Highlights
-
•
BMAA could be detected in seafood which have been imported to Sweden origin from four different continents.
-
•
Quantification of both free and total concentrations BMAA in mussels and scallops.
-
•
The highest concentration of total BMAA was found in imported cooked and canned mussels.
Chemical compounds studied in this article: β-N-Methylamino-l-alanine (BMAA) CID:105089; l-2,4-Diaminobutyric acid (DAB) CID:2724265; N-(2-Aminoethyl) glycine (AEG) CID:428913; 5-Dimethylamino-1-naphthalenesulfonyl chloride/dansyl chloride (DNS) CID:11801
Keywords: β-N-Methylamino-l, -alanine (BMAA), Seafood, Quantification, Dansyl chloride (DNS), UHPLC–MS/MS, Health risks
Abstract
β-N-Methylamino-l-alanine (BMAA) is a potential neurotoxin associated with the aquatic environment. Validated analytical methods for the quantification of both free and total concentrations of BMAA were used in an investigation of seafood purchased from different grocery stores in Uppsala, Sweden. The analysis was performed using ultra high performance liquid chromatography-electrospray ionization-tandem mass spectrometry (UHPLC-ESI–MS/MS) and detection of BMAA as a dansyl derivate. The determined concentrations of free BMAA (after a simple trichloroacetic acid extraction) in mussels and scallops were up to 0.46 μg g−1 wet homogenate. The total BMAA (after hydrochloric acid hydrolysis) levels were between 0.29 and 7.08 μg g−1 wet mussel homogenate. The highest concentration of total BMAA was found in imported cooked and canned mussels which contained about ten times the quantity of BMAA measured in domestic cooked and frozen mussels. In this study it was also concluded that BMAA could be detected in seafood origin from four different continents. The risks associated with human exposure to BMAA through food are unknown today. However, the results of this study show that imported seafood in Sweden contain BMAA, indicating that this area needs more investigation, including a risk assessment regarding the consumption of e.g., mussels, scallops and crab.
1. Introduction
Bioaccumulation of the potential neurotoxin β-N-methylamino-l-alanine (BMAA) in the aquatic food chain and the public health risks associated with exposure to BMAA through consumption of seafood, have been frequently discussed topics in recent years [1], [2], [3], [4], [5], [6]. BMAA was initially reported to be produced by prokaryotic cyanobacteria [7], but during the past year, several studies have suggested new sources of production by eukaryotic organisms such as diatoms [8] and dinoflagellates [9], [10].
The story of BMAA and its assumed connection to the neurological diseases Amyotrophic Lateral Sclerosis (ALS) and Parkinson’s disease (PD) started on the island of Guam in the Pacific Ocean [11]. The indigenous population of the Island, the Chamarro, has had a higher incidence of symptoms reminiscent of amyotrophic lateral sclerosis-parkinsonism dementia complex (ALS–PDC) compared to populations in other areas [12]. The finding that their traditional food contained high levels of BMAA [12] initiated the hypothesis that this toxin could be the causative agent of this disease [13], [14]. The damage BMAA might cause in the body is not fully understood but several studies have indicated that BMAA could be misincorporated into proteins [15], [16], [17], [18], [19] which might disrupt fundamental processes and cause autoimmunity disorders. Studies on the distribution and bioavailability of BMAA in animals and humans have been carried out with varying outcomes [4], [5]. Some conflicting results regarding BMAA content have previously raised questions if the structure isomers to BMAA (e.g., l-2,4-diaminobutyric acid (DAB), N-(2-aminoethyl) glycine (AEG) or β-amino-N-methyl-alanine (BAMA)) could have been erroneous detected as “BMAA” in studies where less selective detection methods were used [4], [6], [20], [21]. Today many published studies concerning BMAA have used selective detection methods separating these isomers. DAB is a known potential neurotoxin which has been investigated since 1960s [22] but the potential toxicity and effects of the BMAA isomers; AEG and BAMA have only sparsely been studied so further investigations needs to be done of these compounds [23].
The detection of BMAA in seafood species e.g., oysters and mussels [6], [23], [24], [25], [26], [27] has also initiated additional studies of uptake, metabolism and possible elimination of BMAA in mussels [28], [29]. Findings in lobsters collected in the Florida Bay [5], and in apex predators such as sharks samples collected in South Florida coastal waters [30] have also confirmed the suspicion of cumulative bioaccumulation in the marine environment.
Depending on the extraction method used, the determined levels of BMAA have been referred to as free or total concentrations [21]. The free form has been defined as the non-protein-associated BMAA and the total as the sum of the non-protein-associated and the protein-associated BMAA. The detection of free BMAA has seldom been reported even though it seems reasonable to assume that biomagnification could more easily originate from the free rather than the bound form of BMAA [16], [23]. A few different methods for the analysis of free BMAA have been published, but a similar approach involving extraction using either diluted trichloroacetic acid or solutions of methanol/water has been used in most of them. For the analysis of total BMAA, the methods have almost exclusively consisted of hydrolysis with 6 N hydrochloric acid at about 110–112 °C for 18–20 h. Most published studies in this area have presented results of total concentrations of BMAA [4], [21].
Previous studies of total concentrations of BMAA in seafood in Sweden have been carried out by three research groups [6], [25], [27]. In all these studies, west coast mussels Mytilus edulis (origin: Kattegat Sea, Sweden) were investigated and the reported levels of BMAA were in the intervals 0.151–0.201 μg g−1 dry weight [25], 0.3–1.6 μg g−1 wet homogenized mussel [27] and 0.08–0.90 μg g−1 wet weight of tissue [6]. M. edulis from the east coast (origin: Baltic Sea, Sweden) have also been studied, but no BMAA was detected [27].
The average value of consumption of fishes and seafood in Sweden (2008–2011) is about 31.2 kg/capita/year (cf. 18.5 kg/capita/year for the world average) according to the Food and Agriculture Organization of the United Nations [31]. Since many seafood products sold today for consumption in Sweden are imported, there is a need for information on whether they contain BMAA, particularly because several international studies have reported higher concentrations of BMAA in seafood [2], [9], [23], [24], [26] than the reported levels in Scandinavian seafood today [6], [25], [27]. The aim of this study was to investigate whether the toxin BMAA could be detected and quantified in different imported seafood sold in grocery stores in Uppsala, Sweden. Since free BMAA could potentially give rise to a greater biomagnification than protein-associated BMAA, another aim was to investigate both free and total BMAA using validated quantification methods with high selectivity and sensitivity. The topic of bioaccumulation of BMAA in fish was also investigated by analysis of different fish species and fish tissues (brain, liver, muscle and kidney) in both imported and domestic fishes.
2. Material and methods
2.1. Chemicals
β-N-Methylamino-l-alanine (BMAA) hydrochloride, l-2,4-diaminobutyric acid (DAB) dihydrochloride, trichloroacetic acid solution 6.1 N (TCA), sodium tetraborate decahydrate ≥99.5% and 5-dimethylamino-1-naphthalenesulfonyl chloride/dansyl chloride (DNS), were purchased from Sigma–Aldrich Corporation, Steinheim, Germany. N-(2-Aminoethyl) glycine (AEG) was obtained from TCI Europe N.V. Deuterated BMAA (2H3-BMAA) was a gift from Dr. Johan Rosén at The National Food Agency in Sweden, and it was synthesized as described in the publication by Rosén and Hellenäs [20]. Formic acid, hydrochloric acid (HCl) (37%), acetone and acetonitrile were obtained from Merck Millipore (Merck KGaA, Dramstadt, Germany). The water was purified with a MilliQ purification system from Merck Millipore (Merck KGaA, Dramstadt, Germany). All other chemicals used were of analytical grade or better.
2.2. Instrumentation
The instrumental setup in this study was the same as that described previously in Salomonsson et al. [27]. The analyses were performed with an Acquity UPLC coupled to a Xevo TQ tandem quadrupole mass spectrometer (Waters Corp. Milford, MA, USA). The chromatographic system used consisted of a C18 guard column (Waters Corp. Milford, MA, USA) and an Acquity BEH C18 column (2.1 × 100 mm [diameter × length], 1.7 μm particle size) (Waters Corp. Milford was, MA, USA) both at 65 °C. The mobile phase consisted of (A) 0.1% formic acid in water and (B) 0.1% formic acid in acetonitrile and it was delivered as a gradient. The gradient started at 10% B from 0 to 0.50 min, was increased linearly over time to 80% B at 4.00 min, and increased to 95% B at 4.50 min. At 5.50 min the gradient switched to 10% B and was held constant for 1.5 min for equilibration. The UHPLC flow was set to “waste” for the first 3 min. The flow-rate was 0.400 mL min−1 and the injection volume 10 μL. The ionization technique was positive electrospray. The ionization parameters were: source voltage 3.80 kV, cone voltage 22.00 V, extractor voltage 3.00 V, source temperature 150 °C, desolvation temperature 500 °C and desolvation gas flow 1000 L h−1. The mass spectrometric mode for qualitative and quantitative analysis was selected reaction monitoring (SRM) (Fig. 1). BMAA, 2H3-BMAA, DAB and AEG were detected by the mass spectrometer as the [M + H]+ of the dansyl derivatized compounds. The SRM transitions for the qualitative detection of BMAA (i.e., BMAA-DNS) were m/z 585 > 277 and m/z 585 > 71 (collision energy 30 eV for both SRM-transitions), m/z 585 > 170 for detection of BMAA-DNS, DAB-DNS and AEG-DNS (collision energy 45 eV) and m/z 585 > 88 for the detection of DAB-DNS and AEG-DNS (collision energy 45 eV). For the quantification, m/z 588 > 280 for 2H3-BMAA-DNS (collision energy 30 eV) and m/z 585 > 277 BMAA-DNS were used. The samples were evaluated using the software TargetLynx (Waters Corp. Milford, MA, USA).
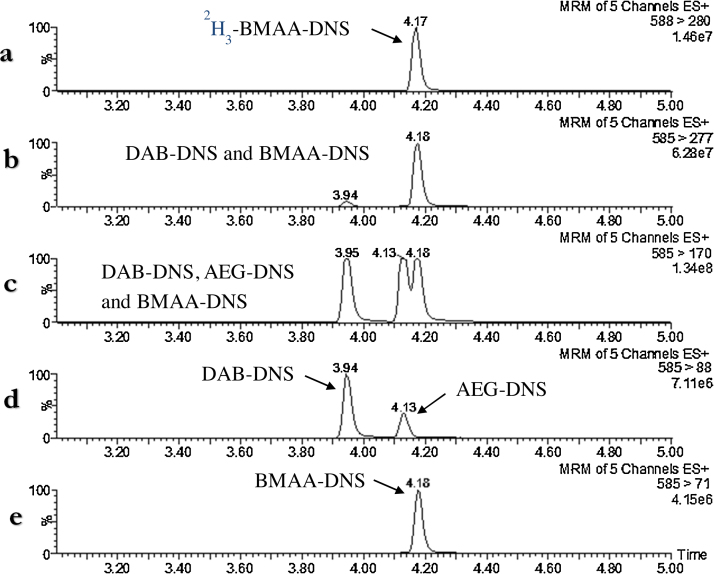
Fig. 1.
UHPLC–MS/MS analysis of a DNS derivatized standard solution containing the four substances 2H3-BMAA, BMAA, DAB and AEG in 20 mM HCl. The mass chromatograms show five SRM-transitions for detection of (a) 2H3-BMAA-DNS; (b) BMAA-DNS and DAB-DNS; (c) BMAA-DNS, DAB-DNS and AEG-DNS; (d) DAB-DNS and AEG-DNS; and (e) BMAA-DNS (not detectable DAB-DNS nor AEG-DNS).
2.3. Sample collection and pre-treatment
Different types of seafood (mussels, shrimps, crayfish, and crab) and six species of fish were chosen for the evaluation. All of the seafood and three of the species of fish were purchased in grocery stores in Uppsala, Sweden. Three fish origin from Sweden were provided by the Section of Fish, Department of Animal Health and Antimicrobial Strategies, National Veterinary Institute (SVA), Sweden. The seafood was of different origin and had been stored in different ways: cooked and canned in water; cooked and stored in salt water solutions; or cooked and then stored in a freezer. Table 1 summarizes a list of samples qualitatively investigated.
Table 1.
Results from the analysis of total concentration of BMAA in seafood and fish.
| Species | Number of containers or animals/numbers of replicates of each animal | Process and preservation method | Storage | Area of origin | BMAA m/585 > 277 and m/z 585 > 71 |
|---|---|---|---|---|---|
| Crab Cancer pagurus | 1 | Cooked | F | Ireland, North East Atlantic | |
| Muscle from claw | 3 | D. | |||
| Crab Portunus haani | 1 | Cooked | RTS | Vietnam | |
| Muscle from claw | 3 | N.D | |||
| Crayfish Procambarus clarkii | 1 | Cooked | RS | China, wild caught | |
| Muscle tissue from tail | 3 | N.D | |||
| Shrimps/Northern prawn Pandalus borealis |
3 | Cooked | Greenland, North East Atlantic | ||
| A (tails, muscle) | 3 | F | N.D | ||
| B (tails, muscle) | 3 | RS | N.D | ||
| C (tails, muscle) | 3 | F | N.D | ||
| Atlantic Salmon Salmo salar | 1 | Raw | RI | Norway, farmed | |
| Brain | 2 | N.D. | |||
| Muscle | 2 | N.D. | |||
| Sea bass Dicentrarchus labrax | 1 | Raw | RI | Italy, farmed | |
| Brain | 2 | N.D. | |||
| Lever | 2 | N.D. | |||
| Muscle | 2 | N.D. | |||
| Kidney | 2 | N.D. | |||
| Sea bream Sparus aurata | 1 | Raw | RI | Greece, farmed | |
| Brain | 2 | N.D. | |||
| Lever | 2 | N.D. | |||
| Muscle | 2 | N.D. | |||
| Kidney | 2 | N.D. | |||
| Whitefish Coregonus spp. | 1 | Raw | F | Sweden, wild caught | |
| Muscle | 2 | N.D | |||
| Pikeperch Sander lucioperca | 1 | Raw | F | Sweden, Baltic Sea, wild caught | |
| Brain | 2 | N.D. | |||
| Lever | 2 | N.D. | |||
| Muscle | 2 | N.D. | |||
| Kidney | 2 | N.D. | |||
| Sea trout Salmo truttae | 2 | Raw | F | Sweden, Bothnain Sea, wild caught | |
| Brain | 2 | N.D. | |||
| Lever | 2 | N.D. | |||
| Muscle | 2 | N.D. | |||
| Kidney | 2 | N.D. | |||
F = Freezer, RI = Refrigerator on ice, RS = Refrigerator in salt water, RTS = Room temperature canned in salt water, D. = Detected and N.D. = Not detectable (i.e., below the methods LOD).
For investigation of the total concentration of BMAA, a previously validated method (validated for mussel matrix) was used for all samples. Three samples of individual mussels/crayfish tails/scrimps from each container were prepared in triplicates. The crab claw was also prepared in triplicate but the fish species were prepared as duplicates. The soft tissue of each sample was placed in a separate plastic bag. Each sample was separately homogenized by rolling a stone rolling-pin over the plastic bag. The samples were stored at −18 °C. Before the sample preparation, each sample was thawed for a few minutes at room temperature before being weighed and placed in a glass tube.
The samples were first analyzed for the total concentration of BMAA and a selection of the sample was then prepared again with a new set of sample matrix for analysis of the free concentration of BMAA. For the latter analysis, the samples were prepared in duplicates.
The sample preparation, derivatization and analysis was performed as described in the sample preparation in Sections 2.5 and 2.6.
2.4. Preparation of calibrators and control samples
For the quantification and preparation of calibrators, two stock solutions of BMAA in 20 mM HCl were prepared. A calibration curve with ten calibrators (0.15–15 μg BMAA g−1 wet mussel homogenate) was constructed using with every second calibrator originating from the first stock solution, and the others from the second one. A third stock solution was prepared and used for the QC samples. The concentrations for the four levels of QC samples were 0.15 μg BMAA g−1 wet mussel homogenate (QC1), 0.19 μg BMAA g−1 wet mussel homogenate (QC2), 0.89 μg BMAA g−1 wet mussel homogenate (QC3) and 4.40 μg BMAA g−1 wet mussel homogenate (QC4).
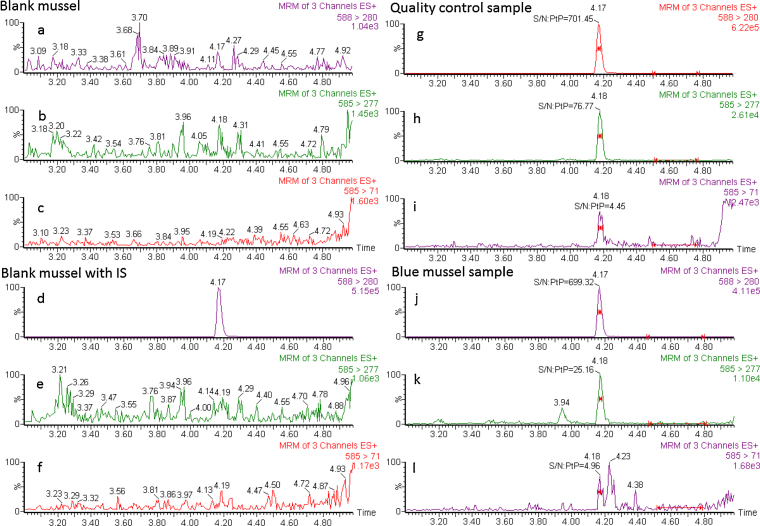
A blank matrix of mussels was used to prepare calibrators and QC samples. The mussels (originating from the Baltic Sea) were first analyzed and their BMAA content was confirmed to be negative (Fig. 4a–c). The mussel tissues were then mixed manually, and homogenized for use as a blank matrix.
Fig. 4.
Example of the results from the analysis blank, blank with IS, quality control sample (0.15 BMAA μg g−1 wet mussel homogenate) and blue mussel sample prepared and analyzed for free concentration BMAA. The UHPLC–MS/MS chromatograms for the three SRM-transitions: (a), (d), (g) and (j) 2H3-BMAA-DNS; (b), (e), (h) and (k) transition for BMAA-DNS; (c), (f), (i) and (l) for selective detection of BMAA-DNS.
One set of calibrators and control samples was prepared for the quantification of the total concentration of BMAA and three sets were prepared for the validation of the method for quantification of the free concentration of BMAA. All calibrators and control samples were prepared and derivatized as described in Sections 2.5 and 2.6.
2.5. Sample preparation
The sample matrix, comprised of about 100 mg homogenized wet material was weighed in a glass tube and the sample preparation for the analysis of total concentration of BMAA was performed using the previously validated method for mussel matrix presented by Salomonsson et al. [27]. All samples were prepared both with and without the internal standard being added.
The samples were also analyzed for the free concentration BMAA. The method used was a slightly modified method of different previously published methods for the detection of free BMAA [21]. Internal standard 75 μL 2H3-BMAA (50 μM, in 20 mM HCl (aq)) was added to about 100 mg homogenized wet material, resulting in 4.50 μg 2H3-BMAA g−1 matrix homogenate. A volume of 75 μL of 20 mM HCl (aq) (or BMAA standard solution for calibrators and control samples) was added. The sample was vortexed and then left on the bench for 30 min for equilibration. TCA (500 μL, 0.1 M) in water was added and the sample was vortexed and frozen using liquid nitrogen. The tube was then alternately vortexed (5 min) and frozen twice, followed by final vortexing for 20 min. TCA (500 μL, 0.1 M) in water was added and the sample was vortexed for 2 h. After that, the sample was centrifuged for 10 min at 3500 rpm and the supernatant was transferred to an Eppendorf tube and evaporated using a vacuum concentrator at 55 °C (RVC 2-18 HCl from Christ®, Osterode am Harz, Germany). The sample was dissolved in 1.5 mL of 20 mM HCl (aq), vortexed, filtrated using a filter Chromatofil® PET-45/15 and an aliquot of 20 μL of the sample was used for derivatization.
2.6. Derivatization
For the analysis of both the free and total concentration of BMAA the derivatization was performed as published previously [27]. Briefly, the derivatization was performed using 20 μL of a diluted extract-solution, adding 80 μL of 0.2 M borate buffer (pH 9.5) and 100 μl DNS (1 mg mL−1 in acetone). The tube was vortexed and placed in a heating block at 60 °C for 4 min. The derivatized sample was cooled to room temperature and then transferred to a vial for UHPLC–MS/MS analysis.
2.7. Identification and quantification criteria
The criteria determined previously for the positive identification of the presence of BMAA [27] were used. The criteria: a peak detected for BMAA-DNS with m/z 585 > 71 must have an S/N above 3, a peak detected for BMAA-DNS with SRM-transition m/z 585 > 277 must have an S/N above 10, the internal standard 2H3-BMAA-DNS (SRM-transition m/z 588 > 280) must have an S/N higher than 10 and, finally, the accuracy of the retention times for both 2H3-BMAA-DNS and BMAA-DNS could not differ by more than ±0.02 min from the retention times of derivatized standard solutions. Furthermore, the ratio of peak areas calculated for the fragments m/z 71 and m/z 277 should not differ by more than ±50% in relative intensity according to the guideline (EC 657/2002) [14] as the m/z 71 response is less than 10 % compared to the fragment m/z 277. In this study, the mean ion ratio for these product ions was determined using a total of 32 calibrators and QC samples and was determined to be 0.049 for detection of total BMAA, and the intra-batch standard deviation was calculated to be 1.2%. Thus for an accepted identification of total BMAA, the ion ratio must be between 0.025 and 0.074. For the detection of free BMAA the mean ion ratio for the product ions was determined to be 0.056 and the intra-batch standard deviation calculated to 1.1%, using a total of 34 calibrators and QC samples. For positive detection of free BMAA the ion ratio must be between 0.028 and 0.084.
3. Results and discussion
3.1. Method development
In this study, BMAA and two of its isomers, L-2,4-diaminobutyric acid (DAB) and N-(2-aminoethyl) glycine (AEG) were detected and mass spectrometrically separated from each other by UHPLC–MS/MS (Fig. 1). The product ion spectra of both BMAA-DNS and DAB-DNS have been presented previously by Salomonsson et al. [27]. AEG-DNS gave a similar fragmentation pattern to that of DAB-DNS (data not shown) and an AEG-DNS peak was detected in the SRM-transition m/z 585 > 88 (Fig. 1d) at retention time 4.13 min. Nevertheless no AEG-DNS peak were detected in the SRM-transition m/z 585 > 277 (Fig. 1b), or in m/z 585 > 71 (Fig. 1e). These findings supported the results that these transitions are more selective for the detection of BMAA-DNS than m/z 585 > 170 (Fig. 1c) this was especially true for the fragment m/z 71.
3.2. Analysis of total BMAA
3.2.1. Determination of the total concentration of BMAA
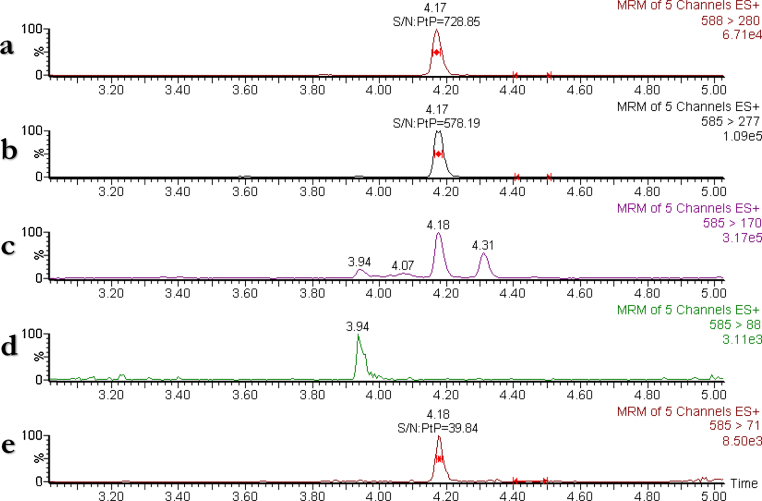
The results of the determination of total BMAA concentration are summarized in Table 1, Table 2 and Fig. 2. The analysis of crab claw Cancer pagurus (Fig. 2) and blue mussels M. edulis and M. edulis platensis, green mussels Perna canaliculus and scallops Placopecten magellanicus (Table 2) shows that all the criteria regarding S/N for the identification of BMAA were fulfilled (c.f. Section 2.7). All calculated ion ratios for the mussel samples were between 0.030 and 0.074, which also confirmed the correct identification of BMAA. BMAA has previously been identified in crustaceans such as lobster collected in Florida Bay [5] and in brain, liver and muscle tissue from carp origin from the Lake Mascoma [32], and it was then reported as a link between ALS and chronic exposure to the neurotoxin via seafood intake. A recently published study Jiang et al. [6] reported the detection of total BMAA in Scandinavian blue mussels, oysters, shrimps and two types of fish, and our study could confirm its presence in blue mussels (originating from Scandinavian) and it demonstrated the presence of the toxin in blue mussels originating from South America, green mussels (originating from Australia), scallops (originating from North America) and crab craw (originating from Europe).
Table 2.
Results from the analysis and quantification of total concentration of BMAA in mussels and scallops. Sample ID A–G refer to different seafood containers, whereas the numbers 1–3 refers to three different individual of same species. Each individual were analyzed in triplicate samples (n = 3). BM1 = blue mussel Mytilus edulis, BM2 = blue mussel Mytilus edulis platensis, GM = green mussel Perna canaliculus and S = Scallop Placopecten magellanicus.
| Sample ID Species (n =3) |
Storagea | Origin | tR (min) (peak m/z 585 > 277) | S/N peak m/z 585 > 277 | S/N peak m/z 585 > 71 | S/N peak m/z 588 > 280 | Ion ratio (peak area m/z 71)/(peak area m/z 277) (average of n = 3) | BMAA (μg g−1 wet mussel homogenate) (average of n = 3) |
|---|---|---|---|---|---|---|---|---|
| A:1 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 254 | 9 | 2298 | 0.030 | 0.56 |
| A:2 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 230 | 21 | 1910 | 0.044 | 0.59 |
| A:3 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 206 | 21 | 1568 | 0.050 | 0.50 |
| B:1 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 66 | 8 | 1804 | 0.040 | 0.29 |
| B:2 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 71 | 7 | 1524 | 0.038 | 0.31 |
| B:3 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 100 | 5 | 2315 | 0.037 | 0.28 |
| C:1 BM2 | CC, RT | South America | 4.17 | 1315 | 74 | 1677 | 0.055 | 4.46 |
| C:2 BM2 | CC, RT | South America | 4.17 | 934 | 156 | 1854 | 0.057 | 4.85 |
| C:3 BM2 | CC, RT | South America | 4.17 | 876 | 93 | 1427 | 0.053 | 4.48 |
| D:1 BM2 | CC, RT | South America | 4.17 | 936 | 107 | 1529 | 0.072 | 6.08 |
| D:2 BM2 | CC, RT | South America | 4.17 | 1676 | 129 | 1112 | 0.064 | 6.98 |
| D:3 BM2 | CC, RT | South America | 4.18 | 961 | 66 | 1284 | 0.057 | 6.52 |
| E:1 BM2 | CC, RT | South America | 4.17 | 1221 | 77 | 1434 | 0.059 | 7.08 |
| E:2 BM2 | CC, RT | South America | 4.17 | 2073 | 121 | 847 | 0.062 | 6.89 |
| E:3 BM2 | CC, RT | South America | 4.17 | 1947 | 263 | 2026 | 0.060 | 6.93 |
| F:1 BM2 | PB, F | South America | 4.17 | 534 | 29 | 1469 | 0.058 | 1.77 |
| F:2 BM2 | PB, F | South America | 4.17 | 507 | 49 | 1372 | 0.068 | 2.28 |
| F:3 BM2 | PB, F | South America | 4.17 | 316 | 58 | 1627 | 0.074 | 1.69 |
| H:1 GM | PB, F | Australia | 4.17 | 531 | 17 | 1001 | 0.051 | 0.85 |
| H:2 GM | PB, F | Australia | 4.17 | 243 | 22 | 2669 | 0.072 | 0.55 |
| H:3 GM | PB, F | Australia | 4.17 | 374 | 21 | 1795 | 0.041 | 1.14 |
| G:1 S | PB, F | North America | 4.18 | 452 | 49 | 2554 | 0.074 | 1.12 |
| G:2 S | PB, F | North America | 4.17 | 608 | 30 | 2693 | 0.069 | 1.12 |
| G:3 S | PB, F | North America | 4.17 | 354 | 36 | 2334 | 0.050 | 1.46 |
Storage: CC = Cooked and Canned, RT = Room temperature, PB = Plastic bag, F = Freezer.
Fig. 2.
Results from the analysis of crab claw Cancer pagurus prepared and analyzed according to the method validated for mussel matrix (method for total concentration BMAA). The UHPLC–MS/MS chromatograms for the five SRM-transitions: (a) 2H3-BMAA-DNS; (b) BMAA-DNS and DAB-DNS; (c) BMAA-DNS, DAB-DNS and AEG-DNS; (d) DAB-DNS and AEG-DNS; and (e) BMAA-DNS (not detectable DAB-DNS nor AEG-DNS).
3.2.2. Quantification of the total concentration of BMAA
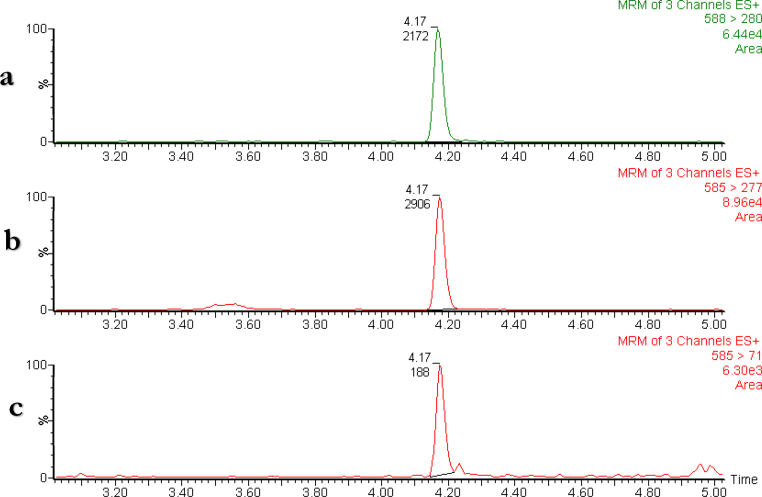
Quantification of the total concentration of BMAA in mussels and scallops was performed. Blank mussel homogenates with and without the internal standard were analyzed together with BMAA-spiked calibrators and quality control samples. BMAA was quantified in all mussel samples and the concentrations determined were between 0.29 and 7.08 μg g−1 wet mussel homogenate (Table 2 and Fig. 3). Since the concentrations reported in literature differ regarding the use of wet weight mussel or dry weight mussel, the dry weight mussel concentration must be multiplied with a dry/wet weight factor of 0.20 (a mussel contain about 80% water [27]) to be able to compare results. The highest total concentrations BMAA found were higher than previously reported concentrations in species of mollusks from the Gonghu Bay (Lake Taihu, China) (0.63–6.72 μg BMAA g−1 dry weight of tissue) Jiao et al. [2] and from Thau Lagoon (France) (0.6–14.4 μg BMAA g−1 dry weight of tissue) [23]. The results in Table 2 also show that the imported cooked and canned blue mussels from South America contained more than 10 times higher concentrations than the blue mussels farmed in Europe (Scandinavian), which also were cooked and canned. The concentrations determined in the cooked and canned Scandinavian blue mussels were comparable to those reported earlier for blue mussels that were raw and frozen from the west coast of Sweden [27]. The results in Table 2 also reveal some differences in the concentrations in mussels originating from same area. These differences could be attributed to many different factors, including how they were caught, the time of harvesting, location, storage etc.
Fig. 3.
Example of the results from the analysis of blue mussel sample Mytilus edulis platensis (method for total concentration BMAA) prepared and analyzed according to the validated method. The UHPLC–MS/MS chromatograms for the three SRM-transitions: (a) 2H3-BMAA-DNS m/z 588 > 280; (b) BMAA-DNS m/z 585 > 277 and (c) BMAA-DNS m/z 585 > 71.
3.3. Analysis of free BMAA
For the sample preparation for extraction of free BMAA a few previously reported methods/ideas [21] were tested. A blue mussel sample previously determined to have a quantifiable total BMAA concentration was used in triplicates for the comparison of three extraction solutions: 80:20 (v/v) methanol: water containing 20 mM HCl, 10% TCA (aq) and 0.1 M TCA (aq). Based on the results of detecting BMAA as a DNS derivate (data not showed) it was concluded that 0.1 M TCA gave the highest BMAA-DNS response and it was therefore used further in the sample preparation for the analysis of free BMAA.
The validation of the method for quantification of free BMAA in seafood was performed in accordance with the FDA Foods Program Guidelines for Chemical Methods [33], the FDA Guidance for Industry in Bioanalytical Method Validation [34] and EC 657/200253 [35]. The validation parameters were selectivity, linearity, intra and inter day precision, accuracy, Limit of Quantification (LOQ) and Limit of Detection (LOD).
3.3.1. Results of the validation
The selectivity of the method for BMAA was evaluated by comparing mass chromatograms obtained from blank mussel with that of a control sample (blank mussel with added BMAA) (Fig. 4a–c and g–i). No background peak above the noise level was detected at retention time 4.18 min for any of the SRM transitions.
For the evaluation of linearity, three calibration curves were constructed, one for each validation day. The regressions coefficients obtained were r2 = 0.99 for all days. On the first day, all calibrators with one exception (located in the middle-range) had back-calculated values that deviated less than 10.6% from the respective nominal value, while the excluded calibrator deviation was 22.8%. On the second day, all of the calibrators had back-calculated values that deviated by less than 6.8% and on the third day, they were less than 11.7%. All these results were within the criteria for acceptance of the validation data.
The results from the three days of validation are presented in Table 3. All three day results for intra- and inter-day precisions and accuracy were within the acceptance criteria, that is, within ±15% of their respective nominal values.
Table 3.
Summary of the validation results for the method of analyzing free concentration of BMAA, for the precision (expressed as the relative standard deviation, %) and accuracy (expressed in%).
| Sample name | Intra-day precision | Intra-day precision | Intra-day precision | Inter-day precision | Accuracy | Accuracy | Accuracy |
|---|---|---|---|---|---|---|---|
| (n = 6) | Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | |
| QC1 (0.15 μg BMAA g−1) | 7.8 | 5.7 | 5.9 | 7.6 | 97 | 104 | 94 |
| QC2 (0.19 μg BMAA g−1) | 6.9 | 3.7 | 6.1 | 6.1 | 101 | 105 | 100 |
| QC3 (0.89 μg BMAA g−1) | 3.9 | 3.3 | 2.8 | 3.4 | 109 | 101 | 102 |
| QC4 (4.40 μg BMAA g−1) | 1.6 | 1.3 | 1.6 | 2.2 | 108 | 100 | 101 |
The LOQ for the method was determined to 0.15 μg g−1 wet homogenized mussel as this was the lowest level of control samples evaluated and with a precision within ±20%. The LOD using m/z 585 > 71 was determined to be approximately 0.10 μg g−1 wet homogenized mussel (comparing Fig. 4f and i) as the S/N for the peak using daughter transition m/z 585 > 71 should be >3 for confirmation of BMAA.
3.3.2. Determination of free concentration BMAA in mussels
It was concluded that the mussel samples evaluated using this approach contained free BMAA (Table 4). Since several samples had an S/N <3 for the selective transition m/z 585 > 71, these results did not meet the criteria. Three samples also gave an ion ratio outside the accepted interval and were therefore excluded. The cause of the unstable ion ratios in these analyses could be the low intensity of the transition m/z 585 > 71 leading to the fact that a slight variation in peak area gave a large influence on the ion ratio. It can also be concluded that the variation in the values determined for the free BMAA concentration for the triplicates and different individuals are higher than the corresponding results for the total concentration of BMAA, which is expected in quantification near the LOQ. The concentration of BMAA, after a simple extraction in ambient temperature and in acidic conditions, still results in quantifiable levels of BMAA in six of the containers evaluated. The discussion in the literature on free BMAA indicates it presumably could have greater biomagnification and therefore these findings are important for the future discussion about what should be detected and evaluated concerning BMAA content in food.
Table 4.
Results from the analysis and quantification of free concentration of BMAA in mussels and scallops. Sample ID A–G refer to different seafood containers, whereas the numbers 1–3 refers to three different individual of same species. Each individual were analyzed in duplicate samples (n = 2). BM1 = blue mussel Mytilus edulis, BM2 = blue mussel Mytilus edulis platensis, GM = green mussel Perna canaliculus and S = Scallop Placopecten magellanicus.
| Sample ID Species (n = 2) |
Storagea | Origin | tR (min) (peak m/z 588 > 277) | S/N peak m/z 585 > 277 | S/N peak m/z 585 > 71 | S/N peak m/z 588 > 280 | Ion ratio (peak area m/z 71)/(peak area m/z 277) (average of n = 3) | BMAA (μg g−1 wet mussel homogenate) (average of n = 3) |
|---|---|---|---|---|---|---|---|---|
| A:1 BM1 | CC, RT | Europe (Scandinavia) | 4.18 | 34 | <3 | 3669 | – | – |
| A:2 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 34 | <3 | 5960 | – | – |
| A:3 BM1 | CC, RT | Europe (Scandinavia) | 4.17 | 192 | <3 | 5248 | – | – |
| B:1 BM1 | CC, RT | Europe (Scandinavia) | 4.18 | 27 | <3 | 7463 | – | – |
| B:2 BM1 | CC, RT | Europe (Scandinavia) | 4.18 | 22 | <3 | 6550 | – | – |
| B:3 BM1 | CC, RT | Europe (Scandinavia) | 4.18 | 17 | <3 | 3401 | – | – |
| C:1 BM2 | CC, RT | South America | 4.17 | 140 | 3 | 5296 | 0.019 | – |
| C:2 BM2 | CC, RT | South America | 4.17 | 247 | 9 | 4680 | 0.039 | 0.24 |
| C:3 BM2 | CC, RT | South America | 4.18 | 193 | 8 | 8407 | 0.048 | <0.15 |
| D:1 BM2 | CC, RT | South America | 4.18 | 101 | 3 | 5613 | 0.018 | – |
| D:2 BM2 | CC, RT | South America | 4.18 | 110 | 12 | 5216 | 0.016 | – |
| D:3 BM2 | CC, RT | South America | 4.17 | 145 | 7 | 7351 | 0.028 | <0.15 |
| E:1 BM2 | CC, RT | South America | 4.18 | 126 | 14 | 5937 | 0.045 | 0.17 |
| E:2 BM2 | CC, RT | South America | 4.18 | 135 | 10 | 5873 | 0.036 | 0.22 |
| E:3 BM2 | CC, RT | South America | 4.18 | 209 | 8 | 4776 | 0.028 | 0.23 |
| F:1 BM2 | PB, F | South America | 4.18 | 181 | 20 | 6800 | 0.059 | 0.38 |
| F:2 BM2 | PB, F | South America | 4.17 | 26 | 5 | 3637 | 0.045 | <0.15 |
| F:3 BM2 | PB, F | South America | 4.17 | 39 | <3 | 6870 | 0.020 | – |
| H:1 GM | PB, F | Australia | 4.18 | 55 | 5 | 4363 | 0.050 | <0.15 |
| H:2 GM | PB, F | Australia | 4.17 | 90 | 6 | 5705 | 0.077 | 0.15 |
| H:3 GM | PB, F | Australia | 4.17 | 121 | 9 | 7123 | 0.082 | <0.15 |
| G:1 S | PB, F | North America | 4.17 | 272 | 22 | 4474 | 0.060 | 0.46 |
| G:2 S | PB, F | North America | 4.17 | 188 | 14 | 6049 | 0.064 | 0.18 |
| G:3 S | PB, F | North America | 4.18 | 283 | 11 | 5512 | 0.032 | 0.40 |
Storage: CC = Cooked and Canned, RT = Room temperature, PB = Plastic bag, F = Freezer.
4. Conclusions
This study presents detection of both free and total concentrations BMAA in mussels and scallops, in seafood which have been imported to Sweden. Total BMAA has also been detected for the first time in crab originating from North East Atlantic and this finding correlated with previously reported findings in another crustaceans; lobster. The study also show that BMAA is present in seafood origin from four different continents. A method involving separation of BMAA from its isomers DAB and AEG using DNS derivatization, a reversed phase chromatographic system and tandem mass spectrometry has been used. The method, using dansyl chloride as the derivatization reagent, is robust, simple and cost efficient. The validation of the detection and quantification of free BMAA was performed in accordance with internationally accepted guidelines. This study is, to the best of our knowledge, the first validated quantitative method for the determination of free BMAA in mussels using DNS derivatization and UHPLC–MS/MS. Today when more knowledge about species containing BMAA is available in the literature an assignment of the risks of toxicity of BMAA would be highly desirable, and if it is determined that the risks are high, a discussion of the limits and restrictions on the times for harvest and the type of species of seafood caught is necessary. A control program for BMAA detection and quantification in seafood might be a solution to avoid humans being exposed to risks of consuming seafood containing BMAA.
Acknowledgments
The authors would like to thank Annelie Hansson for experimental assistance in obtaining the preliminary results regarding the quantification of BMAA in the mussels. The author also would like to thank Dr. Johan Rosén at The National Food Agency, Uppsala, Sweden for the gift of the deuterated internal standard.
References
- 1.Bradley W.G., Borenstein A.R., Nelson L.M., Codd G.A., Rosen B.H., Stommel E.W., Cox P.A. Is exposure to cyanobacteria an environmental risk factor for amyotrophic lateral sclerosis and other neurodegenerative diseases? Amyotroph. Lateral Scler. Frontotemporal. Degener. 2013;14:325–333. doi: 10.3109/21678421.2012.750364. [DOI] [PubMed] [Google Scholar]
- 2.Jiao Y., Chen Q., Chen X., Wang X., Liao X., Jiang L., Wu J., Yang L. Occurrence and transfer of a cyanobacterial neurotoxin β-methylamino-l-alanine within the aquatic food webs of Gonghu Bay (Lake Taihu, China) to evaluate the potential human health risk. Sci. Total Environ. 2014;468–469:457–463. doi: 10.1016/j.scitotenv.2013.08.064. [DOI] [PubMed] [Google Scholar]
- 3.Mondo K., Glover W.B., Murch S.J., Liu G., Cai Y., Davis D.A., Mash D.C. Environmental neurotoxins β-N-methylamino-l-alanine (BMAA) and mercury in shark cartilage dietary supplements. Food Chem Toxicol. 2014;70:26–32. doi: 10.1016/j.fct.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Faassen E.J. Presence of the neurotoxin BMAA in aquatic ecosystems: what do we really know? Toxins. 2014:1109–1138. doi: 10.3390/toxins6031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banack S.A., Metcalf J.S., Bradley W.G., Cox P.A. Detection of cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) within shellfish in diet of an ALS patient in Florida. Toxicon. 2014;90:167–173. doi: 10.1016/j.toxicon.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Jiang L., Kiselova N., Rosén J., Ilag L. Quantification of neurotoxin BMAA (é-N-methylamino-l-alanine) in seafood from Swedish markets. Sci. Rep. 2014;4:6931. doi: 10.1038/srep06931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox P.A., Banack S.A., Murch S. Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13380–13383. doi: 10.1073/pnas.2235808100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang L., Eriksson J., Lage S., Jonasson S., Shams S., Mehine M., Ilag L.L., Rasmussen U. Diatoms: a novel source for the neurotoxin BMAA in aquatic environments. PLoS One. 2014;9:e84578. doi: 10.1371/journal.pone.0084578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lage S., Costa P.R., Moita T., Eriksson J., Rasmussen U., Rydberg S.J. BMAA in shellfish from two Portuguese transitional water bodies suggests the marine dinoflagellate Gymnodinium catenatum as a potential BMAA source. Aquat. Toxicol. 2014;152:131–138. doi: 10.1016/j.aquatox.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 10.Jiang L., Ilag L.L. Detection of endogenous BMAA in dinoflagellate (Heterocapsa triquetra) hints at evolutionary conservation and environmental concern. PubRaw Sci. 2014;1:1–8. [Google Scholar]
- 11.Vega A., Bell B.E. α-Amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry. 1967;6:759–762. [Google Scholar]
- 12.Spencer P.S., Nunn P.B., Hugon J., Ludolph A.C., Ross S.M., Roy D.N., Robertson R.C. Guam amyotrophic lateral sclerosis-parkinsonism-dementia linked to a plant excitant neurotoxin. Science. 1987;237:517–522. doi: 10.1126/science.3603037. [DOI] [PubMed] [Google Scholar]
- 13.Banack S.A., Cox P.A. Biomagnification of cycad neurotoxins in flying foxes. Neurology. 2003;6:387–389. doi: 10.1212/01.wnl.0000078320.18564.9f. [DOI] [PubMed] [Google Scholar]
- 14.Banack S.A., Murc S.J., Cox P.A. Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands. J. Ethnopharmacol. 2006;106:97–104. doi: 10.1016/j.jep.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 15.Andersson M., Karlsson O., Bergström U., Brittebo E.B., Brandt I. Maternal transfer of cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) via milk to suckling offspring. PLoS One. 2013;8:e78133. doi: 10.1371/journal.pone.0078133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlop R.A., Cox P.A., Banack S.A., Rodgers K.J. The non-protein amino acid BMAA is misincorporated into human proteins in place of L-serine causing protein misfolding and aggregation. PLoS One. 2013;8:e75376. doi: 10.1371/journal.pone.0075376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xie X., Basile M., Mash D.C. Cerebral uptake and protein incorporation of cyanobacterial toxin β-N-methylamino-l-alanine. Neuroreport. 2013;24:779–784. doi: 10.1097/WNR.0b013e328363fd89. [DOI] [PubMed] [Google Scholar]
- 18.Karlsson O., Jiang L., Andersson M., Ilag L.L., Brittebo E.B. Protein association of the neurotoxin and non-protein amino acid BMAA (β-N-methylamino-l-alanine) in the liver and brain following neonatal administration in rats. Toxicol. Lett. 2014;226:1–5. doi: 10.1016/j.toxlet.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Glover W.B., Mash D.C., Murch J.M. The natural non-protein amino acid N-β-methylamino-l-alanine (BMAA) is incorporated into protein during synthesis. Amino Acids. 2014;46:2553–2559. doi: 10.1007/s00726-014-1812-1. [DOI] [PubMed] [Google Scholar]
- 20.Rosén J., Hellenäs K.E. Determination of the neurotoxin BMAA (β-N-methylamino-l-alanine) in cycad seed and cyanobacteria by LC–MS/MS (liquid chromatography tandem mass spectrometry) Analyst. 2008;133:1785–1789. doi: 10.1039/b809231a. [DOI] [PubMed] [Google Scholar]
- 21.Cohen S.A. Analytical techniques for the detection of α-amino-β-methylaminopropionic acid. Analyst. 2012;137:1991–2005. doi: 10.1039/c2an16250d. [DOI] [PubMed] [Google Scholar]
- 22.O’Neal R.M., Chen C.-H., Reynolds C.S., Meghal S.K., Koeppe R.E. The ‘neurotoxicity’ of l-2,4-diaminobutyric acid. Biochem. J. 1968;106:699–706. doi: 10.1042/bj1060699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Réveillon D., Abadie E., Séchet V., Brient L., Savar V., Bardouil M., Hess P., Amzil Z. Beta-N-methylamino-l-alanine: LC–MS/MS optimization, screening of cyanobacterial strains and occurrence in shellfish from Thau, a French Mediterranean lagoon. Mar. Drugs. 2014;12:5441–5467. 2014. [Google Scholar]
- 24.Brand L.E., Pablo J., Compton A., Hammerschlaq N., Mash D.C. Cyanobacterial blooms and the occurence of the neurotoxin, beta-N-methylamino-l-alanine (BMAA), in South Florida aquatic food webs. Harmful Algae. 2010;9:620–635. doi: 10.1016/j.hal.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jonasson S., Eriksson J., Berntzon L., Spáčil Z., Ilag L.L., Ronnevi L.O., Rasmussen U., Bergman B. Transfer of a cyanobacterial neurotoxin within a temperate aquatic ecosystem suggests pathways for human exposure. Proc. Natl. Acad. Sci. U. S. A. 2009;107:9252–9257. doi: 10.1073/pnas.0914417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Christensen S.J., Hemscheidt T.K., Trapido-Rosenthal H., Laws E.A., Bidigare R.R. Detection and quantification of beta-methylamino-l-alanine in aquatic invertebrates. Limnol. Oceanogr. Methods. 2012;10:891–898. [Google Scholar]
- 27.Salomonsson M.L., Hansson A., Bondesson U. Development and in-house validation of a method for quantification of BMAA in mussels using dansyl chloride derivatization and ultra performance liquid chromatography tandem mass spectrometry. Anal. Methods. 2013;5:4865–4874. [Google Scholar]
- 28.Downing S., Contardo-Jara V., Pflugmacher S., Downing T.G. The fate of the cyanobacterial toxin β-N-methylamino-l-alanine in freshwater mussels. Ecotoxicol. Environ. Saf. 2014;101:51–58. doi: 10.1016/j.ecoenv.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 29.Baptista M.S., Vasconcelos R.G., Ferreira P.C., Almeida C.M., Vasconcelos V.M. Assessment of the non-protein amino acid BMAA in Mediterranean mussel Mytilus galloprovincialis after feeding with estuarine cyanobacteria. Environ. Sci. Pollut. Res. Int. 2015 doi: 10.1007/s11356-015-4516-5. [DOI] [PubMed] [Google Scholar]
- 30.Mondo K., Hammerschlag N., Basile M., Pablo J., Banack S.A., Mash D.C. Cyanobacterial neurotoxin β-N-methylamino-l-alanine (BMAA) in shark fins. Mar. Drugs. 2012;10:509–520. doi: 10.3390/md10020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Food and Agriculture Organization of the United Nation, Statistics Division, 6th November 2015, http://faostat3fao.org/browse/FB/CL/E.
- 32.Banack S.A., Caller T., Henegan P., Haney J., Murby A., Metcalf J.S., Powell J., Cox P.A., Stomme E. Detection of cyanotoxins, β-N-methylamino-l-alanine and Microcystins, from a lake surrounded by cases of amyotrophic lateral sclerosis. Toxins (Basel) 2015;7(2):322–336. doi: 10.3390/toxins7020322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.FDA Foods Program Guideslines for Chemical Methods, Version 1.0 2/28/2012, http://www.fda.gov/downloads/ScienceResearch/FieldScience/UCM298730 pdf. (accessed 19.03.13.).
- 34.FDA Guidance for Industry, Bioanalytical Method Validation, May 2001, http://www.fda.gov/downloads/Drugs/./Guidances/ucm070107 pdf. (accessed 09.01.13.).
- 35.EC. 657/2002. Commission Decision 2002/657/EC of C1 14 August 2002 Implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results. Off. J. Eur. Communities.