was performed using a spectrophotometer (Thermo Scientific Multiskan® Spectrum, Boston, MA, USA) at 450 nm.
2.10. Malondialdehyde (MDA) activity
About 250 μL of the supernatant were homogenized with 25 μL of butylated hydroxytoluene (4%, v/v) in methanol. Subsequently, 1 mL of trichloroacetic acid (12%, w/v), 1 mL of thiobarbituric acid (0.73%, w/v, in 0.05 M NaOH) and 750 μL of tris/HCl buffer (pH 7.2) were added to the initial mixture and this was followed by incubation in a water bath (100 °C) for 60 min. After that, the samples were placed in a vessel containing ice to block the reaction. A 1.5 mL volume of n-butanol was added to each tube followed by homogenization and centrifugation at 5000 rpm, 25 °C for 10 min. Then optical density reading of the supernatants was performed using a spectrophotometer at 532 and 453 nm. MDA concentrations were obtained by subtracting 20% of the absorbance at 453 nm from the absorbance at 532 nm, using a molar extinction coefficient of 1.56 × 105 M−1 cm−1.
2.11. Statistical analysis
The data were expressed as mean ± standard deviation. The inter group variation was measured by the one- or two-way Analysis of Variance (ANOVA) followed by the Bonferroni test using a GraphPad Prism version 5.01 software for Windows (San Diego, CA, USA). A p < 0.05 was considered to indicate significant differences.
3. Results
3.1. Effect of BPF on body weight using 5-FU-induced intestinal mucositis in mice
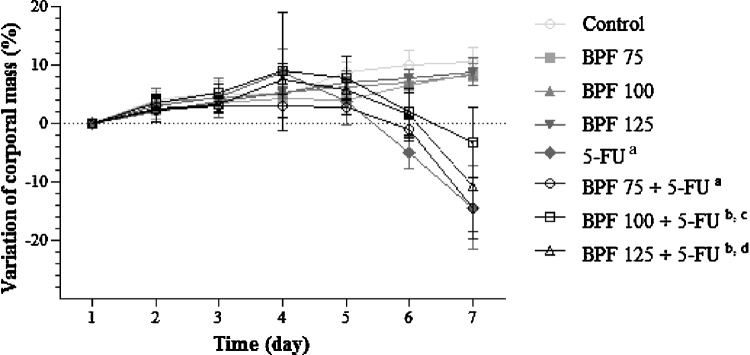
As shown in Fig. 1, control groups with no 5-FU, gained body weight throughout the experimental period, reaching peak weight gains of 10.5 and 8.5%, respectively, on the 7th day. In contrast, animals only treated with 5-FU presented a significant weight loss of 5% on the 6th day and 14.5% on the 7th day of evaluation (p < 0.0001), when compared to controls. When these animals were treated with BPF, the lowest dose (75 mg/kg) did not show any satisfactory effect on mouse body weight. On the other hand, treatment with BPF doses of 100 and 125 mg/kg resulted in a significant gain in body weight of 2 and 4% (p < 0.001), respectively, on the 6th day, as compared to the control group. On the 7th day, these groups showed weight losses of 3.2 and 5.4%, which was significantly lower (p < 0.05) than in the 5-FU group, especially in mice treated with the BPF dose of 100 mg/kg (p < 0.0001).
Fig. 1.
Mice body weight analysis in 5-FU-induced intestinal mucositis and its treatment with BPF. The BPF doses (75, 100 or 125 mg/kg) or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) or sterile water were administered intraperitoneally from 4th to 6th day. The mice were weighed throughout the 7 days of the experiment to obtain the variation of corporal mass (%). Each point presents mean ± SD of five animals (ap < 0.0001 vs. control and BPF groups (6th and 7th days); bp < 0.001 (6th day) and p < 0.0001 (7th day) vs. control and BPF groups; cp < 0.05 (6th day) and p < 0.0001 (7th day) vs. 5-FU; dp < 0.05 (6th day) vs. 5-FU. Two-way ANOVA and Bonferroni test, p < 0.05).
3.2. Effect of BPF on the small intestinal morphometry in 5-FU-induced intestinal mucositis in mice
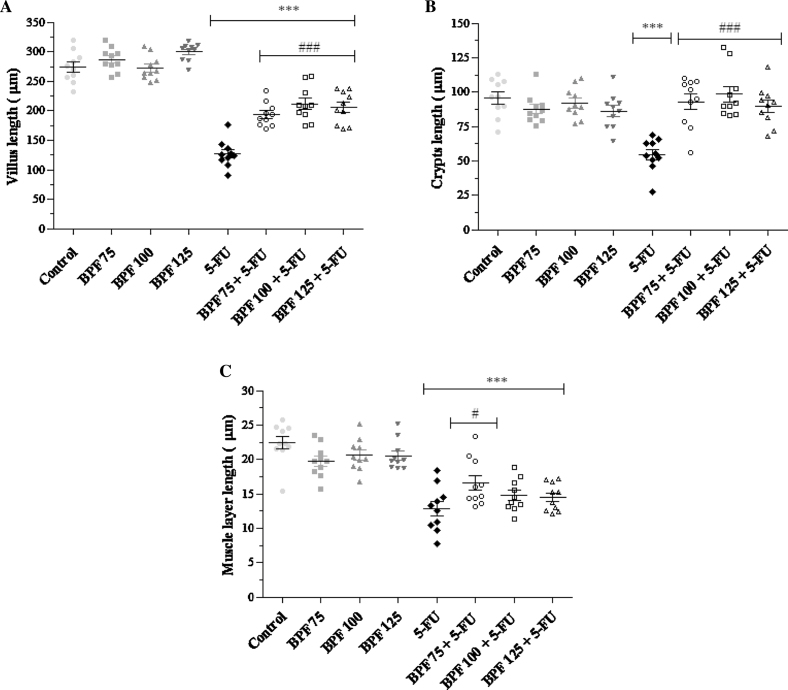
Morphometric evaluation showed that mice without mucositis and treated with BPF doses (75, 100 and 125 mg/kg) presented no changes in the length of their villi, crypts or muscle layers as compared to the control group (Figs. 2A–C). On the other hand, intestinal villi length in animals only exposed to 5-FU showed a marked reduction (54%), when compared with the control groups (p < 0.0001). Moreover, this reduction in intestinal villi length was lower in the animals treated with BPF (p < 0.0001) (30% for 75 mg/kg, 23% for 100 mg/kg and 25% for 125 mg/kg BPF), when compared to 5-FU group (Fig. 2A). In relation to the crypts, the 5-FU group also showed a significant decrease (p < 0.0001) of approximately 43.2%, in relation to the control group (Fig. 2B). Animals with mucositis also showed a significant reduction (p < 0.0001) in the muscular layer. The treatment of mice with BPF protected against the decrease in muscle layer triggered by the 5-FU, increasing the muscle layer in 17% for 75 mg/kg, 8.6% for 100 mg/kg and 7.6% for 125 mg/kg (Fig. 2C).
Fig. 2.
Effect of BPF on the villus (A), crypt (B) and muscle layer (C) morphometry of the small intestine in 5-FU-induced intestinal mucositis in mice. The BPF doses (75, 100 or 125 mg/kg) or blank formulation were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) or sterile water were administered intraperitoneally from 4th to 6th day. On day 7, the animals (5 mice per group) were euthanized and the small intestine of each animal was collected for morphometry analysis (***p < 0.0001 vs. control and BPF groups; #p < 0.05 and ###p < 0.0001 vs. 5-FU group. One-way ANOVA and Bonferroni test, p < 0.05).
Morphometric evaluation was also performed in the small intestine tissue of the mucositis-bearing mice treated with BPE, in comparison with BPF. Although the treatment with BPE showed protective effects against the damage produced by 5-FU, the plant extract alone did not manage to protect against changes in the length of the villi, crypts or muscle layer as found in the group treated with the mucoadhesive formulation (data not shown).
3.3. Effect of BPF on the small intestinal histology in 5-FU-induced intestinal mucositis in mice
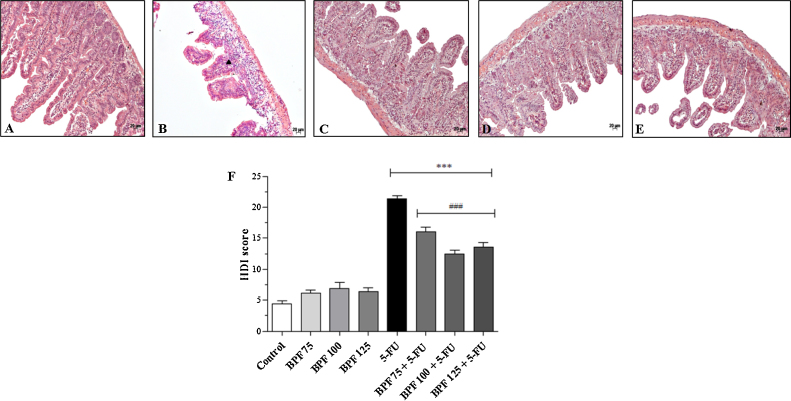
Mice without mucositis treated with different doses of BPF showed no significant histopathological changes (data not shown) in the HDI values similar to those found in the control group (Fig. 3F). However, animals only exposed to 5-FU showed severe disruption of crypts, edema, inflammatory infiltrate, thinning of the muscular layer and vacuolization in the intestinal tissue (Fig. 3B), when compared to animals control (Fig. 3A), with an HDI average of 21.4 (p < 0.0001) (Fig. 3F). This value was approximately 386.4% greater than the control group. In contrast, the BPF treatment protected against these histopathological changes (Fig. 3C–E). Moreover, the groups treated with 75, 100 or 125 mg/kg BPF presented an HDI average of 16.1, 12.5 and 13.6, respectively (Fig. 3F), representing an increase of 266% for 75 mg/kg, 184.1% for 100 mg/kg and 209.1% for 125 mg/kg BPF. In view of these results, associated with body weight and morphometric data, the treatment of mice with the BPF dose of 100 mg/mL was chosen for the subsequent assays.
Fig. 3.
Effect of BPF on small intestinal histological damage in 5-FU-induced intestinal mucositis in mice. The BPF doses (75, 100 or 125 mg/kg, representative figures (C), (D), and (E), respectively) or blank formulation (representative (A)) were administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg, representative (B)) or sterile water were administered intraperitoneally from 4th to 6th day. On day 7, the animals (5 mice per group) were euthanized and the small intestine of each animal was collected for analysis and determination of histological damage index (HDI) (***p < 0.0001 vs. control and BPF groups; ###p < 0.0001 vs. 5-FU group. One-way ANOVA and Bonferroni test, p < 0.05). Symbol represent: ▴ – inflammatory infiltrate.
3.4. Effect of BPF on the expression of Ki-67 in 5-FU-induced intestinal mucositis in mice
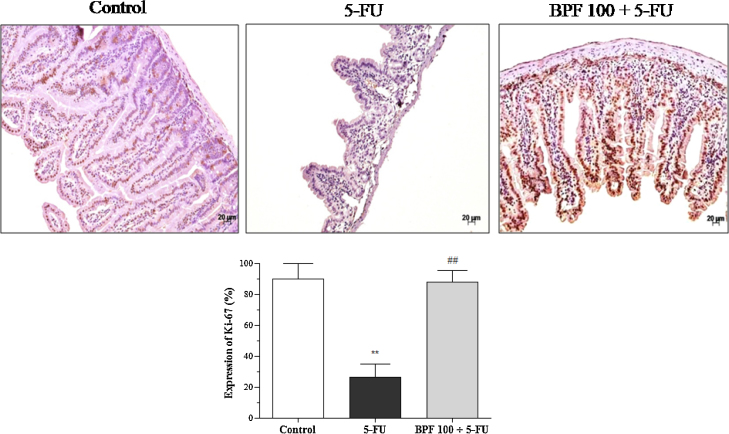
The proliferative activity of BPF in 5-FU-induced intestinal mucositis in mice was evaluated through the expression of Ki-67 by immunohistochemistry. As expected, the control group showed high cytoplasmic and nuclear expression of Ki-67 in the villi and crypts, while the mice treated with only 5-FU demonstrated a reduction (p < 0.001) of 70.8%. On the other hand, the BPF led to the restoration of intestinal proliferative activity, since Ki-67 levels were similar to those found in the control group (Fig. 4).
Fig. 4.
Effect of BPF on the expression of Ki-67 cell proliferation indicator in 5-FU-induced intestinal mucositis. The dose of 100 mg/kg BPF or blank formulation was administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) or sterile water were administered intraperitoneally from 4th to 6th day. On day 7, the animals (5 mice per group) were euthanized and the small intestine of each animal was collected for detection of Ki-67 by immunohistochemistry. The images are a representation of the expression of Ki-67 (**p < 0.001 vs. control; ##p < 0.001 vs. 5-FU group. One-way ANOVA and Bonferroni test, p < 0.05).
3.5. Effect of BPF on the expression of Bcl2, Bax and p53 in 5-FU-induced intestinal mucositis in mice
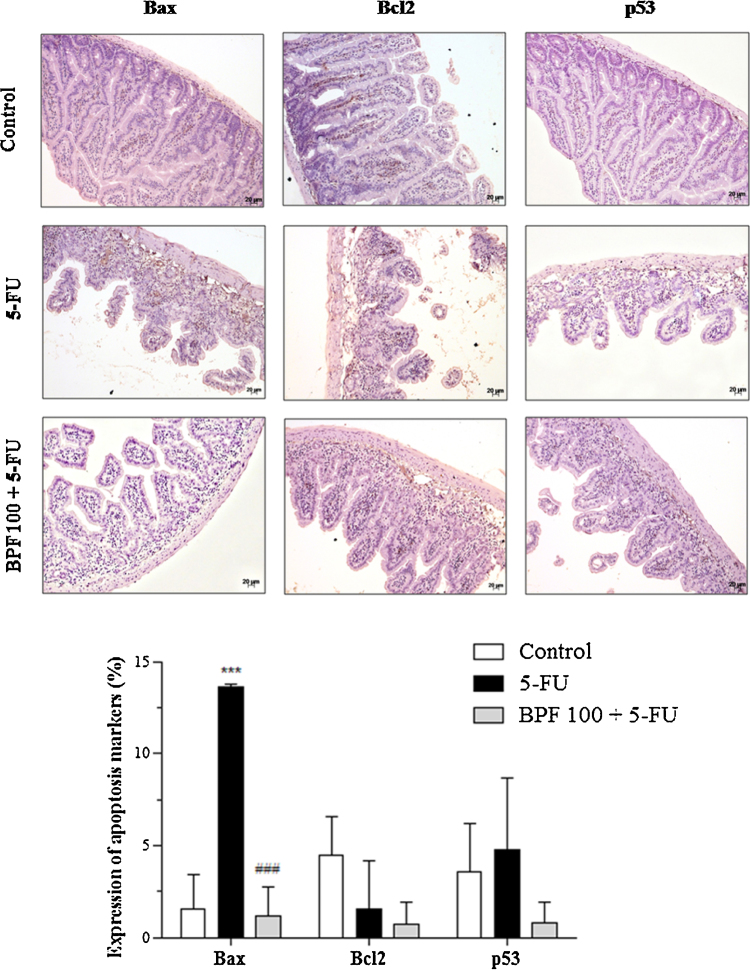
The results of the expressions of Bcl2, Bax and p53 apoptosis markers in 5-FU-induced intestinal mucositis in mice, as well as their treatments with BPF, evaluated by immunohistochemistry, are shown in Fig. 5. These proteins were predominatly expressed in the inflamatory infiltrate of the villi and crypts, with a predominance of nuclear staining for p53 and cytoplasmic staining for the other two markers. The 5-FU led to a significant increase in Bax, as compared to the control group (p < 0.0001). The Bcl2 expression was reduced along with increased expression of p53, but with no statistic significance. The increase of Bax and p53 levels was 777.4 and 35% associated with the decrease of Bcl2 levels of 66%, in comparison to the control group. In contrast, the expression of Bax in mice treated with BPF was similar to that of the control group and statistically significant (p < 0.0001) when compared to 5-FU group and. In addition, Bcl2 and p53 levels were also reduced, although not by statistically significant levels.
Fig. 5.
Effect of BPF on the expression of Bcl2, Bax and p53 apoptosis markers in 5-FU-induced intestinal mucositis. The dose of 100 mg/kg BPF or blank formulation (BF) was administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) or sterile water was administered intraperitoneally from 4th to 6th day. On day 7, the animals (5 mice per group) were euthanized and the small intestine of each animal was collected for detection of Bcl2, Bax, p53 by immunohistochemistry. The images are a representation of the expression of these apoptotic markers (***p < 0.0001 vs. control; ###p < 0.0001 vs. 5-FU group. Two-way ANOVA and Bonferroni test, p < 0.05).
3.6. Effect of BPF on intestinal MPO and MDA activities in 5-FU-induced intestinal mucositis in mice
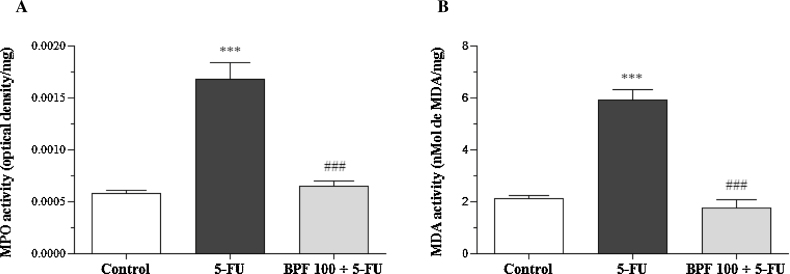
MPO and MDA activities in the 5-FU group showed statistically significant differences (p < 0.0001), as compared to the control group, and reached an increase of 188 and 179.2%, respectively. However, the BPF100 + 5-FU group demonstrated similar MPO and MDA activities in relation to the control group and was statistically significant (p < 0.0001) in relation to 5-FU group, which obtained a decrease of 61.7 and 70.6% for MPO and MDA activities, respectively (Fig. 6).
Fig. 6.
Effect of BPF on intestinal MPO (A) and MDA (B) activity in 5-FU-induced intestinal mucositis in mice. The dose of 100 mg/kg BPF or blank formulation was administered orally (gavage) to mice over 6 days, while doses of 5-FU (200 mg/kg) or sterile water were administered intraperitoneally from 4th to 6th day. On day 7, the animals (5 mice per group) were euthanized and the small intestine of each animal was collected for MPO and MDA analysis. (***p < 0.0001 vs. control; ###p < 0.0001 vs. 5-FU group. One-way ANOVA and Bonferroni test, p < 0.05).
4. Discussion
The pathogenesis of intestinal mucositis due to chemo/radiotherapy is not yet fully understood. Studies have reported a reduction in intestinal cell proliferation associated with an increasing rate of apoptosis, which in turn led to a loss of intestinal arrangement and function, mainly through villus shortening and crypt destruction. Similar findings were reported in this study and by other authors [45], [46]. Additionally, a focus of edema, inflammatory infiltrate and vacuolization caused by 5-FU which reached a high histological damage index were demonstrated here. Simultaneously, a marked body weight loss was observed, since chemotherapy decreases food intake and the inflammatory response causes the loss of the nutrient absorption capacity in the small intestine, thereby resulting in diarrhea, an important dose limiting toxicity in cancer treatment [47], [48].
By contrast, the BPF formulation prevented intestinal tissue injury and loss of body weight, mainly with the dose of 100 mg/kg in mice with 5-FU-induced intestinal mucositis. Similar results were obtained in the treatment of mice with the interleukin 1 receptor antagonist (IL-1Ra) [42]. In addition, interesting results for the treatment of mucositis have been reported using natural products, such as glycoglycerolipids extracted from spinach [49] and Iberogast® [44]. Iberogast® is a herbal formula comprising nine plant extracts used in the treatment of gastrointestinal disorders, and although it protects against mucositis, this phytomedicine does not manage to prevent weight loss [44].
Our preliminary findings showed the beneficial effect of B. pilosa glycolic extract (BPE) on intestinal tissue structure in 5-FU treated mice (data not shown). The incorporation of this plant extract in a mucoadhesive formulation (BPF) has significantly improved the effects of BPE. This could be explained by the more intimate contact of the plant active principles with the damaged mucosa over an extended period. Moreover, poloxamer 407 does not only have mucoadhesive properties [38]. Dumortier et al. [38] reviewed its pharmaceutical applications and reported several studies, in which poloxamer has improved both the cellular and humoral immune response. Thus, the beneficial effects of BPF could result from the anti-inflammatory effects of the plant active extracts associated with the mucoadhesive properties of the poloxamer 407.
Regarding to the histomorphometric evaluation, animals with mucositis and treated with BPF showed a reorganization of their intestinal structure framework. Similar results were obtained in other studies which used an E COG 133 apolipoprotein, a mimetic peptide [5]; minocycline, a semi-synthetic derivative of tetracycline [50]; and chemokine (C-X-C motif) ligand 9 (CXCL9), a molecule produced by interferon-γ stimulated mononuclear cells [2]. However, the magnitude of the in vivo results presented here, and the simplicity and low cost of the BPF make this formulation more attractive than synthetic or biological products. It is also worth mentioning that in vivo toxicological studies reported that B. pilosa extracts showed no sign of toxicity, corroborating with our findings [51], [52], [53]. The data obtained in our study regarding the effects of BPF on normal mice showed that BPF doses did not lead to body mass loss or changes in the intestinal histology of healthy mice.
In order to clarify the protective mechanisms of BPF in the intestinal injury triggered by 5-FU, an evaluation was made of the expression of apoptotic markers of the Bcl2 family, which consists of pro-apoptotic and anti-apoptotic members such as Bax and Bcl2, respectively [54], [55]. Thus, it was observed that the treatment with the dose of 100 mg/kg BPF led to a reduction in the imbalance between the rates of expression of Bax and Bcl2. So the BPF would appear to modulate the expression of anti-apoptotic members at the expense of apoptotic members, and thereby prevent intestinal cell apoptosis. An overexpression of Bax in association with an underexpression of Bcl2 leads to apoptosis in the affected cells [56], [57]. This event is a protective mechanism used by cells to regulate proliferation, which occurs at a reduced rate in a healthy small intestine [1]. In pathological conditions, such as intestinal mucositis, this process is induced mainly by IL-1β, which changes the expression of genes of the Bcl2 family [58], [59]. Additionally, it has been suggested that DNA damage-induced IL-1β secretion may promote an inflammatory cascade via NF-κB-independent mechanism in the intestinal mucositis [60].
The treatment with the BPF also reduced the expression of p53. This stress-induced pro-apoptotic protein is a major determinant of the side effects of chemotherapy, since it is a transcriptional activator of certain pro-apoptotic Bcl2 family proteins, including Bax. It increases transcription of the Fas death receptor gene and inhibits the secretion of growth factors [1], [59], [61]. Thus, as observed in this study, it seems likely that inhibition of apoptotic response triggered by p53 can effectively reduce intestinal damage.
Concurrently, it was seen that the intestinal tissue of animals which only received doses of 5-FU showed a marked reduction in the Ki-67 protein nuclear expression. Contrary data were noted when BPF was administrated, as the nuclear expression of Ki-67 was increased, a fact indicative of proliferative activity [62]. Therefore, these data once again support the protective role of BPF against the intestinal mucositis induced by chemotherapy.
In view of the known anti-inflammatory and antioxidant role of the BPF, and its effect on the regulation of lipid peroxidation and inflammatory infiltration in the intestinal mucositis, MDA and MPO levels were evaluated. MPO is an enzyme involved in inflammatory processes and is mainly present in neutrophils. When released in the injured tissue, it leads to the overproduction of reactive oxygen species (ROS), which results in the peroxidation of cell membranes and causes the release of MDA as a product of this oxidative process [63], [64]. Some studies have reported the involvement of these stress markers in chemotherapy-induced intestinal mucositis [5], [43], [44], [65], [66], [67], corroborating the data obtained here since the BPF treatment decreased inflammatory infiltrate and, consequently, prevented lipid peroxidation. Similar results have been demonstrated using grape seed extract [65], apolipoprotein E COG 133 [5], melatonin [67] and proanthocyanidin [66], a natural antioxidant extracted from Vitis venifera.
The protective activity of B. pilosa has been investigated and correlated with its antioxidant potential. Yang et al. [68] demonstrated that this plant protects human erythrocytes against peroxyl radical-induced oxidative damage in vitro through the reduction of lipid peroxidation and the increase of cellular antioxidants and ATP levels. Additionally, Yuan et al. [19] showed that doses of 50 and 100 mg/kg of B. pilosa extract effectively reduced the carbon tetrachloride-induced liver damage. This effect was associated with decreased hepatic transaminases and MDA content, the restoration of superoxide dismutase and glutathione peroxidase levels and the significant inhibition of NF-κB activation. Another study has shown, in vitro or in mouse skin, that ethyl caffeate, a phenolic compound isolated from B. pilosa, also suppressed the activation of NF-κB and its downstream inflammatory mediators, such as iNOS, COX-2 and PGE2 [39], which supports the use of B. pilosa in inflammatory disorders such as intestinal mucositis.
Given the above, this study demonstrated that the BPF was shown to be safe and efficient against 5-FU-induced intestinal mucositis in mice, corroborating with the popular use of B. pilosa as an anti-inflammatory agent for gastrointestinal disorders. These results showed the involvement of Ki-67, MPO, and MDA in the anti-inflammatory response modulated by the BPF is associated with its ability to regulate Bax, Bcl2 and p53 apoptotic markers. More studies are underway to further characterize the mechanisms involved in the protective effects of BPF in order to make it a clinical tool for the prevention and treatment of intestinal injury in patients undergoing chemotherapy.
Conflict of interest
The authors declare that there are no conflicts of interest in this study.
Transparency document
Acknowledgements
The authors are grateful to Fundação de Apoio à Pesquisa (FUNAPE), Fundação de Apoio à Pesquisa do Estado de Goiás (FAPEG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Financiadora de Estudos e Projetos (FINEP) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for financial support.
References
- 1.Yeoh A.S.J., Gibson R.J., Yeoh E.E.K., Bowen J.M., Stringer A.M., Giam K.A., Keefe D.M.K. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-κB, COX-1, and COX-2. Mol. Cancer Ther. 2007;6:2319–2327. doi: 10.1158/1535-7163.MCT-07-0113. [DOI] [PubMed] [Google Scholar]
- 2.Han X., Wu Z., Di J., Pan Y., Zhang H., Du Y., Cheng Z., Jin Z., Wang Z., Zheng Q., Zhang P., Wang Y. CXCL9 attenuated chemotherapy-induced intestinal mucositis by inhibiting proliferation and reducing apoptosis. Biomed. Pharmacother. 2011;65:547–554. doi: 10.1016/j.biopha.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 3.Touchefeu Y., Montassier E., Nieman K., Gastinne T., Potel G., Bruley des Varannes S., Le Vacon F., de La Cochetière M.F. Systematic review: the role of the gut microbiota in chemotherapy-or radiation-induced gastrointestinal mucositis – current evidence and potential clinical applications. Aliment. Pharmacol. Ther. 2014;40:409–421. doi: 10.1111/apt.12878. [DOI] [PubMed] [Google Scholar]
- 4.Tooley K.L., Saxon B.R., Webster J., Zacharakis B., McNeil Y., Davidson G.P., Butler R.N. A novel non-invasive biomarker for assessment of small intestinal mucositis in children with cancer undergoing chemotherapy. Cancer Biol. Ther. 2006;5:1275–1281. doi: 10.4161/cbt.5.10.3303. [DOI] [PubMed] [Google Scholar]
- 5.Azevedo O.G., Oliveira R.A., Oliveira B., Zaja-Milatovic S., Araujo C.V., Wong D.V., Costa T.B., Lucena H.B., Lima-Junior R.C., Ribeiro R.A., Warren C.A., Lima A.A., Vitek M.P., Guerrant R.L., Oriá R.B. Apolipoprotein E COG 133 mimetic peptide improves 5-fluorouracil-induced intestinal mucositis. BMC Gastroenterol. 2012;12:35. doi: 10.1186/1471-230X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carneiro-Filho B.A., Lima I.P., Araujo D.H., Cavalcante M.C., Carvalho G.H., Brito G.A., Lima V., Monteiro S.M., Santos F.N., Ribeiro R.A., Lima A.A. Intestinal barrier function and secretion in methotrexate-induced rat intestinal mucositis. Dig. Dis. Sci. 2004;49:65–72. doi: 10.1023/b:ddas.0000011604.45531.2c. [DOI] [PubMed] [Google Scholar]
- 7.Keefe D.M. Gastrointestinal mucositis: a new biological model. Support. Care Cancer. 2004;12:6–9. doi: 10.1007/s00520-003-0550-9. [DOI] [PubMed] [Google Scholar]
- 8.Lalla R.V., Schubert M.M., Bensadoun R.J., Keefe D. Anti-inflammatory agents in the management of alimentary mucositis. Support. Care Cancer. 2006;14:558–565. doi: 10.1007/s00520-006-0050-9. [DOI] [PubMed] [Google Scholar]
- 9.Yang W.-C. Botanical, pharmacological, phytochemical, and toxicological aspects of the antidiabetic plant Bidens pilosa L. Evid. Based Complement. Alternat. Med. 2014;2014:1–14. doi: 10.1155/2014/698617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abajo C., Boffill M.A., del Campo J., Méndez M.A., González Y., Mitjans M., Vinardell M.P. In vitro study of the antioxidant and immunomodulatory activity of aqueous infusion of Bidens pilosa. J. Ethnopharmacol. 2004;93:319–323. doi: 10.1016/j.jep.2004.03.050. [DOI] [PubMed] [Google Scholar]
- 11.Bartolome A.P., Villaseñor I.M., Yang W.C. Bidens pilosa L. (Asteraceae): botanical properties, traditional uses, phytochemistry, and pharmacology. Evid. Based Complement. Alternat. Med. 2013;2013:1–51. doi: 10.1155/2013/340215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borges C.C., Matos T.F., Moreira J., Rossato A.E., Zanette V.C., Amaral P.A. Bidens pilosa L. (Asteraceae): traditional use in a community of southern Brazil. Rev. Bras. Plantas Med. 2013;15:34–40. [Google Scholar]
- 13.Costa R.J., Diniz A., Mantovani M.S., Jordão B.Q. In vitro study of mutagenic potential of Bidens pilosa Linné and Mikania glomerata Sprengel using the comet and micronucleus assays. J. Ethnopharmacol. 2008;118:86–93. doi: 10.1016/j.jep.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 14.Brandão M.G.L., Nery C.G.C., Mamão M.A.S., Krettli A.U. Two methoxylated flavone glycosides from Bidens pilosa. Phytochemistry. 1998;48:397–399. [Google Scholar]
- 15.Valdés H.A.L., Rego H.P.L. Bidens pilosa Linné. Ver. Cubana Plant. Med. 2001;6:28–33. [Google Scholar]
- 16.Alvarez A., Pomar F., Sevilla M.A., Montero M.J. Gastric antisecretory and antiulcer activities of an ethanolic extract of Bidens pilosa L. var. radiata Schult. Bip. J. Ethnopharmacol. 1999;67:333–340. doi: 10.1016/s0378-8741(99)00092-6. [DOI] [PubMed] [Google Scholar]
- 17.Tan P.V., Dimo T., Dongo E. Effects of methanol, cyclohexane and methylene chloride extracts of Bidens pilosa on various gastric ulcer models in rats. J. Ethnopharmacol. 2000;73:415–421. doi: 10.1016/s0378-8741(00)00290-7. [DOI] [PubMed] [Google Scholar]
- 18.Chih H.W., Lin C.C., Tanga K.S. The hepatoprotective effects of Taiwan Folk Medicine Ham-Hong-Chho in rats. Am. J. Chin. Med. 1996;24:231–240. doi: 10.1142/S0192415X96000293. [DOI] [PubMed] [Google Scholar]
- 19.Yuan L.P., Chen F.H., Ling L., Dou P.F., Bo H., Zhong M.M., Xia L.J. Protective effects of total flavonoids of Bidens pilosa L. (TFB) on animal liver injury and liver fibrosis. J. Ethnopharmacol. 2008;116:539–546. doi: 10.1016/j.jep.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 20.Ubillas R.P., Mendez C.D., Jolad S.D., Luo J., King S.R., Carlson T.J., Fort D.M. Antihyperglycemic acetylenic glucosides from Bidens pilosa. Planta Med. 2000;66:82–83. doi: 10.1055/s-0029-1243117. [DOI] [PubMed] [Google Scholar]
- 21.Jäger A.K., Hutchings A., van Staden J. Screening of Zulu medicinal plants for prostaglandin-synthesis inhibitors. J. Ethnopharmacol. 1996;52:95–100. doi: 10.1016/0378-8741(96)01395-5. [DOI] [PubMed] [Google Scholar]
- 22.Pereira R.L.C., Ibrahim T., Lucchetti L., Silva A.J.R., Moraes V.L.G. Immunosuppressive and anti-inflammatory effects of methanolic extract and the polyacetylene isolated from Bidens pilosa L. Immunopharmacology. 1999;43:31–37. doi: 10.1016/s0162-3109(99)00039-9. [DOI] [PubMed] [Google Scholar]
- 23.Kviecinski M.R., Felipe K.B., Schoenfelder T., Wiese L.P.L., Rossi M.H., Gonçalez E., Felicio J.D., Filho D.W., Pedrosa R.C. Study of the antitumor potential of Bidens pilosa (Asteraceae) used in Brazilian folk medicine. J. Ethnopharmacol. 2008;117:69–75. doi: 10.1016/j.jep.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Wan Z., Yi J., Wu Y., Peng W., Wu J. Investigation of the extracts from Bidens pilosa Linn. var. radiata Sch. Bip. for antioxidant activities and cytotoxicity against human tumor cells. J. Nat. Med. 2013;67:17–26. doi: 10.1007/s11418-012-0639-x. [DOI] [PubMed] [Google Scholar]
- 25.Rabe T., van Staden J. Antibacterial activity of South African plants used for medicinal purposes. J. Ethnopharmacol. 1997;56:81–87. doi: 10.1016/s0378-8741(96)01515-2. [DOI] [PubMed] [Google Scholar]
- 26.Dimo T., Rakotonirina S.V., Tan P.V., Azay J., Dongo E., Cros G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J. Ethnopharmacol. 2002;83:183–191. doi: 10.1016/s0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- 27.Chiang Y.M., Chuang D.Y., Wang S.Y., Kuo Y.H., Tsai P.W., Shyur L.F. Metabolite profiling and chemopreventive bioactivity of plant extracts from Bidens pilosa. J. Ethnopharmacol. 2004;95:409–419. doi: 10.1016/j.jep.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Brandão M.G.L., Krettli A.U., Soares L.S.R., Nery C.G.C., Marinuzzi H.C. Antimalarial activity of extracts and fractions from Bidens pilosa and other Bidens species (Asteraceae) correlated with the presence of acetylene and flavonoid compounds. J. Ethnopharmacol. 1997;57:131–138. doi: 10.1016/s0378-8741(97)00060-3. [DOI] [PubMed] [Google Scholar]
- 29.Oliveira F.Q., Andrade-Neto V., Krettli A.U., Brandão M.G.L. New evidences of antimalarial activity of Bidens pilosa roots extract correlated with polyacetylene and flavonoids. J. Ethnopharmacol. 2004;93:39–42. doi: 10.1016/j.jep.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Chiang Y.M., Chang C.L.T., Chang S.L., Yang W.C., Shyur L.F. Cytopiloyne, a novel polyacetylenic glucoside from Bidens pilosa, functions as a T helper cell modulator. J. Ethnopharmacol. 2007;110:532–538. doi: 10.1016/j.jep.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Silva F.L., Fischer D.C.H., Tavares J.F., Silva M.S., Athayde-Filho P.F., Barbosa-Filho J.M. Compilation of secondary metabolites from Bidens pilosa L. Molecules. 2011;16:1070–1102. doi: 10.3390/molecules16021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varum F.J.O., McConnell E.L., Sousa J.J.S., Veiga F., Basit A.W. Mucoadhesion and the gastrointestinal tract. Crit. Rev. Ther. Drug Carr. Syst. 2008;25:207–258. doi: 10.1615/critrevtherdrugcarriersyst.v25.i3.10. [DOI] [PubMed] [Google Scholar]
- 33.Rossi S., Marciello M., Bonferoni M.C., Ferrari F., Sandri G., Dacarro C., Grisoli P., Caramella C. Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. Eur. J. Pharm. Biopharm. 2010;74:248–254. doi: 10.1016/j.ejpb.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Desai K.G.H., Mallery S.R., Holpuch A.S., Schwenderman S.P. Development and in vitro–in vivo evaluation of fenretinide-loaded oral mucoadhesive patches for site-specific chemoprevention of oral cancer. Pharm. Res. 2011;28:2599–2609. doi: 10.1007/s11095-011-0489-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Del Fante C., Perotti C., Bonferoni M.C., Rossi S., Sandri G., Ferrari F., Scudeller L., Caramella C.M. Platelet lysate mucohadesive formulation to treat oral mucositis in graft versus host disease patients: a new therapeutic approach. AAPS PharmSciTech. 2011;12:893–899. doi: 10.1208/s12249-011-9649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bautzová T., Rabišková M., Béduneau A., Pellequer Y., Lamprecht A. Bioadhesive pellets increase local 5-aminosalicylic acid concentrationin experimental colitis. Eur. J. Pharm. Biopharm. 2012;81:379–385. doi: 10.1016/j.ejpb.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Ponchel G., Irache J. Specific and non-specific bioadhesive particulate systems for oral delivery to the gastrointestinal tract. Adv. Drug Deliv. Rev. 1998;34:191–219. doi: 10.1016/s0169-409x(98)00040-4. [DOI] [PubMed] [Google Scholar]
- 38.Dumortier G., Grossiord J.L., Agnely F., Chaumeil J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006;23:2709–2728. doi: 10.1007/s11095-006-9104-4. [DOI] [PubMed] [Google Scholar]
- 39.Swain T., Hillis W.E. The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1999;10:63–68. [Google Scholar]
- 40.Roesler R., Catharino R.R., Malta L.G., Eberlin M.N., Pastore G. Antioxidant activity of Caryocar brasiliense (pequi) and characterization of components by electrospray ionization mass spectrometry. Food Chem. 2008;110:711–717. [Google Scholar]
- 41.Hubrecht R., Kirkwood J. 8th ed. Wiley-Blackwell; United Kingdom: 2010. The UFAW Handbook on the Care and Management of Laboratory and Other Research Animals. [Google Scholar]
- 42.Wu Z., Han X., Qin S., Zheng Q., Wang Z., Xiang D., Zhang J., Lu H., Wu M., Zhu S., Yu Y., Han W. Interleukin 1 receptor antagonist reduces lethality and intestinal toxicity of 5-fluorouracil in a mouse mucositis model. Biomed. Pharmacother. 2010;64:589–593. doi: 10.1016/j.biopha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Lima V., Brito G.A.C., Cunha F.Q., Rebouças C.G., Falcão B.A.A., Augusto R.F., Souza M.L.P., Leitão B.T., Ribeiro R.A. Effects of the tumour necrosis factor-α inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur. J. Oral Sci. 2005;113:210–217. doi: 10.1111/j.1600-0722.2005.00216.x. [DOI] [PubMed] [Google Scholar]
- 44.Wright T.H., Yazbeck R., Lymn K.A., Whitford E.J., Cheah K.Y., Butler R.N., Feinle-Bisset C., Pilichiewicz A.N., Mashtoub S., Howarth G.S. The herbal extract Iberogast® improves jejunal integrity in rats with 5-Fluorouracil (5-FU)-induced mucositis. Cancer Biol. Ther. 2009;8:923–929. doi: 10.4161/cbt.8.10.8146. [DOI] [PubMed] [Google Scholar]
- 45.Keefe D.M.K., Brealey J., Goland J.G., Cummins A.G. Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut. 2000;47:632–637. doi: 10.1136/gut.47.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang C.T., Ho T.Y., Lin H., Liang J.A., Huang H.C., Li C.C., Lo H.Y., Wu S.L., Huang Y.F., Hsiang C.Y. 5-Fluorouracil induced intestinal mucositis via nuclear factor-κB activation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS ONE. 2012;7:e31808. doi: 10.1371/journal.pone.0031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keefe D.M.K. Intestinal mucositis: mechanisms and management. Curr. Opin. Oncol. 2007;19:323–327. doi: 10.1097/CCO.0b013e3281214412. [DOI] [PubMed] [Google Scholar]
- 48.Lee C.S., Ryan E.J., Doherty G.A. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J. Gastroenterol. 2014;20:3751–3761. doi: 10.3748/wjg.v20.i14.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiota A., Hada T., Baba T., Sato M., Yamanaka-Okumura H., Yamamoto H., Taketani Y., Takeda E. Protective effects of glycoglycerolipids extracted from spinach on 5-fluorouracil induced intestinal mucosal injury. J. Med. Invest. 2010;57:314–320. doi: 10.2152/jmi.57.314. [DOI] [PubMed] [Google Scholar]
- 50.Huang T.Y., Chu H.C., Lin Y.L., Ho W.H., Hou H.S., Chao Y.C., Liao C.L. Minocycline attenuates 5-fluorouracil-induced small intestinal mucositis in mouse model. Biochem. Biophys. Res. Commun. 2009;389:634–639. doi: 10.1016/j.bbrc.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 51.Cárdenas M.B., Álvarez C.S., Morgado E.B., Gutiérrez M.G., Monteagudo G.L., Suarez O.S. Toxicological evaluation of an infusion of Bidens pilosa. Pharmacologyonline. 2006;3:428–434. [Google Scholar]
- 52.Frida L., Rakotonirina S., Rakotonirina A., Savineau J.P. In vivo and in vitro effects of Bidens pilosa L. (Asteraceae) leaf aqueous and ethanol extracts on primed-oestrogenized rat uterine muscle. Afr. J. Tradit. Complement. Altern. Med. 2008;5:79–91. [PMC free article] [PubMed] [Google Scholar]
- 53.Ezeonwumelu J.O.C., Julius A.K., Muhoho C.N., Ajayi A.M., Oyewale A.A., Tanayen J.K., Balogun S.O., Ibrahim A., Adzu B., Adiukwu C.P., Oloro J., Kiplagat D.M., Goji A.D.T., Okoruwa A.G., Onchweri A.N., Reddy P.M.K. Biochemical and histological studies of aqueous extract of Bidens pilosa leaves from Ugandan rift valley in rats. Br. J. Pharmacol. Toxicol. 2011;2:302–309. [Google Scholar]
- 54.Wyllie A.H. Where, O, death, is thy sting? A brief review of apoptosis biology. Mol. Neurobiol. 2010;42:4–9. doi: 10.1007/s12035-010-8125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Galluzzi L., Bravo-San Pedro J.M., Vitale I., Aaronson S.A., Abrams J.M., Adam D., Alnemri E.S., Andrews D., Annicchiarico-Petruzzelli M., Baehrecke E.H., Bazan N.G., Bertrand M.J., Bianchi K., Blagosklonny M.V., Blomgren K., Borner C., Bredesen D.E., Brenner C., Campanella M., Candi E., Cecconi F., Chan F.K., Chandel N.S., Cheng E.H., Chipuk J.E., Cidlowski J.A., Ciechanover A., Dawson T.M., Dawson V.L., De Laurenzi V., De Maria R., Debatin K.M., Di Daniele N., Dixit V.M., Dynlacht B.D., El-Deiry W.S., Fimia G.M., Flavell R.A., Fulda S., Garrido C., Gougeon M.L., Green D.R., Gronemeyer H., Hajnoczky G., Hardwick J.M., Hengartner M.O., Ichijo H., Joseph B., Jost P.J., Kaufmann T., Kepp O., Klionsky D.J., Knight R.A., Kumar S., Lemasters J.J., Levine B., Linkermann A., Lipton S.A., Lockshin R.A., López-Otín C., Lugli E., Madeo F., Malorni W., Marine J.C., Martin S.J., Martinou J.C., Medema J.P., Meier P., Melino S., Mizushima N., Moll U., Muñoz-Pinedo C., Nuñez G., Oberst A., Panaretakis T., Penninger J.M., Peter M.E., Piacentini M., Pinton P., Prehn J.H., Puthalakath H., Rabinovich G.A., Ravichandran K.S., Rizzuto R., Rodrigues C.M., Rubinsztein D.C., Rudel T., Shi Y., Simon H.U., Stockwell B.R., Szabadkai G., Tait S.W., Tang H.L., Tavernarakis N., Tsujimoto Y., Vanden Berghe T., Vandenabeele P., Villunger A., Wagner E.F., Walczak H., White E., Wood W.G., Yuan J., Zakeri Z., Zhivotovsky B., Melino G., Kroemer G. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong R.S.Y. Apoptosis in cancer: from pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011;30:87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersen J.L., Kornbluth S. The tangled circuitry of metabolism and apoptosis. Mol. Cell. 2013;49:399–410. doi: 10.1016/j.molcel.2012.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonis S.T., Elting L.S., Keefe D., Peterson D.E., Schubert M., Hauer-Jensen M., Bekele B.N., Raber-Durlacher J., Donnelly J.P., Rubenstein E.B. Perspectives on cancer therapy-induced mucosal injury: pathogenesis, measurement, epidemiology, and consequences for patients. Cancer. 2004;100:1995–2025. doi: 10.1002/cncr.20162. [DOI] [PubMed] [Google Scholar]
- 59.Bowen J.M., Gibson R.J., Cummins A.G., Keefe D.M.K. Intestinal mucositis: the role of the Bcl-2 family, p53 and caspases in chemotherapy-induced damage. Support. Care Cancer. 2006;14:713–731. doi: 10.1007/s00520-005-0004-7. [DOI] [PubMed] [Google Scholar]
- 60.Kanarek N., Grivennikov S.I., Leshets M., Lasry A., Alkalay I., Horwitz E., Shaul Y.D., Stachler M., Voronov E., Apte R.N., Pagano M., Pikarsky E., Karin M., Ghosh S., Ben-Neriah Y. Critical role for IL-1β in DNA damage-induced mucositis. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E702–E711. doi: 10.1073/pnas.1322691111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gudkov A.V., Gurova K.V., Komarova E.A. Inflammation and p53: a tale of two stresses. Genes Cancer. 2011;2:503–516. doi: 10.1177/1947601911409747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dowsett M., Nielsen T.O., A’Hern R., Bartlett J., Coombes R.C., Cuzick J., Ellis M., Henry N.L., Hugh J.C., Lively T., McShane L., Paik S., Penault-Llorca F., Prudkin L., Regan M., Salter J., Sotiriou C., Smith I.E., Viale G., Zujewski J.A., Hayes D.F. Assessment of Ki67 in breast cancer: recommendations from the international Ki67 in breast cancer working group. J. Natl. Cancer Inst. 2011;103:1656–1664. doi: 10.1093/jnci/djr393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reaves T.A., Chin A.C., Parkos C.A. Neutrophil transepithelial migration: role of toll-like receptors in mucosal inflammation. Mem. Inst. Oswaldo Cruz. 2005;100:191–198. doi: 10.1590/s0074-02762005000900033. [DOI] [PubMed] [Google Scholar]
- 64.Deng J.S., Chi C.S., Huang S.S., Shie P.H., Lin T.H., Huang G.J. Antioxidant, analgesic, and anti-inflammatory activities of the ethanolic extracts of Taxillus liquidambaricola. J. Ethnopharmacol. 2011;137:1161–1171. doi: 10.1016/j.jep.2011.07.041. [DOI] [PubMed] [Google Scholar]
- 65.Cheah K.Y., Howarth G.S., Yazbeck R., Wright T.H., Whitford E.J., Payne C., Butler R.N., Bastian S.E.P. Grape seed extract protects IEC-6 cells from chemotherapy-induced cytotoxicity and improves parameters of small intestinal mucositis in rats with experimentally-induced mucositis. Cancer Biol. Ther. 2009;8:382–390. doi: 10.4161/cbt.8.4.7453. [DOI] [PubMed] [Google Scholar]
- 66.Gulgun M., Erdem O., Oztas E., Kesik V., Balamtekin N., Vurucu S., Kul M., Kismet E., Koseoglu V. Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp. Toxicol. Pathol. 2010;62:109–115. doi: 10.1016/j.etp.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 67.Kolli V.K., Abraham P., Isaac B., Kasthuri N. Preclinical efficacy of melatonin to reduce methotrexate-induced oxidative stress and small intestinal damage in rats. Dig. Dis. Sci. 2013;58:959–969. doi: 10.1007/s10620-012-2437-4. [DOI] [PubMed] [Google Scholar]
- 68.Yang H.L., Chen S.C., Chang N.W., Chang J.M., Lee M.L., Tsai P.C., Fu H.H., Kao W.W., Chiang H.C., Wang H.H., Hseu Y.C. Protection from oxidative damage using Bidens pilosa extracts in normal human erythrocytes. Food Chem. Toxicol. 2006;44:1513–1521. doi: 10.1016/j.fct.2006.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.