Abstract
Lead poisoning has been known as an important disorder that affects individuals through acute, sub-acute and chronic exposure in environmental and occupational settings. This study was conducted to compare the curative role of garlic combined with silymarin versus dimercaptosuccinic acid (DMSA) in decreasing lead induced nephrotoxicity in adult male albino rats. The period of lead intoxication extended for 3 months followed by either 1 month treatment with garlic and silymarin or 5 days treatment with DMSA. Lead poisoning caused non-significant difference in kidney function tests (BUN and serum creatinine) while, it caused significant elevation in kidney lead level, significant decrease in renal antioxidant enzyme glutathione peroxidase and significant elevation in kidney malondialdehyde. Histologically, lead induced disorganization and shrinkage of glomeruli with sloughing and vaculation of epithelium, widening of Bowman's space and inflammatory infiltration in renal medulla. Treatment by garlic extract combined with silymarin as well as treatment with DMSA resulted in significant improvement in the affected parameters. Also, both methods of treatment resulted in improvement of the histopathological changes. It can be concluded that garlic extract combined to silymarin is comparable to DMSA in amelioration of lead induced nephrotoxicity.
Chemical compounds studied in this article: Lead-acetate (PubChem CID: 19878658), DMSA (PubChem CID: 2724354), Garlic (PubChem CID: 6850761), Silymarin (PubChem CID: 1548994)
Keywords: Lead poisoning, Garlic, Silymarin, DMSA, Kidney
1. Introduction
Lead (Pb) toxicity is probably the most common form of heavy metal intoxication. It is well-documented as one of the most dangerous and insidious poisons [1]. Chronic lead poisoning is responsible for renal failure in experimental animals [2].
The currently approved treatment for lead poisoning is to administer chelating agents that form an insoluble complex with lead and remove it from lead burdened tissue [3]. Meso-2,3-dimercaptosuccinic acid (DMSA) is a sulfhydryl-containing, water-soluble, metal chelator. It is non-toxic and administered orally. It has been in use as an antidote to heavy metal toxicity since the 1950s. Recent clinical use and research establishes this compound as the premier metal chelation compound, based on oral dosing, urinary excretion, and its safety characteristics compared to other chelating substances [4].
A positive correlation has been established between dietary supplementation with certain vegetables and plants and the reduction of toxic effects of various toxicants and environmental agents, including heavy metals [5].
Garlic is one of the plants necessary in everyday life from the past until now. It contains active compounds that are responsible for its effect on almost every part of the human body. Garlic is considered an excellent tonic for the human. It has antioxidant, anti-thrombotic, hypo-cholesterolemic and anti-hypertensive properties [6]. The bulb of the plant has been used as a carminative, anti-septic, expectorant, anti-helmintic and diuretic [7]. Sulfur-containing amino acids like, cysteine in garlic have already been reported for their chemo-prophylactic use in lead poisoning [8].
Silymarin is a drug that has been used for many years in alternate and modern medicine for treating hepatic diseases. Its antioxidant, anti-inflammatory and anti-apoptotic effects make it an interesting herbal medicine, and these properties have implicated this compound as a potential reno-protective agent [9]. Because of the antioxidant activity and radical scavenging effect of silymarin, it has cyto-protection activities. The mechanisms of silymarin protection are most probably due to blockade and adjustment of cell transporters, P-glycoprotein, estrogenic and nuclear receptors [10].
The aim of this study was to compare the role of a combination of garlic extract and silymarin versus DMSA in decreasing lead induced nephropathy in adult albino rats.
2. Materials and methods
This study is a part of a research project funded by the Project Management of Zagazig University.
2.1. Chemicals
-
(1)
Lead-acetate [(C2H3O2)2Pb]: Lead acetate in the form of white crystals manufactured by El Nasr Pharmaceutical Chemical Company – Cairo – Egypt.
-
(2)
DMSA: obtained from Sigma–Aldrich Chemical Company – Cairo – Egypt.
-
(3)
Aqueous garlic extract was prepared at Department of Pharmacognosy, Faculty of Pharmacy – Zagazig University – Egypt.
-
(4)
Silymarin: extraction of silymarin from milk thistle plant was carried out at Department of Pharmacognosy, Faculty of Medicine – Zagazig University – Egypt.
2.2. Animals
Seventy adult male albino rats of average weight between 180 and 200 g were obtained from the animal house of Faculty of Medicine, Zagazig University. Before commencing experimentation, the animals were subjected to 10 days period of passive prelimination in order to adapt themselves to their new environment, to ascertain their physical wellbeing and to exclude diseased animals. Food was offered in equal amounts to all rats in each cage, water was offered in separate clean containers.
The present study was conducted in accordance with the international guidelines for animal research and after the approval of the Institutional Review Board on animal research at Faculty of Medicine – Zagazig University – Egypt (study number 2009/16-3-2014).
2.3. Study protocol
Rats were randomly divided into seven experimental groups:
-
Group I (negative control group):
10 rats received water and food to measure the basic parameters for 4 months.
-
Group II (positive control group):
10 rats, each rat received 1 ml saline orally per day 6 days/week for 4 months.
-
Group III (GS):
10 rats, each received 20 mg/kg/day of garlic in 1 ml saline [11] and silymarin 1000 mg/kg/day (1/10 LD50) in 1 ml saline [12] orally by gavage 6 days/week for 1 month.
-
Group IV (DMSA):
10 rats, each received DMSA in a dose of 30 mg/kg (therapeutic dose) dissolved in 1 ml saline once daily orally by gavage for 5 days [13].
-
Group V (Pb):
10 rats, each received lead acetate in a dose of 20 mg/kg/day orally in 1 ml saline 6 days/week for 3 months [14].
-
Group VI (Pb–GS):
10 rats, each received Pb acetate for 3 months followed by 20 mg/kg/day of garlic in 1 ml saline and 1000 mg/kg/day of silymarin in 1 ml saline for another 1 month.
-
Group VII (Pb–DMSA):
10 rats, each received Pb acetate for 3 months followed by 30 mg/kg/day DMSA dissolved in 1 ml saline for five days.
2.4. Methods
2.4.1. Plant material
The experimental plants (garlic and Silybum marianum) were collected from Al-Kasassin Experimental Farm, Horticulture Research Station, Ismailia Governorate and the soil was sandy and the plants were irrigated from AL-Abbasah canal which is a branch from Nile water.
2.4.2. Preparation of the plant extract
Garlic and silymarin extracts were freshly prepared weekly
-
•
Garlic: 200 mg of fresh peeled garlic cloves were weighted by sensitive balance then homogenized with 10 ml of distillated water to prepare a concentration of 20 mg/ml. The homogenate was centrifuged at 3000 × g for 10 min to remove particulate matter and the supernatant fraction was used for the experiment [11].
-
•
Silymarin: fine powder of seeds of milk thistle was made in a blender. Defatting the seed powder of Silybum marianum in n-hexane 2–3 times and extracted with aqueous acetone. The extract was concentrated to remove acetone by using a rotary evaporator, and then washed by hexane again to remove hydrophobic impurities. The remaining concentrate was treated with 1% NaCl solution to remove water soluble impurities. The precipitate and solid obtained through drying were combined together to form crude silymarin. The crude silymarin was washed with aqueous ethanol and then dried completely to give the refined silymarin [15].
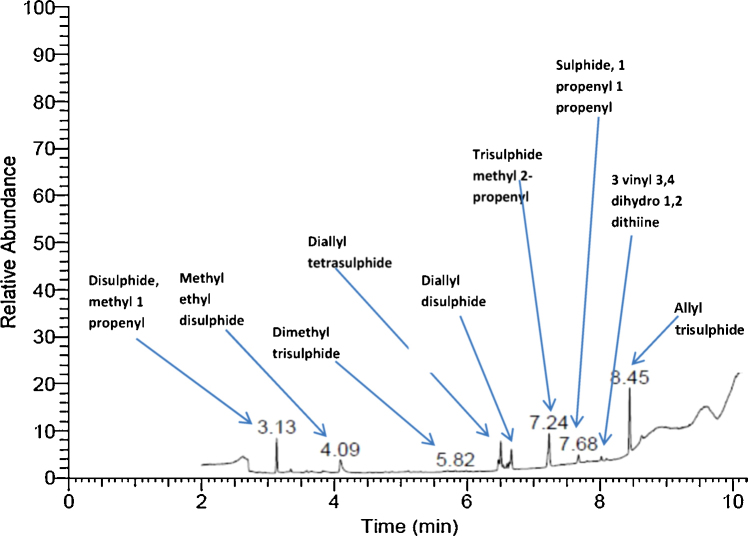
Then gas chromatography–mass spectrometry (GC–MS) analysis of the extracts was performed. The Chromatographic column used was TR. 35 MS column (30 m × 0.25 mm × 0.25 μm). Helium was used as carrier gas at a flow of 1.5 ml/min. Temperature programming: the column temperature was maintained at 50 °C for 1 min, and then raised to 280 °C with a rate of 20 °C/min. The mass spectrometer was operated in electron impact (EI) ionization mode with electron energy of 70 eV and temperature 280 °C. The transfer line temperature was 200 °C and the programmed temperature variance was splitless. Mass list range was 50–500 amu (Fig. 1).
Fig. 1.
GC–MS chromatograph of garlic extract.
2.4.3. Biochemical study
Twenty-four hours after the end of treatment in each group animals were sacrificed and venous blood samples were obtained from the retro-orbital plexus of anaesthetized animals, the blood was left for spontaneous coagulation and serum was separated for estimation of kidney function tests (blood urea nitrogen (BUN) and serum creatinine) using Biomerieux France kit by enzymatic colorimetic method [16].
2.4.4. Kidney examination
After sacrifice, both kidneys of each rat were divided into parts, one part weighed 500 mg was wrapped with aluminum foil and embedded in liquid nitrogen for 1 h then kept frozen in −80 °C till used to asses lead concentration in tissues by the graphite furnace atomic absorption spectrophotometry [17]. Another part was fixed in 10% formalin saline solution for histopathological examination after staining with haematoxylin and eosin according to Bancroft and Stevens [18].
Part of renal tissue was perfused in 0.9% NaCl containing 0.16 mg/ml heparin. Tissue was washed and minced in ice-cold 0.25 M sucrose, then homogenized, diluted and centrifuged at 4000 × g and 4 °C for 2 min. The supernatant was used to measure glutathione peroxidase (GPX) activity following Paglia and Valentine according to the pamphlet of Bio-diagnostic kits using calorimetric method [19].
Renal tissue was perfused with a phosphate buffered saline solution, pH 7.4 containing 0.16 mg/ml heparin to remove any red blood cells and clots, homogenized in 5–10 ml cold buffered per gram tissue then centrifuged at 4000 × g for 15 min. The supernatant is used for measuring malondialdehyde (MDA) using Bio-diagnostic kits of colorimetric method according to Satoh [20].
The collected data of kidney function tests, kidney lead level and renal antioxidant parameters were expressed as mean ± SD. The statistical analysis was done using ANOVA and student's t tests of Epi-info statistical package program version 6.04d, January 2001. Correlation coefficient was done by Excel-Office 2007.
3. Results
3.1. Death rate
Two rats died in group V (Pb), one rat in group VI (Pb–GS) and one rat in group VII (Pb–DMSA).
3.2. Biochemical results
As shown in Table 1, there were no significant differences in all of the measured parameters {the kidney function tests (BUN and serum creatinine), kidney lead level and renal antioxidant parameters (GPX & MDA)} among control groups I (negative control), II (positive control), III (GS) and IV (DMSA) so the values of group II (positive control) was used for comparison with other groups of the study (P > 0.05).
Table 1.
Statistical comparison between groups I (negative control), II (positive control), III (GS) and IV (DMSA) regarding kidney function tests (BUN, serum creatinine), kidney lead level and renal antioxidant parameters (GPX&MDA) by ANOVA test.
| Parameter | Group |
F | P | |||
|---|---|---|---|---|---|---|
| Negative control group (I) n = 10 |
Positive control group (II) n = 10 |
GS group (III) n = 10 |
DMSA group (IV) n = 10 |
|||
| Mean ± SD | ||||||
| BUN (mg/dl) | 27.2 ± 3.31 | 27.14 ± 3.28 | 26.79 ± 2.59 | 27.3 ±3.16 | 0.047 | 0.986# |
| Creatinine (mg/dl) | 0.93 ± 0.21 | 0.95 ± 0.19 | 0.94 ± 0.18 | 0.94 ± 0.20 | 0.025 | 0.995# |
| Kidney lead level (μg/g tissue) | 0.891 ± 0.041 | 0.887 ± 0.049 | 0.884 ± 0.050 | 0.856 ± 0.088 | 0.696 | 0.560# |
| Renal GPX (μ/g tissue) | 0.491 ± 0.053 | 0.464 ± 0.041 | 0.496 ± 0.049 | 0.461 ± 0.049 | 1.380 | 0.265# |
| Renal MDA (nmol/g tissue) | 0.484 ± 0.046 | 0.464 ± 0.041 | 0.481 ± 0.045 | 0.514 ± 0.049 | 2.042 | 0.125# |
# Non-significance (P > 0.05 – ANOVA).
SD: standard deviation.
n = number of rats in each group.
3.3. Kidney function tests (BUN, serum creatinine)
There was no significant difference in the kidney function tests (BUN and serum creatinine) among groups II (positive control), V (Pb), VI (Pb–GS) and VII (Pb–DMSA) by (ANOVA) study (Table 2).
Table 2.
Statistical comparison between group II (positive control), group V (Pb), group VI (Pb–GS) and group VII (Pb–DMSA) regarding kidney function tests (BUN, serum creatinine), kidney lead level and renal antioxidant parameters (GPX&MDA) by ANOVA test.
| Parameter | Group |
F | P | |||
|---|---|---|---|---|---|---|
| Positive control group (II) n = 10 |
Pb group (V) n = 8 |
Pb and GS (VI) n = 9 |
Pb and DMSA (VII) n = 9 |
|||
| Mean ± SD | ||||||
| BUN (mg/dl) | 27.14 ± 3.28 | 29.95 ± 3.12 | 30.08 ± 3.61 | 29.92 ± 3.46 | 1.780 | 0.168# |
| Creatinine (mg/dl) | 0.955 ± 0.193 | 0.941 ± 0.191 | 0.919 ± 0.180 | 0.956 ± 0.179 | 0.086 | 0.967# |
| Kidney lead level (μg/g tissue) | 0.887± 0.049 | 21.45 ± 3.857 | 8.869 ± 1.748 | 8.294 ± 2.133 | 129.412 | 0.000* |
| GPX (μ/g tissue) | 0.464 ± 0.041 | 0.190 ± 0.037 | 0.363 ± 0.074 | 0.401 ± 0.121 | 23.420 | 0.000* |
| MDA (nmol/g tissue) | 0.464 ± 0.041 | 2.011 ± 0.412 | 0.285 ± 0.056 | 0.240 ± 0.052 | 155.145 | 0.000* |
Non-significance (P > 0.05 – ANOVA).
SD: standard deviation.
n = number of rats in each group.
Significance (P < 0.05 – ANOVA).
3.4. Kidney lead level results
Table 2 shows a significant difference in kidney lead level among groups II (positive control), V (Pb), VI (Pb–GS) and VII (Pb–DMSA) by (ANOVA) study (P < 0.05).
The Least significant difference in Table 3 revealed that kidney lead level in group II (positive control group) is significantly lower than that of group V (Pb), VI (Pb–GS) and VII (Pb–DMSA), also there was a significant reduction in kidney lead level after treatment with both garlic and silymarin (group VI) and DMSA (group VII) while there was a non-significant difference between group VI (Pb–GS) and group VII (Pb–DMSA).
Table 3.
Least significant difference between group II (positive control), group V (Pb), VI (Pb–GS) and VII (Pb–DMSA) regarding kidney lead level.
| Kidney lead level (μg/g tissue) | Group |
|||
|---|---|---|---|---|
| Positive Control group (II) n = 10 |
Pb group (V) n = 8 |
Pb and GS group (VI) n = 9 |
Pb and DMSA (VII) n = 9 |
|
| Mean ± SD |
||||
| 0.887 ± 0.049 | 21.45 ± 3.857 | 8.869 ± 1.748 | 8.294 ± 2.133 | |
| Positive control group (II) | – | 0.000* | 0.000* | 0.000* |
| Pb group (V) | – | – | 0.000* | 0.000* |
| Pb & GS group (VI) | – | – | – | 0.591# |
Significance (P < 0.05 – t-test). n: number of rats in each group.
Non-significance (P > 0.05 – t-test). SD: standard deviation.
3.5. Renal anti-oxidant parameters
In Table 2, there was a significant difference in (GPX) and (MDA) among groups II (positive control), V (Pb), VI (Pb–GS) and VII (Pb–DMSA) by (ANOVA) study (P < 0.05).
The least significant difference for GPX revealed that there was a significant decrease in GPX in lead treated group (Pb) when compared to the positive control group while treatment with both garlic combined with silymarin (Pb–GS) and DMSA (Pb–DMSA) caused significant elevation in GPX when compared to lead treated group. There was non-significant difference when comparing DMSA treated group (Pb–DMSA) to positive control group and garlic and silymarin treated group (Pb–GS) while still there was a significant difference between (Pb–GS) and positive control group (Table 4).
Table 4.
Least significant difference between groups II (positive control), V (Pb), VI (Pb–GS) and VII (Pb–DMSA) regarding renal glutathione peroxidase (GPX).
| GPX (μ/gm tissue) | Group |
|||
|---|---|---|---|---|
| Positive control group (II) n = 10 |
Pb group (V) n = 8 |
Pb and GS group (VI) n = 9 |
Pb and DMSA group (VII) n = 9 |
|
| Mean ± SD |
||||
| (0.464 ± 0.041) | (0.190 ± 0.037) | (0.363 ± 0.074) | (0.401 ± 0.121) | |
| Positive control group (II) | – | 0.000* | 0.006* | 0.074# |
| Pb group (V) | – | – | 0.000* | 0.000* |
| Pb and GS (VI) | – | – | – | 0.275# |
Significance (P < 0.05 – t-test). n = number of rats in each group.
SD: standard deviation.
Non-significance (P > 0.05 – ANOVA).
Table 5 showed that the least significant difference for MDA revealed a significant elevation in lead group (Pb) when compared to the positive control group. Treatment with combined garlic and silymarin (Pb–GS) and DMSA (Pb–DMSA) caused significant decrease in MDA when compared to lead treated group to reach a non-significant change when compared to the positive control group. There was a non-significant difference when comparing (Pb–GS) and (Pb–DMSA).
Table 5.
Least significant difference between groups II (positive control), V (Pb), VI (Pb–GS) and VII (Pb–DMSA) regarding renal malondialdehyde (MDA).
| MDA (nmol/g tissue) | Group |
|||
|---|---|---|---|---|
| Positive control group (II) n = 10 |
Pb group (V) n = 8 |
Pb and GS group (VI) n = 9 |
Pb and DMSA group (VII) n = 9 |
|
| Mean ± SD |
||||
| 0.464 ± 0.041 | 2.011 ± 0.412 | 0.285 ± 0.056 | 0.240 ± 0.052 | |
| Positive control group (II) | – | 0.000* | 0.069# | 0.029# |
| Pb group (V) | – | – | 0.000* | 0.000* |
| Pb and GS (VI) | – | – | – | 0.649# |
Significance (P < 0.05 – t-test). SD: standard deviation.
Non-significance (P > 0.05 – ANOVA). n = number of rats in each group.
There was a strong negative correlation between kidney lead level and GPX and a strong positive correlation between kidney lead level and MDA (Table 6).
Table 6.
Correlation co-efficient between kidney lead level and oxidative stress parameters (glutathione peroxidase (GPX) and malondialdehyde (MDA) using Pearson correlation.
Strong correlation.
3.6. Histo-pathological observations
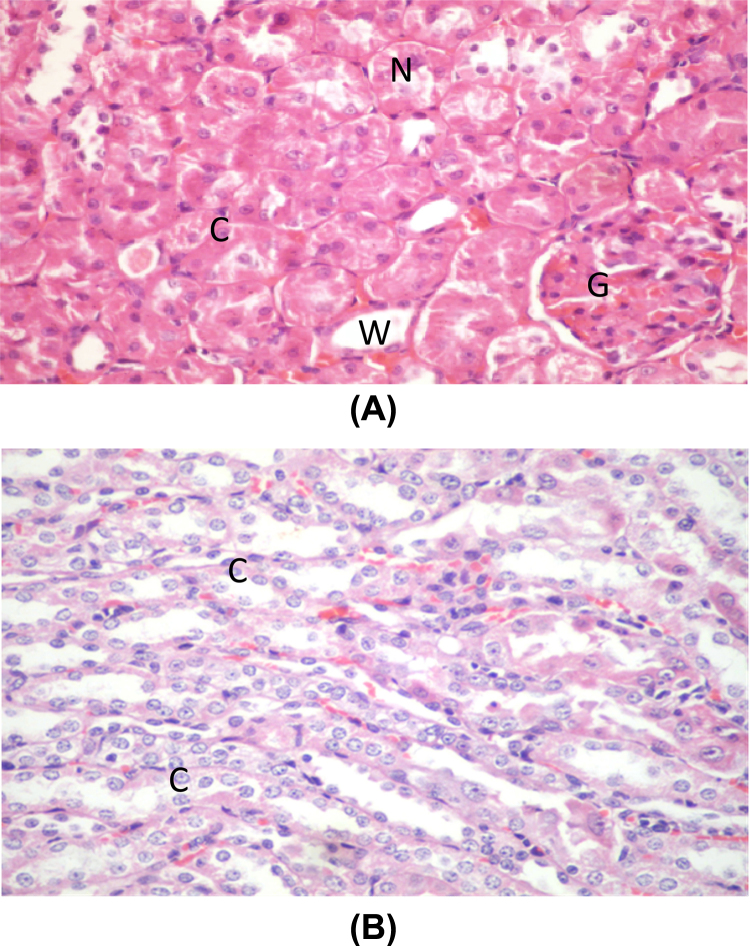
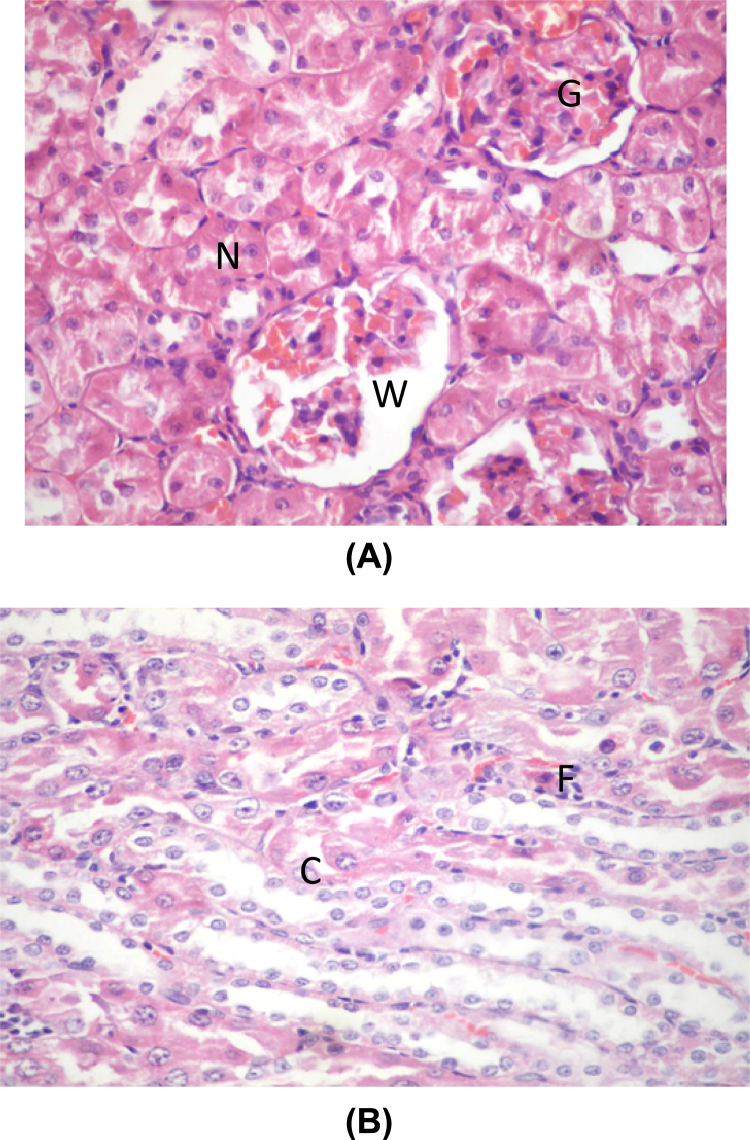
Group I (negative control), II (positive control group), III (GS) and IV (DMSA) showed the normal kidney structure. The cortex showed normal renal corpuscles formed of a tuft of capillaries (glomerulus) surrounded by narrow Bowman's space, renal convoluted tubules are lined by cuboidal epithelium. The proximal tubules have narrow Lumina while distal tubules show wide Lumina (Fig. 2A and B).
Fig. 2.
(A) A photomicrograph of a section in a control rat's kidney showing normal glomerulus surrounded by narrow Bowman's space (G), renal convoluted tubules lined with cuboidal epithelium (C). The proximal tubules have narrow lumina (N) while distal tubules show wide lumina (W) (H&E ×400). (B) A photomicrograph of a section in a control rat's kidney showing normal tubules lined with simple cuboidal epithelium (C) (H&E ×400).
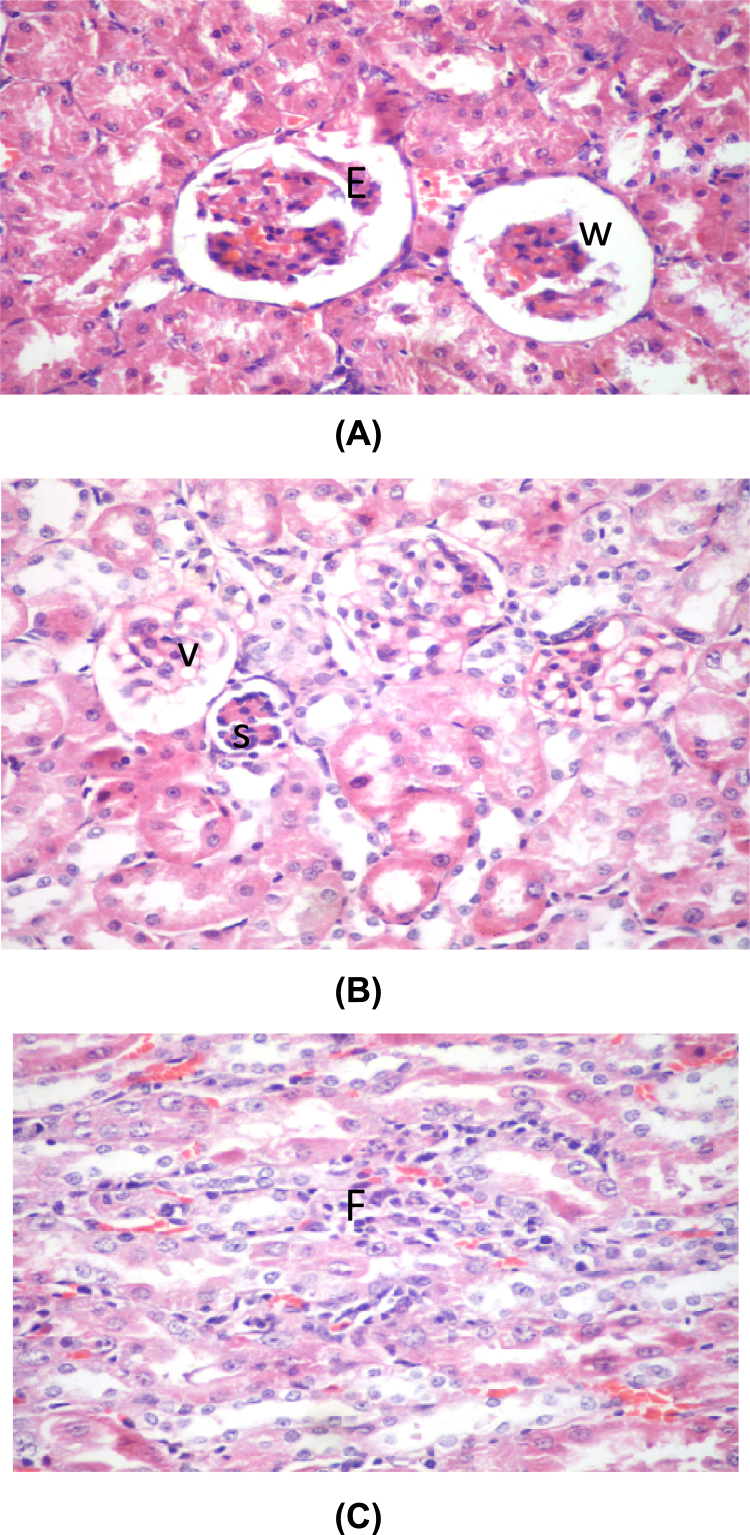
Lead treated group (V) showed disorganization of the glomeruli with sloughing of the epithelium and a widening of Bowman's space (Fig. 3A). The glomerular epithelium showed vaculation and some glomeruli showed shrinkage (Fig. 3B). There was inflammatory infiltration in the renal medulla (Fig. 3C).
Fig. 3.
(A) A photomicrograph of a section in rat's kidney of the lead treated group (V) showing disorganization with sloughing of the epithelium of the glomeruli (E) and widening of Bowman's spaces (W) (H&E ×400). (B) A photomicrograph of a section in the renal cortex of a lead treated rat (group V) showing vaculation of the glomerular epithelium (V) with shrinked glomerulus (S) (H&E ×400). (C) A photomicrograph of a section in the renal cortex of a lead treated rat (group V) showing inflammatory infiltration (F) (H&E ×400).
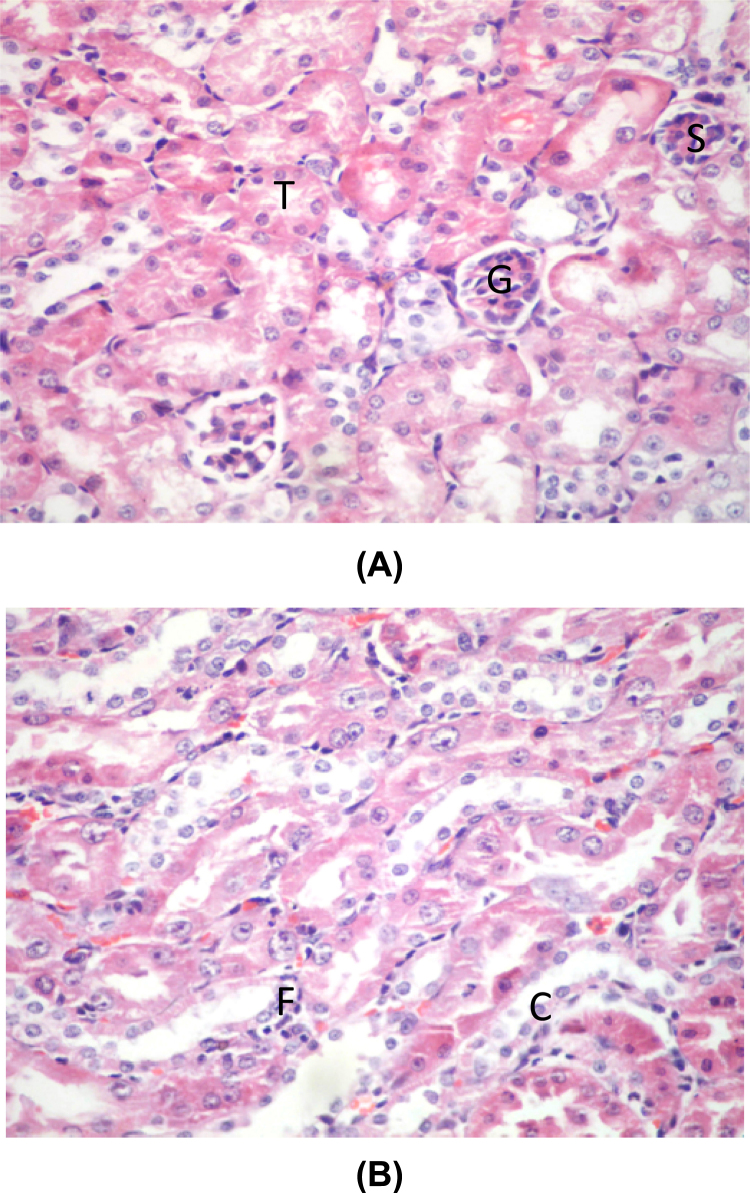
Treatment with garlic and silymarin in group (VI) resulted in improvement in the histopathological changes and recovery of some glomeruli (Fig. 4A) and decrease in the inflammatory infiltration in the medulla (Fig. 4B).
Fig. 4.
(A) A photomicrograph of a section in the renal cortex of lead, garlic and silymarin treated group (VI) showing shrinkage of one glomerulus with disappearance of Bowman's space (S) with appearance of normal glomeruli (G) and tubules (T) (H&E ×400). (B) A photomicrograph of a section in the renal cortex of lead, garlic and silymarin treated group (VI) showing normal tubules lined by simple cuboidal epithelium (C) with slight inflammatory infiltration (F) (H&E ×400).
Also treatment with DMSA in group (VII) has resulted in improvement in the histopathological changes. There was slight congestion and widening of Bowman's space (Fig. 5A). The tubules were normally lined by cuboidal epithelium with minimal inflammatory infiltration (Fig. 5B).
Fig. 5.
(A) A photomicrograph of a section in the renal cortex of lead and DMSA treated group (VII) showing normal glomerulus (G) with slight congestion and widening of Bowman's capsule of another glomerulus (W) and normal tubules (T) (H&E ×400). (B) A photomicrograph of a section in the renal medulla of Lead & DMSA treated group (VII) showing normal tubules with normal simple cuboidal epithelium (C) with minimal inflammatory infiltration (F) (H&E ×400).
4. Discussion
Lead is a common environmental pollutant known to induce chronic renal disease as urinary elimination is the main route of lead excretion [21].
In the present study lead acetate administered to rats in a dose of 20 mg/kg/day for 3 months produced a significant elevation of kidney lead level, a significant decrease in GPX and significant increase in MDA when compared to the positive control group. The absorbed Pb is conjugated in the liver and passed to the kidney, where a small quantity is excreted in urine and the rest accumulates in various body organs and affects many biological activities at the molecular, cellular and intracellular levels and this explains the cause of significant elevation of kidney lead level in lead treated rats [22].
The decreased activity of GPX due to lead exposure was reported in both human [23], and animal studies [24]; since lead exposure is a well-known stimulant of ROS production and it induces a reduction in free radical scavenging enzymes and glutathione [25]. Also, Khan et al. found that lead exposed workers showed a significantly increased serum MDA levels over control which indicated an involvement of oxidative stress in the pathogenesis of lead toxicity [26]. In addition, the current work proved a strong correlation between kidney lead level and anti-oxidant parameters (GPX and MDA) which was reported previously by Ergurhan-Ilhan et al. [27].
The kidney is particularly susceptible to the lead-induced oxidative stress and inflammation due to high concentration and the relatively long residence time of lead in the renal tubular epithelial cells [28]. In the current study, the lead-treated group of animals (V) showed disorganization of the glomeruli with sloughing of the epithelium and widening of Bowman's space. The glomerular epithelium showed vaculation and some glomeruli showed shrinkage. There was inflammatory infiltration in the renal medulla. These data are in accordance with Deveci et al. who reported that chronic lead toxicity induced varying degree of damaged glomeruli and degenerated proximal tubules confirming that lead causes tubulo-interstitial nephritis [29]. The congestion, inflammatory infiltration and atrophied glomeruli in lead acetate treated rats were also reported by Sujatha et al. who explained these histopathological changes by the binding of lead to sulfhydryl groups of essential enzymes of cellular metabolism [30].
Despite the reported effects of lead exposure on kidney lead level, anti-oxidant parameters and histopathology of the kidney, there was no significant effect on the kidney function tests in accordance with Missoun et al. [31]. Meanwhile, Karamala et al. reported significant increase in serum creatinine in a dose dependent manner due to higher dose used resulting in severe renal parenchymal damage with impaired glomerular filtration [32].
In the present study, treatment of lead intoxicated rats with garlic combined to silymarin resulted in a significant decrease in kidney lead level, partial recovery in GPX level and complete improvement in MDA level. Recovery of some glomeruli and decrease in the congestion and inflammatory infiltration in the medulla was also detected. Senapati et al. reported that the efficiency of garlic in reducing kidney lead level was perhaps due to the presence of sulfur-containing compounds. These compounds might have chelated Pb and enhanced its excretion from the body, resulting in reduced Pb accumulation in tissues and blood. In the current study, sulfur containing compounds like allyl trisulphide, trisulphide methyl 2-propenyl, diallyl di and tetrasulphide and disulphide methyl 1 propenylwere actually detected by GC-MS analysis of garlic extract [33]. Shakiba et al. reported that prevention of Pb toxicity by garlic organo-sulfur compounds can be explained by their ability in scavenging of free radical and prevention of GSH depletion [34].
Allicin is a major component of garlic organo-sulfur and its antioxidant properties has been confirmed by many investigations [35]. However, in the current work, the allicin was not detected by GC–MS analysis because allicin itself is an unstable compound and instantly undergoes reactions to form other sulfur derivatives. The presence of disulfides and trisulfides confirms the degradation of allicin [36]. In addition to allicin; other garlic organosulfur compounds, like allyl-triisulfide, also possess antioxidant properties and can neutralize several types of ROS [37].
Silymarin can result in elevation of glutathione levels through the maintenance of GSH homeostasis in the body [11]. The sulfhydryl group of glutathione can bind lead [38].
In this work, treatment with DMSA in lead intoxicated rats resulted in a significant reduction in kidney lead level, reversal of both GPX and MDA to the control levels and improvement in the histopathological changes in the form of recovery of some glomeruli and decrease in the congestion and inflammatory infiltration in the medulla. DMSA is a dithiol (containing two sulfhydryl, or S–H, groups) lipid-soluble compound, used for metal chelation. One of the sulfhydryls in DMSA binds to a cysteine residue on albumin, leaving the other S–H available to chelate metals [39]. DMSA for being an antioxidant and a strong lead chelator has been shown to deplete significantly lead from tissues including kidney [13], and recovery in the oxidative stress [40]. Khalil-Manesh et al. reported that DMSA can improve the renal function and to a lesser degree the pathological alterations induced by lead toxicity. DMSA can improve renal function irrespective of the degree of pathological alterations concluding that the DMSA effect is most likely mediated by hemodynamic changes [41]. Fortunately, in the current study the effect of garlic combined to silymarin in improvement of lead induced nephropathy was comparable to the effect of DMSA, as evidenced by the non-significant difference between them in all the measured parameters of the study.
5. Conclusion
It can be concluded that combined garlic extract and silymarin as well as DMSA can decrease kidney lead level. Also, garlic and silymarin were effective in recovering the level of antioxidant enzymes. But DMSA was more effective in elevating GPX level. Lead causes various histopathological lesions that could be improved by both garlic combined with silymarin and DMSA.
Contributor Information
Iman A. El-Khishin, Email: imanrdy@yahoo.com.
Yara Mohamed Medhat El-fakharany, Email: dryaratox2012@gmail.com.
Omaima I. Abdel Hamid, Email: dr_omaima2006@yahoo.com.
References
- 1.Ashry K.M., El-Sayed Y.S., Khamiss R.M. Oxidative stress and immunotoxic effects of lead and their amelioration with myrrh (Commiphoramolmol) emulsion. Food Chem. Toxicol. 2010;48(1):236–241. doi: 10.1016/j.fct.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed Y.F., Eldebaky H.A.A., Karima G.M. Effects of lead exposure on DNA damage and apoptosis in reproductive and vital organs in female rabbits. Glob. Vet. 2012;9(4):401–408. [Google Scholar]
- 3.Flora S., Kannan G.M., Pant B.P. Combined administration of oxalic acid, succimer and its analogue for the reversal of gallium arsenide-induced oxidative stress in rats. Arch. Toxicol. 2002;76:269–276. doi: 10.1007/s00204-002-0347-5. [DOI] [PubMed] [Google Scholar]
- 4.Alan L., Miller N.D. Dimercaptosuccinic acid (DMSA), a non-toxic, water soluble treatment for heavy metal toxicity. Altern. Med. Rev. 1998;3(3):199–207. [PubMed] [Google Scholar]
- 5.Sharma V., Sharma A., Kansal L. The effect of oral administration of Allium sativum extracts on lead nitrate induced toxicity in male mice. Food Chem. Toxicol. 2010;48(3):928–936. doi: 10.1016/j.fct.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Petrovska B.B., Cekovska S. Extracts from the history and medical properties of garlic. Phcog. Rev. 2010;4(7):106–110. doi: 10.4103/0973-7847.65321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikail H.G. Phytochemical screening, elemental analysis and acute toxicity of aqueous extract of Allium sativum L. bulbs in experimental rabbits. J. Med. Plants Res. 2010;4(4):322–326. [Google Scholar]
- 8.Badiei K., Pourjaafar M., Nowrooziasl A. Effect of dried garlic powder (Allium sativum) on lead content of different tissue following subclinical lead poisoning in goats. Iran. J. Vet. Res. 2005;6(1):12–16. [Google Scholar]
- 9.Dashti-Khavidaki S., Shahbazi F., Khalili H. Potential reno-protective effects of silymarin against nephrotoxic drugs: a review of literature. J. Pharm. Pharm. Sci. 2012;15(1):112–123. doi: 10.18433/j3f88s. [DOI] [PubMed] [Google Scholar]
- 10.Karimi G.H., Vahabzadeh M., Lari P. Silymarin, a promising pharmacological agent for treatment of diseases. Iran. J. Basic Med. Sci. 2011;14(4):308–317. [PMC free article] [PubMed] [Google Scholar]
- 11.Shaarawy S.M., Tohamy A.A., Elgendy S.M. Protective effects of garlic and silymarin on NDEA-induced rat's hepatotoxicity. Int. J. Biol. Sci. 2009;5(6):549–557. doi: 10.7150/ijbs.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraschini F., Dermartini G., Esposti D. Pharmacology of silymarin. Clin. Drug Investig. 2002;22:51–65. [Google Scholar]
- 13.Bradberry S., Vale A. Dimercaptosuccinic acid (succimer; DMSA) in inorganic lead poisoning. Clin. Toxicol. (Phila) 2009;47(7):617–631. doi: 10.1080/15563650903174828. [DOI] [PubMed] [Google Scholar]
- 14.Institóris L., Siroki O., Dési I. Immunotoxicological examination of repeated dose combined exposure by dimethoate and two heavy metals in rats. Hum. Exp. Toxicol. 1999;18(2):88–94. doi: 10.1177/096032719901800205. [DOI] [PubMed] [Google Scholar]
- 15.Polyak S.J., Morishima C., Shuhart M.C. Inhibition of T-cell inflammatory cytokines, hepatocyte NF-kappa B signaling, and HCV infection by standardized silymarin. Gastroenterology. 2007;132:1925–1936. doi: 10.1053/j.gastro.2007.02.038. [DOI] [PubMed] [Google Scholar]
- 16.Bjurosson T.D. Use of serum creatinine concentration to determine renal function. Clin. Pharmacol. 1979;28:477–478. [Google Scholar]
- 17.Miller D.T., Paschal D.C., Gunter E.W. Determination of lead in blood using electro-thermal atomization atomic absorption spectrometry with a L’vov platform and matrix modifier. Analyst. 1987;112(12):1701–1704. doi: 10.1039/an9871201701. [DOI] [PubMed] [Google Scholar]
- 18.Bancroft G., Stevens A. In: Theory and Practice of Histopathological Technique. 4th ed. Edinburgh. Stewart J., Edward B., editors. Churchill Livingstone; London/Melbourne/New York: 1996. pp. 99–112. [Google Scholar]
- 19.Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocytes glutathione peroxidase. J. Lab. Clin. Med. 1967;70(1):158–169. [PubMed] [Google Scholar]
- 20.Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 1978;90:37–43. doi: 10.1016/0009-8981(78)90081-5. [DOI] [PubMed] [Google Scholar]
- 21.Madden E.F., Fowler B.A. Mechanisms of nephrotoxicity from metal combinations: a review. Drug Chem. Toxicol. 2000;23(1):1–12. doi: 10.1081/dct-100100098. [DOI] [PubMed] [Google Scholar]
- 22.Flora S., Flora G., Saxena G. Environmental occurrence, health effects and management of lead poisoning. In: Casas J.S., Sordo J., editors. Lead: Chemistry, Analytical Aspects, Environmental Impact and Health Effects. Elsevier Science; Amsterdam, Netherlands: 2006. pp. 158–228. [Google Scholar]
- 23.Hunaiti A.A., Soud M. Effect of lead concentration on the level of glutathione, glutathione S-transferase, reductase and peroxidase in human blood. Sci. Total Environ. 2000;248:45–50. doi: 10.1016/s0048-9697(99)00548-3. [DOI] [PubMed] [Google Scholar]
- 24.Sivaprasad R., Nagaraj M., Varalakshmi P. Combined efficacies of lipoic acid and 2,3-dimercaptosuccinic acid against lead-induced lipid peroxidation in rat liver. J. Nutr. Biochem. 2004;15(1):18–23. doi: 10.1016/j.jnutbio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Ercal N., Gurer-Orhan H., Aykin-Burns. N. Toxic metals and oxidative stress. Part 1. Mechanisms involved in metal-induced oxidative damage. Curr. Top. Med. Chem. 2001;1:529–539. doi: 10.2174/1568026013394831. [DOI] [PubMed] [Google Scholar]
- 26.Khan D.A., Qayyum1 S., Saleem S. Lead induced oxidative stress adversely affects health of the occupational workers. Toxicol. Indust. Health. 2008;24:611–618. doi: 10.1177/0748233708098127. [DOI] [PubMed] [Google Scholar]
- 27.Ergurhan-Ilhan I., Cadir B., Koyuncu-Arslan M. Level of oxidative stress and damage in erythrocytes in apprentices indirectly exposed to lead. Pediatr. Int. 2008;50(1):45–50. doi: 10.1111/j.1442-200X.2007.02442.x. [DOI] [PubMed] [Google Scholar]
- 28.Bravo Y., Quiroz Y., Ferrebuz A. Mycophenolatemofetil administration reduces renal inflammation, oxidative stress, and arterial pressure in rats with lead-induced hypertension. Am. J. Physiol. Renal Physiol. 2007;293(2):616–623. doi: 10.1152/ajprenal.00507.2006. [DOI] [PubMed] [Google Scholar]
- 29.Deveci E., Söker S., Baran Ö. Ultrastructural changes in the kidney cortex of rats treated with lead acetate. Int. J. Morphol. 2011;29(3):1058–1061. [Google Scholar]
- 30.Sujatha K., Srilatha C., Anjaneyulu Y. Ameliorative efficacy of Ocimum sanctum leaf extract in lead acetate induced toxicity in Wistar albino rats: a pathomorphological study. Indian J. Vet. Pathol. 2011;35(2):147–152. [Google Scholar]
- 31.Missoun F., Slimani M., Aoues A. Toxic effect of lead on kidney function in rat Wistar. Afr. J. Biochem. Res. 2010;4(2):21–27. [Google Scholar]
- 32.Karamala S.K., Seri latha C.H., Anjaneyulu Y. Hematobiochemical changes of lead poisoning and amelioration with Ocimum sanctum in wistar albino rats. Vet. World. 2011;4(6):260–263. [Google Scholar]
- 33.Senapati S.K., Dey S., Dwivedi S.K. Effect of garlic (Allium sativum L.) extract on tissue lead level in rats. J. Ethnopharmacol. 2001;76(3):229–232. doi: 10.1016/s0378-8741(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 34.Shakiba Y., Mostafaie A., Arshadi D. Application of garlic organo-sulfur compounds in prevention of cyclosporine A-induced hepatotoxicity. Iran. J. Med. Hypo. Ideas. 2009;3(3):78. [Google Scholar]
- 35.Leelarungrayub N., Rattanapanone V., Chanarat N. Quantitative evaluation of the antioxidant properties of garlic and shallot preparations. J. Nutr. 2006;22:266–274. doi: 10.1016/j.nut.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Rainy G., Amita S., Preeti M. Study of chemical composition of garlic oil and comparative analysis of Co-trimoxazole in response to in vitro antibacterial activity. Int. Res. J. Pharm. 2014;5(2):97–101. [Google Scholar]
- 37.Chung L.Y. The antioxidant properties of garlic compounds: allyl cysteine, allicin, and allyl disulfide. J. Med. Food. 2006;9(2):205–213. doi: 10.1089/jmf.2006.9.205. [DOI] [PubMed] [Google Scholar]
- 38.Fuhr B.J., Rabenstein D.L. Nuclear magnetic resonance studies of the solution chemistry of metal complexes. IX. Binding of cadmium, zinc, lead, and mercury by glutathione. J. Am. Chem. Soc. 1973;95(21):6944–6950. doi: 10.1021/ja00802a013. [DOI] [PubMed] [Google Scholar]
- 39.Aposhian H.V., Maiorino R.M., Rivera M. Human studies with the chelating agents, DMPS and DMSA. J. Toxicol. Clin. Toxicol. 1992;30:505–528. doi: 10.3109/15563659209017938. [DOI] [PubMed] [Google Scholar]
- 40.Flora S., Mittal M., Mehta A. Heavy metal induced oxidative stress & it's possible reversal by chelation therapy. Indian J. Med. Res. 2008;128:501–523. [PubMed] [Google Scholar]
- 41.Khalil-Manesh F., Gonick H.C., Cohen A. Experimental model of lead nephropathy. II. Effect of removal from lead exposure and chelation treatment with dimercaptosuccinic acid (DMSA) Environ. Res. 1992;58(1):35–54. doi: 10.1016/s0013-9351(05)80203-8. [DOI] [PubMed] [Google Scholar]