Abstract
6,7-Dihydroxycoumarin (6,7-HC) (aesculetin) is a natural and synthetic coumarin derivative of great interest for use by humans due to their potent antioxidant properties. Considering that there are no reports that assess the in vivo genetic toxicity of 6,7-HC, the aim of the present study was to investigate its genotoxic potential in terms of DNA damage in peripheral blood, liver, bone marrow and testicular cells of Swiss albino mice by the comet assay, and its clastogenic/aneugenic potential in bone marrow cells using the micronucleus test. In addition, the ability of 6,7-HC to modulate the genotoxic effects induced by doxorubicin (DXR) was also preliminarily evaluated. Cytotoxicity was assessed by scoring polychromatic (PCE) and normochromatic (NCE) erythrocytes’ ratio. The test compound was administered orally at doses of 25, 50 and 500 mg kg−1 isolated and also simultaneously to DXR (80 mg kg−1). The results showed that 6,7-HC did not induce significant DNA damage in any of the analyzed cells, and also did not show any significant increase in micronucleated PCE at the three tested doses. The PCE/NCE ratio indicated no cytotoxicity. Moreover, the extent of DNA damage induced by DXR decreased significantly only in peripheral blood and testicular cells, and only at the lowest dose of 6,7-HC.
Keywords: Coumarin derivative; Aesculetin; 6,7-Dihydroxycoumarin; Antigenotoxic effects; Comet assay; Micronucleus test
1. Introduction
Coumarins are a large class of heterocyclic molecules found in plants as secondary metabolites, mainly in the fruits, seeds, roots, and leaves [1]. More than 1300 coumarins have been identified considering plants, bacteria, and fungi [38]. Naturally occurring coumarins present a diverse range of pharmacologic/toxicologic properties, that include furanocoumarin-induced photoxicity [2], antimicrobial [3], cytochrome P450 3A4 modulation [4], cancer cell cytotoxicity [5], and anticarcinogenesis/chemoprevention of cancer [6], [7], [8], [9], [10], [11], [12].
The structure of coumarins could bind transition metal ion such as Fe(III), and thus inhibit hydroxyl radical and hydrogen peroxide formation produced by Fenton's reactions. Furthermore, their hydroxyl functions are potent H+ donors for free radical acceptors due to electron delocalization across the molecule [13]. Thus, coumarin derivatives could be potent reactive oxygen species (ROS) scavengers and metal chelating agents.
Coumarin derivative molecules (or coumarin metabolites) are naturally generated by the metabolism of coumarin in the cell, and based on the coumarin chemical structure system (ring) have been synthesized utilizing innovative synthetic techniques. It has been found that these derivatives present numerous therapeutic applications as: antitumor and anti-HIV therapy [14], [15], central nervous system stimulants [16], anti-inflammatory [17], antibacterials [18], and anticoagulants [19], among others. Hydroxycoumarins present powerful chain-breaking antioxidants and can prevent free radical injury by scavenging reactive oxygen species [20]. This is the case of the coumarin metabolite 6,7-dihydroxycoumarin (aesculetin), which may be formed naturally in small quantities or purchased commercially by synthetic techniques [21], [39]. Since some coumarin derivatives can present adverse effects such as mild nausea, diarrhea, hepatotoxicity, and cytotoxicity when used in certain amounts [22], [23], [24], [25], a careful evaluation of its genetic toxicity potential is necessary.
There are few studies evaluating the genetic toxicity of natural and synthetic coumarin derivatives in the literature. 4-Methylesculetin was evaluated in vitro and in vivo and all endpoints investigated showed that this synthetic coumarin derivative had no mutagenic or genotoxic effects. However, cytotoxicity at high concentrations was observed [26], [27]. 6,7-Dihydroxycoumarin had its preliminary genetic toxicity studied only in vitro, and Salmonella/microsome, comet and micronucleus assays showed that this natural coumarin derivative did not present mutagenic, genotoxic or clastogenic/aneugenic action [27].
Most of the incurable and chronic diseases are the outcomes of an overload of free radical attacks in the human system resulting in an oxidative stress-like condition, and DNA and protein-like macro-molecules constitute vulnerable targets for free radical attack [28]. 6,7-HC, due to the presence of two hydroxyl moieties on its benzene rings, affect the formation and scavenging of reactive oxygen species (ROS) and influence free radical-mediated oxidative damage, and is being considered one of the most effective antioxidant coumarins [21], and for this reason, has the potential to be used by humans as an antioxidant, protecting against DNA damage, cancer and aging.
National and international regulatory agencies recommend the in vivo mammalian assessment of genotoxicity/mutagenicity of any agent with therapeutic potential for humans [37], [40]. Considering this, and that the use of 6,7-HC could promote human health, the present study was performed to investigate for the first time, the in vivo genotoxic potential of 6,7-HC in peripheral blood, liver, bone marrow and testicular cells of mice, by the comet and micronucleus tests. In addition, the possible antigenotoxicity of 6,7-HC on doxorubicin-induced DNA damage was preliminarily assessed.
2. Materials and methods
2.1. Chemicals
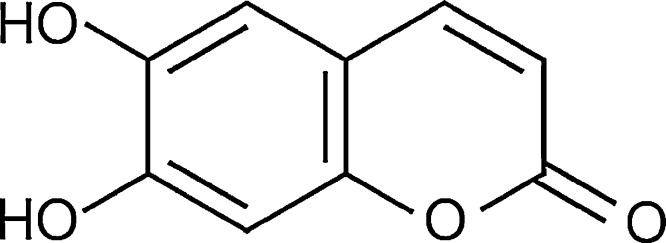
The 6,7-dihydroxycoumarin (6,7-HC) (Fig. 1) used in the experiments was obtained from Sigma–Aldrich (Cat. no. 24,657-3, CAS: 305-01-1, 98% purity) and solubilized in sunflower oil. Doxorubicin (DXR) (IMA S.A.I.C. Laboratories) was used as the positive control substance due to its potential as DNA damaging agent in the comet assay and micronucleus test using Swiss mice. The other main chemicals were obtained from the following suppliers: normal melting point (NMP) agarose (Cat. no. 15510-019 – Invitrogen), low melting point (LMP) agarose (Cat. no. 15517-014 – Invitrogen), sodium salt N-lauroyl sarcosinate (L-5125 – Sigma) and ethylenediaminetetraacetic acid (EDTA – Merck).
Fig. 1.
Chemical structure of 6,7-dihydroxycoumarin (aesculetin).
2.2. Animals and treatments
The experiments were carried out using 12-week-old male Swiss albino mice (Mus musculus), weighing 25–30 g. The animals were acquired from the Universidade Estadual Paulista (UNESP), Botucatu, São Paulo State, Brazil, and housed in polyethylene boxes in a climate-controlled environment (25 ± 4 °C, 55 ± 5% humidity) with a 12 h light/dark cycle (7:00 am to 7:00 pm). Food (Nuvilab CR1, Nuvital) and water were available ad libitum. The mice were divided into 8 experimental groups of 11 animals each. 6,7-HC was dissolved in sunflower oil and administered in a single dose of 0.3 mL by gavage at concentrations of 25, 50 and 500 mg/kg body weight. In this procedure, each animal was weighed individually and then the calculated dose was solubilized in 0.3 mL of the vehicle being administered. These doses were selected based on our preliminary acute toxic studies in mice, which was higher than 500 mg/kg. The negative control group received sunflower oil by gavage, and the positive control group received an intraperitoneal injection of doxorubicin (DXR) at 80 mg/kg body weight. Simultaneous treatments for antigenotoxic assessment were carried out by administering the three selected extract doses and then DXR 1 h later. The animals used in this study were sacrificed by cervical dislocation without anesthesia, to avoid possible alterations in the DNA damage analysis. The Animal Bioethics Committee of the Faculdade de Medicina de Marília (CEP/FAMEMA, Marília, São Paulo state, Brazil) approved the present study on 14 April 2010 (protocol number 085/10), in accordance with federal government legislations on animal care.
2.3. Comet assay
The comet assay (or single cell gel electrophoresis (SCGE)) was carried out by the method described by Speit and Hartmann [29] and reviewed by Burlinson et al. [30]. Peripheral blood samples from the tail vein were obtained from six Swiss mice of each group, at 4 and 24 h after treatment and before euthanasia. After euthanasia, liver, bone marrow and testicular cell samples were collected and washed in saline solution in an ice bath. A small portion of each type (about 4 mm in diameter) was transferred to a Petri dish containing 4 mL phosphate buffer solution (PBS, pH 7.4) and then gently homogenized with a small pair of tweezers and a syringe to remove any clumps of cells. The trypan dye exclusion method was used to determine cell viability immediately before the comet assay; viability was above 80% for all cell types and for all treatments. Another aliquot (20 μl) of each cell types from each animal was mixed with 120 μL of 0.5% low melting point agarose at 37 °C, and rapidly spread onto two microscope slides per animal, pre-coated with 1.5% normal melting point agarose. The slides were coverslipped and allowed to gel at 4 °C for 20 min. The coverslips were gently removed and the slides were then immersed in cold, freshly prepared lysing solution consisting of 89 mL of a stock solution (2.5 M NaCl, 100 mM EDTA, 10 mM Tris, pH set to 10.0 with ∼8 g solid NaOH, 890 mL of distilled water and 1% sodium lauryl sarcosine), plus 1 mL of Triton X-100 (Merck) and 10 mL of dimethyl sulfoxide (Merck). The slides, which were protected from light, were allowed to stand at 4 °C for 1 h and then placed in the gel box, positioned at the anode end, and left in a high pH (>13) electrophoresis buffer (300 mM NaOH-1 mM EDTA, prepared from a stock solution of 10 N NaOH and 200 mM, pH 10.0, EDTA) at 4 °C for 20 min prior to electrophoresis, to allow DNA unwinding. The electrophoresis run was carried out in an ice bath (4 °C) for 20 min at 300 mA and 25 V (0.722 V cm−1). The slides were then submerged in a neutralization buffer (0.4 M Tris–HCl, pH 7.5) for 15 min, dried at room temperature and fixed in 100% ethanol for 10 min. The slides were dried and stored overnight or longer, before staining. For the staining process, the slides were briefly rinsed in distilled water, covered with 30 μL of 1× ethidium bromide staining solution prepared from a 10× stock (200 μg/mL) and coverslipped. The material was evaluated immediately at 400× magnification, using a fluorescence microscope (Olympus BX 50) with a 515–560 nm excitation filter and a 590 nm barrier filter. Only individual nucleoids were scored.
The extent and distribution of DNA damage indicated by the SCGE assay was evaluated by examining at least 100 randomly selected and non-overlapping cells (50 cells per coded slide) per animal in a blind analysis (six mice per group). These cells were scored visually, according to tail size, into the following four classes: class 0 – no tail; class 1 – tail shorter than the diameter of the head (nucleus); class 2 – tail length 1–2 times the diameter of the head; and class 3 – tail length more than twice the diameter of the head. Comets with no heads, with nearly all of the DNA in the tail or with a very wide tail, were being excluded from the evaluation because they probably represented dead cells [31]. The total score for 100 comets, which ranged from 0 (all undamaged) to 300 (all maximally damaged), was obtained by multiplying the number of cells in each class by the damage class.
2.4. Micronucleus test
The assay was carried out following standard protocols as recommended by Schmid [41] and Maistro [32]. Five of the same six male mice of each group used in the comet assay were sacrificed 24 h after treatment and the other five animals were sacrificed 48 h after extract treatment (10 mice per group). The bone marrow from the femur of the other mice was flushed out using 2 mL of saline (0.9% NaCl) and centrifuged at 1000 rev./min for 7 min. The supernatant was discarded. Four drops of formaldehyde (4%) was added to preserve the cell cytoplasm and smears were made on slides. The slides were coded for a “blind” analysis, fixed with methanol and stained with Giemsa. For the analysis of the micronucleated cells, 2000 polychromatic erythrocytes (PCE) per animal were scored to determine the clastogenic/aneugenic property of the extract. To detect possible cytotoxic effects, the PCE/NCE (normochromatic erythrocytes) ratio in 200 erythrocytes/animal was calculated [33]. The cells were blindly scored using a light microscope at 1000× magnification. The mean number of micronucleated polychromatic erythrocytes (MNPCE) in individual mice was used as the experimental unit, with variability (standard deviation) based on differences among animals within the same group.
2.5. Modulation of genotoxic activity
The percentage change of genotoxic (observed by the comet assay) agent-induced damage by 6,7-HC was calculated according to Waters et al. [34], using the following formula:
where A corresponds to the score mean observed in the treatment with CPA (positive control), B corresponds to score mean observed in the antigenotoxic treatment (extract plus DXR) and C corresponds to the score mean in the control.
2.6. Statistical analysis
All data are expressed as the mean ± standard deviation (n = 6/group in the comet assay; n = 5 for each time sample in the MN test). After verifying for normal distribution (normality test KS performed using GraphPad Instat® software), the data obtained from the comet assay were submitted to analysis of variance (ANOVA) and the Tukey–Kramer multiple comparison test, and the data obtained from the micronucleus assay were submitted to the analysis of variance test (ANOVA) with linear regression, both using the GraphPad Prism 5 software (version 3.01). The results were considered statistically significant at P < 0.05.
3. Results
No distinctive toxic clinical signs, deaths or morbidity were observed in the treated animals after 6,7-HC treatments. DNA damage was evaluated in blood leukocytes (4 and 24 h samples), liver, bone marrow and testicular cells of male mice following a single administration of 25, 50 or 500 mg kg−1 by oral gavage. Results of the comet assay are summarized in Table 1. The cell viability observed in the trypan blue staining protocol was over 80% in all treatments (data not shown), which confirms the absence of cytotoxicity found by PCE/NCE ratio analysis in the micronucleus (MN) test (Table 3). As expected, DXR induced a significant increase (P < 0.001) in the total number of cells with damage and scores for all cell types analyzed in comparison with sunflower control, clearly validating the species selected and the study design to detect genotoxic effects. No significant increases in the total number of cells with DNA damage and scores were observed in all cell types over the tested dose range. There was no statistical difference in DNA migration between the three tested doses of 6,7-HC in each cell type. In the few cells that presented DNA damage, it was minor (class 1), as was also observed in the sunflower control.
Table 1.
DNA migration in the comet assay for assessing the genotoxicity of 6,7-dihydroxycoumarin (6,7-HC) in different cells of male Swiss mice in vivo (mean ± SD).
| Treatments and cells analyzed | Totala | Comet class |
Scores | |||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |||
| Peripheral blood (4 h sample) | ||||||
| Control | 14.00 ± 4.43 | 86.00 ± 4.43 | 12.17 ± 4.62 | 1.33 ± 0.52 | 0.50 ± 0.84 | 16.33 ± 4.55 |
| 6,7-HC 25 mg kg−1 | 4.17 ± 1.67 | 95.83 ± 1.67 | 4.17 ± 1.67 | 0.00 ± 0.00 | 0.00 ± 0.00 | 4.17 ± 1.67 |
| 6,7-HC 50 mg kg−1 | 5.33 ± 1.60 | 94.67 ± 1.60 | 5.33 ± 1.60 | 0.00 ± 0.00 | 0.00 ± 0.00 | 5.33 ± 1.60 |
| 6,7-HC 500 mg kg−1 | 6.17 ± 2.67 | 93.83 ± 2.67 | 6.00 ± 2.38 | 0.17 ± 0.37 | 0.00 ± 0.00 | 6.33 ± 2.98 |
| Doxorubicin 80 mg kg−1 | 30.00 ± 3.42* | 72.17 ± 6.94 | 23.83 ± 2.54 | 5.00 ± 1.15 | 1.17 ± 0.37 | 37.33 ± 4.64* |
| Peripheral blood (24 h sample) | ||||||
| Control | 12.33 ± 3.27 | 87.67 ± 3.27 | 11.50 ± 3.56 | 0.83 ± 0.75 | 0.00 ± 0.00 | 17.17 ± 3.13 |
| 6,7-HC 25 mg kg−1 | 17.00 ± 3.27 | 83.00 ± 3.27 | 15.00 ± 2.77 | 2.00 ± 0.58 | 0.00 ± 0.00 | 19.00 ± 3.79 |
| 6,7-HC 50 mg kg−1 | 16.50 ± 2.22 | 83.50 ± 2.22 | 14.00 ± 1.91 | 2.50 ± 0.50 | 0.00 ± 0.00 | 19.00 ± 2.58 |
| 6,7-HC 500 mg kg−1 | 17.00 ± 2.08 | 83.00 ± 2.08 | 14.67 ± 1.80 | 2.33 ± 0.75 | 0.00 ± 0.00 | 19.33 ± 2.56 |
| Doxorubicin 80 mg kg−1 | 36.00 ± 4.43* | 64.00 ± 4.43 | 25.50 ± 6.24 | 8.50 ± 1.71 | 2.00 ± 0.82 | 48.50 ± 3.59* |
| Liver | ||||||
| Control | 33.17 ± 4.36 | 66.83 ± 4.36 | 32.33 ± 4.08 | 083 ± 0.98 | 0.00 ± 0.00 | 34.00 ± 4.82 |
| 6,7-HC 25 mg kg−1 | 21.33 ± 4.19 | 78.67 ± 4.19 | 21.17 ± 3.98 | 0.17 ± 0.37 | 0.00 ± 0.00 | 21.50 ± 4.43 |
| 6,7-HC 50 mg kg−1 | 23.67 ± 3.30 | 76.33 ± 3.30 | 22.17 ± 2.61 | 1.50 ± 0.76 | 0.00 ± 0.00 | 25.17 ± 4.02 |
| 6,7-HC 500 mg kg−1 | 27.83 ± 2.73 | 72.17 ± 2.73 | 26.33 ± 1.97 | 1.50 ± 1.12 | 0.00 ± 0.00 | 29.33 ± 3.68 |
| Doxorubicin 80 mg kg−1 | 88.67 ± 3.14* | 11.33 ± 3.14 | 44.83 ± 2.03 | 30.33 ± 1.97 | 13.50 ± 1.38 | 149.00 ± 7.19* |
| Bone marrow | ||||||
| Control | 21.67 ± 5.54 | 78.83 ± 5.54 | 21.33 ± 5.05 | 0.33 ± 0.82 | 0.00 ± 0.00 | 22.00 ± 6.10 |
| 6,7-HC 25 mg kg−1 | 12.83 ± 2.48 | 87.17 ± 2.48 | 12.50 ± 2.22 | 0.33 ± 0.47 | 0.00 ± 0.00 | 13.17 ± 2.79 |
| 6,7-HC 50 mg kg−1 | 12.17 ± 1.34 | 87.83 ± 1.34 | 11.17 ± 0.69 | 1.00 ± 1.00 | 0.00 ± 0.00 | 13.17 ± 2.27 |
| 6,7-HC 500 mg kg−1 | 12.33 ± 1.80 | 87.67 ± 1.80 | 11.83 ± 1.57 | 0.50 ± 0.50 | 0.00 ± 0.00 | 12.83 ± 2.11 |
| Doxorubicin 80 mg kg−1 | 90.67 ± 0.94* | 9.33 ± 0.94 | 35.00 ± 1.73 | 48.50 ± 1.71 | 7.17 ± 1.07 | 153.50 ± 1.98* |
| Testicular | ||||||
| Control | 27.00 ± 6.93 | 73.00 ± 6.93 | 24.50 ± 6.63 | 2.33 ± 2.42 | 0.17 ± 0.41 | 29.67 ± 8.43 |
| 6,7-HC 25 mg kg−1 | 6.33 ± 2.21 | 93.67 ± 2.21 | 5.83 ± 1.77 | 0.50 ± 0.50 | 0.00 ± 0.00 | 6.83 ± 2.67 |
| 6,7-HC 50 mg kg−1 | 10.33 ± 1.89 | 89.67 ± 1.89 | 10.33 ± 1.89 | 0.00 ± 0.00 | 0.00 ± 0.00 | 10.33 ± 1.89 |
| 6,7-HC 500 mg kg−1 | 13.17 ± 2.61 | 86.83 ± 2.61 | 13.17 ± 2.61 | 0.00 ± 0.00 | 0.00 ± 0.00 | 13.17 ± 2.61 |
| Doxorubicin 80 mg kg−1 | 40.83 ± 4.56* | 60.83 ± 4.18 | 29.33 ± 2.56 | 9.00 ± 1.53 | 2.50 ± 0.76 | 54.83 ± 7.43* |
Total number of damaged cells (class 1 + 2 + 3).
Significantly different from the negative control (P < 0.05).
Table 3.
Number of micronucleated polychromatic erythrocytes (MNPCE) observed in the bone marrow cells of male (M1–5) Swiss mice treated with 4-hydroxycoumarin, and respective controls. For each time period (24 and 48 h) 2000 cells were analyzed. SD = standard deviation of the mean.
| Treatments | Blood collect time | Number of MNPCE per animal |
MNPCE mean ± SD |
PCE/NCE mean ± SD |
||||
|---|---|---|---|---|---|---|---|---|
| M1 | M2 | M3 | M4 | M5 | ||||
| Control (sunflower oil) | 24 h | 1 | 2 | 2 | 1 | 2 | 1.60 ± 0.54 | 1.30 ± 0.12 |
| 48 h | 2 | 3 | 2 | 2 | 1 | 2.00 ± 0.70 | 1.35 ± 0.15 | |
| 6,7-Dihydroxycoumarin (25 mg kg−1) | 24 h | 5 | 3 | 5 | 2 | 5 | 4.00 ± 1.41 | 1.90 ± 0.33 |
| 48 h | 2 | 1 | 1 | 1 | 2 | 1.60 ± 0.54 | 1.50 ± 0.25 | |
| 6,7-Dihydroxycoumarin (50 mg kg−1) | 24 h | 3 | 3 | 4 | 2 | 2 | 2.80 ± 0.83 | 1.60 ± 0.09 |
| 48 h | 3 | 1 | 1 | 2 | 2 | 1.80 ± 0.83 | 1.50 ± 0.40 | |
| 6,7-Dihydroxycoumarin (500 mg kg−1) | 24 h | 4 | 4 | 5 | 5 | 6 | 4.80 ± 0.83 | 1.30 ± 0.20 |
| 48 h | 4 | 4 | 7 | 6 | 7 | 5.60 ± 1.51 | 1.35 ± 0.16 | |
| Doxorubicin (DXR) (80 mg kg−1) | 24 h | 7 | 27 | 28 | 24 | 35 | 24.20 ± 10.43* | 1.20 ± 0.20 |
| 48 h | 24 | 23 | 9 | 19 | 18 | 18.60 ± 5.94* | 1.30 ± 0.16 | |
Significantly different from the negative control (P < 0.001).
The potential chemoprotection of 6,7-HC against DXR DNA damage administered simultaneously to the test compound was also investigated by the comet assay (Table 2). The results showed a significant reduction in the extent of DNA damage for peripheral blood and testicular cells exposed to 25 mg kg−1 of 6,7-HC plus DXR, when compared with the DXR-treated group alone. In the other high doses of all cell types and in all doses tested in liver and bone marrow cells, the chemoprevention was not observed. In the cells with significant chemoprevention, the percentage of reductions was high and ranged from 80.91% to 87.50%.
Table 2.
DNA migration in the comet assay for assessing the antigenotoxicity of 6,7-dihydroxycoumarin (6,7-HC) in different cells of male Swiss mice in vivo (mean ± SD).
| Treatments and cells analyzed | Totala | Comet class |
Scores | Reduction (%)b | |||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | ||||
| Peripheral blood (4 h sample) | |||||||
| 6,7-HC 25 mg kg−1+ DXR 80 mg kg−1 | 13.00 ± 2.38* | 87.00 ± 2.38 | 11.50 ± 1.71 | 1.50 ± 0.76 | 0.00 ± 0.00 | 14.50 ± 3.10* | 80.91 |
| 6,7-HC 50 mg kg−1 + DXR 80 mg kg−1 | 28.17 ± 3.24 | 71.83 ± 3.24 | 22.67 ± 2.49 | 5.00 ± 0.58 | 0.50 ± 0.50 | 34.17 ± 4.49 | 9.87 |
| 6,7-HC 500 mg kg−1 + DXR 80 mg kg−1 | 28.33 ± 3.50 | 71.67 ± 3.50 | 22.67 ± 2.49 | 5.17 ± 0.69 | 0.50 ± 0.50 | 34.50 ± 4.99 | 9.12 |
| Doxorubicin (DXR) 80 mg kg−1 | 30.00 ± 3.42 | 72.17 ± 6.94 | 23.83 ± 2.54 | 5.00 ± 1.15 | 1.17 ± 0.37 | 37.33 ± 4.64 | |
| Peripheral blood (24 h sample) | |||||||
| 6,7-HC 25 mg kg−1+ DXR 80 mg kg−1 | 18.17 ± 2.85* | 81.83 ± 2.85 | 15.33 ± 2.21 | 2.17 ± 0.69 | 0.67 ± 0.47 | 21.67 ± 3.99* | 85.63 |
| 6,7-HC 50 mg kg−1 + DXR 80 mg kg−1 | 34.17 ± 2.54 | 65.83 ± 2.54 | 29.00 ± 2.00 | 4.00 ± 0.82 | 1.17 ± 0.37 | 40.50 ± 3.30 | 27.11 |
| 6,7-HC 500 mg kg−1 + DXR 80 mg kg−1 | 32.67 ± 2.43 | 67.33 ± 2.43 | 27.00 ±1.29 | 4.00 ± 1.00 | 1.67 ± 0.75 | 40.00 ± 4.73 | 29.13 |
| Doxorubicin (DXR) 80 mg kg−1 | 36.00 ± 4.43 | 64.00 ± 4.43 | 25.50 ± 6.24 | 8.50 ± 1.71 | 2.00 ± 0.82 | 48.50 ± 3.59 | |
| Liver | |||||||
| 6,7-HC 25 mg kg−1+ DXR 80 mg kg−1 | 88.33 ± 1.34 | 11.17 ± 1.34 | 47.33 ± 2.75 | 29.33 ± 1.25 | 12.17 ± 0.69 | 142.50 ± 1.98 | 5.09 |
| 6,7-HC 50 mg kg−1 + DXR 80 mg kg−1 | 88.67 ± 2.43 | 11.33 ± 2.43 | 47.17 ± 2.41 | 29.33 ± 2.49 | 12.17 ± 2.91 | 142.33 ± 3.59 | 5.38 |
| 6,7-HC 500 mg kg−1 + DXR 80 mg kg−1 | 89.67 ± 1.89 | 10.33 ± 1.89 | 50.17 ± 1.95 | 27.33 ± 1.60 | 12.17 ± 1.77 | 141.33 ± 6.47 | 6.40 |
| Doxorubicin (DXR) 80 mg kg−1 | 88.67 ± 3.14 | 11.33 ± 3.14 | 44.83 ± 2.03 | 30.33 ± 1.97 | 13.50 ± 1.38 | 149.00 ± 7.19 | |
| Bone marrow | |||||||
| 6,7-HC 25 mg kg−1+ DXR 80 mg kg−1 | 87.67 ± 1.37 | 12.33 ± 1.37 | 39.17 ± 3.53 | 42.50 ± 1.89 | 6.00 ± 0.82 | 142.17 ± 2.41 | 8.07 |
| 6,7-HC 50 mg kg−1 + DXR 80 mg kg−1 | 90.83 ± 1.34 | 9.17 ± 1.34 | 35.83 ± 1.86 | 47.17 ± 1.77 | 7.83 ± 0.69 | 153.67 ± 2.21 | 0.12 |
| 6,7-HC 500 mg kg−1 + DXR 80 mg kg−1 | 88.67 ± 1.60 | 10.83 ± 1.77 | 35.17 ± 2.67 | 45.50 ± 2.43 | 8.00 ± 1.29 | 150.17 ± 3.48 | 2.36 |
| Doxorubicin (DXR) 80 mg kg−1 | 90.67 ± 0.94 | 9.33 ± 0.94 | 35.00 ± 1.73 | 48.50 ± 1.71 | 7.17 ± 1.07 | 153.50 ± 1.98 | |
| Testicular | |||||||
| 6,7-HC 25 mg kg−1+ DXR 80 mg kg−1 | 11.67 ± 2.81* | 88.33 ± 2.81 | 10.50 ± 2.22 | 1.17 ± 0.69 | 0.00 ± 0.00 | 12.83 ± 3.44* | 87.50 |
| 6,7-HC 50 mg kg−1 + DXR 80 mg kg−1 | 37.33 ± 2.43 | 62.67 ± 2.43 | 28.83 ± 1.77 | 6.00 ± 1.29 | 2.50 ± 0.50 | 48.33 ± 3.90 | 14.60 |
| 6,7-HC 500 mg kg−1 + DXR 80 mg kg−1 | 39.50 ± 5.19 | 60.50 ± 5.19 | 29.50 ± 2.36 | 7.83 ± 2.27 | 2.17 ± 0.69 | 51.67 ± 8.77 | 7.58 |
| Doxorubicin (DXR) 80 mg kg−1 | 40.83 ± 4.56 | 60.83 ± 4.18 | 29.33 ± 2.56 | 9.00 ± 1.53 | 2.50 ± 0.76 | 54.83 ± 7.43 | |
Significantly different from doxorubicin (P < 0.05).
Total number of damaged cells (class 1 + 2 + 3).
How much 6,7-HC decreased the genotoxicity of the DXR, according to Waters et al. [34].
The clastogenicity/aneugenicity of 6,7-HC was evaluated by the micronucleus test. The frequency of MNPCE in bone marrow cells collected 24 and 48 h after the administration of the test substance and the PCE/NCE ratio are presented in Table 3. The number of micronucleated cells did not increase after treatment with 25, 50 and 500 mg kg−1 b.w. of 6,7-HC, demonstrating that this coumarin derivative has no effects on these mutagenic endpoints at the tested doses. As expected, the administration of DXR as positive control drug led to a significant increase in the number of micronucleated cells when compared to the control (P < 0.001). Bone marrow cytotoxicity was evaluated by quantifying the PCE/NCE ratio in 200 erythrocytes. The results revealed that 6,7-HC or DXR treatment did not decrease the PCE/NCE ratio compared with sunflower oil control.
4. Discussion
To improve the protection and safety of humans and the environment, international agencies determine the requirement of in vivo mammalian studies to assess the genotoxicity of chemicals intended for human use to its registration and authorization as therapeutic agents [35]. On the other hand, other international agencies, as the European Commission Cosmetic Products Directive, determine an increase in efforts to reduce the use of animals in safety testing. To address both goals, in the present study we combined the in vivo comet and micronucleus (MN) assays without need to use additional number of animals.
In both the assays, the endpoints measured are different. The alkaline version of the comet assay detects double and single strand breaks in DNA and maximizes the expression of alkali-labile sites (apurinic sites) transforming them quickly to strand breaks under alkaline condition [36]. In comparison with other genotoxicity tests, the advantages of the comet assay include the sensitivity for detecting low levels of DNA damage, requirement of small number of cells per sample, its relative low costs, flexibility, easy application, and the short time to complete a study. In our present contribution, comet assay performed in peripheral blood leukocytes, liver, bone marrow and testicular cells showed that the test compound 6,7-HC presented no genotoxic effects in any of the tested doses. Likewise, Maistro et al. [27] showed preliminarily, with in vitro studies, also using the comet assay, absence of cytotoxic and genotoxic effects of 6,7-HC in human lymphocytes. The same authors also observed absence of point mutations by the Salmonella/microsome test. Therefore, our in vivo study using comet assay confirms the absence of genotoxic effects of 6,7-HC. Another coumarin derivative with chemical structure very similar to the 6,7-HC that had its genotoxic and mutagenic potential analyzed was 4-methylesculetin (4-ME). In vitro and in vivo studies using the comet assay also showed absence of genotoxicity and mutagenicity by the Ames test; however, 4-ME showed greater cytotoxicity at high concentrations than 6,7-HC [26], [27].
The other contribution of this study was to investigate the in vivo aneugenic/clastogenic potential of 6,7-HC by micronucleus test. This assay detects gross mutations, like structural and numerical chromosome aberrations [32], [42]. The results obtained for us revealed that 6,7-HC presented absence of clastogenic/aneugenic effects in bone marrow cells of mice. These results confirm the in vitro data obtained on human lymphocytes as well as by the Ames test [27]. Similarly, Fedato and Maistro [26] also observed that 4-methylesculetin has no aneugenic/clastogenic effect in polychromatic erythrocytes from bone marrow of mice.
Considering that coumarin derivative 6,7-HC displays a high antioxidant activity with potential use to humans, the present study also investigated its potential chemoprotection on doxorubicin (DXR)-induced DNA damage. The results obtained showed that only the lower tested dose of 6,7-HC (25 mg kg−1) reduced significantly the DNA damage of DXR. This chemoprotection occurred only in peripheral blood leukocytes and testicular cells detected by the comet assay. From the antigenotoxic point of view, the coumarin derivative 4-ME, that also exerts a high antioxidant activity, demonstrated higher protective effects against DXR-induced DNA damage than 6,7-HC. All tested doses of 4-ME showed antigenotoxic effects in all analyzed cell types, with chemoprotection ranging from 34.1% to 93.3% in the comet assay, and 54.4% to 65.9% in the micronucleus test [26].
In conclusion, the results obtained in this investigation showed that 6,7-dihydroxycoumarin (6,7-HC) administered by gavage was not genotoxic in peripheral blood leukocytes, liver, bone marrow and testicular cells of mice by the comet assay, and was not clastogenic/aneugenic in bone marrow cells of mice by the micronucleus test. These results along with the findings from other studies on 6,7-HC available in the literature attest its safety relative to damage in the genetic material of mammals. 6,7-HC also plays a role in inhibiting the genotoxicity induced by the antitumoral agent DXR, but the protective effects were observed only in a low dose of simultaneous treatment. Therefore, further studies are needed to better understand the possible antigenotoxic effects of this hydroxycoumarin derivative.
Transparency document
Acknowledgment
This research was supported by the FAPESP – Fundação de Amparo à Pesquisa do Estado de São Paulo, Brazil (grants 2010/07577-3).
References
- 1.Murray R.D.H. Progress in the Chemistry of Organic Natural Products. Spinger; New York, NY, USA: 1997. Naturally occurring plant coumarins; pp. 2–105. [Google Scholar]
- 2.Berenbaum M. Phototoxicity of plant secondary metabolites: insect and mammalian perspectives. Arch. Insect Biochem. Physiol. 1995;29:119–134. doi: 10.1002/arch.940290204. [DOI] [PubMed] [Google Scholar]
- 3.Ngwendson J.N., Bedir E., Efange S.M., Okunji C.O., Iwu M.M., Schuster B.G., Khan I. Constituents of Peucedanum zenkeri seeds and their antimicrobial effects. Pharmazie. 2003;58:587–589. [PubMed] [Google Scholar]
- 4.Guo L.Q., Yamazoe Y. Inhibition of cytochrome P450 by furanocoumarins in grapefruit juice and herbal medicines. Acta Pharmacol. Sin. 2004;25:129–136. [PubMed] [Google Scholar]
- 5.Setzer W.N., Setzer M.C., Schmidt J.M., Moriarity D.M., Vogler B., Reeb S., Holmes A.M., Haber W.A. Cytotoxic components from the bark of Stauranthus perforatus from Monteverde, Costa Rica. Planta Med. 2000;66:493–494. doi: 10.1055/s-2000-8595. [DOI] [PubMed] [Google Scholar]
- 6.Wattenberg L.W., Lam L.K., Fladmoe A.V. Inhibition of chemical carcinogen-induced neoplasia by coumarins and alpha-angelicalactone. Cancer Res. 1979;39:1651–1654. [PubMed] [Google Scholar]
- 7.Cai Y., Kleiner H., Johnston D., Dubowski A., Bostic S., Ivie W., DiGiovanni J. Effect of naturally occurring coumarins on the formation of epidermal DNA adducts and skin tumors induced by benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 1997;18:1521–1527. doi: 10.1093/carcin/18.8.1521. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka T., Kawabata K., Kakumoto M., Hara A., Murakami A., Kuki W., Takahashi Y., Yonei H., Maeda M., Ota T., Odashima S., Yamane T., Koshimizu K., Ohigashi H. Citrus auraptene exerts dose-dependent chemopreventive activity in rat large bowel tumorigenesis: the inhibition correlates with suppression of cell proliferation and lipid peroxidation and with induction of phase II drug-metabolizing enzymes. Cancer Res. 1998;58:2550–2556. [PubMed] [Google Scholar]
- 9.Kelly V.P., Ellis E.M., Manson M.M., Chanas S.A., Moffat G.J., McLeod R., Judah D.J., Neal G.E., Hayes J.D. Chemoprevention of aflotoxin B1 hepatocarcinogenesis by coumarin, a natural benzopyrone that is a potent inducer of aflatoxin B1-aldehyde reductase, the glutathione S-transferase A5 and P1 subunits, and NAD(P)H:quinone oxidoreductase in rat liver. Cancer Res. 2000;60:957–969. [PubMed] [Google Scholar]
- 10.Baba M., Jin Y., Mizuno A., Suzuki H., Okada Y., Takasuka N., Tokuda H., Nishino H., Okuyama T. Studies on cancer chemoprevention by traditional folk medicines. XXIV. Inhibitory effect of a coumarin derivative, 7-isopentenyloxycoumarin, against tumor-promotion. Biol. Pharm. Bull. 2002;25:244–246. doi: 10.1248/bpb.25.244. [DOI] [PubMed] [Google Scholar]
- 11.Kleiner H.E., Vulimiri S.V., Starost M.F., Reed M.J., DiGiovanni J. Oral administration of the citrus coumarin, isopimpinellin, blocks DNA adduct formation and skin tumor initiation by 7,12-dimethylbenz[a]anthracene in SENCAR mice. Carcinogenesis. 2002;23:1667–1675. doi: 10.1093/carcin/23.10.1667. [DOI] [PubMed] [Google Scholar]
- 12.Prince M., Campbell C.T., Robertson T.A., Wells A.J., Kleiner H.E. Naturally occurring coumarins inhibit 7,12-dimethylbenz[a]anthracene DNA adduct formation in mouse mammary gland. Carcinogenesis. 2006;27:1204–1213. doi: 10.1093/carcin/bgi303. [DOI] [PubMed] [Google Scholar]
- 13.Sharma S.D., Rajor H.K., Chopra S., Sharma R.K. Studies on structure activity relationship of some dihydroxy-4-methylcoumarin antioxidants based on their interaction with Fe(III) and ADP. Biometals. 2005;18:143–154. doi: 10.1007/s10534-004-4256-3. [DOI] [PubMed] [Google Scholar]
- 14.Harvey R.G., Cortex C., Ananthanarayan T.P., Schmolka S.J. A new coumarin synthesis and its utilization for the synthesis of polycyclic coumarin compounds with anticarcinogenic properties. J. Org. Chem. 1988;53:3936–3943. [Google Scholar]
- 15.Kostova I., Raleva S., Genova P., Argirova R. Structure-activity relationships of synthetic coumarins as HIV-1 inhibitors. Bioinorg. Chem. Appl. 2006;2006:1–9. doi: 10.1155/BCA/2006/68274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffet R.S. Central nervous system depressants. VII. Pyridyl coumarins. J. Med. Chem. 1964;7:446–449. doi: 10.1021/jm00334a010. [DOI] [PubMed] [Google Scholar]
- 17.Fylaktakidou K.C., Hadipavlou-Litina D.J., Litinas K.E., Nicolaides D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004;10:3813–3833. doi: 10.2174/1381612043382710. [DOI] [PubMed] [Google Scholar]
- 18.Al-Haiza M.A., Mostafa M.S., El-Kady M.Y. Synthesis and biological evaluation of some new coumarin derivatives. Molecules. 2003;8:275–286. [Google Scholar]
- 19.Jung J., Kin J., Park O.S. A Convenient one-pot synthesis of 4-hydroxycoumarin, 4-hydroxythiocoumarin and 4-hydroxyquinolin-2(1H)-one. Synth. Commun. 2001;31:1195–1200. [Google Scholar]
- 20.Paya M., Halliwell B., Hoult J.R. Interactions of a series of coumarins with reactive oxygen species. Scavenging of superoxide, hypochlorous acid and hydroxyl radicals. Biochem. Pharmacol. 1992;44:205–214. doi: 10.1016/0006-2952(92)90002-z. [DOI] [PubMed] [Google Scholar]
- 21.Vianna D.R., Bubols G., Meirelles G., Silva B.V., Rocha A., Lanznaster M., Monserrat J.M., Garcia S.C., Poser G.V., Eifler-Lima V.L. Evaluation of the antioxidant capacity of synthesized coumarins. Int. J. Mol. Sci. 2012;13:7260–7270. doi: 10.3390/ijms13067260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lake B.G., Grasso P. Comparison of the hepatotoxicity of coumarin in the rat, mouse, and Syrian hamster: a dose and time response study. Fundam. Appl. Pharm. 1996;97:311–323. doi: 10.1006/faat.1996.0181. [DOI] [PubMed] [Google Scholar]
- 23.Lake B. Coumarin metabolism, toxicity, and carcinogenicity: relevance for human risk assessment. Food Chem. Toxicol. 1999;37:423–453. doi: 10.1016/s0278-6915(99)00010-1. [DOI] [PubMed] [Google Scholar]
- 24.Casley-Smith J.R. Benzo-pyrones in the treatment of lymphoedema. Int. Angiol. 1999;18:31–41. [PubMed] [Google Scholar]
- 25.Born S.L., Hu J.K., Lehman-McKeeman L.D. o-Hydroxyphenylacetaldehyde is a hepatotoxic metabolite of coumarin. Drug Metab. Dispos. 2000;28:218–223. [PubMed] [Google Scholar]
- 26.Fedato R.P., Maistro E.L. Absence of genotoxic effects of the coumarin derivative 4-methylesculetin in vivo and its potential chemoprevention against doxorubicin-induced DNA damage. J. Appl. Toxicol. 2014;34:33–39. doi: 10.1002/jat.2823. [DOI] [PubMed] [Google Scholar]
- 27.Maistro E.L., Marques E.S., Fedato R.P., Tolentino F., Silva C.A.C., Tsuboy M.S.F., Resende F.A., Varanda E.A. In vitro assessment of mutagenic and genotoxic effects of coumarin derivatives 6,7-dihydroxycoumarin and 4-methylesculetin. J. Toxicol. Environ. Health A. 2015;78:109–118. doi: 10.1080/15287394.2014.943865. [DOI] [PubMed] [Google Scholar]
- 28.Evans M.D., Cooke M.S. Factors contributing to the outcome of oxidative damage to nucleic acids. Bioassays. 2004;26:533–542. doi: 10.1002/bies.20027. [DOI] [PubMed] [Google Scholar]
- 29.Speit G., Hartmann A. The comet assay (single-cell gel test) In: Henderson D.S., editor. Methods in Molecular Biology, vol. 113, DNA Repair Protocols: Eukaryotic Systems. Humana Press Inc.; Totowa, NJ: 1999. pp. 203–212. [DOI] [PubMed] [Google Scholar]
- 30.Burlinson B., Tice R.R., Speit G., Agurell E., Brendler-Schwaab S.Y., Collins A.R., Escobar P., Honma M., Kumaravel T.S., Nakajima M., Sasaki Y.F., Thybaud V., Uno Y., Vasquez M., Hartmann A. Fourth international workgroup on genotoxicity testing: results of the in vivo comet assay workgroup. Mutat. Res. 2007;627:31–35. doi: 10.1016/j.mrgentox.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Hartmann A., Speit G. The contribution of cytotoxicity to DNA – effects in the single cell gel test (comet assay) Toxicol. Lett. 1997;90:183–188. doi: 10.1016/s0378-4274(96)03847-7. [DOI] [PubMed] [Google Scholar]
- 32.Maistro E.L. The in vivo rodent micronucleus test. In: María Sierra L., Isabel Gaivão, editors. Genotoxicity and DNA Repair: A Practical Approach, Methods in Pharmacology and Toxicology. Springer Science+Business Media; New York: 2014. pp. 103–113. [Google Scholar]
- 33.Gollapudi B.B., McFadden L.G. Sample size for the estimation of polychromatic to normochromatic erithrocyte ratio in the bone marrow micronucleus test. Mutat. Res. 1995;347:97–99. doi: 10.1016/0165-7992(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 34.Waters M.D., Brady A.L., Stack H.F., Brockman H.E. Antimutagenicity profiles for some model compounds. Mutat. Res. 1990;238:57–85. doi: 10.1016/0165-1110(90)90039-e. [DOI] [PubMed] [Google Scholar]
- 35.EMEA . EMEA; London, UK: 2006. ICH Topic S2A Genotoxicity. Note for Guidance on Genotoxicity: Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals (CPMP/ICH/141/95) [Google Scholar]
- 36.Tice R.R., Agurell E., Anderson D., Burlinson B., Hartmann A., Kobayashi H., Miyamae Y., Rojas E., Ryu J.C., Sasaki Y.F. Single cell gel/comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000;35:206–221. doi: 10.1002/(sici)1098-2280(2000)35:3<206::aid-em8>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 37.Conselho Nacional de Saúde . 1997. Resolution No. 251. http://conselho.saude.gov.br/docs/Resolucoes/Reso251.doc (accessed 22.09.14) [Google Scholar]
- 38.Iranshahi M., Askari M., Sahebkar A., Hadjipavlou-Litina D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU J. Pharm. Sci. 2009;17:99–103. [Google Scholar]
- 39.Mitra I., Saha A., Roy K. Predictive modeling of antioxidant coumarin derivatives using multiple approaches: descriptor-based QSAR, 3D-pharmacophore mapping, and HQSAR. Sci. Pharm. 2013;81:57–80. doi: 10.3797/scipharm.1208-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Organization for Economic Co-Operation and Development . 2001. Harmonized Integrated Classification System for Human Health and Environmental Hazards of Chemical Substances and Mixtures. www.oecd.org/dataoecd/48/51/37182285.pdf (accessed 22.09.14) [Google Scholar]
- 41.Schmid W. The micronucleous test. Mutat. Res. 1976 [Google Scholar]
- 42.Krishna G., Hayashi M. In vivo rodent micronucleus assay: protocol, conduct and data interpretation. Mutat. Res. 2000;455:155–166. doi: 10.1016/s0027-5107(00)00117-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.