Abstract
The prototype dioxin congener 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is known to exert anti-estrogenic effects via activation of the aryl hydrocarbon receptor (AhR) by interfering with the regulation of oestrogen homeostasis and the estrogen receptor α (ERα) signalling pathway. The AhR/ER cross-talk is considered to play a crucial role in TCDD- and E2-dependent mechanisms of carcinogenesis, though the concerted mechanism of action in the liver is not yet elucidated. The present study investigated TCDD's impact on the transcriptional cross-talk between AhR and ERα and its modulation by 17β-estradiol (E2) in the human hepatoma cell line HepG2, which is AhR-responsive but ERα-negative. Transient transfection assays with co-transfection of hERα and supplementation of receptor antagonists showed anti-estrogenic action of TCDD via down-regulation of E2-induced ERα signaling. In contrast, enhancement of AhR signaling dependent on ERα was observed providing evidence for increased cytochrome P450 (CYP) induction to promote E2 metabolism. However, relative mRNA levels of major E2-metabolizing CYP1A1 and 1B1 and the main E2-detoxifying catechol-O-methyltransferase were not affected by the co-treatments. This study provides new evidence of a TCDD-activated AhR-mediated molecular AhR/ERα cross-talk mechanism at transcriptional level via indirect inhibition of ERα and enhanced transcriptional activity of AhR in HepG2 cells.
Abbreviations: AhR, aryl hydrocarbon receptor; COMT, catechol-O-methyltransferase; CPRG, chlorophenol red β-d-galactopyranoside; Ct, cycle threshold; CYP, cytochrome P450; DMSO, dimethyl sulfoxide; E2, 17β-estradiol; E, strogen receptor; ERE, estrogen response element; α-NF, α-naphthoflavone; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin; XRE, xenobiotic response element
Keywords: TCDD, Dioxin, 17β-estradiol, Estrogen receptor, AhR, Human hepatoma cell line HepG2, Gene reporter assay
1. Introduction
The prototype dioxin congener 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) is a highly toxic and persistent organic pollutant, which is ubiquitously found in the environment. There is extensive evidence in vivo and in vitro that TCDD exerts anti-estrogenic effects via activation of the arylhydrocarbon receptor (AhR) by interfering with the regulation of estrogen homeostasis and the estrogen receptor α (ERα) signaling pathway (reviewed in [1]). A number of mechanisms were proposed to describe dioxin-mediated AhR/ERα cross-talk ([2], [3]).
It was hypothesized that TCDD may interfere with the regulation of estrogen homeostasis resulting in reduced concentrations of circulating estrogens. This is thought to result from enhanced oxidative metabolism of 17β-estradiol (E2) via AhR-mediated induction of cytochromes P450 (CYPs), particularly CYP1A1 and CYP1B1 [4]. The latter also serve as general surrogate markers for AhR activation [5].
Furthermore, TCDD may also prevent binding of the E2/ER-complex to the estrogen response element (ERE) and instead recruit the hormone receptor to AhR target genes via an indirect protein-protein interaction [6], [7]. It was shown that E2-dependent expression of genes and proteins such as pS2, cathepsin D and vitellogenin were inhibited by the action of TCDD [8]. Furthermore, TCDD was reported to inhibit E2-induced cell proliferation and DNA synthesis by specifically blocking the E2-induced transition from G1 to S phase [9]. TCDD also induced the degradation of ERα through activation of the proteasome as observed in breast cancer cell lines [10] and it mediated the down-regulation of ER levels via a repressor site in the promoter region of ER-regulated genes [3]. Most of these studies were performed using breast cancer cell lines or other hormone-related cells and focused on AhR agonists which directly affected ERα-dependent pathways [11], [12], [13]. In contrast, TCDD did not show direct activation of ERα in a competitive binding assay [14]. TCDD has been classified as a human carcinogen by the International Agency for Research on Cancer [15], its carcinogenic effect in rodent liver being most probably related to its mode of action as a liver tumour promoter [5]. AhR signaling-dependent suppression of apoptosis of preneoplastic hepatocytes seems to play a central role in this effect [16].
Interestingly, TCDD was found to be a more potent liver carcinogen in female rats compared to male rats and it reduced age-related spontaneous hormone-dependent tumours, suggesting a role of estrogens [17], [18]. Exposure to E2 is primarily associated with increased risk of breast cancer [19]. However, E2 was also related to liver carcinogenesis and it has been postulated to promote ER-mediated growth stimulation via co-mitogenic effects [20]. In rat primary hepatocytes in culture a co-mitogenic action of TCDD and estrogens was reported [21].
Altogether, the AhR/ER cross-talk is considered to play a crucial role in TCDD- and E2-dependent mechanisms of liver carcinogenesis, though the exact mechanism of action in the liver is not yet elucidated. Furthermore, the metabolism of estrogens via CYPs primarily occurs in the liver [4]. In this study TCDD's impact on the transcriptional cross-talk between AhR and ERα and its modulation by E2 was investigated in the human hepatoma cell line HepG2, which is AhR responsive but deficient for ERα [22]. Transient transfection assays were performed using the luciferase gene regulated by either the ERE or the dioxin response element (XRE) with or without co-transfection of a human ERα expression vector. Furthermore, differential mRNA expression of major E2-metabolizing CYPs and the main E2-detoxifying gene catechol-O-methyltransferase (COMT) was assessed in the presence or absence of ERα.
2. Materials and methods
2.1. Cell culture and treatments
The human hepatoma cell line HepG2 (European Collection of Cell Cultures, ECACC No 85011430) was grown in phenol red-free Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% foetal bovine serum (FBS), 1% penicillin/streptomycin, and 4 mM l-glutamine (cell culture media and supplementations were obtained from PAA Laboratories) maintained at 37 °C and 5% CO2. Cells were seeded in culture medium with 10% FBS (or 10% dextran-coated charcoal treated FBS (DCC-FBS) for transfection assays) for 24 h. HepG2 cells were either placed on 60 mm-diameter plates (0.375 × 106 cells/mL) for RNA extraction or on 24 well-plates (0.12 × 106 cells/mL) for transfection assays and RNA extraction from transfected cells. Cells were treated with TCDD 1 nM (Promochem) and/or E2 10 nM (Sigma–Aldrich) dissolved in dimethyl sulfoxide (DMSO, max. 0.25%; Sigma–Aldrich) in complete phenol red-free DMEM with 0.5% FBS (or without FBS for transfection assays). Additionally, simultaneous treatments with the AhR antagonist α-naphthoflavone (α-NF, Sigma–Aldrich) or the pure anti-estrogen ZK 191 703 (kindly provided by Dr. Karl-Heinrich Fritzemeier, Bayer-Schering, Germany) were performed.
2.2. Transient transfection
HepG2 cells were transiently transfected with XRE- or ERE-dependent luminescent reporter genes (ERE-TK-Luc or XRE-Luc) using ExGen 500 transfection agent (Euromedex) and co-transfected or not with a hERα expression vector. Plasmids pCMVβ-Gal and pSG5 served as control plasmids (kindly supplied by Dr. M. Cherkaoui-Malki, LBMN, University of Burgundy, Dijon, France). Plasmids ERE-TK-Luc and pRST7-hERα were kindly provided by Dr. D. McDonnell (Ligand Pharmaceutical, San Diego, USA). The reporter gene plasmid pGL3-XRE-Luc was previously described [23], [24].
Transient transfections were performed following manufacturer's instructions. Briefly, plasmid mixes were prepared as follows: 100 ng ERE-TK-Luc or XRE-Luc, 100 ng hERα, 100 ng of pCMVβ and pSG5 to a final concentration of 0.5 μg DNA. For transfections omitting hERα, the amount of pSG5 was adjusted accordingly. The Exgen 500/DNA mixture was added to appropriate amounts of phenol red-free Opti-MEM (Invitrogen) and transferred to the wells. After transfection medium was removed and replaced by fresh DMEM medium (without DCC-FBS) containing test substances or the solvent control. E2 10 nM and TCDD 1 nM served as the positive controls for ERE- or XRE-mediated luciferase activity, respectively. After 20 h treatment cells were lysed with Reporter Lysis Buffer 1× (Promega). The microplate was then frozen at −80 °C for at least 30 min. Cells were scraped off, transferred into microtubes, and submitted to three sequential freezing-thawing cycles in liquid nitrogen and at 37 °C. Microtubes were centrifuged (5 min, 10,000 × g, room temperature) and 10 μL of the lysate were pipetted into an opaque white 96-well plate. A volume of 50 μL luciferase assay reagent (Promega) was added to each well, the plate covered with an adhesive seal and immediately read in a microplate luminometer (TopCountNT, Packard). The β-galactosidase activity was determined using chlorophenol-red β-d-galactopyranoside (CPRG) (Roche), and the chlorophenol red product was measured on a microplate spectrophotometer at 570 nm (MRX Dynex). Protein levels were measured on a spectrophotometer at 595 nm (MRX Dynex) according to the Bradford method [25]. Luciferase activity was normalized against β-galactosidase activity and protein contents and related to the respective positive controls.
2.3. RNA extraction and real-time RT PCR
Total RNA was isolated with the RNeasy Mini Kit (Qiagen). Samples were quantified spectrophotometrically via a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific). RNA (0.5 μg) was reverse-transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad) following DNase treatment (Desoxyribonuclease I, Amplification Grade, Invitrogen). Real-time PCR was performed in a total volume of 25 μL per reaction on an iCycler iQ Real-Time PCR Detection System with iCycler Software version 3.1 (Bio-Rad). Each PCR reaction contained 25 ng of the diluted cDNA, 12.5 μL of AbsoluteTM QPCR SYBR® Green Fluorescein Mix (Thermo Fisher Scientific), 200 nM of forward and reverse primers and pure water (qsp 25 μl). When a fluorogenic probe was used qPCR Master Mix no ROX (Eurogentec, Belgium) with a primer mix containing the primer pairs (300 nM/well) and the fluorogenic probe (100 nM/well) were added instead. Primer sets were designed using the free software primer 3 (http://frodo.wi.mit.edu/) and purchased from MWG. Fluorogenic probes were designed and obtained from Eurogentec (primer sequences see Table 1). Optimized PCR consisted of 45 cycles at 95 °C for 15 s followed by amplification at 58–60 °C for 30 or 60 s. For real-time PCR using SYBR Green mix, a melting curve emerging in a gradient starting at the respective annealing temperature up to 90 °C in increasing steps of 0.5 °C verified the single PCR product. Expression of the target gene relative to the estradiol-independent 36B4 reference gene [26] normalized to the solvent control was calculated using the ΔΔCt comparative method [27].
Table 1.
Sequences of primers and fluorogenic probes used for amplification of mRNA gene products in Real-time RT PCR.
| Gene | Primer sequence (5′–3′) |
|---|---|
| hCYP1B1 | Sense: GCT AAA CCC GCT GTC CAT CC |
| Anti-sense: CCG CCT CCG TTG CCT CAG | |
| Probe: 6-FAM-ACC ACG CTC CTG CTA CTC CTG TCG G-TAMRA | |
| hCYP1A1 | Sense: CCT CTT TGG AGC TGG GTT TG |
| Anti-sense: CCT GTG GGG GAT GGT GAA | |
| h36B4 | Sense: ACT TGC TGA AAA GGT CAA GG |
| Anti-sense: TTC CTT GGC TTC AAC CTT AG | |
| hAhR | Sense: TCC ACA GTT GGC TTT GTT TGC |
| Anti-sense: TGT GAA GTC CAG TTT GTG TTT GG | |
| Probe: 6-FAM-CTA CTC CAC TTC AGC CAC CGT CCATCCT-TAMRA | |
| hERα | Sense: AAG ATC AAC GAC ACT TTG ATC CAC |
| Anti-sense: ATG CCT TTG TTA CTC ATG TGC C | |
| Probe: 6-FAM-CTG GCC CAG CTC CTC CTC ATC CT-TAMRA | |
| hCOMT | Sense: ACA GTG CTA CTG GCT GGC TGA CAA |
| Anti-sense: GGC TGT CTT GGA ACT CAC TT | |
2.4. Statistical analysis
Experiments were performed at least in triplicates. Results are presented as mean ± SD. The One-way ANOVA followed by Tukey's post test was performed to analyze the reporter gene data. For the statistical analysis of the gene expression data the Two-way ANOVA followed by the Bonferroni post test was applied. For graphs and statistics the software GraphPad Prism 5 for Windows was used.
3. Results
3.1. Effects off ERα on ERE-and XRE-dependent transcriptional activity
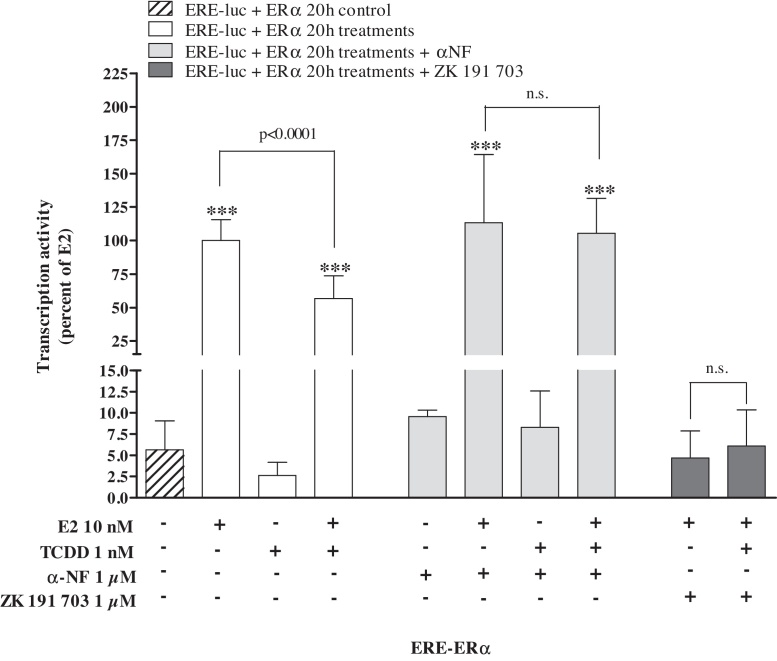
HepG2 were transiently co-transfected with ERE-TK-LUC and the hERα expression vector. E2 (10 nM) resulted in a significant induction of reporter gene activity. TCDD (1 nM) significantly decreased E2-induced ERE-mediated activity by about 50%, whereas TCDD alone had no effect on ERE-mediated transcription (Fig. 1). The partial AhR antagonist α-naphthoflavone reversed TCDD's anti-estrogenic action and the pure estrogen antagonist ZK 191 703 completely blocked the estrogenic action of E2.
Fig. 1.
Effects of ERα on ER-mediated transcription. HepG2 cells were transiently transfected with ERE-TK-LUC and co-transfected with human ERα expression plasmid as described in Section 2. Cells were treated with TCDD and/or E2 over a period of 20 h. An induction equal to 100% was attributed to the luciferase activity of treatment with E2 and all results were expressed as a percentage of the luciferase activity of this positive control (corresponding to 111,133 ± 7564 cps). Mean ± SD (n ≥ 3); One-way ANOVA followed by Tukey's post test: significantly different from solvent control (DMSO 0.2% max.): ***p ≤ 0.0001; significant difference between respective E2 and E2/TCDD treatments as indicated; n.s.: not significant.
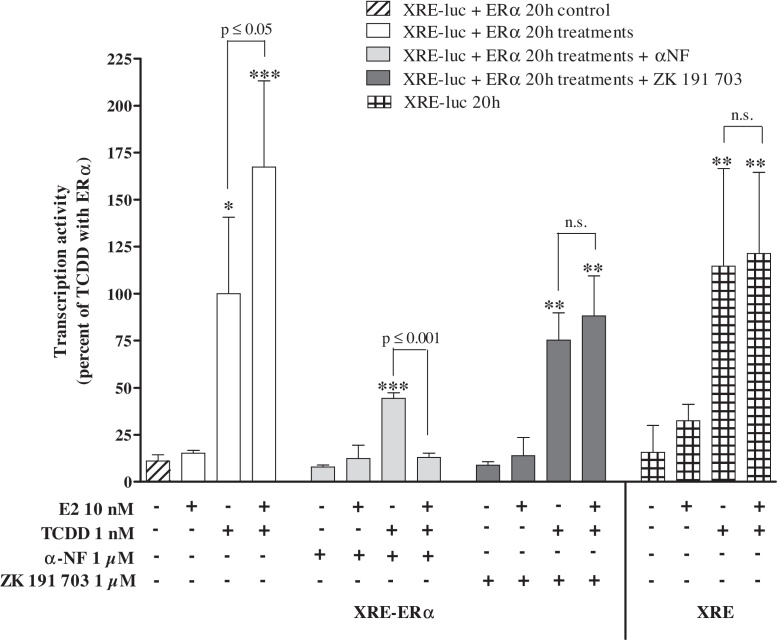
In cells lacking the transfected ERα none of the tested compounds had any effect on reporter gene expression (data not shown). In the same way, XRE-luc reporter was co-transfected or not with hERα into HepG2 cells (Fig. 2). E2 (10 nM) significantly enhanced TCDD-induced AhR-regulated transcription up to 1.6-fold in co-transfected cells, whereas E2 alone had no effect on transcriptional activity via the AhR. By adding the anti-estrogen ZK 191 703, this enhancement by the co-treatment was abolished, while the XRE-driven increase by TCDD was still observed. The AhR-mediated action of TCDD was partially inhibited by the AhR antagonist α-naphthoflavone, while addition of E2 to TCDD/α-naphthoflavone further enhanced this inhibitory effect. Application of the anti-oestrogen ZK 191 703 or experiments with XRE-luc without exogenous ERα reversed the potentiating effect by E2. In any case basal levels of reporter plasmid (ERE or XRE)-mediated activity were not influenced by transfection of ERα or solvent treatment.
Fig. 2.
Effects of ERα on AhR-mediated transcription. HepG2 cells were transiently transfected with pGL3-XRE and co-transfected or not with human ERα expression plasmid as described in Section 2. Cells were treated with TCDD and/or E2 over a period of 20 h. An induction equal to 100% was attributed to the luciferase activity of treatment with TCDD in ERα-transfected cells and all results were expressed as a percentage of the luciferase activity of this positive control (corresponding to 1,740,574 ± 484,008 cps). Mean ± SD (n ≥ 3); One-way ANOVA followed by Tukey's post test: significantly different from solvent control (DMSO 0.2% max.): *p ≤ 0.01, **p ≤ 0.001, ***p ≤ 0.0001; significant difference between respective TCDD and E2/TCDD treatments as indicated; n.s.: not significant.
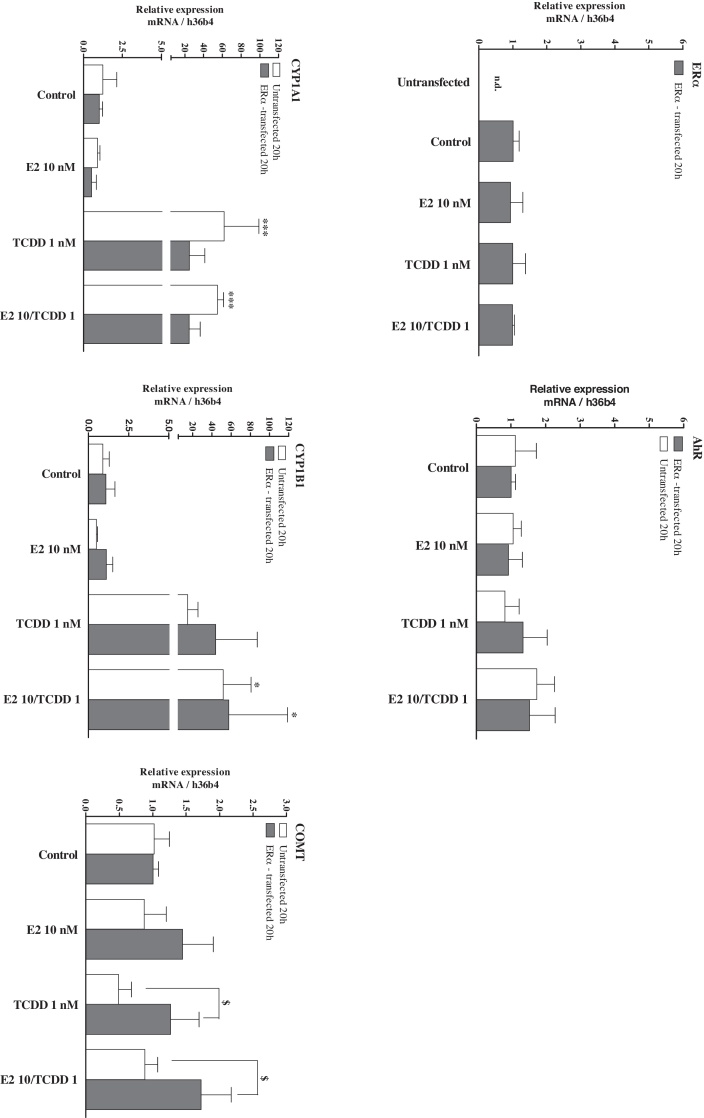
3.2. Relative expression of major E2-metabolizing CYPs and detoxifying COMT after treatment with TCDD and/or E2 in the presence and absence of ERα
Receptor transcript levels for ERα and AhR were not changed with treatments (Fig. 3). With regard to relative CYP expression (normalized to respective controls) there was no difference in response to TCDD between non-transfected and ERα-transfected HepG2 cells. TCDD (1 nM) induced both CYP1A1 and CYP1B1 mRNA, whereas the latter response was less pronounced. E2 alone had no impact on CYP1A1 and CYP1B1 mRNA compared with solvent control. Furthermore, E2 showed no modulating effect on TCDD-induced CYP expression.
Fig. 3.
Real-time RT PCR analysis of ERα, AhR, CYP1A1, CYP1B1, and COMT mRNA in non-transfected and ERα-transfected HepG2. Effects of TCDD 1 nM and/or E2 10 nM were assayed after 20 h exposure. Mean ± SD (n ≥ 3); Two-way ANOVA followed by the Bonferroni post test: statistically significant from respective transfected or untransfected solvent control (DMSO 0.25%): *p ≤ 0.05, ***p ≤ 0.001; significant difference between respective treatment of transfected and non-transfected cells: $p ≤ 0.05; n.d.: not detected.
The treatments had no significant influence on COMT mRNA levels (Fig. 3). However, transcript levels were significantly different in the TCDD treatment and the co-treatment with and without ERα transfection.
4. Discussion
4.1. Relevance of AhR/ER cross-talk for the liver
In this study a well-known in vitro human liver cancer cell model, the HepG2 hepatoma cell line, was used to investigate the mode of action of the cross-talk between ERα and AhR following treatment with E2 and/or TCDD. Transient transfection assay responses and mRNA analysis confirmed HepG2 cell line to be AhR-responsive. However, cells were ERα deficient, which is in accordance with Iwanari et al. [22]. Thus, to analyze receptor interaction in detail, the study was performed in hERα overexpressed HepG2 cells after transient transfection.
Though for some less potent AhR agonists such as 3-methylcholanthrene a direct activation of ERα resulted in an estrogenic response, TCDD has been reported to be an indirect ERα inhibitor and exert anti-estrogenic effects [6], [11], [12], [13], [14]. Previous studies on the AhR/ER cross-talk mainly focused on investigating these effects in breast cancer cell lines. However, the liver is one of the major target organs of TCDD's toxic action mediated via AhR. Thus, the focus of this research work was put on the liver since the liver is also one major site of estradiol metabolism and the ERα is highly expressed [28].
4.2. AhR/ER cross-talk: TCDD's anti-estrogenic action on transcriptional activity of ER
In HepG2 cells TCDD led to anti-estrogenic activity by reducing E2-mediated ERα signalling in the ERE-regulated reporter gene activity assay only in the presence of ERα. The complete ER antagonist ZK 191 703 totally blocked the estrogenic response and application of the partial AhR antagonist α-naphthoflavone [29] reversed TCDD's anti-estrogenic repression of AhR-dependent reporter gene activity in HepG2 cells. Thus, these results support the hypothesis that the ligand-activated AhR interacts with ERα and represses E2-bound ERα-mediated transcription upon ERE similarly to what is reported in hormone-dependent cell lines [6], [7], [30]. The activation of AhR by TCDD is supposed to be a crucial step in the interaction of AhR/ER, since various experiments in AHR-deficient cell models have failed to demonstrate the modulation of ERα functional activity. In multiple ER-positive breast and endometrial cancer cells TCDD was shown to be strongly anti-estrogenic, such as in MCF-7 breast cancer cells, but also in ER-negative Hepa-1 mouse hepatoma cells transfected with an ERα expression vector [3], [10], [30], [31], [32]. In contrast, in a non-functional AhR mutant Hepa-1 cell line TCDD failed to exert an effect on E2-dependent ER signalling, suggesting an interaction between AhR and ER pathways [30]. Similarly, the expression of E2-responsive genes/proteins and their related activities was decreased in multiple ER-positive breast and endometrial cancer cells after co-treatment of E2 and TCDD and the identification of so-called inhibitory XREs (iXREs) in the critical promoter regions of these E2-responsive genes provided further evidence for the inhibition of E2-dependent target genes via interaction with the activated AhR [3], [31], [32], [33], [34], [35].
4.3. AhR/ER cross-talk: E2-enhanced transcriptional activity of AhR
Reciprocally, HepG2 cells transiently transfected with a XRE-luc reporter showed enhanced TCDD-mediated luciferase activity upon E2 treatment only in the presence of constitutively over-expressed ERα. TCDD alone resulted in increased luciferase activity independent of the ERα. Thus, E2-activated ERα is suggested to interact with XRE-bound AhR, playing a role in the enhancement of AhR signalling. One proposed mechanism of the AhR/ERα cross-talk is the enhanced metabolism of E2 by CYPs, particularly CYP1A1 and 1B1, which are induced by the potent AhR ligand TCDD [3]. Our data are consistent with reports in other cell lines showing a positive effect of ERα on the modulation of AHR-responsiveness [36], [37], [38]. The repression of TCDD-induced luciferase activity by the AhR antagonist α-naphthoflavone suggests a partial inhibition which was however further inhibited in the presence of E2, suggesting an enhancement of the antagonist effect by the co-treatment. Data from Matthews and co-workers in ERα- and AhR-positive MCF-7 and T47D human breast cancer cells revealed that TCDD (10 nM)-bound AhR recruited ERα to the CYP1A1 and CYP1B1 promoters [7]. This promoter occupancy was enhanced by additional treatment with E2 (10 nM). Studies with the AhR-responsive HuH7 human hepatoma cell line lacking ERα revealed that increasing amounts of ERα expression resulted in a concentration-dependent potentiation of TCDD-induced XRE-driven luciferase reporter gene activity after 24 h co-treatment of TCDD with E2 1 nM and 10 nM [7], [39].
4.4. Impact of AHR/ER cross-talk on AhR-responsive gene expression
In a recent study stable siRNA-mediated knockdown of ERα in non-tumorigenic HC11 mouse mammary epithelial cells revealed reduced TCDD-induced CYP1A1 mRNA expression [40]. Despite the increasing effects of TCDD-induced CYP1A1 luciferase activity by E2 in ERα-transfected HepG2 cells neither TCDD-induced CYP1A1 nor CYP1B1 mRNA levels were affected by the co-treatments upon ERα transfection in the present study. This result is an apparent contradiction to the XRE-driven reporter gene data and may be due to the large degree of CYP induction which may activate signalling pathways limiting the overall response or due to the higher sensitivity of the luciferase assay. Alternatively, the ER-dependent added interaction with AhR may be target gene-dependent which requires further clarification.
4.5. Impact of AHR/ER cross-talk on COMT gene expression
In addition to the CYPs, COMT was investigated in HepG2 since changes in its expression may be a limiting factor in the balance of estradiol metabolism. COMT is the major E2 detoxifying enzyme, which catalyses inactivation of the two main reactive catechol estradiol metabolites [41]. In HepG2 cells TCDD alone and the co-treatment slightly increased COMT mRNA in the presence of ERα compared to controls. An estrogenic down regulation of human COMT requiring the presence of ERs was previously described. It has been reported that E2 reduced COMT transcription in ER-positive MCF-7 cells but not in ER-negative HeLA cells [42]. In the AhR-positive but ERα-negative human breast cancer cell line MCF-10F, under conditions in which E2 metabolism has been enhanced by TCDD, increased levels of catechol estrogens and depurinating DNA adducts were detected in the presence of a COMT inhibitor, suggesting the balance of COMT activity as an important factor in cancer initiation [43].
5. Conclusion
A complex AhR/ERα cross-talk at the transcriptional level was demonstrated in the human hepatoma cell line HepG2 applying specifically designed transient transfection assays with co-transfection of hERα and the supplementation of antagonists of both the ERα and AhR receptors. TCDD demonstrated an anti-estrogenic action via down-regulation of the E2-mediated induced ERα-signalling. This anti-estrogenic action is supposed to occur via an indirect activation of ERα since TCDD alone had no effect on ERα-dependent transcriptional activity. At the same time enhanced AhR activation was observed dependent on ERα resulting in enhanced XRE-driven reporter gene expression but not in enhanced expression of the AhR target genes CYP1A1 and 1B1. Thus, concomitant effects of TCDD and E2 resulted in anti-estrogenic activity and an enhancement of certain but not all AhR-dependent transcriptional activities. This study provides further evidence that AhR/ERα cross-talk can play a crucial role in the regulation of estrogen-mediated and TCDD-related mechanism of action in the liver. Different responses in HepG2 cells compared to cells derived from mainly hormone-regulated tissues may indicate that the involved molecular mechanisms of the ER and AhR signaling differ in cell- or tissue-dependent manner such as receptor levels or available co-regulatory proteins that may interact with the receptors.
Overall, HepG2 cell line is an appropriate tool to further elucidate the molecular mechanisms in the liver which are involved in the nuclear receptor interactions. The mechanism of estrogen receptor signaling alteration by TCDD-activated AhR is important to understand the estrogen-related adverse effects of TCDD on the liver as one of its target organs.
Acknowledgement
The authors thank Dr. Hans-Joachim Schmitz at the University of Kaiserslautern for proof reading the article.
Footnotes
Available online 22 October 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.09.016.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Swedenborg E., Pongratz I. AhR and ARNT modulate ER signaling. Toxicology. 2010;268:132–138. doi: 10.1016/j.tox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Matthews J., Gustafsson J.A. Estrogen receptor and aryl hydrocarbon receptor signaling pathways. Nucl. Recept. Signal. 2006;4:e016. doi: 10.1621/nrs.04016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Safe S., Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem. Res. Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- 4.Cavalieri E., Chakravarti D., Guttenplan J., Hart E., Ingle J., Jankowiak R., Muti P., Rogan E., Russo J., Santen R., Sutter T. Catechol estrogen quinones as initiators of breast and other human cancers: implications for biomarkers of susceptibility and cancer prevention. Biochim. Biophys. Acta. 2006;1766:63–78. doi: 10.1016/j.bbcan.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Knerr S., Schrenk D. Carcinogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in experimental models. Mol. Nutr. Food Res. 2006;50:897–907. doi: 10.1002/mnfr.200600006. [DOI] [PubMed] [Google Scholar]
- 6.Beischlag T.V., Perdew G.H. ER alpha-AHR-ARNT protein-protein interactions mediate estradiol-dependent transrepression of dioxin-inducible gene transcription. J. Biol. Chem. 2005;280:21607–21611. doi: 10.1074/jbc.C500090200. [DOI] [PubMed] [Google Scholar]
- 7.Matthews J., Wihlen B., Thomsen J., Gustafsson J.A. Aryl hydrocarbon receptor-mediated transcription: ligand-dependent recruitment of estrogen receptor alpha to 2,3,7,8-tetrachlorodibenzo-p-dioxin-responsive promoters. Mol. Cell. Biol. 2005;25:5317–5328. doi: 10.1128/MCB.25.13.5317-5328.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Safe S. Molecular biology of the Ah receptor and its role in carcinogenesis. Toxicol. Lett. 2001;120:1–7. doi: 10.1016/s0378-4274(01)00301-0. [DOI] [PubMed] [Google Scholar]
- 9.Wang W., Smith R., 3rd, Safe S. Aryl hydrocarbon receptor-mediated antiestrogenicity in MCF-7 cells: modulation of hormone-induced cell cycle enzymes. Arch. Biochem. Biophys. 1998;356:239–248. doi: 10.1006/abbi.1998.0782. [DOI] [PubMed] [Google Scholar]
- 10.Wormke M., Stoner M., Saville B., Walker K., Abdelrahim M., Burghardt R., Safe S. The aryl hydrocarbon receptor mediates degradation of estrogen receptor alpha through activation of proteasomes. Mol. Cell. Biol. 2003;23:1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdelrahim M., Ariazi E., Kim K., Khan S., Barhoumi R., Burghardt R., Liu S., Hill D., Finnell R., Wlodarczyk B., Jordan V.C., Safe S. 3-Methylcholanthrene and other aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha. Cancer Res. 2006;66:2459–2467. doi: 10.1158/0008-5472.CAN-05-3132. [DOI] [PubMed] [Google Scholar]
- 12.Liu S., Abdelrahim M., Khan S., Ariazi E., Jordan V.C., Safe S. Aryl hydrocarbon receptor agonists directly activate estrogen receptor alpha in MCF-7 breast cancer cells. Biol. Chem. 2006;387:1209–1213. doi: 10.1515/BC.2006.149. [DOI] [PubMed] [Google Scholar]
- 13.Shipley J.M., Waxman D.J. Aryl hydrocarbon receptor-independent activation of estrogen receptor-dependent transcription by 3-methylcholanthrene. Toxicol. Appl. Pharmacol. 2006;213:87–97. doi: 10.1016/j.taap.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Matthews J., Wihlen B., Heldring N., MacPherson L., Helguero L., Treuter E., Haldosen L.A., Gustafsson J.A. Co-planar 3,3′,4,4′,5-pentachlorinated biphenyl and non-co-planar 2,2′,4,6,6′-pentachlorinated biphenyl differentially induce recruitment of oestrogen receptor alpha to aryl hydrocarbon receptor target genes. Biochem. J. 2007;406:343–353. doi: 10.1042/BJ20070585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IARC IARC working group on the evaluation of Carcinogenic risks to humans: polychlorinated dibenzo-para-dioxins and polychlorinated dibenzofurans. Lyon, France, 4–11 February 1997. IARC Monogr. Eval. Carcinog. Risks Hum. 1997;69:1–631. [PMC free article] [PubMed] [Google Scholar]
- 16.Chopra M., Schrenk D. Dioxin toxicity, aryl hydrocarbon receptor signaling, and apoptosis-persistent pollutants affect programmed cell death. Crit. Rev. Toxicol. 2011;41:292–320. doi: 10.3109/10408444.2010.524635. [DOI] [PubMed] [Google Scholar]
- 17.Kociba R.J., Keyes D.G., Beyer J.E., Carreon R.M., Wade C.E., Dittenber D.A., Kalnins R.P., Frauson L.E., Park C.N., Barnard S.D., Hummel R.A., Humiston C.G. Results of a two-year chronic toxicity and oncogenicity study of 2,3,7,8-tetrachlorodibenzo-p-dioxin in rats. Toxicol. Appl. Pharmacol. 1978;46:279–303. doi: 10.1016/0041-008x(78)90075-3. [DOI] [PubMed] [Google Scholar]
- 18.Lucier G.W., Tritscher A., Goldsworthy T., Foley J., Clark G., Goldstein J., Maronpot R. Ovarian hormones enhance 2,3,7,8-tetrachlorodibenzo-p-dioxin-mediated increases in cell proliferation and preneoplastic foci in a two-stage model for rat hepatocarcinogenesis. Cancer Res. 1991;51:1391–1397. [PubMed] [Google Scholar]
- 19.Clemons M., Goss P. Estrogen and the risk of breast cancer. N. Engl. J. Med. 2001;344:276–285. doi: 10.1056/NEJM200101253440407. [DOI] [PubMed] [Google Scholar]
- 20.Ni N., Yager J.D. Comitogenic effects of estrogens on DNA synthesis induced by various growth factors in cultured female rat hepatocytes. Hepatology. 1994;19:183–192. [PubMed] [Google Scholar]
- 21.Schrenk D., Karger A., Lipp H.P., Bock K.W. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and ethinylestradiol as co-mitogens in cultured rat hepatocytes. Carcinogenesis. 1992;13:453–456. doi: 10.1093/carcin/13.3.453. [DOI] [PubMed] [Google Scholar]
- 22.Iwanari M., Nakajima M., Kizu R., Hayakawa K., Yokoi T. Induction of CYP1A1, CYP1A2, and CYP1B1 mRNAs by nitropolycyclic aromatic hydrocarbons in various human tissue-derived cells: chemical-cytochrome P450 isoform-, and cell-specific differences. Arch. Toxicol. 2002;76:287–298. doi: 10.1007/s00204-002-0340-z. [DOI] [PubMed] [Google Scholar]
- 23.Baumgart A., Schmidt M., Schmitz H.J., Schrenk D. Natural furocoumarins as inducers and inhibitors of cytochrome P450 1A1 in rat hepatocytes. Biochem. Pharmacol. 2005;69:657–667. doi: 10.1016/j.bcp.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Racky J., Schmitz H.J., Kauffmann H.M., Schrenk D. Single nucleotide polymorphism analysis and functional characterization of the human Ah receptor (AhR) gene promoter. Arch. Biochem. Biophys. 2004;421:91–98. doi: 10.1016/j.abb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Laborda J. 36B4 cDNA used as an estradiol-independent mRNA control is the cDNA for human acidic ribosomal phosphoprotein PO. Nucleic Acids Res. 1991;19:3998. doi: 10.1093/nar/19.14.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfaffl M.W. Real-time RT-PCR: Neue Ansätze zur exakten mRNA Quantifizierung. BIOspektrum. 2004;1:92. [Google Scholar]
- 28.Rossini G.P., Baldini G.M., Villa E., Manenti F. Characterization of estrogen receptor from human liver. Gastroenterology. 1989;96:1102–1109. doi: 10.1016/0016-5085(89)91629-6. [DOI] [PubMed] [Google Scholar]
- 29.Merchant M., Arellano L., Safe S. The mechanism of action of alpha-naphthoflavone as an inhibitor of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced CYP1A1 gene expression. Arch. Biochem. Biophys. 1990;281:84–89. doi: 10.1016/0003-9861(90)90416-v. [DOI] [PubMed] [Google Scholar]
- 30.Kharat I., Saatcioglu F. Antiestrogenic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin are mediated by direct transcriptional interference with the liganded estrogen receptor Cross-talk between aryl hydrocarbon- and estrogen-mediated signaling. J. Biol. Chem. 1996;271:10533–10537. doi: 10.1074/jbc.271.18.10533. [DOI] [PubMed] [Google Scholar]
- 31.Duan R., Porter W., Samudio I., Vyhlidal C., Kladde M., Safe S. Transcriptional activation of c-fos protooncogene by 17beta-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol. Endocrinol. 1999;13:1511–1521. doi: 10.1210/mend.13.9.0338. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan V., Porter W., Santostefano M., Wang X., Safe S. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol. Cell. Biol. 1995;15:6710–6719. doi: 10.1128/mcb.15.12.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gierthy J.F., Lincoln D.W., Gillespie M.B., Seeger J.I., Martinez H.L., Dickerman H.W., Kumar S.A. Suppression of estrogen-regulated extracellular tissue plasminogen activator activity of MCF-7 cells by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Res. 1987;47:6198–6203. [PubMed] [Google Scholar]
- 34.Zacharewski T.R., Bondy K.L., McDonell P., Wu Z.F. Antiestrogenic effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on 17 beta-estradiol-induced pS2 expression. Cancer Res. 1994;54:2707–2713. [PubMed] [Google Scholar]
- 35.Gillesby B.E., Stanostefano M., Porter W., Safe S., Wu Z.F., Zacharewski T.R. Identification of a motif within the 5’ regulatory region of pS2 which is responsible for AP-1 binding and TCDD-mediated suppression. Biochemistry. 1997;36:6080–6089. doi: 10.1021/bi962131b. [DOI] [PubMed] [Google Scholar]
- 36.Jana N.R., Sarkar S., Ishizuka M., Yonemoto J., Tohyama C., Sone H. Role of estradiol receptor-alpha in differential expression of 2,3,7, 8-tetrachlorodibenzo-p-dioxin-inducible genes in the RL95-2 and KLE human endometrial cancer cell lines. Arch. Biochem. Biophys. 1999;368:31–39. doi: 10.1006/abbi.1999.1288. [DOI] [PubMed] [Google Scholar]
- 37.Thomsen J.S., Wang X., Hines R.N., Safe S. Restoration of aryl hydrocarbon (Ah) responsiveness in MDA-MB-231 human breast cancer cells by transient expression of the estrogen receptor. Carcinogenesis. 1994;15:933–937. doi: 10.1093/carcin/15.5.933. [DOI] [PubMed] [Google Scholar]
- 38.Vickers P.J., Dufresne M.J., Cowan K.H. Relation between cytochrome P450IA1 expression and estrogen receptor content of human breast cancer cells. Mol. Endocrinol. 1989;3:157–164. doi: 10.1210/mend-3-1-157. [DOI] [PubMed] [Google Scholar]
- 39.MacPherson L., Lo R., Ahmed S., Pansoy A., Matthews J. Activation function 2 mediates dioxin-induced recruitment of estrogen receptor alpha to CYP1A1 and CYP1B1. Biochem. Biophys. Res. Commun. 2009;385:263–268. doi: 10.1016/j.bbrc.2009.05.060. [DOI] [PubMed] [Google Scholar]
- 40.Wihlen B., Ahmed S., Inzunza J., Matthews J. Estrogen receptor subtype- and promoter-specific modulation of aryl hydrocarbon receptor-dependent transcription. Mol. Cancer Res. 2009;7:977–986. doi: 10.1158/1541-7786.MCR-08-0396. [DOI] [PubMed] [Google Scholar]
- 41.Rogan E.G., Badawi A.F., Devanesan P.D., Meza J.L., Edney J.A., West W.W., Higginbotham S.M., Cavalieri E.L. Relative imbalances in estrogen metabolism and conjugation in breast tissue of women with carcinoma: potential biomarkers of susceptibility to cancer. Carcinogenesis. 2003;24:697–702. doi: 10.1093/carcin/bgg004. [DOI] [PubMed] [Google Scholar]
- 42.Xie T., Ho S.L., Ramsden D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 1999;56:31–38. doi: 10.1124/mol.56.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Lu F., Zahid M., Saeed M., Cavalieri E.L., Rogan E.G. Estrogen metabolism and formation of estrogen-DNA adducts in estradiol-treated MCF-10F cells. The effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin induction and catechol-O-methyltransferase inhibition. J. Steroid Biochem. Mol. Biol. 2007;105:150–158. doi: 10.1016/j.jsbmb.2006.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.