Abstract
There is increasing evidence that metals have a role in the etiology of diverse neurological diseases. This study used PC12 cells as an in vitro model to examine the toxicity of tungsten alloys that have important military applications. Initially, the relative concentrations of tungsten (W), nickel (Ni), and cobalt (Co) mobilized from pellets of a weapons-grade tungsten alloy incubated in physiologically relevant solutions were determined. Dosing solutions of soluble metal salts that were equivalent in ratio to those mobilized from these alloy pellets were used to treat nerve growth factor (NGF) differentiated PC12 cells. Treatments consisted of single (W, Ni or Co), paired (W/Ni, W/Co or Ni/Co) or complete (W/Ni/Co) metal exposures for 24 h followed by measurement of cytotoxicity, viability, and microarray analysis to examine their impact on survival and viability, global gene expression, and biological processes. Gene expression changed dramatically with addition of NGF. Addition of Ni or Co either singly or in combination further impacted gene expression. An observed additive effect of Ni and Co on gene expression was unaffected by the addition of W. The work showed that tungsten, as found in this tungsten alloy, had minimal relative toxicity as compared to the other alloy components when used either alone or in combination.
Keywords: Tungsten, Metal alloy, Tungstate, Microarray, Metal mixture, Cobalt
1. Introduction
Tungsten (W) has numerous consumer, industrial, and military applications; its high melting point, density, and hardness making it useful in products ranging from tungsten carbide (machinery and tools) to alloys of tungsten used in armor piercing weaponry [14]. Naturally occurring tungsten is most often found as tungsten oxide in soil or as tungstate (WO42⿿) in water. In addition, WO42⿿ in water aggregates into polymeric forms under acidic conditions, with the formation of paratungstate (HW6O215⿿) at pH 6 [6], making the underlying chemistry of tungsten an important variable in exposure studies.
The Department of Defense has an interest in the potential toxicity of tungsten alloys relating to Soldier exposure during combat [4] as well as occupational exposure during manufacture. In a chronic rodent study, a tungsten alloy pellet formulation containing nickel and cobalt (WNiCo) caused a 100% incidence of sarcoma when implanted into the leg muscles of Fischer rats [11]. Further work showed that tungsten alloy containing iron (WNiFe) as a replacement for cobalt [19] was not carcinogenic in the same animal model. In rat leg muscle, WNiFe pellets were resistant to corrosion and did not cause tumors; by contrast, WNiCo was extensively corroded, with aggressively metastasizing tumors seen within six months [17], [19]. In these studies, the impact of high serum levels of W, Ni, and Co [13] on the brain was not assessed. However, it has recently been hypothesized that translocation of metals to the brain could potentially exacerbate pre-existing traumatic brain injury [12]. In addition, metals have been proposed to contribute to chronic neurodegenerative disease [3], [15]. Tungsten has been detected in rat brain tissue following intravenous or intraperitoneal injection [1], [26] indicating systemic delivery to the brain is possible under certain conditions. However, the toxicity of tungsten alloys or their component metals to neuronal tissue or in vitro neuronal cell culture models has not been assessed. Exposure of PC12 cells to NiCl2, chlorpyrifos, diazinon, and dieldrin resulted in changes to the expression profile; however, NiCl2 induced changes were separable from other tested substances [21]. As there are considerable data on PC12 cell responses to CoCl2 and NiCl2, the in vitro PC12 cell-line model was selected to explore the toxicology and transcriptional profile of cells exposed to tungsten alloy surrogates containing salts of W, Ni and Co. When induced with nerve growth factor (NGF) PC12 cells differentiate, form neurite projections, and are phenotypically similar to acetylcholinesterase and catecholamine neurons [7], [25].
In this work, the effect of single tungsten alloy metals W, Ni and Co, the complete mixture (W⿿Ni⿿Co) and metal combinations (W⿿Ni, W⿿Co, and Ni⿿Co) on NGF stimulated PC12 cell gene expression was investigated. All test conditions were performed at physiological pH and in buffered media with soluble salts of W (Na2WO4), Ni (Ni(CH3CO2)2) and Co (Co(CH3CO2)2). The concentrations of soluble metal ions released from tungsten alloy pellets incubated in media were used to set the doses for the for exposure studies. By using metal concentrations that leached from the alloy pellets, a realistic tissue level exposure scenario was created, resulting in better estimates of toxicity. Prior to microarray analysis, pilot dose-ranging experiments identified the maximum tolerated concentration of the soluble metal salts. Metal concentrations were maintained at sufficiently low levels to avoid overt cytotoxicity. The results indicated little or no measureable toxicity was associated with acute exposure to tungstate, while nickel and cobalt had varying degrees of toxicity.
2. Materials and methods
2.1. Reagents/chemicals
Metal salts were purchased from Sigma, (St. Louis, MO, USA): 10ÿ PBS (D1408), sodium tungstate (379751), sodium acetate (59929), nickel acetate (379883), and cobalt acetate (399973). Test kits were purchased from Promega (Madison, WI, USA): Multitox Cytotoxicity Assay (G9270), and Caspase Glo 3/7 Assay (G8091). Tissue culture supplies (unless otherwise noted) were purchased from Fisher Scientific (Pittsburg, PA, USA): Hyclone molecular biology grade water (SH30538), Roswell Park Memorial Institute (RPMI-1640) cell culture medium supplemented with l-glutamine (SH30027FS), Neuronal Growth Factor (NGF) 2.5S natural mouse (356004), rat tail Collagen I (CB-40236), 100ÿ penicillin/streptomycin solution (SV30010), equine serum (donor herd defined and heat inactivated, SH3007403HI), fetal bovine serum (FBS-heat inactivated, SH3008803HI), and nitric acid (PN A467-1 Optima grade). Buffer RLT (PN-79216) was purchased from Qiagen (Germantown, MD, USA).
2.2. Mass spectrocopy
Tungsten alloy pellets (3 pellets in 3 mL of solution) were incubated in distilled water, RPMI 1640 medium, or complete culture medium (RPMI + glutamine, 10% equine serum, 5% FBS, 1ÿ penicillin/ streptomycin), for a total of 11 days at 5% CO2/37 °C. Aliquots for analysis were taken on days 3 and 11 and stabilized in 2% nitric acid prior to analysis. The concentrations of the metal salts in the media used for the PC12 exposure experiments were sampled before and after PC12 cell incubation. Analytical reference standards containing the elements of interest were purchased from Spex Certiprep (Metuchen, NJ, USA). Samples were analyzed using an Agilent 7500ce Inductively coupled Plasma-Mass Spectrometer (ICP-MS). The Agilent ICP-MS was operated using the Octopole Reaction System (ORS) in the Helium collision mode. The instrument was calibrated using standards prepared in 2% nitric acid at concentrations near the expected concentration range of the samples. Internal standards were added using a mixing tee prior to the introduction of sample into the plasma. Continuing check standards and blanks were run at a 10% frequency during the run. The lowest analyzed calibration standard was 0.5 μg/L, which was defined as the detection limit.
2.3. Cell culture
PC12 cell (a rat pheochromocytoma cell-line) stocks were a gift from the laboratory of Fred Seidler (Duke University, NC, USA). Cells were maintained according to previously published methods [16], [21], [23]. Briefly, a frozen aliquot of cells was thawed and immediately transferred drop-wise to a collagen treated T-75 tissue culture flask containing 25 mL of 37 °C complete media (RPMI + glutamine, 10% equine serum, 5% FBS, 1ÿ penicillin/ streptomycin) and incubated for 5 days at 5% CO2/37 °C/100% humidity. The manufacturer's recommended coating procedure was used to treat the plates and flasks with collagen. Adherent cells were collected, centrifuged for 5 min (300 RCF), washed via resuspension in RPMI, and centrifuged as described above. The subsequent pellet of cells was resuspended in complete media to a stock density of either 1 ÿ 105 cells/mL for experiments performed in 96-well plates or 2 ÿ 106 cells/mL for experiments performed in 12 well plates. Where appropriate, cells were allowed to adhere to the plate surface in complete media prior to inducing differentiation by replacing the complete media with low serum supplemented medium (RPMI 1640 + glutamine, 1.4% equine serum, 0.7% FBS, 1ÿ penicillin/streptomycin) and adding 2.5 S NGF (60 ng/mL, final concentration). The negative controls for differentiation were cultured in low serum medium without NGF.
2.4. Viability/cytotoxicity
For this study, the ratio of the individual metals used were equivalent to the ratio of solubilized metals that were mobilized from manufactured pellets (1 mm diameter ÿ 2 mm) of weapons grade tungsten alloy as measured by mass spectroscopy. The manufacturer specified the concentrations of W (91%), Ni (6%), and Co (3%). The soluble ionic forms of the metal species were selected based on the predominant oxidation state, and the acetate species were selected for convenience: Tungsten as tungstate WO42⿿ (sodium tungstate), nickel as Ni2+ (nickel acetate), and cobalt as Co2+ (cobalt acetate). The non-toxic cation/anion was used as a parallel negative control (i.e., sodium acetate).
PC12 cells were plated onto collagen coated 96 well plates at a density of 2 ÿ 104 cells/well and allowed to adhere overnight prior to metal salt exposure in low serum-supplemented media. Cells were incubated with metal salts (W = 20 and 100 μM; Ni = 10 μM; Co = 20 and 100 μM) or negative controls for 18 h. Hydrogen peroxide (H2O2, 1 mM) was the positive control for cytotoxicity and was added to specified wells for the final 4 h of the 18 h incubation. The assays were performed in duplicate wells. The manufacturer⿿s protocols for the detection of cytotoxicity using a luminescent endpoint (AAF-Glo) and viability using a fluorescent endpoint (AFC) were followed. Luminescence and fluorescence were detected using a Synergy HT instrument. (AAF-Glo is a trademark of Promega Corporation; Synergy HT is a trademark of BioTek Instruments, Inc.).
2.5. Microarray expriment
The exposure experiment was performed on four independent occasions to generate four replicates (N = 4 for each condition) for the microarray experiments. For each replicate, PC12 cells were plated at a density of 2 ÿ 105 cells/well in collagen treated 12 well plates and incubated for 24 h to allow for attachment in high serum (10% equine/5% FBS) supplemented complete medium. The complete media was then aspirated and replaced with low serum medium containing the appropriate metal(s). NGF (60 ng/mL final) was added to all wells except the negative control wells. The negative and positive control wells received sodium acetate (100 μM final). Each test condition was performed in triplicate wells, which were then pooled for microarray analysis. Cells were incubated for 24 h (37 °C/5% CO2) before collection of the cells. Adherent cells were harvested by first chilling the plate on ice then aspirating the media away from the attached cells. Adherent cells were dislodged by adding 400 μL of Buffer RLT to each well and incubating the plate on ice for 5 min followed by gently pipetting the cell/buffer mixture across the well attachment surface three times. The cell mixture was then transferred to a chilled microcentrifuge tube with triplicate wells pooled into a single tube for each condition (final volume approximately 1.2 mL per tube). The samples were stored at ⿿80 °C until RNA extraction.
2.6. Microarray hybridization and scanning
40 Affymetrix GeneChip® Rat Genome 230 2.0 chips were used for the experiment. As described above, each test condition (metal treatment) was performed in triplicate wells and the cells from these triplicate wells were pooled at the time of collection. For biological replicates, experiments were carried out on four independent occasions, resulting in four Affymetrix chips per test condition. RNA extraction and microarray processing were conducted by Expression Analysis Inc. (Durham, NC, USA). Total RNA was isolated from the cells using RNeasy (Qiagen) and the quality of the RNA was assessed by an Agilent 2100 Bioanalyzer (260/280 ratios >2.0), measuring the RIN score, and other internal metrics. cRNA was synthesized and subsequently hybridized to the Affymetrix GeneChip Rat Genome 230 v.2.0 containing 31,042 probe sets using protocols that were previously established by Expression Analysis Inc. (GeneChip® is a registered trademark of Affymetrix, Inc.).
2.7. Microarray and probe set analysis
Raw ⿿cell⿿ data files were imported into JMP® Genomics 4.1, log base 2 (log2) transformed, and merged with an experimental design file for analysis. Interquartile (IQR) normalization was followed by one-way analysis of variance (ANOVA) analysis (p = 0.05) using a False Discovery Rate (FDR) of 0.05. An ANOVA was first run to compare the positive (ContP) and the negative (ContN) controls only. A second ANOVA model was then run using the positive controls (ContP) alone and the metal treatment combinations. Volcano plots, showing the fold-change vs. the statistical significance of the change for each probe, were generated by comparing treatments to positive control. All volcano plots (with the exception of ContP vs. ContN) were plotted on identical X and Y-axis scales for clarity. Lists of significant genes from the ANOVA analysis were first saved as SAS files, then exported to Excel as saved ⿿.txt⿿ files and finally imported into Ingenuity® Pathway Analysis (IPA®) (Ingenuity Systems, Redwood City, CA, USA). Standard IPA analysis was applied to these genes to generate the analytical reports shown. Asterisks indicate genes that had more than one probe contributing to the analysis. (JMP® is a registered trademark of SAS Institute, Inc.; Ingenuity® and IPA® is a registered trademark of Ingenuity Systems, Inc.).
3. Results
3.1. Assessment of metal composition in alloy leachate
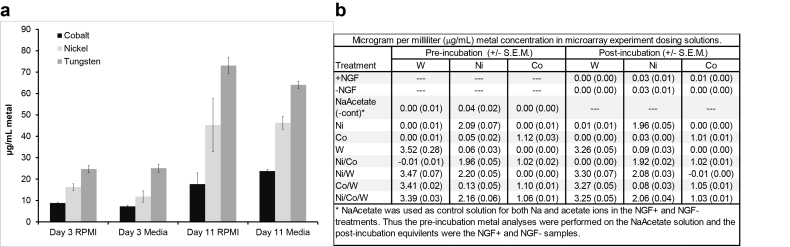
The data for the pellet leaching experiment are presented in Fig. 1a. Over the experimental period of 11 days, the metals were recovered from the physiological buffer (RPMI) or the complete PC12 cell medium at an approximate ratio of 3:2:1 W/Ni/Co. Based on our findings, the incubation ratios of metal ions for the microarray were selected at 3:2:1. Although the percentage composition of the alloy is 91:6:3 for W, Ni, and Co respectively, solubilization is influenced by the two-phase composition of the alloy such that the matrix is the main contributor of solubilized metals. Prior work has established that the alloy used here has a matrix ratio of 43:38:19⿿values that are more aligned to the measured 3:2:1 ratio found in animal models [19]. The concentrations of metals in the treatments used for the microarray experiment were evaluated at the beginning and end of the incubation period to verify the intended concentrations. The results of the treatment concentration verification in the PC12 exposure media are presented in Fig. 1b.
Fig. 1.
Metal analysis. (a) Leachate analysis from alloy pellets. The mean concentration (±SD) of leached soluble metals from three tungsten alloy pellets incubated for either 3 or 11 days in either RPMI or complete culture medium (Media). (b) Pre and post treatment concentration verification of metals (microarray experiment). The concentration of metals in the treatment media before and after the 24 h PC12 cell incubation was measured. The nominal (calculated) concentrations were 1, 2 and 3 μg/mL Co:Ni:W, and the averaged measured concentrations were 1, 2.1 and 3.4 μg/mL Co:Ni:W. In the text, the nominal exposures are used for consistency.
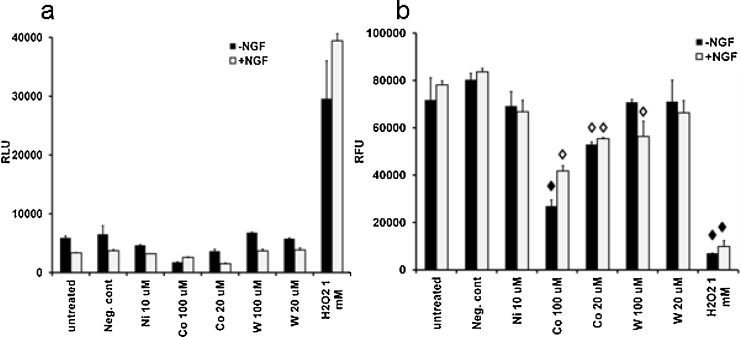
Cytotoxicity and viability screening for PC12 Cells Exposed to Soluble Alloy Surrogates: commercially available cytotoxicity and viability assays were used to screen the response of PC12 cells to the metal treatments; the results are shown in Fig. 2(a and b.) The endpoint measured with the cytotoxicity assay was compromised membrane permeability (i.e., non-viable cells). The endpoint measured with the viability assay was intact cell membranes (i.e., viable cells). Assessment of cell health uses both endpoints to determine viability. When compared to the positive control (i.e., H2O2), the data presented in Fig. 2 are consistent with the published findings that cobalt acetate concentrations below 100 μM are not overtly cytotoxic and nickel acetate at or below 10 μM is not overtly cytotoxic after an 18 h incubation. Additionally, the data show that Na2WO4 was not cytotoxic at or below 100 μM. Reductions in cell viability (Fig. 2b) were observed in PC12 cells treated with cobalt above 20 μM (either with or without NGF) and tungstate at 100 μM (NGF treated cells only).
Fig. 2.
Cytotoxicity and viability screen of PC12 cells exposed to alloy surrogates. (a) Cytotoxicity. PC12 cells treated with and without NGF were exposed to selected concentrations of soluble metal ions and incubated for 18 h in duplicate. The luminescent is reported as relative luminance units (RLU ± SD). Negative control was 200 μM sodium acetate. (b) Viability. PC12 cells treated with or without NGF were exposed to soluble metalions for 18 h in duplicate wells. The fluorescent signal was measured using a plate reader and is reported as mean relative fluorescence units (RFU ± SD). In cells treated with NGF to stimulate differentiation, the cytotoxicity of PC12 cells exposed to the metals was similar to the cells not treated with NGF. Exposure conditions that resulted in a greater than 20% loss in viability are indicated with an open diamond; treatments that resulted in a greater than 60% loss in viability are indicated with closed diamonds.
Based on the cytotoxicity and viability observations, a cobalt concentration less than 20 μM was identified as suitable for the microarray experiment since cytotoxicity was minimal (i.e., less than 20% non-viable cells and >80% viable cells) within the first 24 h of exposure⿿the relevant time-frame for the microarray experiment. The 3:2:1 μg/mL ratio W:Ni:Co identified in the leachate experiment was used to set the Co value. The W and Ni concentrations were then defined as 3 and 2 μg/mL, respectively. These μg/mL concentrations are equivalent to molarities of 16 μM W5+, 34 μM Ni2+ and 17 μM Co2+.
3.2. Microarray analysis
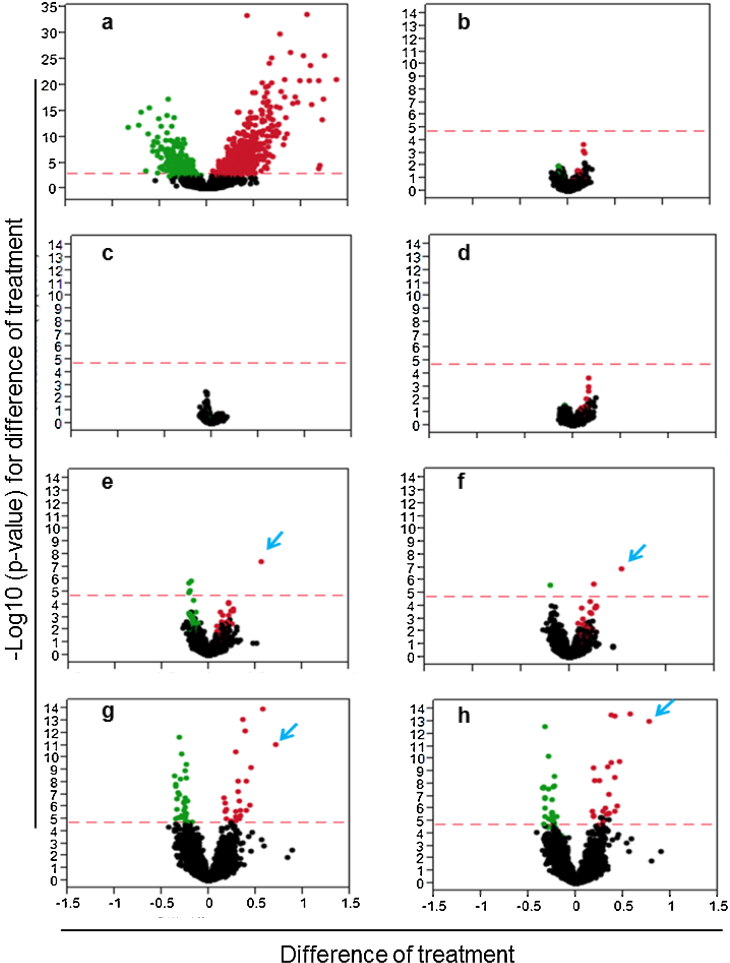
To capture transcriptional changes early in the differentiation process, cells were exposed to the soluble metal surrogates for 24 h. Microarray results provided information on the global gene response to exposure (using ANOVA). The list of the significant genes (76) found in all treatments (ANOVA) is provided in Supplementary Table 1. The differential expression of genes between treatment groups (after False Discovery correction) was visualized by volcano plots (Fig. 3a⿿h). At the 24 h time-point, there were 1028 significant probes due to NGF treatment (ContP) as compared to naïve controls (ContN) while the additional change in gene expression due to the presence of Ni, W, or W/Ni in cells exposed to NGF (Fig.3b⿿d) was significantly less (76 probes) as compared to the response due to NGF alone. The expression profile in cells exposed to Co was marked by an increased expression of heme oxygenase (HMOX1), which is a gene response biomarker to Co [18] that remained a consistently high responder when Co was in the mixture (Fig.3e and f, blue arrow). Significantly, cells exposed to the combination of Ni/Co up- and down-regulated the same groups of transcripts as did the triple metal exposed cells (Fig.3g and h), indicating that W contributes little to the signature gene expression profiles that were generated by Ni and Co. Increased expression of metallothionein 1a (Mt1a) was observed as well.
Fig. 3.
Differences in PC12Cell Gene Expression After 24-h exposure to single metal salts and metal salt combinations. Red horizontal dashed line indicates the significance cutoff for the False Discovery Rate (FDR) analysis. Transcripts that are shared between the treatment scenarios are labeled green (down regulated) and red (up-regulated). (a) A volcano plot comparing the genes expressed in the untreated negative control to the NGF treated positive control. (b)⿿(h) Volcano plots comparing the gene expression differences between the metal treated and positive control (NGF only) treatments. Metals are at the following concentrations: 3 μg W/mL, 2 μg Ni/mL and 1 μg Co/mL. (b) Ni; (c) W; (d) W/Ni; (e) Co; (f) W/Co; (g) Ni/Co; (h) W/Ni/Co. Blue arrow indicates heme oxygenase 1 (HMOX1).
3.3. IPA analysis
IPA uses a database of biological interactions and functional annotations that are created from millions of modeled or experimentally described molecular relationships to infer connectivity and linkages (i.e., functional pathways). We examined the affected pathways and systems due to NGF treatment (Table 1, Table 2). The pathways identified were consistent with early changes in PC12 cell differentiation. Any additional changes due to specific metal treatments could not be analyzed with IPA due to the low number of significant probes in the metal treated cells. It should be noted that IPA analysis depends on a large pool of genes (i.e., 100⿿s) for robust interpretation of pathways; in this experiment, low numbers of significant genes (<100) may have somewhat weakened interpretation of the pathway results.
Table 1.
Top IPA identified genes and functions following NGF treatment for 24 h.
| Log 2 ratio values of genes with largest change in expression compared to control | |||
|---|---|---|---|
| Up-regulated | Down-regulated | ||
| EGR1 | 1.731 | C5ORF13 | ⿿0.835 |
| DUSP6 | 1.378 | NCALD | ⿿0.721 |
| VGF | 1.234 | CA12 | ⿿0.617 |
| CRH | 1.227 | RAMP1 | ⿿0.566 |
| MMP10 | 1.203 | ULK1 | ⿿0.529 |
| SPRR1A | 1.188 | MT1F | ⿿0.525 |
| MMP3 | 1.184 | DIXDC1 | ⿿0.507 |
| CGA | 1.112 | SCTR | ⿿0.501 |
| SCN7A | 1.097 | PPP2R2B | ⿿0.491 |
| S100A4 | 1.089 | CXCR4 | ⿿0.486 |
Table 2.
IPA functional and system assignments for PC12 cell response to NGF for 24 h.
| Top molecular and cellular functions | P-value | # Molecules |
|---|---|---|
| Cellular assembly and organization | 8.16 ÿ 10⿿6 ⿿ 4.94 ÿ 10⿿2 | 38 |
| Cellular movement | 9.71 ÿ 10 ⿿6 ⿿ 4.94 ÿ 10⿿2 | 32 |
| Cell-to-cell signaling and interaction | 1.18 ÿ 10⿿5 ⿿ 4.94 ÿ 10⿿2 | 28 |
| Cell morphology | 4.42 ÿ 10⿿5 ⿿ 3.91 ÿ 10⿿2 | 31 |
| Cell death | 2.18 ÿ 10⿿4 ⿿ 4.44 ÿ 10⿿2 | 41 |
| Top physiological system development and function | P-value | # Molecules |
|---|---|---|
| Nervous system | 8.16 ÿ 10⿿6 ⿿ 4.61 ÿ 10⿿2 | 40 |
| Renal/urological | 1.28 ÿ 10⿿4 ⿿ 3.39 ÿ 10⿿2 | 9 |
| Tumor morphology | 6.73 ÿ 10⿿4 ⿿ 4.52 ÿ 10⿿2 | 8 |
| Hematological system | 7.81 ÿ 10⿿4 ⿿ 4.52 ÿ 10⿿2 | 20 |
| Immune cell trafficking | 7.81 ÿ 10⿿4 ⿿ 3.58 ÿ 10⿿2 | 11 |
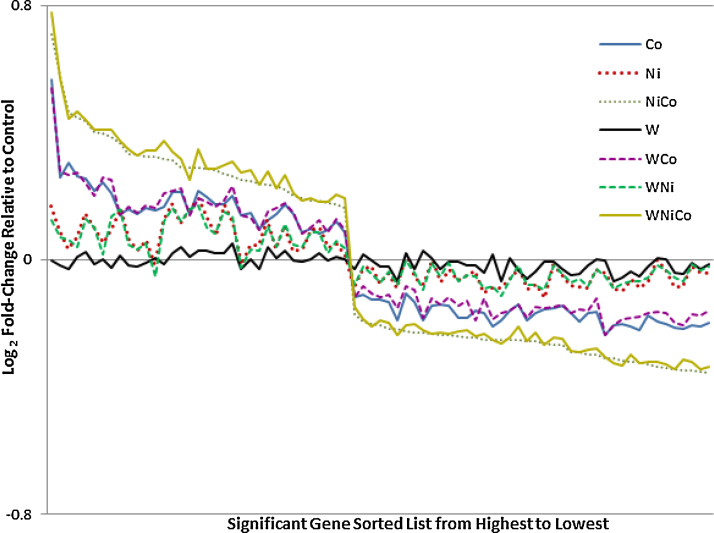
The list of significant genes (77) that were found in all treatments (ANOVA) is shown in Supplementary Table 1. The gene lists were sorted for log2 fold-change and the relative profile of fold expression is shown in Fig. 4. Treatments containing Ni and Co caused the highest range of differential changes in expression (with HMOX-1 being the highest). Tungsten treatments showed little or no departure from baseline while Ni and Ni/W treatments were similar to each other, indicating the response to Ni was not affected by the presence of W. Cobalt or Co/W showed relatively higher changes in expression than did Ni combinations. The largest impacts were from treatments that contained Co combined with Ni, while W had no impact either alone or in any combination with the other metals. Overall, these data show an interaction between Ni and Co that result in increased registry of significant genes.
Fig. 4.
Nickel and cobalt have additive effects on PC12 cell gene expression. 77 genes were identified as up or down regulated by alloy exposure. These data were plotted (left to right) from high (increased expression) to low (decreased expression). No significant changes in expression were identified in the W (tungstate) only exposure while all significant changes were identified in exposures containing Co (cobalt acetate). In co-exposures containing Co and Ni the expression pattern shifts in a manner that suggests additivity.
4. Discussion
The goal of this study was to investigate the toxic and transcriptional responses of PC12 cells in a manner that recapitulates an in vivo exposure where alloy fragments would release constituent metals. As such, the soluble ionic forms of the alloy were used and the ratio of the constituents in physiological solutions was identified experimentally by incubating the solid alloy in media and measuring the concentration of released metals over several days. While the alloy consists of 90% tungsten by weight, the matrix, from which almost all of the metals are leached, consists of 43% tungsten [19] and this was reflected in the relative concentrations of tungsten, cobalt, and nickel that leached from the pellets.
To test physiologically relevant responses, these leached concentrations were then used to derive the exposure conditions for the microarray experiment. Additionally, prior to the microarray experiments, the sensitivity of PC12 cells to the individual metal ions were explored to avoid exposure of cells to metal ion concentrations that were acutely toxic. Although high levels of cobalt and nickel were found in the serum of animals implanted with alloy pellets [13], a review of the literature indicated that acceptable in vitro exposure conditions were in the low micromolar range for both cobalt and nickel [8], [10], [21], [22], [24], [28]. In our dose-ranging experiments, the low micromolar range was suitable, as it did not provoke a strong cytotoxic response⿿an effect we wished to minimize as we did not want apoptotic signals to overwhelm the transcriptional profile. Additionally, we used mixtures of the alloy constituents to examine the collective effect of Co, Ni, and W exposure.
Similar to other reports, our findings showed that Co and Ni decreased cell viability [8], [21] but had minimal acute cytotoxicity at the concentrations used for the microarray study. For the microarray experiment, the addition of NGF to the culture medium was necessary to initiate neuritogenesis and, as expected, resulted in dramatic increases and decreases in global gene expression. The molecular pathways and cellular functions that were identified using IPA (e.g., cellular assembly, morphology and movement) were consistent with the early changes involved in PC12 cells that were stimulated to differentiate and undergo neuritogenesis. Further, the top IPA pathway was nervous system development and function. Taken together, these results show that the PC12 cell model was behaving as expected after NGF exposure.
While changes in gene expression due to the presence of the metals were minimal when controlled for NGF, it was clear that treatments that contained Co provoked more changes in gene expression as compared to treatments that did not contain Co. Cobalt, as cobalt chloride (CoCl2) is a known hypoxia-mimetic and induces apoptosis in numerous cell types including rat glioma cells [27]. Indeed, CoCl2 is used as a positive control reagent for cellular oxidative stress. Likewise, nickel exposure as NiCl2 generates intracellular reactive oxygen species that results in cytotoxicity [9].
The 77 significant transcriptional changes in the alloy treatments were compared (Fig. 4). Nickel and Co might have had at least an additive effect when dosed in combination as evidenced by the fold-change shift in the Ni/Co treatment compared to the Ni and Co treatments (Fig. 4). By comparison, W had a minimal effect on PC12 cell gene expression at the time-point tested. Additionally, the expression pattern of mixtures that included W (i.e., W/Co, W/Ni, and W/Ni/Co) overlaid the expression pattern of the treatments that did not contain W (i.e., W/Co = Co, W/Ni = Ni and W/Ni/Co = Ni/Co). A report by Bardack et al. [2] examined the transcriptional responses to W/Ni/Co and W/Ni/Fe (iron) alloys in rodent myoblast cell lines (L6 (rat) and C2C12 (mouse)). There were no correlations in the expression profiles between the Bardack study and the results reported here; however, there were substantial differences in the concentrations used for the experiments. Principally, the dosing design used here represented the ratio of alloy constituents that solubilize from the pellet in aqueous solution (i.e., 3:2:1 W/Ni/Co), while the study design reported by Bardack et al. represented the ratio of alloy constituents, by weight (i.e., 91:6:3 W/Ni/Co), of the pellet.
This study was designed to investigate early changes in neuronal function as a result of alloy exposure; however, the results have implications for the alloy⿿s potential to initiate tumors as well. Although the gene set is small (76 genes), the coordinated changes between treatments containing Co and/or Ni suggest that the PC12 cell response to metal cations (e.g., oxidative stress response) is at least additive. Although the mechanisms of Co and Ni toxicity are presumed independent ⿿Co ions can stabilize the transcription factor hypoxia-inducible factor (HIF) and thereby mimic hypoxia [20] while divalent Ni has been demonstrated to inactivate iron-and 2-oxoglutarate dioxygenases involved in epigenetic regulation and DNA repair [5]. When combined, both mechanisms may contribute to tumorigenesis because of increased oxidative DNA damage and a reduced capacity to repair damaged DNA.
Tungsten alloys are used in such a manner that inhalation of alloy dust or an embedment of alloy fragments is possible. Subsequently, ionic species of the constituents may dissolve into the surrounding tissues. These results suggest that the adverse effects observed in tissues because of embedded tungsten alloy fragments, will most likely occur from the Ni and/or Co content of the fragments, whereas W will have minimal impact at these doses.
Disclaimer
The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as official Department of the Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
Conflict of interest
Author has declared no conflict of interest.
Transparency document
Acknowledgements
The authors thank Drs. Michael Gargas, Michael G. Stockelman, and Vishwesh P. Mokashi for their valuable perspectives and input on the design and initiation of the study. The authors also thank Drs. Michael J. Quinn, Mark A. Williams, and Mark S. Johnson for their review of the manuscript. Special thanks to Dr. Ed Perkins for technical advice. This project was funded via Military Interdepartmental Purchase Request (MIPR)N4181709MP9T022.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.toxrep.2015.09.005.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Ando A., Ando I., Hiraki T., Hisada K. Relation between the location of elements in the periodic table and various organ-uptake rates. Int. J. Rad. Appl. Instrum. B. 1989;16:57–80. doi: 10.1016/0883-2897(89)90216-x. [DOI] [PubMed] [Google Scholar]
- 2.Bardack S., Dalgard C.L., Kalinich J.F., Kasper C.E. Genotoxic changes to rodent cells exposed in vitro to tungsten, nickel, cobalt and iron. Int. J. Environ. Res. Public Health. 2014;11:2922–2940. doi: 10.3390/ijerph110302922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnham K.J., Bush A.I. Metals in Alzheimer⿿s and Parkinson⿿s diseases. Curr. Opin. Chem. Biol. 2008;12:222–228. doi: 10.1016/j.cbpa.2008.02.019. [DOI] [PubMed] [Google Scholar]
- 4.Centeno J.A., Rogers D.A., van der Voet G.B., Fornero E., Zhang L., Mullick F.G., Chapman G.D., Olabisi A.O., Wagner D.J., Stojadinovic A., Potter B.K. Embedded fragments from U.S. military personnel-chemical analysis and potential health implications. Int. J. Environ. Res. Public Health. 2014;11:1261–1278. doi: 10.3390/ijerph110201261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H., Giri N.C., Zhang R., Yamane K., Zhang Y., Maroney M., Costa M. Nickel ions inhibit histone demethylase JMJD1A and DNA repair enzyme ABH2 by replacing the ferrous iron in the catalytic centers. J. Biol Chem. 2010;285:7374–7383. doi: 10.1074/jbc.M109.058503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton F.A., Wilkinson G. In Advanced Inorganic Chemistry. Interscience Publishers (John Wiley and Sons); New York: 1972. The elements of the second and third transition series; pp. 946–957. [Google Scholar]
- 7.Fujita K., Lazarovici P., Guroff G. Regulation of the differentiation of PC12 pheochromocytoma cells. Environ. Health Perspect. 1989;80:127–142. doi: 10.1289/ehp.8980127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gopalakrishna R., Gundimeda U., Schiffman J.E., McNeill T.H. A direct redox regulation of protein kinase C isoenzymes mediates oxidant-induced neuritogenesis in PC12 cells. J. Biol. Chem. 2008;283:14430–14444. doi: 10.1074/jbc.M801519200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jia J., Chen J. Chronic nickel-induced DNA damage and cell death: the protection role of ascorbic acid. Environ. Toxicol. 2008;23:401–406. doi: 10.1002/tox.20346. [DOI] [PubMed] [Google Scholar]
- 10.Jung J.Y., Kim W.J. Involvement of mitochondrial- and Fas-mediated dual mechanism in CoCl2-induced apoptosis of rat PC12 cells. Neurosci. Lett. 2004;371:85–90. doi: 10.1016/j.neulet.2004.06.069. [DOI] [PubMed] [Google Scholar]
- 11.Kalinich J.F., Emond C.A., Dalton T.K., Mog S.R., Coleman G.D., Kordell J.E., Miller A.C., McClain D.E. Embedded weapons-grade tungsten alloy shrapnel rapidly induces metastatic high-grade rhabdomyosarcomas in F344 rats. Environ. Health Perspect. 2005;113:729–734. doi: 10.1289/ehp.7791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalinich J.F., Kasper C.E. Do metals that translocate to the brain exacerbate traumatic brain injury? Med. Hypotheses. 2014;82:558–562. doi: 10.1016/j.mehy.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Kalinich J.F., Vergara V.B., Emond C.A. Urinary and serum metal levels as indicators of embedded tungsten alloy fragments. Mil. Med. 2008;173:754–758. doi: 10.7205/milmed.173.8.754. [DOI] [PubMed] [Google Scholar]
- 14.Lassner E., Schubert W.D., Luderitz E., Wulf H.U. Ullmans Encyclopedia of Industrial Chemistry. Wiley-VCH; 2000. Tungsten: properties, chemistry, technology of the element, alloys, and chemical compounds. [Google Scholar]
- 15.Marchetti C. Interaction of metal ions with neurotransmitter receptors and potential role in neurodiseases. Biometals. 2014;27:1097–1113. doi: 10.1007/s10534-014-9791-y. [DOI] [PubMed] [Google Scholar]
- 16.Qiao D., Seidler F.J., Slotkin T.A. Developmental neurotoxicity of chlorpyrifos modeled in vitro: comparative effects of metabolites and other cholinesterase inhibitors on DNA synthesis in PC12 and C6 cells. Environ. Health Perspect. 2001;109:909–913. doi: 10.1289/ehp.01109909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roszell L.E., Hess-Ruth A., Beall P., Catherine P. In SOT Annual Meeting (The Toxicologist, ed.) Society of Toxicology; Seattle, WA: 2008. Health effects of embedded fragments of tungsten and tungsten alloys. [Google Scholar]
- 18.Salinas M., Diaz R., Abraham N.G., Ruiz de Galarreta C.M., Cuadrado A. Nerve growth factor protects against 6-hydroxydopamine-induced oxidative stress by increasing expression of heme oxygenase-1 in a phosphatidylinositol 3-kinase-dependent manner. J. Biol. Chem. 2003;278:13898–13904. doi: 10.1074/jbc.M209164200. [DOI] [PubMed] [Google Scholar]
- 19.Schuster B.E., Roszell L.E., Murr L.E., Ramirez D.A., Demaree J.D., Klotz B.R., Rosencrance A.B., Dennis W.E., Bao W., Perkins E.J., Dillman J.F., Bannon D.I. In vivo corrosion, tumor outcome, and microarray gene expression for two types of muscle-implanted tungsten alloys. Toxicol. Appl. Pharmacol. 2012;265:128–138. doi: 10.1016/j.taap.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 20.Shrivastava K., Shukla D., Bansal A., Sairam M., Banerjee P.K., Ilavazhagan G. Neuroprotective effect of cobalt chloride on hypobaric hypoxia-induced oxidative stress. Neurochem. Int. 2008;52:368–375. doi: 10.1016/j.neuint.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Slotkin T., Seidler F. Transcriptional profiles reveal similarities and differences in the effects of developmental neurotoxicants on differentiation into neurotransmitter phenotypes in PC12 cells. Brain Res. Bull. 2009;78:211–225. doi: 10.1016/j.brainresbull.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slotkin T.A., MacKillop E.A., Ryde I.T., Tate C.A., Seidler F.J. Screening for developmental neurotoxicity using PC12 cells: comparisons of organophosphates with a carbamate, an organochlorine, and divalent nickel. Environ. Health Perspect. 2007;115:93–101. doi: 10.1289/ehp.9527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song X., Violin J.D., Seidler F.J., Slotkin T.A. Modeling the developmental neurotoxicity of chlorpyrifos in vitro: macromolecule synthesis in PC12 cells. Toxicol. Appl. Pharmacol. 1998;151:182–191. doi: 10.1006/taap.1998.8424. [DOI] [PubMed] [Google Scholar]
- 24.Tan X.L., Huang X.Y., Gao W.X., Zai Y., Huang Q.Y., Luo Y.J., Gao Y.Q. CoCl2-induced expression of p300 promotes neuronal-like PC12 cell damage. Neurosci. Lett. 2008;441:272–276. doi: 10.1016/j.neulet.2008.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Teng K.K., Greene L.A. KT5926 selectively inhibits nerve growth factor-dependent neurite elongation. J. Neurosci. 1994;14:2624–2635. doi: 10.1523/JNEUROSCI.14-05-02624.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wase A.W. Absorption and distribution of radio-tungstate in bone and soft tissues. Arch. Biochem. Biophys. 1956;61:272–277. doi: 10.1016/0003-9861(56)90349-6. [DOI] [PubMed] [Google Scholar]
- 27.Yang S.J., Pyen J., Lee I., Lee H., Kim Y., Kim T. Cobalt chloride-induced apoptosis and extracellular signal-regulated protein kinase 1/2 activation in rat C6 glioma cells. J. Biochem. Mol. Biol. 2004;37:480–486. doi: 10.5483/bmbrep.2004.37.4.480. [DOI] [PubMed] [Google Scholar]
- 28.Zou W., Yan M., Xu W., Huo H., Sun L., Zheng Z., Liu X. Cobalt chloride induces PC12 cells apoptosis through reactive oxygen species and accompanied by AP-1 activation. J. Neurosci. Res. 2001;64:646–653. doi: 10.1002/jnr.1118. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.