Abstract
The cytotoxicity of quercetin is not well understood. Using an ICR murine model, we unexpectedly found that mice exposed to 7 Gy total body irradiation (TBI) exhibited general in vivo toxicity after receiving quercetin (100 mg/kg PO), whereas this result was not observed in mice that received TBI only. In order to understand the involvement of alterations in mitochondrial biogenesis, we used a real-time qPCR to analyze the mitochondrial DNA copy number (mtDNAcn) by amplifying the MTRNR1 (12S rRNA) gene in murine bone marrow. We also utilized reverse transcription qPCR to determine the mRNA amounts transcribed from the polymerase gamma (POLG), POLG2, and mammalian mitochondrial transcription factor A (TFAM) genes in the tissue. In the mice exposed to TBI combined with quercetin, we found: (1) the radiation-induced increase of mtDNAcn was inhibited with a concurrent significant decrease in POLG expression; (2) TFAM expression was significantly increased; and (3) the expression of POLG2 was not influenced by the treatments. These data suggest that the overall toxicity was in part associated with the decrease in mtDNAcn, an effect apparently caused by the inhibition of POLG expression and overexpression of TFAM; unaltered POLG2 expression did not seem to contribute to toxicity.
Chemical compounds studied in this article: Quercetin (PubChem CID: 5280343)
Keywords: Quercetin, Radiation, Mitochondrial DNA copy number, POLG, POLG2, TFAM
1. Introduction
Many natural components in our diet have a wide variety of beneficial health effects and are widely consumed by the general population [1], [2], [3]. Epidemiological studies have found that an increased intake of dietary flavonoids is associated with a decreased risk of chronic diseases, including certain cancers and cardiovascular diseases [4], [5], [6]. Quercetin, one such flavonoid ubiquitously present in various vegetables, fruits, seeds, nuts, tea, and red wine, has received considerable attention due to its antiproliferative, chemopreventive, anti-inflammatory, and anticarcinogenic activities and gene expression-modulating potential [7], [8]. However, studies have also shown that quercetin has potentially toxic effects, including its mutagenicity, prooxidant activity, mitochondrial toxicity, and inhibition of key enzymes involved in hormone metabolism [9], [10]. Such scientific uncertainty has prompted further investigations of the pharmacological properties of quercetin to better ascertain its safety as a dietary supplement.
Since numerous studies have reported that quercetin possesses strong antioxidant properties, we recently carried out a study to compare the radioprotective effects of a few other natural potential antioxidants, such as curcumin and resveratrol, with quercetin on ICR mice exposed to 7 Gy total body irradiation (TBI). We unexpectedly observed that mice exposed to TBI in combination with quercetin displayed more severe overall health status and weight loss than the mice that only received TBI. Considering the established roles of mitochondria in the metabolism of reactive oxygen species (ROS), the extensively described antioxidant and pro-oxidant properties of quercetin, and the finding that mitochondria can accumulate large amounts of quercetin [11], we speculated that mitochondria-related changes might be associated with the observed toxicity.
This paper reports the effects of quercetin on mitochondrial DNA copy number (mtDNAcn) and the concurrent expression alterations of 3 key factors involved in mitochondrial replication, polymerase gamma (POLG), POLG2, and mammalian mitochondrial transcription factor A (TFAM). Polg and Polg2 are 2 subunits that constitute the mitochondria-specific DNA polymerase gamma (Polγ), which is the only polymerase responsible for mtDNA replication. Tfam is a protein that triggers mtDNA transcription and is a necessary component for the initiation of mtDNA replication in mammals [12]. In light of the finding that an increase in mtDNAcn is a compensatory response after irradiation [13], [14], [15], we reasoned that the harmful effects of quercetin on mice could be associated with the repression of mtDNAcn and the accompanying expression changes in the key factors related to mtDNA anabolism.
2. Materials and methods
2.1. Chemicals and solutions
Quercetin was purchased from Sigma (St. Louis, MO). Platinum SYBR Green qPCR SuperMix-UDG, M-MLV First Strand cDNA synthesis kit, and TRIzol reagent were obtained from Invitrogen (Grand Island, NY). All other chemicals were reagent grade or the highest available grade and were purchased from commercial vendors.
2.2. Animals and treatments
Since in most species, including humans, mtDNA is maternally inherited, and studies have shown that female mice are more sensitive than male mice to mitochondrial toxicities when the animals are exposed to chemicals [16], our present study thus only used female ICR mice (8–10 weeks old). They were divided into groups of 6 mice. Normal control mice were not irradiated. Mice in the irradiated groups were immobilized with the aid of a plastic restrainer and received a single dose of 7 Gy TBI via a 6 MV X-ray source (600 CD Accelerator, Varian Oncology Systems, Palo Alto, CA) at the dose rate of 400 MU/min with a homogeneity of ±3.0%. One hour after irradiation, mice were gavaged with quercetin dissolved in physiological saline (a volume of 0.02 ml/g body weight after well suspension so as to deliver 100 mg/kg body weight) or with the same unit volume of saline. Mice were maintained in a temperature-controlled room with a 12-h light/dark cycle and provided with standard laboratory chow and tap water ad libitum. Mice were weighed one day before irradiation and every other day after exposure. They were euthanized 17 days after treatment. Bone tissues were rapidly dissected on ice and collected into vials after soft tissues were removed. The collected bone marrow tissues were frozen immediately at −70 °C until use. All procedures involving animals were in accordance with protocols established by the World Health Organization and the Chinese National Science Academy.
2.3. DNA extraction from mouse tissues
Total DNA was extracted from the stored bone marrow tissues by standard proteolytic digestion followed by phenol/chloroform/isoamyl alcohol purification. To extract the DNA, the bone marrow tissues were first crushed by repetitive strikes with a hammer immediately after they underwent snap-freezing in liquid nitrogen vapor in a clean, disposable plastic bag. Absorbance at 260 nm was used for quantification of the extracted DNA.
2.4. RNA extraction from mouse tissues and cDNA synthesis
After bone marrow tissues were crushed (as described above), TRIzol reagent was used to extract the total RNA, and the RNA pellet from each sample was dissolved in diethylpyrocarbonate (DEPC) water at approximately 1 ng/μl. Reverse transcription PCR was performed with a M-MLV First Strand cDNA synthesis kit, according to manufacturer's instructions, using oligo(dT)20 as a primer.
2.5. Primer design
The 2 oligonucleotide PCR primers for detecting the MTRNR1 (12S rRNA) gene of the murine mitochondrial genome were designed based on GenBank nucleotide sequence NC_005089.1, Mus musculus domesticus mitochondrion. The sequences for the primers were 5′-ACC GCG GTC ATA CGA TTA AC-3′ (forward) and 5′-CCC AGT TTG GGT CTT AGC TG-3′ (reverse). They directed the synthesis of the 177-bp mtDNA fragment from an mtDNA region in which deletions are rare. As such, alterations in mtDNA content or copy number in cells could be detected quantitatively by comparing them to a nuclear-encoded housekeeping gene. The housekeeping gene used for this purpose was the mouse nuclear 18S rRNA gene. The primer sequences amplifying this housekeeping gene were designed based on GeneBank sequence BK000964.3, Mus musculus 18SrRNA. Their sequences were 5′-CGC GGT TCT ATT TTG TTG GT-3′ (forward) and 5′-AGT CGG CAT CGT TTA TGG TC-3′ (reverse), producing a 219-bp product. The primers for detecting POLG gene expression were designed based on GeneBank sequence BC061472.1, Mus musculus POLG cDNA. Their sequences were 5′-AGA AGC AGA GCC TGC CTT AC-3′ (forward) and 5′-CTC TGC CAA GCA GAC CTC C-3′(reverse), which produced a 125-bp amplicon. The primers for determining POLG2 gene expression were designed based on GeneBank sequence NM_015810.1, Mus musculus POLG2 cDNA. Their sequences were 5′-CTT GCA AGA CAG AGA GCC G-3′ (forward) and 5′-CTG GGT GTC TGA TTG CTG TTC-3′ (reverse), which resulted in a 210-bp PCR product. The sequences of the primers amplifying the murine TFAM cDNA were 5′-GGA ATG TGG AGC GTG CTA AAA-3′ (forward) and 5′-TGC TGG AA A AAC ACT TCG GAA TA-3′(reverse); these were chosen from a publication [17], and they produced a 118-bp amplicon. The ACTB gene was used as an internal control for quantifying the transcription levels of these genes, and the primers were designed based on GeneBank sequence NM_007393.3, Mus musculus ATCB cDNA. Their sequences were 5′-GAA GGA CTC CTA TGT GGG TGA C-3′ (forward) and 5′-TTC ACG GTT GGC CTT AGG-3′ (reverse), which produced a 205-bp product.
2.6. Gene amplification and quantification by real-time quantitative PCR (qPCR)
Real-time qPCR was performed using a combination of the 2 primers noted above and the extracted genomic DNA or synthesized cDNA as the DNA template sources. The total volume of the qPCR mixture was 20 μl, consisting of 2× PlatinumSYBR Green qPCR SuperMix-UDG (1× final), 0.5 μM of each oligonucleotide primer, and 1–3 μl of the genomic DNA or cDNA template source. Each reaction was subjected to an initial denaturation of 5 min at 95 °C, followed by 40 amplification cycles of denaturation at 95 °C for 30 s and combined annealing and extension at 60 °C for 1 min on an Applied Biosystems7500 real-time PCR detection system. Preliminary conventional PCR and qPCR were first run to develop qPCR conditions using the same primers. All the qPCRs were run in duplicate and repeated at least twice to eliminate technical bias after averaging the obtained cycle number (Ct) data. The Ct was the cycle number at which the fluorescent signal of a given reaction well crossed the threshold value and was used as a basis for quantification of mtDNA and nuclear DNA (nDNA) copy numbers as well as gene expression profiles. Under the assumption of equal efficiencies during qPCR amplifications, the difference in Ct resulting from the different reactions was calculated to estimate the mtDNA:nDNA ratio and to normalize the gene expression levels using 2−ΔCt and 2−ΔΔCt mathematical models [18], [19].
2.7. Data analysis
The data were expressed as mean ± SE and plotted with Microsoft Excel (Microsoft Corporation, Redmond, WA) and the GraphPad Prism 5 statistical package (GraphPad Software, Inc., La Jolla, CA). When appropriate, analysis of variance (ANOVA) was first performed by using the package to ascertain whether there were significant differences among the multiple means; the data were scrutinized further by performing unpaired two-tailed t tests. The significance level was set at P < 0.05.
3. Results
3.1. Body weight changes in mice
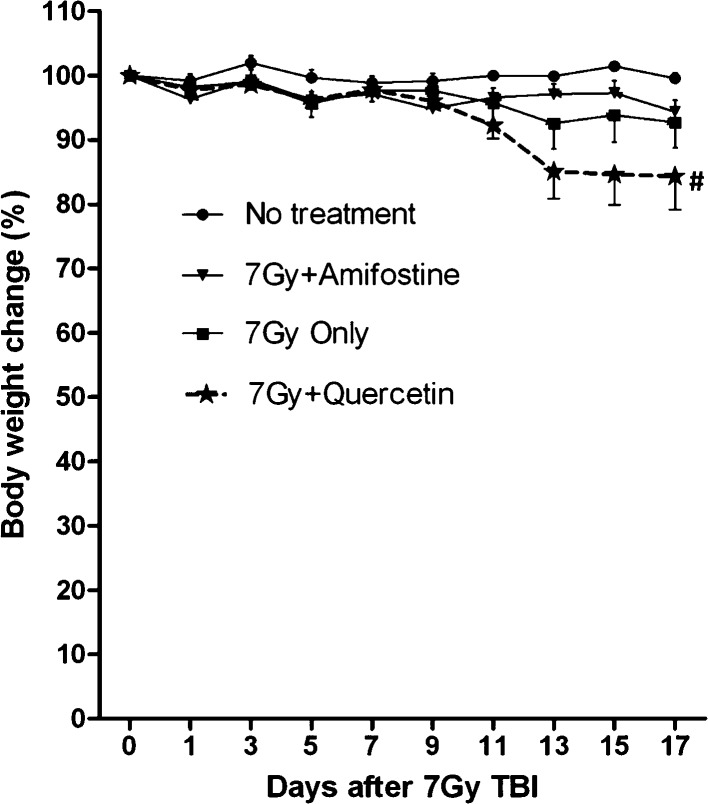
Fig. 1 shows the changes in body weight over 17 days after different treatments. As it has been established, amifostine treatment 30 min prior to TBI protected the mice against apparent weight loss; thus, no significant weight difference was observed between this group and the nonirradiated control group. We also observed that the mice receiving nonlethal 7 Gy TBI started losing weight 9 days after irradiation, and the loss sustained during the subsequent days until the end of the experiment was a maximum of approximately 7% of the pre-TBI value. However, we unexpectedly observed a rapid weight decrease at 9 days after TBI in the group of mice administered quercetin. Although the loss slowed 13 days after irradiation, the maximum decrease reached up to 17% of the pre-treatment value; one death was observed in this group on day 17 just prior to the experiment being terminated.
Fig. 1.
Changes in body weight of mice administered quercetin. Mice received intraperitoneal injections of amifostine dissolved in physiological saline (a volume of 0.02 ml/g body weight to deliver 200 mg/kg body weight) 30 min before irradiation or were orally administered quercetin 1 h after 7 Gy TBI, then observed for 17 days for body weight changes and survival. Data represented are the mean ± SE of 6 animals per treatment and time point. # represents the death of one mouse in the group at the time indicated.
3.2. Development and optimization of qPCR
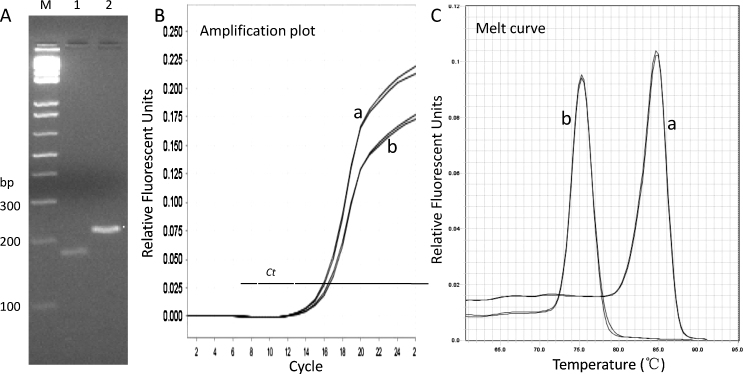
To reliably detect the possible differences in mtDNAcn in bone marrow in the different groups, we first optimized our qPCR conditions using MTRNR1 (12S rRNA)-specific and 18S rRNA-specific primers and preliminarily operating the conventional PCR and qPCR. When we ran the PCR products, which were generated with a normal control sample DNA template by the optimized conventional PCR, we found that the intensity of the MTRNR1 (12S rRNA)-derived band was slightly lighter than that of the 18S rRNA-derived band in 4% agarose gel when the equal volume of PCR product solution was directly loaded, whereas the size of the former was 177 bp and the latter was 219 bp, respectively (Fig. 2A). Correspondingly, the Ct values that were presented in the qPCR amplification curve for the same sample DNA template (Fig. 2B) were also lower for the 12S rRNA-derived amplicons than for the 18S rRNA-derived amplicons, indicating that the copy number for 18S rRNA gene was marginally higher than that of the mtDNA in the ICR bone marrow tissues. In addition, as shown in the accompanying melt curves (Fig. 2C), the expected single, narrow peaks indicate that the mtDNA-derived and nDNA-derived PCR products in our qPCR system were sufficiently pure and specific.
Fig. 2.
Normal sample profile for mtDNA and nDNA products. In panel A, PCR conditions were optimized to produce amplicons of different sizes and intensities for 12SmtDNA (177 bp, lane 1) and 18S nDNA (219 bp, lane 2), where the same volume of PCR solutions were loaded in the gel. Panels B and C show the optimized qPCR profiles for the same sample amplification curves and melt-curves with (a) and (b) denoting the qPCR product of nDNA and mtDNA, respectively.
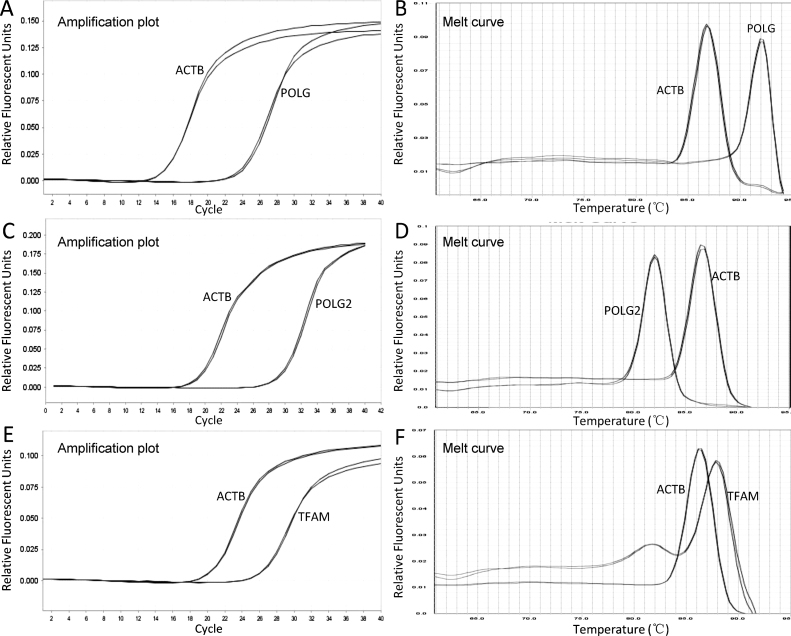
To detect the expression levels of the POLG, POLG2, and TFAM genes in the bone marrow tissues, we also optimized our qPCR conditions using the primer sets specific to each of the 3 genes. Although we initially experienced a few inferior primer sets that produced multiple peaks in the melt curves, we found good conditions by constructing new primer sets. As shown in Fig. 3A–F, the finalized conditions generated amplicons that presented good amplification and melt curves; thus, they were used in the final assay to quantitatively reveal the transcriptional differences between each of the 3 genes and ACTB (the internal control gene). The amplification curves for each gene showed the typical two phases – an exponential phase followed by a plateau phase. The corresponding melt curves for the POLG, POLG2, and ACTB genes exhibited single and narrow peaks, which are the typical features of sufficiently pure and specific PCR products. Although the melt curve for the TFAM gene had an additional smaller peak before the gene-specific peak, this was likely due to the formation of primer–dimer. The endpoint calculation, which was performed based on the Ct values, was not affected.
Fig. 3.
Representative reverse transcription qPCR (RT-qPCR) profiles of 3 target genes. The mRNA amounts transcribed from POLG, POLG2, and TFAM in bone marrow samples were determined under the optimal conditions to reflect the expression levels of these genes. Each pair of panels (A/B, C/D, and E/F) in this figure denote the acquired corresponding amplification and melt curve profiling for detecting POLG, POLG2, and TFAM genes, respectively.
As the above results confirmed that the qPCR conditions developed were reliable, these conditions were used for subsequent determinations.
3.3. Effect of quercetin on mtDNA copy number
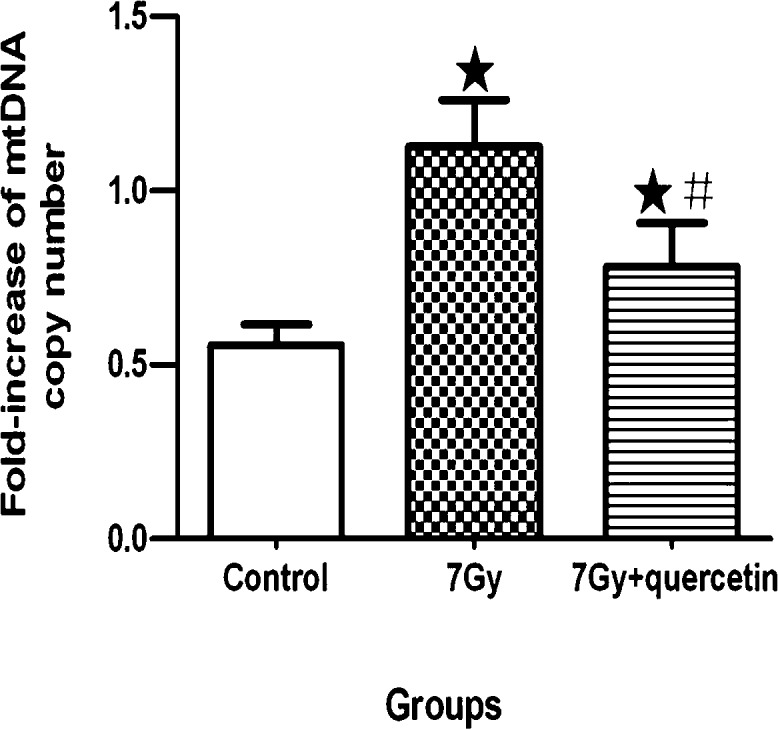
Fig. 4 shows the effects of quercetin treatment on mtDNAcn in bone marrow. Although the mtDNAcn in the bone marrow of the TBI group was higher than the nonirradiated normal controls, it did not change significantly in the group treated with 7 Gy TBI and quercetin (100 mg/kg) relative to the control. These results indicate that quercetin inhibited the radiation-stimulated increase of mtDNAcn.
Fig. 4.
Effect of quercetin on mtDNAcn. Alterations in mtDNAcn are shown with the data points denoting the mean ± SE of 6 mice per group. ★ indicates a significant difference in the average mtDNAcn between the nonirradiated normal control and each of the groups exposed to TBI alone and to quercetin after TBI; # indicates a significant difference in the average mtDNAcn between the two treatment groups.
3.4. Expression levels of target genes involved in mtDNA amplification
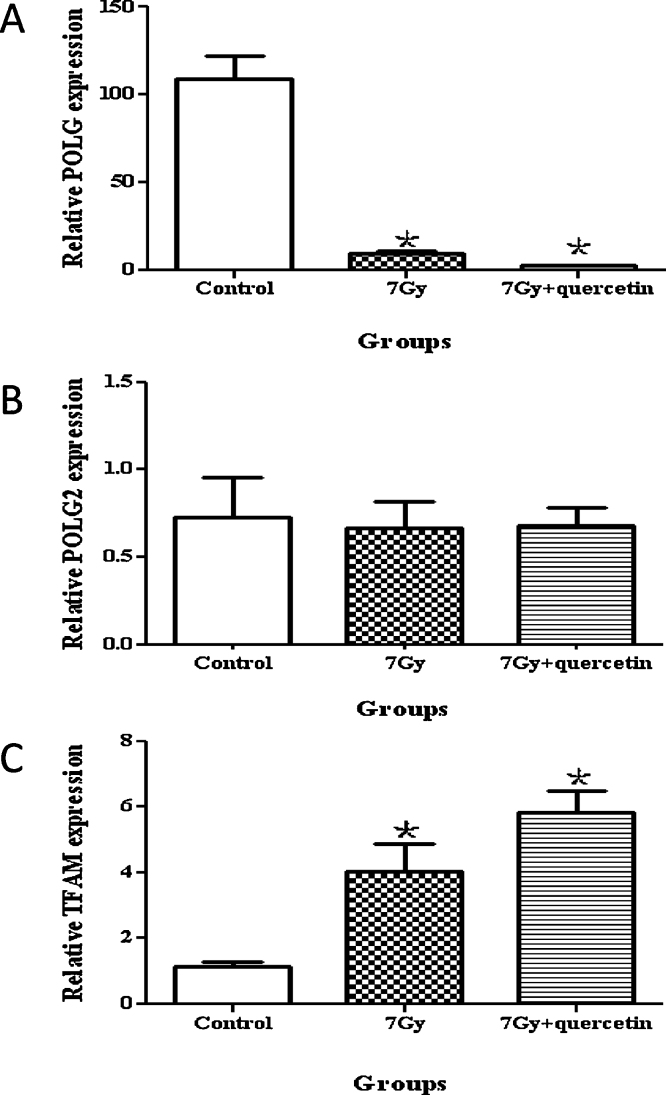
The transcription levels of POLG, POLG2, and TFAM in bone marrow tissues were measured with our optimized qPCR using the gene-specific primer sets and the synthesized cDNA templates. The results showed that in the TBI group POLG expression decreased markedly, whereas TFAM expression increased significantly up to 4 fold compared to the nonirradiated control group. These changes were also observed in the group treated with quercetin after TBI; the changes were even more dramatic than those observed in the TBI group, with up to a 6-fold increase observed for the expression of the TFAM gene. No difference was found in POLG2 expression among the 3 groups (Fig. 5A–C).
Fig. 5.
Effect of quercetin on the expression of POLG, POLG2, and TFAM. The expression levels of POLG (A), POLG2 (B), and TFAM (C) in the 3 groups are shown with the data points representing the mean ± SE of 6 mice per group. * indicates a significant difference between the normal control and treatment groups.
4. Discussion
Although previous studies have reported that radiation induces an increase in mtDNAcn [13], [14], to the best of our knowledge we are the first to report that quercetin inhibits the radiation-induced increase of mtDNAcn, which results from decreased POLG expression and the overexpression of TFAM. Quercetin is widely consumed as a dietary supplement and has been studied for several decades. Unfortunately, most of the literature focuses on its beneficial health properties, and its potentially harmful effects have been understudied or largely ignored [1], inevitably raising potential health concerns.
The beneficial health effects of quercetin are believed to be due to its strong antioxidative and free radical scavenging properties [20], [21]. Such activities are largely a function of its chemical structure, which has been shown to contain all the structural elements characteristic of an effective antioxidant [20], [22]. However, when quercetin exerts its antioxidant effect, it may be converted into potentially harmful oxidation products or subjected to in vitro oxidative degradation, resulting in the formation of an ortho-quinone and the subsequent production of ROS, such as superoxide and hydrogen peroxide (H2O2) [23]. The resultant prooxidant properties of quercetin are responsible for its in vitro mutagenic and cytotoxic effects [24], [25]. Whether quercetin exerts antioxidant and prooxidant effects largely relates to its dose in a given biological system [26] and can be revealed by determining changes in the level of oxidative stress in the system when quercetin is added at different doses. The combination of ROS level and endogenous antioxidant protective activity as well as the repair or removal rate of the oxidized molecular targets, including nucleic acids, proteins, and lipids, in a system determine the level of oxidative stress. This stress can be detected by several available approaches [27], [28].
Mitochondrial DNA replication is reliant on nuclear-encoded transcription and replication factors that are translocated to the mitochondria. It is well established that heterotrimeric Polγ and Tfam are crucial for mtDNA replication [12], [29]. The Polγ holoenzyme is composed of a single catalytic subunit encoded by the POLG gene and a homodimeric accessory subunit encoded by the POLG2 gene. The catalytic subunit possesses DNA polymerase 3′–5′ exonuclease and 5′ deoxyribose phosphate lyase activities that are responsible for mtDNA replication and repair, whereas the accessory subunit is a DNA-binding factor that confers high processivity on the protein complex by increasing its affinity to DNA, which is responsible for enhancing DNA binding and promoting processivity of DNA synthesis. Tfam is another key factor in regulating mtDNA amplification and mitochondrial biogenesis [12], [29]. Several studies have reported that the increase in mtDNAcn is associated with upregulated TFAM expression and that TFAM knockdown inhibits the enhancement of relative mtDNAcn [12], [30]. Others have also reported that POLG homozygous knockout in mice produces severe mtDNA depletion and respiratory-chain deficiency and that mutations in POLG and POLG2 are associated with a variety of clinical features [31], [32].
Research has well documented that mitochondria are the major intracellular sources and primary targets of ROS [33], [34], [35]. Each human cell contains between several hundred and over a thousand mitochondria with each carrying 2–10 copies of mtDNA. Mitochondrial DNAcn is correlated with the size and number of mitochondria, which have been shown to change under different energy demands and physiological conditions. Due to the elevated free radical production following radiation exposure and the close relationship between mitochondria and ROS metabolism, mtDNA is more susceptible to radiation damage [35]. Radiation-induced mtDNA alterations are exhibited through mtDNAcn changes and other damage, such as mutation and depletion. As mtDNA has few repair mechanisms, mitochondrial function is preserved primarily by increasing mtDNAcn after irradiation. This notion is supported by our present data and other studies [13], [36], [37], [38], [39], which indicate that enhanced mtDNA replication and the resulting elevation of mtDNAcn after irradiation or elevation in the intracellular level of ROS may be radioprotective mechanisms or the result of the feedback response that compensates for defective mitochondria in cells. The molecular events responsible for this radioprotection or feedback response may be related to the coordinated alterations of mitochondrial membrane potential and protein synthesis in cytoplasm [37]. Recently, another study demonstrated that POLG is expressed at normal levels in cells lacking mtDNA, suggesting that POLG level is not responsive or parallel to the abundance of mtDNA in a cell [38]. This may explain in part why the mtDNAcn was increased in mice exposed to only TBI, whereas the expression of POLG was reduced in our present study.
While an oxidative stress-induced increase in mtDNAcn may compensate for the decline of mitochondrial respiratory function, ROS are also generated from the increased mitochondria in the affected cells. These ROS cause more oxidative damage to mitochondria and other intracellular constituents, including DNA, RNA, proteins, and lipids, and consequently lead the cell to enter the process of senescence or apoptosis [40]. This scenario of oxidative stress response plays two roles in the affected cells. On one hand, it stimulates mitochondrial proliferation to supply the energy needs for cell survival; on the other hand, it causes excess ROS production and further oxidative damage, thereby initiating cell death. The outcome of this increase in mitochondrial abundance and mtDNAcn is dependent on the level of oxidative stress, the capacity of the intracellular antioxidant system, and the quality of mitochondria and mtDNA. When cells have higher antioxidant capacity and good quality mitochondria and mtDNA, the response to mild oxidative stress will lead to an increase in the abundance of mitochondria and mtDNA molecules. However, a compromise in the capacity of the antioxidant system leads to an increase in defective mitochondria and mutated mtDNA, which in turn leads to an increase in ROS production and oxidative damage. Once beyond a certain threshold, mitochondria drive the cell into an irreversible cell death process. Studies have extensively documented the complicated scenario of mitochondrial functions under oxidative stress [33], [34], [35], [36], [37], [38], [39]. While we acknowledge that we did not conduct relevant measurements in our present study, a wealth of literature supports our presumption that oxidative stress contributes to our current observations. We not only observed an increase in mtDNAcn and a decrease in POLG expression in the TBI mice but also found that mice gavaged with quercetin after TBI presented with poor overall health status (matted fur and up to17% loss of body weight) and a pronounced reduction in average mtDNAcn relative to the TBI-only group. These results, which potentially represent an adverse effect resulting from the quercetin treatment, were contrary to what we predicted at the beginning of the study. This outcome might be attributable to the following: (1) the repressed expression of the POLG gene resulted in the decline of mitochondrial biogenesis and energy production; (2) the more affected cells underwent a gradual yet irreversible cell death process due to the other potential toxicities of quercetin, such as its prooxidant activity, apoptosis-inducing properties, and interactions with some metabolizing enzymes [1]; and/or (3) the approximately 6-fold overexpression of the TFAM gene contributed to the repression of the mtDNAcn and the overall toxicity observed in our study. Previous studies indicated that the increased Tfam:mtDNA ratio may inhibit mitochondrial transcription [41] and that the overexpression of TFAM decreases the amount of a D-loop form of mtDNA in cells [42]. These studies support our notion that TFAM overexpression could also cause the observed potential toxicity of quercetin.
Our study further demonstrated that the expression of POLG2 did not change markedly in the 2 treated groups relative to the normal control. This finding indicates that radiation-induced oxidative stress can preferentially inhibit the transcription of POLG while maintaining the transcription of POLG2. This finding is in keeping with the in vitro observation that the catalytic subunit Polg is a target of oxidative stress and that this oxidation inactivates the subunit, whereas the function of the accessory subunit Polg2 is not compromised when it is oxidized [43]. In addition, we observed statistically insignificant differences in the expression of POLG and POLG2 between the 2 treated groups although the expression of POLG was seemingly reduced more in the mice exposed to quercetin after TBI, indicating that the effects of quercetin on the expression of the 2 genes were undetectable. Taken together, these results suggest that the responses of the POLG, POLG2, and TFAM genes to oxidative stress and quercetin treatment are complex. Further studies are needed to clarify the detailed mechanisms that are involved.
Notably, quercetin was administered to mice in saline in our present study. Although quercetin lacks easy solubility in aqueous solutions [44], [45], its absorption after oral supplementation ranges from 0 to over 50% of the administered dose in human or the total oral bioavailability is 17% of the applied dose in pigs [46]. We acknowledge that, due to the lack of direct measurements, we do not know how much quercetin is actually absorbed into the blood and delivered to the bone marrow of the mice in our study. As such, our comparisons are likely to be more qualitative than quantitative. With regard to the scientific basis for the quercetin dose used in the present study, previous animal studies used oral treatments of quercetin at doses ranging from 10 to 3000 mg/kg body weight [47], [48]. Moreover, animal toxicity studies have demonstrated that quercetin is tolerated at oral dose levels exceeding its oral LD50 value 160 mg/kg body weight by several fold [49]. Further, since curcumin or vesveratrol, two natural potential antioxidants tested simultaneously in the present study, was orally given to mice at 100 mg/kg body weight in several earlier studies [50], [51], the same dose of quercetin (100 mg/kg body weight) was thus used in the present study in order to make comparisons. In addition, we found discrepancies between the data presented in this study and in an earlier study [52]. Previously, we observed that the ratio of mtDNA:nDNA was clearly higher when the small bowel DNA of nonirradiated male BALB/c mice was amplified with the 2 sets of identical primers, as evidenced by the difference in the resulting band intensity in 4% gel; however, this result is contrary to what we found in this most recent study (Fig. 2A). Moreover, another study showed that Polg protein levels were significantly increased in the intestines of mice receiving 5 Gy TBI, whereas the Tfam protein levels were clearly decreased [39]. This result is also contrary to our present data. We propose that differences in animal species and tissue types or other variables might account for our most recent findings. Further studies are warranted.
In summary, our results demonstrate that quercetin can repress mtDNAcn via selectively affecting the expression levels of POLG and TFAM genes, whereas it has no apparent effect on POLG2 gene expression. This finding reveals that quercetin not only affects mitochondrial anabolism but also differentially affects the expression levels of the genes that jointly encode the same mitochondria-specific polymerase. These effects should be taken into consideration in drug development programs for quercetin. Further studies are required to determine if quercetin interacts with other effect or molecules to achieve deeper insight into its in vivo biological activities.
Ethical standard
All procedures used in the present study followed the “Principles of Laboratory Animal Care” of the National Institutes of Health and were approved by the local Ethical Committee.
Conflict of interest
None.
Acknowledgements
This study was supported in part by the hospital start-up funds. We thank Xiuying Chen in the Department of Radiotherapy for irradiating the mice in this experiment. We also thank Kate Casey-Sawicki in the University of Florida for her thoughtful editorial work.
Footnotes
Available online 30 July 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.07.014.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Galati G., O’Brien P.J. Potential toxicity of flavonoids and other dietary phenolics: significance for their chemopreventive and anticancer properties. Free Radic. Biol. Med. 2004;37:287–303. doi: 10.1016/j.freeradbiomed.2004.04.034. [DOI] [PubMed] [Google Scholar]
- 2.Skibola C.F., Smith M.T. Potential health impacts of excessive flavonoid intake. Free Radic. Biol. Med. 2000;29:375–383. doi: 10.1016/s0891-5849(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 3.Duthie G.G., Gardner P.T., Kyle J.A. Plant polyphenols: are they the new magic bullet? Proc. Nutr. Soc. 2003;62:599–603. doi: 10.1079/PNS2003275. [DOI] [PubMed] [Google Scholar]
- 4.Arts I.C., Hollman P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005;81:317S–325S. doi: 10.1093/ajcn/81.1.317S. [DOI] [PubMed] [Google Scholar]
- 5.Hertog M.G., Feskens E.J., Hollman P.C., Katan M.B., Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–1011. doi: 10.1016/0140-6736(93)92876-u. [DOI] [PubMed] [Google Scholar]
- 6.Hollman P.C., Geelen A., Kromhout D. Dietary flavonol intake may lower stroke risk in men and women. J. Nutr. 2010;140:600–604. doi: 10.3945/jn.109.116632. [DOI] [PubMed] [Google Scholar]
- 7.Chirumbolo S. The role of quercetin, flavonols and flavones in modulating inflammatory cell function. Inflamm. Allergy Drug Targets. 2010;9:263–285. doi: 10.2174/187152810793358741. [DOI] [PubMed] [Google Scholar]
- 8.Jung C.H., Cho I., Ahn J., Jeon T.I., Ha T.Y. Quercetin reduces high-fat diet-induced fat accumulation in the liver by regulating lipid metabolism genes. Phytother. Res. 2013;27:139–143. doi: 10.1002/ptr.4687. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto T. Safety of quercetin for clinical application. Int. J. Mol. Med. 2005;16:275–278. [PubMed] [Google Scholar]
- 10.Zhang Q., Zhao X.H., Wang Z.J. Cytotoxicity of flavones and flavonols to a human esophageal squamous cell carcinoma cell line (KYSE-510) by induction of G2/M arrest and apoptosis. Toxicol. In Vitro. 2009;23:797–807. doi: 10.1016/j.tiv.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Fiorani M., Guidarelli A., Blasa M., Azzolini C., Candiracci M., Piatti E., Cantoni O. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010;21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Ekstrand M.I., Falkenberg M., Rantanen A., Park C.B., Gaspari M., Hultenby K., Rustin P., Gustafsson C.M., Larsson N.G. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum. Mol. Genet. 2004;13:935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 13.Malakhova L., Bezlepkin V.G., Antipova V., Ushakova T., Fomenko L., Sirota N., Gaziev A.I. The increase in mitochondrial DNA copy number in the tissues of gamma-irradiated mice. Cell Mol. Biol. Lett. 2005;10:721–732. [PubMed] [Google Scholar]
- 14.Wen Q., Hu Y., Ji F., Qian G. Mitochondrial DNA alterations of peripheral lymphocytes in acute lymphoblastic leukemia patients undergoing total body irradiation therapy. Radiat. Oncol. 2011;6:133–139. doi: 10.1186/1748-717X-6-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X., Li N., Wang Y., Wang Y., Zhang X., Zhang H. Effects of X-irradiation on mitochondrial DNA damage and its supercoiling formation change. Mitochondrion. 2011;11:886–892. doi: 10.1016/j.mito.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Desai V.G., Lee T., Moland C.L., Branham W.S., Mittelstaedt R.A., Lewis S.M., Leakey J.E., Fuscoe J.C. Evaluation of hepatic mitochondria and hematological parameters in zidovudine-treated B6C3F mice (1) AIDS Res. Treat. 2012;2012:317695. doi: 10.1155/2012/317695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon-Areces J., Dietrich M.O., Hermes G., Garcia-Segura L.M., Arevalo M.A., Horvath T.L. UCP2 induced by natural birth regulates neuronal differentiation of the hippocampus and related adult behavior. PLOS ONE. 2012;7:e42911. doi: 10.1371/journal.pone.0042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pfaffl M.W. A new mathematical model for relative quantification in real time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 20.Rietjens I.M., Boersma M.G., van der Woude H., Jeurissen S.M., Schutte M.E., Alink G.M. Flavonoids and alkenylbenzenes: mechanisms of mutagenic action and carcinogenic risk. Mutat. Res. 2005;574:124–138. doi: 10.1016/j.mrfmmm.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Ružić I., Skerget M., Knez Z. Potential of phenolic antioxidants. Acta Chim. Slov. 2010;57:263–271. [PubMed] [Google Scholar]
- 22.Silva M.M., Santos M.R., Caroco G., Rocha R., Justino G., Mira L. Structure–antioxidant activity relationships of flavonoids: a re-examination. Free Radic. Res. 2002;36:1219–1227. doi: 10.1080/198-1071576021000016472. [DOI] [PubMed] [Google Scholar]
- 23.Boots A.W., Kubben N., Haenen G.R.M.M., Bast A. Oxidized quercetin reacts with thiols rather than with ascorbate: implication for quercetin supplementation. Biochem. Biophys. Res. Commun. 2003;308:560–565. doi: 10.1016/s0006-291x(03)01438-4. [DOI] [PubMed] [Google Scholar]
- 24.Sahu S.C., Washington M.C. Quercetin-induced lipid peroxidation and DNA damage in isolated rat-liver nuclei. Cancer Lett. 1991;58:75–79. doi: 10.1016/0304-3835(91)90026-e. [DOI] [PubMed] [Google Scholar]
- 25.Soria E.A., Eynard A.R., Bongiovanni G.A. Cytoprotective effects of silymarin on epithelial cells against arsenic-induced apoptosis in contrast with quercetin cytotoxicity. Life Sci. 2010;87:309–315. doi: 10.1016/j.lfs.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Robaszkiewicz A., Balcerczyk A., Bartosz G. Antioxidative and prooxidative effects of quercetin on A549 cells. Cell Biol. Int. 2007;31:1245–1250. doi: 10.1016/j.cellbi.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Lee J.C., Kim J., Park J.K., Chung G.H., Jang Y.S. The antioxidant, rather than prooxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp. Cell Res. 2003;291:386–397. doi: 10.1016/s0014-4827(03)00410-5. [DOI] [PubMed] [Google Scholar]
- 28.Alía M., Mateos R., Ramos S., Lecumberri E., Bravo L., Goya L. Influence of quercetin and rutin on growth and antioxidant defense system of a human hepatoma cell line (HepG2) Eur. J. Nutr. 2006;45:19–28. doi: 10.1007/s00394-005-0558-7. [DOI] [PubMed] [Google Scholar]
- 29.Ylikallio E., Tyynismaa H., Tsutsui H., Ide T., Suomalainen A. High mitochondrial DNA copy number has detrimental effects in mice. Hum. Mol. Genet. 2010;19:2695–2705. doi: 10.1093/hmg/ddq163. [DOI] [PubMed] [Google Scholar]
- 30.Campbell C.T., Kolesar J.E., Kaufman B.A. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim. Biophys. Acta. 2012;1819:921–929. doi: 10.1016/j.bbagrm.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Copeland W.C. Defects in mitochondrial DNA replication and human disease. Crit. Rev. Biochem. Mol. Biol. 2012;47:64–74. doi: 10.3109/10409238.2011.632763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stewart J.D., Schoeler S., Sitarz K.S., Horvath R., Hallmann K., Pyle A., Yu-Wai-Man P., Taylor R.W., Samuels D.C., Kunz W.S., Chinnery P.F. POLG mutations cause decreased mitochondrial DNA repopulation rates following induced depletion in human fibroblasts. Biochim. Biophys. Acta. 2011;1812:321–325. doi: 10.1016/j.bbadis.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Nickel A., Kohlhaas M., Maack C. Mitochondrial reactive oxygen species production and elimination. J. Mol. Cell Cardiol. 2014 doi: 10.1016/j.yjmcc.2014.03.011. pii:S0022-2828(14)00088-1. [DOI] [PubMed] [Google Scholar]
- 34.Ayala-Peña S. Role of oxidative DNA damage in mitochondrial dysfunction and Huntington's disease pathogenesis. Free Radic. Biol. Med. 2013;62:102–110. doi: 10.1016/j.freeradbiomed.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muftuoglu M., Mori M.P., Souza-Pinto N.C. Formation and repair of oxidative damage in the mitochondrial DNA. Mitochondrion. 2014 doi: 10.1016/j.mito.2014.03.007. pii:S1567-7249(14)00033-6. [DOI] [PubMed] [Google Scholar]
- 36.Bartoletti-Stella A., Mariani E., Kurelac I., Maresca A., Caratozzolo M.F., Iommarini L., Carelli V., Eusebi L.H., Guido A., Cenacchi G., Fuccio L., Rugolo M., Tullo A., Porcelli A.M., Gasparre G. Gamma rays induce a p53-independent mitochondrial biogenesis that is counter-regulated by HIF1α. Cell Death Dis. 2013;4:e663. doi: 10.1038/cddis.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee H.C., Yin P.H., Chi C.W., Wei Y.H. Increase in mitochondrial mass in human fibroblasts under oxidative stress and during replicative cell senescence. J. Biomed. Sci. 2002;9:517–526. doi: 10.1007/BF02254978. [DOI] [PubMed] [Google Scholar]
- 38.Lee H.C., Wei Y.H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 2005;37:822–834. doi: 10.1016/j.biocel.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H.S., David D.J., Zhang M., Zhang L.R., Okunieff P. Elevated mitochondrial DNA copy number and POL-γ expression but decreased expression of TFAM in murine intestine following therapeutic dose irradiation. Adv. Exp. Med. Biol. 2011;701:201–206. doi: 10.1007/978-1-4419-7756-4_27. [DOI] [PubMed] [Google Scholar]
- 40.Bladier C., Wolvetang E.J., Hutchinson P., de Haan J.B., Kola I. Response of a primary human fibroblast cell line to H2O2: senescence-like growth arrest or apoptosis? Cell Growth Differ. 1997;8:589–598. [PubMed] [Google Scholar]
- 41.Matsushima Y., Goto Y., Kaguni L.S. Mitochondrial Lon protease regulates mitochondrial DNA copy number and transcription by selective degradation of mitochondrial transcription factor A (TFAM) Proc. Natl. Acad. Sci. U. S. A. 2010;107:18410–18415. doi: 10.1073/pnas.1008924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohgaki K., Kanki T., Fukuoh A., Kurisaki H., Aoki Y., Ikeuchi M., Kim S.H., Hamasaki N., Kang D. The C-terminal tail of mitochondrial transcription factor a markedly strengthens its general binding to DNA. J. Biochem. 2007;141:201–211. doi: 10.1093/jb/mvm020. [DOI] [PubMed] [Google Scholar]
- 43.Graziewicz M.A., Day B.J., Copeland W.C. The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic Acids Res. 2002;30:2817–2824. doi: 10.1093/nar/gkf392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sak K. Site-specific anticancer effects of dietary flavonoid quercetin. Nutr. Cancer. 2014;66:177–193. doi: 10.1080/01635581.2014.864418. [DOI] [PubMed] [Google Scholar]
- 45.Graefe E.U., Wittig J., Mueller S., Riethling A.K., Uehleke B., Drewelow B., Pforte H., Jacobasch G., Derendorf H., Veit M. Pharmacokinetics and bioavailability of quercetin glycosides in humans. J. Clin. Pharmacol. 2001;41:492–499. doi: 10.1177/00912700122010366. [DOI] [PubMed] [Google Scholar]
- 46.Egert S., Wolffram S., Bosy-Westphal A., Boesch-Saadatmandi C., Wagner A.E., Frank J., Rimbach G., Mueller M.J. Daily quercetin supplementation dose-dependently increases plasma quercetin concentrations in healthy humans. J. Nutr. 2008;138:1615–1621. doi: 10.1093/jn/138.9.1615. [DOI] [PubMed] [Google Scholar]
- 47.García-Saura M.F., Galisteo M., Villar I.C., Bermejo A., Zarzuelo A., Vargas F., Duarte J. Effects of chronic quercetin treatment in experimental renovascular hypertension. Mol. Cell. Biochem. 2005;270:147–155. doi: 10.1007/s11010-005-4503-0. [DOI] [PubMed] [Google Scholar]
- 48.Ruiz M.J., Fernández M., Estela J.M., Asensi M., Mañes J., Picó Y. Short-term oral toxicity of quercetin and pterostibene in Swiss mice. Toxicol. Lett. 2006;164S:S1–S324. (Abstract P22-15) [Google Scholar]
- 49.Harwood M., Danielewska-Nikiel B., Borzelleca J.F., Flamm G.W., Williams G.M., Lines T.C. A critical review of the data related to the safety of quercetin and lack of evidence of in vivo toxicity, including lack of genotoxic/carcinogenic properties. Food Chem. Toxicol. 2007;45:2179–2205. doi: 10.1016/j.fct.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 50.Noorafshan A., Karbalay-Doust S. Curcumin protects the seminal vesicles from metronidazole-induced reduction of secretion in mice. Acta Med. (Hradec Kralove) 2012;55:32–36. doi: 10.14712/18059694.2015.72. [DOI] [PubMed] [Google Scholar]
- 51.Gordon B.S., Delgado Díaz D.C., Kostek M.C. Resveratrol decreases inflammation and increases utrophin gene expression in the mdx mouse model of Duchenne muscular dystrophy. Clin. Nutr. 2013;32:104–111. doi: 10.1016/j.clnu.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H.S., David D.J., Swarts S., Sun W., Yang S., Wang W., Liu C., Zhang M., Zhang D., Zhang L., Zhang K., Keng P., Zhang L., Okunieff P. Replication of murine mitochondrial DNA following irradiation. Adv. Exp. Med. Biol. 2009;645:43–48. doi: 10.1007/978-0-387-85998-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.