Abstract
Lepidium sativum seed (LSS) (family: Cruciferae) has been used in traditional medicine for the treatment of jaundice, liver problems, spleen diseases and gastrointestinal disorders. It was also reported to possess antihypertensive, diuretic, anti-asthmatic, antioxidant, and anti-inflammatory activities. Attempt has been made to study hepatoprotective potential of LSS available in Saudi Arabian Market. The aim of the present study was to determine the hepatoprotective effect of ethanolic extracts of LSS against carbon tetrachloride (CCl4) induced acute liver injury in rats. The bioactive compounds responsible for this activity have been analyzed by GC⿿MS.
To evaluate the hepatoprotective activity, six groups (n = 6) of rats were taken. First group was control, second was toxic and other groups received oral test solutions: 100 mg/kg silymarin, or LSS (100, 200, and 400 mg/kg), once daily for 7 consecutive days, followed by hepatotoxicity induction with CCl4. Blood and liver tissues were collected for biochemical, antioxidant and microscopic analyses. The bioactive constituents present in the extract were analyzed by GC⿿MS. Results showed that pretreatment with LSS and silymarin significantly reduced the level of serum alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and bilirubin (BIL), which was increased significantly in toxic group treated with only CCl4. Histological analysis of liver tissues in groups pretreated with LSS and silymarin showed mild necrosis and inflammation of the hepatocytes compared to the toxic group. GC⿿MS analysis of LSS showed the presence of twelve major fatty acids including alpha-linolenic acid as a major constituent. These results indicated that LSS exerts enhance hepatoprotective activity that could be attributed towards its antioxidant activity, coupled together with the presence of anti-inflammatory compounds in LSS extract.
Keywords: Lepidium sativum, Carbon tetrachloride, Antioxidant, Hepatoprotective, GC⿿MS
1. Introduction
Lepidium sativum Linn. (Family: Cruciferae) locally known as ⿿El-Rshad⿿ is a fast-growing, edible herb with tangy flavor and aroma. In traditional system of medicine various parts of this plant have been used for the treatment of jaundice, liver problems, spleen diseases, gastrointestinal disorders, menstrual problems, fracture, arthritis, and other inflammatory conditions [1], [2], [3]. The seeds of L. sativum (LSS) are also used to cure throat diseases, asthma, headache, uterine tumour, nasal polyps and breast cancer [1]. Chemically, the seeds of L. sativum contain essential oils, fatty oils, carbohydrate, protein, fatty acids, vitamins, flavonoids and isothiocynates glycoside [4]. Experimental evidences showed that L. sativum has antihypertensive [5], diuretic [6], anti-asthmatic [7], hypoglycaemic [8], antioxidant [4], [9], [10] and anti-inflammatory [11] properties.
Due to reported antioxidant and anti-inflammatory activity, the present study was designed to investigate the protective effect of LSS against CCl4 induced toxicity. Additionally, the compound present in the extract was also investigated that could be responsible for the activity of the extract.
Previously the hepatoprotective activity of LSS against CCl4 [12], and doxorubicin [13] induced toxicity has been reported but as per our knowledge no work has been done on the LSS available in Saudi Arabia. In addition to this, the novelty of this work is that the constituent of the bioactive extract has been investigated that may be responsible for the biological activity of the extract.
2. Materials and methods
2.1. Collection of plant materials
LSS were collected from local market of Riyadh, Kingdom of Saudi Arabia and were stored in air tight containers.
2.2. Preparation of the extract
The extraction was performed using Soxhlet apparatus. Sixty grams of dried seeds were grinded into coarse powder. Coarsely powdered seeds were taken and 600 mL of ethanol was added. The extraction was continued for 6⿿8 h until all the soluble constituents dissolved in the solvent. The extract was filtered and evaporated in rotary evaporator (Buchi, Switzerland; temp: 60 °C; pressure 175 mbar) to yield semi solid masses. Extract thus obtained, were collected and stored at 4 °C until further use.
2.3. Hepatoprotective activity of the extract
2.3.1. Animals
Thirty six male Wistar rats (190⿿200 g) were provided by the central animal house facility, Research Center, Prince Sultan Military Medical City, Riyadh. They were kept in standard cages at room temperature (25 ± 3 °C) with a 12 h dark⿿light cycle. They were allowed to consume standard laboratory food and water. Rats were equally divided into six groups. Group I was considered as control and administered with olive oil of 1 mL/kg body weight (b.w.) via the intraperitoneal route. Group II received 1 mL/kg b.w. of CCl4 (50% in olive oil) via the intraperitoneal route 24 h prior to sacrifice. Groups III, IV and V were given 100 mg/kg b.w., 200 mg/kg b.w. and 400 mg/kg b.w. of LSS respectively via the oral route for one week. Group VI received 100 mg/kg b.w. of silymarin for one week. All animals except the control group were challenged with CCl4. After 24 h, the animals were sacrificed under mild anesthesia. The blood was collected and used for biochemical analysis. Livers were also removed and half of the liver was snapped freeze for enzymatic analyses, whereas the other portion was processed for histological analyses.
2.3.2. Assessment of levels of liver marker enzymes and biochemical parameters
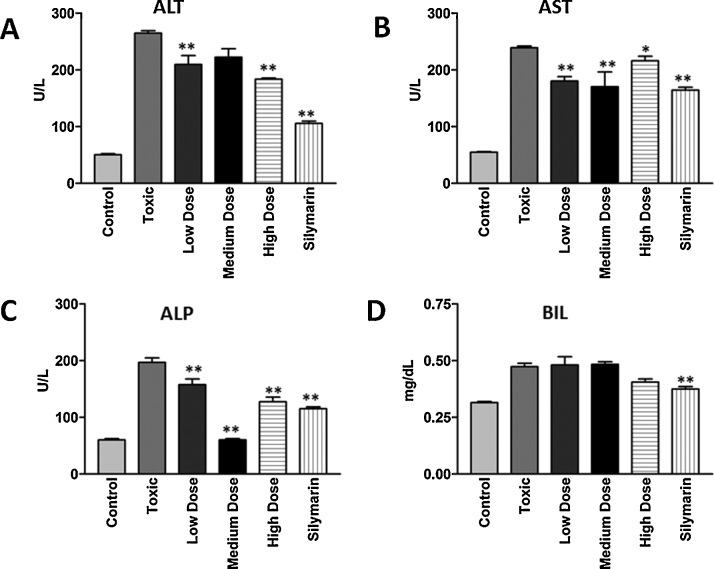
Serum analysis of various liver enzymes including alanine transaminase (ALT), aspartate transaminase (AST), alkaline phosphatase (ALP) and Bilirubin (BIL) was estimated using standard kits (Human Diagnostics, Germany). Results are shown in Fig. 1A⿿D.
Fig. 1.
Effect of Lipidium sativum seed extract on serum marker enzymes (A) alanine transaminase (ALT), (B) aspartate transaminase (AST), (C) alkaline phosphatase (ALP), and (D) bilirubin (BIL) after CCl4 intoxication in rats. * Statistically significant (P 0.05).
Control: animals with no treatment; toxic: animals receiving only CCl4; low dose: animals receiving ethanolic extract of the Lipidium sativum seed at a dose of 100 mg/kg followed by challenging with CCl4; medium dose: animals receiving seed extract at a dose of 200 mg/kg followed by challenging with CCl4; high dose: animals receiving ethanolic extract of the L. sativum seed at a dose of 400 mg/kg followed by challenging with CCl4; animals receiving silymarin at a dose of 100 mg/kg followed by challenging with CCl4.
2.3.3. Assessment of Myeloperoxidase (MPO) enzyme
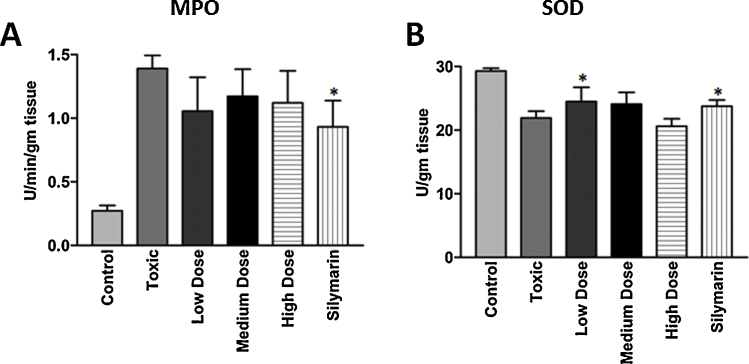
MPO activity was assayed by the method of Bradley et al. [14]. Tissue homogenate was prepared in potassium phosphate buffer (50 mM; pH 6.0) containing hexadecyl⿿trimethyl ammonium bromide (0.5%). Samples were subjected to three cycles of sonication freeze-thaw process followed by centrifugation (@14000 rpm; 4 °C) for 25 min. Supernatant was collected and to one hundred microliter of supernatant, added 2.9 mL phosphate buffer (50 mM, pH 6.0) containing 0.167 mg/mL o-dianisidine dihydrochloride and 1% hydrogen peroxide as substrates. Kinetics of MPO activity was measured at 460 nm for three min. The result was shown in Fig. 2A.
Fig. 2.
Effect of Lipidium sativum seed extract on marker enzymes (A) myeloperoxidase (MPO) and (B) superoxide dismutase (SOD) in liver tissues after CCl4 intoxication in rats. * Statistically significant (P 0.05).
2.3.4. Assessment of Superoxide dismutase (SOD) enzyme
SOD activity was analyzed by the method of Marklund and Marklund [15]. Tissue homogenate was prepared in ice cold sucrose solution (0.25 M; pH 8.5) containing Triton x-100 (0.5%). The homogenate solution was centrifuged (@17000 rpm; 4 °C) for 30 min. Supernatant was collected for analysis. Five micro liter of supernatant was added to 1395 μL of ice cold sucrose solution (0.25 M; pH 8.5) and 100 μL of pyraogallol (2.6 mM) containing Tris-HCl. SOD activity was measured at 420 nm over 3 min. The results were presented in Fig. 2B.
2.3.5. Histopathological study
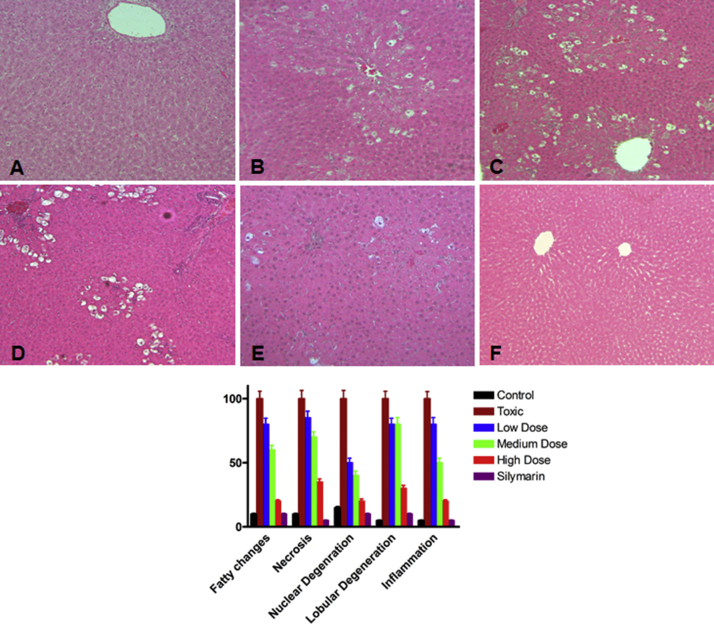
For microscopic evaluation, liver samples were fixed in a fixative (formalin solution neutral buffered, 10%) and embedded in paraffin sectioned at 5 μm thickness. The sections were subsequently stained with Mayers⿿ hematoxylin and eosin (Sigma⿿Aldrich, Germany). The stained samples were read under light microscope at 10ÿ magnification. The Slides were photographed, and documented in Fig. 3A⿿F.
Fig. 3.
Photomicrographs of histological changes of rat liver at magnification of 10ÿ. (A) normal control showed well maintained histology of rat liver. (B) CCl4 treated rats showed, fatty changes, necrosis, nuclear and lobular degeneration and inflammation of the cells. (C) LSE (100 mg/kg, p.o.) + CCl4 (1.0 mL/kg in olive oil 1:1, i.p.) treated group showed less nuclear degeneration as compared to toxic group. (D) LSE (200 mg/kg, p.o.) + CCl4 (1.0 mL/kg in olive oil 1:1, i.p.) treated rat liver showed improved structure with mild nuclear degeneration. (E) LSE (400 mg/kg, p.o.) + CCl4 (1.0 mL/kg in olive oil 1:1, i.p.) treated rat liver showed minimum fatty acid changes and retain the general structure. (F) Silymarin (100 mg/kg, p.o.) + CCl4 (1.0 mL/kg in olive oil 1:1, i.p.) treated rat liver showed preserved cell architecture. (G) Graph showing the level of deformity in each group. Scoring was performed as follows; scale: 1⿿100; 1: minimum deformity and 100: maximum deformity.
2.3.6. Statistical analyses
The data were analyzed by one way analysis of variance (ANOVA) using computer software Graph Prism, version 3.02 (Graph Pad Software Inc, CA, USA) and statistically significant effects were further analyzed by comparing means using Dunnett⿿s test. The level of significance was considered at P 0.05.
2.4. Preliminary phytochemical screening of the plant
Preliminary phytochemical screening were carried out on the LSS using standard procedure to identify the phyto constituents as described by Trease and Harborne [16], [17], [18].
2.5. Gas chromatography⿿mass spectrometry (GC⿿MS) analysis
2.5.1. Sample preparation for FAME (Fatty acid methyl ester) analysis
FAME sample was prepared as per modified method of Metcalfe et al. [19]. Briefly, one gram of extract was taken and added 2 mL of isooctane and 100 μL of methanolic potassium hydroxide solution (2 M). The mixture was shaken/vortexed exactly for one minute and allowed to settled down under gravity. Next, 2 mL of 40 % (w/v) aqueous sodium chloride solution was added into it. The mixture was again shaken briefly and allowed the layers to be separated. Upper layer (isooctane layer) was taken in another tube and added a pinch of anhydrous sodium sulphate to remove moisture. It was filtered through 0.22 μM filter and transferred to GC vial for analysis.
2.5.2. Instrumentation for FAME analysis of LSS
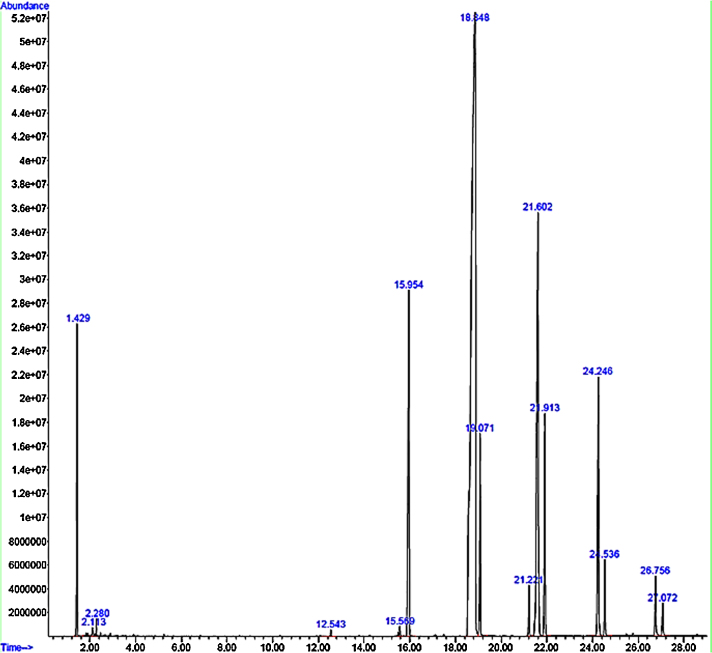
GC⿿MS analysis were carried out in a GC system (Agilent 7890A series, USA) equipped with split/splitless injector and auto-sampler attached to an apolar 5-MS (5% phenyl polymethyl siloxane) capillary column (Agilent 19,091S-43; 30 m ÿ 0.25 mm i.d. and 0.25 μm film thickness) and fitted to mass detector (Agilent 5975C series, USA). The flow rate of the carrier gas, helium (He) was set to be at 1 mL min⿿1, split ratio was 1:50. The injector temperature was adjusted at 250 °C, while the detector temperature was fixed to 280 °C. The column temperature was kept at 40 °C for 1 min followed by linear programming to raise the temperature from 40° to 120 °C (at 4 °C min⿿1 with 2 min hold time), 120 °C to 170 °C (at 6 °C min⿿1 with 1 min hold time) and 170 °C to 200 °C (at 10 °C min⿿1 with 1 min hold time). The transfer line was heated at 280 °C. Two microliter of FAME sample was injected for analysis. Mass spectra were acquired in scan mode (70 eV); in the range of 50⿿550 m/z. The chromatogram is presented in Fig. 4.
Fig. 4.
GC⿿MS analyses of Lepidium sativum extract. Typical TIC-GC/MS chromatogram of L. sativum seed extract analyzed on GC system (Agilent 7890A series, USA) equipped with an apolar 5-MS (5% phenyl polymethyl siloxane) capillary column (Agilent 19,091S-43; 30 m ÿ 0.25 mm i.d. and 0.25 μm film thickness) attached with mass detector. After comparing with the spectra in NIST Library, It showed the presence of twelve compounds.
2.5.3. Identification of compounds
Interpretation of mass spectrum was conducted using the database of National Institute of Standards and Technology (NIST, USA). The database consists of more than 62,000 patterns of known compounds. The spectrum of the extract was matched with the spectrum of the known components stored in the NIST library.
3. Results
3.1. Hepatoprotective activity of LSS
3.1.1. Effect of LSS on parameters of liver markers enzymes
As shown in Fig. 1A⿿D, administration of CCl4 markedly increased the activity of liver enzymes AST, ALT, ALP and bilirubin as compared to the control group. Secretion of these enzymes was found significantly low (P 0.05) due to the treatment of LSS and silymarin as compared to the CCl4 group.
3.1.2. Effect of the plant extract on parameters of oxidative stress in the liver
The activity of MPO in various concentrations of LSS pretreated rats and silymarin was significantly decreased as compared to the group of rats treated with CCl4 only (Fig. 2A). In addition to this, the activity of SOD was also found to be significantly low when treated with CCl4. Furthermore, treatment of rats with various concentrations of LSS and silymarin (100 mg/kg b.w.) in combination with CCl4 significantly maintained the levels of antioxidant enzymes as stated above (Fig. 2B).
3.1.3. Histopathological study
The livers of the normal control rats showed normal histological architecture as shown in Fig. 3A. The liver sections of normal rats showed the presence of distinct hepatic cells with well-preserved cytoplasm, prominent nucleus, hepatocytes radially arranged around the central vein, and well-defined sinusoidal line. However, the section of rats treated with CCl4 showed necrosis, severe cytoplasmic vacuolation, microvesicular and macrovesicular fatty changes, loss of cellular boundaries, infiltration of inflammatory cells around the central vein and in the portal areas and congestion in the sinusoids (Fig. 3B). These observations were markedly ameliorated in the groups of rats which were pretreated with LSS or silymarin indicating a significant reduction in the number of degenerated hepatocytes and a significant decrease in necrosis (Fig. 3C⿿F). In the group of animals pretreated with 100 mg/kg of LSS followed by CCl4 administration, showed less nuclear degeneration as compared to toxic group. Inconspicuous fatty acid changes and infiltration of lymphocytes and spotty and focal necrosis were observed (Fig. 3C). Pretreatment with 200 mg/kg of LSS followed by CCl4 administration showed rescue in structural architects with moderate nuclear degeneration, some fatty layer changes and mild leucocytes infiltration around the centrilobular region (Fig. 3D). The group which was pretreated with 400 mg/kg LSS showed minimum fatty acid changes, mild nuclear degeneration and slight necrosis. Overall this group retained the original structure of hepatocytes (Fig. 3E). The group pretreated with 100 mg/kg silymarin was devoid of any structural alteration as compared to toxic group and retain the normal cellular architecture (Fig. 3F). The histopatholgical scoring of all the groups are shown in Fig. 3G. Scoring was performed on the scale of 1⿿100; considering 1 as minimum deformity and 100 as maximum deformities in hepatocytes in terms of fatty layer changes, necrosis, nuclear degeneration, lobular degeneration and inflammation of the cells. To sum-up the histological findings, our results showed that the LSS extract as well as silymarin remarkably decrease the signs of CCl4-induced toxicity.
3.2. Preliminary phytochemical screening
Preliminary phytochemical screening of LSS revealed the presence of alkaloids, carbohydrates, saponins, tannins, phenolics and flavonoids. Interestingly, the glycosides were found to be absent.
3.3. GC⿿MS analysis of LSS
LSS profile obtained on GC⿿MS showed 12 major fatty acids namely tetradecanoic acid (myristic acid), 9-hexadecenoic acid (palmitoleic acid), hexadecanoic acid (palmitic Acid), 9,12,15-octadecatrienoic acid (alpha-linolenic acid), octadecenoic acid (stearic acid), 9-octadecen-12-ynoic acid, cis-13-eicosenoic acid (paullinic acid), eicosanoic acid (arachidic acid), 13-docosenoic acid (erucic acid), docosanoic acid (behenic acid), 15-tetracosenoic acid (nervonic acid) and tetracosanoic acid (lignoceric acid) (Fig. 4). The retention time, chemical/formulae and chemical structures of all fatty acids found in LSS are depicted in Table 1.
Table 1.
Compounds present in ethanolic extract of Lepidium sativum seed.
| S. No. | RTa | Formula | RPb | IUPAC name |
Common name | LNc | Chemical structure |
|---|---|---|---|---|---|---|---|
| 1 | 12.54 | C14H28O2 | 0.1 | Tetradecanoic acid | Myristic acid | C14:0 | 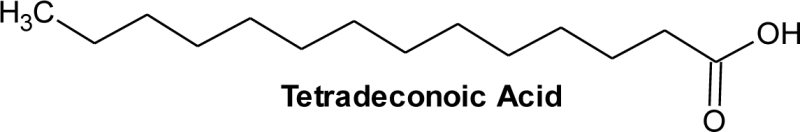 |
| 2 | 15.56 | C16H30O2 | 0.22 | 9-Hexadecenoic acid, | Palmitoleic acid | C16:1 | 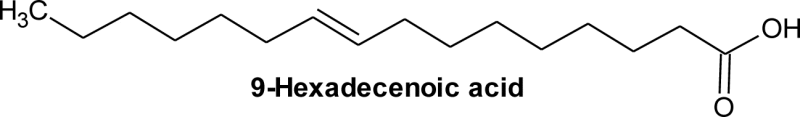 |
| 3 | 15.95 | C16H32O2 | 8.54 | Hexadecanoic acid, | Palmitic acid | C16:0 | 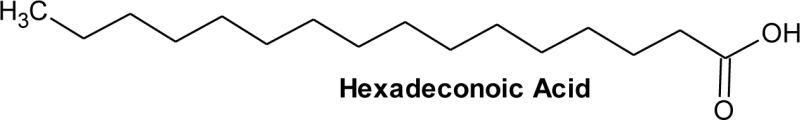 |
| 4 | 18.84 | C18H30O2 | 55.47 | 9,12,15-Octadecatrienoic acid, | Alpha-linolenic acid | C18:3 | 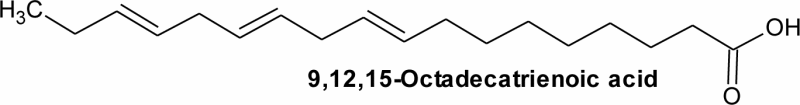 |
| 5 | 19.07 | C18H36O2 | 3.29 | Octadecenoic acid | Stearic acid | C18:0 | 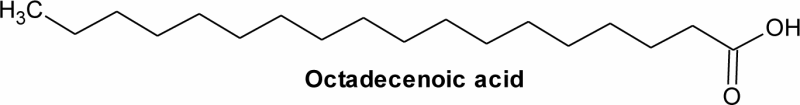 |
| 6 | 21.22 | C18H32O2 | 0.84 | 9-Octadecen-12-ynoic acid | NA | C18:2 | 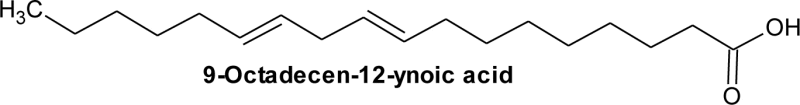 |
| 7 | 21.60 | C20H38O2 | 14.21 | Cis-13-eicosenoic acid | Paullinic acid (omega-7 fatty acid) |
20:1 | 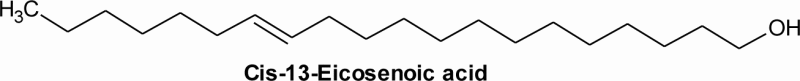 |
| 8 | 21.91 | C20H40O2 | 4.16 | Eicosanoic acid | Arachidic acid | 20:0 | 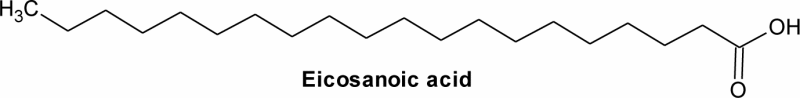 |
| 9 | 24.24 | C22H42O2 | 5.65 | 13-Docosenoic acid | Erucic acid (monounsaturated omega-9 fatty acid) | 22:1 | 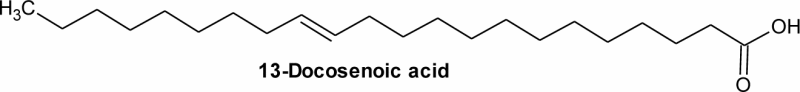 |
| 10 | 24.53 | C22H44O2 | 1.34 | Docosanoic acid | Behenic acid | 22:0 | 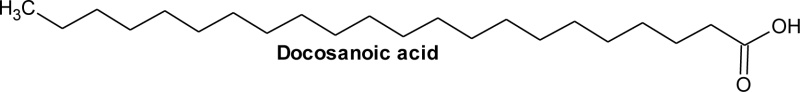 |
| 11 | 26.75 | C24H46O2 | 1.15 | 15-Tetracosenoic acid | Nervonic acid (monounsaturated omega-9 fatty acid) |
24:1 |  |
| 12 | 27.07 | C24H48O2 | 0.84 | Tetracosanoic acid | Lignoceric acid | 24:0 | 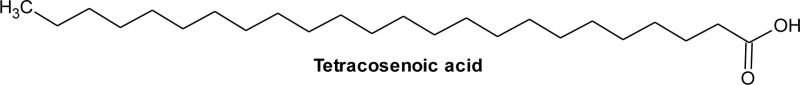 |
Retention time.
Relative percentage.
Lipid numbers.
4. Discussion
Liver disease is becoming an important public health concerns due to rising incidence of obesity in all age groups [20]. The disease effects millions of the people in the United Sates [21]. The conventional treatments of hepatic problems, such as cirrhosis, fatty liver and chronic hepatitis, are frequently inadequate due to adverse effects caused by hepatotoxic chemical drugs [22]. To overcome this problems, medicinal plants, due to their natural richness in hepatoprotective compounds, have been extensively studied to cure the liver ailments.
In the present study, hepatoprotective activity of ethanolic extract of L. sativum was investigated using hepatotoxic model induced by CCl4.
In biological system, CCl4 is activated by cytochrome P-450 dependent mixed oxidase in the endoplasmic reticulum to form trichloromethyl free radical (.CCl3 and .Cl3COO). This leads to changes in the endoplasmic reticulum and other membrane hence to increase in plasma membrane permeability to calcium ion resulting in a severe disturbances of calcium homeostasis and consequently necrotic cell death [23], [24]. Carbon tetrachloride causes multi organ disorder including the fatty layer degeneration of liver and centrilobular necrosis [25]. This phenomenon confirms that CCl4 is a potent hepatotoxin and can be used for the characterization of the liver disease curing medicines. The hepatotoxicity of CCl4 is further evidenced by elevation of liver damaging markers enzymes (AST, ALT, APT and bilirubin), increased MPO activity in tissues and histopathological anomalies. Two of the marker enzymes; namely AST and ALT which are present in the hepatocyte can easily leaks into the blood stream once the liver cells were damaged [24]. A high level of AST indicates the loss of functional integrity of the liver, which mimics the effects seen in viral hepatitis, cardiac infraction, and muscle injury. The ALT enzymes catalyzes the conversion of alanine to pyruvate and glutamate and is released from hepatocytes to blood in a similar manner as AST [26]. ALP catalyzes the hydrolysis of phosphate esters. Elevation of ALP indicates intrahepatic, extrahepatic biliary obstruction or infiltrative disease of the liver. Serum concentration of bilirubin is a marker of the liver⿿s ability to take up bilirubin from the plasma into the hepatocyte. Elevated level of serum bilirubin is duo its leakage from hepatocytes to plasma, usually because of hepatic obstruction to bile outflow and cholestasis [27].
In this study, we assessed the hepatoprotective role of L. sativum seed extract. The results suggested that pretreatment with LSS extract significantly restored the liver damaging marker enzymes, hence confirming its hepatoprotective potential. Our results are in accordance with the previously published work by Afaf et al. [12] where they reported the role of LSS as hepatoprotective agent. In our study we found the improved histological structure in extract treated group that also supports the hepatoprotective effect of the LLS.
Although the exact molecular mechanism of action of LSS is not fully understood, however, Lee et al. [28] have reported the role of LSS in preventing oxidative stress related liver damage. This noble finding could be attributed towards the presence of phenolic compound in the LSS extract. Similar to this finding our preliminary phytochemical screening studies also showed the presence of phenolic compound in the extract which we have used in this study. Our findings correlates with the previous finding [28] and can be linked to the same conclusion as the role of phenolic compounds which play significant role in oxidative stress related liver damage.
Liver inflammation also plays an important role in drug induced acute hepatitis [26]. In our study the histopathology of the LS pretreated group of rats showed less inflammation, well restored hepatocytes and less area of necrosis as compared to severe necrosis and inflammation in CCl4 treated group of animals. These findings suggest the possibility of the presence of anti-inflammatory compounds in LSS extract which could also be a contributing factor towards the hepatoprotective action of LSS.
Our findings in the GC/MS analysis showed the presence of lipid compounds including alpha linoleic acid which has anti-inflammatory activity [29], [30], [31], [32]. The role of alpha linoleic acid is to down regulate the expression of inflammatory cytokines such as IL-6, IL-1β and TNF-α [33]. We have reported a significant down trained in hepatic tissues inflammation in the animal group treated with LSS extract. Therefore, our current findings can also be well correlated with the previous published work where alpha linoleic acid showed anti- inflammatory effect [33].
5. Conclusion
In brief, it can be concluded that L. sativum seed extract exerts hepatoprotective activity that could be partly contributed by its antioxidant activity and presence of antioxidant and anti-inflammatory compounds like Alpha linoleic acid. However the mechanistic studies on underlying molecular signaling pathways, like effects of seed extract on inflammatory cytokines, redox sensitive transcription factors including nuclear factor-kappa B (NF-kappaB) and NF-E2 related factor 2 (Nrf2) and apoptotic pathways can be further studied to understand the changes at molecular level. A detail study needs to be done for isolation and characterization of individual bioactive compounds followed by assessment of its biological activity so that the constituents responsible and the mechanism involved for the efficacy of L. sativum seed as a hepatoprotective agent can be confirmed.
Disclosure
The authors have no conflict of interest.
Acknowledgment
The authors thank the Research Center, Prince Sultan Military Medical City (PSMMC) for providing all necessary facilities to conduct this study. Authors also thank Mr. Mohamed Ismail for assisting in extract preparation, and Mr. Kumar Pulamadan for biochemical analysis.
References
- 1.Al-Yahya M., Mossa J., Ageel A., Rafatullah S. Pharmacological and safety evaluation studies on Lepidium sativum L., seeds. Phytomedicine. 1994;1:155–159. doi: 10.1016/S0944-7113(11)80035-8. [DOI] [PubMed] [Google Scholar]
- 2.Ahsan S., Tariq M., Ageel M., Alyahya M., Shah A. Studies on some herbal drugs used in fracture healing. Pharm. Biol. 1989;27:235–239. [Google Scholar]
- 3.Al-Asmari A.K., Al-Elaiwi A.M., Athar M.T., Tariq M., Al Eid A., Al-Asmary S.M. A review of hepatoprotective plants used in saudi traditional medicine. J. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/890842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav Y.C., Srivastav D.N., Seth A.K., Saini V., Balaraman R., Ghelani T.K. In vivo antioxidant potential of Lepidium sativum seeds in albino rats using cisplatin induced nephrotoxicity. Int. J. Phytomed. 2010;2:292–298. [Google Scholar]
- 5.Maghrani M., Zeggwagh N.-A., Michel J.-B., Eddouks M. Antihypertensive effect of Lepidium sativum L. in spontaneously hypertensive rats. J. Ethnopharmacol. 2005;100:193–197. doi: 10.1016/j.jep.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 6.Patel U., Kulkarni M., Undale V., Bhosale A. Evaluation of diuretic activity of aqueous and methanol extracts of Lepidium sativum garden cress (Cruciferae) in rats. Trop. J. Pharm. Res. 2009;8 [Google Scholar]
- 7.Paranjape A.N., Mehta A.A. A study on clinical efficacy of Lepidium sativum seeds in treatment of bronchial asthma. Iran. J. Pharmacol. Ther. 2006;5:55–59. [Google Scholar]
- 8.Patole A., Agte V., Phadnis M. Effect of mucilaginous seeds on in vitro rate of starch hydrolysis and blood glucose levels of NIDDM subjects with special reference to garden cress seeds. J. Med. Aromat. Plant Sci. 1998;20:1005–1008. [Google Scholar]
- 9.Zia-Ul-Haq M., Ahmad S., Calani L., Mazzeo T., Del Rio D., Pellegrini N., De Feo V. Compositional study and antioxidant potential of Ipomoea hederacea Jacq. and Lepidium sativum L. seeds. Molecules. 2012;17:10306–10321. doi: 10.3390/molecules170910306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agarwal J., Verma D.L. Antioxidant activity-guided fractionation of aqueous extracts from Lepidium sativum and identification of active flavonol glycosides. Acad. Arena. 2011;3:14–18. [Google Scholar]
- 11.Raval N.D., Ravishankar B., Ashok B.K. Anti-inflammatory effect of Chandrashura (Lepidium sativum Linn.) an experimental study. AYU. 2013;34:302–304. doi: 10.4103/0974-8520.123132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afaf I., Abuelgasim N.H., Mohammed A. Hepatoprotective effect of Lepidium sativum against carbon tetrachloride induced damage in rats. Res. J. Anim. Vet. Sci. 2008;3:20–23. [Google Scholar]
- 13.Issabeagloo E. Hepatoprotective effects of Lepidium Sativum on doxorubicin mediated toxicity in rat. Magnt. Res. Rep. 2014;2:557–565. [Google Scholar]
- 14.Bradley P.P., Priebat D.A., Christensen R.D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982:206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- 15.Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 16.Trease G., Evans W. Brailliar Tridel Can. 11th ed. Macmillan publishers; 1989: 2015. Pharmacognsy. [Google Scholar]
- 17.Harborne J. JB Harborne; 1973. Phytochemical Methods a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 18.Edeoga H., Okwu D., Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. Afr. J. Biotechnol. 2005;4:685–688. [Google Scholar]
- 19.Metcalfe L., Schmitz A.A., Pelka J. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis. Anal. Chem. 1966;38:514–515. [Google Scholar]
- 20.Than N.N., Newsome P.N. A concise review of non-alcoholic fatty liver disease. Atherosclerosis. 2015;239:192–202. doi: 10.1016/j.atherosclerosis.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Anonymous, Liver Disease Overview, http://www.healthcommunities.com/liver-disease/liver-disease-overview.shtml, in: S.J. Swierzewski (Ed.), 2015.
- 22.Pereira C., Barros L., Ferreira I.C. Extraction, identification, fractionation and isolation of phenolic compounds in plants with hepatoprotective effects. J. Sci. Food Agric. 2015 doi: 10.1002/jsfa.7446. [DOI] [PubMed] [Google Scholar]
- 23.Weber L.W., Boll M., Stampfl A. Hepatotoxicity and mechanism of action of haloalkanes: carbon tetrachloride as a toxicological model. Crit. Rev. Toxicol. 2003;33:105–136. doi: 10.1080/713611034. [DOI] [PubMed] [Google Scholar]
- 24.Alqasoumi S.I., Abdel-Kader M.S. INTECH Open Access Publisher; 2012. Screening of Some Traditionally Used Plants for their Hepatoprotective Effect. [Google Scholar]
- 25.Recknagel R.O., Glende E.A., Dolak J.A., Waller R.L. Mechanisms of carbon tetrachloride toxicity. Pharmacol. Ther. 1989;43:139–154. doi: 10.1016/0163-7258(89)90050-8. [DOI] [PubMed] [Google Scholar]
- 26.Grespan R., Aguiar R.P., Giubilei F.N., Fuso R.R., Damião M.J., Silva E.L., Mikcha J.G., Hernandes L., Bersani Amado C., Cuman R.K.N. Hepatoprotective effect of pretreatment with Thymus vulgaris essential oil in experimental model of acetaminophen-induced injury. J. Evid. Based Complement. Altern. Med. 2014;2014 doi: 10.1155/2014/954136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Field K.M., Dow C., Michael M. Part I: liver function in oncology: biochemistry and beyond. Lancet Oncol. 2008;9:1092–1101. doi: 10.1016/S1470-2045(08)70279-1. [DOI] [PubMed] [Google Scholar]
- 28.Lee C.-C., Shen S.-R., Lai Y.-J., Wu S.-C. Rutin and quercetin, bioactive compounds from tartary buckwheat, prevent liver inflammatory injury. Food Funct. 2013;4:794–802. doi: 10.1039/c3fo30389f. [DOI] [PubMed] [Google Scholar]
- 29.Calder P.C. Polyunsaturated fatty acids and inflammation. Prostaglandins Leukot. Essent. Fatty Acids. 2006;75:197–202. doi: 10.1016/j.plefa.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 30.Rallidis L.S., Paschos G., Liakos G.K., Velissaridou A.H., Anastasiadis G., Zampelas A. Dietary α-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin-6 in dyslipidaemic patients. Atherosclerosis. 2003;167:237–242. doi: 10.1016/s0021-9150(02)00427-6. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Garcia E., Schulze M.B., Manson J.E., Meigs J.B., Albert C.M., Rifai N., Willett W.C., Hu F.B. Consumption of (n-3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J. Nutr. 2004;134:1806–1811. doi: 10.1093/jn/134.7.1806. [DOI] [PubMed] [Google Scholar]
- 32.Ferrucci L., Cherubini A., Bandinelli S., Bartali B., Corsi A., Lauretani F., Martin A., Andres-Lacueva C., Senin U., Guralnik J.M. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J. Clin. Endocrinol. Metab. 2006;91:439–446. doi: 10.1210/jc.2005-1303. [DOI] [PubMed] [Google Scholar]
- 33.Zhao T.D., Etherton K.R., Martin P.J., Gillies S.G. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am. J. Clin. Nutr. 2007;85:385–391. doi: 10.1093/ajcn/85.2.385. [DOI] [PubMed] [Google Scholar]