Abstract
Epidemiological and toxicological studies have suggested that the health effects associated with exposure to particulate matter (PM) are related to the different physicochemical properties of PM. These effects occur through the initiation of differential cellular responses including: the induction of antioxidant defenses, proinflammatory responses, and ultimately cell death. The main objective of this study was to investigate the effects of size-fractionated ambient PM on epithelial cells in relation to their physicochemical properties. Concentrated ambient PM was collected on filters for three size fractions: coarse (aerodynamic diameter [AD] 2.5–10 μm), fine (0.15–2.5 μm), and quasi-ultrafine (<0.2 μm), near a busy street in Toronto, Ontario, Canada. Filters were extracted and analyzed for chemical composition and redox activity. Chemical analyses showed that the coarse, fine, and quasi-ultrafine particles were comprised primarily of metals, water-soluble species, and organic compounds, respectively. The highest redox activity was observed for fine PM. After exposure of A549 cells to PM (10–100 μg/ml) for 4 h, activation of antioxidant, proinflammatory and cytotoxic responses were assessed by determining the expression of heme oxygenase (HMOX-1, mRNA), interleukin-8 (IL-8, mRNA), and metabolic activity of the cells, respectively. All three size fractions induced mass-dependent antioxidant, proinflammatory, and cytotoxic responses to different degrees. Quasi-ultrafine PM caused significant induction of HMOX-1 at the lowest exposure dose. Correlation analyses with chemical components suggested that the biological responses correlated mainly with transition metals and organic compounds for coarse and fine PM and with organic compounds for quasi-ultrafine PM. Overall, the observed biological responses appeared to be related to the combined effects of size and chemical composition and thus both of these physicochemical properties should be considered when explaining PM toxicity.
Keywords: Ambient particulate matter, Physicochemical properties, Reactive oxygen species, Antioxidant enzyme, Inflammation, Cytotoxicity
1. Introduction
Among the common ambient air pollutants, epidemiological studies have identified that exposures to particulate matter (PM) correlate most consistently with pulmonary and cardiovascular morbidity and mortality [10], [14], [22], [39]. While ambient PM range from 0.001 to 100 μm in aerodynamic diameter (AD) [7], particles larger than 10 μm AD are generally trapped in the nasal passages [19]. Based on size, PM smaller than 10 μm AD are divided into three fractions: PM10 (AD < 10 μm), PM2.5 (AD < 2.5 μm) and ultrafine (UF) (AD < 0.1 μm) [51]. PM10 is further divided into coarse (AD 2.5–10 μm) and fine (AD < 2.5 μm) PM [51]. The fate of inhaled particles depends on their size, which determines their site of deposition in the lung, which in turn influences their rate of clearance. While larger particles are primarily deposited in the extrathoracic and thoracic regions, smaller particles (<1 μm) deposit deep in the lung, particularly in the alveolar region [19]. Of further concern, ultrafine particles also have the potential to cross the lung alveolar-capillary border and gain access to the circulation [6], [33], which can have serious implications in terms of systemic toxicity and cardiovascular disease. Several studies have reported translocation of ultrafine particles to secondary target organs, including the liver, spleen, heart, and brain in rats [37], [46]. However, to date, translocation of particles has only been observed in spleens and livers of coal mine workers after long-term exposure to very high doses [25]. While exposure standards for PM10 and PM2.5 have already been established in North America, an ambient standard for ultrafine particles has yet to be established.
Recent studies have suggested that the presence of excessive reactive oxygen species (ROS), i.e., hydrogen peroxide (H2O2), superoxide (O2•−), and hydroxyl radical (OH−) in PM, may lead to oxidative stress in the pulmonary system [26]. ROS can be generated from soluble transition metals (Cu, Cr, Fe, Zn, etc.) or organic compounds (OCs), i.e., polycyclic aromatic hydrocarbons (PAHs), that are present on the surface of particles [47], [52]. Li et al. [26], [27] proposed that oxidative stress may initiate a specific sequence of cellular responses. At lower levels of oxidative stress, antioxidant enzymes are activated in order to protect the lung. If this antioxidant response of the cell fails to provide protection against the generation of ROS, an inflammatory response may be induced to attract inflammatory cells to the site of “injury”. Finally, at toxic levels, cell death occurs through both apoptosis and necrosis [26], [27], [29], [56]. Thus, the progressive increase in the severity of the response to PM suggests a hierarchical dose-effect relationship, whereby the activation of antioxidant mechanisms precedes inflammatory responses and in turn ultimately cell death. Long-term exposure to PM resulting in chronic pulmonary inflammation may also impair lung development, and increase the risk of developing pulmonary diseases, including asthma and chronic obstructive pulmonary diseases [14], [15], [44].
An intensive, collaborative, multidisciplinary field campaign ‘Health Effects of Aerosols in Toronto (HEAT)’ was conducted in Toronto in 2010. The main objectives of this campaign were to: (1) characterize the physicochemical properties of Toronto's size-fractionated concentrated ambient PM [40]; (2) measure different cellular responses (i.e., antioxidant defense, proinflammatory changes, and cytotoxicity) of airway epithelial cells to PM; (3) identify relationships between the physicochemical characteristics of the PM and the cellular responses; and (4) assess the differential effects of different PM size fractions on both pulmonary and cardiovascular functions [3]. We hypothesized that particle exposure would induce dose-dependent differential toxicological effects (i.e., antioxidant defense, inflammatory changes, and ultimately cell death) on the cells and that these cellular responses would vary depending on the diverse physicochemical properties of the different size-fractionated PM.
2. Materials and methods
2.1. Collection of ambient particles
Concentrated ambient PM was collected using the high volume ambient particulate concentrator system located at the Gage Occupational and Environmental Health Unit, University of Toronto, which is located on a busy downtown street. The concentrator facility consists of three Concentrated Ambient Particle Systems (CAPS) that fractionate and concentrate ambient PM in the coarse (2.5 < AD < 10 μm), fine (1.5 < AD < 2.5 μm), or quasi-ultrafine (AD < 0.2 μm) size ranges. On average, coarse, fine, and quasi-ultrafine particles were concentrated (by mass) by factors of ∼103, 36, and 28, respectively. We have previously reported a detailed description of the effectiveness of these three systems [31], [40].
Size-fractionated PM was collected on filters on weekdays during the winter of 2010 (February 19–March 19). Samples were simultaneously collected over 4–8 h on 47 mm quartz (Whatman, USA), 47 mm Teflon (Pall Gelman, USA), and 37 mm Teflon filters (Pall Gelman, USA) for chemical characterization, determination of redox activity, and in vitro analyses, respectively. The number of filters used for this study was 7 for coarse, 6 for fine, and 8 for quasi-ultrafine PM. All filters were weighed gravimetrically before and after sampling to measure the total collected PM mass. To prevent photo-degradation and evaporation loss, the filter samples were then sealed and kept in the dark at -20 °C until further analyses. Information regarding the PM collection times and collected mass is described in Supplemental Table S.1. The filters were extracted using cell culture medium, as described previously [2]. The extraction efficiency varied from 65% to 90% for the three different particle sizes. Extraction efficiency was the highest for fine particles with the highest water-soluble fraction.
During the study period, the number concentrations of the size-distributed ultrafine particles (diameter: 10–800 nm) were also measured online using a Scanning Mobility Particle Sizer (SMPS) (Model 3080, TSI) (Supplemental Fig. S.1.A). The particle surface area was calculated from the number concentration data, assuming the particles were spherical (Supplemental Fig. S.1.B). Particle-bound polycyclic aromatic hydrocarbons (p-PAHs) concentrations were determined using a Photoelectric Aerosol Sensor (PAS, Model PAS2000CE, EcoChem Analytics) (Supplemental Fig. S.2).
2.2. Chemicals and assay kits
Dulbecco's modified Eagle's medium (DMEM), penicillin–streptomycin, dl-dithiothreitol (DTT, 98%) and 1, 2-naphthoquinone were obtained from Sigma–Aldrich (Oakville, ON, Canada). l-Glutamine, 0.05% trypsin, and superscript II reverse transcriptase kits were obtained from Invitrogen (Carlsbad, CA, USA). Fetal bovine serum (FBS) and phosphate buffer saline (PBS) were obtained from Wisent Inc. (St-Bruno, QC, Canada). Tris–HCl, tetrasodium EDTA and trichloroacetic acid were obtained from Fisher Scientific (Whitby, ON, Canada), and 5, 5′-dithio-bis (2-nitrobenzoic acid) was obtained from Alfa Aesar (Ward Hill, MA, USA). The MTT assay was performed using a kit from the American Type Culture Collection (ATCC) (Manassas, VA, USA). RNA extraction kits and TaqMan master mix were obtained from Qiagen® (Germantown, MD, USA) and Applied Biosystems® (Life Technologies Inc., Burlington, ON), respectively. Plasmids and primers for HMOX-1 and IL-8 were obtained from OriGene (Rockville, MD, USA) and Integrated DNA Technologies Inc. (Coralville, IA, USA), respectively.
2.3. Cell culture and particle exposure
A549 cells (American Type Culture Collection, Rockville, MD, USA), a cell line of human adenocarcinomatous cells derived from lung cancer that exhibits characteristics of alveolar epithelial cells, were used in this study [16]. Cell culture and in vitro PM exposures were performed, as described previously [2] with several modifications. Cells were seeded at 2.5 × 105 cells/ml in 96-well plates for the MTT assay and at 1 × 106 cells/ml in 6-well plates for real-time polymerase chain reaction (RT-PCR). PM suspensions were prepared in cell culture medium containing 10% FBS and were added to the cells at final concentrations of 10, 50, and 100 μg/ml (mass/volume) for 4 h along with appropriate media-treated controls. The exposure duration was selected based on the expression of IL-8 (protein) in our previous study [2]. Cells were exposed to PM isolated from each filter in triplicate at each PM concentration.
2.4. PM chemical characterization
For chemical speciation, the quartz filters were cut into three pieces: two quarters and one half portions. One quarter portion was used for the analyses of acid-soluble elements. From the half portion, 1.7 cm diameter punches were taken for elemental carbon (EC) and organic carbon (OC) analyses using a Sunset EC-OC analyzer, according to the National Institute of Occupational Health and Safety (NIOSH) 5040 protocol [5]. The mass of the OC fractions was converted to the organic mass (OM) by multiplying by a factor of 1.4 to account for the mass of oxygen in addition to the OC mass. The fractions of OM measured at various temperatures (310, 475, 615, and 870 °C) were named as OM-1, OM-2, OM-3, and OM-4, respectively, representing their volatility order. The remaining filter portion was extracted with deionized water (Millipore DQ-3, resistivity > 18.3 MΩ) for analysis of water-soluble cations, including amines, anions, metals, and organic carbon (WSOC). Acid and water-soluble extracts were analyzed for 26 elements (Ag, Al, As, B, Ba, Be, Ca, Cd, Co, Cr, Cu, Fe, K, Mg, Mn, Mo, Na, Ni, Pb, Sb, Se, Si, Ti, Tl, V, and Zn) using Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES, Perkin Elmer Optima 3700 DV) in the axial mode, at the Analytical Laboratory for Environmental Science Research and Training (ANALEST) facility of University of Toronto. Anions and cations in the size-fractionated PM sample water extracts were measured by Ion Chromatography (Dionex ICS-2000). WSOC was measured using a Total Carbon Analyzer system (Shimadzu TOC-VCPH/CPN) equipped with an 8-port sampler. The DTT assay was used to measure the redox activity of the PM samples, as previously described [2]. Redox activity was expressed as the rate of DTT consumption per minute normalized to the quantity of PM used.
2.5. Analyses of biological endpoints
In this study, heme oxygenase (HMOX-1) was selected as the biomarker of induction of antioxidant enzymes. HMOX-1 is known to be highly responsive to oxidative stress and plays a protective role by producing the antioxidant bilirubin through the degradation of heme. To evaluate the proinflammatory response to PM, IL-8 expression was measured at both the mRNA and protein levels. The results for IL-8 (protein) are presented in Supplemental Materials (S.1). IL-8 acts as a chemoattractant factor and is a ubiquitous early response marker of inflammation in many cell types. Following 4 h-exposure of cells to PM, cell lysates and supernatants were collected from each well. Total RNA was extracted from cell lysates using a RNeasy mini kit according to the manufacturer's instructions (Cat. No. 74104, Qiagen) and was quantified using a Nanodrop-1000 system (Thermo Scientific). First strand cDNA was synthesized from 1 μg of mRNA for each sample using SuperScript® II Reverse Transcriptase (Invitrogen™). Expression of HMOX-1 and IL-8 mRNA was quantified using an Applied Biosystems 7900HT Fast Real-Time PCR System. The copy number of the target gene was normalized to 18S RNA, as a housekeeping gene. The primers and probe sets for HMOX-1, IL-8, and 18S are reported in Table 1. MTT assays were performed as described previously [2]. All assays were conducted in triplicate.
Table 1.
Primers and probes used for real-time RT-PCR.
| Gene target | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| HMOX-1 | 5′-TCAGGCAGAGGGTGATAGAAG-3′ | 5′-TTGGTGTCATGGGTCAGC-3′ | 5′-TGGATGTTGAGCAGGAACGCAGT-3′ |
| IL-8 | 5′-ATACTCCAAACCTTTCCACCC-3′ | 5′-TCTGCACCCAGTTTTCCTTG-3′ | 5′-CCACACTGCGCCAACACAGAAA-3′ |
| 18S | 5′-GGACATCTAAGGGCATCACAG-3′ | 5′-GAGACTCTGGCATGCTAACTAG-3′ | 5′-TGCTCAATCTCGGGTGGCTGAA-3′ |
2.6. Statistical analyses
Statistical analyses were performed using GraphPad Prism 4.0c (La Jolla, CA, USA). All biological response results are reported as the mean ± standard error of the mean (SEM) and relative to controls. Non-parametric statistical analyses were used because the data were not normally distributed. Kruskal–Wallis one-way analysis of variance with post hoc Dunn's multiple comparison test were used to compare the redox-activities of different sized PM and to determine the difference in biological responses between controls and different PM concentrations. Two-way ANOVA was used to investigate the effects of size and concentration on the biological endpoints. Biological response data for all three size fractions were also plotted on the same graph with elemental concentration to test for the effect of size on the responses. Spearman correlations (ρ) were used to assess the strength of relationships between composition and biological responses. Correlation tests were also used to examine the relationship between quasi-ultrafine particle number concentration and surface area (4 h averaged data) and biological responses. Statistical differences were considered significant at p < 0.05.
3. Results
3.1. PM characterization
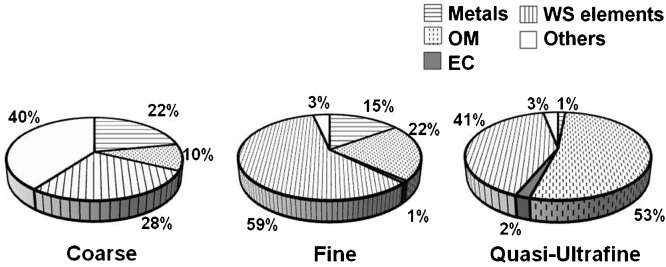
Each of the PM size fractions exhibited distinct chemical compositions (Fig. 1). The metal concentrations (including water-soluble metals) were the highest in the coarse fraction (22%), whereas quasi-ultrafine PM was dominated by OM (53%). On average, water-soluble components (ions, amines, and OC) comprised 59% of the fine PM, compared with 41% and 28% in the ultrafine and coarse PM, respectively. Among the water-soluble compounds, a significant contribution of anions (i.e., NO3−, SO42+, C2O42−, PO43−, Cl−) was observed for both fine and coarse PM (Supplemental Fig. S.3). While ultrafine PM was comprised of the highest proportion of amines (38%) and OC (24%), coarse PM contained the highest proportion of water-soluble metals (17%). In ultrafine PM, trimethyl amine (TMA) contributed about 41% of the total amine concentration. Detailed information regarding the chemical compositions are provided in Supplemental Tables S.2–S.4.
Fig. 1.
Average chemical composition of concentrated ambient coarse, fine, and quasi-ultrafine PM collected from Toronto (EC = elemental carbon, OM = organic mass, WS = water-soluble elements (anions, cations, amines, organic carbon (OC))).
3.2. Redox activity measurement
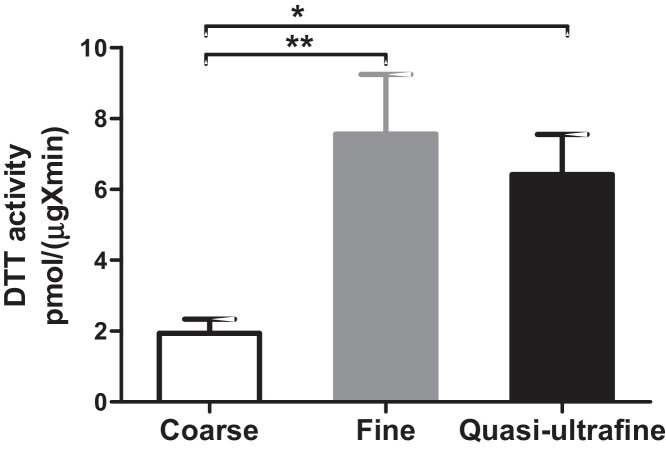
The ROS generation potential of the collected coarse, fine, and ultrafine PM samples was analyzed using the DTT assay. This assay demonstrated that each of the PM size fractions exhibited measurable redox activity (Fig. 2). The activity was higher for fine and quasi-ultrafine particles compared with coarse PM (p < 0.05).
Fig. 2.
Redox activity of coarse, fine, and quasi-ultrafine PM, as measured by the DTT assay. Data are expressed as the mean ± SEM (n = 7, 6, and 8 for coarse, fine, and quasi-ultrafine, respectively). Kruskal–Wallis ANOVA followed by Dunn's multiple comparison test showed that redox activity for fine and quasi-ultrafine PM were significantly higher than coarse PM (* p < 0.05, ** p < 0.01).
3.3. Effect of different size-fractionated PM on biological endpoints
3.3.1. Activation of antioxidant defense
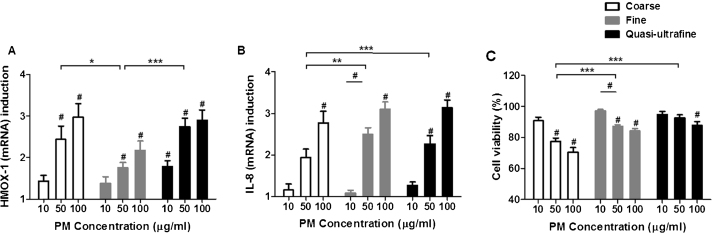
HMOX-1 mRNA expression increased in a dose-dependent manner in response to all three size fractions (Fig. 3A). Significant induction of HMOX-1 expression was observed as compared with controls at 10 μg/ml for ultrafine PM and at 50 μg/ml for the other size fractions. At exposure levels of 100 μg/ml, the order of response was coarse ≥ ultrafine > fine. HMOX-1 expression was significantly lower for fine PM compared with both coarse (p < 0.05 by two-way ANOVA) and ultrafine PM (p < 0.001), but was similar between the coarse and ultrafine PM.
Fig. 3.
Effect of different size-fractionated ambient PM on different biological endpoints. A549 cells were exposed to coarse, fine, and ultrafine PM at mass concentrations of 10, 50, and 100 μg/ml (mass/volume) for 4 h. (A) HMOX-1 (mRNA), (B) IL-8 (mRNA), and (C) cytotoxic responses. HMOX-1 and IL-8 expressions are presented as fold change and cytotoxic responses are presented as the percentage of cell viability relative to controls. The data are presented as mean ± SEM (n = 21, 18, and 24 for coarse, fine, and ultrafine, respectively, for HMOX-1 and IL-8 expression, n = 7 for coarse, 6 for fine, and 8 for ultrafine for cytotoxicity). Statistically significant differences from control and in between concentrations # (p < 0.05) and between sizes of PM *, **, *** p < 0.05, 0.01, 0.001, respectively).
3.3.2. Activation of the proinflammatory response
Significant induction of IL-8 (mRNA) expression was observed as compared to controls at 50 μg/ml for ultrafine PM and at 100 μg/ml for the other size fractions (Fig. 3B). At exposure levels of 100 μg/ml, the order of response was ultrafine = fine > coarse. IL-8 expression was significantly lower for coarse PM compared with both fine (p < 0.01) and ultrafine PM (p < 0.001), but was similar between the fine and ultrafine PM.
3.3.3. Cell viability
The cytotoxicity profiles for coarse, fine, and ultrafine PM were determined using the MTT assay (Fig. 3C). Similar to the antioxidant and proinflammatory responses, a PM concentration-dependent decrease in cell viability was observed. For coarse and fine PM, a statistically significant decrease was observed starting at PM mass concentrations of 50 μg/ml, whereas significant cytotoxicity for ultrafine PM was observed at 100 μg/ml. At 100 μg/ml, cell viability decreased to 70%, 84%, and 87% for coarse, fine, and ultrafine particles, respectively. Two-way ANOVA analyses showed that the coarse PM were more cytotoxic than fine and ultrafine PM (p < 0.001).
3.4. Linear regression and correlation analyses
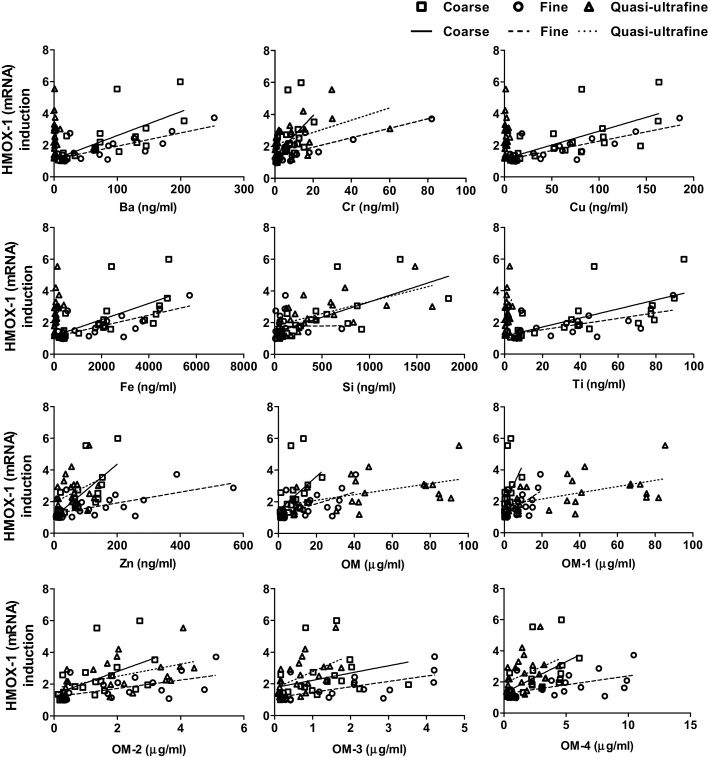
Scatter plots of selected chemical constituents with biological endpoints were used to examine whether the differences in the responses were due to the mass concentrations of different elements vs. particle size (Fig. 4, Fig. 5, Fig. 6). If the responses were governed by mass concentration alone, all data for the three PM size fractions would be expected to be fit along the same regression line. Three distinct and independent linear regression lines were observed for most of the elements, indicating that for the same elemental mass concentration, the responses were different between the different PM sizes. The slopes of the biological response vs. total PM mass concentration and the different PM constituent masses were also determined for the different size fractions (Supplemental Tables S.5). Among the 17 elements, the slopes of the dose-response relationships were significantly different among the sizes for 4 (HMOX-1), 15 (IL-8 mRNA), and 12 (cell viability) elements. Greater slope values were generally observed for metals in ultrafine PM and for OM for coarse PM. Although metal and OM concentrations were lower in ultrafine and coarse PM, respectively, the high slope values indicated that small increments of these PM constituents could have greater influence on the biological responses. Slope values were higher for Cr, Ti, and OM for all size fractions and biological endpoints than for total PM mass, suggesting that these two metals and OM impose comparatively greater effects compared to other chemical elements.
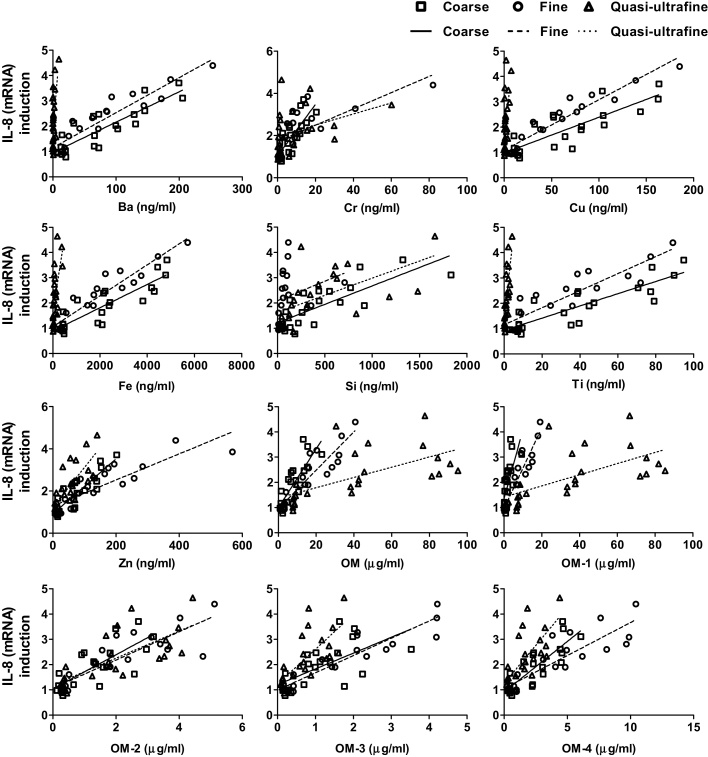
Fig. 4.
Scatter plots of selected chemical elements vs. HMOX-1 upregulation for coarse, fine, and quasi-ultrafine PM.
Fig. 5.
Scatter plots of selected chemical elements with IL-8 (mRNA) upregulation for coarse, fine, and quasi-ultrafine PM.
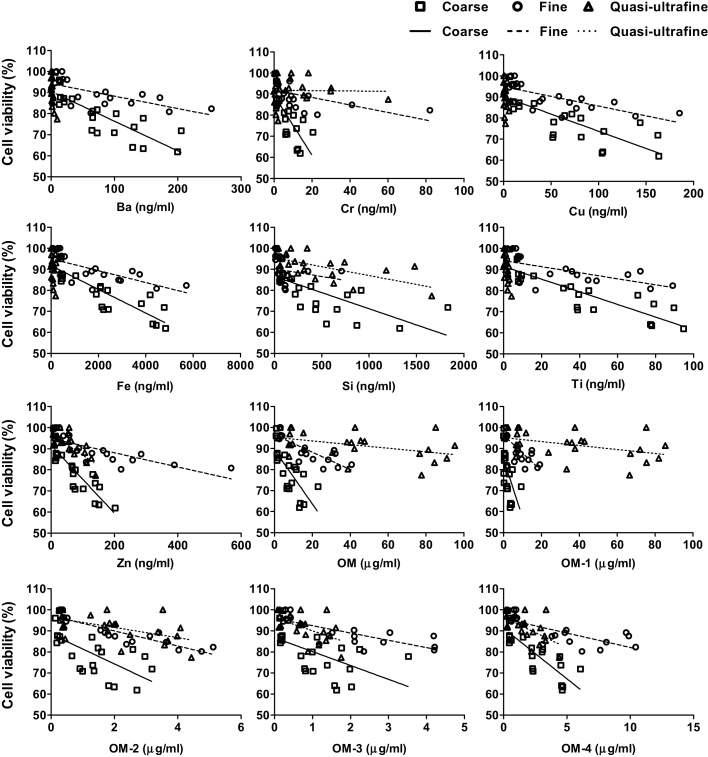
Fig. 6.
Scatter plots of selected chemical elements with cell viability for coarse, fine, and quasi-ultrafine PM.
The Spearman correlation coefficients (ρ) of the biological endpoints with different chemical constituents, redox activity, and between the endpoints are reported in Supplemental Tables S.6–S.9. All the correlation values mentioned hereafter were statistically significant (p < 0.05). In the coarse size range, all three biological endpoints (HMOX-1 and IL-8 (mRNA) positively, and cell viability negatively) correlated with total Al, Ba, Ca, Cr, Cu, Fe, Mg, Mn, Na, Si, Ti, Zn, and OC fractions. For fine PM, Ba, Cr, Cu, Fe, Mg, Mn, Ti, Zn, and OC fractions correlated positively with HMOX-1 and IL-8 (mRNA), and inversely with cell viability. For ultrafine PM, total Mn, Na, Si, Ti, Zn, and OC fractions correlated positively with the upregulation of HMOX-1 and IL-8 (mRNA), and inversely with cell viability. Redox activities correlated with all biological responses (inversely with cell viability) for all size fractions. For ultrafine particles, all the biological responses were also correlated with trimethyl amine (TMA) and dimethyl amine (DME) (Supplemental Table S.7). No significant correlations were found between number concentration, surface area, and biological responses for ultrafine PM; except for cell viability, which correlated positively with particle number concentration (Supplemental Table S.8). Among the biological responses, IL-8 expression consistently correlated inversely with cell viability in all size fractions (Supplemental Table S.9).
4. Discussion
This in vitro study investigated the antioxidant, proinflammatory, and cytotoxic effects of coarse, fine, and quasi-ultrafine PM collected in Toronto, Ontario, Canada. Size-dependant cellular responses were observed after exposure to PM. All the size fractions exhibited differential biological effects with respect to oxidative stress and inflammatory responses, and cell death. Coarse PM exhibited higher cytotoxic responses than fine and ultrafine PM, whereas antioxidant and inflammatory responses for quasi-ultrafine PM were as high as coarse and fine PM, respectively. Based on the correlations of the chemical elements with biological endpoints, and the inter-correlations among the elements, it appeared that the observed responses for all size fractions were associated with traffic-related sources (exhaust and non-exhaust). The sampling site was located next to a busy road with ∼20,000 automobiles/weekday. At this site, 25–51% of the PM2.5 mass comes from local sources and the main local source is vehicular traffic [21].
4.1. Redox activity and biological responses
Since urban ultrafine particles are associated with traffic emissions, they are generally high in organic matter and contain higher mass loadings of PAHs than coarse or fine particles [9], [13]. Quinone species are highly active redox-cycling catalysts [47] and are present in gasoline and diesel engine exhaust particles [20]. Quinones may be generated during the oxidation of PAHs associated with exhaust particles [43], [54]. The DTT assay used in this study is particularly reactive to organic species, specifically to quinones. This assay measures quinone-catalyzed production of O2•− by the transfer of electrons from DTT to oxygen [23]. Semi-continuous measurement of p-PAHs during the HEAT campaign showed that ultrafine PM had higher concentrations of p-PAHs than coarse and fine PM, in both ambient and concentrated PM samples (Supplemental Fig. S.2). Therefore, it was expected that ultrafine particles enriched with PAHs would exhibit greater redox activity than fine and coarse particles [9], [34], [42]. However, in this study fine PM was found to be more redox active than ultrafine and coarse PM. HMOX-1 is a sensitive biomarker of oxidative stress and its expression would thus be expected to be related to the redox potential of the particles. The increased expression of HMOX-1 following exposure to coarse and ultrafine particles compared with fine PM also suggested that the DTT assay did not comprehensively measure the redox potential of the coarse and ultrafine particles. The lack of an association between HMOX-1 expression and the redox activity, as measured by the DTT assay, could be due to two reasons. Firstly, there is evidence suggesting that aqueous chemistry within the CAPS can promote adsorption of condensable vapors, which likely adds a large amount of OM to the concentrated ultrafine particles [31]. For example, the abundance of amine compounds in these samples was much higher than that typical of urban ultrafine PM; concentrations of amine containing particles are usually quite low, but short-term exposure spikes do occur in cities around the world. The addition of these amine compounds and other OM may have skewed the observed redox activities, since these are expressed per unit PM mass. Thus, if the condensable material deposited on the particles exhibited lower redox activity than the original particles it would decrease the DTT activity per unit mass observed for the ultrafine particles. Secondly, the contribution of transition metals in coarse particles, i.e., Fe and Cu, to the redox activity could not be determined by this assay, as these metals catalyze ROS production following a different reaction pathway (Fenton reaction, metals reduce H2O2 producing OH•) [17]. Thus, this approach did not allow us to differentiate between metal and organic-based redox activity, and could limit the interpretation of these findings.
4.2. Differential responses for different sized PM
Dose-dependent increases in HMOX-1 and IL-8 expression and decreases in cell viability were observed in this study for all three PM size fractions. However, only the ultrafine particle data supported the differential cellular responses proposed by Li et al. [27]. Significant activation of the antioxidant defense response, i.e., upregulation of HMOX-1, was observed at a lower ultrafine PM concentration (10 μg/ml), whereas proinflammatory response (IL-8) and cell death were induced at 50 and 100 μg/ml particle mass concentrations, respectively. This finding was consistent with other studies using diesel exhaust particles (size <1 μm) that have shown that cytoprotective pathways are induced at relatively low levels of oxidative stress and that cellular apoptosis or necrosis occurs at a higher level of oxidative stress [27], [56]. Li et al. [27] suggested that the hierarchical oxidative stress model was governed by the OC content of particles, especially PAHs. The ultrafine samples collected in Toronto were also highly enriched in PAHs compared with coarse and fine PM. By contrast, for coarse PM, while cytotoxic effects were observed at the lowest PM dose (10 μg/ml), significant upregulation of HMOX-1 was not observed until a higher dose was reached. Thus, the results for the coarse PM were inconsistent with the hierarchical oxidative stress model proposed by Li et al. [27] and hence this model is likely not applicable for all particle sizes.
More generally, it appears that the cells were responding differently when exposed to the coarse vs. quasi-ultrafine PM. The possibility exists that metals, different water-soluble or organic species, and endotoxins could trigger different or a combination of biological pathways leading to different responses. For example, several studies have also suggested that the presence of endotoxins on the particle surface may initiate inflammatory responses in the cells, particularly for larger particles [8], [30], [48], [49], [53]. A recent study from our group measured the endotoxin levels in concentrated coarse (293 EU/mg) PM at this site, and found that human exposure to these concentrations was associated with inflammatory responses [4]. The possibility that particles of differing size or composition may elicit alternate or additional biological pathways further complicates interpretation.
4.3. Effects of different physicochemical properties
Exposure to specific components within the different size fractions showed different degrees of responses (Fig. 4, Fig. 5, Fig. 6). If size has no impact on the biological responses, then similar responses should have been observed following exposure to the equivalent mass concentrations of the components of different size fractions. Thus, the different linear regression lines for the different components indicated that the observed responses were not caused by total or component mass alone; initial particle size may also play a role in inducing the response.
Similarly, the variability in our observed responses cannot be explained based on size alone; otherwise the order of response for different sizes would be expected to always be the same across studies. In vitro studies conducting spatial and temporal PM toxicity studies have not reported any consistent relationships between biological responses and PM sizes [8], [12], [24], [38], [45]. It is likely that the differences in composition within different PM size-fractions played an important role on the observed responses. Higher slope values for transition metals (Cr and Ti) and OM compared with total PM mass and other chemical elements suggested their stronger influence on the observed responses. The relative abundance of transition metals in coarse PM might be related to the observed cytotoxic effects [32], whereas ultrafine PM with higher OCs could induce greater activation of inflammatory response [28].
A third important physical property that can play an important role in the extent of PM toxicity is particle surface area, particularly in the case of the smaller particles. Several studies have suggested that the surface area of particles is the critical determinant of their biological effects and should be used as a dose metric when comparing particle-induced effects [11], [18], [35], [55]. Depending on the size of the particles, equal masses of PM could be comprised of quite different numbers of particles, which would lead to significant differences in surface area. For monodispersed particles with unit density, only one particle/cm3 with a diameter of 2.5 μm would constitute a mass concentration of 10 μg/m3 compared with the same mass concentration of particles 20 nm in diameter would comprise about 2.4 million particles/cm3 with 100 times more surface area [36]. In this study of size-fractionated particles, all experiments were conducted based on an equal mass exposure. Thus, the quasi-ultrafine particles were comprised of the greatest number of particles and total surface area for a given mass. Given the differential responses to the three size fractions, it would not be appropriate to assume that the observed biological effects could be fully described by a single property, i.e., size or surface area. It is most likely that the observed biological effects were the combined effect of mass, size, surface area, and chemical composition. Furthermore, correlation coefficients calculated between surface area and biological responses did not support this hypothesis; suggesting that the complexity of the cellular responses is much greater than originally hypothesized.
4.4. Potential emission sources related to the biological responses
The correlation analyses between chemical elements and biological responses helped to determine the toxicological contribution of different constituents toward the biological endpoints for the different particle sizes. For coarse and fine particles, HMOX-1 expression and cell viability correlated significantly with transitional metals (Cr, Cu, Fe, and Ti) and OC fractions, whereas for ultrafine particles, correlations were found mainly with the OC fractions. IL-8 mRNA upregulation was affected by both transition metals and OC for all size fractions. Correlation analyses among elements also helped to identify potential PM sources responsible for the observed effects (data not shown). For coarse and fine particles, Ba, Cr, Cu, Fe, Mn, Si, Ti, and Zn, were significantly inter-correlated, indicating that the elements might be coming from similar sources. As the study site is located next to a busy street, potential sources for these metals could be traffic exhaust and non-exhaust emission sources, i.e., brake and tire wear [50]. Contaminated road dust and roadside soils could also contribute to the metal concentrations [41]. Further, these metals have all been found to exhibit diurnal and weekday/weekend patterns at this site, similar to other traffic-related pollutants. OC, which correlated significantly with Fe, Mn, and Ti for both coarse and fine particles, could also be produced by the same traffic sources [50]. In the ultrafine size range, higher EC and OC concentrations were observed in the morning and afternoon, respectively, indicating that the primary emission (in the morning) and secondary particle formation (in the afternoon) could be the sources of ultrafine in that location [1]. The particle size distribution of the ambient air (maximum number concentrations for 10–30 nm particles) also suggested that primary emissions from vehicles were a major source of ultrafine particles [40].
4.5. Limitations of the study
There are several limitations to this study. Firstly, filter extracts were used for the cell exposure experiments. The filter extraction methodology used in this study emphasizes the mass/composition-related responses rather than those associated with the particle number or surface area, as the filter collection/extraction process was presumed to not conserve the original number concentration, size distribution or surface area properties of the particles. Secondly, particles were added to the cells as a suspension in culture medium, which differs substantially from the actual deposition of airborne particles onto the respiratory cell surface. The quantity (i.e., mass or number) of particles that actually interacts with the cells could, therefore, not be determined. Thus, it is not surprising that the relationships between the physicochemical properties (number concentration, surface area, and chemical composition) and biological responses were significant when considering composition alone. Thirdly, particles for the chemical analysis and biological tests were collected on different filters and were extracted in different media. Therefore, the particle extraction efficiency could be different among the filters used. Fourthly, the concentrating process for the ultrafine PM causes a shift in the size distribution for small particles (20–40 nm) [40]. Composition of the ultrafine particles was also altered by the addition of excess OM during the condensation process [31]. These factors could have led to a reduction in the redox activity of ultrafine particles. Furthermore, as noted above, the method of assessment of redox activity did not allow the differentiation between metal and organic-based redox activity. Finally, the biological responses observed in the A549 cell line limits the interpretation of these findings to whole organism exposures. Future studies using primary cells will be helpful to confirm and translate these findings into higher models (i.e., whole animal/human exposures).
5. Conclusions
This in vitro study investigated the comparative biological effects of size-fractionated ambient PM on human alveolar epithelial cells. This study demonstrated that exposure to ambient PM can initiate mass-dependent antioxidant, proinflammatory, and cytotoxic responses. The biological responses correlated strongly with transition metals and OC fractions for the coarse and fine particles, and with OC fractions for the quasi-ultrafine particles. For all three size fractions, traffic-related emissions appeared to trigger the biological responses. However, the biological responses did not correlate consistently with any specific particle size fraction. These observations indicate that these responses are dependent upon physicochemical properties, such as size and composition, and that these yield complex patterns that preclude making simple generalizations. It appears that the observed biological responses were caused by the combined effects of different physicochemical properties of the particles. Therefore, it is important to consider the combined influences of these physicochemical properties when explaining PM toxicity.
Acknowledgements
The authors thank Drs. Jeffrey R. Brook and Frances Silverman for supporting our collaboration in the HEAT campaign. Thanks to Dr. Mike Fila for his assistance with the particle collection. Operational funding was provided by Natural Sciences and Engineering Research Council of Canada (NSERC) and Canadian Institutes of Health Research (CIHR) through the Collaborative Health Research Projects (CHRP) and the AllerGen Network of Centres of Excellence (07-A5). Infrastructure for the Southern Ontario Centre for Atmospheric Aerosol Research (SOCAAR) was funded by the Canada Foundation for Innovation (CFI) and the Ontario Research Fund (ORF). Umme Akhtar was funded by Ontario Graduate Scholarship (OGS), Queen Elizabeth II/Richard Quittenton Scholarship, McAllister Graduate Fellowship, and Edward Jarvis Tyrell Fellowship.
Footnotes
Available online 16 May 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.05.002.
Appendix B. [{(Appendix A)}]Supplementary data
The following are the supplementary data to this article:
References
- 1.Ahlm L., Liu S., Day D.A., Russell L.M., Weber R., Gentner D.R., Goldstein A.H., DiGangi J.P., Henry S.B., Keutsch F.N., VandenBoer T.C., Markovic M.Z., Murphy J.G., Ren X., Scheller S. Formation and growth of ultrafine particles from secondary sources in Bakersfield, California. J. Geophys. Res.-Atmos. 2012;11:7. [Google Scholar]
- 2.Akhtar U.S., McWhinney R.D., Rastogi N., Abbatt J.P.D., Evans G.J., Scott J.A. Cytotoxic and proinflammatory effects of ambient and source-related particulate matter (PM) in relation to the production of reactive oxygen species (ROS) and cytokine adsorption by particles. Inhal. Toxicol. 2010;22:37–47. doi: 10.3109/08958378.2010.518377. [DOI] [PubMed] [Google Scholar]
- 3.Amatullah H., North M.L., Akhtar U.S., Rastogi N., Urch B., Silverman F.S., Chow C.W., Evans G.J., Scott J.A. Comparative cardiopulmonary effects of size-fractionated airborne particulate matter. Inhal. Toxicol. 2012;24:161–171. doi: 10.3109/08958378.2011.650235. [DOI] [PubMed] [Google Scholar]
- 4.Behbod B., Urch B., Speck M., Scott J.A., Liu L., Poon R., Coull B., Schwartz J., Koutrakis P., Silverman F., Gold D.R. Endotoxin in concentrated coarse and fine ambient particles induces acute systemic inflammation in controlled human exposures. Occup. Environ. Med. 2013;70:761–767. doi: 10.1136/oemed-2013-101498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birch M.E., Cary R.A. Elemental carbon-based method for monitoring occupational exposures to particulate diesel exhaust. Aerosol Sci. Technol. 1996;25:221–241. doi: 10.1039/an9962101183. [DOI] [PubMed] [Google Scholar]
- 6.Brook R.D. Cardiovascular effects of air pollution. Clin. Sci. 2008;115:175–187. doi: 10.1042/CS20070444. [DOI] [PubMed] [Google Scholar]
- 7.Brook R.D., Brook J.R., Rajagopalan S. Air pollution: “The heart” of the problem. Curr. Hypertens. Rep. 2003;5:32–39. doi: 10.1007/s11906-003-0008-y. [DOI] [PubMed] [Google Scholar]
- 8.Camatini M., Corvaja V., Pezzolato E., Mantecca P., Gualtieri M. PM10-biogenic fraction drives the seasonal variation of proinflammatory response in A549 cells. Environ. Toxicol. 2012;27:63–73. doi: 10.1002/tox.20611. [DOI] [PubMed] [Google Scholar]
- 9.Cho A.K., Sioutas C., Miguel A.H., Kumagai Y., Schmitz D.A., Singh M., Eiguren-Fernandez A., Froines J.R. Redox activity of airborne particulate matter at different sites in the Los Angeles Basin. Environ. Res. 2005;99:40–47. doi: 10.1016/j.envres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Dockery D.W., Pope C.A., Xu X.P., Spengler J.D., Ware J.H., Fay M.E., Ferris B.G., Speizer F.E. An association between air-pollution and mortality in 6 United-States cities. N. Eng. J. Med. 1993;329:1753–1759. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 11.Duffin R., Tran C.L., Clouter A., Brown D.M., MacNee W., Stone V., Donaldson K. The importance of surface area and specific reactivity in the acute pulmonary inflammatory response to particles. Ann. Occup. Hyg. 2002;46(S1):242–245. [Google Scholar]
- 12.Duvall R.M., Norris G.A., Dailey L.A., Burke J.M., McGee J.K., Gilmour M.I., Gordon T., Devlin R.B. Source apportionment of particulate matter in the US and associations with lung inflammatory markers. Inhal. Toxicol. 2008;20:671–683. doi: 10.1080/08958370801935117. [DOI] [PubMed] [Google Scholar]
- 13.Fine P.M., Chakrabarti B., Krudysz M., Schauer J.J., Sioutas C. Diurnal variations of individual organic compound constituents of ultrafine and accumulation mode particulate matter in the Los Angeles basin. Environ. Sci. Technol. 2004;38:1296–1304. doi: 10.1021/es0348389. [DOI] [PubMed] [Google Scholar]
- 14.Gauderman W.J., Avol E., Gilliland F., Vora H., Thomas D., Berhane K., McConnell R., Kuenzli N., Lurmann F., Rappaport E., Margolis H., Bates D., Peters J. The effect of air pollution on lung development from 10 to 18 years of age. N. Eng. J. Med. 2004;351:1057–1067. doi: 10.1056/NEJMoa040610. [DOI] [PubMed] [Google Scholar]
- 15.Gehring U., Wijga A.H., Brauer M., Fischer P., de Jongste J.C., Kerkhof M., Oldenwening M., Smit H.A., Brunekreef B. Traffic-related air pollution and the development of asthma and allergies during the first 8 years of life. Am. J. Respir. Crit. Care Med. 2010;181:596–603. doi: 10.1164/rccm.200906-0858OC. [DOI] [PubMed] [Google Scholar]
- 16.Giard D.J., Aaronson S.A., Todaro G.J., Arnstein P., Kersey J.H., Dosik H., Parks W.P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J. Nat. Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- 17.Halliwell B., Gutteridge J.M. 4th ed. Oxford University Press; Oxford, UK: 1999. Free Radicals in Biology and Medicine. [Google Scholar]
- 18.Hetland R.B., Schwarze P.E., Johansen B.V., Myran T., Uthus N., Refsnes M. Silica-induced cytokine release from A549 cells: importance of surface area versus size. Hum. Exp. Toxicol. 2001;20:46–55. doi: 10.1191/096032701676225130. [DOI] [PubMed] [Google Scholar]
- 19.International Commission on Radiological Protection (ICRP) Elsevier Science Ltd.; Oxford: 1994. Human Respiratory Tract Model for Radiological Protection. Publication 66. Annals of the ICRP 24(1-3) [PubMed] [Google Scholar]
- 20.Jakober C.A., Riddle S.G., Robert M.A., Destaillats H., Charles M.J., Green P.G., Kleeman M.J. Quinone emissions from gasoline and diesel motor vehicles. Environ. Sci. Technol. 2007;41:4548–4554. doi: 10.1021/es062967u. [DOI] [PubMed] [Google Scholar]
- 21.Jeong C.H., McGuire M.L., Herod D., Dann T., Dabek-Zlotorzynska E., Wang D., Ding L., Celo V., Mathieu D., Evans G. Receptor model based identification of PM2.5 sources in Canadian cities. Atmos. Pollut. Res. 2011;2:158–171. [Google Scholar]
- 22.Katsouyanni K., Touloumi G., Samoli E., Gryparis A., Le Tertre A., Monopolis Y., Rossi G., Zmirou D., Ballester F., Boumghar A., Anderson H.R., Wojtyniak B., Paldy A., Braunstein R., Pekkanen J., Schindler C., Schwartz J. Confounding and effect modification in the short-term effects of ambient particles on total mortality: results from 29 European cities within the APHEA2 project. Epidemiology. 2001;12:521–531. doi: 10.1097/00001648-200109000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Kumagai Y., Koide S., Taguchi K., Endo A., Nakai Y., Yoshikawa T., Shimojo N. Oxidation of proximal protein sulfhydryls by phenanthraquinone, a component of diesel exhaust particles. Chem. Res. Toxicol. 2002;15:483–489. doi: 10.1021/tx0100993. [DOI] [PubMed] [Google Scholar]
- 24.Lauer F.T., Mitchell L.A., Bedrick E., McDonald J.D., Lee W.Y., Li W.W., Olvera H., Amaya M.A., Berwick M., Gonzales M., Currey R., Pingitore N.E., Burchiel S.W. Temporal-spatial analysis of US-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2009;238:1–10. doi: 10.1016/j.taap.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefevre M.E., Green F.H.Y., Joel D.D., Laqueur W. Frequency of black pigment in livers and spleens of coal workers: correlation with pulmonary pathology and occupational information. Hum. Pathol. 1982;13:1121–1126. doi: 10.1016/s0046-8177(82)80250-5. [DOI] [PubMed] [Google Scholar]
- 26.Li N., Hao M.Q., Phalen R.F., Hinds W.C., Nel A.E. Particulate air pollutants and asthma – a paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 2003;109:250–265. doi: 10.1016/j.clim.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 27.Li N., Kim S., Wang M., Froines J., Sioutas C., Nel A. Use of a stratified oxidative stress model to study the biological effects of ambient concentrated and diesel exhaust particulate matter. Inhal. Toxicol. 2002;14:459–486. doi: 10.1080/089583701753678571. [DOI] [PubMed] [Google Scholar]
- 28.Li R., Ning Z., Majumdar R., Cui J., Takabe W., Jen N., Sioutas C., Hsiai T. Ultrafine particles from diesel vehicle emissions at different driving cycles induce differential vascular pro-inflammatory responses: implication of chemical components and NF-kappa B signaling. Part. Fibre Toxicol. 2010:7. doi: 10.1186/1743-8977-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li X.Y., Gilmour P.S., Donaldson K., MacNee W. In vivo and in vitro proinflammatory effects of particulate air pollution (PM10) Environ. Health Perspect. 1997;105:1279–1283. doi: 10.1289/ehp.97105s51279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mantecca P., Farina F., Moschini E., Gallinotti D., Gualtieri M., Rohr A., Sancini G., Palestini P., Camatini M. Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 2010;198:244–254. doi: 10.1016/j.toxlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 31.McWhinney R.D., Rastogi N., Urch B., Silverman F., Brook J.R., Evans G.J., Abbatt J.P.D. Characterization of the University of Toronto Concentrated Aerosol Particle Exposure Facility (CAPEF)-effects on fine and ultrafine nonrefractory aerosol composition. Aerosol Sci. Technol. 2012;46:697–707. [Google Scholar]
- 32.Monn C., Becker S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2,5) and coarse particles (PM10-2.5) in outdoor and indoor air. Toxicol. Appl. Pharmacol. 1999;155:245–252. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- 33.Nemmar A., Hoet P.H.M., Vanquickenborne B., Dinsdale D., Thomeer M., Hoylaerts M.F., Vanbilloen H., Mortelmans L., Nemery B. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 34.Ntziachristos L., Froines J.R., Cho A.K., Sioutas C. Relationship between redox activity and chemical speciation of size-fractionated particulate matter. Part. Fibre Toxicol. 2007;4 doi: 10.1186/1743-8977-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oberdörster G. Pulmonary effects of inhaled ultrafine particles. Int. Arch. Occup. Environ. Health. 2001;74:1–8. doi: 10.1007/s004200000185. [DOI] [PubMed] [Google Scholar]
- 36.Oberdörster G., Gelein R.M., Ferin J., Weiss B. Association of particulate air-pollution and acute mortality: involvement of ultrafine particles? Inhal. Toxicol. 1995;7:111–124. doi: 10.3109/08958379509014275. [DOI] [PubMed] [Google Scholar]
- 37.Oberdörster G., Sharp Z., Atudorei V., Elder A., Gelein R., Kreyling W., Cox C. Translocation of inhaled ultrafine particles to the brain. Inhal. Toxicol. 2004;16:437–445. doi: 10.1080/08958370490439597. [DOI] [PubMed] [Google Scholar]
- 38.Perrone M.G., Gualtieri M., Consonni V., Ferrero L., Sangiorgi G., Longhin E., Ballabio D., Bolzacchini E., Camatini M. Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environ. Pollut. 2013;176:215–227. doi: 10.1016/j.envpol.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Pope C.A., Thun M.J., Namboodiri M.M., Dockery D.W., Evans J.S., Speizer F.E., Heath C.W. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am. J. Respir. Crit. Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 40.Rastogi N., McWhinney R.D., Akhtar U.S., Urch B., Fila M., Abbatt J.P.D., Scott J.A., Silverman F.S., Brook J.R., Evans G.J. Physical characterization of the University of Toronto coarse, fine, and ultrafine high-volume particle concentrator systems. Aerosol Sci. Technol. 2012;46:1015–1024. [Google Scholar]
- 41.Rehbein P.J.G., Jeong C.H., McGuire M.L., Evans G.J. Strategies to enhance the interpretation of single-particle ambient aerosol data. Aerosol Sci. Technol. 2012;46:584–595. [Google Scholar]
- 42.Rossner P., Jr., Topinka J., Hovorka J., Milcova A., Schmuczerova J., Krouzek J., Sram R.J. An acellular assay to assess the genotoxicity of complex mixtures of organic pollutants bound on size segregated aerosol. Part II: Oxidative damage to DNA. Toxicol. Lett. 2010;198:312–316. doi: 10.1016/j.toxlet.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki J., Aschmann S.M., Kwok E.S.C., Atkinson R., Arey J. Products of the gas-phase OH and NO3 radical-initiated reactions of naphthalene. Environ. Sci. Technol. 1997;31:3173–3179. [Google Scholar]
- 44.Schikowski T., Sugiri D., Ranft U., Gehring U., Heinrich J., Wichmann H.E., Kramer U. Long-term air pollution exposure and living close to busy roads are associated with COPD in women. Respir. Res. 2005:6. doi: 10.1186/1465-9921-6-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seagrave J., McDonald J.D., Bedrick E., Edgerton E.S., Gigliotti A.P., Jansen J.J., Ke L., Naeher L.P., Seilkop S.K., Zheng M., Mauderly J.L. Lung toxicity of ambient particulate matter from southeastern US sites with different contributing sources: relationships between composition and effects. Environ. Health Perspect. 2006;114:1387–1393. doi: 10.1289/ehp.9234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Semmler M., Seitz J., Erbe F., Mayer P., Heyder J., Oberdorster G., Kreyling W.G. Long-term clearance kinetics of inhaled ultrafine insoluble iridium particles from the rat lung, including transient translocation into secondary organs. Inhal. Toxicol. 2004;16:453–459. doi: 10.1080/08958370490439650. [DOI] [PubMed] [Google Scholar]
- 47.Squadrito G.L., Cueto R., Dellinger B., Pryor W.A. Quinoid redox cycling as a mechanism for sustained free radical generation by inhaled airborne particulate matter. Free Radic. Biol. Med. 2001;31:1132–1138. doi: 10.1016/s0891-5849(01)00703-1. [DOI] [PubMed] [Google Scholar]
- 48.Steenhof M., Gosens I., Strak M., Godri K.J., Hoek G., Cassee F.R., Mudway I.S., Kelly F.J., Harrison R.M., Lebret E., Brunekreef B., Janssen N.A.H., Pieters R.H.H. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential – the RAPTES project. Part. Fibre Toxicol. 2011;8 doi: 10.1186/1743-8977-8-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steerenberg P.A., van Amelsvoort L., Lovik M., Hetland R.B., Alberg T., Halatek T., Bloemen H.J.T., Rydzynski K., Swaen G., Schwarze P., Dybing E., Cassee F.R. Relation between sources of particulate air pollution and biological effect parameters in samples from four European cities: an exploratory study. Inhal. Toxicol. 2006;18:333–346. doi: 10.1080/08958370500515913. [DOI] [PubMed] [Google Scholar]
- 50.Thorpe A., Harrison R.M. Sources and properties of non-exhaust particulate matter from road traffic: a review. Sci. Total Environ. 2008;400:270–282. doi: 10.1016/j.scitotenv.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 51.United States Environmental Protection Agency (USEPA) U.S. Environmental Protection Agency, Office of Research and Development; Research Triangle Park, North Carolina, USA: 2004. Air Quality Criteria for Particulate Matter: Volume II of II. [Google Scholar]
- 52.Verma V., Shafer M.M., Schauer J.J., Sioutas C. Contribution of transition metals in the reactive oxygen species activity of PM emissions from retrofitted heavy-duty vehicles. Atmos. Environ. 2010;44:5165–5173. [Google Scholar]
- 53.Wang B., Li K., Jin W., Lu Y., Zhang Y., Shen G., Wang R., Shen H., Li W., Huang Y., Zhang Y., Wang X., Li X., Liu W., Cao H., Tao S. Properties and inflammatory effects of various size fractions of ambient particulate matter from Beijing on A549 and J774A.1 cells. Environ. Sci. Technol. 2013;47:10583–10590. doi: 10.1021/es401394g. [DOI] [PubMed] [Google Scholar]
- 54.Wang L., Atkinson R., Arey J. Formation of 9,10-phenanthrenequinone by atmospheric gas-phase reactions of phenanthrene. Atmos. Environ. 2007;41:2025–2035. [Google Scholar]
- 55.Wittmaack K. In search of the most relevant parameter for quantifying lung inflammatory response to nanoparticle exposure: particle number, surface area, or what? Environ. Health Perspect. 2007;115:187–194. doi: 10.1289/ehp.9254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao G.G., Wang M.Y., Li N., Loo J.A., Nel A.E. Use of proteomics to demonstrate a hierarchical oxidative stress response to diesel exhaust particle chemicals in a macrophage cell line. J. Biol. Chem. 2003;278:50781–50790. doi: 10.1074/jbc.M306423200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.