Abstract
Damage to the mucous membrane is a serious issue associated with chemotherapy. Gastrointestinal (GI) toxicity is complex and multistep process and unregulated production of reactive oxygen species (ROS) and inflammatory mediators play vital role in the development of GI toxicity. In the present study we have investigated the attenuating potential of vitamin C (vit. C) on 5 fluorouracil (5-FU) induced GI toxicity by targeting oxidative stress and inflammatory markers in Sprague Dawley (SD) rats. Rats were gavaged with vit. C (500 mg/kg b. wt.) or vehicle daily (day 1–10) and were given intraperitoneal injection of 5-FU (150 mg/kg b. wt.) or saline (control) on day 8 to induce mucositis. We found that vit. C supplementation attenuated 5-FU induced lipid peroxidation, myeloperoxidase (MPO) activity, activation of NF-kB and expression of COX-2. Histological observations further supported the protective potential of vit. C against 5-FU induced intestinal anomalies such as neutrophil infiltration, loss of cellular integrity, villus and crypt deformities. Thus the biochemical, molecular and histological findings of the present study demonstrate that oxidative stress and inflammation play vital role in 5-FU induced GI toxicity and the inhibitory potential of vit. C is may be due to the modulation of oxidative stress, activation of redox sensitive transcription factor and also its downstream target molecules.
Keywords: Antineoplastic drugs, Intestinal toxicity, Oxidative stress and inflammation
1. Introduction
Gastrointestinal damage is one of the important adverse effects of anti-neoplastic drug on the cancer patients undergoing chemotherapy characterized by ulceration of the entire gastrointestinal tract. Unbearable and painful symptoms such as pain, nausea, vomiting, diarrhoea, heart burden and constipation develops [15], [26]. Mucositis is also known to increase the mortality, morbidity and poses a huge economic burden on the patient receiving chemotherapy [11], [17].
A Number of anti-neoplastic drugs are used for the chemotherapy. 5-Fluorouracil (5-FU) is the most commonly used chemotherapeutic drug for the treatment of various forms of cancer because of its ability to improve tumor-free status and survival rates [24]. However, more than fifty percent of patients receiving 5-FU develops mucositis in addition to other gastrointestinal (GI) complications because 5-FU is not specifically acts on cancer or tumor cells, but also normal proliferating cells which ultimately result into the severe toxicity [34].
5-Fu induced GI toxicity is a complex and multistep phenomenon. It includes direct damage to cellular macromolecules, over production of harmful reactive oxygen species (ROS), alteration of various signaling pathways, over expression of inflammatory agants like nuclear factor kappa-B (NF-kB), cyclooxygenase-2 (COX-2) and proinflammatory cytokines [34].
While use of chemotherapeutic drugs for the treatment of cancer is one of the commonly used approaches by the clinicians but this approach is associated with serious adverse effects on patients and GI toxicity is one of them. Available measures to treat GI toxicity are not adequate and mostly symptomatic. To overcome this potential problem and to make chemotherapeutic measures more effective attention has been given to combination therapy with natural or synthetic agents to reduce the overall burden of cancer worldwide such as targeted intervention, antioxidants, herbal products, growth factors and anti-inflammatory agents [20], [22].
Vit. C, a hydrophilic vitamin, is a very important cellular antioxidant and radical scavenger which protects cellular architecture and maintains homeostasis from different toxic insults. Vit. C has well known antioxidant, anti-inflammatory, anti-carcinogenic, immunomodulatory, antimicrobial, cytoprotective and many other beneficial properties [3], [9], [14], [16]. Various findings demonstrate that vit. C has strong attenuative characteristics against the pathogenesis of various diseases and their complications [5], [13], [33], [40].
Taking the above mentioned facts into account, in the present study we have investigated the preventive potential of vit. C on 5-FU induced GI toxicity in Sprague Dawley (SD) rats by analyzing the biochemical, molecular and histological parameters.
2. Materials and methods
2.1. Chemicals and other reagents
Hexadecyl trimethyl ammonium bromide (HTAB), hydrogen peroxide, potassium di-hydrogen phosphate, di-potassium hydrogen phosphate, o-dianisidine dihyderochloride, vitamin C, tris and Tween 20 were purchased from Sigma–Aldrich. 5-Fluorouracil was supplied by ICN Biomedicals Inc., Ohio, USA. Trichloroacetic acid (TCA), thiobarbituric acid (TBA) and all other reagents used were of analytical grade.
2.2. Animals
Male Sprague Dawley (SD) rats (150–200 g), 6–8 weeks old, were obtained from animal house facility of research center, Prince Sultan Military Medical City (PSMMC), Riyadh, Saudi Arabia. All animals were housed in the animal care facility under room temperature at 22–25 °C with 12 h light/12 h dark cycle in standard cages and were given free access to standard laboratory diet and water ad libitum. The animals received humane care in accordance with the Guide for the Care and Use of Laboratory Animals, published by the ethical scientific research committee of PSMMC, Riyadh, Saudi Arabia.
2.3. Treatment regimen
Rats were randomly divided in to four groups. Each group comprises of six animals. All four group animals received normal standard diet and water throughout the experimental period. Animals in group one received only vehicle (0.9% saline intraperitonially) on day eighth and serve as the control group. Animals of the group second were given single intraperitoneal injection of 5-FU (150 mg/kg b. wt.) on day eight and served as positive control. Animals in group third were given oral gavage of vit. C (500 mg/kg. b. wt.) for ten days (day one to day ten) and single intraperitoneal injection of F-5U (150 mg/kg b. wt.) on day eighth. Animals of the group four received only oral gavage of vit. C (500 mg/kg. b. wt.) for ten days. All four sets (group) of experimental animals were sacrificed under mild anaesthesia 72 h after 5-FU injection: that is on day eleven.
2.4. Tissue processing
At the termination of treatments, animals were sacrificed by cervical dislocation under mild anaesthesia. Small intestine was excised and washed with ice cold sodium chloride (0.9). A piece of small intestinal tissue was preserved in 10% neutral buffered formalin for histological observation.
2.4.1. Myeloperoxidase activity
MPO activity was assayed by the method of Bradley et al. [1]. Tissue homogenate was prepared in 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyl- trimethylammonium bromide. Samples were subjected to three cycles of sonication freezing-thawing procedures followed by spinning at 14,000 rpm for 25 min at 4 °C.In 0.1 ml of supernatant, MPO activity was measured using 2.9 ml phosphate buffer (50 mM, pH 6.0) containing 0.167 mg/ml O-dianisidine dihydrochloride and 1% hydrogen peroxide as substrates, at 460 nm over three min. MPO activity was expressed in a unit which is defined as the quantity of enzyme degrading 1 μ mol of H2O2 / min at 25 °C. The result was expressed as Units/min/gm.
2.4.2. Lipid peroxidation
The rate of lipid peroxidation (LPO) was determined according to the method of Utley et al., [35] by estimating malondialdehyde (MDA) with slight modification. Briefly, 0.25 ml of tissue homogenate (in 10% phosphate buffer, pH 7.4) was incubated at 37 °C in water bath. After an hour of incubation, 0.25 ml of TCA(5%) and 0.5 ml of TBA (0.67%) was added to the sample and centrifuged at 3000 × g for 10 min. The clear supernatant was transferred to another tube and placed in a boiling water bath for 10 min. Finally, the tubes were cooled and the color intensity was measured was recorded at 535 nm. The malondialdehyde concentration in the samples was calculated using the molar extinction coefficient of 1.56 × 10–5 M-1 cm-1 for MDA-TBA colored complex and expressed as μmol of malondialdehyde formed/g tissue.
2.5. Alcian blue-safronin staining for mucin analysis
Sections of 5 μm from paraffin embedded intestinal tissue were fixed on poly-l-lysine coated glass slides. Next, were dewaxed in xylene and rehydrated through graded series of ethanol to water. The staining was performed in 1% Alcian blue prepared in 3% acetic acid solution (pH 2.5) for 30 min. To avoid any non-specific staining, a quick dip of the slides were given in 3% acetic acid solution. After thorough washing of slides, sections were counterstained with 1% safronin followed by washing and dehydration of the slides in alcohol and xylene. Slides were mounted using DPX mountant and observed under light microscope (BX53 microscope, Olympus, Japan).
2.6. Immunohistochemical detection of NF-kB and COX-2 expression
Intestine sections of 5 μm thickness were cut onto poly l-Lysine coated glass slides. Sections were deparaffinized 3 times (5 min each) in xylene followed by dehydration in graded ethanol and finally rehydrated in running tap water. For antigen retrieval, the sections were boiled in 10 mM citrate buffer (pH 6.0) for 15–20 min. Next, the sections were incubated with protein block (abcam) for 10 min at room temperature followed by TBST wash. Normal goat serum (ab138478) was used as the blocker for 2 h prior to primary antibodies treatment for overnight at 4 °C. Further processing was done by using Mouse and Rabbit specific HRP (ABC) Detection IHC kit (ab93697) from Abcam. The peroxides complex was visualized with 3,3-diaminobenzidine. Lastly the slides were counterstained with haematoxylin, cleaned in water, xylene and ethanol. After DPX mounting microscopic (BX53 microscope, Olympus, Japan) analysis was done. Primary antibodies were rabbit anti-NF-kB p105/p50 (phosphor S927) (dilution1:150, Abcam, ab131493) and rabbit anti-COX-2 (Abcam, ab21704).
Evaluation of immunohistochemical expression of NF-kB and COX-2 was done by two independent observers on a binocular upright microscope (Olympus BX 53, Japan) randomly at least in six different focuses per slide. For evaluation of the immunohistochemical expression of NF-kB and COX-2, we used a score (immuno-reactive) including intensity and quantity of stained cells. Intensity of the staining was scored as negative or no staining (0), weak but detectible (1), moderate or distinct (2+) or strong or intense (3) by eyes in a blinded fashion. The average number of cells used for the analysis of NF-kB and COX-2 staining were approximately five hundred. Thereafter, we counted the number of positively stained cells and percentage of NF-kB and COX-2 stained cells were scored on a scale of 0–4 (0 for <5% immunopositive cells; 1 for <10% immunopositive cells; 2 for 10–50% immunopositive cells; 3 for 51–80% immunopositive cells; or 4 for >80% immunopositive cells). Immunoreactive score was calculated by multiplying the intensity and number of stained cells.
2.7. Histological analysis
For histopathological studies, a portion from small intestine was fixed in freshly prepared 10% neutral-buffered formalin (NBF) immediately after sacrifice. Then, each portion of small intestine tissue was embedded in paraffin wax and blocks were prepared. Sections of 5 μm thickness were cut on to poly-l-lysine coated microscopic slides and stained with haematoxylin and eosin (H&E). Histological changes were monitored under light microscope (BX53 microscope, Olympus, Japan) at least in six different regions.
We have also assessed the role of vit. C in healing the antineoplastic drug induced intestinal damage by measuring the villus hight. Intestinal epithelial cell renewal was also assessed by the microscopic analysis of mitotic cells (mitotic figures). All the analysis was performed randomly at least in six different regions per section and the microscopy analysis was double-blinded.
2.8. Statistical analysis
The data from individual treatment groups are presented as the means ± standard error of the mean (SEM). Differences between groups were analyzed by using one way analysis of variance (ANOVA) followed by Tukey–Kramer multiple comparisons test. Data were considered statistically significant when the p values were p < 0.05.
3. Results
3.1. Vit. C inhibits 5-FU induced intestinal MPO activity
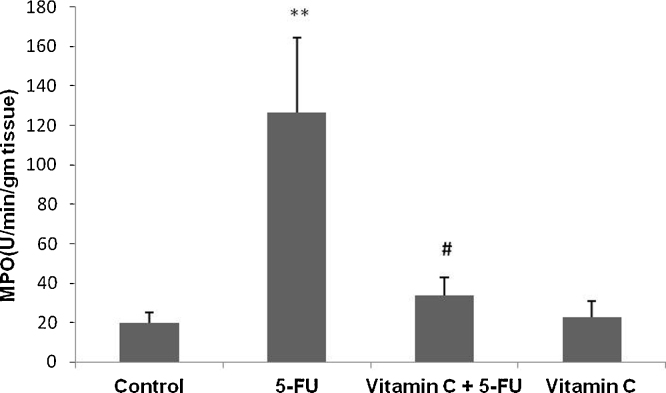
MPO is one of the key abundant enzymes secreted by inflammatory cells and known as important marker of inflammation. We observed sigficantly increased intestinal MPO activity in only 5-FU treated animals as compared to only vehicle treated control animals (p < 0.01). However, treatment with vit. C in addition to 5-FU significantly attenuated MPO activity as compared to only 5-FU treated animals (p < 0.05). No significant difference was observed in MPO activity between vehicle treated and only vit. C administered animals (Fig. 1).
Fig. 1.
Effect of vit. C and 5-FU on MPO activity of small intestine. Bars represent the mean ± SEM per treatment group (n = 6). **p < 0.001 shows significant difference in the animals treated with only 5-FU as compared with vehicle treated control animals. #p < 0.05, shows the statistically significant difference in the animals treated with vit. C along with 5-FU when compared with the animals injected with 5-FU only.
3.2. Vit. C abrogates 5-FU induced intestinal lipid peroxidation (MDA level)
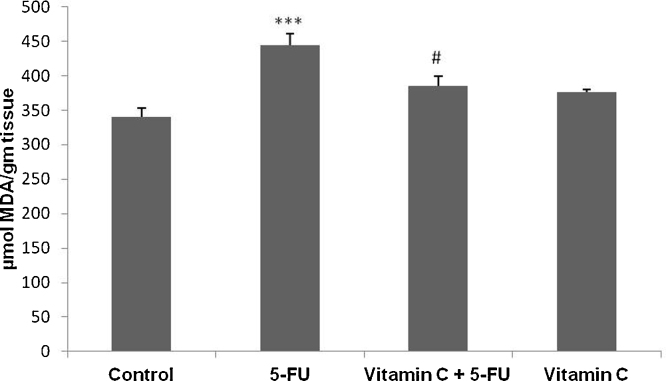
Attenuative potential of vit.C was further strengthened by MDA level estimation. We found that there was a significantly increased level of MDA in 5-FU treated animals as compared to vehicle treated animals (p < 0.001). Supplementation with vit. C along with 5-FU has showed significant reduction of MDA level as compared to 5-FU treated animals (p < 0.05). There was no significant difference observed in MDA level between vehicle and vit. C treated animals (Fig. 2).
Fig. 2.
Effect of vit. C and 5-FU on MDA level of small intestine. Bars represent the mean ± SEM per treatment group (n = 6). ***p < 0.001 shows significant difference in only 5-FU treated animals when compared with vehicle treated animals. #p < 0.05, shows the statistically significant difference in the animals treated with vit. C along with 5-FU when compared with the animals injected with 5-FU only.
3.3. Vit. C modulates the 5-FU induced expression of NF-kB (p105/p50) and COX-2
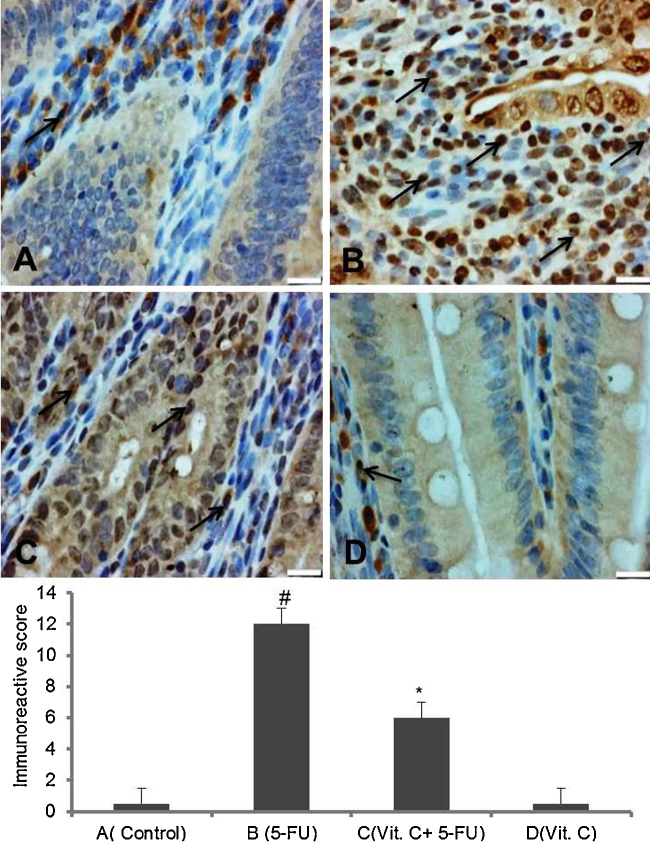
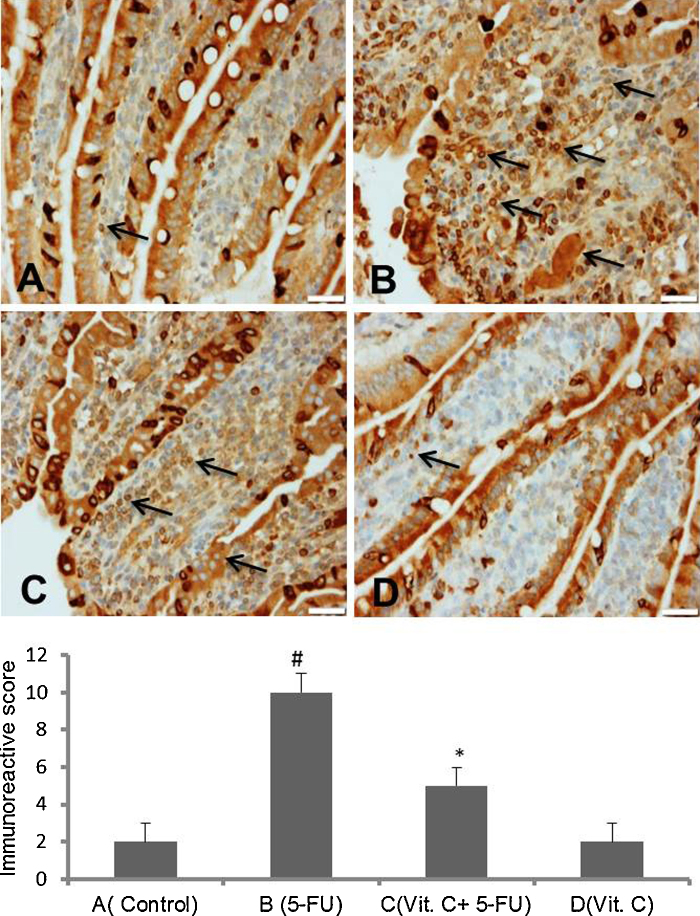
Modulatory action of vit.C was further evaluated by checking the expression of molecular markers of pathogenesis. Immunohistochemical staining for the expression of NF-kB and COX-2 in small intestine has been shown in Fig. 3, Fig. 4, respectively. Elevated expression of NF-kB and COX-2 in small intestine sections was observed to the animals injected with only 5-FU as compared to vehicle treated animals. While vit. C treated animals have showed reduce immunopositive staining of NF-kB and COX-2 as compared to only 5-FU treated animals. However, there was no marked difference in the intensity of brown color was observed in the immunostaining of NF-kB and COX-2 in only vit. C treated animals as compared to vehicle treated animals. For immunohistochemical analyses, brown color indicates specific immunostaining of NF-kB and COX-2 and light blue color indicates haematoxylin staining. Original magnification: 20× and 40× for COX-2 and NF-kB, respectively.
Fig. 3.
Effect of vit. C on 5-FU induced NF-kB activation in intestine. Representative photomicrographs (magnification 40×; scale bar-100 μm) depicting immunohistochemical activation of NF-kB, (A) vehicle treated animals showing minimal or no staining, (B) 5-FU treated animals showing remarkably high intense staining as shown by arrows, (C) administration of vit. C + 5-FU treated animals showing moderate staining (arrows) and (D) vit. C treated animals showing minimal or no staining. Brown color indicates immunopositive staining of NF-kB and blue color indicates haematoxylin staining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 4.
Effect of vit. C on 5-FU induced expression of COX-2 in small intestine. Representative photomicrographs (magnification 20×; scale bar-100 μm)) depicting immunohistochemical activation of COX-2, (A) vehicle treated animals showing minimal or no immunopositive staining, (B) 5-FU treated animals showing remarkable intense staining as shown by arrows, (C) Vit. C + 5-FU treated animals showing low staining (arrow) and (D) Vit. C only treated animals showing minimal or no staining. Brown color indicates immunopositive staining of COX-2 and blue color depicts haematoxylin staining. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
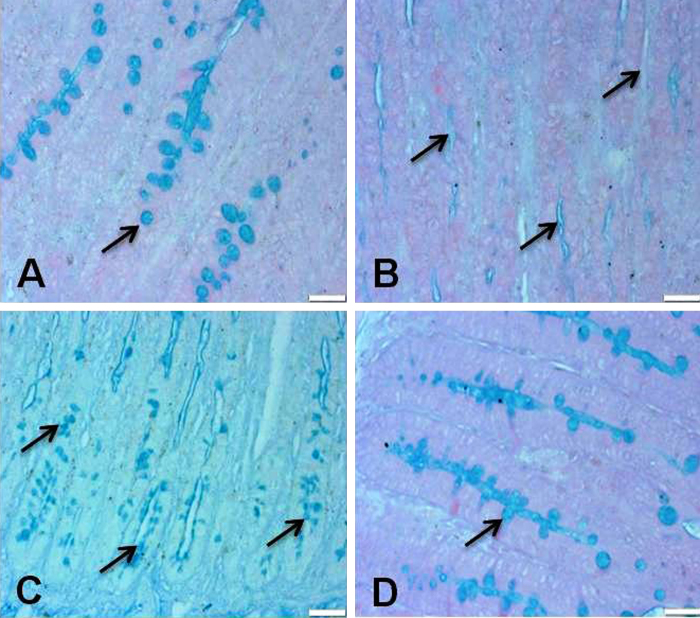
3.4. Vit. C attenuates mucin depletion in small intestine after 5-FU injection
Mucin plays an important role in proper functioning of small intestine. We found that 5-FU injection causes complete depletion of mucin in small intestine as compared to vehicle treated animals. However, supplementation of vit. C with 5-FU reduced the level of mucin depletion when compared with only 5-FU treated animals. However, there is no depletion of mucin layer was observed in only vit. C treated animals as compared to vehicle treated control animals (Fig. 5). Original magnification: 20×.
Fig. 5.
Effect of vit. C treatment on 5-FU induced mucin depletion in small intestine. Representative photomicrographs (magnification 20×; scale bar-100 μm) depicting mucin staining, (A) vehicle treated animals showing normal mucin layer (arrows), (B) 5-FU treated animals showing complete mucin depletion as shown by arrows, (C) Vit. C + 5-FU treated animals showing moderate mucin depletion (arrows) and (D) Vit. C only treated animals showing normal mucin layer.
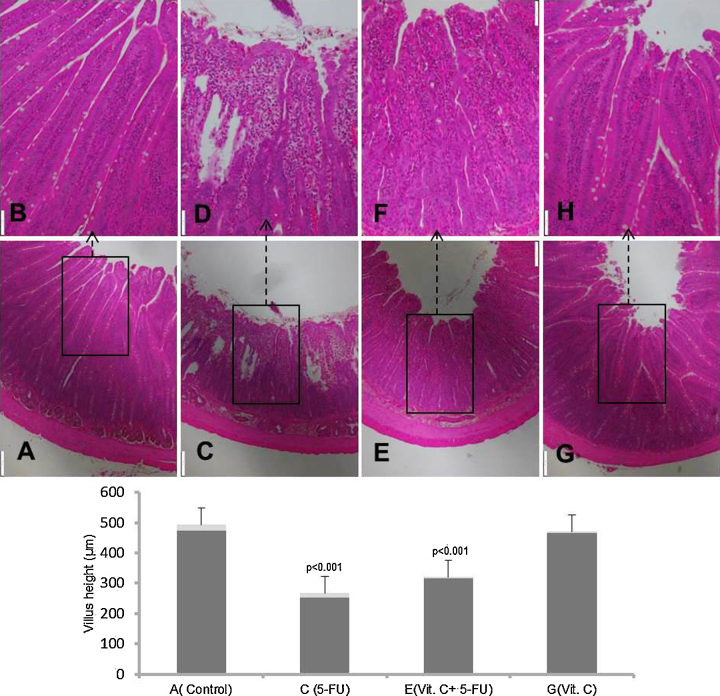
3.5. Vit. C abrogates 5-FU induced histological ailments in small intestine
Modulatory potential of vit.C was further supported by histological observation in small intestine. Photo-micrographic analysis of small intestine depicts that 5-FU treatment resulted in massive inflammatory response characterized by increased inflammatory cell infiltration in both mucosal and sub-mucosal regions. Additionally, massive deformities of villi including shortening of height, loss and atrophy, and marked disorganization of crypts and accumulation of fluids was also observed in 5-FU treated animals as compared to vehicle treated animals. Supplementation of vit.C along with 5-FU attenuated the above mentioned histological deformities of small intestine induced by 5-FU injection. There was no remarkable histological change observed in the animals treated with only vit.C and also in the animals treated with vehicle (Fig. 6). Original magnification: 4× & 10×.
Fig. 6.
Effect of vit. C treatment on 5-FU induced histological alterations in small intestine. Representative photomicrographs (magnification 4× & 10×; scale bar-100 μm) depicting histology analysis, (A) only vehicle treated animals showing normal histology, (C) 5-FU treated animals showing marked histological deformities, (E) Vit. C + 5-FU treated animals showing suppressed histological alterations and (G) Vit. C only treated animals showing normal histology. Insets (B, D, F & H) at the upper panel show a magnified view (10× magnifications) of the insets (A, C, E & G) showed at the lower panel (4× magnifications).
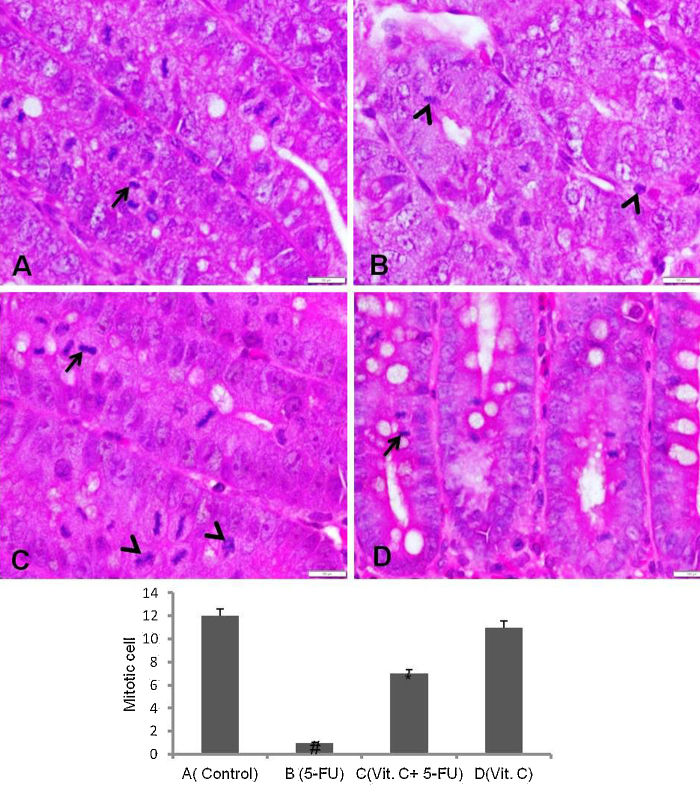
5-FU administration in rats showed intense cell degeneration or proliferation impairment as reflected by the mitotic cells (mitotic bodies) analysis in the crypts of intestine. Number of apoptotic bodies and loss of usual proliferating cells were seen in the intestinal crypts of 5-FU injected rats. Vit. C supplementation attenuated the impairment of cell regeneration as demonstrated by the presence of normal mitotic cells and suppressed apoptosis (Fig. 7).
Fig. 7.
Showing the mitotic and apoptotic bodies of the H & E stained small intestine crypts (magnification 40×, scale bar 100 μm). (A), vehicle treated animals showing normal mitotic cells (arrow), (B) 5-FU treated animals depicting no intact mitotic cells and number of apoptotic bodies (arrow head), (C) Vit. C + 5-FU treated animals showing renewal of mitotic cells (arrow) and suppressed apoptotic bodies (arrow head) and (D) only Vit. C treated animals showing normal mitotic cells with no apoptotic bodies.
4. Discussion
The findings of this study showed that vit. C has strong preventive potential on gastrointestinal ailments caused by anti-neoplastic drug 5-FU as evident by its inhibitory action on biochemical, molecular and histological markers.
GI mucositis is one of the most frequent dose limiting and costly toxic effects of cancer therapy because it causes major hindrance in tumor treatment and also play vital role in cancer related morbidity and mortality [11], [17], [26]. Although, extensive efforts have been made to explore the pathogenesis, the underlying mechanism of mucositis is not fully revealed. Gastro intestinal damage induced by anti-neoplastic drugs is a multistep process which includes direct DNA damage to normal intestinal proliferating cells, over production of reactive oxygen species, up regulation of different transcription factors, ulceration and healing [31]. Reactive oxygen species and inflammatory mediators are the key elements responsible for the initial injuries to intestinal mucosa [22], [32].
Anti-neoplastic drug induced oxidative stress plays major role in the development of most of the adverse effects including mucositis via modulating different signaling pathways which ultimately lead to abnormal cellular integrity and functioning [6], [27], [32], [39]. Here in the current study we observed the elevated level of oxidative stress marker after 5-FU injection which supports the previous findings. Here we found that administration with vit. C inhibits 5-FU induced lipid peroxidation, a well-known marker of oxidative stress suggesting that supplementation with antioxidant could enhance the therapeutic potential of chemotherapy.
Since, role of redox sensitive transcription factors like NF-kB have been well explored in the development of adverse effects associated with chemotherapy so, in the current study we have investigated the modulatory potential of vit. C on nuclear translocation of NF-kB.
NF-kB, a ubiquitous redox-sensitive transcription factor which regulates the functioning of almost all the cellular activities including the genes controlling inflammation, apoptosis, cell division, differentiation and development [37]. In normal or resting state NF-kB localized in cytoplasm but under stress conditions it translocates into nucleus and regulates the expression of downstream targets. A number of stimuli can activate the nuclear translocation of NF-kB. Activation of NF-kB modulates the expression of more than hundred genes resulting in accumulation of various type of biologically active proteins including COX-2 and proinflammatory cytokines and ultimately leads to gastrointestinal damage [4], [29], [30], [32].
Here we have observed that administration of vit. C has diminished the activation of NF-kB which reflects the anti-inflammatory potential of vit. C. Previous research findings also support the modulatory effect of vit. C on NF-kB activation [2], [28], [36]. The exact underlying mechanism about the modulatory action of vit.C on the nuclear translocation of NF-kB is not well explained. NF-kB is a redox sensitive transcription factor and alteration in redox state leads to its activation. Vit. C has well known anti-oxidant potential and the modulatory action of vit.C on NF-kB may be due to its quenching effect on ROS and so the inhibition of IkB kinase (IKK) activation which lead to dissociation of IkB (Inhibitor of kB).
Inhibition of COX-2, a stress response protein, by vit. C administration further supports its preventive potential against anti-neoplastic drug induced gastrointestinal toxicity. Role of COX-2 has been well documented in the development of most of the chronic human diseases and pathological conditions. Elevated expression of COX-2 in the intestine was detected after 5-FU injection which is in agreement with previous findings [12]. Since COX-2 is a stress response protein, a number of stimuli such as ROS, proinflammatory cytokines generated due to 5-FU also up regulate the expression of COX-2 [21]. Up regulation of COX-2 leads to overproduction of prostaglandins and expression of matrix metalloproteinases (MMPs) which results in breakdown of collagen, epithelial basement membrane, edema formation and ultimately tissue injury [8], [32], [38]. Here we found that vit. C administration has suppressed 5-FU induced COX-2 expression in small intestine which support it's anti-inflammatory and thus anti-mucositis potential which is in accordance with previous findings [18], [23].
Evaluation of MPO activity further supported the anti-inflammatory potential of vit.C. MPO is a key proinflammatory enzyme secreted by inflammatory cells play important role in cellular protection from microbial infection by forming hypochlorous acid/hypochlorite and other toxic moieties. Various research findings demonstrate that elevated MPO activity is linked with development of most of the chronic human diseases [7], [19], [25]. Remarkably increased MPO activity after 5-FU injection suggests its role in the gastrointestinal damage and vit. C supplementation attenuated the elevated MPO activity which shows its strong anti-inflammatory and anti-oxidant potential.
Histopatholical observations further supported the attenuative potential of vit.C against anti-neoplastic drug 5-FU induced intestinal damage. Massive histological deformities like loss of villus architecture including shortening of villi with blunting and fusion, disorganization of crypts, abundance of inflammatory cells, cellular loss and impairment of cellular renewal or regeneration was seen in small intestine after 5-FU injection. While vit.C administration suppressed 5-FU induced histological deformities and this action of vit.C supports its preventive potential. In addition, biochemical and molecular findings also supports the histological observations. The overall findings of the current study provides important clue about the attenuative role of vit.C against 5-FU induced GI toxicity. Dissection of deep underlying molecular mechanism is important to further strengthen the data for preventive potential of vit. C by studying various redox sensitive transcription factors, cytokines, apoptotic and anti-apoptotic markers.
In conclusion, findings of the current study demonstrate that vit.C has shown strong potential against antineoplastic drug 5-FU induced gastrointestinal damage. Additional studies are warranted to further support the attenuative potential of vit.C against the anti-neoplastic drug 5-FU induced gastrointestinal damage by studying deep molecular mechanisms.
Conflict of interest
The Author(s) declare(s) that there is no conflict of interest.
Acknowledgement
Authors thank the Research Centre, PSMMC, kingdom of Saudi Arabia for financial support to carry out this work.
References
- 1.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982;78:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 2.Cárcamo J.M., Pedraza A., Bórquez-Ojeda O., Golde D.W. Vitamin C suppresses TNFα-induced NFB activation by inhibiting I(Bα phosphorylation. Biochemistry. 2002;41:12995–13002. doi: 10.1021/bi0263210. [DOI] [PubMed] [Google Scholar]
- 3.Chambial S., Dwivedi S., Shukla K.K., John P.J., Sharma P. Vitamin C in disease prevention and cure: an overview. Indian J. Clin. Biochem. 2013;28:314–328. doi: 10.1007/s12291-013-0375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang C.-T., Ho T.-Y., Lin H., Liang J.-A., Huang H.-C., Li C.-C., Lo H.-Y., Wu S.-L., Huang Y.-F., Hsiang C.-Y. 5-Fluorouracil induced intestinal mucositis via nuclear factor-(B ctivation by transcriptomic analysis and in vivo bioluminescence imaging. PLoS One. 2012;7:e31808. doi: 10.1371/journal.pone.0031808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y., Luo G., Yuan J., Wang Y., Yang X., Wang X., Li G., Liu Z., Zhong N. Vitamin C mitigates oxidative stress and tumor necrosis factor-alpha in severe community-acquired pneumonia and LPS-induced macrophages. Mediators Inflamm. 2014;2014:426740. doi: 10.1155/2014/426740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conklin K.A. Chemotherapy-associated oxidative stress: impact on chemotherapeutic effectiveness. Integr. Cancer Ther. 2004;3:294–300. doi: 10.1177/1534735404270335. [DOI] [PubMed] [Google Scholar]
- 7.Davies M.J., Hawkins C.L., Pattison D.I., Rees M.D. Mammalian heme peroxidases: from molecular mechanisms to health implications. Antioxid. Redox Signaling. 2008;10:1199–1234. doi: 10.1089/ars.2007.1927. [DOI] [PubMed] [Google Scholar]
- 8.Dubois R.N., Abramson S.B., Crofford L., Gupta R.A., Simon L.S., Van De Putte L.B., Lipsky P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- 9.Durak D., Uzun F.G., Kalender S., Ogutcu A., Uzunhisarcikli M., Kalender Y. Malathioninduced oxidative stress in human erythrocytes and the protective effect of vitamins C and E in vitro. Environ. Toxicol. 2009;24:235–242. doi: 10.1002/tox.20423. [DOI] [PubMed] [Google Scholar]
- 11.Elting L.S., Cooksley C., Chambers M., Cantor S.B., Manzullo E., Rubenstein E.B. The burdens of cancer therapy. Cancer. 2003;98:1531–1539. doi: 10.1002/cncr.11671. [DOI] [PubMed] [Google Scholar]
- 12.Freitas A.P.F., Bitencourt F.S., Brito G.A.C., de Alencar N.M.N., Ribeiro R.A., Lima-Júnior R.C.P., Ramos M.V., Vale M.L. Protein fraction of Calotropis procera latex protects against 5-fluorouracil-induced oral mucositis associated with downregulation of pivotal pro-inflammatory mediators. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2012;385:981–990. doi: 10.1007/s00210-012-0778-3. [DOI] [PubMed] [Google Scholar]
- 13.Fritz H., Flower G., Weeks L., Cooley K., Callachan M., McGowan J., Skidmore B., Kirchner L., Seely D. Intravenous Vitamin C and cancer: a systematic review. Integr. Cancer Ther. 2014;13:280–300. doi: 10.1177/1534735414534463. [DOI] [PubMed] [Google Scholar]
- 14.Fukui H., Iwahashi H., Endoh S., Nishio K., Yoshida Y., Hagihara Y., Horie M. Ascorbic acid attenuates acute pulmonary oxidative stress and inflammation caused by zinc oxide nanoparticles. J. Occup. Health. 2015;57:118–125. doi: 10.1539/joh.14-0161-OA. [DOI] [PubMed] [Google Scholar]
- 15.Harris D.J. Cancer treatment-induced mucositis pain: strategies for assessment and management. Ther. clinical Risk Manage. 2006;2:251. doi: 10.2147/tcrm.2006.2.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoda Q., Sinha S. Vitamin C-mediated minimisation of Rogor-induced genotoxicity. Mutat. Res. Genet. Toxicol. 1993;299:29–36. doi: 10.1016/0165-1218(93)90116-u. [DOI] [PubMed] [Google Scholar]
- 17.Keefe D.M., Gibson R.J. The combination of oral and small intestinal mucositis, pediatrics and biomarkers: a particularly tricky problem! Cancer Biol. Ther. 2006;5:1282–1284. doi: 10.4161/cbt.5.10.3508. [DOI] [PubMed] [Google Scholar]
- 18.Kim N., Kim H., Kong M., Bae S., Kim S., Lee N., Cho J., Lee K., Kim R., Hwang Y.i. Vitamin C down‐regulates VEGF production in B16F10 murine melanoma cells via the suppression of p42/44 MAPK activation. J. Cell. Biochem. 2011;112:894–901. doi: 10.1002/jcb.22997. [DOI] [PubMed] [Google Scholar]
- 19.Klebanoff S.J. Myeloperoxidase: friend and foe. J. Leukocyte Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 20.Lalla R.V., Peterson D.E. Treatment of mucositis, including new medications. Cancer J. 2006;12:348–354. doi: 10.1097/00130404-200609000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Lalla R.V., Pilbeam C.C., Walsh S.J., Sonis S.T., Keefe D.M., Peterson D.E. Role of the cyclooxygenase pathway in chemotherapy-induced oral mucositis: a pilot study. Supportive Care Cancer. 2010;18:95–103. doi: 10.1007/s00520-009-0635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalla R.V., Schubert M.M., Bensadoun R.-J., Keefe D. Anti-inflammatory agents in the management of alimentary mucositis. Supportive Care Cancer. 2006;14:558–565. doi: 10.1007/s00520-006-0050-9. [DOI] [PubMed] [Google Scholar]
- 23.Lee S.K., Kang J.S., Jung D.J., Hur D.Y., Kim J.E., Hahm E., Bae S., Kim H.W., Kim D., Cho B.J. Vitamin C suppresses proliferation of the human melanoma cell SK-MEL-2 through the inhibition of cyclooxygenase-2 (COX-2) expression and the modulation of insulin-like growth factor II (IGF-II) production. J. Cell. Physiol. 2008;216:180–188. doi: 10.1002/jcp.21391. [DOI] [PubMed] [Google Scholar]
- 24.Longley D.B., Harkin D.P., Johnston P.G. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 25.Mashtoub S., Tran C.D., Howarth G.S. Emu oil expedites small intestinal repair following 5-fluorouracil-induced mucositis in rats. Exp. Biol. Med. 2013;238:1305–1317. doi: 10.1177/1535370213493718. [DOI] [PubMed] [Google Scholar]
- 26.Peterson D.E., Keefe D.M., Sonis S.T. American Society of Clinical Oncology Educational Book/ASCO. American Society of Clinical Oncology; 2011. New frontiers in mucositis; pp. 545–551. Meeting. [DOI] [PubMed] [Google Scholar]
- 27.Shiota A., Hada T., Baba T., Sato M., Yamanaka-Okumura H., Yamamoto H., Taketani Y., Takeda E. Protective effects of glycoglycerolipids extracted from spinach on 5-fluorouracil induced intestinal mucosal injury. J. Med. Invest. 2010;57:314–320. doi: 10.2152/jmi.57.314. [DOI] [PubMed] [Google Scholar]
- 28.Son E.-W., Mo S.-J., Rhee D.-K., Pyo S. Vitamin C blocks TNF-α-induced NF-kB activation and ICAM-1 expression in human neuroblastoma cells. Arch. Pharmacal Res. 2004;27:1073–1079. doi: 10.1007/BF02975434. [DOI] [PubMed] [Google Scholar]
- 29.Sonis S., O’Donnell K., Popat R., Bragdon C., Phelan S., Cocks D., Epstein J. The relationship between mucosal cyclooxygenase-2 (COX-2) expression and experimental radiation-induced mucositis. Oral Oncol. 2004;40:170–176. doi: 10.1016/s1368-8375(03)00148-9. [DOI] [PubMed] [Google Scholar]
- 30.Sonis S.T. The biologic role for nuclear factor-kappaB in disease and its potential involvement in mucosal injury associated with anti-neoplastic therapy. Crit. Rev. Oral Biol. Med. 2002;13:380–389. doi: 10.1177/154411130201300502. [DOI] [PubMed] [Google Scholar]
- 31.Sonis S.T. A biological approach to mucositis. J. Support Oncol. 2004;2:21–32. [PubMed] [Google Scholar]
- 32.Sonis S.T. The pathobiology of mucositis. Nat. Rev. Cancer. 2004;4:277–284. doi: 10.1038/nrc1318. [DOI] [PubMed] [Google Scholar]
- 33.Sorice A., Guerriero E., Capone F., Colonna G., Castello G., Costantini S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev. Med. Chem. 2014;14:444–452. doi: 10.2174/1389557514666140428112602. [DOI] [PubMed] [Google Scholar]
- 34.Tooley K.L., Howarth G.S., Butler R.N. Mucositis and non-invasive markers of small intestinal function. Cancer Biol. Ther. 2009;8:753–758. doi: 10.4161/cbt.8.9.8232. [DOI] [PubMed] [Google Scholar]
- 35.Utley H.G., Bernheim F., Hochstein P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967;118:29–32. [Google Scholar]
- 36.Wang W.G., Xiu R.J., Xu Z.W., Yin Y.X., Feng Y., Cao X.C., Wang P.S. Protective effects of Vitamin C against spinal cord injury-induced renal damage through suppression of NF-κB and proinflammatory cytokines. Neurol. Sci. 2015;36:521–526. doi: 10.1007/s10072-014-1965-4. [DOI] [PubMed] [Google Scholar]
- 37.Wu J.T., Kral J.G. The NF-(B/I(B signaling system: a molecular target in breast cancer therapy. J. Surg. Res. 2005;123:158–169. doi: 10.1016/j.jss.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 38.Yeoh A.S., Gibson R.J., Yeoh E.E., Bowen J.M., Stringer A.M., Giam K.A., Keefe D.M. A novel animal model to investigate fractionated radiotherapy-induced alimentary mucositis: the role of apoptosis, p53, nuclear factor-(B, COX-1, and COX-2. Mol. Cancer Ther. 2007;6:2319–2327. doi: 10.1158/1535-7163.MCT-07-0113. [DOI] [PubMed] [Google Scholar]
- 39.Yoshino F., Yoshida A., Nakajima A., Wada-Takahashi S., Takahashi S.-s., Lee M.C.-i. . Alteration of the redox state with reactive oxygen species for 5-fluorouracil-induced oral mucositis in hamsters. PloS One. 2013;8:e82834. doi: 10.1371/journal.pone.0082834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang K., Li Y., Cheng X., Liu L., Bai W., Guo W., Wu L., Zuo L. Cross-over study of influence of oral vitamin C supplementation on inflammatory status in maintenance hemodialysis patients. BMC Nephrol. 2013;14:252. doi: 10.1186/1471-2369-14-252. [DOI] [PMC free article] [PubMed] [Google Scholar]