Highlights
-
•
Silica nanoparticles (225 nm) induced ER stress and unfolded protein response.
-
•
MAPK pathway and associated genes are induced.
-
•
PP2Ac, TNFα, NFкB and interferon stimulated genes are up-regulated.
-
•
p53 is down-regulated, indicating inhibition of apoptosis.
-
•
The data suggest hepatotoxic, inflammatory and tumorigenic action of SiO2-NPs.
Abbreviations: SiO2-NPs, silica nanoparticles; Huh7, human hepatoma cells; UPR, unfolded protein response; PERK, protein kinase like ER kinase; IRE-1, inositol-requiring protein 1; ATF-6, activating transcription factor 6; eIF2α, eukaryotic initiation factor 2α; PP2A, protein phosphatase 2a; MAPK, mitogen-activated protein kinase signaling pathway; ISGs, interferon stiulated genes; IFN α, interferon α; IFN β, interferon β; TNFα, tumor necrosis factor α; NFκB, nuclear factor ‘kappa-light-chain-enhancer’ of activated B-cells; BiP, binding immunoglobulin protein; XBP-1, X-box binding protein 1; ATF-4, Activating transcription factor 4; CHOP, CCAAT/enhancer binding protein-homologous protein; CREB, cAMP response element-binding protein; p53, TP53-tumorsuppressor-gene; IP-10, interferon gamma-induced protein 10; ISG-15, interferon-induced 17 kDa protein; IRF-9, interferon regulatory factor 9; STAT1, signal transducer and activator of transcription 1; Noxa, phorbol-12-myristate-13-acetate-induced protein 1
Keywords: Tumor necrosis factor alpha, Human hepatoma cells, Proinflammatory response ;Iinterferon-stimulated genes
Abstract
Application of silica nanoparticles (SiO2-NPs) may result in human exposure. Here we investigate unexplored modes of action by which SiO2-NPs with average size of 225 nm act on human hepatoma cells (Huh7). We focused on the endoplasmic (ER) stress response and on mitogen-activated protein kinase (MAPK) signaling pathways. Both pathways were induced. ER stress and the associated three unfolded protein response (UPR) pathways were activated as demonstrated by significant inductions of BiP and XBP-1s and a moderate but significant induction of ATF-4 at 0.05 and 0.5 mg/ml. In addition to activation of NFкB interferon stimulated genes IP-10, IRF-9, and ISG-15 were up-regulated. As a consequence of ER stress, the pro-inflammatory cytokine TNFα and PP2Ac were induced following exposure to 0.05 mg/ml SiO2-NPs. Additionally, this occurred at 0.005 mg/ml SiO2-NPs for TNFα at 24 h. This in turn led to a strong transcriptional induction of MAP-kinases and its target genes cJun, cMyc and CREB. A strong transcriptional down-regulation of the proapoptotic gene p53 occurred at 0.05 and 0.5 mg/ml SiO2-NP. Exposure of Huh7 cells to the anti-oxidant N-acetyl cysteine reduced transcriptional induction of ER stress markers demonstrating a link between the induction of oxidative stress and ER stress. Our study demonstrates that SiO2-NPs lead to strong ER stress and UPR induction, oxidative stress, activation of MAPK signaling and down-regulation of p53. All of these activated pathways, which are analyzed here for the first time in detail, inhibit apoptosis and induce cell proliferation, which may contribute to a hepatotoxic, inflammatory and tumorigenic action of SiO2-NPs.
1. Introduction
Engineered silica nanoparticles (SiO2-NPs) find widespread application leading to exposure of humans via oral intake and inhalation. Despite their widespread use, the potential toxicological implications and molecular modes of action are not well known. In mice, SiO2-NPs occurred in mononuclear phagocytic cells in liver and spleen and induced hepatocytic necrosis, increased serum aminotransferase, and inflammatory cytokines [1]. The clearance from bloodstream and tissues can be very slow [2]. SiO2-NPs enter cells and induce time- and size-dependent cytotoxicity at high doses by induction of oxidative stress, membrane damage, as well as disturbed calcium homeostasis [3], [4]. Recently, we have shown that SiO2-NPs also lead to induction of ER stress in human hepatoma cells [5].
The ER stress response is triggered when folding and export of proteins is perturbed under different cellular stress conditions. Accumulation of non- or misfolded proteins in the ER triggers an adaptive response, the unfolded protein response (UPR) that attenuates protein de novo synthesis and enhances the production of chaperones that facilitate protein folding [6] Additionally, enhanced proteosomal degradation of misfolded proteins (proteotoxicity) and apoptosis is induced after a cascade of molecular reactions. There are three distinct pathways triggered by ER stress, all of which induce the expression of different genes aiming to restore the normal function of the ER, and in case it fails, apoptosis will be induced ([7]). The pathways are based on activation of the chaperone BiP (or also called GRP78) that dissociates from transmembrane proteins (ER-resident signaling proteins), such as protein kinase like ER kinase (PERK), inositol-requiring protein 1 (IRE1) and activating transcription factor 6 (ATF6). PERK then phosphorylates eukaryotic elongation factor 2α (eIF2α), which leads to a general translation block, but also to a specific translation of ATF4 [8]. IRE1 turns X-box binding protein 1 (XBP-1) mRNA into the transcription factor XBP-1s. ATF6 gets phosphorylated and turns into a transcription factor. XBP-1s and ATF6 positively lead to up-regulation of a wide variety of ER stress target genes, including chaperones BiP (GRP78). ATF4 and ATF6 result in up-regulation of CCAAT/enhancer-binding protein-homologous protein CHOP (or also called GADD153), which is a pro-apoptotic marker gene. Overexpression of CHOP promotes cell death.
On this basis, the ER stress response can be assessed by selective markers such as induction of the chaperone BiP, splicing of XBP-1 mRNA, and phosphorylation of eIF2α⋅ The ER stress response and associated UPR has important consequences, including apoptosis. It accompanies acute and chronic liver diseases and plays a significant role in liver pathogenesis [9]. Additionally ER stress can activate NFκB [10] leading to the expression of interferons (INFs) Type I and inflammatory cytokines like TNF-α [11]. Interferons have a wide variety of biological activities including antiviral, immunomodulatory, antiangiogenic and antiproliferative and promote apoptosis [12]. IFNs stimulate the expression of anti-viral genes (ISG) [11] and induce several hundreds of genes [13]. Most prominent is ISG-15, a broadly active non-specific antiviral effector and an ubiquitin-like protein that conjugates to over 150 cellular target proteins [14]. TNF-α is involved in the inflammatory response, apoptosis, cell proliferation and cell differentiation.
Inflammatory and immune responses are regulated by multiple signaling pathways. Among them are the NFκB and mitogen-activated protein kinase (MAPK) signaling pathways, which include many proteins including MAPK (originally called the extracellular signal-regulated kinase1/2 ERK1/2), p38, CREB, cMyc and c-Jun N-terminal kinase (JNK) pathways. MAPK regulates the activities of many transcription factors. MAPK phosphorylates cMyc and activates MNK, which phosphorylates CREB. By altering transcription factors, MAPK leads to altered transcription of genes important for the cell cycle. Thus, the MAPK pathway is important in the cellular stress response and modulates a variety of inflammatory responses [15], apoptosis and plays a role in cancer development.
Based on our previous demonstration that by SiO2-NPs induced expression of BiP and splicing of XBP-1 mRNA as two markers of ER stress [5], here we aimed to deepen our understanding on ER stress and associated UPR induction and its consequences as well as on oxidative stress and MAPK signaling. By focusing on these important cellular signaling pathways, here we demonstrate that SiO2-NPs up-regulates PP2Ac, induces three pathways of ER stress reaction, activates NFκB, and induces the expression of TNF-α, IFN-α and some of its downstream genes, and thus establish an anti-viral response in human hepatoma cells. We demonstrate that up-regulation of ER stress and associated UPR and interference with IFN and MAPK signaling are important modes of action of SiO2-NPs.
2. Materials and methods
2.1. SiO2-NP preparation
Fumed SiO2-NPs were purchased from Sigma–Aldrich, Buchs, Switzerland. NPs were weighted, mixed with nano pure water to obtain a stock solution of 1 mg/ml and stirred for 1 h and sonicated in a water bath for 5 min. NP suspensions were subsequently diluted with nano pure water and finally a 1:2 dilution with the cell culture medium (without FBS) was done to achieve the final assay concentrations. Before adding the NP dilutions to the cells, the dilutions were mixed again to distribute the NPs as homogenously as possible.
2.2. Nanoparticle tracking analysis (NTA)
SiO2-NPs at a concentration of 1 mg/ml were dispersed in cell culture medium, stirred for 1 h and sonicated in a water bath for 5 min. Afterwards the particle size distribution was determined by NanoSight LM10 (NanoSight Ltd., U.K.) followed by evaluation using the Nanoparticle Tracking Analysis (NTA) software.
2.3. Huh7 cells
The human hepatoma cell line Huh7 was kindly provided by Markus Heim, University Hospital Basel, Switzerland. Cells were grown in DMEM with GlutaMAX™ (LuBioScience, Lucerne, Switzerland) supplemented with 10% FBS in a humidified incubator with 5% CO2 at 37 °C. Cells were usually split every 4 days and sub-cultured at split ratios of about 1:6.
2.4. RNA isolation, reverse transcription, and quantitative (q)PCR
Total RNA was isolated from Huh7 cells using Trizol reagent according to the manufacturer's instructions. RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Promega Biosciences, Inc., Wallisellen, Switzerland) in the presence of random hexamers (Roche) and deoxynucleoside triphosphate. The reaction mixture was incubated for 5 min at 70 °C and then for 1 h at 37 °C. The reaction was stopped by heating at 95 °C for 5 min. qPCR was performed based on SYBR green fluorescence (SYBR green PCR master mix; Roche, Switzerland). The sequences of the used primers are shown in Table 1. The amplification conditions were 95 °C for 5 min for initial denaturing, 40 cycles of 95 °C for 30 s for denaturing, 61 °C for 60 s for annealing and elongation. A melting curve was run afterwards. The difference in the cycle threshold (ΔCT) value was derived by subtracting the CT value for GAPDH, which served as an internal control, from the CT value for the target genes. All reactions were run in duplicates using a BioRad real time PCR machine (CFX 96 Real Time System). mRNA expression levels of target genes were expressed as a several fold increase according to the formula 2ΔCT (not exposed) −ΔCT (exposed).
Table 1.
Sequences of used qPCR primers.
| Primer | Forward (5 ′→ 3′) | Reverse (5 ′→ 3′) |
|---|---|---|
| GAPDH | GAAGGTGAAGGTCGGAGTC | GAAGATGGTGATGGGATTTC |
| ATF4 | AGT GGC ATC TGT ATG AGC CCA | GCT CCT ATT TGG AGA GCC CCT |
| BiP | CGA GGA GGA GGA CAA GAA GG | GAC CTT GAA CGG CAA GAA CT |
| XBP-1s | TGC TGA GTC CGC AGC AGG TG | GCT GGC AGG CTC TGG GGA AG |
| Noxa | ATT ACC GCT GGC CTA CTG TG | GTG CTG AGT TGG CAC TGA AA |
| PP2Ac | CCA CAG CAA GTC ACA G/CAT TGG | CAGAGCACTTGATCGCCTACAA |
| TNF-α | CAG CCT CTT CTC CTT CCT GA | TGAGGTACAGACCCTCTGAT |
| IP-10 | CGA TTC TGA TTT GCT GCC TTA TC | GCAGGTACAGCGTAGATTTC |
| IRF-9 | CCCGAAAACTCCGGAACTG | CAGCACACTCCGGGAAACT |
| ISG-15 | GGTGGACAAATGCGACGAA | ATG CTG GTG GAG/C GCC CTT A |
| CREB | ATT CAC AGG AGT CAG TGG ATA GT | CAC CGT TAC AGT GGT GAT GG |
| c-Jun | TCC AAG TGC CGA AAA AGG AAG | CGA GTT CTG AGC TTT CAA GGT |
| STAT1 | ATC AGG CTC AGT CGG GGA ATA | TGG TCT CGT GTT CTC TGT TCT |
| c-Myc | TGA GGA GAC ACC GCC CAC | CAA CAT CGA TTT CTT CCT CAT CTT |
2.5. Preparation of cell extracts and immunoblotting
Cells were homogenized in 50 μl of lysis buffer (50 mM Tris, 150 mM NaCl, 15 mM EDTA, 0.1% Triton X-100 and 1 mM phenylmethylsulfonyl fluoride) incubated for 20 min on ice, centrifuged at 14,000 rpm for 5 min. Protein concentrations were determined with Thermo Scientific BCA™ protein assay kit (Fish Scientific, Wohlen, Switzerland). Immunoblotting was performed as described [49]. To detect the PP2Ac and BiP band, the membranes were scanned with a Fujifilm FLA-9000 scanner (Bucher biotec, Basel, Switzerland). Membranes were stained after scanning with Ponceau S solution (Sigma–Aldrich, Buchs, Switzerland) to check for equal loading.
2.6. ROS assay for assessment of reactive oxygen species (ROS) production
Huh7 cells were plated at a density of 50,000 cells per well in 96-well plates. After a 24 h recovery cells were treated with either non-toxic or toxic concentrations of SiO2-NPs (0.005, 0.05 and 0.5 mg/ml). After 24 h incubation, the medium was aspirated and each well was washed with PBS. Thereafter, cells were incubated with 100 μM H2DCFDA for 30 min and washed again with PBS. H2DCFDA is a non-fluorescent, cell permeable substrate that is converted into a fluorescent product by reactive oxygen species. The fluorescence (extinction at 485 nm and emission at 530 nm) was measured by an automatic microplate reader (Tecan Infinite M200, Tecan, Männedorf, Switzerland).
2.7. MTT assay for cytotoxicity assessment
Huh7 were plated at a density of 50,000 cells per well in 96-well plates. After 24 h, cells were treated with 0.005, 0.05 and 0.5 mg/ml SiO2-NPs for 24 h. Before adding 25 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT, 5 mg/mL in PBS, Sigma–Aldrich, Buchs, Switzerland) to each well, the medium containing the SiO2-NPs was soaked off, each well was washed once with PBS and 200 μL medium were added. Subsequently the plates were incubated at 37 °C for 3 h. At the end of this period, the medium, containing the MTT solution, was replaced with 150 μL dimethyl sulfoxide and shaken for 10 min to solubilize the crystals. The absorbance was measured by an automatic microplate reader (GENios Tecan reader, Tecan, Männedorf, Switzerland) at 570 nm. The results were expressed as percent living cells compared to untreated control cells.
2.8. TNF-α ELISA
In the supernatant of Huh7 cells, the levels of TNF-α were measured according to the manufacturer's instructions (Bioscience, San Diego, USA).
2.9. NFκB activation assay
The activation of NFκB was investigated using the TransAM-NFκB p65 assay according to the manufacturer's instructions (Active Motif, LaHulpe, Belgium)
3. Results
3.1. Characterization of SiO2-NPs
The employed SiO2-NPs previously analyzed by [5] were characterized by heterogeneous size distribution of the SiO2-NPs with a mean size of 273 nm, a BET of 115 m2/g and a Zeta potential of −12.7 mV. For confirmation, SiO2-NPs were measured again. The heterogeneous size distribution with particles with a size smaller than 100 nm and particles bigger than 500 nm were determined. The majority of particles showed a size between 100 and 300 nm with an average of 225 ± 32 nm (Fig. S1).
3.2. Induction of ER stress in Huh7 cells
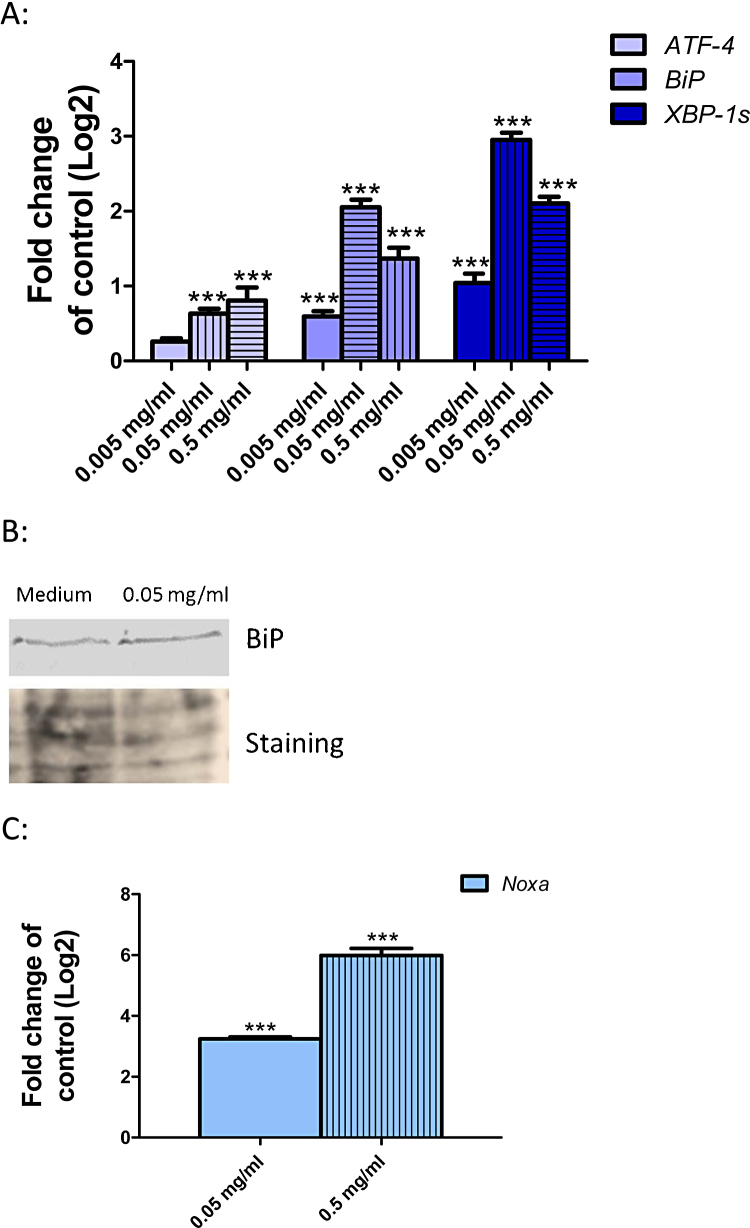
In our previous study, we demonstrated the uptake of the SiO2-NPs into Huh7 cells by transmission electron microscopy [5]. Based on our previous data demonstrating an induction of ER stress in Huh7 cells after exposure to SiO2-NP, here we made a more detailed analysis of ER stress and induction of the UPR. We investigated three well known ER stress markers associated with three distinct branches of the UPR, namely ATF-4, BiP and XBP-1s. Huh7 cells were exposed to 0.005, 0.05 and 0.5 mg/ml SiO2-NPs for 24 h followed by quantification of ATF-4, BiP and XBP-1s mRNA. SiO2-NPs lead to a strong induction of BiP and XBP-1s at all concentrations and a moderate but significant induction of ATF-4 at 0.05 and 0.5 mg/ml (Fig. 1A). In addition to the transcript BiP protein was induced at 0.05 mg/ml SiO2-NPs (Fig. 1B). These data clearly demonstrate that exposure to SiO2-NP lead to ER stress and associated induction of UPR. In addition we analyzed the expression of Noxa, a gene up-regulated in response to ER stress. We found a strong up-regulation of Noxa after exposure to 0.05 and 0.5 mg/ml SiO2-NPs (Fig. 1C).
Fig. 1.
Induction of ER stress in Huh7 cells after SiO2-NP exposure. (A) Huh7 cells were exposed to 0.005 (blank bars), 0.05 (vertical strips) and 0.5 mg/ml (horizontal strips) SiO2-NPs for 24 h followed by the investigation of mRNA level of ATF-4, BiP and XBP-1s. (B) Huh7 cells were exposed to 0.05 mg/ml SiO2-NPs for 24 h followed by Western blot analysis for BiP (upper panel). As loading control the Western blot membrane was stained with Ponceau S solution (lower panel). (C) Huh7 cells were exposed to 0.05 (blank bar) and 0.5 mg/ml (vertical strips) SiO2-NPs for 24 h followed by the detection of mRNA levels of Noxa. Shown are the results of three independent experiments. Significant differences with p-value of ≤0.05 are marked with asterisks.
3.3. Induction of TNF-α and PP2Ac as consequences of ER stress
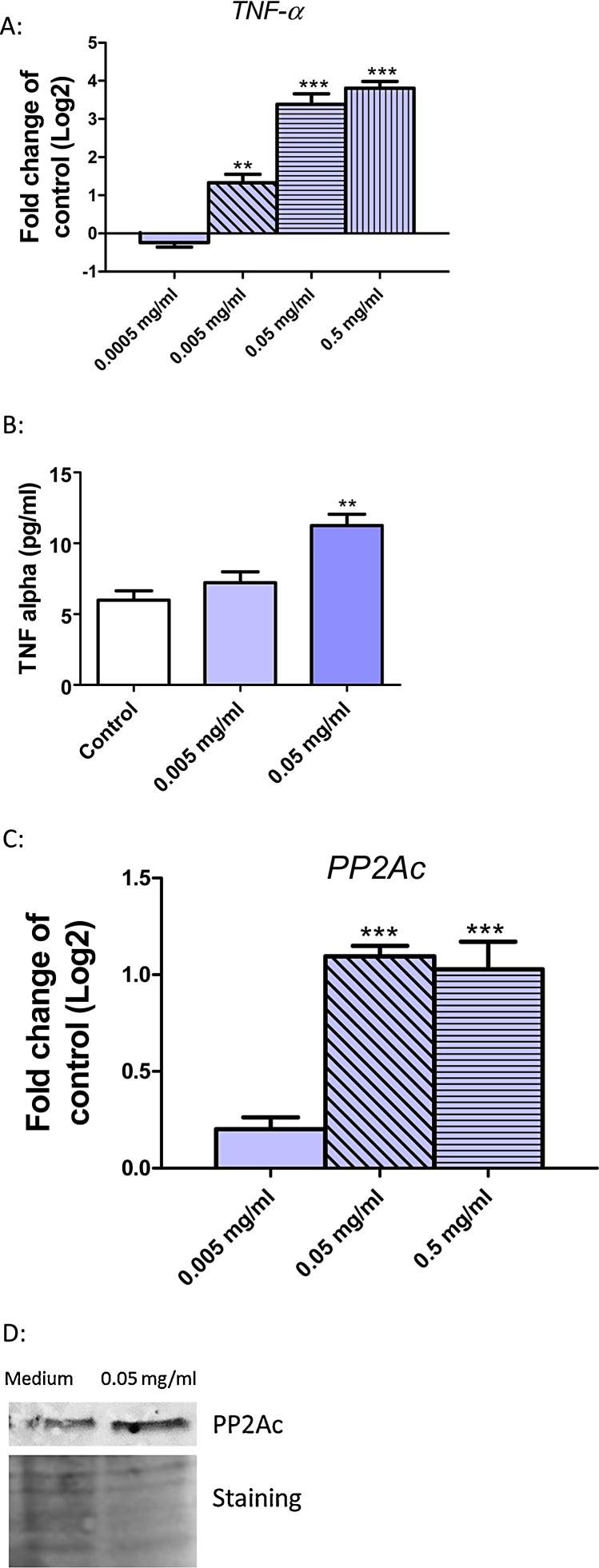
One consequence of ER stress is the induction of TNF-α. Therefore we analyzed the expression of TNF-α on the mRNA and protein level in Huh7 cells after 24 h exposure to SiO2-NPs. Fig. 2A shows a significant and dose-dependent induction of TNF-α mRNA. In addition, we analyzed the TNF-α protein level in the supernatant of Huh7 cells. An induction of TNF-α protein occurred after a 24 h exposure to SiO2-NPs at 0.005 mg/ml, which was significant at 0.05 mg/ml (Fig. 2B). Another known consequence of ER stress is the induction of PP2Ac. A significant induction of PP2Ac mRNA was detected after exposure of Huh7 cells to 0.05 and 0.5 mg/ml SiO2-NPs (Fig. 2C). PP2Ac was also induced at the protein level (Fig. 2D).
Fig. 2.
Induction of TNF-α and PP2Ac in Huh7 cells after exposure to SiO2-NPs. (A) Huh7 cells were exposed to 0.0005 (blank bars), 0.005 (diagonal strips), 0.05 (horizontal strips) and 0.5 mg/ml (vertical strips) SiO2-NPs for 24 h followed by determination of mRNA levels of TNF-α. (B) Huh7 cells were exposed to 0.005 and 0.05 mg/ml SiO2-NPs for 24 h followed by the investigation of TNF-α protein level in the cell culture supernatant. (C) Huh7 cells were exposed to 0.005 (blank bars), 0.05 (diagonal strips) and 0.5 mg/ml (horizontal strips) SiO2-NPs for 24 h followed by detection of mRNA level of PP2Ac. (D) Huh7 cells were exposed to 0.05 mg/ml SiO2-NPs for 24 h followed by Western blot analysis for PP2Ac (upper panel). As loading control the Western blot membrane was stained with Ponceau S solution (lower panel). Shown are the results of three independent experiments. Significant differences with p-value of ≤0.05 are marked with asterisks.
3.4. Activation of NFκB and interferon stimulated genes as consequence of ER stress
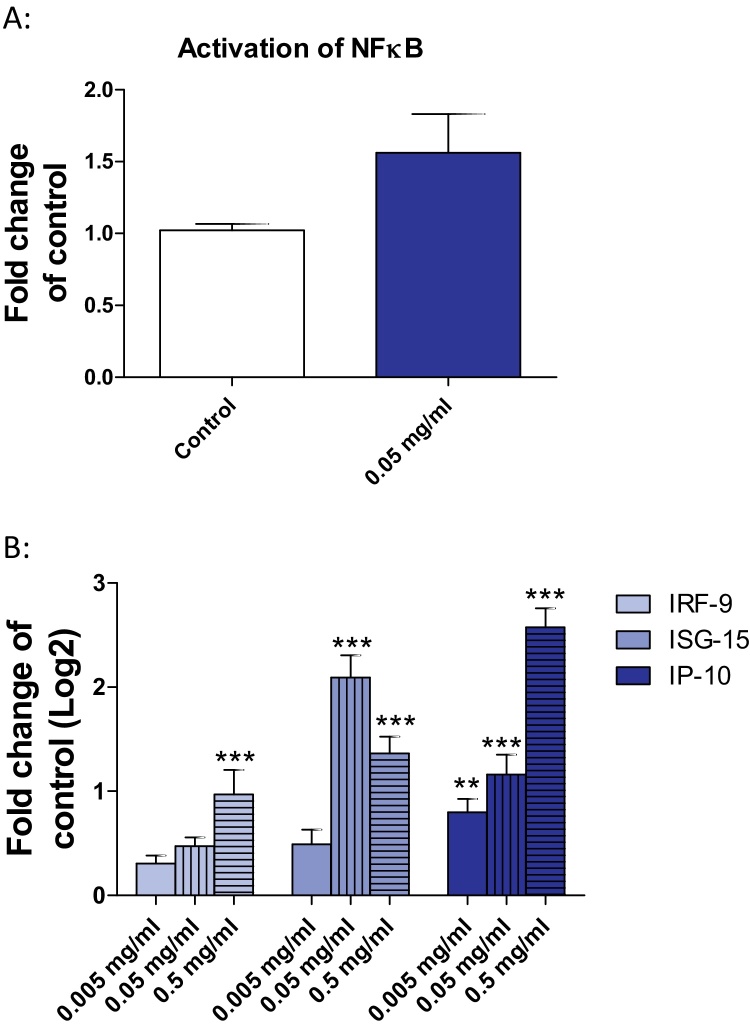
ER stress and TNF-α can both lead to an activation of NFκB. We investigated the phosphorylation of p65, one member of the NFκB family. Upon exposure of Huh7 cells to 0.05 mg/ml SiO2-NPs p65 showed a weak activation (Fig. 3A). As NFκB is a transcription factor regulating interferon-α and interferon-β, we analyzed the expression of interferon stimulated genes. The IP-10 transcript showed a dose-dependent and significant induction (Fig. 3B). ISG-15 was significantly induced at 0.05 and 0.5 mg/ml SiO2-NPs and IRF-9 was weakly but significantly induced at the highest concentration (Fig. 3B).
Fig. 3.
Activation of NFκB and induction of interferon stimulated genes in Huh7 cells after exposure to SiO2-NPs. (A) Huh7 cells were exposed to 0.05 mg/ml SiO2-NPs for 24 h followed by NFκB activation assay. (B) Huh7 cells were exposed to 0.005 (blank bars), 0.05 (vertical strips) and 0.5 mg/ml (horizontal strips) SiO2-NPs for 24 h followed by the investigation of mRNA level of IRF-9, ISG-15 and IP-10. Shown are the results of three independent experiments. Significant differences with p-value of ≤0.05 are marked with asterisks.
3.5. Activation of MAP-kinase pathway
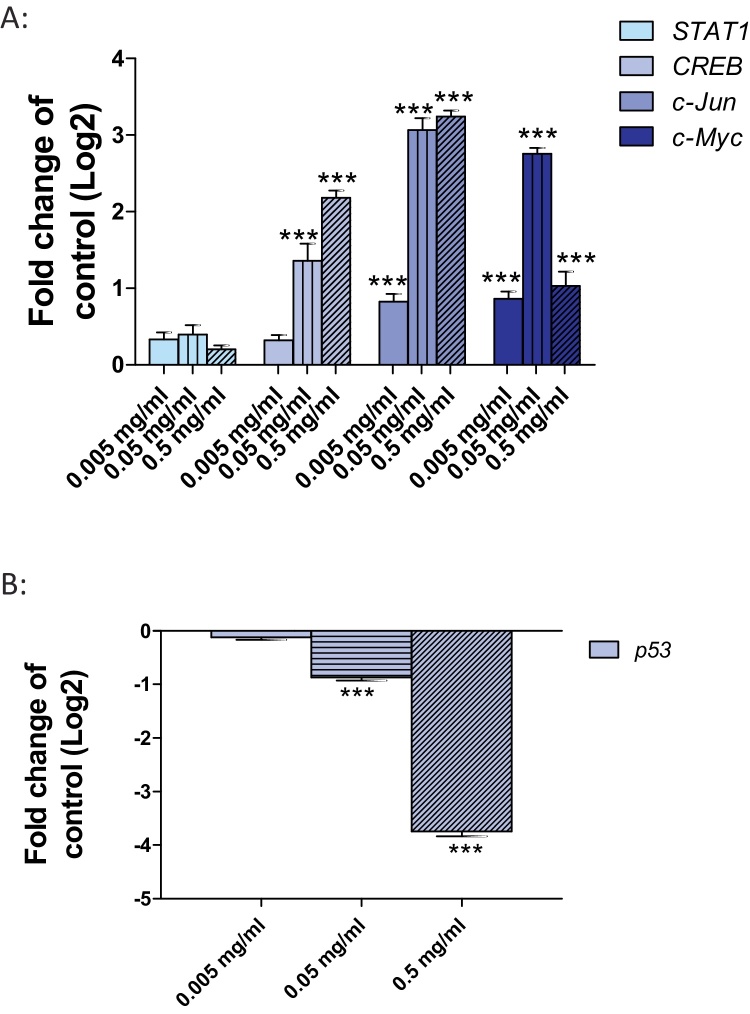
As TNF-α leads to an activation of the MAP-kinases, the expression of four different MAP-kinases target genes, including STAT1, CREB, c-Jun and c-Myc was analyzed. A significant induction of CREB was observed after exposure of Huh7 cells to 0.05 and 0.5 mg/ml SiO2-NPs. c-Jun and c-Myc were weakly but significant induced after exposure to 0.005 mg/ml and strongly induced after exposure to 0.05 and 0.5 mg/ml SiO2-NPs (Fig. 4A). No induction of STAT1 was detected (Fig. 4A). Additionally, the MPK-kinases target gene p53, which is negatively regulated through c-Jun, was analyzed. A significant down-regulation of p53 occurred after exposure of Huh7 cells to 0.05 mg/ml SiO2-NPs and a very strong down-regulation after exposure to 0.5 mg/ml (Fig. 4B).
Fig. 4.
Activation of MAPK signaling pathway and inhibition of p53 in Huh7 cells after exposure to SiO2-NPs. (A) Huh7 cells were exposed to 0.005 (blank bars), 0.05 (vertical strips) and 0.5 mg/ml (diagonal strips) SiO2-NPs for 24 h followed by the investigation of mRNA level of STAT1, CREB, c-Jun and c-Myc. (B) Huh7 cells were exposed to 0.005 (blank bars), 0.05 (horizontal strips) and 0.5 mg/ml (diagonal strips) SiO2-NPs for 24 h followed by the investigation of mRNA level of p53. Shown are the results of three independent experiments. Significant differences with p-value of ≤0.05 are marked with asterisks.
3.6. Link between oxidative stress and ER stress
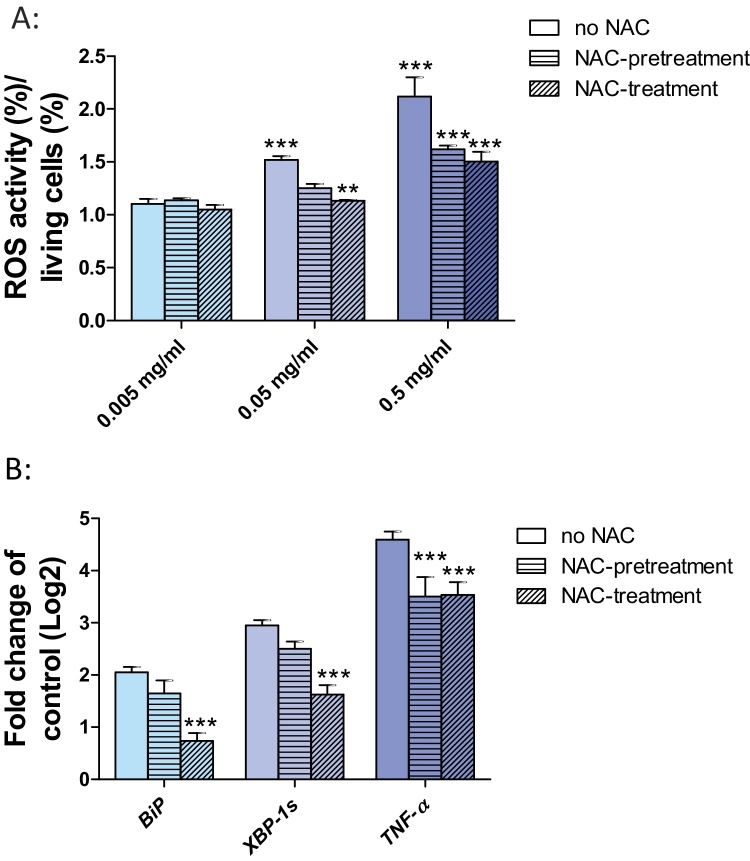
To analyze the potential induction of oxidative stress in Huh7 cells after exposure to SiO2-NPs, we determined ROS induction. To further demonstrate a mitigation of oxidative stress induction, we pre-treated Huh7 cells with the antioxidant N-acetyl-l-cysteine (NAC) for 30 min prior to the exposure to SiO2-NPs. In addition, we pre-treated Huh7 cells for 30 min with NAC followed by co-exposure to SiO2-NPs and NAC. The aim was to test, whether SiO2-NP related oxidative stress and associated expression of ER stress genes are lowered or prevented by NAC. Exposure to 0.05 and 0.5 mg/ml SiO2-NPs lead to the induction of oxidative stress (Fig. 5A). Pre-treatment or co-exposure with NAC clearly reduced oxidative stress (Fig. 5A). As there is evidence that oxidative stress causes ER stress, we analyzed the expression of two ER stress markers BiP and XBP-1s as well as the expression of TNF-α in Huh7 cells after pre-treatment with NAC and co-exposure with NAC and SiO2-NPs. Co-exposure of Huh7 cells with SiO2-NPs and NAC significantly reduced transcriptional expression of BiP and XBP-1s (Fig. 5B). The TNF-α transcript was also significantly reduced when Huh7 cells were treated with NAC prior to the exposure to SiO2-NPs and when co-exposed to SiO2-NPs and NAC (Fig. 5B).
Fig. 5.
Induction of oxidative stress in Huh7 cells upon exposure to SiO2-NPs. (A) Huh7 cells were exposed to 0.005, 0.05 and 0.5 mg/ml SiO2-NPs alone (blank bars), or pretreated for 30 min with NAC followed by 24 h exposure to SiO2-NP (horizontal bars) or co-exposed to NAC and SiO2-NPs for 24 h (diagonal bars) followed by the investigation of oxidative stress. (B) Huh7 cells were exposed to 0.05 mg/ml SiO2-NPs alone (blank bars), or pretreated for 30 min with NAC followed by 24 h exposure to SiO2-NP (horizontal bars) or co-exposed to NAC and SiO2-NPs for 24 h (diagonal bars) followed by the investigation of the mRNA of BiP, XBP-1s and TNF-α. Shown are the results of three independent experiments. Significant differences with p-value of ≤0.05 are marked with asterisks.
4. Discussion
4.1. Induction of ER stress, activation of UPR and consequences
Our present work deepened the understanding of the molecular effects of SiO2-NPs by focusing on ER stress response previously detected [5]. Here we showed that exposure of Huh7 cells to SiO2-NPs lead to ER stress and activation of the UPR. After 24 h, all three ER stress pathways were activated; the IRE1 pathway, resulting in the splicing of XBP-1, the PERK pathway, resulting in elevated transcription and translation of the transcription factor ATF-4 and the ATF-6 pathway, resulting in elevated transcription and translation of BiP (Fig. 1A and B). The activation of the IRE1 and ATF-6 pathways occurred at all SiO2-NP concentrations, whereas the activation of the PERK pathway occurred at the two higher concentrations.
The induction of ER stress can have several consequences for the cell. Either the cell can cope with the stress and restore normal cellular functions, or it will undergo apoptosis. To restore cellular functions and remove the unfolded proteins from the ER, chaperons become up-regulated, protein translation is inhibited and protein degradation increases. In case the ER stress is too strong and the cell cannot restore normal ER function, apoptotic pathways will be activated [7]. Therefore, ER stress is one mechanism contributing to the cytotoxicity of NPs.
One important consequence of ER stress is the release of calcium from the ER lumen into the cytosol [16]. Increased calcium concentration in turn can have important consequences. One effect is the phosphorylation of the transcription factor CREB, which induces the transcription of protein phosphatase 2Ac (PP2Ac). Our data demonstrate the up-regulation of PP2Ac on the mRNA and on the protein level by SiO2-NPs (Fig. 2C and D). PP2Ac is involved in a wide range of cellular processes including cell cycle regulation, cell morphology, development, signal transduction, apoptosis and stress response [17]. Therefore, the induction of ER stress followed by up-regulation of PP2A has marked cellular effects. Previously, increased cytosolic calcium concentrations were reported in neuronal cells after silica NP exposure [3], and interpreted as an influence of the nanoparticles on influx pumps. However, based on our data, the increased calcium concentration may also originate from the ER stress response. Induction of intracellular calcium transients was also found in human lung fibroblasts after exposure to silver nanoparticles [18]. Additionally, an increase in intracellular free calcium was observed after exposure of cells with TiO2-NPs [19]. Consequently, ER stress and associated alteration of calcium homeostasis triggering cellular toxicity may be an important effect underlying the cytotoxicity of NPs.
Furthermore, ER stress was also shown for other nanoparticles, including ZnO-NPs in human umbilical vein endothelial cells [20], poly(lactic-co-glycolic acid)-nanoparticles [21] and gold nanoparticles in human chronic myelogenous leukemia cells [22].
Activation of both the PERK and IRE1 pathways leads to regulation of the NFκB-IKK signaling pathway during ER stress through activation of IκB kinase (IKK) or degradation of the p65 unit [11]. The ATF6 branch of the ER stress response can also regulate NFκB activity [23]. We could also show the activation of NFκB in Huh7 cells after SiO2-NP exposure (Fig. 3A). Consequences of the activation of NFκB are the induction of INF-α [11] and TNF-α [10]. This was also observed in our experiments (Fig. 2A and B, Fig. 3B). There is also a direct link between ER stress and TNF-α. Silencing of ATF4 and CHOP prevented the upregulation of TNF-α in cells [24]. Similarly, the induction of TNF-α was observed in human bronchial epithelial cells after exposure to titanium dioxide nanoparticles [25]. ZnO-NPs induced the expression of TNF-α in human keratinocytes. The up-regulation of TNF-α was dependent on the activation of the extracellular signal-regulated kinase (ERK) of MAPK pathways [26]. TNF-α belongs to the group of proinflammatory cytokines involved in the pathogenesis of several diseases including cancer [27], rheumatoid arthritis, diabetes and inflammatory bowel disease [28]. TNF-α is known as an endogenous tumor promoter [29]. Therefore, chronic human exposure to SiO2-NPs may ultimately result in adverse effects on human health.
Our data further corroborate on previous results the induction of ER stress by SiO2-NPs [5]. We therefore hypothesize that ER stress and up-regulation of UPR may be considered as a more general effect induced by nanoparticles.
4.2. Consequences of prolonged ER stress and activation of UPR
Chronic and severe ER stress results in the activation of apoptotic pathways. Expression of CHOP, an important proapoptotic marker gene, is induced by ATF-4. CHOP itself induces the expression of the apoptotic genes BIM (member of the Bcl-2 family) and p53 upregulated modulator of apoptosis (PUMA). The IRE1 pathway may induce apoptosis by the activation of the apoptosis signaling kinase 1 (ASK1) and through interaction with tumor necrosis factor-associated factor 2 (TRAF2). Therefore, SiO2-NPs may show hepatotoxic activity through ER stress and induction of UPR. Another important gene transcript up-regulated in response to ER stress is Noxa [30], which induces apoptosis by the Usp9x-Mcl-1 pathway [31]. This could also contribute to the hepatotoxic action of SiO2-NPs.
Constant ER stress contributes to the development of the metabolic syndrome, is linked with hepatic steatosis and ER stress also inhibits hepatic lipoprotein secretion [32], [33], [34]. UPR activation including eIF2α phosphorylation and splicing of XBP-1 mRNA was detected during adipogenesis. [35].
Additionally, the UPR plays also a role in cancer development. Activation of ATF-4 is critical for tumor cell proliferation and tumor growth [36]. The IRE1α-XBP-1 pathway is important for tumor cell survival and growth [37]. Therefore, it is conceivable that chronic exposure to SiO2-NPs may result in the induction of these alterations in the liver.
4.3. Inhibition of p53
P53 is important for apoptosis, genomic stability, DNA repair, inhibition of angiogenesis and inhibition of growth by stopping the cells cycle in the G1/S phase. In case of irreversible DNA damage, p53 leads to induction of apoptosis [38]. In more than 50% cancers the p53 protein is either absent or non-functional due to various other reasons [39]. We found a significant down-regulation of p53 in Huh7 cells after exposure to SiO2-NPs (Fig. 4B). One reason for this down-regulation could be the up-regulation of c-Jun, as it is known that overexpression of c-Jun in cells results in decreased level of p53 [40]. Another reason for the down-regulation of p53 could be the activation of NFκB. It is known that NFκB can suppress p53 levels by upregulating mouse double minute 2 homolog (MDM2) expression mediated through B-cell CLL/lymphoma 3 (Bcl3) [41]. Transcriptional down-regulation of the tumor suppressor p53 could contribute to the cancerogenic activity of SiO2-NPs.
4.4. Link between oxidative stress and ER stress
In addition to induction of ER stress, we observed the induction of oxidative stress by SiO2-NPs (Fig. 5A). Oxidative stress is a common reaction of cells to the exposure to nanoparticles [5], [42]. It is known that oxidative stress mediated Ca2+ release induces ER stress and UPR [43]. To investigate the link between oxidative stress and ER stress, we pre-treated Huh7 cells with the antioxidant NAC before exposure to SiO2-NPs. Pre-treatment of Huh7 cells with NAC reduced the SiO2-NP induced oxidative stress and the expression of ER stress genes and TNF-α (Fig. 5A and B). But even when there was no oxidative stress (because of the NAC treatment) XBP-1s and TNF-α were still induced (Fig. 5B). These data show that oxidative stress contributes to the induction of ER stress, but it is not the only factor leading to ER stress.
4.5. Mitogen-activated protein kinases (MAP kinases) signaling cascade
Three groups of MAP kinases belong to the MAP signaling cascade. Their function is to transduce a variety of extracellular signals that regulate cellular responses implicated in proliferation, differentiation and death [44]. The three most predominant members of the MAPK family are the extracellular signal-regulated kinase (ERK), c-Jun N-terminal kinase (JNK), and p38 [45]. The MAP signaling cascade can be activated by TNF-α [44]. Here we demonstrate the activation of three MAP signaling cascade target genes, namely CREB, c-Jun and c-Myc by SiO2-NPs (Fig. 4A). Therefore, we propose that the MAP signaling cascade is activated in response to ER stress. The activation of MAPK signaling cascade in cells following SiO2-NP exposure was previously observed in human bronchial epithelial cells [46]. Cerium oxide nanoparticles activate the MAP signaling cascade in human hepatoma SMMC-7721 cells [47]. Silver nanoparticles at non-cytotoxic concentration induced the expression of c-Jun in human hepatoma cells (HepG2 cells). This activation of the MAPK signaling cascade was linked to an increased proliferation of the HepG2 cells [48].
5. Conclusions
We investigated the ER stress response in Huh7 cells upon exposure to SiO2-NPs as well as down-stream events triggered by ER stress. SiO2-NPs lead to activation of NFκB and induction of interferon stimulated genes. We also monitored the activation of TNF-α and the activation of the MAP kinase target genes CREB, c-Jun and c-Myc. All these genes contribute to the activation of a proinflammatory response. Furthermore, we showed the up-regulation of PP2A in response to ER stress. Induction of PP2Ac and TNF-α and down-regulation of p53 induced by SiO2-NPs can contribute to the development of cancer after chronic exposure. Therefore we assume that chronic exposure to SiO2-NPs may lead to adverse health effects in the liver.
Transparency document
Acknowledgment
We thank Sebastian Müller for assistance and the School of Life Sciences for initial funding of the work.
Footnotes
Available online 4 November 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.10.023.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Liu T., Li L., Teng X., Huang X., Liu H., Chen D., Ren J., He J., Tang F. Single and repeated dose toxicity of mesoporous hollow silica nanoparticles in intravenously exposed mice. Biomat. 2011;32:1657–1668. doi: 10.1016/j.biomaterials.2010.10.035. [DOI] [PubMed] [Google Scholar]
- 2.Cho M., Cho W.S., Choi M., Kim S.J., Han B.S., Kim S.H., Kim H.O., Sheen Y.Y., Jeong J. The impact of size on tissue distribution and elimination by single intravenous injection of silica nanoparticles. Toxicol. Lett. 2009;189:177–183. doi: 10.1016/j.toxlet.2009.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Ariano P., Zamburlin P., Gilardino A., Mortera R., Onida B., Tomatis M., Ghiazza M., Fubini B., Lovisolo D. Interaction of spherical silica nanoparticles with neuronal cells: size-dependent toxicity and perturbation of calcium homeostasis. Small. 2011;7:766–774. doi: 10.1002/smll.201002287. [DOI] [PubMed] [Google Scholar]
- 4.Napierska D., Thomassen L.C., Lison D., Martens J.A., Hoet P.H. The nanosilica hazard: an-other variable entity. Part. Fibre Toxicol. 2010;7:39. doi: 10.1186/1743-8977-7-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Christen V., Fent K. Silica nanoparticles and silver-doped silica nanoparticles induce en-doplasmatic reticulum stress response and alter cytochrome P4501A activity. Chemosphere. 2012;87:423–434. doi: 10.1016/j.chemosphere.2011.12.046. [DOI] [PubMed] [Google Scholar]
- 6.Harding H.P., Calfon M., Urano F., Novoa I., Ron D. Transcriptional and translational control in the mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- 7.Rutkowski D.T., Kaufman R.J. A trip to the ER: coping with stress. Trends Cell. Biol. 2004;1:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Harding H.P., Zhang Y., Ron D. Protein translation and folding are coupled by an endo-plasmic-reticulum-resident kinase. Nature. 1999;397:271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 9.Dara L., Ji C., Kaplowitz N. The contribution of ER stress to liver diseases. Hepatology. 2011;53:1752–1763. doi: 10.1002/hep.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin W.C., Chuang Y.C., Chang Y.S., Lai M.D., Teng Y.N., Su I.J. Endoplasmic reticulum stress stimulates p53 expression through NF-κB activation. PLoS ONE. 2012;7:e39120. doi: 10.1371/journal.pone.0039120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Stark G.R., Kerr I.M., Williams B.R., Silverman R.H., Schreiber R.D. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 13.de Veer M.J., Holko M., Frevel M., Walker E., Der S., Paranjape J.M. Functional classification of interferon-stimulated genes identified using microarrays. J. Leukoc. Biol. 2001;69:912–920. [PubMed] [Google Scholar]
- 14.Ritchie K.J., Hahn C.S., Kim K.I., Yan M., Rosario D., Li L. Role of ISG15 protease UBP43 (USP18) in innate immunity to viral infection. Nat. Med. 2004;10:1374–1378. doi: 10.1038/nm1133. [DOI] [PubMed] [Google Scholar]
- 15.DiDonato J.A., Mercurio F., Karin M. NFκB and the link between inflammation and cancer. Immunol. Rev. 2012;246:379–400. doi: 10.1111/j.1600-065X.2012.01099.x. [DOI] [PubMed] [Google Scholar]
- 16.Christen V., Treves S., Duong F.H., Heim M.H. Activation of endoplasmic reticulum stress response by hepatitis viruses up-regulates protein phosphatase 2A. Hepatology. 2007;46:558–565. doi: 10.1002/hep.21611. [DOI] [PubMed] [Google Scholar]
- 17.Janssens V., Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asharani P.V., Hande M.P., Valiyaveettil S. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol. 2009;10:65. doi: 10.1186/1471-2121-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koeneman B.A., Zhang Y., Westerhoff P., Chen Y., Crittenden J.C., Capco D.G. Toxicity and cellular responses of intestinal cells exposed to titanium dioxide. Cell Biol. Toxicol. 2010;26:225–238. doi: 10.1007/s10565-009-9132-z. [DOI] [PubMed] [Google Scholar]
- 20.Chen R., Huo L., Shi X., Bai R., Zhang Z., Zhao Y., Chang Y., Chen C. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano. 2014;8:2562–2574. doi: 10.1021/nn406184r. [DOI] [PubMed] [Google Scholar]
- 21.Hou C.C., Tsai T.L., Su W.P., Hsieh H.P., Yeh C.S., Shieh D.B., Su W.C. Pronounced induction of endoplasmic reticulum stress and tumor suppression by surfactant-free poly(lactic-co-glycolic acid) nanoparticles via modulation of the PI3K signaling pathway. Int. J. Nanomed. 2013;8:2689–26707. doi: 10.2147/IJN.S47208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai Y.Y., Huang Y.H., Chao Y.L., Hu K.Y., Chin L.T., Chou S.H., Hour A.L., Yao Y.D., Tu C.S., Liang Y.J., Tsai C.Y., Wu H.Y., Tan S.W., Chen H.M. Identification of the nanogold particle-induced endoplasmic reticulum stress by omic techniques and systems biology analysis. ACS Nano. 2011;5:9354–9369. doi: 10.1021/nn2027775. [DOI] [PubMed] [Google Scholar]
- 23.Yamazaki H., Hiramatsu N., Hayakawa K., Tagawa Y., Okamura M. Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J. Immunol. 2009;183:1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Y., Gao H., Yin Q., Chen L., Dong P., Zhang X., Kang J. ER stress activating ATF4/CHOP-TNF-α signaling pathway contributes to alcohol-induced disruption of osteogenic lineage of multipotential mesenchymal stem cell. Cell. Physiol. Biochem. 2013;32:743–754. doi: 10.1159/000354476. [DOI] [PubMed] [Google Scholar]
- 25.Val S., Hussain S., Boland S., Hamel R., Baeza-Squiban A., Marano F. Carbon black and titanium dioxide nanoparticles induce pro-inflammatory responses in bronchial epithelial cells: need for multiparametric evaluation due to adsorption artifacts. Inhal. Toxicol. 2009;1:115–122. doi: 10.1080/08958370902942533. [DOI] [PubMed] [Google Scholar]
- 26.Jeong S.H., Kim H.J., Ryu H.J., Ryu W.I., Park Y.H., Bae H.C., Jang Y.S., Son S.W. ZnO nanoparticles induce TNF-α expression via ROS-ERK-Egr-1 pathway in human keratinocytes. J. Dermatol. Sci. 2013;72:263–273. doi: 10.1016/j.jdermsci.2013.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Locksley R.M., Killeen N., Lenardo M.J. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 28.Wong V.W., Wong G.L., Tsang S.W., Hui A.Y., Chan A.W., Choi P.C., So W.Y., Tse A.M., Chan F.K., Sung J.J., Chan H.L. Genetic polymorphisms of adiponectin and tumor necrosis factor-alpha and nonalcoholic fatty liver disease in Chinese people. J. Gastroenterol. Hepatol. 2008;6:914–921. doi: 10.1111/j.1440-1746.2008.05344.x. [DOI] [PubMed] [Google Scholar]
- 29.Fujiki H., Suganuma M. Tumor necrosis factor-alpha, a new tumor promoter, engendered by biochemical studies of okadaic acid. J. Biochem. 1994;115:1–5. doi: 10.1093/oxfordjournals.jbchem.a124282. [DOI] [PubMed] [Google Scholar]
- 30.Rosebeck S., Sudini K., Chen T., Leaman D.W. Involvement of Noxa in mediating cellular ER stress responses to lytic virus infection. Virology. 2011;417:293–303. doi: 10.1016/j.virol.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yan J., Zhong N., Liu G., Chen K., Liu X., Su L., Singhal S. Usp9x- and Noxa-mediated Mcl-1 downregulation contributes to pemetrexed-induced apoptosis in human non-small-cell lung cancer cells. Cell Death Dis. 2014;5:e1316. doi: 10.1038/cddis.2014.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fu S., Watkins S.M., Hotamisligil G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Kammoun H.L., Chabanon H., Hainaul I., Luquet S., Magnan C., Koike T. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J. Clin. Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ota T., Gayet C., Ginsberg H.N. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J. Clin. Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sha H., He Y., Chen H., Wang C., Zenno A., Shi H. The IRE1alpha-XBP1 pathway of the unfolded protein response is required for adipogenesis. Cell Metab. 2009;9:556–564. doi: 10.1016/j.cmet.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye J., Kumanova M., Hart L.S., Sloane K., Zhang H., De Panis D.N. GCN2-ATF4 pathway is critical for tumour cell survival and proliferation in response to nutrient deprivation. EMBO J. 2010;29:2082–2096. doi: 10.1038/emboj.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auf G., Jabouille A., Guérit S., Pineau R., Delugin M., Bouchecareilh M. Inositol-requiring enzyme 1alpha is a key regulator of angiogenesis and invasion in malignant glioma. Proc. Natl. Acad. Sci. U.S.A. 2010;107:15553–15558. doi: 10.1073/pnas.0914072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amaral J.D., Castro R.E., Steer C.J., Rodrigues C.M. p53 and the regulation of hepatocyte apoptosis: implications for disease pathogenesis. Trends Mol. Med. 2009;11:531–541. doi: 10.1016/j.molmed.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 39.Gudkov A.V., Komarova E.A. Pathologies associated with the p53 response. Cold Spring Harb. Perspect. Biol. 2010;7:a001180. doi: 10.1101/cshperspect.a001180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schreiber M., Kolbus A., Piu F., Szabowski A., Möhle-Steinlein U., Tian J., Karin M., Angel P., Wagner E.F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999;13:607–619. doi: 10.1101/gad.13.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kashatus D., Cogswell P., Baldwin A.S. Expression of the Bcl-3 proto-oncogene suppresses p53 activation. Genes Dev. 2006;20:225–235. doi: 10.1101/gad.1352206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brun N.R., Christen V., Furrer G., Fent K. Indium and indium tin oxide induce endoplasmic reticulum stress and oxidative stress in zebrafish (Danio rerio) Environ. Sci. Technol. 2014;48:11679–11687. doi: 10.1021/es5034876. [DOI] [PubMed] [Google Scholar]
- 43.Farrukh M.R., Nissar U.A., Afnan Q., Rafiq R.A., Sharma L., Amin S., Kaiser P., Sharma P.R., Tasduq S.A. Oxidative stress mediated Ca(2+) release manifests endoplasmic reticulum stress leading to unfolded protein response in UV-B irradiated human skin cells. J. Dermatol. Sci. 2014;75:24–35. doi: 10.1016/j.jdermsci.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Sabio G., Davis R.J. TNF and MAP kinase signalling pathways. Semin. Immunol. 2014;3:237–245. doi: 10.1016/j.smim.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jorge A.A., Malaquias A.C., Arnhold I.J., Mendonca B.B. Noonan syndrome and related disorders: a review of clinical features and mutations in genes of the RAS/MAPK pathway. Horm. Res. 2009;71:185–193. doi: 10.1159/000201106. [DOI] [PubMed] [Google Scholar]
- 46.Skuland T., Ovrevik J., Låg M., Schwarze P., Refsnes M. Silica nanoparticles induce cytokine responses in lung epithelial cells through activation of a p38/TACE/TGF-α/EGFR-pathway and NF-κB signalling. Toxicol. Appl. Pharmacol. 2014;279:76–86. doi: 10.1016/j.taap.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 47.Cheng G., Guo W., Han L., Chen E., Kong L., Wang L., Ai W., Song N., Li H., Chen H. Cerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathways. Toxicol. In Vitro. 2013;3:1082–1088. doi: 10.1016/j.tiv.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Jiao Z.H., Li M., Feng Y.X., Shi J.C., Zhang J., Shao B. Hormesis effects of silver nanoparticles at non-cytotoxic doses to human hepatoma cells. PLoS ONE. 2014;9:e102564. doi: 10.1371/journal.pone.0102564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duong F.H., Filipowicz M., Tripodi M., La Monica N., Heim M.H. Hepatitis C virus inhibits interferon signaling through up-regulation of protein phosphatase 2A. Gastroenterology. 2004;126:263–277. doi: 10.1053/j.gastro.2003.10.076. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.