Abstract
Trichloroethylene (TCE) is widely used as a metal degreaser in industrial processes. The present study reports on the effects of TCE exposure on workers employed in the lock industries. To ensure exposure of the workers to TCE, its toxic metabolites, trichloroacetic acid (TCA), dichloroacetic acid (DCA) and trichloroethanol (TCEOH) were detected in the plasma of the subjects through solid phase microextraction-gas chromatography-electron capture detection. TCA, DCA and TCEOH were detected in the range of 0.004–2.494 μg/mL, 0.01–3.612 μg/mL and 0.002–0.617 μg/mL, respectively. Quantitative reverse transcription polymerase chain reaction analysis revealed up-regulated expression of p53 (2.4-fold; p < 0.05), p21 (2-fold; p < 0.01), bax (2.9-fold; p < 0.01) mRNAs and down-regulated expression of bcl-2 (67%; p < 0.05) mRNAs, indicating DNA damaging potential of these metabolites. No effects were observed on the levels of p16 and c-myc mRNAs. Further, as TCA and DCA, the ligand of peroxisome proliferator activated receptor alpha (PPARA), are involved in the process of hepatocarcinogenesis in rodents, we examined expression of PPARA mRNA and let-7c miRNA in the workers. No statistically significant differences in expression of PPARA mRNA and let-7c miRNA in patients were observed as compared to values in controls. Dehydroepiandosterone sulfate (DHEAS) is a reported endogenous ligand of PPARA so its competitive role was also studied. We observed decreased levels of DHEAS hormone in the subjects. Hence, its involvement in mediation of the observed changes in the levels of various mRNAs analyzed in this study appears unlikely.
Keywords: Trichloroethylene, Trichloroacetic acid, Dichloroacetic acid, Dehydroepiandosterone sulfate, Peroxisome proliferator activated receptor alpha, miRNA let-7c
1. Introduction
Increasing rate of industrialization is posing potential health hazards to workers due to accumulation of harmful chemicals in the environment. Trichloroethylene (TCE) is a lipophilic and volatile organic solvent, which is haphazardly used in India as a part of commercial enterprises for metal degreasing processes. Epidemiological studies have suggested a correlation between TCE exposure and non-Hodgkin's lymphoma, liver, renal, and biliary tract cancers [1]. TCE has become an occupational hazard. This study was performed on workers of small scale industries of Aligarh city located in Uttar Pradesh, an Indian state where locks and keys are manufactured.
The U.S. Environmental Protection Agency (EPA) characterized TCE as “carcinogenic to humans” in 2011 [46], although there is no convincing evidence indicating its genotoxicity in vitro [2]. TCE is known to exert its hepatotoxic and carcinogenic effects primarily through metabolic activation [3]. TCE metabolism occurs through two major pathways: cytochrome P450 (CYP) dependent oxidation and glutathione (GSH) conjugation pathway. GSH-derived metabolites of TCE are known to be mutagenic [4]. Moore and Harrington-Brock [5] advocated requirement of very high doses of its metabolites to induce genotoxic effects. Different studies have shown that the oxidative metabolites of TCE, specifically trichloroacetic acid (TCA), dichloroacetic acid (DCA) and trichloroethanol (TCEOH) also cause DNA damage [6], [7], [8], [9]. DNA damaging agents have been documented to alter the expression of several genes associated with cell cycle, tumor suppression or induction (oncogenes) and apoptosis. DNA damage induces expression of p53, a tumor suppressor protein that regulates cell cycle progression check point G1-S transition. p53 can arrest the cell cycle at the G1 check point to avoid replication of damaged DNA and/or to lead to apoptosis [10], [11]. p53 also induces the expression of the cyclin-dependent kinase inhibitor p21. p53-controlled p21 expression in response to DNA damage blocks cell cycle progression into the S-phase [12]. p16, a cyclin-dependent-kinase inhibitor, is also a tumor suppressor protein that checks the cell cycle in the early G1 phase and inhibits transition of the cell cycle from G1 to S phase, as a component of a multi-protein regulatory complex. c-myc, a proto-oncogene, is a transcription factor that participates in the control of cell proliferation and apoptosis. p53 and c-myc are involved in many similar cellular processes, affecting similar targets and regulating one another's action [13]. Apoptosis in cells is also regulated through bcl-2 family protein members. Among these protein members, bax and bcl-2 physically interact with each other and form the homo- and heterodimers bcl-2/bcl-2, bcl-2/bax and bax/bax. The bax homodimers promote apoptosis, and bax-mediated cell death is checked by bcl-2/bax heterodimerization [14]. p53 up-regulates bax gene expression and down-regulates bcl-2 gene expression in association with genotoxic stress.
TCE is reported to have similar metabolic pathways in humans, rats, and mice. However sex-, species-, and strain-specific differences in the rates of metabolism determine the susceptibility to TCE [15], [16]. The acidic metabolites of TCE, TCA and DCA, act via peroxisome proliferator-activated receptor (PPAR), a ligand-activated transcription factor belonging to the steroid receptor superfamily. They interact with the alpha isoform of PPAR, resulting into peroxisome proliferation in rodents and liver carcinoma. In spite of the fact that TCA and DCA are considered hepatocarcinogens in rodents [42], [17], [18], [19], [20], [21], the aspects of their impact on humans are obscure. Dehydroepiandosterone sulfate (DHEAS) is acknowledged as an endogenous PPAR activator. DHEAS, a sulfate ester of DHEA and having peroxisome proliferative potential in rodents, is one of the most abundant and multifunctional steroids in humans [22]. However, the underlying cellular mechanisms of DHEAS in peroxisome proliferation in rats are unclear.
Micro RNAs represent a class of noncoding, small RNAs that regulate gene expression. They mostly induce mRNA degradation and translational repression by binding to the 3′-untranslated regions (3′UTR) of the target mRNAs [23], [24]. In recent studies, miRNAs have been noted to participate in hepatocarcinogenesis [25], [26]. Among all human cancer-related miRNAs, the let-7 family of miRNAs has been observed to be aberrantly expressed and play pivotal roles in tumorigenesis [44]. Among members of the let-7 family miRNAs, let-7c has been found to be associated with PPARA-agonist incited liver proliferation and tumorigenesis in mice [27].
We have focused our studies toward the occupational health hazard of TCE and investigated the underlying mechanism of the interaction between TCE metabolites (TCA/DCA) and PPARA and the potential role of miRNA let-7c in these events. In addition, we have investigated a connection between TCE exposure and levels of the DHEAS hormone in lock industry workers.
2. Materials and methods
2.1. Chemicals
Trichloroacetic acid (TCA; CAS No. 76-03-9; ≥99%pure), chloroform, isopropanol and ethanol were purchased from Sigma Aldrich (St. Louis, MO, USA). Dichloroacetic acid (DCA; CAS No.79-43-6; ≥98.0% pure), trichloroethanol (TCEOH; CAS No.115-20-8), methyl chloroformate (MCF; CAS No. 79-22-1), pyridine (CAS No. 110-86-1), methanol, and NaCl were procured from Merck Chemicals (Darmstandt, Germany). Trizol reagent was purchased from Invitrogen Bioservices India Pvt. Ltd. (Bangalore, India). Material related to solid-phase microextraction (SPME), such as polydimethylsiloxane (PDMS) fiber of 100 μm film thickness, SPME vials, septa, holder and heating block were supplied by Supelco (Bellefonte, PA, USA).
2.2. Selection of subjects
The study population consisted of 68 male workers. A total of 49 workers were employed in the metal degreasing process in the lock industries of Aligarh (West UP, India) and were exposed to trichloroethylene (TCE). This group of workers was considered an exposed group. Out of 49 workers 19 workers with comparable socioeconomic status and demographic profile, employed in assembling of locks, were included in the study as the control group. Detailed information regarding socioeconomic status, family history, education, personal habits, health status and exposure to TCE were recorded for each subject in a pre-tested questionnaire. The questionnaire was both in English and Hindi (local language) and investigators recorded all the information. The purpose of the study was explained to all the participants and prior to the study, written consent was obtained from each subject. Ethical clearance from the Institutional Human Ethics committee of Indian Institute of Toxicology Research (IITR) was also obtained.
2.3. Lung function studies
Lung function tests, including Peak Expiratory Flow rate (PEFR) and Forced Expiratory Volume in one sec (FEV1), were performed using a calibrated spirometer (PIKO-1, UK, under the recommendation of the American Thoracic Society standards) in a standing position for each subject. The volunteer performed the lung function test three times, allowing for sufficient rest between repetitions. The best values for PEFR and FEV1 from three tests for each subject were recorded.
2.4. Body composition monitoring
Height was measured without shoes in an erect position using a stadiometer and rounded down to the nearest centimeter. Body mass index (BMI), weight and body fat % were measured using body fat monitor HBF 352 (Omron Co., Ltd., Kyoto, Japan) and rounded up to the nearest kilogram based on bioelectric impedance analysis (BIA).
2.5. Blood sample collection
The sampling of biological material was carried out according to the Helsinki Declaration and under the supervision of experts. Approximately 2–3 mL of blood was collected from both the control and exposed groups. Blood samples were collected in EDTA-coated vacutainers, BD serum vacutainers (BD Biosciences, Becton Drive Franklin Lakes, New Jersey, USA), tubes containing Trizol and lysis buffer to perform various analyses. Samples kept in ice were immediately transported to the biochemical laboratory of the Aligarh Muslim University of Aligarh. Plasma and serum separation was carried out on the same day and stored. All the samples were stored at −80 °C until processing was started.
2.6. Measurement of TCE metabolites in plasma
The TCE metabolites TCA, DCA and TCEOH were measured in the plasma of control and exposed groups. Plasma was separated from the blood after centrifugation at a speed of 1200 rpm (241 × g) and collected in separate centrifuge tubes for analysis. Stock solutions of TCE metabolites were individually prepared in ultrapure water produced from a Milli-Q water purification system (Millipore, Bedford, MA, USA) at a concentration of 1 mg/mL. A working mix standard was prepared by diluting stock solutions of DCA, TCEOH and TCA accordingly.
2.7. In-matrix derivatization and SPME
In-matrix derivatization was based on a previously reported procedure [28]. Briefly, 100 μL of plasma sample was diluted up to 1 mL with methanol/water (1:1; v/v). To this mixture, 200 μL of pyridine was added as a catalyst for in-matrix derivatization followed by addition of 300 μL of MCF (150 μL × 2 times). The reaction mixture was mixed for 30 s on a vortex mixer to ensure complete derivatization. The derivatized sample was then transferred to a SPME (solid-phase microextraction) vial and diluted up to 3 mL with ultrapure water. At this stage 562.5 mg of NaCl was added to enhance the ionic strength of the sample. Prior to extraction, the SPME fibers were conditioned at 250 °C for 30 min as recommended by the manufacturer. The sample was then exposed to SPME fiber (PDMS, 100 μm) for 22 min at 50 °C. After the adsorption of non-polar MCF derivatives of TCE metabolites, the SPME fibers were injected into the GC-ECD injector port for desorption of analytes at 200 °C for 1 min.
2.8. Chromatographic conditions
Analysis of TCE metabolites was carried out on a Perkin Elmer Clarus 500 gas chromatograph equipped with an electron capture detector (ECD). Separation was achieved on a DB-5 capillary column (5% phenyl–95% methylpolysiloxane; 30 m length × 0.25 mm I.D. × 0.25 μm film thickness). Nitrogen (99.99%) was used as a carrier gas at a flow rate of 1 mL/min and also as a makeup gas for ECD at a flow rate of 30 mL/min. ECD was operated at 375 °C. Oven temperature programming was as follows: initial 80 °C (no hold), increased to 100 °C at the rate of 2 °C/min (hold for 3 min), and further increased to 280 °C at the rate of 45 °C/min (hold for 5 min; total run time 22 min). Desorption of analytes from SPME fibers was achieved at an injector port temperature of 200 °C (split 1:5).
2.9. Quantitative determination of DHEAS in human serum
For serum separation, the blood was collected in a BD serum vacutainer and allowed to coagulate by keeping the vials in a slanted position at room temperature. The whitish-colored upper layer that contained serum was aspirated. DHEAS (Dehydroepiandosterone sulphate) hormone level was measured in serum of both the control and exposed subjects with a DHEAS Elisa kit (Alpha diagnostic International, San Antonio, Texas, USA) as per the manufacturer's instructions.
2.10. PPARA and cell cycle regulating gene expression analyses using reverse transcriptase polymerase chain reaction
Each subject was analyzed three times for gene expression study following the procedures described below.
2.10.1. RNA extraction
Total RNA was extracted from blood of control (n = 19) and TCE-exposed persons (n = 49) with Trizol reagent using the standard protocol as described by Chomczynski and Sacchi [29]. Briefly, in 2 mL centrifuge tubes 100 μL of blood and 600 μL of Trizol was added. Then 120 μL of chloroform was added and the contents were incubated for 15 min at 37°C. Contents were centrifuged at 12,000 rpm at 4°C for 10 min. Addition of chloroform followed by centrifugation separated the solution into an aqueous phase and an organic phase. The aqueous phase was transferred to a new tube and 500 μL of isopropanol was added and then incubated for 10 min at room temperature. The solution was centrifuged at 12,000 rpm at 4°C for 10 min. Centrifugation resulted in the formation of a white pellet that contained RNA. Supernatant was discarded and the pellet was washed with 70% ethanol. The pellet was air-dried and dissolved in DEPC-treated water. Quality and quantity of RNA samples were checked with a Picodrop spectrophotometer.
2.10.2. Quantitative RT PCR (qRT-PCR) analysis
cDNA was synthesized using total RNA (500 ng) and a High Capacity cDNA Reverse Transcriptase kit (ABI, Staley Road, Grand Island, New York, USA) according to the manufacturer's protocol. 2X RT master mixes were prepared on ice according to the manufacturer's instructions. The reaction mixture, containing 1X RT buffer, 8 mM dNTP mix, 1X RT random primers and 1 μL each of Multiscribe reverse transcriptase and RNAs inhibitor in a total volume of 10 μL, was mixed gently. RNA (10 μL, 500 ng) was added to 10 μL of 2X RT master mix. The total volume of the reaction mixture in the tube was 20 μL. Briefly, the tube was centrifuged and loaded on to a thermal cycler (DNA Engine, M. J. Research, Minnesota, USA) programmed for 10 min at 25°C, 120 min at 37°C and 5 min at 85°C. The amplification of genes was carried out in a real-time PCR machine using SYBR green-based chemistry. The reaction mixture (20 μL) was prepared containing 1X SYBR green master mix, 0.5 μM of both forward primers (FP) and reverse primers (RP), and 1 μL of cDNA. The β-actin gene was used as an internal control. Sequences of the primers used for various genes were as follows: β-actin, FP 5′-CCTCTATGCCAACACAGT-3′, RP 5′-AGCCACCAATCCACACAG-3′; PPARA, FP 5′-ATCAGCCACACCTTTTCCAG-3′, RP 5′-ACCCCCAGCATTTGAGTTC-3′; p53, FP 5′-TGGCCCCTGTCATCTTCTG-3′, RP 5′-CCGTCATGTGCTGTGACTGC-3′; p21,FP 5′-TGAGCGATGGAACTTCGACT-3′, RP 5′-GACAGTGACAGGTCCACATGG-3′; bax, FP 5′-ATGGACGGGTCCGGGGAG-3′, RP 5′-ATCCAGCCCAACAGCCGC-3′; bcl-2, FP 5′-AAGCCGGCGACGACTTCT-3′, RP 5′-GGTGCCGGTTCAGGTACTCA-3′; p16, FP 5′-CCCGCTTTCGTAGTTTTCAT-3′, RP 5′-TTATTTGAGCTTTGGTTCTG-3′, and c-myc FP 5′-CCAACAGGAGCTATGACCTC-3′, RP 5′-CTCGGTCACCATCTCCAG-3′. Samples were loaded in the real-time PCR machine and the program was run with thermal cycling conditions as follows: For the β-actin gene – 10 min at 95°C, 30 cycles of 30 s at 95°C, 40 s at 59°C and 30 s at 72°C; for the PPARA gene – 10 min at 95°C, 45 cycles of 15 s at 95°C, 60 s at 62°C and 15 s at 72°C; for the p53 and bcl-2 genes – 10 min at 95°C and 32 cycles of 45 s at 95°C, 35 s at 65°C and 60 s at 72°C; for the p21 gene – 10 min at 95°C and 32 cycles of 45 s at 95°C, 30 s at 63°C and 50 s at 72°C; for the bax gene – 10 min at 95°C and 32 cycles of 45 s at 95°C, 45 s at 71°C and 15 s at 72°C; for the p16 gene – 10 min at 95°C and 32 cycles of 15 s at 95°C, 15 s at 62°C and 15 s at 72°C; and for the c-myc gene – 10 min at 95°C and 32 cycles of 45 s at 95°C, 35 s at 52°C and 60 s at 72°C. Relative quantification was done by means of the −ΔΔCt method.
2.11. let-7c miRNA expression profiling
The total RNA containing small RNAs was isolated using mirVana miRNA isolation kit (Invitrogen Bioservices India Pvt. Ltd., Bangalore, India) as described by the manufacturer. After RNA isolation, reverse transcription was carried out using reverse transcriptase primers of hsa-let-7c assay ID 000379 and RNU 44 assay ID 001094 (ABI, Staley Road, Grand Island, New York, USA). RNU 44 miRNA was used as reference control. RT was performed with the Taqman miRNA reverse transcription kit from ABI, as described by the manufacturer. In brief, 15 μL of reaction mixture contained 1X RT buffer, 15 mM dNTPs, 3.8 units of RNAs inhibitor, 50 cells of Multiscribe RT enzyme and 50 ng total RNA. The thermal cycling conditions used for reverse transcription were 30 min at 16°C, 30 min at 42°C and 5 min at 85°C. After reverse transcription, amplification was carried out using TaqMan probe base chemistry. The PCR reaction mixture (20 μL) contained 1X Taqman microRNA assay, Taqman 1X universal PCR master mix without UNG and 1.33 μL RT reaction product. The thermal cycling conditions used for amplification were 10 min at 95°C, 40 cycles of 15 s at 95°C and 60 s at 60°C. Relative quantification was carried out by means of −ΔΔCt method considering the levels of RNU 44 as endogenous control.
2.12. Statistical analysis
A student's t-test and χ2 test were applied to compare the socio-demographic variables between TCE exposed persons and control group. The student's t-test was employed to compare the level of hormone and expression of gene. The correlation was detected between age and DHEAS level in the exposed group. The results were analyzed using Prism version 5, Graph Pad Software Inc. Values of p < 0.05 were considered statistically significant.
3. Results
3.1. Study population characteristics
Physical characteristics of the study population are depicted in Table 1 and no significant differences were found between the exposed and control group except the weight of the exposed workers that was lower than that of workers in control group (p ≤ 0.05). Workers in the factory were exposed to TCE for an average of 36.6 ± 15.3 years (mean ± SD). Table 2 illustrates outcomes of physical tests performed in both groups to be acquainted with health status. BMI and body fat percent of study subjects was in the normal range, although there was a significant difference between exposed and controls (p < 0.05). Lung function test was performed using an electronic spirometer. Pulmonary function measurements included peak expiratory flow rate (PEFR) and forced expiratory volume after 1 s (FEV1). A highly significant decline in PEFR was observed among exposed subjects compared to control subjects (p < 0.001). FEV1 also showed significant reduction (p < 0.01) in the exposed subjects compared to the controls.
Table 1.
Distribution of physical and socio-demographic variables in TCE exposed and control group.
| Variables | Control (n = 19) | Exposed (n = 49) | p-value |
|---|---|---|---|
| Age (years) {Mean ± SD} | 36.57 ± 15.3 | 34.59 ± 15.6 | 0.36a |
| Height (cm) {Mean ± SD} | 168.94 ± 7.09 | 164.57 ± 6.02 | 0.06a |
| Weight (kg) {Mean ± SD} | 65.26 ± 13.62 | 53.9 ± 10.6 | 0.0017a*** |
| Alcohol intake {n(%)} | |||
| Non-alcoholic | 15 (78.95) | 30 (61.22) | 0.16b |
| Alcoholic | 4 (21.05) | 19 (38.78) | |
| Marital status {n(%)} | |||
| Bachelor | 12 (63.16) | 21 (42.86) | 0.13b |
| Married | 7 (36.84) | 28 (57.14) | |
| Education {n(%)} | |||
| Literate | 16 (22.2) | 29 (34.28 | 0.05b* |
| Illiterate | 3 (77.78) | 20 (65.72) | |
| Socioeconomic status {n(%)} | |||
| High | 0 | 0 | 0.16b |
| Middle | 14 (84.21) | 27 (55.1) | |
| Low | 5 (15.79) | 22 (44.9) | |
| Dietary habit {n(%)} | |||
| Vegetarian | 4 (21.05) | 9 (18.37) | 0.80b |
| Non-vegetarian | 15 (78.95) | 40 (81.63) | |
| Exposure (years) {Mean ± SD} | 0 | 12 ± 9.88 | |
p-value based on Student's t test for difference in the distributions between groups.
p-value based on χ2 test for difference in the distributions between groups.
Number in () refer to percentage.
*p ≤ 0.05, ***p ≤ 0.001 statistically significant from control.
Table 2.
Characteristics of selected exposure variables in TCE exposed and controls.
| Variable | Control (n = 19) | Exposed (n = 49) | p-value# |
|---|---|---|---|
| BMI | 22.84 ± 4.16 | 20.09 ± 4.09 | 0.0157* |
| Body fat% | 23.20 ± 7.09 | 18.23 ± 7.1 | 0.0129* |
| PEFR(Lt/min) | 440.08 ± 102.28 | 318.19 ± 120.53 | 0.0057** |
| FEV1 (Lt) | 1.83 ± 0.58 | 1.47 ± 0.40 | 0.0378* |
Abbreviations: BMI – Basal metabolic index, PEFR – Peak expiratory flow rate, FEV1 – Forced expiratory volume after 1 s. Results are mean ± SD. #p-value based on Student's t-test, *p ≤ 0.05, **p ≤ 0.01 statistically significant from control.
3.2. TCE metabolites in blood
The TCE metabolites TCA, DCA and TCEOH were detected in the plasma of all subjects (Table 3). The control group showed the absence of metabolites in plasma while TCA was detected in 15 exposed workers and the TCA concentration ranged from 0.004 to 2.494 μg/mL. DCA was detected in the plasma of 15 exposed workers in the range of 0.01–3.612 μg/mL. TCEOH was found in the plasma of 13 workers ranging from 0.002 to 0.617 μg/mL. Five of the exposed workers were identified as having TCA, DCA and TCEOH traces in their blood, and the level was comparatively higher than other metabolites. Substantial levels of these metabolites were present in the plasma sample of subjects 4 and 10, having 5 and 9 years of TCE exposure, respectively. On the other hand, subjects 8 and 17 had the highest concentration of TCA and DCA of those that showed the presence of either TCA or DCA.
Table 3.
Plasma was separated from the blood samples. They were extracted and derivatized for analysis by gas chromatography with electron capture detector.
| Subject | Exposure (years) | TCE metabolites (μg/mL) |
||
|---|---|---|---|---|
| TCA | DCA | TCEOH | ||
| 1 | 35 | 0.216 | 0 | 0.475 |
| 2 | 8 | 0.004 | 0 | 0 |
| 3 | 6 | 0.012 | 0 | 0 |
| 4 | 9 | 1.969 | 1.262 | 0.617 |
| 5 | 22 | 0.233 | 0.057 | 0.149 |
| 6 | 6 | 0.332 | 0 | 0.023 |
| 7 | 7 | 0.123 | 0 | 0 |
| 8 | 5 | 2.494 | 0 | 0 |
| 9 | 7 | 0.04 | 0 | 0 |
| 10 | 5 | 1.417 | 0.534 | 0.26 |
| 11 | 6 | 0 | 0.249 | 0.106 |
| 12 | 11 | 0.02 | 0 | 0.311 |
| 13 | 15 | 0.517 | 0 | 0 |
| 14 | 4 | 0.246 | 0.161 | 0 |
| 15 | 4 | 0.333 | 0.163 | 0.114 |
| 16 | 40 | 0 | 0.747 | 0 |
| 17 | 20 | 0 | 3.612 | 0 |
| 18 | 7 | 0 | 0.194 | 0 |
| 19 | 25 | 0 | 0.07 | 0.065 |
| 20 | 6 | 0.022 | 0.01 | 0.007 |
| 21 | 5 | 0 | 0.448 | 0 |
| 22 | 20 | 0 | 0.063 | 0 |
| 23 | 17 | 0 | 0.163 | 0 |
| 24 | 14 | 0 | 0.112 | 0 |
| 25 | 4 | 0 | 0 | 0.94 |
| 26 | 35 | 0 | 0 | 0.002 |
| 27 | 12 | 0 | 0 | 0.548 |
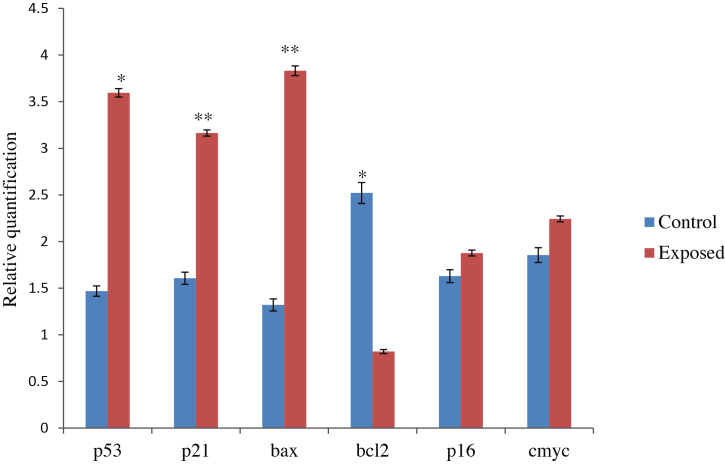
3.3. Effect of TCE exposure on p53, p21, bax, bcl-2, p16 and c-myc mRNA expression
mRNA expression analysis of p53, p21, bax, bcl-2, p16 and c-myc genes was carried out in TCE exposed and control groups. Quantitative reverse transcription (qRT)-PCR analysis revealed up-regulation of expression of p53 (2.4-fold; p < 0.05), p21 (2-fold; p < 0.01), and bax (2.9-fold; p < 0.01) concomitant with the down-regulation of bcl-2 (67%; p < 0.05) in the exposed individuals in comparison to the controls as shown in Fig. 1. In contrast, the expression level of p16 and c-myc genes in the exposed group was not statistically significant as compared to the control values.
Fig. 1.
Expression profiling of cell cycle regulating genes by qRT-PCR in control and TCE exposed persons.
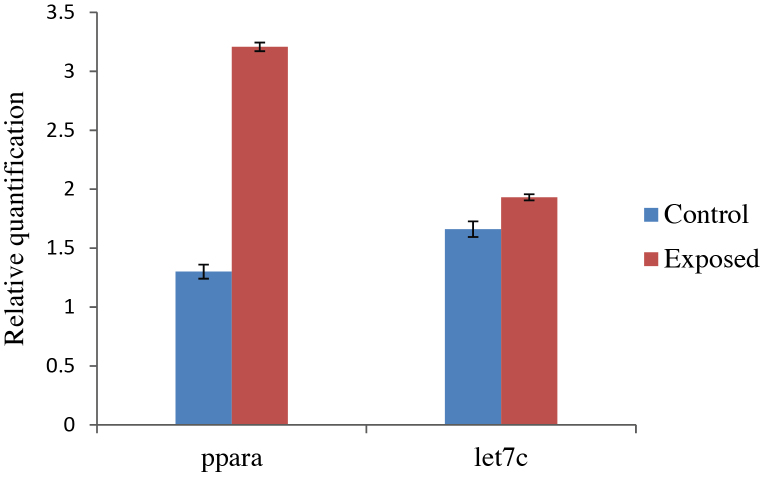
3.4. Effect of TCE exposure on mRNA levels of PPARA and miRNA let-7c expression
The level of PPARA mRNA expression was nearly 2.5-fold higher in the exposed group in comparison to the control group but the difference was statistically non-significant due to the extent of variation (Fig. 2). miRNA expression analysis was also carried out in both groups. No significant difference was observed in miRNA let-7c expression level in the exposed workers and control persons.
Fig. 2.
Expression profiling of PPARA mRNA and miRNA let-7c by qRT-PCR in control and TCE exposed persons.
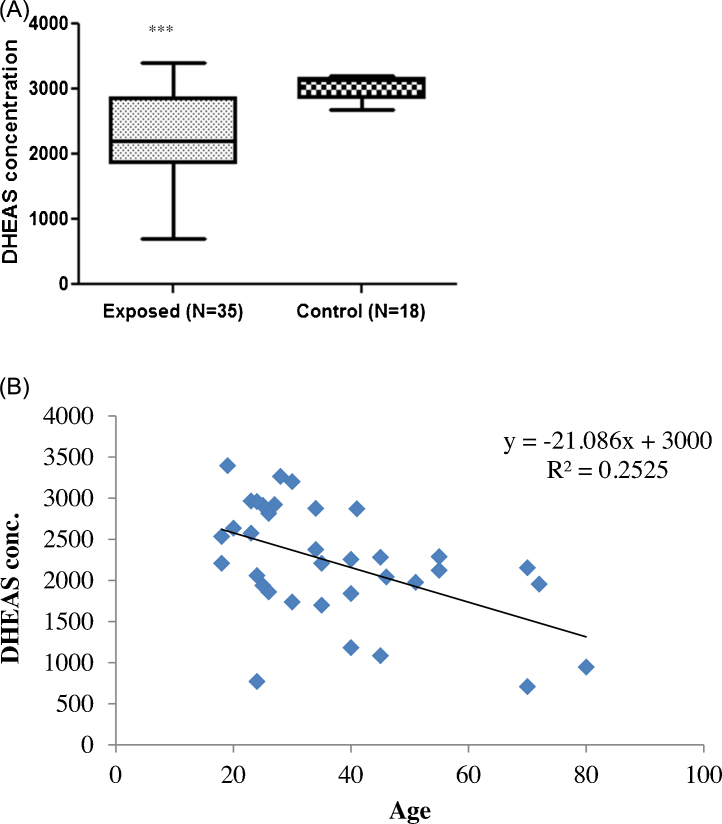
3.5. TCE-induced endocrine disruption
Inhibition of DHEAS production in TCE-exposed workers and its comparison with the controls is summarized in Fig. 3 (A and B). After restricting statistical analysis to the exposed group, the age of the workers and DHEAS levels were found to be significantly different but with a negative correlation (R2 = 0.252, p < 0.001, Fig. 3). There was a significant decrease in the DHEAS level of the exposed group in comparison to the control group. Table 4 shows the mean hormonal levels with years of exposure. Decrease in DHEAS concentration was significantly correlated with the years of TCE exposure. Workers exposed to TCE for ≤5 years to 40 years showed consistent decline in DHEAS level from 2334.20 ng/mL to 1767.32 ng/mL, respectively.
Fig. 3.
Effect of trichloroethylene on DHEAS hormone level. (A) The decrease in DHEAS level in the blood of TCE exposed workers in comparison to control is shown (Mean ± SD). DHEAS level decreases to 2218.03 ± 698.3 ng/mL in TCE exposed group of workers in comparison to 3035.2 ± 195.8 ng/mL in control group of workers. (B) The correlation between logarithmically transformed DHEAS concentration (ng/mL) in the blood of TCE exposed workers and their age is shown (p < 0.001, R = −0.50, Spearman, R2 = 0.25).
Table 4.
Mean DHEAS hormone level with years of exposure to TCE.
| Subjects | Years of exposure |
||||
|---|---|---|---|---|---|
| Control (n = 18) | ≤5 years (n = 13) | 5–10 years (n = 8) | 10–20 years (n = 7) | 20–40 years (n = 7) | |
| DHEAS (ng/mL) | 3035.20 (2692.47–3207.40) | 2334.20 (1738.39–3265.18) | 1822.81* (709.3–2876.37) | 1810.41* (771.41–3396.01) | 1767.32* (947.26–3201.67) |
Results are geometric mean. *p < 0.05, p-value compared with control group of workers. Number in () refer to range.
4. Discussion
We assessed the effects of TCE on the health of the workers employed in lock industries through lung function tests and body composition monitoring. To the best of our knowledge, there are no earlier studies that have been carried out on the occupational exposure to TCE leading to lung function abnormalities, especially PEFR and FEV1. The influence of body composition factors on the lung function test parameters was ruled out among the study subjects based on the normal BMI and body fat (%) observed among them. The considerable decline in PEFR and FEV1 denote upper and smaller airway obstruction among the exposed subjects. The exposure to TCE can lead to bronchiolar damage in workers. In the rodent model, TCE was observed to produce bronchiolar damage when administered to mice [30]. TCE is carcinogenic to the mouse lung and the toxicity is confined to the accumulation of TCE metabolite in non-ciliated Clara cells of the lung. Metabolic capacity of lung determines the extent of toxicity of TCE in mouse and human [31]. Pulmonary surfactant prevents alveolar collapse at maximal expiration. Inhalation of TCE may damage the enzymes that are responsible for synthesizing the pulmonary surfactant, resulting in lower amounts of surfactant being stored and available for secretion into the alveolus [32].
In the present study effects of TCE on the cell cycle regulating genes in TCE-exposed workers were investigated. None of the previous studies reported the possible role of miRNA let-7c in regulation of PPARA gene expression in lymphocytes of people occupationally exposed to TCE. In this study we observed the effect of TCE exposure on cell cycle regulating genes along with a possible mechanism of action and regulation of TCE metabolites in 49 TCE-exposed workers. We examined exposure-related differences in mRNA levels of p53, p21, bax, bcl2, p16 and c-myc among individuals occupationally exposed to TCE and controls. Various DNA-damaging agents that trigger programmed cell death also induce p53 expression. p53 mediated cell death may involve activation or suppression of other genes like p21, bcl-2 and bax. Both p53 and p21 play crucial roles in the DNA damage response. We observed lower expression of mRNAs for p53 and p21 in the control group, whereas they were nearly twofold up-regulated in the exposed individuals, suggesting DNA damaging potential of TCE or its metabolites. An in vitro study also reported elevated levels of p53 and Bax proteins and a decreased level of Bcl-2 protein in TCE-treated, p53-WT H460 cells [33]. A known p53 inducer, γ-radiation, has been reported to decrease bcl-2 mRNA expression in a human leukemia cell line [34]. TCE and its metabolites TCA and DCA have been reported to decrease the methylation of promoter regions of the c-myc gene, leading to an increased mRNA expression in the liver of B6C3F1 mice [35]. In the present study there was no significant change in the mRNA expression level of c-myc and p16 genes in the TCE-exposed group. It would be difficult to conclude that TCE and/or its metabolites cause modulation in expression of c-myc and p16 genes in human lymphocytes.
We measured the levels of TCA and DCA in the blood of both exposed and control groups through gas chromatography using electron capture detection [28]. TCA and DCA were present in detectable amounts in the exposed group. Further, we observed no statistically significant change in the expression of PPARA transcript in the control and the TCE-exposed groups. The TCE metabolites TCA and DCA are weak PPARA ligands. They activate PPARA receptor; however, their binding affinity is fairly low among all known PPARA ligands [45]. Both metabolites are known to cause hepatocarcinogenesis in rodent models through peroxisome proliferation and activation of the PPARA receptor. Walgren et al. [1] investigated their impact on human PPARA activation in cultured human hepatocytes and suggested that humans may not be susceptible to TCA- and DCA-induced hepatocarcinogenesis. Numerous studies on hepatocarcinogenesis caused by ligand activation of PPARA showed that receptor specificity, species differences in receptor activity, and relative ligand binding or activation are the critical factors that influence the ligand or receptor mediated effect [36], [43]. There are no mechanistic studies that have reported a direct role of TCA and DCA in modulating expression of the PPARA transcript in humans. There may be involvement of other downstream regulating factors. Therefore, we investigated the role of miRNAs in its regulation. The let-7 family has been reported as a tumor suppressor family of miRNAs [37], [38]. qRT-PCR analysis showed up-regulation of miRNA let-7c in the exposed group in comparison to the control group. It has been reported that let-7 miRNA directly or indirectly regulates multiple cell proliferation genes and let-7c is a key regulator of cell cycle progression [39]. Thus, the potentially important role of let-7c miRNA in control of fundamental processes such as the cell cycle must be further investigated.
TCA and DCA are known as exogenous PPARA ligands while DHEAS is an endogenous PPARA ligand in rodents. DHEAS is a naturally occurring adrenal steroid with known peroxisome proliferation potential [22]. Therefore, we measured DHEAS concentration in serum of the control and exposed group. Age of workers was significantly and negatively correlated with their DHEAS hormone level. Our findings are in conflict with those reported by Chia et al. [40], where occupational exposure to TCE was related to elevated levels of DHEAS in the serum of the workers. Previous studies hypothesized that the elevated level of DHEAS hormone in TCE exposed people might be a result of possible competition between TCE metabolites and DHEAS as ligands of the PPARA receptor [41]. The present study showed that with increasing years of TCE exposure. DHEAS hormone level significantly declined. Thus, a possibility of DHEAS competition with TCA and DCA for binding to PPARA receptor and its activation, if any, would diminish.
Results of the present study substantiate that occupational exposure to TCE causes respiratory ailments, which is evident by the decline of pulmonary function. TCE exposure also reduces body fat%. The study also supports the association between TCE exposure and disturbances in cell cycle regulation, which is indicated by increasing expression levels of p53, p21 and bax mRNAs together with decreasing levels of bcl-2 mRNA. Nonetheless, involvement of DHEAS as an endogenous ligand of PPARA is uncertain in the observed changes in the levels of various mRNAs. However, the significant decrease in the DHEAS level of the exposed workers in comparison to the controls indicates its potential for endocrine disruption. Further, no difference was observed in PPARA mRNA and let-7c miRNA expression in exposed workers. Further studies are essential to explore the entire mechanism of TCE toxicity by attempting additional human studies.
Funding
A senior research fellowship from the Council of Scientific and Industrial Research (CSIR, New Delhi, India) No. ITRC/CSIR (SRF)/112 (51)/08 to Meenu Varshney.
Conflict of interest
None declared.
Acknowledgments
The authors would like to thank Dr. K. C. Gupta, Director of the Indian Institute of Toxicology Research, for his continuous support. The authors wish to acknowledge the technical assistance of Mr. Fareed Mohammad and Mr. Praveen Kumar.
References
- 1.Walgren J.E., Kurtz D.T., McMillan J.M. Expression of PPAR(alpha) in human hepatocytes and activation by trichloroacetate and dichloroacetate. Res. Commun. Mol. Pathol. Pharmacol. 2000;108:116–132. [PubMed] [Google Scholar]
- 2.Kumar M., Tewari S., Sharma P., Verma V.K., Chauhan L.K., Agarwal S.K., Dwivedi U.N., Goel S.K. Study of genetic polymorphism in solvent exposed population and its correlation to in vitro effect of trichloroethylene on lymphocytes. J. Environ. Biol. 2009;30:685–691. (Academy of Environmental Biology, India) [PubMed] [Google Scholar]
- 3.NTP . National Toxicology Program; 2015. Report on Carcinogens Monograph on Trichloroethylene: Cancer Evaluation. [PubMed] [Google Scholar]
- 4.Lash L.H., Chiu W.A., Guyton K.Z., Rusyn I. Trichloroethylene biotransformation and its role in mutagenicity, carcinogenicity and target organ toxicity. Mutat. Res. Rev. Mutat. Res. 2014;762:22–36. doi: 10.1016/j.mrrev.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore M.M., Harrington-Brock K. Mutagenicity of trichloroethylene and its metabolites: implications for the risk assessment of trichloroethylene. Environ. Health Perspect. 2000;108:215–223. doi: 10.1289/ehp.00108s2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plewa M.J., Simmons J.E., Richardson S.D., Wagner E.D. Mammalian cell cytotoxicity and genotoxicity of the haloacetic acids, a major class of drinking water disinfection by-products. Environ. Mol. Mutagen. 2010;51:871–878. doi: 10.1002/em.20585. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Xu L., Zeng Q., Zhang S.H., Xie H., Liu A.L., Lu W.Q. Comparison of DNA damage in human-derived hepatoma line (HepG2) exposed to the fifteen drinking water disinfection byproducts using the single cell gel electrophoresis assay. Mutat. Res. 2012;741:89–94. doi: 10.1016/j.mrgentox.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Varshney M., Chandra A., Chauhan L.K., Goel S.K. Micronucleus induction by oxidative metabolites of trichloroethylene in cultured human peripheral blood lymphocytes: a comparative genotoxicity study. Environ. Sci. Pollut. Res. Int. 2013;20:8709–8716. doi: 10.1007/s11356-013-1806-7. [DOI] [PubMed] [Google Scholar]
- 9.Varshney M., Chandra A., Chauhan L.K., Goel S.K. In vitro cytogenetic assessment of trichloroacetic acid in human peripheral blood lymphocytes. Environ. Sci. Pollut. Res. Int. 2014;21:843–850. doi: 10.1007/s11356-013-1949-6. [DOI] [PubMed] [Google Scholar]
- 10.Lane D.P. Cancer. A death in the life of p53. Nature. 1993;362:786–787. doi: 10.1038/362786a0. [DOI] [PubMed] [Google Scholar]
- 11.el-Deiry W.S., Harper J.W., O’Connor P.M., Velculescu V.E., Canman C.E., Jackman J., Pietenpol J.A., Burrell M., Hill D.E., Wang Y. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res. 1994;54:1169–1174. [PubMed] [Google Scholar]
- 12.Gartel A.L., Tyner A.L. Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp. Cell Res. 1999;246:280–289. doi: 10.1006/excr.1998.4319. [DOI] [PubMed] [Google Scholar]
- 13.Ho J.S., Ma W., Mao D.Y., Benchimol S. p53-Dependent transcriptional repression of c-myc is required for G1 cell cycle arrest. Mol. Cell. Biol. 2005;25:7423–7431. doi: 10.1128/MCB.25.17.7423-7431.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreeff M., Jiang S., Zhang X., Konopleva M., Estrov Z., Snell V.E., Xie Z., Okcu M.F., Sanchez-Williams G., Dong J., Estey E.H., Champlin R.C., Kornblau S.M., Reed J.C., Zhao S. Expression of Bcl-2-related genes in normal and AML progenitors: changes induced by chemotherapy and retinoic acid. Leukemia. 1999;13:1881–1892. doi: 10.1038/sj.leu.2401573. [DOI] [PubMed] [Google Scholar]
- 15.IARC . vol. 63 (February) World Health Organization; Lyon, France: 1995. Dry cleaning, some chlorinated solvents and other industrial chemicals; pp. 1017–1606. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). 33-477. [PMC free article] [PubMed] [Google Scholar]
- 16.IARC . Vol. 106. International Agency for Research on Cancer; 2014. Trichloroethylene, tetrachloroethylene, and some other chlorinated agents. (IARC Monographs on the Evaluation of Carcinogenic Risks to Humans). [PMC free article] [PubMed] [Google Scholar]
- 17.DeAngelo A.B., George M.H., House D.E. Hepatocarcinogenicity in the male B6C3F1 mouse following a lifetime exposure to dichloroacetic acid in the drinking water: dose-response determination and modes of action. J. Toxicol. Environ. Health A. 1999;58:485–507. doi: 10.1080/009841099157115. [DOI] [PubMed] [Google Scholar]
- 18.DeAngelo A.B., Daniel F.B., Wong D.M., George M.H. The induction of hepatocellular neoplasia by trichloroacetic acid administered in the drinking water of the male B6C3F1 mouse. J. Toxicol. Environ. Health A. 2008;71:1056–1068. doi: 10.1080/15287390802111952. [DOI] [PubMed] [Google Scholar]
- 19.George M.H., Moore T., Kilburn S., Olson G.R., DeAngelo A.B. Carcinogenicity of chloral hydrate administered in drinking water to the male F344/N rat and male B6C3F1 mouse. Toxicol. Pathol. 2000;28:610–618. doi: 10.1177/019262330002800415. [DOI] [PubMed] [Google Scholar]
- 20.Leakey J.E., Seng J.E., Latendresse J.R., Hussain N., Allen L.J., Allaben W.T. Dietary controlled carcinogenicity study of chloral hydrate in male B6C3F1 mice. Toxicol. Appl. Pharmacol. 2003;193:266–280. doi: 10.1016/j.taap.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Richmond R.E., Carter J.H., Carter H.W., Daniel F.B., DeAngelo A.B. Immunohistochemical analysis of dichloroacetic acid (DCA)-induced hepatocarcinogenesis in male Fischer (F344) rats. Cancer Lett. 1995;92:67–76. doi: 10.1016/0304-3835(94)03756-9. [DOI] [PubMed] [Google Scholar]
- 22.Waxman D.J. Role of metabolism in the activation of dehydroepiandrosterone as a peroxisome proliferator. J. Endocrinol. 1996;150(Suppl.):S129–S147. [PubMed] [Google Scholar]
- 23.Zamore P.D., Haley B. Ribo-gnome: the big world of small RNAs. Science. 2005;309:1519–1524. doi: 10.1126/science.1111444. [DOI] [PubMed] [Google Scholar]
- 24.Johnson S.M., Grosshans H., Shingara J., Byrom M., Jarvis R., Cheng A., Labourier E., Reinert K.L., Brown D., Slack F.J. RAS is regulated by the let-7 microRNA family. Cell. 2005;120:635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Sassen S., Miska E.A., Caldas C. MicroRNA: implications for cancer. Virchows Arch.: Int J. Pathol. 2008;452:1–10. doi: 10.1007/s00428-007-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinhart B.J., Slack F.J., Basson M., Pasquinelli A.E., Bettinger J.C., Rougvie A.E., Horvitz H.R., Ruvkun G. The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature. 2000;403:901–906. doi: 10.1038/35002607. [DOI] [PubMed] [Google Scholar]
- 27.Shah Y.M., Morimura K., Yang Q., Tanabe T., Takagi M., Gonzalez F.J. Peroxisome proliferator-activated receptor alpha regulates a microRNA-mediated signaling cascade responsible for hepatocellular proliferation. Mol. Cell. Biol. 2007;27:4238–4247. doi: 10.1128/MCB.00317-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mudiam M.K., Jain R., Varshney M., Ch R., Chauhan A., Goyal S.K., Khan H.A., Murthy R.C. In matrix derivatization of trichloroethylene metabolites in human plasma with methyl chloroformate and their determination by solid-phase microextraction-gas chromatography-electron capture detector. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2013;925:63–69. doi: 10.1016/j.jchromb.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Chomczynski P., Sacchi N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: twenty-something years on. Nat. Protoc. 2006;1:581–585. doi: 10.1038/nprot.2006.83. [DOI] [PubMed] [Google Scholar]
- 30.Forkert P.G., Sylvestre P.L., Poland J.S. Lung injury induced by trichloroethylene. Toxicology. 1985;35:143–160. doi: 10.1016/0300-483x(85)90028-9. [DOI] [PubMed] [Google Scholar]
- 31.Green T. Pulmonary toxicity and carcinogenicity of trichloroethylene: species differences and modes of action. Environ. Health Perspect. 2000;108(Suppl. 2):261–264. doi: 10.1289/ehp.00108s2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott J.E., Forkert P.G., Oulton M., Rasmusson M.G., Temple S., Fraser M.O., Whitefield S. Pulmonary toxicity of trichloroethylene: induction of changes in surfactant phospholipids and phospholipase A2 activity in the mouse lung. Exp. Mol. Pathol. 1988;49:141–150. doi: 10.1016/0014-4800(88)90028-7. [DOI] [PubMed] [Google Scholar]
- 33.Chen S.J., Wang J.L., Chen J.H., Huang R.N. Possible involvement of glutathione and p53 in trichloroethylene- and perchloroethylene-induced lipid peroxidation and apoptosis in human lung cancer cells. Free Radic. Biol. Med. 2002;33:464–472. doi: 10.1016/s0891-5849(02)00817-1. [DOI] [PubMed] [Google Scholar]
- 34.Zhan Q., Lord K.A., Alamo I., Jr., Hollander M.C., Carrier F., Ron D., Kohn K.W., Hoffman B., Liebermann D.A., Fornace A.J., Jr. The gadd and MyD genes define a novel set of mammalian genes encoding acidic proteins that synergistically suppress cell growth. Mol. Cell. Biol. 1994;14:2361–2371. doi: 10.1128/mcb.14.4.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tao L., Yang S., Xie M., Kramer P.M., Pereira M.A. Hypomethylation and overexpression of c-jun and c-myc protooncogenes and increased DNA methyltransferase activity in dichloroacetic and trichloroacetic acid-promoted mouse liver tumors. Cancer Lett. 2000;158:185–193. doi: 10.1016/s0304-3835(00)00518-8. [DOI] [PubMed] [Google Scholar]
- 36.Peters J.M., Cheung C., Gonzalez F.J. Peroxisome proliferator-activated receptor-alpha and liver cancer: where do we stand? J. Mol. Med. (Berl.) 2005;83:774–785. doi: 10.1007/s00109-005-0678-9. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y.S., Dutta A. MicroRNAs: small but potent oncogenes or tumor suppressors. Curr. Opin. Investig. Drugs. 2006;7:560–564. [PubMed] [Google Scholar]
- 38.Zhang B., Pan X., Cobb G.P., Anderson T.A. microRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007;302:1–12. doi: 10.1016/j.ydbio.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 39.Johnson C.D., Esquela-Kerscher A., Stefani G., Byrom M., Kelnar K., Ovcharenko D., Wilson M., Wang X., Shelton J., Shingara J., Chin L., Brown D., Slack F.J. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 40.Chia S.E., Goh V.H., Ong C.N. Endocrine profiles of male workers with exposure to trichloroethylene. Am. J. Ind. Med. 1997;32:217–222. doi: 10.1002/(sici)1097-0274(199709)32:3<217::aid-ajim6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 41.Zhou Y.C., Waxman D.J. Activation of peroxisome proliferator-activated receptors by chlorinated hydrocarbons and endogenous steroids. Environ. Health Perspect. 1998;106(Suppl. 4):983–988. doi: 10.1289/ehp.98106s4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bull R.J., Orner G.A., Cheng R.S., Stillwell L., Stauber A.J., Sasser L.B., Lingohr M.K., Thrall B.D. Contribution of dichloroacetate and trichloroacetate to liver tumor induction in mice by trichloroethylene. Toxicol. Appl. Pharmacol. 2002;182:55–65. doi: 10.1006/taap.2002.9427. [DOI] [PubMed] [Google Scholar]
- 43.Klaunig J.E., Babich M.A., Baetcke K.P., Cook J.C., Corton J.C., David R.M., DeLuca J.G., Lai D.Y., McKee R.H., Peters J.M., Roberts R.A., Fenner-Crisp P.A. PPARalpha agonist-induced rodent tumors: modes of action and human relevance. Crit. Rev. Toxicol. 2003;33:655–780. doi: 10.1080/713608372. [DOI] [PubMed] [Google Scholar]
- 44.Lan F.F., Wang H., Chen Y.C., Chan C.Y., Ng S.S., Li K., Xie D., He M.L., Lin M.C., Kung H.F. Hsa-let-7g inhibits proliferation of hepatocellular carcinoma cells by downregulation of c-Myc and upregulation of p16(INK4A) Journal International du CancerInt. J. Cancer. 2011;128:319–331. doi: 10.1002/ijc.25336. [DOI] [PubMed] [Google Scholar]
- 45.Maloney E.K., Waxman D.J. trans-Activation of PPARalpha and PPARgamma by structurally diverse environmental chemicals. Toxicol. Appl. Pharmacol. 1999;161:209–218. doi: 10.1006/taap.1999.8809. [DOI] [PubMed] [Google Scholar]
- 46.U.S. EPA Toxicological Review of Trichloroethylene (CAS No. 79-01-6): In Support of Summary Information on the Integrated Risk Information System (IRIS); Washington, DC; 2011. EPA/635/R-09/011F. [Google Scholar]