Abstract
Dichlorodiphenyltrichloroethane (DDT) and its metabolites accumulate in adipose tissue through dietary exposure, and have been proposed to contribute to the development of abdominal obesity, insulin resistance and dyslipidemia. Toxicity may also result when DDT and its metabolites are released from adipose tissue into the bloodstream as a result of rapid weight loss. We hypothesized that DDT-exposed rats fed a high fat diet (HFD) followed by 60% calorie restriction would have an adverse metabolic response to rapid weight loss. To test this, we exposed obese Sprague-Dawley (SD) rats to DDT and a HFD over one month followed by 60% calorie restricted diet for two weeks, and examined metabolic parameters throughout the study. During the HFD feeding period, DDT-exposed rats had significantly elevated postprandial non-esterified fatty acids (NEFAs) and decreased body temperature compared with control rats. During calorie restriction, DDT-exposed rats had lowered food efficiency (weight gained/calories consumed), body temperature, and circulating TSH. Our findings suggest that exposure to DDT may impairs metabolic substrate utilization in rats during dynamic periods of weight gain and weight loss.
Abbreviations: DDT, dichlorodiphenyltrichloroethane; DDE, dichlorodiphenyldichloroethylene; HFD, high fat diet; CR, caloric restriction; SD, Sprague Dawley; NEFA, non esterified fatty acid; T2DM, type 2 diabetes mellitus; CVD, cardiovascular disease; TG, triglyceride; OLTT, oral lipid tolerance test; TSH, thyroid-stimulating hormone; T4, thyroxine; T3, triiodothyronine
Keywords: DDE, DDT, Food efficiency, Dyslipidemia, Thermoregulation, Thyroid hormone
1. Introduction
Obesity is increasing worldwide, and can lead to a number of serious health conditions, including hypertriglyceridemia [18] and type 2 diabetes mellitus (T2DM) [6], which are major risk factors for cardiovascular disease (CVD) [28], [43]. One of the most effective ways to achieve a reduction of excess weight in obese individuals is bariatric surgery, which restricts caloric intake and decreases nutrient absorption [13]. Bariatric surgical procedures have also been shown to improve lipid profiles including decreases of elevated triglyceride (TG) levels and prevention or resolution of T2DM [1], [7], [36], [42]. However, whether toxicants released from diminishing adipose tissue depots can modify metabolic outcomes following weight loss resulting from bariatric surgery is suggested but not known [21].
Still recommended for use by the World Health Organization [44], the insecticide dichlorodiphenyltrichloroethane (DDT) and its primary metabolite dichlorodiphenyldichloroethylene (DDE) accumulate persistently in the adipose tissue of humans and other animals [20], [33]. When DDT and DDE are stored in adipose tissue, other tissues are protected from their exposure and resulting toxicity; however, these chronic exposures within adipose tissue raise the possibility of specific toxicity to adipose tissue [22]. Consistent with adipose as a target tissue of toxicity, DDT and DDE are reported to have disruptive effects on lipid homeostasis associated with obesity observed in rodents and humans [23], [24], [30], [34], [37], [41]. Because endocrine disruption has been implicated in these adverse metabolic effects of exposure to DDT and DDE [23], [34], it is possible that the mobilization of DDT and DDE from adipose tissue during weight loss could also contribute to DDT and DDE metabolic toxicity by targeting non-adipose tissues [2], [19], [21], [22], [31].
One study in humans indicated that DDT and DDE mobilized during weight loss are paradoxically associated with the adverse metabolic profiles typically observed in obesity. During a period of weight loss, obese humans with higher levels of circulating plasma DDT and DDE had lower serum thyroid hormone (triiodothyronine (T3)) and lower resting metabolic rate, two factors that adversely influence energy homeostasis and body weight [31]. However a well-controlled experimental study has not yet been performed to evaluate whether the presence of DDT and DDE during weight loss is sufficient to attenuate the metabolic benefits of weight loss.
The present study begins to address this knowledge gap by evaluating the relationships between DDT and DDE exposure and (1) the adverse effects of weight gain and (2) the beneficial effects of weight loss. To achieve this, the aims of this study were to first examine whether exposure to DDT and DDE leads to altered lipid levels and energy expenditure in obese rats exposed to a high fat diet, and second whether DDT and DDE-exposed rats would have attenuated improvements of lipid and energy homeostasis resulting from weight loss compared with unexposed rats.
2. Methods
2.1. Exposures
This report combines data from two experimental cohorts of Charles River Sprague Dawley (SD) rats, which are substantially heavier than Harlan SD rats (403 g vs. 251 g 2-month old males, respectively). SD (Charles River) breeders weighing between 380 g and 420 g have been selectively bred at UC Davis for 31 generations to maintain their obesity. Compared with lean SD rats (Harlan), notable differences of this line of obese SD rats include: rapid weight gain and visceral adipose accumulation in the first month of life, impaired insulin sensitivity and altered glucose and lipid metabolism evidenced by increased fasting insulin concentrations and liver TG content at 3 months, and increased free fatty acids and skeletal muscle TG content at six months of age [38], [39]. While these obese rats exhibit a progressive increase of insulin resistance over their life, they do not develop overt diabetes [8].
At five months of age, obese SD rats were randomized into two treatment groups (control or DDT mixture) matched for body weight and fasting lipid concentrations. In the initial cohort, three rats in the control group died during the calorie restriction period at weeks five, six and eight. Hence, these rats were excluded from the data analysis, which was truncated at six weeks. In the 2nd study cohort, core body temperature measurements were added based on our previous observation of decreased body temperature resulting from DDT exposure in mice [23]. When these two cohorts of animals were combined, the total number of rats in the analyses for body weight and lipids was 17 (n = 7 control rats and n = 10 DDT mixture rats), and for body temperature was 10 (n = 5 control rats and n = 5 DDT mixture rats). The rats were individually housed with a 12-h light/dark cycle (light 5:00–17:00). The room was maintained at 22 ± 1 °C.
Until five months of age, all rats had access to water and standard rat chow (3.1 kcal/g, 24% protein, 58% carbohydrate, and 18% fat per kcal, 2018; Teklad, Madison, WI) ad libitum. Beginning at five months of age, from study day 1 to day 30, rats were provided water and fed a high fat/moderate sugar diet (HFD, 4.73 kcal/g, 20% protein, 35% carbohydrate, of which 17% was sugar/sucrose, and 45% fat per kcal, D12451, Research Diets, New Brunswick, NJ) ad libitum. In the two weeks following HFD feeding, all animals received a calorically restricted (60% caloric reduction) standard rat chow at 16:00 based on their average energy intake during the 30 day HFD feeding period to model a 20–25% reduction of body weight similar to the rapid short term weight loss typically achieved with bariatric surgery [1]. Throughout the study, body weight and food intake were recorded three times weekly.
The cumulative dose of the DDT mixture used in this study was based on the cumulative dose of a DDT mixture contained in a salmon oil based-HFD administered for 30 days to SD rats in a previously published study [34]. During the HFD feeding period, rats in the DDT mixture group (n = 10) received 5.60 μg DDT mixture/kg body weight/day (1.12 μg DDT mixture/mL of certified organic olive oil vehicle) once a week for four weeks by oral gavage. The 5.60 μg DDT mixture was comprised of 1.05 μg p,p′-DDT (accounting for 97.4% purity neat, AccuStandard, New Haven, CT), 0.31 μg o,p′-DDT (accounting for 99.7% purity neat, AccuStandard), and 4.24 μg p,p′-DDE (100% purity neat, AccuStandard). During the same dosing interval, rats in the control group (n = 7) received an identical dose volume of 5 ml certified (Quality Assurance International) organic olive oil/kg body weight vehicle. The procedures of this study were approved by University of California Davis Animal Care and Use Committee protocol #17627.
2.2. Lipid parameters (n = 17)
Blood was collected in the morning after food was withheld from rats overnight (13 h) on study day one (baseline), study day 31 (after four weeks of HFD), and study day 45 (after two weeks of calorie-restricted diet). Fasting plasma TG (Thermo Fisher Scientific, Waltham, MA) and NEFA (Wako Chemicals, Richmond, VA) concentrations were evaluated enzymatically. An oral lipid tolerance test (OLTT) was performed as described [9] after four weeks of HFD and two weeks of caloric restriction and TG concentrations were measured as above.
2.3. Core body temperature (n = 10)
Core body temperature was measured as described [23], when between 16:00 and 17:00 weekly the thermocoupled probe was gently inserted 2.5 cm into the rectum of rats of the second cohort.
2.4. Thyroid hormone and thyroid-stimulating hormone (n = 17)
Total plasma thyroid hormone (T4, Calbiotech Spring Valley, CA) and thyroid-stimulating hormone (TSH, Alpco diagnostics, Boston, MA) levels were evaluated using enzyme-linked immunosorbent assay (ELISA) tests after four weeks of HFD and after two weeks of calorie restriction.
2.5. Statistics
Daily caloric intake was calculated from the difference in food weight per single housed rat cage divided by the number of days rats had access (e.g., two–three days) and multiplied by the caloric density of the diet. The caloric content of gavaged oil was added to the daily caloric intake from food on the days when rats were gavaged. We defined food efficiency as body weight gained (grams) divided by total energy (kilocalories) consumed per week [10] as an index of energy efficiency. Body mass, caloric intake, food efficiency, lipids, thyroid hormones, and temperature were evaluated in general linear models stratified by diet to calculate least square means, standard errors, and p-values (PROC GLM, SAS version 9, Cary, NC). Longitudinal analysis of body mass and of temperature was conducted using the model described above, with the addition of study day and a study week × treatment term as fixed effects, and individual rat identifiers as random effects while stratified by diet. In all models, we used a significance threshold of p < 0.05 for main effects.
3. Results
3.1. Rats fed the DDT mixture had reduced food efficiency
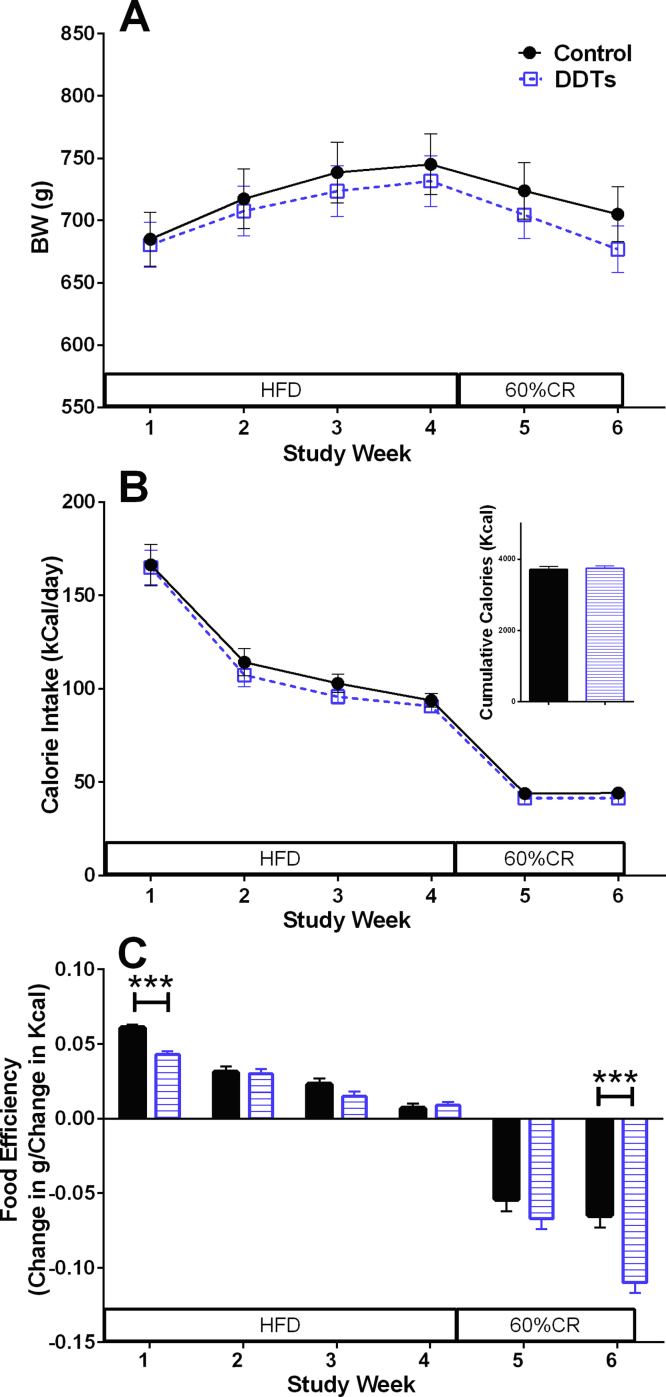
In order to examine effects of the DDT mixture on energy intake and obesity, body weight and food intake were measured. Initiation of HFD feeding resulted in transient hyperphagia consistent with other studies [11], [32]. There was no difference in energy intake between the treatments; however, compared with the control animals, the rate of weight gain in the exposed rats during HFD was significantly lower (p < 0.01), and their rate of weight loss during dietary restriction of was significantly higher (p < 0.001; Fig. 1A and B).
Fig. 1.
Effect of DDT mixture on body weight, energy intake, and food efficiency. (A) Body weight, (B) daily caloric intake, cumulative caloric intake, and (C) food efficiency in 7 control and 10 DDT mixture exposed rats. ***p < 0.001 vehicle control vs. DDT mixture. Data are shown as mean ± SEM. HFD: high fat diet, CR: calorie restriction.
As expected during any weight loss, food efficiency declined to negative values in the control rats [16], [15]. Across the study, compared to controls, food efficiency tended to be lower in rats exposed to the DDT mixture. This difference was significant during the initial week of HFD (p < 0.001; Fig. 1C), and the second week of calorie restriction (p < 0.001; Fig. 1C), when the food efficiency of rats exposed to the DDT mixture decreased by 64% from the first week of caloric restriction, compared with a 22% decrease in the food efficiency of the control group.
3.2. DDT mixture administration increases postprandial NEFA after HFD
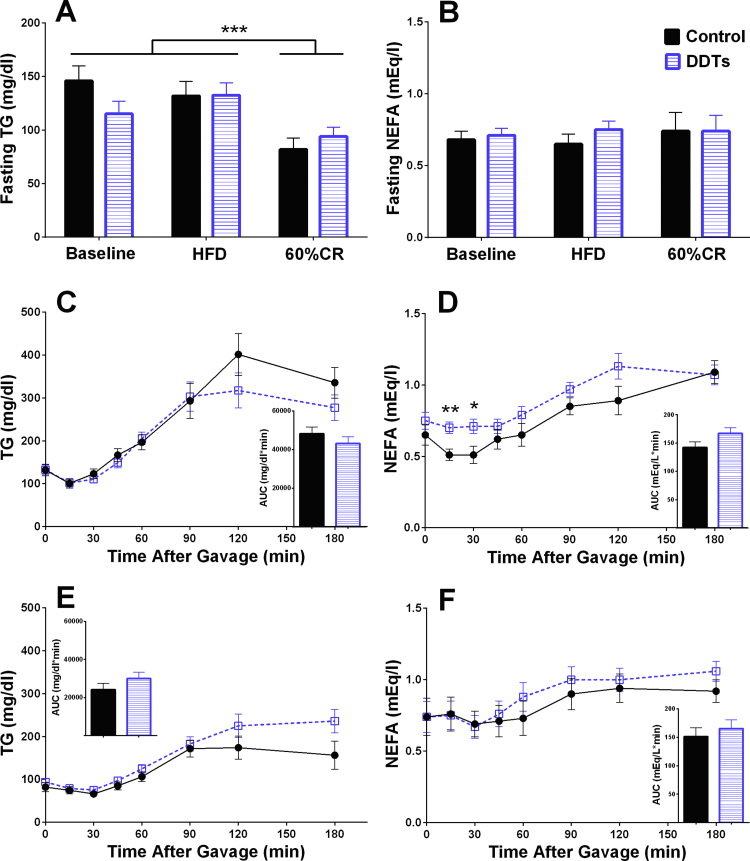
In order to examine the potential impact of DDT exposure on lipid metabolism, we measured fasting TG and NEFA levels and performed OLTT (Fig. 2). Fasting TG and NEFA levels were similar across treatments (Fig. 2A and B). In both treatment groups fasting TG levels remained unchanged after one month of HFD feeding, but were significantly decreased (p < 0.001) after two weeks of caloric restriction relative to the HFD feeding period (Fig. 2A). Further, there were no changes of fasting NEFA levels in animals receiving the DDT mixture or in response to the HFD (Fig. 2B).
Fig. 2.
Effect of DDT mixture on lipid homeostasis. Fasting plasma (A) triglycerides and (B) NEFAs. Plasma (C) triglyceride and (D) NEFA excursions and their respective AUC during OLTT after 1 month of HF feeding. Plasma (E) triglyceride and (F) NEFA excursions and their respective AUC during OLTT after 2 weeks of 60% calorie restriction. (n = 7 control and n = 10 DDT mixture exposed rats), *p < 0.05, **p < 0.01, ***p < 0.001 vehicle control vs. DDT mixture. Data are shown as mean ± SEM. HFD: high fat diet, CR: calorie restriction.
While there was no effect of DDT mixture on postprandial TG after the HFD feeding period, the postprandial plasma NEFA levels in the DDT mixture exposed rats were significantly higher compared with control rats during the first 30 min after this OLTT began (p < 0.05, Fig. 2D). There were no significant differences between treatment groups in the results from the OLTT performed after 2 weeks of caloric restriction (Fig. 2E and F).
3.3. Rats exposed to the DDT mixture had lower core body temperatures
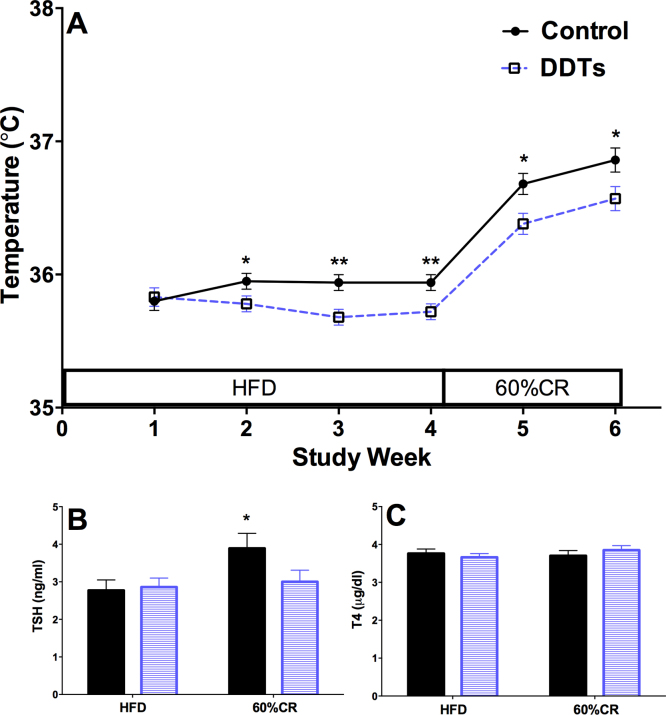
In order to assess energy expenditure, we measured core body temperature because maintaining body temperature is the largest component of energy expenditure in rodents [25]. As rats progressed through the DDT mixture dosing period, the core body temperature of rats exposed to the DDT mixture decreased to lower levels than observed in the control rats (p < 0.05; Fig. 3A). During the calorie restriction period, the DDT treated rats continued to have lower core body temperatures than control rats (p < 0.05; Fig. 3A).
Fig. 3.
Effect of DDT mixture on core body temperature and thyroid hormones. (A) Core body temperature (p = 0.15 and 0.10 at study weeks 2 and 5, respectively) in 5 rats/treatment). Fasting plasma (B) TSH and (C) T4 in 7 control and 10 DDT mixture exposed rats. *p < 0.05, ***p < 0.001 vehicle control vs. DDT mixture. Data are shown as mean ± SEM. HFD: high fat diet, CR: calorie restriction.
3.4. Rats exposed to DDT mixture had lower plasma TSH levels after calorie restriction
Hypothesizing that lower core body temperatures resulted from impaired thyroid hormone regulation of thermogenesis [12], [14], we measured levels of TSH and T4 in the plasma of fasted rats on study day 31 (after one month of HFD feeding), and on study day 45 (after two weeks of calorie restriction). Consistent with our hypothesis, the transition of plasma TSH levels from HFD to caloric restriction was attenuated in rats exposed to the DDT mixture (p = 0.04; Fig. 3B). However, there was no effect of DDT mixture on TSH levels during HFD or on T4 levels during either HFD or caloric restriction (Fig. 3B and C).
4. Discussion
In the present study, we investigated whether exposure to DDT and DDE at levels expected through the human food supply [34], in combination with HFD feeding and caloric restriction, had effects on energy balance, lipid metabolism and thermogenesis. Our results suggest that exposure to DDT and DDE during HFD feeding impairs food efficiency, thermogenesis and postprandial lipid utilization. Our data also suggests that DDT and DDE released from adipose tissue in response to dynamic weight loss during calorie restriction may decrease food efficiency, thermogenesis, and circulating TSH.
The results of the study reveal that during HFD feeding, core body temperature decreased as exposure to the DDT mixture increased. This effect was sustained through the caloric restriction period. Impaired thermogenesis and reduced energy expenditure was also observed when a higher dose of a DDT mixture was administered to mice [23]. Their findings support the possibility that the observed impairment of thermogenesis may have been due to a reduction of T3 mediated activation of thermogenesis in brown adipose tissue [23]. This is aligned with a number of human studies, which found levels of thyroid hormones in serum and cord blood, and resting metabolic rate were negatively associated with circulating DDT and DDE concentrations [26], [27], [31]. Consistent with our hypothesis that DDT and DDE released from adipose tissue during weight loss result in toxicity in a non-adipose target tissue, caloric restriction did not increase TSH in rats previously exposed to the DDT mixture despite increased TSH in control rats. This may be linked to decreased body temperature in DDT exposed rats, since reduced TSH signaling decreases body temperature [12] and DDT reduces TSH stimulation of cAMP [35], a known mediator of thermogenesis [5]. Future studies in our present model are needed to mechanistically confirm whether DDT and DDE reduced brown adipose thermogenesis and whole-body energy expenditure.
Exposure to the DDT mixture also resulted in increased postprandial plasma NEFA levels during OLTT after a period of HF feeding. This was consistent with a previously reported effect of DDT on NEFA homeostasis, in which a high DDT exposure increased hepatic NEFA content in rats [30]. Unfortunately, despite the lipid kinetics expected with insulin-mediated suppression of lipolysis [4], [40], we know of no other studies that examined NEFA levels during early periods in OLTTs. The impaired suppression of postprandial NEFA concentrations in obese rats exposed to the DDT mixture here is consistent with a greater degree of insulin resistance [3], [29] than in obese unexposed rats, however further mechanistic studies are needed to confirm these findings. The lack of effect of the DDT mixture exposure on fasting TG and NEFA concentrations is consistent with two recent studies of the effects of DDT exposure in mice [17], [23].
Because the calorically restricted diet resulted in only 6–8% loss of body weight over a 2 week period, a primary limitation of the present study is its inability to achieve the 20–25% loss of body weight observed after bariatric surgery [1]. With this lower degree of weight loss, the DDT and DDE mobilization here may not have been comparable to that exhibited clinically in patients following bariatric surgery, and this could have diminished the power of the study to detect the endocrine disruptive effects of mobilized DDT and DDE. Nevertheless, rats exposed to the DDT mixture had decreased food efficiency as calorie restriction proceeded. This was consistent with our hypothesis of altered metabolism during weight loss among DDT exposed rats given healthy rats undergoing weight loss would be expected to have a ‘starvation response,’ where weight loss is opposed by an increased food efficiency [16].
In conclusion, this study suggests that rats exposed to levels of DDT, DDE, and HFD found in human diets have decreased core body temperature and a modest lipid intolerance. This is the first study in rats to show DDT can depress thermogenesis and at a much lower DDT dose than previously observed [23]. This is also the first experimental study to illustrate that mobilization of a low dose DDT mixture during weight loss may have unexpected metabolic consequences, specifically an impairment of food efficiency and in thyroid hormone regulation. Given the potential importance of these observations and their unclear mechanisms, further research is needed to investigate the specific mechanisms leading to the effects of DDT and DDE exposure on substrate utilization and energy metabolism.
Acknowledgements
This research was support by NIH (ES019919 to ML; DK095980, HL091333, HL107256, HL107256 to PH), a University of California, Davis College of Agricultural and Environmental Sciences Programmatic Initiative (to ML and PH), and a Multi-campus grant from the University of California, Office of the President (#142691 to PH).
References
- 1.Asztalos B.F., Swarbrick M.M., Schaefer E.J., Dallal G.E., Horvath K.V., Ai M., Stanhope K.L., Austrheim-Smith I., Wolfe B.M., Ali M., Havel P.J. Effects of weight loss, induced by gastric bypass surgery, on HDL remodeling in obese women. J. Lipid Res. 2010;51:2405–2412. doi: 10.1194/jlr.P900015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Backman L., Kolmodinhedman B. Concentration of Ddt and Dde in plasma and subcutaneous adipose-tissue before and after intestinal-bypass operation for treatment of obesity. Toxicol. Appl. Pharm. 1978;46:663–669. doi: 10.1016/0041-008x(78)90311-3. [DOI] [PubMed] [Google Scholar]
- 3.Borel A.L., Boulet G., Nazare J.A., Smith J., Almeras N., Tremblay A., Bergeron J., Poirier P., Carpentier A.C., Despres J.P. Improved plasma FFA/insulin homeostasis is independently associated with improved glucose tolerance after a 1-year lifestyle intervention in viscerally obese men. Diabetes Care. 2013;36:3254–3261. doi: 10.2337/dc12-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boston R.C., Moate P.J. A novel minimal model to describe NEFA kinetics following an intravenous glucose challenge. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1140–R1147. doi: 10.1152/ajpregu.00749.2007. [DOI] [PubMed] [Google Scholar]
- 5.Bukowiecki L.J., Follea N., Lupien J., Paradis A. Metabolic relationships between lipolysis and respiration in rat brown adipocytes — the role of long-chain fatty-acids as regulators of mitochondrial respiration and feedback inhibitors of lipolysis. J. Biol. Chem. 1981;256:2840–2848. [PubMed] [Google Scholar]
- 6.Colditz G.A., Willett W.C., Rotnitzky A., Manson J.E. Weight gain as a risk factor for clinical diabetes mellitus in women. Ann. Intern. Med. 1995;122:481–486. doi: 10.7326/0003-4819-122-7-199504010-00001. [DOI] [PubMed] [Google Scholar]
- 7.Cummings B.P., Bettaieb A., Graham J.L., Stanhope K.L., Kowala M., Haj F.G., Chouinard M.L., Havel P.J. Vertical sleeve gastrectomy improves glucose and lipid metabolism and delays diabetes onset in UCD-T2DM rats. Endocrinology. 2012;153:3620–3632. doi: 10.1210/en.2012-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings B.P., Digitale E.K., Stanhope K.L., Graham J.L., Baskin D.G., Reed B.J., Sweet I.R., Griffen S.C., Havel P.J. Development and characterization of a novel rat model of type 2 diabetes mellitus: the UC Davis type 2 diabetes mellitus UCD-T2DM rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;295:R1782–R1793. doi: 10.1152/ajpregu.90635.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cummings B.P., Strader A.D., Stanhope K.L., Graham J.L., Lee J., Raybould H.E., Baskin D.G., Havel P.J. Ileal interposition surgery improves glucose and lipid metabolism and delays diabetes onset in the UCD-T2DM rat. Gastroenterology. 2010;138:2437–2446. doi: 10.1053/j.gastro.2010.03.005. e2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Densmore V.S., Morton N.M., Mullins J.J., Seckl J.R. 11 Beta-hydroxysteroid dehydrogenase type 1 induction in the arcuate nucleus by high-fat feeding: a novel constraint to hyperphagia? Endocrinology. 2006;147:4486–4495. doi: 10.1210/en.2006-0106. [DOI] [PubMed] [Google Scholar]
- 11.Dess N.K., Choe S., Minor T.R. The interaction of diet and stress in rats: high-energy food and sucrose treatment. J. Exp. Psychol. Anim. Behav. Process. 1998;24:60–71. doi: 10.1037//0097-7403.24.1.60. [DOI] [PubMed] [Google Scholar]
- 12.Endo T., Kobayashi T. Thyroid-stimulating hormone receptor in brown adipose tissue is involved in the regulation of thermogenesis. Am. J. Physiol. Endocrinol. Metab. 2008;295:E514–E518. doi: 10.1152/ajpendo.90433.2008. [DOI] [PubMed] [Google Scholar]
- 13.Gloy V.L., Briel M., Bhatt D.L., Kashyap S.R., Schauer P.R., Mingrone G., Bucher H.C., Nordmann A.J. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. doi: 10.1136/bmj.f5934. (Clinical research ed.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Golozoubova V., Gullberg H., Matthias A., Cannon B., Vennstrom B., Nedergaard J. Depressed thermogenesis but competent brown adipose tissue recruitment in mice devoid of all hormone-binding thyroid hormone receptors. Mol. Endocrinol. 2004;18:384–401. doi: 10.1210/me.2003-0267. [DOI] [PubMed] [Google Scholar]
- 15.Guijarro A., Osei-Hyiaman D., Harvey-White J., Kunos G., Suzuki S., Nadtochiy S., Brookes P.S., Meguid M.M. Sustained weight loss after Roux-en-Y gastric bypass is characterized by down regulation of endocannabinoids and mitochondrial function. Ann. Surg. 2008;247:779–790. doi: 10.1097/SLA.0b013e318166fd5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guijarro A., Suzuki S., Chen C., Kirchner H., Middleton F.A., Nadtochiy S., Brookes P.S., Niijima A., Inui A., Meguid M.M. Characterization of weight loss and weight regain mechanisms after Roux-en-Y gastric bypass in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R1474–R1489. doi: 10.1152/ajpregu.00171.2007. [DOI] [PubMed] [Google Scholar]
- 17.Howell G.E., 3rd, Mulligan C., Meek E., Chambers J.E. Effect of chronic p,p′-dichlorodiphenyldichloroethylene (DDE) exposure on high fat diet-induced alterations in glucose and lipid metabolism in male C57BL/6H mice. Toxicology. 2015;328:112–122. doi: 10.1016/j.tox.2014.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hubert H.B., Feinleib M., McNamara P.M., Castelli W.P. Obesity as an independent risk factor for cardiovascular-disease — a 26-year follow-up of participants in the framingham heart-study. Circulation. 1983;67:968–977. doi: 10.1161/01.cir.67.5.968. [DOI] [PubMed] [Google Scholar]
- 19.Hue O., Marcotte J., Berrigan F., Simoneau M., Dore J., Marceau P., Marceau S., Tremblay A., Teasdale N. Increased plasma levels of toxic pollutants accompanying weight loss induced by hypocaloric diet or by bariatric surgery. Obes Surg. 2006;16:1145–1154. doi: 10.1381/096089206778392356. [DOI] [PubMed] [Google Scholar]
- 20.Inmaculada Sanz-Gallardo M., Guallar E., van Tveer P., Longnecker M.P., Strain J.J., Martin B.C., Kardinaal A.F., Fernandez-Crehuet J., Thamm M., Kohlmeier L., Kok F.J., Martin-Moreno J.M. Determinants of p,p-dichlorodiphenyldichloroethane (DDE) concentration in adipose tissue in women from five European cities. Arch. Environ. Health. 1999;54:277–283. doi: 10.1080/00039899909602486. [DOI] [PubMed] [Google Scholar]
- 21.Kim M.J., Marchand P., Henegar C., Antignac J.P., Alili R., Poitou C., Bouillot J.L., Basdevant A., Le Bizec B., Barouki R., Clement K. Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss. Environ. Health Perspect. 2011;119:377–383. doi: 10.1289/ehp.1002848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.La Merrill M., Emond C., Kim M.J., Antignac J.P., Le Bizec B., Clement K., Birnbaum L.S., Barouki R. Toxicological function of adipose tissue: focus on persistent organic pollutants. Environ. Health Perspect. 2013;121:162–169. doi: 10.1289/ehp.1205485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.La Merrill M., Karey E., Moshier E., Lindtner C., La Frano M.R., Newman J.W., Buettner C. Perinatal exposure of mice to the pesticide DDT impairs energy expenditure and metabolism in adult female offspring. PloS One. 2014;9:e103337. doi: 10.1371/journal.pone.0103337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee D.H., Steffes M.W., Sjodin A., Jones R.S., Needham L.L., Jacobs D.R., Jr. Low dose organochlorine pesticides and polychlorinated biphenyls predict obesity, dyslipidemia, and insulin resistance among people free of diabetes. PloS One. 2011;6:e15977. doi: 10.1371/journal.pone.0015977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lichtenbelt W.D.V.M., Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;301:R285–R296. doi: 10.1152/ajpregu.00652.2010. [DOI] [PubMed] [Google Scholar]
- 26.Lopez-Espinosa M.J., Vizcaino E., Murcia M., Llop S., Espada M., Seco V., Marco A., Rebagliato M., Grimalt J.O., Ballester F. Association between thyroid hormone levels and 4,4′-DDE concentrations in pregnant women (Valencia, Spain) Environ. Res. 2009;109:479–485. doi: 10.1016/j.envres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Maervoet J., Vermeir G., Covaci A., Van Larebeke N., Koppen G., Schoeters G., Nelen V., Baeyens W., Schepens P., Viaene M.K. Association of thyroid hormone concentrations with levels of organochlorine compounds in cord blood of neonates. Environ. Health Perspect. 2007;115:1780–1786. doi: 10.1289/ehp.10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBride P.E. Triglycerides and risk for coronary heart disease. JAMA J. Am. Med. Assoc. 2007;298:336–338. doi: 10.1001/jama.298.3.336. [DOI] [PubMed] [Google Scholar]
- 29.McLaughlin T., Yee G., Glassford A., Lamendola C., Reaven G. Use of a two-stage insulin infusion study to assess the relationship between insulin suppression of lipolysis and insulin-mediated glucose uptake in overweight/obese, nondiabetic women. Metab. Clin. Exp. 2011;60:1741–1747. doi: 10.1016/j.metabol.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narayan S., Dani H.M., Misra U.K. Changes in lipid profiles of liver microsomes of rats following intratracheal administration of DDT or endosulfan. J. Environ. Sci. Health B. 1990;25:243–257. doi: 10.1080/03601239009372687. [DOI] [PubMed] [Google Scholar]
- 31.Pelletier C., Doucet E., Imbeault P., Tremblay A. Associations between weight-loss induces changes in plasma organochlorine concentrations, serum T-3 concentration and resting metabolic rate. Obes Res. 2001;9:199S. doi: 10.1093/toxsci/67.1.46. [DOI] [PubMed] [Google Scholar]
- 32.Ramirez I. High-fat diets stimulate transient hyperphagia whereas wet diets stimulate prolonged hyperphagia in Fischer rats. Physiol. Behav. 1991;49:1223–1228. doi: 10.1016/0031-9384(91)90355-r. [DOI] [PubMed] [Google Scholar]
- 33.Rivero-Rodriguez L., Borja-Aburto V.H., Santos-Burgoa C., Waliszewskiy S., Rios C., Cruz V. Exposure assessment for workers applying DDT to control malaria in Veracruz, Mexico. Environ. Health Perspect. 1997;105:98–101. doi: 10.1289/ehp.9710598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruzzin J., Petersen R., Meugnier E., Madsen L., Lock E.J., Lillefosse H., Ma T., Pesenti S., Sonne S.B., Marstrand T.T., Malde M.K., Du Z.Y., Chavey C., Fajas L., Lundebye A.K., Brand C.L., Vidal H., Kristiansen K., Froyland L. Persistent organic pollutant exposure leads to insulin resistance syndrome. Environ. Health Perspect. 2010;118:465–471. doi: 10.1289/ehp.0901321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santini F., Vitti P., Ceccarini G., Mammoli C., Rosellini V., Pelosini C., Marsili A., Tonacchera M., Agretti P., Santoni T., Chiovato L., Pinchera A. In vitro assay of thyroid disruptors affecting TSH-stimulated adenylate cyclase activity. J. Endocrinol. Invest. 2003;26:950–955. doi: 10.1007/BF03348190. [DOI] [PubMed] [Google Scholar]
- 36.Sjostrom C.D., Lissner L., Wedel H., Sjostrom L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obes Res. 1999;7:477–484. doi: 10.1002/j.1550-8528.1999.tb00436.x. [DOI] [PubMed] [Google Scholar]
- 37.Skinner M.K., Manikkam M., Tracey R., Guerrero-Bosagna C., Haque M., Nilsson E.E. Ancestral dichlorodiphenyltrichloroethane (DDT) exposure promotes epigenetic transgenerational inheritance of obesity. BMC Med. 2013;11:16. doi: 10.1186/1741-7015-11-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanhope K.L., Kras K.M., Moreno-Aliaga M.J., Havel P.J. A comparison of adipocyte size and metabolism in Charles river and Harlan Sprague Dawley rats (abstract) Obes Res. 2000;8:66S. [Google Scholar]
- 39.Stanhope K.L., Sinha M., Graham J.L., Havel P.J. Low circulating adiponectin levels and reduced adipocyte adiponectin production in obese, insulin-resistant Sprague-Dawley rats (abstract) Diabetes. 2002;52:A404. [Google Scholar]
- 40.Sumner A.E., Bergman R.N., Vega G.L., Genovese D.J., Cochran C.S., Pacak K., Watanabe R.M., Boston R.C. The multiphasic profile of free fatty acids during the intravenous glucose tolerance test is unresponsive to exogenous insulin. Metab. Clin. Exp. 2004;53:1202–1207. doi: 10.1016/j.metabol.2004.03.020. [DOI] [PubMed] [Google Scholar]
- 41.Thayer K.A., Heindel J.J., Bucher J.R., Gallo M.A. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ. Health Perspect. 2012;120:779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vest A.R., Heneghan H.M., Agarwal S., Schauer P.R., Young J.B. Bariatric surgery and cardiovascular outcomes: a systematic review. Heart. 2012;98:1763–1777. doi: 10.1136/heartjnl-2012-301778. [DOI] [PubMed] [Google Scholar]
- 43.Wilson P.W., Bozeman S.R., Burton T.M., Hoaglin D.C., Ben-Joseph R., Pashos C.L. Prediction of first events of coronary heart disease and stroke with consideration of adiposity. Circulation. 2008;118:124–130. doi: 10.1161/CIRCULATIONAHA.108.772962. [DOI] [PubMed] [Google Scholar]
- 44.World Health Organization . World Health Organization; Geneva: 2011. The Use of DDT in Malaria Vector Control. WHO Position Statement. [Google Scholar]