Abstract
Alterations in liver vascular tone play an important role in chronic liver disease. The hepatic stellate cell (HSC) and mediators such as nitric oxide (NO) and hydrogen sulfide (H2S) have been implicated in regulation of vascular tone and intra-hepatic pressure. Though these have been studied in chronic liver damage, changes in response to acute liver injury induced by hepatotoxins such as dimethyl nitrosamine are not well understood. Liver injury was induced in mice by a single intra-peritoneal injection of dimethylnitrosamine (DMN), following which animals were sacrificed at 24, 48 and 72 h. Changes in vascular mediators such as NO and H2S as well as stellate cell activation was then examined. It was found that a single low dose of DMN in mice is sufficient to induce activation of hepatic stellate cells within 24 h, accompanied by oxidative stress, compromised metabolism of H2S and decreased levels of the von Willebrand factor (vWF) cleaving protease; a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13), which functions in intravascular thrombosis. A suppression of hepatic NO levels is also initiated at this time point, which progresses further and is sustained up to 72 h, at which point the HSC activation is still present. Compromised levels of ADAMTS13 and H2S metabolism however, begin to recover by 48 h and are almost similar to control by 72 h. In conclusion, these data suggest that even moderate acute insults in the liver can have far reaching consequences on a number of mediators of vascular flow in the liver.
Keywords: Liver injury, Nitric oxide, Hydrogen sulfide, Stellate cell, Rhodanese, Oxidant stress
1. Introduction
The liver is a highly vascular organ, where regulation of blood flow plays a critical role in synthetic and metabolic functions. Changes in liver vasculature and the resulting alterations in blood flow have far reaching consequences not only in the liver, but also in other organs such as the lungs [1]. These alterations are typically seen in chronic liver injury, where the resulting portal hypertension leads to complications of liver disease [2]. A number of molecular mediators have been implicated in regulation of hepatic vascular tone with nitric oxide being one of the most important. Nitric oxide (NO) has been shown to modulate hepatic vascular tone in the normal rat liver [3] and NO is one of several vasoactive substances, which are activated in portal hypertension and are responsible for the marked splanchnic vasodilatation seen [2]. Nitric oxide deficiency in the liver can lead to increased intra-hepatic resistance, while increased NO in the circulation contributes to the hyperdynamic systemic/splanchnic circulation [4]. It has recently been demonstrated that NO acts in conjunction with hydrogen sulfide (H2S) to carry out regulation of angiogenesis and endothelium-dependent vasorelaxation [5] and cytoprotective signaling mediated by H2S was found to be dependent on NO generated by endothelial nitric oxide synthase (eNOS) [6].
Hydrogen sulfide is a water soluble small molecule, which is synthesized by the action of the enzymes cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfur transferase (3MST) [7]. CBS and CSE are expressed in many tissues, including the liver [8] and H2S has been suggested to be a potent modulator of vascular tone and also a stimulator of angiogenesis [9]. Hydrogen sulfide has also been shown to modulate sinusoidal constriction and contribute to hepatic microcirculatory dysfunction during endotoxemia [10].
The liver is also an important source of proteins involved in the coagulation cascade, and plays a critical role in regulation of clotting. The organ's importance is illustrated by the impact on coagulation in liver disease, where patients with advanced liver diseases tend to bleed because of reduced plasma levels of several clotting factors and thrombocytopenia [11]. In addition to various coagulation factors produced in the liver, stellate cells in the organ are also the site of production of a disintegrin and metalloprotease with a thrombospondin type 1 motif, member 13 (ADAMTS13), a protease which specifically cleaves multimeric von Willebrand factor (vWF) [12]. Circulating levels of ADAMTS13 levels have been shown to be significantly lower in patients in a variety of liver diseases, including alcoholic hepatitis [13] and idiopathic non cirrhotic intrahepatic portal hypertension [14], [15]. Circulating VWF antigen levels were also found to be highly elevated in patients with acute liver injury/acute liver failure accompanied by low ADAMTS13 levels [16].
Though alterations in these vascular factors in circulation and liver have been studied after chronic liver injury and development of cirrhosis, information on their modulation at early time points induced by acute liver injury is lacking. This is important, since understanding these early changes would provide insight into key mechanistic features which may be amenable to therapeutic interventions, before irreversible changes in liver vasculature take place. To investigate this, we evaluated the temporal course of these vascular mediators in response to a single dose of the hepatotoxin dimethylnitrosamine (DMN).
2. Materials and methods
2.1. Animals and induction of liver injury by DMN
Black C57BL6 mice of both sexes (18–25 g), exposed to a daily 12-h light–dark cycle and fed water and rat chow ad libitum were used for the study. The study was approved by the Institutional Animal Ethics Committee (IAEC). For induction of liver injury, mice were administered a single intraperitoneal injection of DMN (10 mg/kg, ip), while control mice received the vehicle methanol alone. Mice were then sacrificed 24, 48 or 72 h after DMN treatment and blood was taken from the vena cava into a heparinized syringe for measurement of alanine aminotransferase (ALT) activities (Autospan ALT kit, Span Diagnostics Ltd., Surat, India). The liver was removed and rinsed in saline; liver sections were fixed in 10% phosphate-buffered formalin for histological analyses. The remaining liver was snap-frozen in liquid nitrogen and stored at −80 °C for further analysis.
2.2. Liver histology and immunofluorescence detection
Formalin-fixed tissue sections were embedded in paraffin and 4 μm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for evaluation of liver injury. For immunofluorescence, sections were placed on poly-l-lysine coated slides, following which the slides were deparaffinized and antigen retrieval performed (20 min at 95 °C in 10 mmol/L sodium citrate buffer, pH 6). Slides were then incubated either with a mouse monoclonal anti-nitrotyrosine antibody (1:100 dilution, Santa Cruz), a polyclonal rabbit anti-glial fibrillary acidic protein (GFAP) (1:400 dilution, DAKO) or a polyclonal rabbit anti-ADAMTS13 (1:100 dilution, Santa Cruz) overnight at 4 °C. The sections were then incubated with the corresponding secondary antibody for one hour in the dark. The nuclei were stained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (1:1000 dilution, Sigma). The immunostained sections were then mounted using Fluorogel with Tris buffer (Electron Microscopy Sciences) and visualized under a confocal microscope (Olympus FluoView™ FV1000).
2.3. Nitrate measurements
Nitrate in the samples was first reduced to nitrite using a copper–cadmium alloy, which was then measured by the Griess reaction as described [17]. Samples were incubated with the alloy filings in carbonate buffer for 1 h at room temperature with shaking. The reaction was stopped by addition of 0.35 M NaOH and 120 mM ZnSO4 solution, followed by vortexing. After standing for 10 min, the samples were centrifuged at 4000 × g for 10 min. 0.5 ml aliquots of the clear supernatant were treated with 0.25 ml of 1% sulfanilamide and 0.25 ml of 0.1% N-naphthylethylenediamine. After 10 min, the absorbance was read at 545 nm in a spectrophotometer. Amount of nitrate formed was calculated using a standard curve prepared from sodium nitrate and expressed as nmoles/mg protein.
2.4. Measurement of arginase activity
Arginase activity was measured by the conversion of l-arginine to l-ornithine as described [18], with slight modifications. Briefly, liver homogenates were incubated with 10 mM manganese chloride at 55 °C to activate available arginase. Samples were then incubated at 37 °C for 10 min in carbonate buffer (100 mM, pH 10) containing 100 mM l-arginine hydrochloride, followed by addition of acetic acid to stop the reaction. The color developed after a further incubation at 100 °C for 60 min in presence of ninhydrin solution was then measured at 515 nm on a spectrophotometer.
2.5. Quantitative real-time polymerase chain reaction (qRT-PCR)
Expression of the eNOS gene was quantified using qRT-PCR analysis as previously described [19] using the following primers – Fwd: 5′-TGTCACTATGGCAACCAGCGT-3′, Rev: 5′-GCGCAATGTGAGTCCGAAAA-3′. Briefly, mice liver RNA was extracted using the QIAGEN RNeasy mini kit and cDNA synthesized using the QIAGEN Quantitect-Reverse transcription kit according to the manufacturer's instructions. The SYBR green PCR Master Mix (KAPA Biosystems, Boston, MA) was used for real-time PCR analysis. The relative differences in expression between groups were expressed using cycle time (Ct) values. Ct values for the gene was first normalized with that of RPL19, as a housekeeping gene, in the same sample, and relative differences between groups were expressed as relative decrease, setting control as 100%.
2.6. Measurement of oxidant stress parameters
Malondialdehyde levels were estimated in liver samples as an indication of lipid peroxidation by measuring thiobarbituric acid reactive substances (TBARS) as described [20]. Protein carbonyl content was used as a marker for protein oxidation and was measured in samples using dinitrophenyl hydrazine (DNPH) as described [21].
2.7. Isolation of liver mitochondria
The liver was excised and homogenized with 8 volumes of homogenization buffer containing 230 mmol/L mannitol, 70 mmol/L sucrose, and 3 mmol/L HEPES and 1 mmol/L EDTA, pH-7.4 using a Porter–Elvehjem homogenizer. Mitochondria were prepared by differential centrifugation as described [22]. Briefly, the liver homogenate was first centrifuged at 600 × g for 10 min to remove cell debris and mitochondria were pelleted at 15,000 × g for 5 min and washed twice with mitochondrial suspension buffer containing 230 mmol/L mannitol, 70 mmol/L sucrose and 3 mmol/L HEPES at pH 7.4.
2.8. Western blotting
Inducible nitric oxide synthase (iNOS) protein levels were quantitated by western blot analysis. Liver homogenates (20 μg protein) were separated on a 5–16% gradient SDS-PAGE gel using Tris–Glycine–SDS buffer. After electroblotting, the PVDF membranes were blocked with 5% skimmed milk in TBS/Tween 20 (0.02%) (TBST), following which the membrane was probed with a rabbit polyclonal anti-iNOS primary antibody (Santa Cruz Biotechnology) for 12 h at 4 °C. The secondary antibody used was an anti-rabbit (1:5000 dilution) IgG conjugated to horse radish peroxidase (HRP), followed by enhanced chemiluminescence detection. As a loading control, membranes were stripped and reprobed with mouse monoclonal anti-β-actin (1:5000 dilution, Sigma) for 12 h at 4 °C. The secondary antibody used was anti-mouse IgG conjugated to horse radish peroxidase (HRP). Densitometric analysis of the bands was performed using Image J software (NIH).
2.9. Assay of liver H2S synthesis and rhodanese activity
Hydrogen sulfide biosynthesis in the liver was measured as described previously [23]. Briefly, liver homogenates in phosphate buffer were incubated with l-cysteine and pyridoxal 5 phosphate, followed by trapping of the released H2S with zinc acetate. After treatment with N,N-dimethyl-phenylenediamine sulfate and FeCl3, the absorbance at 670 nm was determined and the H2S concentration calculated against a calibration curve of NaHS. Rhodanese activity in liver samples was determined as described previously [24].
2.10. In vitro oxidant exposure
For examining the direct effect of oxidative stress on mitochondrial rhodanese activity, normal liver mitochondria (200 μg protein) was treated with hydrogen peroxide (100 μM) for 30 min at 37 °C. Following treatment, the activity of rhodanese was estimated as mentioned above. To confirm the role, if any, of hydrogen peroxide, experiments were also carried out in the presence of catalase (1000 units).
2.11. Statistical analysis
Results are expressed as mean ± SEM. Student's t-test was used for statistical analysis and a p value of less than 0.05 was taken to indicate statistical significance.
3. Results
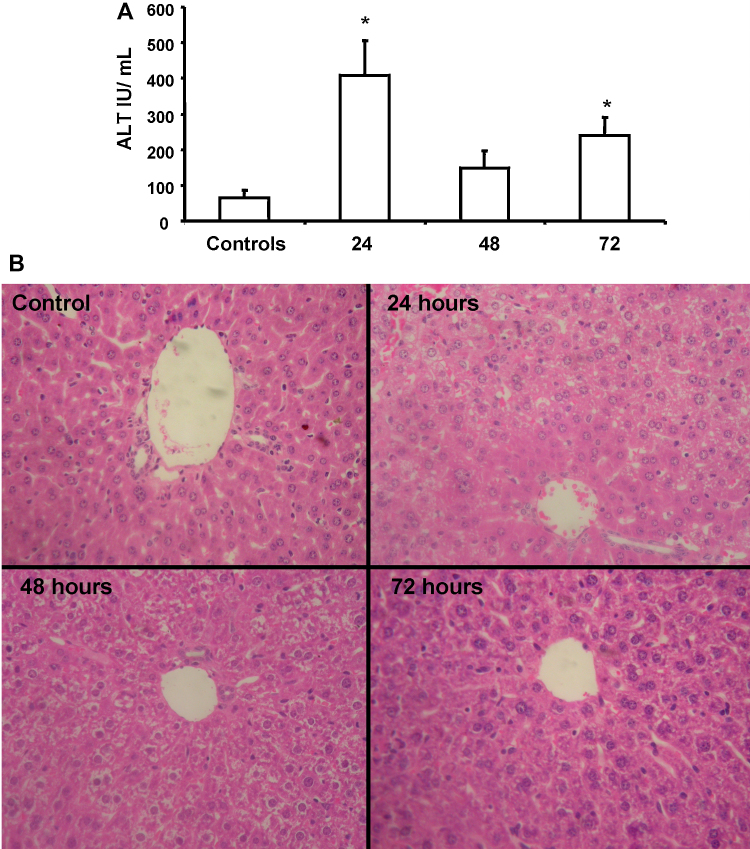
Treatment of C57BL6J mice with a single dose of dimethylnitrosamine (DMN) at 10 mg/kg, resulted in moderate hepatocyte injury by 24 h as seen by the elevation in ALT levels (Fig. 1A). This injury seems to be transient, since ALT levels were found to be lowered by 48 and 72 h after DMN administration. Liver histology indicated normal hepatocellular architecture in controls. By 24 h, perivenular necrosis was evident with hepatocytes showing microvesicular change. Cells around the portal tract were spared. Scattered neutrophils and lymphocytes were seen. By 48 h, these changes were resolving, with only scattered necrotic cells and a decrease in inflammatory cells. By 72 h, recovery was more pronounced with only occasional inflammatory cells being seen (Fig. 1B).
Fig. 1.
(A) Liver injury as assessed by circulating ALT levels at various time points after administration of a single intra-peritoneal dose of 10 mg/kg body weight dimethyl nitrosamine (DMN). Values are mean ± SEM, n = 4 (*p < 0.05). (B) Liver histology at various time points after a single intra-peritoneal dose of 10 mg/kg body weight DMN.
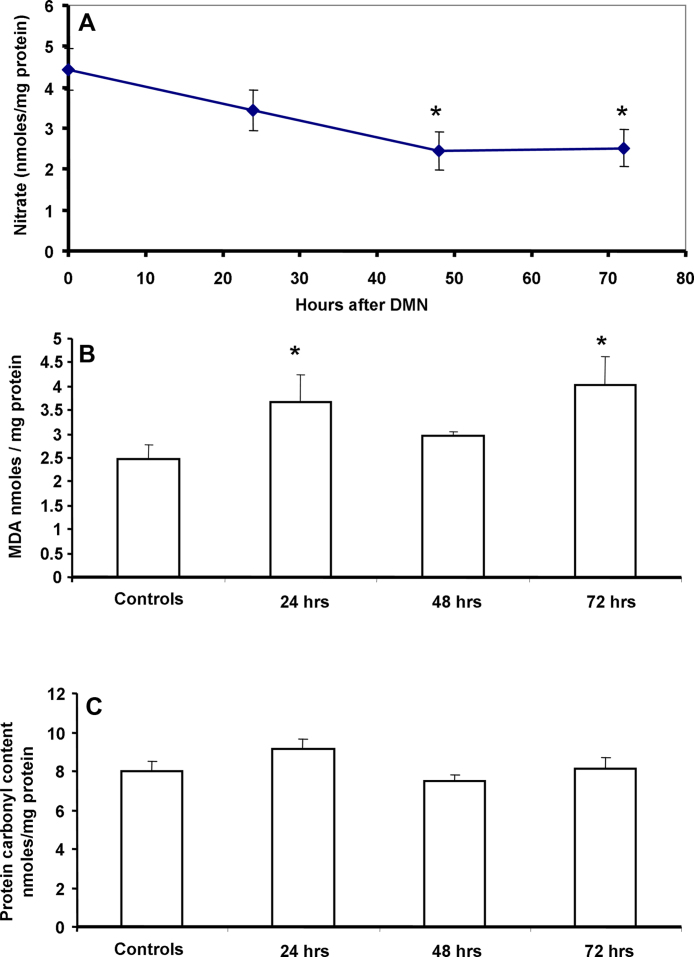
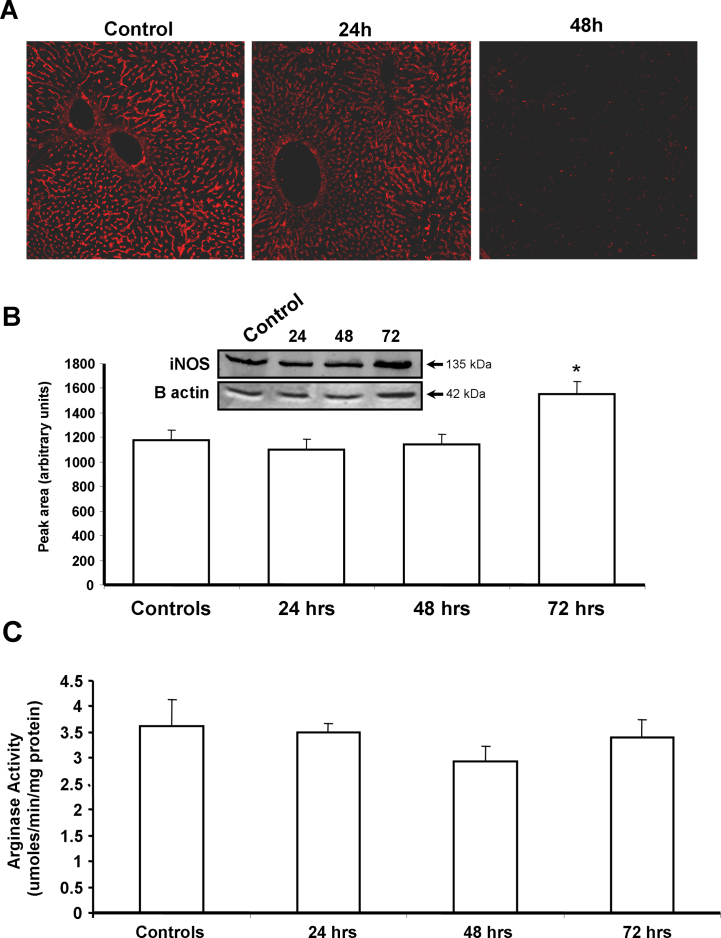
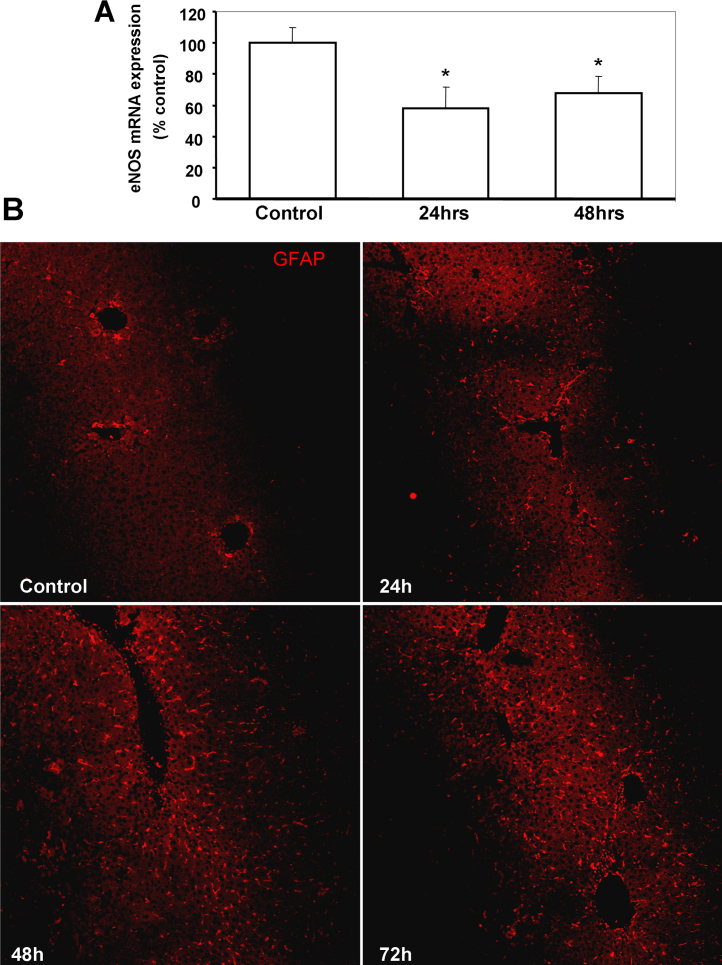
Nitric oxide is a well known physiological vasodilator and has been shown to be involved in modulation of intra-hepatic vascular tone. To evaluate if the low dose DMN administration affected this mediator, the temporal course of liver nitrate was evaluated. As seen in Fig. 2A, a decrease in NO levels was evident as early as 24 h and reached statistical significance by 48 and 72 h after DMN treatment. DMN administration has been shown to induce generation of ROS [25] as well as iNOS [26], albeit when multiple doses were used. To evaluate if a single injection could also induce oxidative stress, parameters of lipid peroxidation and protein oxidation were examined in the liver at various time points after DMN treatment. A moderate but significant increase in malondialdehyde levels was evident within 24 h, which was sustained also at 72 h (Fig. 2B), though no change in liver protein carbonyl content was seen (Fig. 2C). Since the lowering of nitric oxide levels began by 24 h and reaction with reactive oxygen species such as superoxide can scavenge NO due to formation of peroxynitrite [27], this was evaluated next by examination of N-tyrosine formation. Interestingly, immunofluorescence staining of liver sections for N-tyrosine showed that the intensity of nitration was slightly lower by 24 h when compared to constitutive levels in controls and decreased further by 48 h (Fig. 3A). To further examine if the decrease in NO was due to alterations in iNOS levels, this was examined by Western blotting. As seen in Fig. 3B, no significant change was seen in iNOS protein levels at the earlier time points after DMN treatment, though a slight increase was evident by 72 h. This is probably due to the very low DMN dosage in our study compared to earlier studies using DMN. Another cause of modulation of NO levels could be activity of liver arginase, which could compete with NOS for arginine and shunt it to citrulline production instead of NO generation. But this also does not seem to be the case, since liver arginase enzyme activity was not altered after DMN administration (Fig. 3C). These data indicate that the reduction in NO levels subsequent to DMN administration was not due to increased consumption by ROS or inhibition of iNOS, but probably due to lower steady-state levels of the molecule. The NOS isoform responsible for constitutive levels of NO is endothelial NOS, which has been shown to be compromised during DMN-induced liver cirrhosis [28]. eNOS mRNA levels were then examined and as seen in Fig. 4A, a significant decrease in eNOS expression was evident by 24 h after DMN, which remained suppressed even at 48 h.
Fig. 2.
(A) Temporal course of nitrate levels in the liver after a single intra-peritoneal dose of 10 mg/kg body weight DMN. Values are mean ± SEM, n = 4. (B) Lipid peroxidation in the liver at various time points after DMN treatment as estimated by hepatic levels of malondialdehyde. Values are mean ± SEM, n = 4 (*p < 0.05). (C) Protein carbonyl content in the liver at various time points after DMN treatment. Values are mean ± SEM, n = 4 (*p < 0.05).
Fig. 3.
(A) Immunofluorescence staining for nitro-tyrosine in liver sections from mice at various time points after a single intra-peritoneal dose of 10 mg/kg body weight DMN. (B) Representative Western blot and densitometric quantitation of inducible nitric oxide synthase (iNOS) in liver homogenate at 24, 48 and 72 h after DMN administration. Values are mean ± SEM, n = 3 (*p < 0.05). (C) Arginase activity in the liver at various time points after DMN treatment. Values are mean ± SEM, n = 4.
Fig. 4.
(A) eNOS mRNA expression in the liver at various time points after treatment with DMN. Values are mean ± SEM, n = 3 (*p < 0.05). (B) Immunofluorescence staining for glial fibrillary acidic protein (GFAP) as a marker for stellate cells in liver sections from mice at various time points after a single intra-peritoneal dose of 10 mg/kg body weight DMN.
Hepatic stellate cells have been implicated in modulation of vascular tone due to their close proximity to sinusoidal endothelial cells and treatment with higher doses of DMN has been shown to induce HSC proliferation [25]. To evaluate if stellate cells were induced by the moderate liver injury seen after a single 10 mg/kg dose of DMN, immunofluorescence staining for glial fibrillary acidic protein (GFAP), a marker for stellate cells in the liver [29] was carried out in liver sections of mice after DMN treatment. An elevation in stellate cell proliferation was evident by 24 h after induction of liver injury, which was amplified by 48 h and further increased by 72 h after DMN administration (Fig. 4B). This suggests that a single dose of DMN is capable of inducing stellate cell proliferation within 24 h, which persists 3 days later, similar to that seen after multiple doses as described earlier [25].
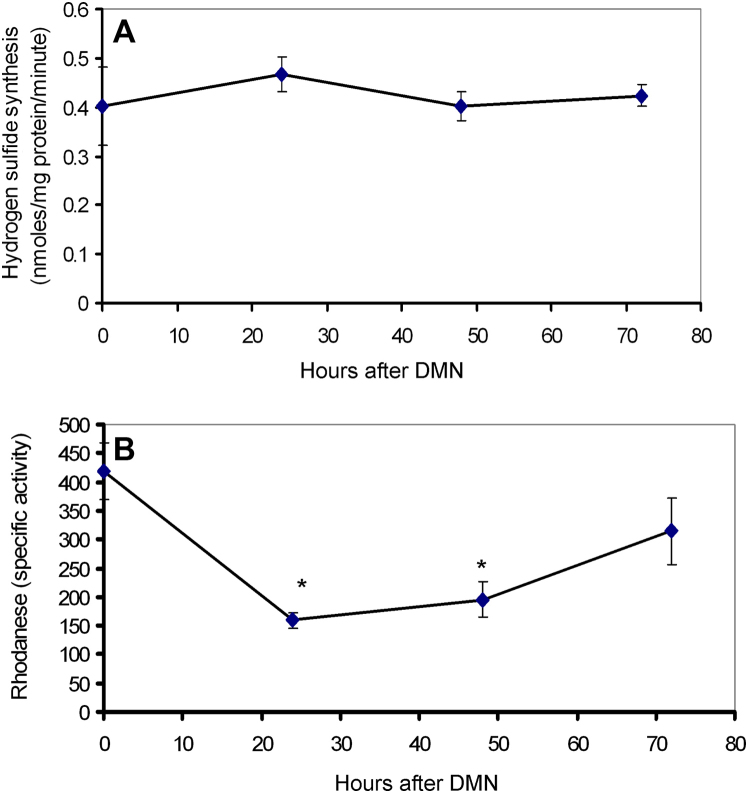
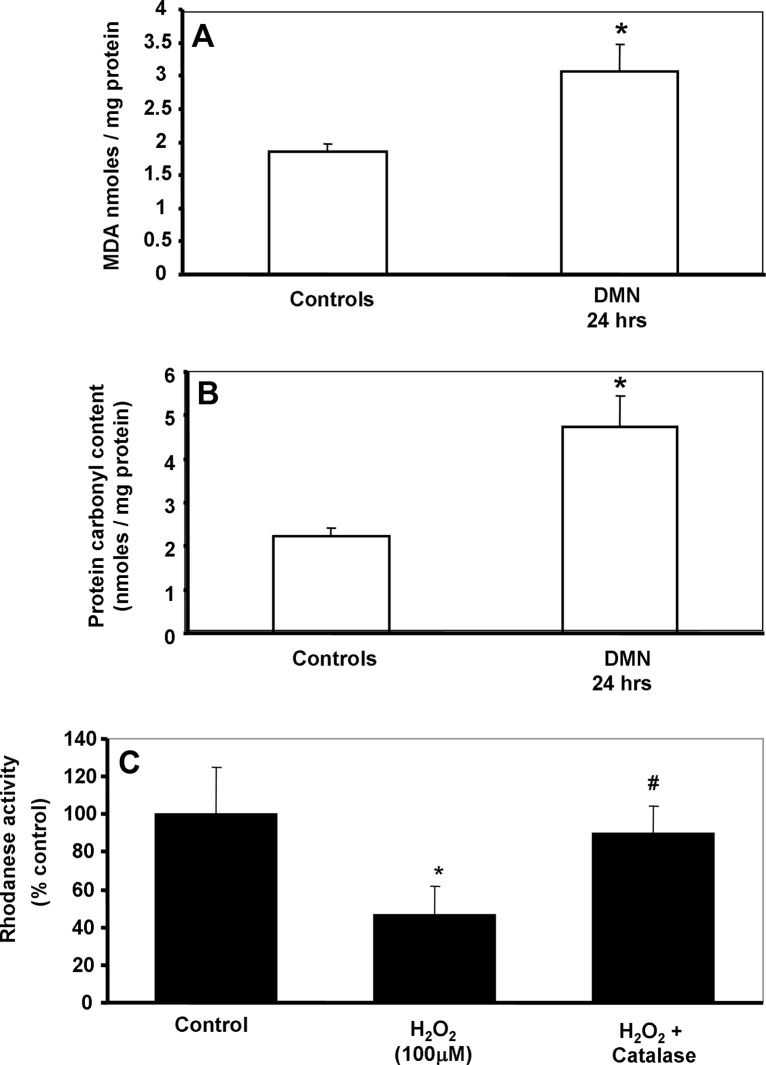
Stellate cell activation has been suggested to be involved in regulation of vascular tone by modulating synthesis of H2S [30], which is now recognized as a vasoconstrictor in the liver [10]. CBS and CSE are the two main enzymes involved in hydrogen sulfide synthesis, while mitochondrial rhodanese plays an important role in H2S degradation. As seen in Fig. 5, examination of H2S synthesis and degradation activity in the liver after DMN administration indicates that while no significant change is seen in the synthetic reactions (Fig. 5A), H2S degradation could be compromised due to the initial loss of rhodanese activity by 24 h, which recovers within 72 h (Fig. 5B). This could produce a transient elevation of H2S levels after DMN administration. To examine potential reasons for inhibition of mitochondrial rhodanese activity within 24 h after DMN, oxidant stress parameters were then examined in mice liver mitochondria isolated after DMN treatment. A significant increase in both malondialdehyde and protein carbonyl content (Fig. 6A and B) clearly indicates an oxidant stress in the mitochondria after DMN. But does oxidant stress affect rhodanese activity? This was then examined in a series of in vitro experiments, where normal mouse liver mitochondria was incubated with hydrogen peroxide (H2O2) to stimulate oxidant stress, following which rhodanese activity was measured. As seen in Fig. 6C, a significant inhibition of rhodanese activity was evident on exposure to H2O2, indicating that the enzyme is susceptible to oxidative damage. The role of hydrogen peroxide in the process was confirmed by repeating the experiments with the H2O2 scavenger, catalase, which completely reversed inhibition of rhodanese, indicating the decrease in activity was due to oxidant stress.
Fig. 5.
Activity of hydrogen sulfide synthetic enzymes (A) and rhodanese (B) in liver homogenates at different time points after a single intraperitoneal dose of 10 mg/kg body weight DMN. Values are mean ± SEM, n = 3 (*p < 0.05).
Fig. 6.
Malondialdehyde (A) and protein carbonyl content (B) in liver mitochondrial isolated 24 h after a 10 mg/kg dose of DMN in mice. Values are mean ± SEM, n = 4 (*p < 0.05). (C) Mitochondrial rhodanese activity after in vitro exposure of normal mitochondria to either 100 μM H2O2 or 100 μM H2O2 + catalase. Values are mean ± SEM, n = 3 (*p < 0.05 when compared to control, #p < 0.05 when compared to H2O2).
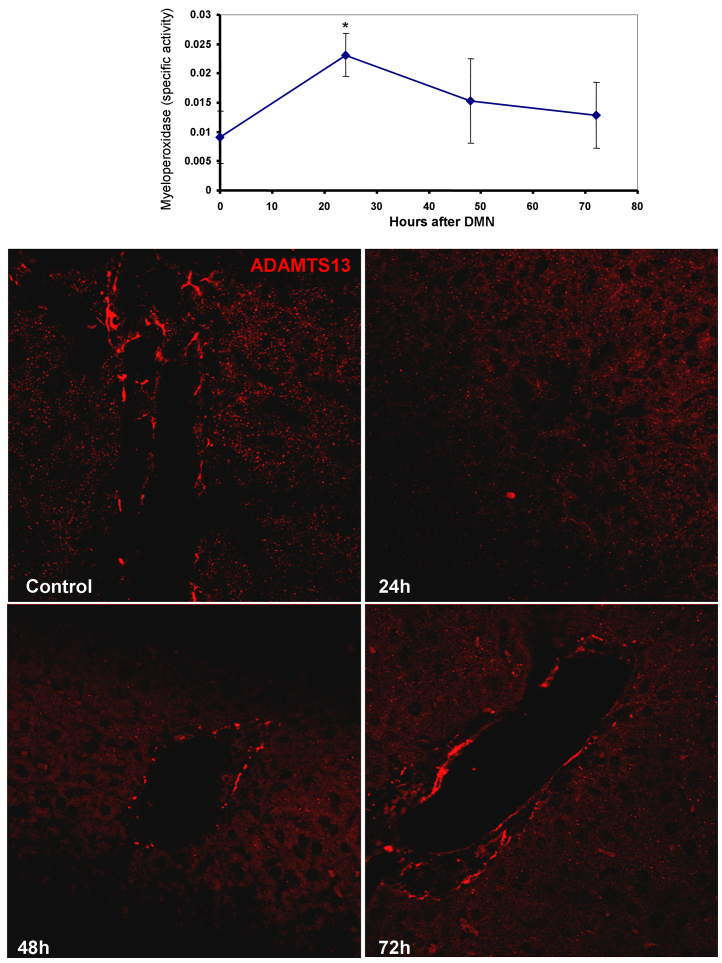
Secretion of pro-inflammatory cytokines such as IL1β, IL6 and TNFα has been noted in activated rat HSCs [31] and the presence of inflammation was then examined in liver samples after DMN treatment. As seen in Fig. 7A, a transient elevation in myeloperoxidase activity was evident, with a peak at 24 h, which reverted to control levels by 48 h. This suggests that some pro-inflammatory cytokine release was occurring after HSC activation at 24 h. Cytokines such as IL-1β have been shown to influence expression of proteins involved in hemodynamics such as the von Willebrand factor cleaving protease, ADAMTS13. Immunofluorescence staining revealed a significant decrease in ADAMTS13 levels within 24 h after DMN administration, which started recovering by 48 h and almost reached control levels by 72 h (Fig. 7B).
Fig. 7.
(A) Temporal course of myeloperoxidase activity in liver homogenates after DMN administration as a marker of inflammation. Values are mean ± SEM, n = 3 (*p < 0.05). (B) Immunofluorescence staining for ADAMTS13 in liver sections from mice at various time points after a single intra-peritoneal dose of 10 mg/kg body weight DMN.
4. Discussion
Dimethylnitrosamine is an alkylating hepatotoxin which has been used extensively to induce chronic liver injury, where doses are typically repeated for a number of weeks to develop severe liver damage such as cirrhosis and hepatocarcinogenesis [32]. Dimethyl-nitrosamine induces liver necrosis and carcinogenesis by a number of mechanisms, which include calcium activated DNA fragmentation [33] and activation of metalloproteases [34]. Microcirculatory differences have been noted in DMN-induced cirrhotic livers during ischemia–reperfusion [35] and intravascular coagulation has been shown to occur in DMN-induced liver injury [36]. Our ALT and histology data suggests that a single 10 mg/kg body weight dose of DMN results in moderate liver injury within 24 h, which begins to resolve by 48 h. This is probably expected, since only a single administration was carried out, with no additional treatments which could have sustained the injury. It has been shown earlier that 3 repeated daily administrations of 10 mg/kg DMN in rats resulted in ALT levels similar to our data, 24 h after the third injection [25]. This suggests that the initial injury is similar with a either a single injection or 3 repeated doses. But can this single moderate insult affect intrahepatic vascular mediators such as nitric oxide?
This seems to be the case, since liver nitrate levels began to drop at 24 h and a significant decrease was obvious at the 48 h time point, NO levels being suppressed up to 72 h. But what was responsible for this continual suppression in steady state NO levels after DMN treatment? Dimethylnitrosamine has been shown to elevate reactive oxygen species in the liver [25] and our data also demonstrated lipid peroxidation by 24 h after DMN administration. Though NO could be scavenged by radicals such as superoxide to produce peroxynitrite [27], this does not seem to be the case in this instance, where interestingly, constitutive nitrotyrosine levels were reduced after DMN. Accompanied by the finding that iNOS levels or arginase activity were also unaltered, this suggests that the low dose DMN treatment alters steady-state NO levels early during injury. The NOS isoform involved in constitutive NO generation is endothelial NOS (eNOS), the hepatic levels of which have been shown to be altered in various liver disorders including NAFLD [37] and hepatic ischemia [38]. The sustained decrease in eNOS mRNA beginning at 24 h after DMN treatment indicates that the lowering of NO levels after DMN induced liver injury could be due to eNOS down regulation. These data suggest that at least at this dose of DMN, NO production is a target and probably not a cause of toxicity. A reduction in nitric oxide production by endothelial cells has been shown in cirrhotic rat livers [39] and it has been demonstrated that activation of hepatic stellate cells is accompanied by decreased eNOS mRNA expression as well as protein [40].
Hepatic stellate cells are critical mediators of liver injury, especially fibrosis. Stellate cells are involved in regulating sinusoidal vascular tone, and are also implicated in the pathogenesis of intrahepatic portal hypertension [41]. In addition, the stellate cell is the main location for synthesis of the ADAMTS13 protein [12], [42], which is a critical mediator in maintaining intrahepatic blood flow and preventing a hypercoagulatory state. GFAP expression in the liver is an early marker of stellate cell activation [29], and HSC activation has been shown with repeated administrations of DMN [25]. Our data now shows that these cells are exquisitely sensitive to even a single moderate insult, since sustained HSC activation, was evident for up to 72 h after a single DMN dose. Activated hepatic stellate cells have been shown to be crucially involved in portal hypertension in cirrhosis, and targeted inhibition of stellate cell Rho-kinase, which is involved in cellular contraction, decreased fibrosis and lowered portal pressure acutely without major systemic effects [43].
So what consequences does this early activation of stellate cells have on other vascular mediators? Stellate cell activation has been shown to modulate levels of H2S in the liver [30], which is now recognized to be involved in modulating vascular tone [10]. Though H2S has been suggested to have a vasodilatory effect in a number of organs including the placenta [44], it has been recently shown to induce a vasoconstrictor effect on the hepatic sinusoid and contribute to vascular resistance in the liver [10]. Portal infusion of H2S has been shown to increase portal pressure in vivo and inhibition of cystathionine γ lyase significantly attenuated the sinusoidal sensitization to endothelin-1 in endotoxin-treated animals [10]. Our data with DMN administration suggests that the decrease in rhodanese activity at 24 h, which would compromise H2S metabolism, coupled with the stable generation of H2S, could result in a transient elevation of the molecule at that time point. This, coupled with the decrease in the vasodilator NO could provide a milieu for vascular resistance in the liver early after DMN. But why is rhodanese activity selectively affected initially after DMN? This is probably due to its localization in the mitochondria [45], an organelle in which oxidant stress was evident within 24 h after DMN as indicated by the MDA and protein carbonyl data. This is further supported by our in vitro studies, which clearly indicate that rhodanese activity is susceptible to oxidant stress. It has been shown that drugs such as atorvastatin increase perivascular adipose tissue-derived H2S by suppressing its mitochondrial oxidation [46] and a lower rhodanese protein expression has been shown to be significantly associated with higher mitochondrial superoxide production in monocytes [47].
The data so far clearly indicates that even moderate liver injury induced by a single administration of DMN results in activation of stellate cells, along with modulation of vasoactive molecules such as NO and H2S. However, another hallmark of stellate cell activation is inflammation [48], and the next question was whether this moderate liver insult could affect inflammatory mediators induced by HSC activation? This seems to be the case, since an elevation in myeloperoxidase activity was evident 24 h after DMN, when HSC proliferation was initiated, along with oxidative stress and compromised rhodanese activity. Cytokines such as IFN-γ, IL-4 and TNF-a dramatically inhibit biosynthesis and secretion of ADAMTS13 protease in rat primary hepatic stellate cells and human endothelial cells [49]. It has also been demonstrated that IL-1β significantly reduced ADAMTS-13 mRNA expression in human microglia and astroglioma cells suggesting their role in the haemostasis of the local microenvironment under inflammatory conditions [50].
A few earlier studies have demonstrated that treatment with 50 mg/kg dimethylnitrosamine induced significant changes in intravascular coagulation within the liver, with observation of fibrin clots in the hepatic sinusoids at 12 h and soluble fibrin monomer complexes detected at 24 h [36]. An extensive reduction in plasma factor VIIIC levels and peripheral platelets was seen after 18 and 24 h, respectively, only in the DMN model [51]. These changes were unique to DMN treatment and not seen in animals treated with CCl4 [51]. Our data now shows that significant alterations in additional coagulation factors such as ADAMTS13 are also evident, even after much smaller doses of DMN. The effect of DMN on NO levels is also relevant in this context, since NO is known to have a protective role against thrombosis, by decreasing vascular resistance and inhibiting platelet aggregation and adhesion [52]. It has also been demonstrated that inhibition of nitric oxide production increases DMN-induced liver injury in rats by accelerating intravascular coagulation [53].
In conclusion, as shown in Fig. 8, we show that moderate liver injury induced by a single low dose of dimethylnitrosamine has far reaching consequences on vascular mediators in the liver. DMN-induced liver injury results in activation of hepatic stellate cells, and induces a drop in steady-state NO levels due to inhibition of eNOS expression. These changes are accompanied by early induction of oxidant stress, which is probably responsible for compromised metabolism of another vasoactive mediator, H2S, along with a decrease in levels of the VWF-cleaving protease ADAMTS13. The compromised degradation of H2S could result in its elevation in the liver, which, combined with the loss of NO as well as ADAMTS13 could contribute to elevations in vascular resistance and a hyper-coagulatory state.
Fig. 8.
Hypothetical model based on the observations of the present study showing modulation of vasoactive mediators in the liver after DMN-induced liver injury. A single low dose DMN administration results in activation of hepatic stellate cells, which induces a drop in steady-state NO levels through down regulation of eNOS expression. This is accompanied by an induction of oxidant stress, which, probably through mitochondrial dysfunction, inhibits the H2S degrading enzyme rhodanese. These changes are accompanied by a decrease in levels of the VWF-cleaving protease ADAMTS13, probably due to activation of inflammatory mediators. The compromised degradation of H2S could result in its elevation in the liver, which, combined with the loss of NO as well as ADAMTS13 could contribute to elevations in vascular resistance and a hyper-coagulatory state.
Transparency document
Acknowledgements
GJA was funded by a Senior Research Fellowship from the Council of Scientific and Industrial Research, Govt. of India. Fluid research funding from the Christian Medical College is gratefully acknowledged. We would also like to acknowledge Prof. Elwyn Elias and Dr. Ian Mackie for helpful discussions.
Footnotes
Available online 16 September 2014
References
- 1.Grace J.A., Angus P.W. Hepatopulmonary syndrome: update on recent advances in pathophysiology, investigation, and treatment. J. Gastroenterol. Hepatol. 2013;28:213–219. doi: 10.1111/jgh.12061. [DOI] [PubMed] [Google Scholar]
- 2.Bolognesi M., Di Pascoli M., Verardo A., Gatta A. Splanchnic vasodilation and hyperdynamic circulatory syndrome in cirrhosis. World J. Gastroenterol. 2014;20:2555–2563. doi: 10.3748/wjg.v20.i10.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittal M.K., Gupta T.K., Lee F.Y., Sieber C.C., Groszmann R.J. Nitric oxide modulates hepatic vascular tone in normal rat liver. Am. J. Physiol. 1994;267:G416–G422. doi: 10.1152/ajpgi.1994.267.3.G416. [DOI] [PubMed] [Google Scholar]
- 4.Hu L.S., George J., Wang J.H. Current concepts on the role of nitric oxide in portal hypertension. World J. Gastroenterol. 2013;19:1707–1717. doi: 10.3748/wjg.v19.i11.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Modis K., Panopoulos P., Asimakopoulou A., Gero D., Sharina I., Martin E., Szabo C. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc. Natl. Acad. Sci. U. S. A. 2012;109:9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King A.L., Polhemus D.J., Bhushan S., Otsuka H., Kondo K., Nicholson C.K., Bradley J.M., Islam K.N., Calvert J.W., Tao Y.X., Dugas T.R., Kelley E.E., Elrod J.W., Huang P.L., Wang R., Lefer D.J. Hydrogen sulfide cytoprotective signaling is endothelial nitric oxide synthase-nitric oxide dependent. Proc. Natl. Acad. Sci. U. S. A. 2014;111:3182–3187. doi: 10.1073/pnas.1321871111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen C.Q., Xin H., Zhu Y.Z. Hydrogen sulfide: third gaseous transmitter, but with great pharmacological potential. Acta Pharmacol. Sin. 2007;28:1709–1716. doi: 10.1111/j.1745-7254.2007.00629.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang R. Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16:1792–1798. doi: 10.1096/fj.02-0211hyp. [DOI] [PubMed] [Google Scholar]
- 9.Kohn C., Dubrovska G., Huang Y., Gollasch M. Hydrogen sulfide: potent regulator of vascular tone and stimulator of angiogenesis. Int. J. Biomed. Sci. 2012;8:81–86. [PMC free article] [PubMed] [Google Scholar]
- 10.Norris E.J., Feilen N., Nguyen N.H., Culberson C.R., Shin M.C., Fish M., Clemens M.G. Hydrogen sulfide modulates sinusoidal constriction and contributes to hepatic microcirculatory dysfunction during endotoxemia. Am. J. Physiol.: Gastrointest. Liver Physiol. 2013;304:G1070–G1078. doi: 10.1152/ajpgi.00395.2012. [DOI] [PubMed] [Google Scholar]
- 11.Marks P.W. Hematologic manifestations of liver disease. Semin. Hematol. 2013;50:216–221. doi: 10.1053/j.seminhematol.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhou W., Inada M., Lee T.P., Benten D., Lyubsky S., Bouhassira E.E., Gupta S., Tsai H.M. ADAMTS13 is expressed in hepatic stellate cells. Lab. Invest. 2005;85:780–788. doi: 10.1038/labinvest.3700275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uemura M., Fujimura Y., Matsuyama T., Matsumoto M., Ishikawa M., Ishizashi H., Kato S., Tsujimoto T., Fujimoto M., Yoshiji H., Morioka C., Fukui H. Potential role of ADAMTS13 in the progression of alcoholic hepatitis. Curr. Drug Abuse Rev. 2008;1:188–196. doi: 10.2174/1874473710801020188. [DOI] [PubMed] [Google Scholar]
- 14.Mackie I., Eapen C.E., Neil D., Lawrie A.S., Chitolie A., Shaw J.C., Elias E. Idiopathic noncirrhotic intrahepatic portal hypertension is associated with sustained ADAMTS13 deficiency. Dig. Dis. Sci. 2011;56:2456–2465. doi: 10.1007/s10620-011-1729-4. [DOI] [PubMed] [Google Scholar]
- 15.Goel A., Alagammai P.L., Nair S.C., Mackie I., Ramakrishna B., Muliyil J., Keshava S.N., Eapen C.E., Elias E. ADAMTS13 deficiency, despite well-compensated liver functions in patients with noncirrhotic portal hypertension. Indian J. Gastroenterol. 2014;33:355–363. doi: 10.1007/s12664-014-0460-4. [DOI] [PubMed] [Google Scholar]
- 16.Hugenholtz G.C., Adelmeijer J., Meijers J.C., Porte R.J., Stravitz R.T., Lisman T. An unbalance between von Willebrand factor and ADAMTS13 in acute liver failure: implications for hemostasis and clinical outcome. Hepatology. 2013;58:752–761. doi: 10.1002/hep.26372. [DOI] [PubMed] [Google Scholar]
- 17.Sastry K.V., Moudgal R.P., Mohan J., Tyagi J.S., Rao G.S. Spectrophotometric determination of serum nitrite and nitrate by copper–cadmium alloy. Anal. Biochem. 2002;306:79–82. doi: 10.1006/abio.2002.5676. [DOI] [PubMed] [Google Scholar]
- 18.Bernard A.C., Fitzpatrick E.A., Maley M.E., Gellin G.L., Tsuei B.J., Arden W.A., Boulanger B.R., Kearney P.A., Ochoa J.B. Beta adrenoceptor regulation of macrophage arginase activity. Surgery. 2000;127:412–418. doi: 10.1067/msy.2000.104115. [DOI] [PubMed] [Google Scholar]
- 19.Novella S., Dantas A.P., Segarra G., Vidal-Gomez X., Mompeon A., Garabito M., Hermenegildo C., Medina P. Aging-related endothelial dysfunction in the aorta from female senescence-accelerated mice is associated with decreased nitric oxide synthase expression. Exp. Gerontol. 2013;48:1329–1337. doi: 10.1016/j.exger.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Lahoti T.S., Patel D., Thekkemadom V., Beckett R., Ray S.D. Doxorubicin-induced in vivo nephrotoxicity involves oxidative stress-mediated multiple pro- and anti-apoptotic signaling pathways. Curr. Neurovasc. Res. 2012;9:282–295. doi: 10.2174/156720212803530636. [DOI] [PubMed] [Google Scholar]
- 21.Sohal R.S., Agarwal S., Dubey A., Orr W.C. Protein oxidative damage is associated with life expectancy of houseflies. Proc. Natl. Acad. Sci. U. S. A. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guerra F.C. Rapid isolation techniques for mitochondria: technique for rat liver mitochondria. Methods Enzymol. 1974;31:299–305. doi: 10.1016/0076-6879(74)31031-2. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Bhatia M., Zhu Y.Z., Zhu Y.C., Ramnath R.D., Wang Z.J., Anuar F.B., Whiteman M., Salto-Tellez M., Moore P.K. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J. 2005;19:1196–1198. doi: 10.1096/fj.04-3583fje. [DOI] [PubMed] [Google Scholar]
- 24.Aminlari M., Gilanpour H. Comparative studies on the distribution of rhodanese in different tissues of domestic animals. Comp. Biochem. Physiol. B. 1991;99:673–677. doi: 10.1016/0305-0491(91)90353-f. [DOI] [PubMed] [Google Scholar]
- 25.Svegliati-Baroni G., Saccomanno S., van Goor H., Jansen P., Benedetti A., Moshage H. Involvement of reactive oxygen species and nitric oxide radicals in activation and proliferation of rat hepatic stellate cells. Liver. 2001;21:1–12. doi: 10.1034/j.1600-0676.2001.210101.x. [DOI] [PubMed] [Google Scholar]
- 26.Hwang Y.P., Choi J.H., Yun H.J., Han E.H., Kim H.G., Kim J.Y., Park B.H., Khanal T., Choi J.M., Chung Y.C., Jeong H.G. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011;49:93–99. doi: 10.1016/j.fct.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Shiva S., Crawford J.H., Ramachandran A., Ceaser E.K., Hillson T., Brookes P.S., Patel R.P., Darley-Usmar V.M. Mechanisms of the interaction of nitroxyl with mitochondria. Biochem. J. 2004;379:359–366. doi: 10.1042/BJ20031758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu B., Zhu G.H., Weng J.F., Cai W.S., Xia J.T., Li S.H. The roles of caveolin-1 and endothelial nitric oxide synthase in the development of portal hypertension in rats with liver cirrhosis. Zhonghua Gan Zang Bing Za Zhi. 2008;16:184–187. [PubMed] [Google Scholar]
- 29.Morini S., Carotti S., Carpino G., Franchitto A., Corradini S.G., Merli M., Gaudio E. GFAP expression in the liver as an early marker of stellate cells activation. Ital. J. Anat. Embryol. 2005;110:193–207. [PubMed] [Google Scholar]
- 30.Fiorucci S., Antonelli E., Mencarelli A., Orlandi S., Renga B., Rizzo G., Distrutti E., Shah V., Morelli A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology. 2005;42:539–548. doi: 10.1002/hep.20817. [DOI] [PubMed] [Google Scholar]
- 31.Wang C.M., Li S.J., Wu C.H., Hu C.M., Cheng H.W., Chang J.S. Transient knock down of Grp78 reveals roles in serum ferritin mediated pro-inflammatory cytokine secretion in rat primary activated hepatic stellate cells. Asian Pac. J. Cancer Prev. 2014;15:605–610. doi: 10.7314/apjcp.2014.15.2.605. [DOI] [PubMed] [Google Scholar]
- 32.Ray S.D., Parikh H., Bagchi D. Proanthocyanidin exposure to B6C3F1 mice significantly attenuates dimethylnitrosamine-induced liver tumor induction and mortality by differentially modulating programmed and unprogrammed cell deaths. Mutat. Res. 2005;579:81–106. doi: 10.1016/j.mrfmmm.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 33.Ray S.D., Sorge C.L., Kamendulis L.M., Corcoran G.B. Ca(++)-activated DNA fragmentation and dimethylnitrosamine-induced hepatic necrosis: effects of Ca(++)-endonuclease and poly(ADP-ribose) polymerase inhibitors in mice. J. Pharmacol. Exp. Ther. 1992;263:387–394. [PubMed] [Google Scholar]
- 34.Syed I., Rathod J., Parmar M., Corcoran G.B., Ray S.D. Matrix metalloproteinase-9, -10, and -12, MDM2 and p53 expression in mouse liver during dimethylnitrosamine-induced oxidative stress and genomic injury. Mol. Cell Biochem. 2012;365:351–361. doi: 10.1007/s11010-012-1277-z. [DOI] [PubMed] [Google Scholar]
- 35.Shimamoto T., Mori Y., Takagi H., Yamada T., Sakamoto K., Matsuo H., Nitta T., Mizutani T., Iwata H., Hirose H. Experimental studies on morphological changes of microcirculation of DMN-induced liver cirrhosis after normothermic ischemia with charge-coupled device microscope. J. Gastroenterol. Hepatol. 2003;18:1071–1075. doi: 10.1046/j.1440-1746.2003.03121.x. [DOI] [PubMed] [Google Scholar]
- 36.Fujiwara K., Ogata I., Ohta Y., Hirata K., Oka Y., Yamada S., Sato Y., Masaki N., Oka H. Intravascular coagulation in acute liver failure in rats and its treatment with antithrombin III. Gut. 1988;29:1103–1108. doi: 10.1136/gut.29.8.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheldon R.D., Laughlin M.H., Rector R.S. Reduced hepatic eNOS phosphorylation is associated with NAFLD and type 2 diabetes progression and is prevented by daily exercise in hyperphagic OLETF rats. J. Appl. Physiol. 2014;116(1985):1156–1164. doi: 10.1152/japplphysiol.01275.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miyake T., Yokoyama Y., Kokuryo T., Mizutani T., Imamura A., Nagino M. Endothelial nitric oxide synthase plays a main role in producing nitric oxide in the superacute phase of hepatic ischemia prior to the upregulation of inducible nitric oxide synthase. J. Surg. Res. 2013;183:742–751. doi: 10.1016/j.jss.2013.01.048. [DOI] [PubMed] [Google Scholar]
- 39.Rockey D.C., Chung J.J. Reduced nitric oxide production by endothelial cells in cirrhotic rat liver: endothelial dysfunction in portal hypertension. Gastroenterology. 1998;114:344–351. doi: 10.1016/s0016-5085(98)70487-1. [DOI] [PubMed] [Google Scholar]
- 40.Wang W., Zhao C., Zhou J., Zhen Z., Wang Y., Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis-related liver fibrosis. PLoS ONE. 2013;8:e76538. doi: 10.1371/journal.pone.0076538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rockey D.C. Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin. Liver Dis. 2001;21:337–349. doi: 10.1055/s-2001-17551. [DOI] [PubMed] [Google Scholar]
- 42.Uemura M., Tatsumi K., Matsumoto M., Fujimoto M., Matsuyama T., Ishikawa M., Iwamoto T.A., Mori T., Wanaka A., Fukui H., Fujimura Y. Localization of ADAMTS13 to the stellate cells of human liver. Blood. 2005;106:922–924. doi: 10.1182/blood-2005-01-0152. [DOI] [PubMed] [Google Scholar]
- 43.Klein S., Van Beuge M.M., Granzow M., Beljaars L., Schierwagen R., Kilic S., Heidari I., Huss S., Sauerbruch T., Poelstra K., Trebicka J. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J. Hepatol. 2012;57:1220–1227. doi: 10.1016/j.jhep.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 44.Cindrova-Davies T., Herrera E.A., Niu Y., Kingdom J., Giussani D.A., Burton G.J. Reduced cystathionine gamma-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am. J. Pathol. 2013;182:1448–1458. doi: 10.1016/j.ajpath.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smirnov A., Comte C., Mager-Heckel A.M., Addis V., Krasheninnikov I.A., Martin R.P., Entelis N., Tarassov I. Mitochondrial enzyme rhodanese is essential for 5 S ribosomal RNA import into human mitochondria. J. Biol. Chem. 2010;285:30792–30803. doi: 10.1074/jbc.M110.151183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Beltowski J. Endogenous hydrogen sulfide in perivascular adipose tissue: role in the regulation of vascular tone in physiology and pathology. Can. J. Physiol. Pharmacol. 2013;91:889–898. doi: 10.1139/cjpp-2013-0001. [DOI] [PubMed] [Google Scholar]
- 47.Krueger K., Koch K., Juhling A., Tepel M., Scholze A. Low expression of thiosulfate sulfurtransferase (rhodanese) predicts mortality in hemodialysis patients. Clin. Biochem. 2010;43:95–101. doi: 10.1016/j.clinbiochem.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 48.Zimmermann H.W., Tacks F. Modification of chemokine pathways and immune cell infiltration as a novel therapeutic approach in liver inflammation and fibrosis. Inflamm. Allergy Drug Targets. 2011;10:509–536. doi: 10.2174/187152811798104890. [DOI] [PubMed] [Google Scholar]
- 49.Cao W.J., Niiya M., Zheng X.W., Shang D.Z., Zheng X.L. Inflammatory cytokines inhibit ADAMTS13 synthesis in hepatic stellate cells and endothelial cells. J. Thromb. Haemost. 2008;6:1233–1235. doi: 10.1111/j.1538-7836.2008.02989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frentzou G.A., Bradford C., Harkness K.A., Haddock G., Woodroofe M.N., Cross A.K. IL-1beta down-regulates ADAMTS-13 mRNA expression in cells of the central nervous system. J. Mol. Neurosci. 2012;46:343–351. doi: 10.1007/s12031-011-9591-6. [DOI] [PubMed] [Google Scholar]
- 51.Hirata K., Ogata I., Ohta Y., Fujiwara K. Hepatic sinusoidal cell destruction in the development of intravascular coagulation in acute liver failure of rats. J. Pathol. 1989;158:157–165. doi: 10.1002/path.1711580211. [DOI] [PubMed] [Google Scholar]
- 52.Toda N., Toda H. Coronary hemodynamic regulation by nitric oxide in experimental animals: recent advances. Eur. J. Pharmacol. 2011;667:41–49. doi: 10.1016/j.ejphar.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 53.Nagase S., Isobe H., Ayukawa K., Sakai H., Nawata H. Inhibition of nitric oxide production increases dimethylnitrosamine-induced liver injury in rats. J. Hepatol. 1995;23:601–604. doi: 10.1016/0168-8278(95)80068-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.