Graphical abstract
Keywords: Givotia rottleriformis, Gallic acid, Methyl gallate, A431 cells, Cyclooxygenases, HaCaT
Highlights
-
•
We isolated gallic acid (GA) and methyl gallate (MG) from the seed coats of G. rottleriformis.
-
•
We investigated the effect of purified GA and MG on A431 skin cancer cell proliferation in a dose and time dependent manner.
-
•
We analyzed the apoptosis enhancer/suppressor regulatory marker proteins in GA/MG treated A431 cells in a dose or time dependent manner.
-
•
We found that GA/MG is an effective natural molecule to avoid the risk of skin cancers.
Abstract
Gallic acid (GA) and its derivative methyl gallate (MG) are well studied plant phenolics. They have exhibited anticancer effects in several cancer cell lines. However, the presence of GA/MG in the seed coats of Givotia rottleriformis and their inhibitory effect on human epidermoid carcinoma (A431) skin cancer cells were not reported. In this study we have isolated and chemically characterized the bioactive compounds GA and MG from the bioassay guided methanolic (MeOH) seed coat extracts of G. rottleriformis. The fractions obtained from open silica column chromatography were subjected to in vitro enzymatic assays. Among seven fractions we found that only fractions 5 and 6 showed significant inhibition activity toward COX-1 with an IC50 value of 28 μg/mL and 9.3 μg/mL and COX-2 with an IC50 value of 35 μg/mL and 7.0 μg/mL respectively. However, we could not find 5-LOX enzyme inhibition activity. MG (10 mg/g DW) and GA (6 mg/g DW) were the major compounds of seed coats. Cell viability was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, which showed that GA/MG significantly reduced the growth of A431 cells with an IC50 value of 25 μg/mL and 53 μg/mL and 11 μg/mL and 43 μg/mL at 24 h and 48 h, respectively. However the cytotoxic effect of GA/MG on HaCaT normal skin keratinocyte cell line was found to be less. Western blot analysis has shown that GA/MG treatment down regulated Bcl-2 and up regulated cleaved caspase-3 with respect to increasing doses. Our results conclude that GA and MG have potential anticancer effects and can be used as therapeutic agents for skin cancers.
1. Introduction
Skin diseases are becoming more common worldwide, because of increasing environmental pollutants, such as chemical hazards and radiations. Diverse factors influence skin cancers, e.g. solar radiation, UV irradiation. More than one million new skin cancer cases were reported annually in USA [1]. Increasing incidence of skin cancers was reported in AIDS (Acquired immune deficiency syndrome) patients [2]. The cancer chemoprevention by natural agents such as phytochemicals, minerals and vitamins were shown to have effective results on various malignancies [3], [4]. The curcumin isolated from plants inhibits UV-irradiation-induced oxidative stress and enhanced apoptosis in A431 cells [1], [5]. The isolated resveratrol from plants was reported to have potential anti-cancer effect that enhanced apoptosis in A431 cells [6].
In this study we used the seed coats of Givotia rottleriformis, a tree species that belongs to Euphorbiaceae family. This plant grows in limited areas and particularly in the forests of Andhra Pradesh, Karnataka, Tamil Nadu and West Bengal in India. In our previous study we have observed that the seeds of this plant were rich in phenolics [7] and were reported to have anti-rheumatic, anti-psoriatic and anti-dandruff medicinal properties [8].
The gallic acid is isolated from plants [9], [10] and the antioxidant and anticancer effect of GA were well reported in most of the cancer cells [9], [11], [12], [13], [14], [15], [16], [17]. Many of GA derived chemical derivatives were also shown to have good anti-cancer properties [18]. Methyl gallate, a methyl ester of GA, was also isolated from several plants [19], [20]. The biosynthesis of GA and MG takes place via dihydroshikimate, a derivative of phenylpropanoid biosynthesis [21]. The anti-cancer and anti oxidant effects of MG was reported in different cancer cells [22], [23], [24], [25], [26], [27]. In addition MG was also shown to have anti-bacterial and anti-viral properties [28], [29], [30].
Cyclooxygenases (COX-1 and COX-2) and lipoxygenases (5-LOX, 12-LOX, 15-LOXa and 15-LOXb) are the key enzymes of arachidonic acid (AA) metabolism [31]. Previous reports suggested that inducible form of COX-1 and COX-2 leads to the biosynthesis of prostaglandins and thus causes inflammation and cancer [32], [33], [34]. Inhibition of 5-LOX blocks production of 5-LOX metabolites and triggers apoptosis in prostate cancer cells [35]. Therefore, identification of natural dual COX-2/5-LOX inhibitors is an interesting area of research to control cancer progression [36].
The over expression of Bcl-2 protein causes reduction of apoptosis in cancer cells [37], [38], [39], [40]. In contrast, the up-regulation of cleaved caspase-3 activated by other caspases [41], [42], enhances apoptosis and thus reduces cancer cell survival [43], [44], [45].
In the present study, we have isolated GA and MG from the seed coats of G. rottleriformis using chromatographic techniques and chemically characterized them using IR, NMR and LC–MS analysis (Fig. 1). The inhibitory effect of GA or MG on COX-1, COX-2 and 5-LOX was determined. We further studied the cytotoxic effect of purified GA and MG on human epidermoid carcinoma (A431) skin cancer cell line and normal human skin keratinocyte cell line HaCaT using MTT assay. The apoptotic effect of GA/MG was studied by western blotting analysis of Bcl-2 and cleaved caspase-3 in a dose dependent manner.
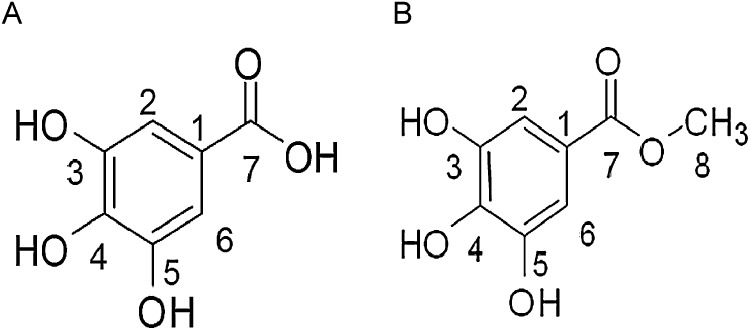
Fig. 1.
Chemical structure of (A) gallic acid and (B) methyl gallate.
2. Materials and methods
2.1. General procedure
IR spectra was determined in the KBr pellet using a JASCO FT-IR model 5300 spectrophotometer with polystyrene as reference. NMR spectra were recorded at 400 MHz for 1H NMR and 100 MHz for 13C NMR on Bruker-Avance-400 spectrometer with chloroform-d as solvent and tetramethylsilane (TMS) as reference (δ = 0 ppm) in DMSO-d6 at 25 °C. The chemical shift was expressed in δ, downfield from the signal of internal TMS. Mass spectra were recorded using LC–MS-2010 (Shimadzu).
2.2. Plant materials
Mature and dry seeds of G. rottleriformis were collected during summer from Regional Forest Research Centre (RFRC), Rajahmundry, Andhra Pradesh, India.
2.3. Cell lines and reagents
A431 skin cancer cell line and normal HaCaT skin cell line were obtained from National Centre for Cell Science (NCCS), Pune, India. Dulbeco's modified Eagle's medium (DMEM), fetal bovine serum (FBS), phosphate buffered saline (PBS), penicillin and streptomycin were purchased from GIBCO, Ltd. (BRL Life Technologies, Inc., Grand Island, NY). MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] and dimethyl sulfoxide (DMSO) were obtained from Sigma; poly-l-lysine, glutaraldehyde, proteinase inhibitor K, propidium iodide (PI), phenyl methyl sulfonyl fluoride (PMSF), luepeptin, aprotinin, pepstatin A, trypsin, Tween 20, Triton X-100, TMPD (N,N,N,N′-tetramethyl-p-phenylenediamine) were purchased from Sigma Chemical Company (St. Louis, USA). The primary antibodies for Bcl-2 and cleaved caspase-3 were obtained from Upstate Biotechnology (Charlottesville, VA, USA). The GA and MG were purified from the seed coats of G. rottleriformis. All other chemicals and reagents used in the study were obtained either from Merck or Sigma.
2.4. Extraction and reverse phase HPLC analysis of crude extract
The seed coats were macerated into fine powder and were extracted in methanol (MeOH). The crude extract was filtered using Whatman No.1 filter paper and was concentrated using Rotavapor (R3, Buchi). The extract was further analyzed with RP-HPLC (Shimadzu) using C18 reverse phase column (Shim-pack column with dimensions 250 mm × 4.6 mm, particle size 5 μM), with flow rate of 6 mL/min and detection at 280 nm. The mobile phase used was a complex gradient of solvent-A (water:acetic acid, 1000:1), and solvent-B (MeOH:acetic acid, 1000:1).
2.5. Open silica column and RPHPLC
The crude extract (10 g) was chromatographed using (Acme's) silica gel (100–200 mesh). Hexane and ethyl acetate was used as a mobile phase. Each fraction was passed through a thin layer chromatography (TLC) using silica gel plates (Merck 60F254) and visualized by irradiation with UV light and/or by iodine vapors. COX-1, COX-2 and 5-LOX assays were performed for all the fractions (see below). Only fractions that showed significant bioactivity were resolved using RP-HPLC (method mentioned above), isolated and concentrated by lyophilizer. The purified compounds were stored at room temperature and were used for bioassays and further analysis.
2.6. Isolation of COX-1 enzyme
We isolated COX-1 enzyme from Ram seminal vesicles using Hemler and Lands [46] method with slight modifications. In brief, Ram seminal vesicles were minced and homogenized in Tris–HCl (pH 8.0) buffer for 1 min; the homogenate was filtered through cheese cloth and centrifuged at 13,000 × g at 4 °C for 30 min. Finally 0.01% sodium azide was added to the supernatant. It was stored as small aliquots at −80 °C and was used as COX-1 enzyme source.
2.7. Isolation of COX-2 enzyme
The enzyme COX-2 was isolated according to the method of Reddy et al. [47] with slight modifications. In brief, human recombinant COX-2 enzyme was expressed in Sf-9 cells. Cells were pelleted down by centrifugation at 2000 rpm and the cells were further resuspended in 50 mM Tris–HCl buffer (pH 7.2) and sonicated for 3 min followed by centrifugation at 90,000 × g at 4 °C for 1 h 20 min using ultracentrifuge (Hitachi, Himac CP-100α). The obtained pellet was re-suspended in 2.5 mM Tris–HCl buffer (pH 7.2), 0.8% Tween 20, 1 mM phenol, and 0.5% glycerol. It was stored as small aliquots at −80 °C and used as COX-2 enzyme source.
2.8. COX-1 and COX-2 enzyme activity
We used the method of Copeland et al. [48] with slight modifications to measure the enzymatic activities of both COX-1 and COX-2, based on the chromogenic assay and oxidation of TMPD during the reduction of prostaglandin G2 (PGG2) to prostaglandin H2 (PGH2). In brief, the assay mixture contained Tris–HCl buffer (100 mM, pH 8.0), hematin (15 μM), EDTA (3 μM), enzyme (COX-1 or COX-2) and test compound. The assay mixture was pre-incubated at 25 °C for 15 min and the reaction was initiated by addition of AA and TMPD to a final volume of 1 mL. The activity was measured by estimating the rate of TMPD oxidation with an increase in absorbance at 603 nm during 1 min. A low rate of non-enzymatic TMPD oxidation observed in the absence of COX-1 or COX-2 enzymes was used as control reaction and was subtracted from the test experimental values while calculating the percent inhibition.
2.9. 5-LOX assay
The 5-LOX enzyme was extracted from potato tubers and assayed according to Reddanna et al. [49]. The enzyme activity was measured using polarographic method with a Clark's oxygen electrode on Strathkelvin instruments, model 782, RC-300. The typical reaction mixture contained 50–100 μL of enzyme and 10 μL of substrate (133 μM of AA), 100 mM phosphate buffer (pH 6.3) in a total volume of 3 mL. The rate of decrease in the oxygen concentration was taken as a measure of enzyme activity. The stock solutions of the test compounds were freshly prepared before use and were dissolved in DMSO. Various concentrations of the test compound were prepared and the 5-LOX was initiated by the addition of the substrate. The assay was performed at 25 °C and the maximum slope generated was taken for calculating the activity. The percent inhibition was calculated by the comparison of 5-LOX activity in the presence or absence of the inhibitor and IC50 value was calculated from the concentration–inhibition response curve.
2.10. Cell culture
A431 cells and HaCaT cells were grown as a monolayer in petriplates and supplemented with DMEM that contains 10% heat inactivated FBS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine and maintained at 37 °C in a humidified atmosphere with 5% CO2. The cells were sub-cultured twice in a week and the exponentially growing cells were used for the analysis.
2.11. MTT assay
A431 cells and HaCaT cells (5 × 103 cells per well) were seeded in 96 well plates and were treated with or without GA/MG at various concentrations (0.1, 10, 25, 50 and 100 μg/mL) in a final volume of 100 μL and incubated for 24 h and 48 h. Later 20 μL of MTT (5 mg/mL in PBS) was added to each well and incubated for an additional 3 h at 37 °C. The culture medium was removed and 50 μL of DMSO was added to each well to dissolve the purple-blue formazan crystals and optical density was measured at 570 nm using ELISA multi mode plate reader (SYNERGYMX, Bioteck).
2.12. Preparation of whole cell extract and Western blot analysis
The cells were treated with or without GA or MG at various concentrations (25, 50 and 100 μg/mL) for 24 h. Then the cells were washed with PBS and harvested by trypsinization and finally cell pellets were resuspended in RIPA buffer containing 1× protease inhibitor cocktail followed by incubation for 30 min at 4 °C with frequent vortex. The lysate was centrifuged at 10,000 rpm for 30 min and the supernatant was collected as whole-cell extract [50]. The protein concentration was estimated by Bradford method Bradford [51]. The total protein was resolved on 12% SDS-PAGE and transferred onto nitrocellulose (NC) membrane. The NC membrane was then incubated in 5% (w/v) non-fat dry milk powder at room temperature for 1 h to block non-specific sites. The NC membrane was incubated with the primary antibody of interest for 12 h at 4 °C under shaking (cleaved caspase-3, and Bcl-2) and washed thrice with TBST. The NC membrane was again incubated with the respective secondary antibody conjugated with HRP for 1 h at room temperature, and again washed thrice with TBST. The blot was developed by adding HRP substrate (PerkinElmer; Western lightning Plus-ECL).
2.13. Statistical analyses
The data was presented graphically as the mean ± standard deviation (SD). Statistical analysis was done using Sigma Plot 12.0 software and the graphs were plotted using Graph pad prism 6.0 software.
3. Results
3.1. RP-HPLC analysis and column chromatography of seed coat extract
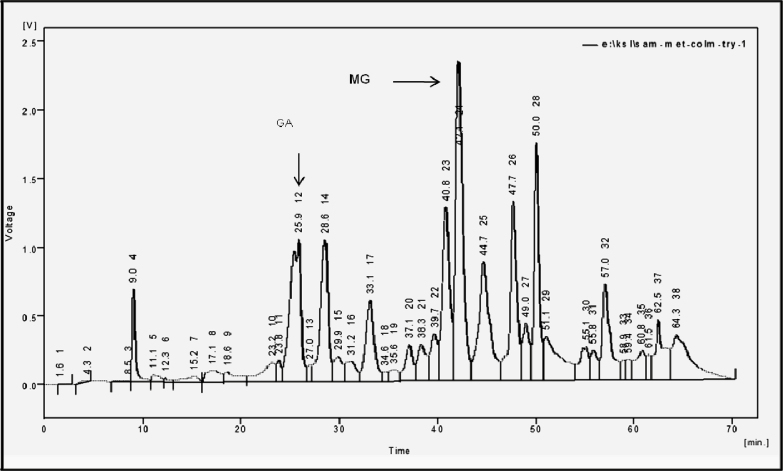
The crude seed coat extract subjected to RP-HPLC and the resolved chromatogram showed 10 major peaks at different retention times (RTs). Peak-1 eluted at 9.0 min, peak-2 at 25.9 min, peak-3 at 28.6 min, peak-4 at 33.1 min, peak-5 at 40.8 min, peak-6 at 42.1 min, peak-7 at 44.7 min, peak-8 at 47.7 min, peak-9 at 50.0 min and peak-10 at 57.0 min (Fig. 2). The crude seed coat extract (10 g) was fractionated into one to seven fractions, fraction 1 (0.6 g), fraction 2 (5.0 g), fraction 3 (1 g), fraction 4 (0.8 g), fraction 5 (1.0 g), fraction 6 (1.2 g), fraction 7 (0.4 g) according to the polarity using open silica chromatography and the purity of each fraction was monitored with TLC.
Fig. 2.
High-performance liquid chromatography (HPLC) analysis of crude extract of G. rottleriformis seed coats using a reverse phase C18 column. High-performance liquid chromatography (HPLC) chromatogram showing crude compounds resolved at their respective retention times (RTs).
3.2. COX-1, COX-2 and 5-LOX enzymatic essays
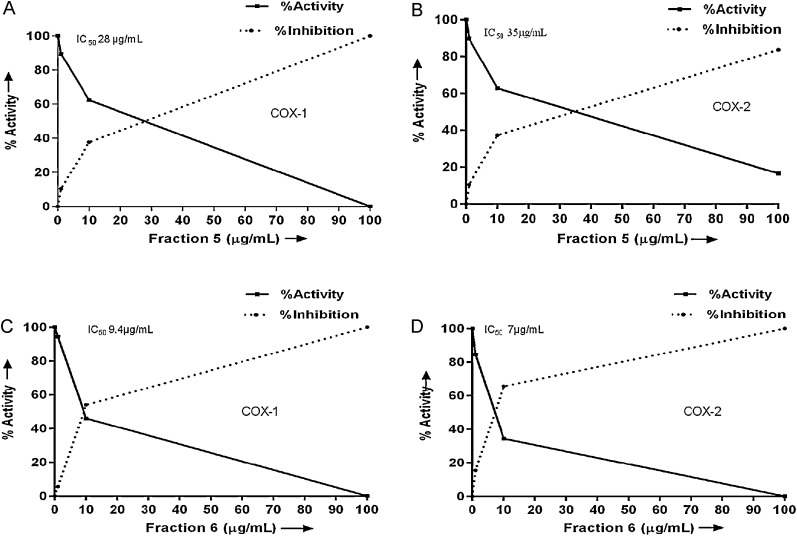
COX-1, COX-2 and 5-LOX activity were estimated for fractions 1–7. However, only fractions 5 and 6 inhibited COX-1 with an IC50 value of 7 μg/mL and 5.1 μg/mL and COX-2 with an IC50 value of 9 μg/mL and 6.44 μg/mL respectively (Fig. 3A–D). None of the fractions showed 5-LOX inhibition activity.
Fig. 3.
COX-1 and COX-2 inhibition activity of fractions 5 and 6. (A) COX-1 inhibition of fraction 5, (B) COX-2 inhibition of fraction 5, (C) COX-1 inhibition of fraction 6 and (D) COX-2 inhibition of fraction 6.
3.3. Isolation and chemical characterization of active compound from fraction 5
The bioassay guided fraction 5 that showed only a single peak at RT of 41.5 min, when resolved by RP-HPLC was collected (Fig. 4A). The isolated compound was subjected to IR, 1H, 13C NMR and LC–MS analysis and identified as MG (Supplementary Fig. S1–S4). IR (KBr) cm−1: 3506 (O—H), 1697 (●C O), 1618 (C C), 1541, 1471, 1439, 1313, 1251, 1195, 1037, 767, 746 (Supplementary Fig. S1). 1H NMR (400 MHz, CDCl3) δ ppm: 7.26 (S, 2H, A), 4.01 (S, 2H, B) (Supplementary Fig. S2). 13C NMR (200 MHz, CDCl3) δ ppm: 165.67 (C7, ●C O) 144.10 (C3, C5), 136.99 (C4), 118.82 (C1) 107.86 (C2, C5) 50.36 (C8) (Supplementary Fig. S3). The molecular mass of isolated MG was determined as 184 using LC–MS analysis (Supplementary Fig. S4).
Fig. 4.
Purification of methyl gallate (MG) from fraction 5 and gallic acid (GA) from fraction 6 by high-performance liquid chromatography (HPLC) using a reverse phase C18 column. (A) MG from fraction 5. (B). GA from fraction 6.
3.4. Isolation and chemical characterization of active compound from fraction 6
RP-HPLC chromatogram of fraction 6 that showed a mixture of 3 compounds eluted at different RTs, C1 (600 mg) at RT 27.49 min, C2 (250 mg) at RT 40.8 min and C3 (350 mg) at RT 51.4 min were isolated separately (Fig. 4B). MTT assay results showed that significant inhibition of A431 cell proliferation was seen when treated with C1, whereas treatment with C2 or C3 did not show any inhibitory effect on cell proliferation. The active C1 was subjected to IR, 1H, 13C NMR and LC–MS analysis and its chemical structure was identified as GA. IR (KBr) cm−1 33.71 (—COOH), 3057 (C—H), 1705 ( C O), 1620 (C C), 1452, 1338, 1248, 1026, 702 (Supplementary Fig. S5). 1H NMR δ ppm: 12.52 (S, H acid OH), 7.22 (S, 2H) (Supplementary Fig. S6). 13C NMR δ ppm: 167.01 (C7 C O), 143.98 (C3, C5), 136.61 (C4), 119.87 (C1) 108.14 (C2, C6); (Supplementary Fig. S7). The mass of the purified GA was determined as 169 by LC–MS (Supplementary Fig. S8).
3.5. MTT assay
Dose and time dependent effect of GA and MG on A431 and HaCaT cell proliferation was investigated. A431 cells that were treated with various concentrations of GA/MG (1, 10, 25, 50 and 100 μg/mL) showed significantly reduced cell proliferation due to enhanced cell death (Fig. 5A and B). GA treatment enhanced A431 cell death in a dose dependent manner with calculated IC50 value of 25 μg/mL at 24 h. The cell death was increased further when the GA treatment time increased to 48 h with an IC50 value of 11 μg/mL (Fig. 5Ai–iv). Similarly MG treatment also reduced A431 cell proliferation in a dose dependent manner. MG treatment showed reduced A431 cell proliferation with an IC50 value of 53 μg/mL at 24 h. However when the MG treatment time increased to 48 h the rate of cell death also increased further with an IC50 value of 43 μg/mL (Fig. 5Bi–iv). HaCaT cells that were treated with same GA concentrations (1, 10, 25, and 100 μg/mL) showed low level of reduction in cell proliferation with the calculated IC50 values of 84.2 μg/mL at 24 h and 64.4 μg/mL at 48 h, respectively (Fig. 6Ai–iv). MG treatment also showed reduced effect on HaCaT cell proliferation with an IC50 value of 79.4 μg/mL at 48 h (Fig. 6Bi–iv).
Fig. 5.
Gallic acid (GA) and methyl gallate (MG) reduced the cell viability of A431 cells. Cells were seeded at a density of 1 × 106 cells/mL in 96 well polystyrene culture plates and maintained at 37 °C with 5% (v/v) CO2 for 1 day. After 24 h the cells were incubated with GA/MG at the indicated concentrations for 24 h and 48 h and then processed for MTT assay. (Ai–iv) Percentage of cell death and viability of A431 cells treated with GA in a dose dependent manner at 24 h and 48 h. (Bi–iv) Percentage of cell death and viability of A431 cells after treatment with MG at 24 h and 48 h. The data presented as the mean ± SD of three independent experiments. The bars represent statistical significance over control (P < 0.05).
Fig. 6.
Gallic acid (GA) and methyl gallate (MG) reduced the cell viability of HaCaT normal skin keratinocyte cell line. Cells were seeded at a density of 1 × 106 cells/mL in 96 well polystyrene culture plates at 37 °C with 5% (v/v) CO2 for 1 day. After 24 h the cells were incubated with GA/MG at the indicated concentrations for 24 h and 48 h and then processed for MTT assay. (Ai–iv) Percentage of cell death and viability in HaCaT cells when treated with GA in a dose dependent manner at 24 h and 48 h. (Bi-iv) Percentage of cell death and viability in HaCaT cells when treated with MG in a dose dependent manner at 24 h and 48 h. The data presented as the mean ± SD of three independent experiments. Bars represent statistical significance over control (P < 0.05).
3.6. Western blot analysis
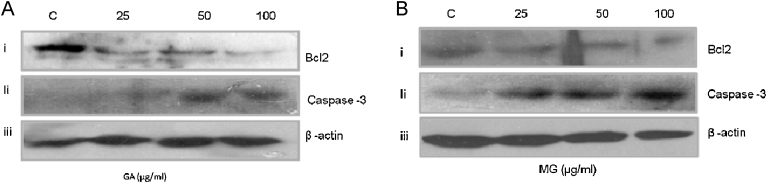
Treatment of A431 cells with GA and MG down regulated Bcl-2 and thus enhanced apoptosis in dose (25, 50 and 100 μg/mL) dependent manner (Fig. 7Ai and Bi). The cleaved caspase-3 was also induced in a dose dependent manner with increasing doses of GA and MG treatment (Fig. 6, Fig. 7). β-Actin was used as a loading control (Fig. 7Aiii and Biii).
Fig. 7.
Western blotting analysis showing Bcl-2 and cleaved caspase-3 protein up regulation in A431 cells that were treated with purified gallic acid (GA)/methyl gallate (MG). (Ai) A431 cells treated with GA down regulated Bcl-2 expression with respect to increasing doses. (Aii) Up regulation of the expression of cleaved caspase-3 in GA treated A431 cells. (Bi) Down regulation of the expression of Bcl-2 levels with increasing doses of MG treated A431 cells. (Bii) Up regulation of the expression of cleaved caspase-3 with increasing doses of MG treated A431 cells.
4. Discussion
Plants synthesize a wide range of metabolites for their survival, growth, development and protection from a broad spectrum of bacterial, fungal and viral pathogens. Some of the plant metabolites work as anti-oxidant and anti-cancer drugs because of their toxic effect toward cancer cells [6], [26], [52]. In this study we isolated GA and MG as major metabolites from the seed coats of G. rottleriformis. The total seed coat extract was fractionated and subjected to COX-1/COX-2/5-LOX assays and cytotoxic efficiency was conducted on A431 cells and HaCaT cells. The HPLC chromatogram data of MeOH seed coat extract revealed a group of peaks that were fractionated from one to seven by open silica column chromatography. Among all the fractions, only 5 and 6 fractions significantly inhibited COX-1 and COX-2 enzyme activity (Fig. 3a–d). However, these fractions did not show any significant inhibition on 5-LOX enzyme activity. Earlier studies have demonstrated that COXs and LOXs are the key enzymes that play an important role in inflammation and carcinogenesis [10], [11], [52]. Only 5 and 6 chromatographed fractions that inhibited the A431 cell proliferation was further subjected to active compound isolation by RP-HPLC. The active compounds of fractions 5 and 6 were purified by RP-HPLC (Fig. 4A and B) and were identified as GA and MG using IR, NMR and LC–MS analysis. GA and MG are natural constituents isolated from different plants [19], [25], [29]. Phenylpropanoid metabolism produce enormous array of secondary metabolites. The biosynthesis of GA and its derivative MG takes place via phenylpropanoid metabolism [21]. Our results suggest that MG (10 mg/g DW) and GA (6 mg/g DW) are the major compounds of the seed coats of this plant. We have also isolated several phenylpropanoid metabolites both known and unknown and studied their role in inducing plant immunity (Samuel et al., unpublished data). It was well demonstrated that some plant compounds such as curcumin, resveratrol and α-santalol suppressed cell proliferation and enhanced apoptosis in A431 cells [1], [5], [6].
Treatment of A431 cells with GA/MG suppressed cell survival by enhanced apoptosis in a dose and time dependent manner (Fig. 5Ai–iv and Bi–iv). The cytotoxic effect of GA and MG was investigated in various cancer cells [11], [26], [53], [54], [55]. The GA isolated from Terminalia bellerica potentially inhibited COX-1 and COX-2 enzymes in a concentration dependent manner [10]. Similarly, MG also showed dual COX-2/5-LOX inhibitory activity; however, 5-LOX inhibition was based on the leukotriene C4 [36]. Similar results were observed with our data with GA inhibiting both COX-1 and COX-2 enzymes in a dose dependent manner [10]. The inhibition of COX-2 enzyme by MG was similar as that mentioned by Kim et al. [36]. The COX-1 activity of fraction 5 and the purified MG were the same (IC50 value of 28 μg/mL). Here we report for the first time the COX-1 inhibition activity of MG. We have not observed any effect of GA on 5-LOX activity and our results were consistent with the findings from previous reports [10]. Whereas the 5-LOX activity of MG could not be observed even at increasing concentrations though it was earlier reported that MG exhibits 5-LOX inhibition activity [36].
The anti-oxidant and anti-proliferative effects of GA/MG were well studied [11], [19], [20], [22], [26], [28], [30], [53]. In our results, the treatment of A431 cells with purified GA suppressed the growth of A431 cells in a dose dependent manner with an IC50 value of 25 μg/mL and 11 μg/mL at 24 h and 48 h, respectively (Fig. 5Ai–iv). On the other hand, GA has shown much less cytotoxic effect on HaCaT normal skin keratinocyte cell line and showed IC50 value of 84.2 μg/mL and 62.4 μg/mL at 24 h and 48 h, respectively (Fig. 6Ai–iv). The anti-proliferative and apoptotic properties of phytochemicals were demonstrated in A431 cells [1], [5], [6]. However this is the first observation that reported the effect of GA on A431 cells. Similarly MG reduced the cell proliferation with an IC50 value of 53 μg/mL and 48 μg/mL at 24 h and 48 h respectively (Fig. 5Bi–iv). In the same way, normal skin HaCaT cells that were treated with MG showed less cytotoxicity with an IC50 value of 79.4 μg/mL than A431 cancer cells (Fig. 6Bi–iv). It was suggested by previous reports that MG inhibits cancer cells [25], [53]. Our data showed that the treatment of A431 cells with GA and MG down regulated Bcl-2 (Fig. 7Ai and Bi). Bcl-2 promotes cell proliferation and reduced apoptosis in cancer cells [56], [57], [58], [59]. Treatment of A431 cells with GA or MG also upregulated the protein levels of cleaved caspase-3 in a dose dependent manner (Fig. 7Aii and Bii). Caspase-3 was reported as an apoptotic marker protein, the expression of which enhances apoptosis, cleaves key cellular proteins and promotes cell death in cancer cells [56], [60], [61], [62]. Apart from cancer, because of anti-oxidant property of GA, GA and its ester derivatives were widely used as additives in food industry [63]. In summary, this study demonstrates that the seeds coats of G. rottleriformis are a rich source of GA/MG and may play significant role in treatment of various cancers. These results demonstrate and encourage the need of further studies to establish the role of GA or MG as potential anti-inflammatory drugs to avoid the risk of various skin and other cancer problems.
Conflict of interest
The author has received grants from University Grants commission, Council of Scientific and Industrial Research, and Department of Science and Technology, during the conduct of the study.
Transparency document
Acknowledgements
The authors acknowledge the support of Prof. Pallu Reddanna, UOH for lab facilities. The study was supported by the fellowship received from University Grants Commission (UGC), New Delhi; Council of Scientific and Industrial Research (CSIR), New Delhi, India; Young Scientist grant from Department of Science and Technology under Science and Engineering Research Board (DST-SERB). The infrastructural facilities at School of Life Sciences, University of Hyderabad established with the support of UGC-SAP-CAS, DST-FIST and DBT-CREBB have been utilized in the present study.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2015.03.001.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Kaur M., Agarwal C., Singh R.P., Guan X., Dwivedi C., Agarwal R. Skin cancer chemopreventive agent, {alpha}-santalol, induces apoptotic death of human epidermoid carcinoma A431 cells via caspase activation together with dissipation of mitochondrial membrane potential and cytochrome c release. Carcinogenesis. 2005;26:369–380. doi: 10.1093/carcin/bgh325. [DOI] [PubMed] [Google Scholar]
- 2.Saladi R.N., Persaud A.N. The causes of skin cancer: a comprehensive review. Drugs Today (Barc.) 2005;41:37–53. doi: 10.1358/dot.2005.41.1.875777. [DOI] [PubMed] [Google Scholar]
- 3.Gescher A., Pastorino U., Plummer S.M., Manson M.M. Suppression of tumour development by substances derived from the diet – mechanisms and clinical implications. Br. J. Clin. Pharmacol. 1998;45:1–12. doi: 10.1046/j.1365-2125.1998.00640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manson M.M. Cancer prevention – the potential for diet to modulate molecular signalling. Trends Mol. Med. 2003;9:11–18. doi: 10.1016/s1471-4914(02)00002-3. [DOI] [PubMed] [Google Scholar]
- 5.Chan W.H., Wu C.C., Yu J.S. Curcumin inhibits UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermoid carcinoma A431 cells. J. Cell. Biochem. 2003;90:327–338. doi: 10.1002/jcb.10638. [DOI] [PubMed] [Google Scholar]
- 6.Ahmad N., Adhami V.M., Afaq F., Feyes D.K., Mukhtar H. Resveratrol causes WAF-1/p21-mediated G(1)-phase arrest of cell cycle and induction of apoptosis in human epidermoid carcinoma A431 cells. Clin. Cancer Res. 2001;7:1466–1473. [PubMed] [Google Scholar]
- 7.Samuel K., Debashish D., Madhumita B., Padmaja G., Siva Ram Prasad, Bhaskara R.M.V., Rao P.S. In vitro germination and micropropagation of Givotia rottleriformis Griff. In Vitro Cell. Dev. Biol. Plant. 2009;45:466–473. [Google Scholar]
- 8.Thammanna, Narayana Rao K. Tirumala Tirupati Devasthanam (TTD); Tirupati, Andhra Pradesh: 1990. Medicinal Plants of Tirumala. [Google Scholar]
- 9.Chen H.M., Wu Y.C., Chia Y.C., Chang F.R., Hsu H.K., Hsieh Y.C., Chen C.C., Yuan S.S. Gallic acid, a major component of Toona sinensis leaf extracts, contains a ROS-mediated anti-cancer activity in human prostate cancer cells. Cancer Lett. 2009;286:161–171. doi: 10.1016/j.canlet.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 10.Reddy T.C., Aparoy P., Babu N.K., Kumar K.A., Kalangi S.K., Reddanna P. Kinetics and docking studies of a COX-2 inhibitor isolated from Terminalia bellerica fruits. Protein Pept. Lett. 2010;17:1251–1257. doi: 10.2174/092986610792231537. [DOI] [PubMed] [Google Scholar]
- 11.Chandramohan Reddy T., Bharat Reddy D., Aparna A., Arunasree K.M., Gupta G., Achari C., Reddy G.V., Lakshmipathi V., Subramanyam A., Reddanna P. Anti-leukemic effects of gallic acid on human leukemia K562 cells: downregulation of COX-2, inhibition of BCR/ABL kinase and NF-kappaB inactivation. Toxicol. In Vitro. 2012;26:396–405. doi: 10.1016/j.tiv.2011.12.018. [DOI] [PubMed] [Google Scholar]
- 12.Hou A.J., Peng L.Y., Liu Y.Z., Lin Z.W., Sun H.D. Gallotannins and related polyphenols from Pistacia weinmannifolia. Planta Med. 2000;66:624–626. doi: 10.1055/s-2000-8633. [DOI] [PubMed] [Google Scholar]
- 13.Inoue M., Suzuki R., Sakaguchi N., Li Z., Takeda T., Ogihara Y., Jiang B.Y., Chen Y. Selective induction of cell death in cancer cells by gallic acid. Biol. Pharm. Bull. 1995;18:1526–1530. doi: 10.1248/bpb.18.1526. [DOI] [PubMed] [Google Scholar]
- 14.Kaur M., Velmurugan B., Rajamanickam S., Agarwal R., Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenograft growth in nude mice. Pharm. Res. 2009;26:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veluri R., Singh R.P., Liu Z., Thompson J.A., Agarwal R., Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 16.Yeh R.D., Chen J.C., Lai T.Y., Yang J.S., Yu C.S., Chiang J.H., Lu C.C., Yang S.T., Yu C.C., Chang S.J., Lin H.Y., Chung J.G. Gallic acid induces G(0)/G(1) phase arrest and apoptosis in human leukemia HL-60 cells through inhibiting cyclin D and E, and activating mitochondria-dependent pathway. Anticancer Res. 2011;31:2821–2832. [PubMed] [Google Scholar]
- 17.Zuo Y., Chen H., Deng Y. Simultaneous determination of catechins, caffeine and gallic acids in green, Oolong, black and pu-erh teas using HPLC with a photodiode array detector. Talanta. 2002;57:307–316. doi: 10.1016/s0039-9140(02)00030-9. [DOI] [PubMed] [Google Scholar]
- 18.Saxena H.O., Faridi U., Srivastava S., Kumar J.K., Darokar M.P., Luqman S., Chanotiya C.S., Krishna V., Negi A.S., Khanuja S.P. Gallic acid-based indanone derivatives as anticancer agents. Bioorg. Med. Chem. Lett. 2008;18:3914–3918. doi: 10.1016/j.bmcl.2008.06.039. [DOI] [PubMed] [Google Scholar]
- 19.Abou-Zaid M.M., Lombardo D.A., Nozzolillo C. Methyl gallate is a natural constituent of maple (Genus Acer) leaves. Nat. Prod. Res. 2009;23:1373–1377. doi: 10.1080/14786410802420457. [DOI] [PubMed] [Google Scholar]
- 20.Kane C.J., Menna J.H., Yeh Y.C. Methyl gallate, methyl-3,4,5-trihydroxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. I. Purification and characterization of methyl gallate from Sapium sebiferum. Biosci. Rep. 1988;8:85–94. doi: 10.1007/BF01128975. [DOI] [PubMed] [Google Scholar]
- 21.Vogt T. Phenylpropanoid biosynthesis. Mol. Plant. 2010;3:2–20. doi: 10.1093/mp/ssp106. [DOI] [PubMed] [Google Scholar]
- 22.Chaubal R., Deshapande V.H., Deshpande N.R. Methyl gallate, the medicinally important compound: a review. J. Environ. Agric. Food Chem. 2005;4:956–962. [Google Scholar]
- 23.Da Silva S.L., Chaar J.daS., Yano T. Chemotherapeutic potential of two gallic acid derivative compounds from leaves of Casearia sylvestris Sw (Flacourtiaceae) Eur. J. Pharmacol. 2009;608:76–83. doi: 10.1016/j.ejphar.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 24.Fiuza S.M., Gomes C., Teixeira L.J., Girao da Cruz M.T., Cordeiro M.N., Milhazes N., Borges F., Marques M.P. Phenolic acid derivatives with potential anticancer properties – a structure-activity relationship study. Part 1: Methyl, propyl and octyl esters of caffeic and gallic acids. Bioorg. Med. Chem. 2004;12:3581–3589. doi: 10.1016/j.bmc.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh T.J., Liu T.Z., Chia Y.C., Chern C.L., Lu F.J., Chuang M.C., Mau S.Y., Chen S.H., Syu Y.H., Chen C.H. Protective effect of methyl gallate from Toona sinensis (Meliaceae) against hydrogen peroxide-induced oxidative stress and DNA damage in MDCK cells. Food Chem. Toxicol. 2004;42:843–850. doi: 10.1016/j.fct.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 26.Lee H., Kwon Y., Lee J.H., Kim J., Shin M.K., Kim S.H., Bae H. Methyl gallate exhibits potent antitumor activities by inhibiting tumor infiltration of CD4+CD25+ regulatory T cells. J. Immunol. 2010;185:6698–6705. doi: 10.4049/jimmunol.1001373. [DOI] [PubMed] [Google Scholar]
- 27.Lizarraga D., Tourino S., Reyes-Zurita F.J., de Kok T.M., van Delft J.H., Maas L.M., Briede J.J., Centelles J.J., Torres J.L., Cascante M. Witch hazel (Hamamelis virginiana) fractions and the importance of gallate moieties – electron transfer capacities in their antitumoral properties. J. Agric. Food Chem. 2008;56:11675–11682. doi: 10.1021/jf802345x. [DOI] [PubMed] [Google Scholar]
- 28.Choi J.G., Kang O.H., Lee Y.S., Oh Y.C., Chae H.S., Jang H.J., Kim J.H., Sohn D.H., Shin D.W., Park H., Kwon D.Y. In vitro activity of methyl gallate isolated from galla rhois alone and in combination with ciprofloxacin against clinical isolates of salmonella. J. Microbiol. Biotechnol. 2008;18:1848–1852. doi: 10.4014/jmb.0800.025. [DOI] [PubMed] [Google Scholar]
- 29.Kane C.J., Menna J.H., Sung C.C., Yeh Y.C. Methyl gallate, methyl-3,4,5-trihydoxybenzoate, is a potent and highly specific inhibitor of herpes simplex virus in vitro. II. Antiviral activity of methyl gallate and its derivatives. Biosci. Rep. 1988;8:95–102. doi: 10.1007/BF01128976. [DOI] [PubMed] [Google Scholar]
- 30.Kang M.S., Oh J.S., Kang I.C., Hong S.J., Choi C.H. Inhibitory effect of methyl gallate and gallic acid on oral bacteria. J. Microbiol. 2008;46:744–750. doi: 10.1007/s12275-008-0235-7. [DOI] [PubMed] [Google Scholar]
- 31.Greene E.R., Huang S., Serhan C.N., Panigrahy D. Regulation of inflammation in cancer by eicosanoids. Prostaglandins Other Lipid Mediat. 2011;96:27–36. doi: 10.1016/j.prostaglandins.2011.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dannhardt G., Kiefer W. Cyclooxygenase inhibitors – current status and future prospects. Eur. J. Med. Chem. 2001;36:109–126. doi: 10.1016/s0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 33.Rizzo M.T. Cyclooxygenase-2 in oncogenesis. Clin. Chim. Acta. 2011;412:671–687. doi: 10.1016/j.cca.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 34.Tang X., Sun Y.J., Half E., Kuo M.T., Sinicrope F. Cyclooxygenase-2 overexpression inhibits death receptor 5 expression and confers resistance to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human colon cancer cells. Cancer Res. 2002;62:4903–4908. [PubMed] [Google Scholar]
- 35.Sarveswaran S., Thamilselvan V., Brodie C., Ghosh J. Inhibition of 5-lipoxygenase triggers apoptosis in prostate cancer cells via down-regulation of protein kinase C-epsilon. Biochim. Biophys. Acta. 2011;1813:2108–2117. doi: 10.1016/j.bbamcr.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim S.J., Jin M., Lee E., Moon T.C., Quan Z., Yang J.H., Son K.H., Kim K.U., Son J.K., Chang H.W. Effects of methyl gallate on arachidonic acid metabolizing enzymes: cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived mast cells. Arch. Pharm. Res. 2006;29:874–878. doi: 10.1007/BF02973908. [DOI] [PubMed] [Google Scholar]
- 37.Cory S., Adams J.M. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez Y., Gu B., Martinez A., Torregrosa A., Sierra A. Inhibition of apoptosis in human breast cancer cells: role in tumor progression to the metastatic state. Int. J. Cancer. 2002;101:317–326. doi: 10.1002/ijc.10628. [DOI] [PubMed] [Google Scholar]
- 39.Huang D.S., Shen K.Z., Wei J.F., Liang T.B., Zheng S.S., Xie H.Y. Specific COX-2 inhibitor NS398 induces apoptosis in human liver cancer cell line HepG2 through BCL-2. World J. Gastroenterol. 2005;11:204–207. doi: 10.3748/wjg.v11.i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joensuu H., Pylkkanen L., Toikkanen S. Bcl-2 protein expression and long-term survival in breast cancer. Am. J. Pathol. 1994;145:1191–1198. [PMC free article] [PubMed] [Google Scholar]
- 41.Enari M., Talanian R.V., Wong W.W., Nagata S. Sequential activation of ICE-like and CPP32-like proteases during Fas-mediated apoptosis. Nature. 1996;380:723–726. doi: 10.1038/380723a0. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes-Alnemri T., Litwack G., Alnemri E.S. CPP32, a novel human apoptotic protein with homology to Caenorhabditis elegans cell death protein Ced-3 and mammalian interleukin-1 beta-converting enzyme. J. Biol. Chem. 1994;269:30761–30764. [PubMed] [Google Scholar]
- 43.Dai D., Liang Y., Xie Z., Fu J., Zhang Y., Zhang Z. Survivin deficiency induces apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Oncol. Rep. 2012;27:621–627. doi: 10.3892/or.2011.1544. [DOI] [PubMed] [Google Scholar]
- 44.Fan Y., Bergmann A. The cleaved-caspase-3 antibody is a marker of caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 2010;17:534–539. doi: 10.1038/cdd.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu L.F., Ye Y.Q., Huang G.Y., Li H.B., Li G.P., Pu Z.J., Wei B.L., Feng J.L. Involvement of endoplasmic reticulum stress in adenosine-induced human hepatoma HepG2 cell apoptosis. Oncol. Rep. 2011;26:73–79. doi: 10.3892/or.2011.1247. [DOI] [PubMed] [Google Scholar]
- 46.Hemler M., Lands W.E. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J. Biol. Chem. 1976;251:5575–5579. [PubMed] [Google Scholar]
- 47.Reddy C.M., Bhat V.B., Kiranmai G., Reddy M.N., Reddanna P., Madyastha K.M. Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulina platensis. Biochem. Biophys. Res. Commun. 2000;277:599–603. doi: 10.1006/bbrc.2000.3725. [DOI] [PubMed] [Google Scholar]
- 48.Copeland R.A., Williams J.M., Giannaras J., Nurnberg S., Covington M., Pinto D., Pick S., Trzaskos J.M. Mechanism of selective inhibition of the inducible isoform of prostaglandin G/H synthase. Proc. Natl. Acad. Sci. U. S. A. 1994;91:11202–11206. doi: 10.1073/pnas.91.23.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reddanna P., Whelan J., Maddipati K.R., Reddy C.C. Purification of arachidonate 5-lipoxygenase from potato tubers. Methods Enzymol. 1990;187:268–277. doi: 10.1016/0076-6879(90)87031-w. [DOI] [PubMed] [Google Scholar]
- 50.Sambrook J., Fritsch E.F., Maniatis T. 1st ed. Cold Spring Harbor Laboratory Press; 1989. Molecular Cloning: A Laboratory Manual. [Google Scholar]
- 51.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 52.Reddy D.B., Reddy T.C., Jyotsna G., Sharan S., Priya N., Lakshmipathi V., Reddanna P. Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J. Ethnopharmacol. 2009;124:506–512. doi: 10.1016/j.jep.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 53.Crispo J.A., Piche M., Ansell D.R., Eibl J.K., Tai I.T., Kumar A., Ross G.M., Tai T.C. Protective effects of methyl gallate on H2O2-induced apoptosis in PC12 cells. Biochem. Biophys. Res. Commun. 2010;393:773–778. doi: 10.1016/j.bbrc.2010.02.079. [DOI] [PubMed] [Google Scholar]
- 54.Hsu J.D., Kao S.H., Ou T.T., Chen Y.J., Li Y.J., Wang C.J. Gallic acid induces G2/M phase arrest of breast cancer cell MCF-7 through stabilization of p27(Kip1) attributed to disruption of p27(Kip1)/Skp2 complex. J. Agric. Food Chem. 2011;59:1996–2003. doi: 10.1021/jf103656v. [DOI] [PubMed] [Google Scholar]
- 55.Lee S.H., Kim J.K., Kim D.W., Hwang H.S., Eum W.S., Park J., Han K.H., Oh J.S., Choi S.Y. Antitumor activity of methyl gallate by inhibition of focal adhesion formation and Akt phosphorylation in glioma cells. Biochim. Biophys. Acta. 2013;1830:4017–4029. doi: 10.1016/j.bbagen.2013.03.030. [DOI] [PubMed] [Google Scholar]
- 56.Chang C., Zhu Y., Tang X., Tao W. The anti-proliferative effects of norcantharidin on human HepG2 cells in cell culture. Mol. Biol. Rep. 2011;38:163–169. doi: 10.1007/s11033-010-0090-6. [DOI] [PubMed] [Google Scholar]
- 57.Korsmeyer S.J. BCL-2 gene family and the regulation of programmed cell death. Cancer Res. 1999;59:1693–1700. [PubMed] [Google Scholar]
- 58.McDonnell T.J., Troncoso P., Brisbay S.M., Logothetis C., Chung L.W., Hsieh J.T., Tu S.M., Campbell M.L. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–6944. [PubMed] [Google Scholar]
- 59.Raffo A.J., Perlman H., Chen M.W., Day M.L., Streitman J.S., Buttyan R. Overexpression of bcl-2 protects prostate cancer cells from apoptosis in vitro and confers resistance to androgen depletion in vivo. Cancer Res. 1995;55:4438–4445. [PubMed] [Google Scholar]
- 60.Devarajan E., Sahin A.A., Chen J.S., Krishnamurthy R.R., Aggarwal N., Brun A.M., Sapino A., Zhang F., Sharma D., Yang X.H., Tora A.D., Mehta K. Down-regulation of caspase 3 in breast cancer: a possible mechanism for chemoresistance. Oncogene. 2002;21:8843–8851. doi: 10.1038/sj.onc.1206044. [DOI] [PubMed] [Google Scholar]
- 61.Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 62.Winter R.N., Kramer A., Borkowski A., Kyprianou N. Loss of caspase-1 and caspase-3 protein expression in human prostate cancer. Cancer Res. 2001;61:1227–1232. [PubMed] [Google Scholar]
- 63.Serrano A., Palacios C., Roy G., Cespon C., Villar M.L., Nocito M., Gonzalez-Porque P. Derivatives of gallic acid induce apoptosis in tumoral cell lines and inhibit lymphocyte proliferation. Arch. Biochem. Biophys. 1998;350:49–54. doi: 10.1006/abbi.1997.0474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.