Abstract
Hexavalent chromium, Cr(VI), is an environmental toxicant and is associated with hepatotoxicity. However, very little is known about the intracellular antioxidant defense mechanism against Cr(VI)-induced cytotoxicity in hepatocytes. In the present study, we cultured human liver (HepG2) cells in the absence or presence of Cr(VI) and determined its effect on cellular oxidative stress, mitochondrial damage, apoptosis and the expression of the transcription factor Nrf2 and the Nrf2-dependent antioxidant enzymes. Cr(VI) intoxication at a dose of 0, 3.125, 6.25, 12.5, 25, or 50 μM for 24 h exhibited a dose dependent cytotoxic effects in hepatocytes. Besides, Cr(VI) induced oxidative stress and subsequent mitochondrial damage. Cr(VI) also induced caspase 3-dependent apoptosis in HepG2 cells. In addition, Cr(VI) induced the translocation of Nrf2 into the nucleus and up-regulated the expression of Nrf2-dependent antioxidant enzymes, including SOD2, GCLC, and HO1. Our present experimental data support the notion that Cr(VI) caused mitochondrial damage, apoptosis, oxidative stress, and subsequently lead to a strong induction of HO1, GCLC and SOD2 via the Nrf-2 signaling pathway in hepatocytes.
Abbreviations: Cr(VI), hexavalent chromium; GCLC, glutamyl cysteine ligase catalytic subunit; HO1, heme oxygenase-1; MDA, malonaldehyde; Nrf2, nuclear erythroid 2-related factor 2; ROS, reactive oxygen species; SOD, superoxide dismutase; TUNEL, terminal deoxynucleotidyl transferase (TdT) nick end labeling
Keywords: Chromium, Hepatocytes, Oxidative stress, Mitochondrial damage, Apoptosis, Antioxidant signaling, Nrf2
1. Introduction
Chromium (Cr), is a naturally occurring element that exist in a variety of oxidation states (−2 to +6). Among the several ionic forms of Cr, hexavalent chromium [Cr(VI)] is the most toxic that can readily cross cellular membranes via nonspecific anion transporters. After entering into the cell, Cr(VI) is reduced by cellular reductants to produce reactive intermediates, including Cr(V), Cr(IV), Cr(III) and reactive oxygen species (ROS). These species can cause DNA strand breaks, base modification and lipid peroxidation, thereby disrupting cellular integrity and inducing toxic as well as mutagenic effects [1]. Cr(VI) is being used in more than 50 different industries worldwide. It has been reported that Cr(VI) has many uses in pigment production, textile, leather tannery, wood processing, chrome plating, metallurgical and chemical industries, stainless steel factory, welding, cement manufacturing factory, ceramic, glass, photographic industries, catalytic converter for automobile, heat resistance and as an anti-rusting agent in cooling plant, etc. [2], [3]. Owing to the increased use by the industries and improper disposal of these Cr(VI) waste in the environment, Cr(VI) levels continue to increase in the soil, water and air that creates severe environmental pollution [4], [5], [6], [7]. Besides, occupational exposure (via inhalation and skin contact) to Cr(VI) has also been found among approximately half a million workers in the United States and several millions throughout the world [5].
Due to environmental or occupational exposure to Cr(VI), people are suffering from increased risk of asthma, nasal septum, skin ulcerations and respiratory cancers [5]. Cr(VI) is also known to cause allergic dermatitis, cytotoxic, genotoxic, immunotoxic and carcinogenic effects both in humans and laboratory animals [2], [8], [9]. Besides, Cr(VI) exposure also induces hepatotoxicity associated with oxidative stress, tissue injury, mitochondrial damage and apoptosis [10], [11], [12], [13], [14]. Although chromium and chromium-containing compounds has gained much interest in the field of toxicology research, appropriate in vitro model is warranted to fully understand the mechanism of cytotoxicity and oxidative stress. Moreover, the intracellular antioxidant defense mechanism against Cr(VI)-induced cytotoxicity is not clearly understood and not yet been studied in details. The intracellular antioxidant defense mechanism is composed by high cellular level of glutathione (GSH), and a family of phase II detoxification enzymes, including glutamyl cysteine ligase catalytic subunit (GCLC), heme oxygenase-1 (HO1) and Mn-superoxide dismutase (SOD2) which are controlled by a transcription factor nuclear erythroid 2-related factor 2 (Nrf2). Under oxidative stress condition, Nrf2 is translocated to the nucleus from cytosol, where it binds to the antioxidant response element (ARE), resulting in a cytoprotective response by inducing the transcription of antioxidant genes [15], [16], [17], [18], [19], [20].
Therefore, the objectives of the present in vitro study were to: (i) determine the cytotoxic effect of Cr(VI) on human liver (HepG2) cells; (ii) evaluate the effect of Cr(VI) on oxidative stress and mitochondrial damage; and (iii) explore the effect of Cr(VI) on Nrf2-dependent antioxidant signaling pathways.
2. Materials and methods
2.1. Chemicals
Anti caspase 3, anti HO1, anti Nrf2, anti SOD2, anti actin, anti lamin B1 and anti GCLC antibodies were purchased from Abcam (Cambridge, MA, USA). K2Cr2O7 and all other reagents were bought from Sisco Research Laboratory (Mumbai, India).
2.2. Cell culture and treatment
Human liver (HepG2) cells were obtained from National Center for Cell Science (NCCS), Pune, India. The cells were cultured in DMEM supplemented with 10% FBS and 100 U/mL penicillin–streptomycin in a humidified incubator maintained at 37 °C and 5% CO2. For in vitro cytotoxicity experiments, cells were treated with different concentrations of K2Cr2O7 (0, 3.125, 6.25, 12.5, 25 and 50 μM) for 24 h in DMEM media supplemented with 1% FBS and 100 U/mL penicillin–streptomycin. The reason for the selection of these doses is as follows.
The maximum permissible limit of Cr(VI) in drinking water is 0.05 mg/L (0.17 μM) as recommended by WHO. Since our aim was to evaluate the cytotoxic effect of Cr(VI) on HepG2 cells, we planned to investigate that effect at a dose of 3.125–50 μM by performing a dose-dependent study following the report of Patlolla et al. [10].
For the NAC pre-treatment experiments, cells were pre-treated with NAC (5 mM) for 1 h followed by K2Cr2O7 treatment at different doses (0, 3.125, 6.25, 12.5, 25 and 50 μM) for 24 h.
2.3. Cell viability assay
Cells were seeded (5 × 105 cells/well) onto 96-well flat bottom culture plates and incubated for 24 h at 37 °C in a 5% CO2 incubator. The old medium was replaced by fresh DMEM media containing 1% FBS. Cells were then treated with different concentrations of K2Cr2O7 (0, 3.125, 6.25, 12.5, 25 and 50 μM) for 24 h in a humidified incubator at 37 °C and 5% CO2. Cell viability assay was performed using the MTT {3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide} method. The absorbance was read at a wavelength of 570 nm using microtiter plate reader [21].
2.4. Intracellular ROS measurement
Cells were incubated with 10 μM 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA) in a humidified incubator at 37 °C for 30 min. Cells were then washed with phosphate-buffered saline (PBS) and resuspended in PBS. Fluorescence emission was measured by flow cytometry using a 525 nm band pass filter. In addition, for microscopic measurement cells were first grown on glass slide, treated with K2Cr2O7 followed by incubation with 10 μM H2-DCFDA at 37 °C for 30 min. Then cells were washed with PBS, mounted with fluorescent medium, covered with glass cover slip and observed under a fluorescent microscope [22].
2.5. Cell cycle analysis
After treatment, cells were washed with PBS, trypsinized and fixed with 95% ethanol for 24 h at −20 °C. Cells were then washed and incubated with 0.05 mg/mL PI and 1 μg/mL RNase A at 37 °C for 30 min, and analyzed by flow cytometry. The cells belonging to the sub-G1 population were considered to be apoptotic cells. The percentage of cells in each phase of the cell cycle was determined.
2.6. Assessment of lipid peroxidation
After treatment, cells were washed with PBS and resuspended in a buffer containing Hepes (10 mM), KCl (3 mM), NaCl (130 mM), NaH2PO4 (1 mM) and glucose (10 mM), and pH 7.4. After that cells were lysed by sonication. Intracellular lipid peroxidation was measured in the form of malondialdehyde (MDA) content following the methods of Esterbauer and Cheeseman [23]. In brief, samples containing 1 mg of protein was mixed with 1 mL trichloro acetic acid (20%) and 2 mL thiobarbituric acid (0.67%). The content was heated for 1 h at 100 °C. After cooling, the precipitate was removed by centrifugation. Thee absorbance of the sample was measured at 535 nm, against a suitable blank.
2.7. JC1 staining
Cells were first grown on glass slide, treated with K2Cr2O7 followed by incubation with 10 μM JC1 at 37 °C for 15 min. Then cells were washed with PBS, mounted with fluorescent medium, covered with glass cover slip and observed under a fluorescent microscope.
2.8. TUNEL assay
Cells were first grown on glass slides and after getting 80% cell confluency the cells were treated with K2Cr2O7. Then the cells were washed with PBS, fixed with 4% paraformaldehyde, washed again and incubated with 0.1% Triton X-100. TdT-mediated dUTP nick-end labeling (TUNEL) detection of apoptotic cells was performed in the treated cells according to the manufacturer's instructions (Invitrogen, USA).
2.9. Immunocytochemistry
Cells were first grown on glass slides and after getting 80% cell confluency the cells were treated with K2Cr2O7. Then the cells were washed with PBS, fixed with 4% paraformaldehyde, washed again and incubated with 0.1% Triton X-100. Cells were then incubated with the appropriate primary antibodies in 1% bovine serum albumin at room temperature. After that cells were again washed and incubated with an appropriate fluorescence-conjugated secondary antibody at room temperature. Finally, the cells were mounted with fluorescent medium, covered with glass cover slip and observed under a fluorescent microscope [22].
2.10. Immunoblotting
HepG2 cells were lysed in RIPA lysis buffer containing protease inhibitors. Equal amounts of protein were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and proteins were then electrophoretically transferred to a PVDF. Membranes were blocked at room temperature with 5% non-fat dry milk for 2 h to prevent non specific binding and then incubated with primary antibodies overnight at 4 °C. Immunoreactivity was detected through sequential incubation with horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence reagents.
2.11. Quantification of images
Relative intensity of all fluorescent images and densitometry of western blot were carried out by Imagej software.
2.12. Statistical analysis
All the values are expressed as mean ± SEM (n = 3). Significant differences between the groups were determined with SPSS 10.0 software (SPSS Inc., Chicago, IL, USA) for Windows using one-way analysis of variance (ANOVA) and the group means were compared by Student–Newman–Keuls post hoc tests. A difference was considered significant at the P < 0.05 level.
3. Results
3.1. Cr(VI) induced cytotoxicity in HepG2 cells
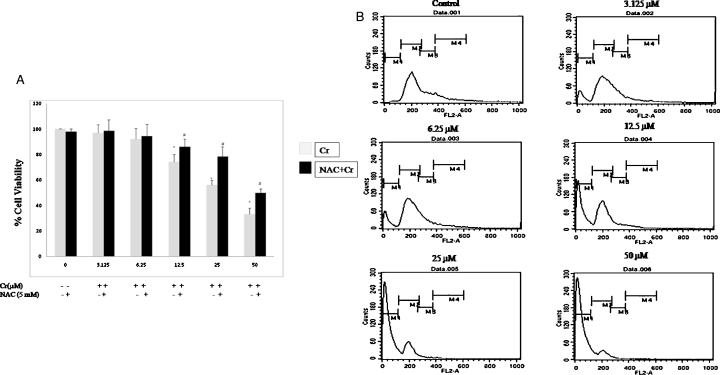
To determine the cytotoxic effect of Cr(VI), cell viability was measured after culturing the cells in the presence of Cr(VI) in different concentrations (0, 3.125, 6.25, 12.5, 25, and 50 μM) for 24 h. As shown in Fig. 1A, Cr(VI) decreased the cell viability in HepG2 cells. To further quantify the dead cells, a flow cytometric analysis was performed by staining with PI after treatment with Cr(VI) at different doses for 24 h. The population of dead cells was represented as a hypodiploid sub-G1 DNA peak, which was increased by stimulation with Cr(VI) in a dose-dependent manner (Fig. 1B). However, pre-treatment with NAC (5 mM, 1 h) significantly reduced Cr(VI)-induced cell viability loss (Fig. 1A) in HepG2 cells.
Fig. 1.
Cytotoxicity of Cr(VI) in HepG2 cells. (A) Cell viability relative to the control (100%) in HepG2 cells was measured using the MTT. Cells were pre-treated with 5 mM NAC for 1 h and then exposed to different concentrations of Cr(VI) in μM for 24 h. All values were expressed as mean ± S.E.M. (n = 3).*P < 0.05 compared to the control. #P < 0.05 compared with the Cr(VI)-treated groups. (B) Cell cycle analysis after treatment with different concentrations of Cr(VI) in μM for 24 h. M1 represents the percentage of sub-G1g populations.
3.2. Cr(VI) induced oxidative stress and disrupted mitochondrial membrane potential in HepG2 cells
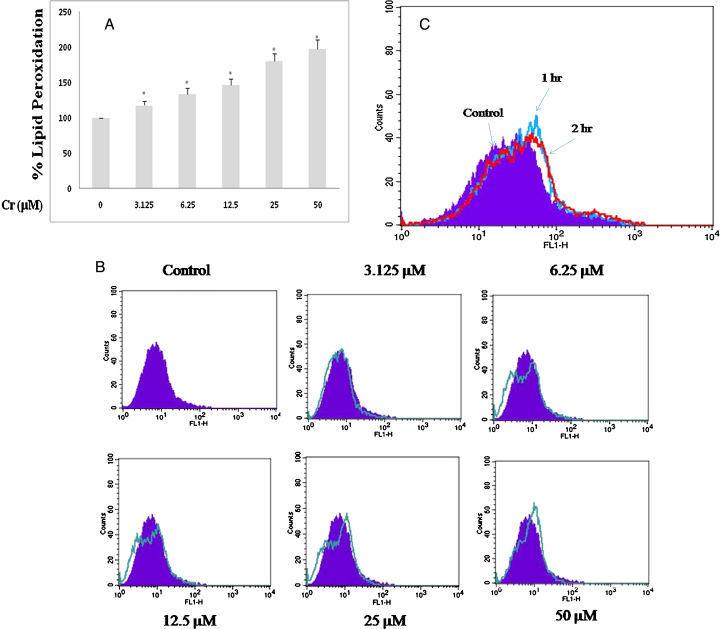
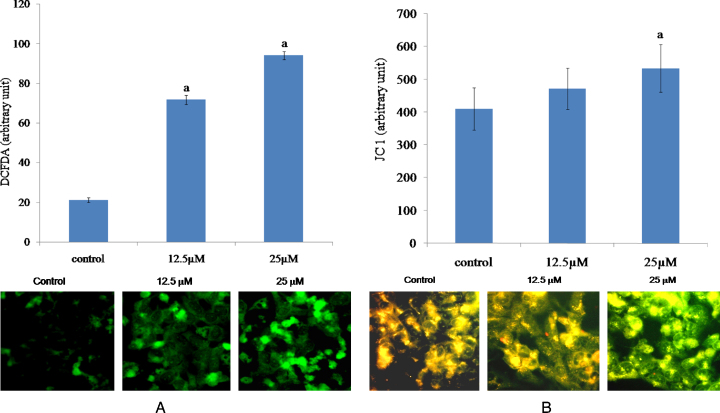
To investigate the involvement of oxidative stress in Cr(VI)-induced cell death, we measured the cellular MDA level (indicative of lipid peroxidation) and formation of reactive oxygen species (ROS) in HepG2 cells exposed to Cr(VI). After 24 h of Cr(VI) intoxication, we observed that both the MDA and ROS levels increased dose dependently (Fig. 2A and B). However, we also observed that production of intracellular ROS started between 1 and 2 h when the cells were exposed to 12.5 μM Cr(VI) (Fig. 2C). Cr(VI) induced ROS formation was also confirmed by the fluorescence microscopic analysis. As shown in Fig. 3A, the cells exposed to Cr(VI) with increasing doses showed enhanced green fluorescence. The alteration of mitochondrial membrane potential, as evident by JC1 dye straining, was depicted in Fig. 3B. Compared with the untreated control, a significant increase in the green fluorescence intensity was observed in the cells exposed to increasing dose of Cr(VI), indicating that mitochondria experience a loss of membrane polarization, which corresponds to a loss in function. These results indicated that Cr(VI)-induced cytotoxicity was mediated through oxidative stress and subsequent mitochondrial damage.
Fig. 2.
Oxidative stress induced by Cr(VI) in HepG2 cells. (A) Lipid peroxidation relative to the control (100%) in HepG2 cells exposed to different concentrations of Cr(VI) in μM for 24 h. All values are expressed as mean ± S.E.M. (n = 3). *P < 0.05 compared to the control. (B) ROS production in HepG2 cells after treatment with different concentrations of Cr(VI) for 24 h. (C) ROS production in HepG2 cells after treatment with 12.5 μM Cr(VI) for 1 and 2 h respectively. ROS production in cells was measured flow cytometrically by using a cationic fluorescent dye, 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA).
Fig. 3.
Effects of Cr(VI) on ROS and MMP in HepG2 cells. (A) Representative fluorescent images of HepG2 cells, after staining with cationic fluorescent dye, 2′,7′-dichlorodihydrofluorescein diacetate (H2-DCFDA). (B) Representative fluorescent images of HepG2 cells, after staining with fluorescent dye, JC1. Cells were first grown on glass slide, treated with 12.5 μM and 25 μM Cr(VI) for 24 h followed by incubation with 10 μM H2-DCFDA or 10 μM JC1 at 37 °C for 15 min at 37 °C for 30 min. Then cells were washed with PBS, mounted with fluorescent medium, covered with glass cover slip and observed under a fluorescent microscope. For JC1 staining, the merged images captured under green and red filter were provided. Graphs represent the average green fluorescence intensity ± SEM of separate experiments in each group. “a” indicates the significant difference between the control and Cr(VI) exposed groups where Pa < 0.05.
3.3. Cr(VI) induced apoptotic cell death in HepG2 cells
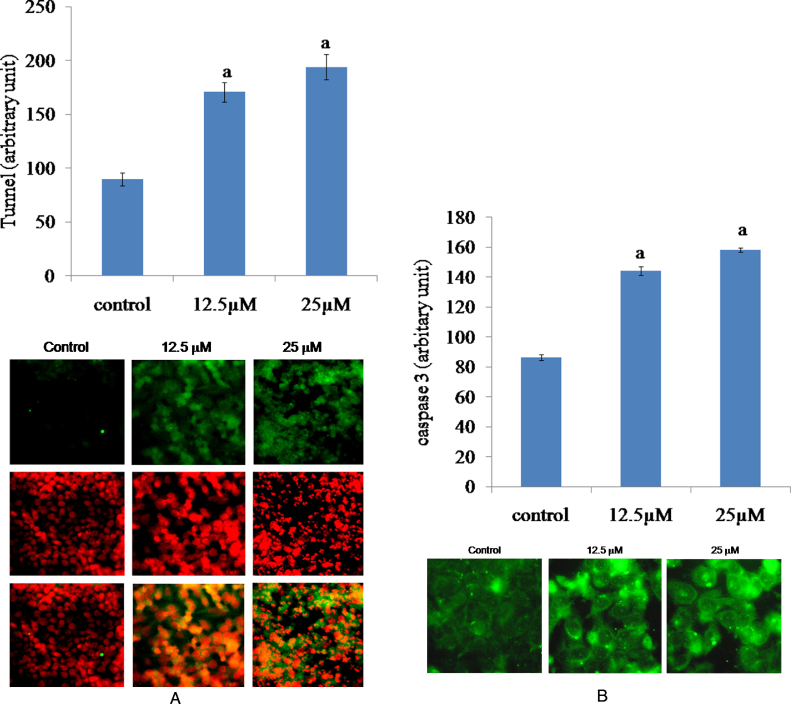
Next, the mode of cell death due to Cr(VI) intoxication has been investigated. Cr(VI) induced apoptosis in HepG2 cells as was evidenced from both TUNEL staining and immunocytochemical localization of cleaved caspase 3. Treatment with 25 and 50 μM Cr(VI) for 24 h increased the proportion of TUNEL-positive cells significantly compared to that observed in the control cells (Fig. 4A). In addition, immunofluorescence study showed that Cr(VI) increased the cleaved caspase 3 expression in HepG2 cells when the cells were exposed to 12.5 and 25 μM Cr(VI) for 24 h compared with untreated control (Fig. 4B).
Fig. 4.
Cr(VI) induced apoptosis in HepG2 cells. (A) Representative fluorescent images of TUNEL-positive (green) cells after exposure to 25 μM and 50 μM Cr(VI) for 24 h. Cell nuclei were counterstained with PI (red). (B) Immunocytochemical localization of active caspase 3 by fluorescent microscope after exposure to 12.5 μM and 25 μM Cr(VI) for 24 h. Graphs represent the average green fluorescence intensity ± SEM of separate experiments in each group. “a” indicates the significant difference between the control and Cr(VI) exposed groups where Pa < 0.05.
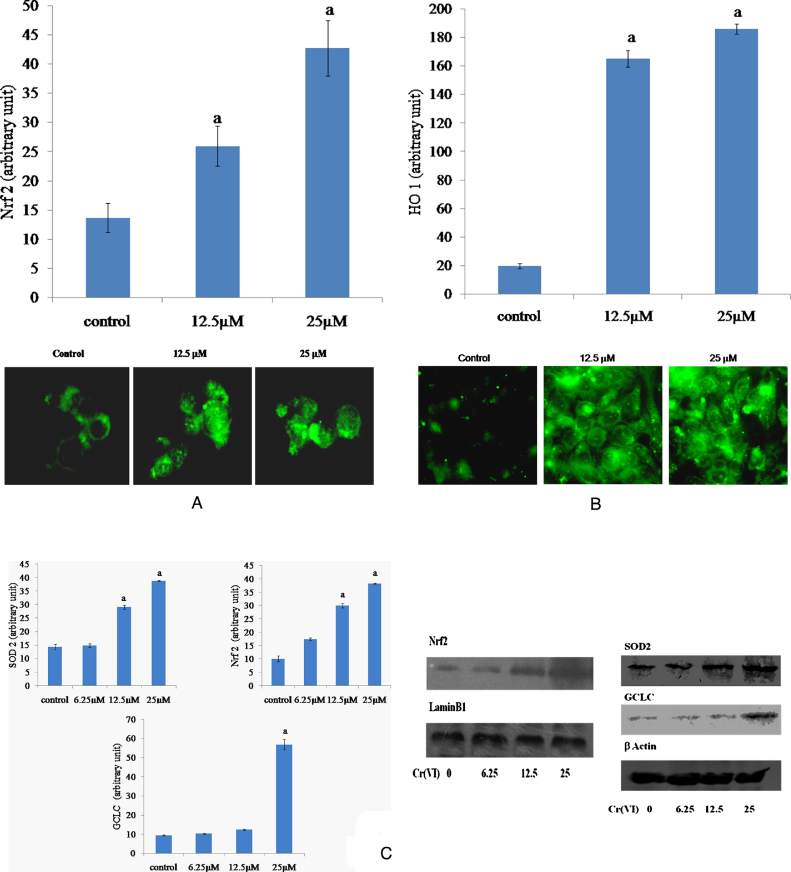
3.4. Cr(VI) activated the antioxidant signaling pathways in HepG2 cells
To investigate whether Nrf2-dependent antioxidant defense mechanism is activated during Cr(VI)-induced oxidative stress mediated cytotoxicity in HepG2 cells, we examined the Nrf2 nuclear translocation and the expressions of HO1, GCLC and SOD2. Immunofluorescence experiments showed a weak, diffuse staining of Nrf2 in untreated control. However, Cr(VI) intoxication resulted in a marked increase in nuclear Nrf2 staining (Fig. 5A). The nuclear accumulation of Nrf2 by Cr(VI) was also supported by western blot analysis (Fig. 5C). In addition, immunofluorescence study also showed that Cr(VI) increased the HO1 expression in HepG2 cells when exposed to 12.5 and 25 μM Cr(VI) for 24 h compared with untreated control (Fig. 5B). Furthermore, Cr(VI) also augmented the expression of GCLC and SOD2 proteins compared to the untreated cells (Fig. 5B).
Fig. 5.
Effects of Cr(VI) on the antioxidant signaling pathways in HepG2 cells. (A) Immunocytochemical localization of Nrf2 by fluorescent microscope after exposure to 12.5 μM and 25 μM Cr(VI) for 24 h. (B) Immunocytochemical localization of HO1 by fluorescent microscope after exposure to 12.5 μM and 25 μM Cr(VI) for 24 h. (C) Western blot analysis of Nrf2, Gclc and SOD2 proteins in HepG2 cells after treatment with different concentrations of Cr(VI) for 24 h. GCLC, SOD2 and HO1 proteins were analyzed in the whole cell protein lysate. Nrf2 was analyzed in the nuclear protein fraction. Graphs represent the average green fluorescence intensity ± SEM for immunocytochemical localization of Nrf2 and HO1 and average ± SEM for Western blot of separate experiments in each group. “a” indicates the significant difference between the control and Cr(VI) exposed groups where Pa < 0.05.
4. Discussion
This study was undertaken to determine the in vitro cytotoxic effects of Cr(VI) on human liver (HepG2) cells. We have further checked the effects of Cr(VI) on oxidative stress, mitochondrial damage, apoptosis and antioxidant signaling mechanisms in HepG2 cells. To achieve these, cells were first treated with Cr(VI) at a dose of 0, 3.125, 6.25, 12.5, 25, or 50 μM for 24 h, and then biochemical, flow cytometric, fluorescence microscopic and immunoblotting analyses were performed.
Our results showed that Cr(VI) exhibited a dose dependent cytotoxic effects in HepG2 cells, as evident from reduced cell viability and increased hypodiploid sub-G1 DNA populations. Next we investigated whether oxidative stress and mitochondrial dysfunction played any role in our current experimental model. We observed that Cr(VI) intoxication dose dependently increased lipid peroxidation and the production of intracellular reactive oxygen species (ROS) when the cells were exposed to Cr(VI) for 24 hr. We have also found that ROS production has been one of the earliest phenomena for the Cr(VI)-induced cytotoxicity, as the ROS production has started to increase between 1 and 2 h when the cells were exposed to 12.5 μM Cr(VI). Besides, mitochondrial membrane potential (MMP) was also decreased dose dependently after Cr(VI) intoxication for 24 h. We observed a significant increase in the green fluorescence intensity in the cells exposed to Cr(VI), indicating mitochondria membrane depolarization. Studies with ROS inhibitor, NAC showed that it could reduce Cr(VI)-induced cell death. Therefore, oxidative stress and subsequent mitochondrial damage play a crucial role in Cr(VI) induced cytotoxicity. These results are also supported by previous reports where the authors have shown that Cr(VI)-induced cytotoxicity in hepatocytes has been mediated through oxidative stress and mitochondrial dysfunction [10], [11], [12].
After that we have investigated whether Cr(VI)-induced cytotoxic effect was mediated through apoptotic cell death pathway in HepG2 cells. We observed that intoxication with Cr(VI) for 24 hr significantly increased the proportion of TUNEL-positive cells and cleaved caspase 3 expression in HepG2 cells compared to that observed in the control cells. These results are in agreement with previous reports where the authors have demonstrated that Cr(VI) induced ROS-dependent and caspase 3-mediated apoptosis in hepatocytes [13], [14].
The fate of cells undergoing death or survival under oxidative stress condition depends on the balance between the formation of ROS and the enzymatic as well as non-enzymatic antioxidant molecules. Therefore, we have investigated the role of the redox-sensitive transcription factor, Nrf2 and its associated antioxidant defense mechanism as a target of Cr(VI) toxicity. In our present study, we observed that Cr(VI) induced the translocation of Nrf2 into the nucleus, as evident by the results of the immunofluorescence study and western blot analysis. We have also observed that Cr(VI) induced the upregulation of several antioxidant enzyme expression that are regulated by the Nrf2-ARE dependent pathways. These enzymes include SOD2 (scavenges mitochondrial superoxide; [24]), GCLC (is the rate limiting enzyme for GSH biosynthesis; [25]) and HO1 (a novel cytoprotective antioxidant enzyme; [26], [27]). Therefore, the importance of Nrf2 signaling in terms of a contribution to antioxidant response, such as HO1, SOD2 and GCLC up regulation, may merit further investigation to develop an improved Cr(VI) antagonists in the future to combat Cr(VI)-induced hepatotoxicity.
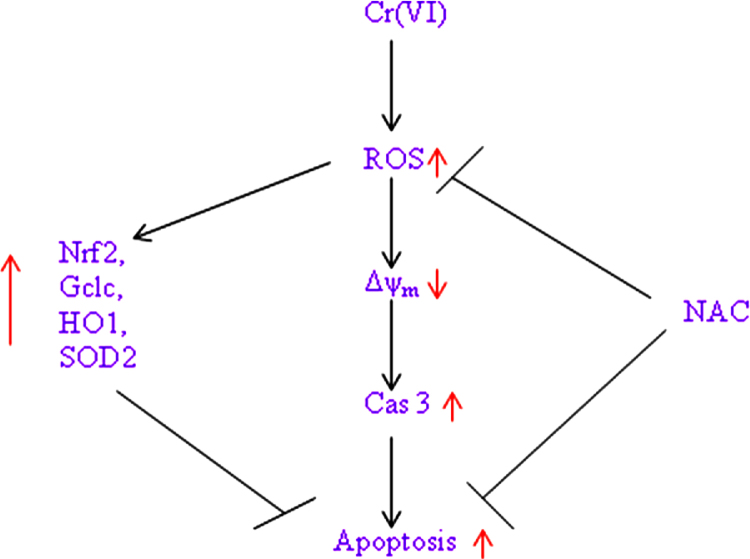
In conclusion, we say that Cr(VI) induces mitochondrial damage, apoptosis as well as cellular oxidative stress, and subsequently leads to a strong induction of HO1, GCLC and SOD2 via the Nrf-2 signaling pathway in hepatocytes (Fig. 6).
Fig. 6.
Schematic diagram of Cr(VI) induced cytotoxicity in HepG2 cells.
Conflicts of interest statement
The authors declare no conflicts of interest.
Transparency document
Acknowledgements
The authors are extremely grateful to Ms. Sayantani Chowdhury and Ms. Sharmistha Banerjee for their excellent experimental assistance.
References
- 1.Mattia G.D., Bravi M.C., Laurenti O., Luca O.D., Palmeri A., Sabatucci A., Mendico G., Ghiselli A. Impairment of cell and plasma redox state in subjects professionally exposed to chromium. Am. J. Ind. Med. 2004;46:120–125. doi: 10.1002/ajim.20044. [DOI] [PubMed] [Google Scholar]
- 2.Stohs J.S., Bagchi D., Hassoun E., Bagchi M. Oxidative mechanism in the toxicity of chromium and cadmium ions. J. Environ. Pathol. Toxicol. Oncol. 2001;20(2):77–88. [PubMed] [Google Scholar]
- 3.Costa M., Klein C.B. Toxicity and carcinogenicity of chromium compounds in humans. Crit. Rev. Toxicol. 2006;36:155–163. doi: 10.1080/10408440500534032. [DOI] [PubMed] [Google Scholar]
- 4.OSHA . Department of Labor, Occupational Safety and Health Administration; Washington, DC: 2006. Occupational Exposure to Hexavalent Chromium: Final Rule. [Google Scholar]
- 5.Salnikow K., Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic, and chromium. Chem. Res. Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honeycutt M.E. Hexavalent chromium in Texas drinking water. Toxicol. Sci. 2010;119:423–424. doi: 10.1093/toxsci/kfq347. [DOI] [PubMed] [Google Scholar]
- 7.Layton L. The Washington Post; Washington, DC: 2010. Probable Carcinogen Hexavalent Chromium found in Drinking Water of 31 U.S. Cities. [Google Scholar]
- 8.Li Z.H., Li P., Randak T. Evaluating the toxicity of environmental concentrations of waterborne chromium (VI) to a model teleost, Oncorhynchus mykiss: a comparative study of in vivo and in vitro. Comp. Biochem. Physiol. C. 2011;153:402–407. doi: 10.1016/j.cbpc.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 9.Von Burg R., Liu D. Chromium and hexavalent chromium. J. Appl. Toxicol. 1993;13:225–230. doi: 10.1002/jat.2550130315. [DOI] [PubMed] [Google Scholar]
- 10.Patlolla A.K., Barnes C., Hackett D., Tchounwou P.B. Potassium dichromate induced cytotoxicity, genotoxicity and oxidative stress in human liver carcinoma (HepG2) cells. Int. J. Environ. Res. Public Health. 2009;6:643–653. doi: 10.3390/ijerph6020643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu K.J., Shi X. In vivo reduction of chromium (VI) and its related free radical generation. Mol. Cell. Biochem. 2001;222(1–2):41–47. [PubMed] [Google Scholar]
- 12.Soudani N., BenAmara I., Sefi M., Boudawara T., Zeghal N. Effects of selenium on chromium (VI)-induced hepatotoxicity in adult rats. Exp. Toxicol. Pathol. 2011;63(6):541–548. doi: 10.1016/j.etp.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 13.Yuan Y., Ming Z., Gong-Hua H., Lan G., Lu D., Peng L., Feng J., Cai-Gao Z. Cr(VI) induces the decrease of ATP level and the increase of apoptosis rate mediated by ROS or VDAC1 in L-02 hepatocytes. Environ. Toxicol. Pharmacol. 2012;34(2):579–587. doi: 10.1016/j.etap.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 14.Xiao F., Li Y., Dai L., Deng Y., Zou Y., Li P., Yang Y., Zhong C. Hexavalent chromium targets mitochondrial respiratory chain complex I to induce reactive oxygen species-dependent caspase-3 activation in L-02 hepatocytes. Int. J. Mol. Med. 2012;30(3):629–635. doi: 10.3892/ijmm.2012.1031. [DOI] [PubMed] [Google Scholar]
- 15.Baird L., Dinkova-Kostova A.T. The cytoprotective role of the Keap1–Nrf2 pathway. Arch. Toxicol. 2011;85:241–272. doi: 10.1007/s00204-011-0674-5. [DOI] [PubMed] [Google Scholar]
- 16.Surh Y.J., Kundu J.K., Na H.K. Nrf2 as a master redox switch in turning on the cellular signaling involved in the induction of cytoprotective genes by some chemopreventive phytochemicals. Planta Med. 2008;74:1526–1539. doi: 10.1055/s-0028-1088302. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda H., Nishi S., Sakai M. Transcription factor Nrf2/MafK regulates rat placental glutathione S-transferase gene during hepatocarcinogenesis. Biochem. J. 2004;380:515–521. doi: 10.1042/BJ20031948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun Z., Huang Z., Zhang D.D. Phosphorylation of Nrf2 at multiple sites by MAP kinases has a limited contribution in modulating the Nrf2-dependent antioxidant response. PLoS ONE. 2009;4:e6588. doi: 10.1371/journal.pone.0006588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim A.N., Jeon W.K., Lee J.J., Kim B.C. Up-regulation of heme oxygenase-1 expression through CaMKII-ERK1/2-Nrf2 signaling mediates the anti-inflammatory effect of bisdemethoxycurcumin in LPS-stimulated macrophages. Free Radic. Biol. Med. 2011;49:323–331. doi: 10.1016/j.freeradbiomed.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 21.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application 867 to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 22.Sinha K., Pal P.B., Sil P.C. Cadmium exposure differentially elicits both cell proliferation and cell death related responses in SKRC cell line. Toxicol. In Vitro. 2014;28:307–318. doi: 10.1016/j.tiv.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol. 1990;186:407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- 24.Bannister J., Bannister W., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit. Rev. Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- 25.Franklin C.C., Backos D.S., Mohar I., White C.C., Forman H.J., Kavanagh T.J. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol. Aspects Med. 2009;30(1–2):86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morse D., Choi A.M. Heme oxygenase-1: the emerging molecule has arrived. Am. J. Respir. Cell Mol. Biol. 2002;27:8–16. doi: 10.1165/ajrcmb.27.1.4862. [DOI] [PubMed] [Google Scholar]
- 27.Otterbein L.E., Soares M.P., Yamashita K., Bach F.H. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.