Abstract
Monoamine oxidase-A (MAO-A) is the main enzyme in the metabolism of the neurotransmitter serotonin (5-hydroxytryptamine). Elevated activity of MAO-A in the brain may contribute to the pathogenesis of depressive disorders. Plant flavonoids, such as flavonol quercetin and flavone luteolin, have been suggested to be potential antidepressant compounds because they exert a suppressive effect on the MAO-A reaction. We evaluated the effects of these flavonoids on MAO-A activity and protein level using SH-SY5Y as model serotoninergic nerve cells. Quercetin and luteolin were incorporated into SH-SY5Y cells rapidly and converted to O-methylated derivatives. Luteolin accumulated in cells after 24-h incubation, whereas quercetin disappeared completely from cell fractions and culture medium. Addition of ascorbic acid prevented the disappearance of quercetin and allowed it to exert its cytotoxicity (similar to luteolin) at >10 μM. Luteolin and quercetin were incorporated into mitochondria fractions within 1-h incubation and attenuated MAO-A activity slightly but significantly. After 24-h incubation, luteolin attenuated MAO-A activity, but quercetin needed ascorbic acid for its attenuation. Neither luteolin nor quercetin significantly affected MAO-A protein level. These data suggest that luteolin and quercetin can be direct inhibitors of MAO-A in nerve cells by targeting mitochondria.
Keywords: Monoamine oxidase-A, Quercetin, Luteolin, SH-SY5Y cells, Serotonin metabolism
Abbreviations: MAO-A, monoamine oxidase-A; CNS, central nervous system; DMSO, dimethyl sulfoxide; DMEM, Dulbecco's modified Eagle's medium; PBS, phosphate-buffered saline; SDS, sodium dodecyl sulfate; BCA, bicinchoninic acid
Chemical compounds studied in this article: Quercetin (PubChem ID: 5280343, Luteolin (PubChem ID: 5280445), Isorhamnetin (PubMed ID: 5281654), Diosmetin (PubMed ID: 5281612)
1. Introduction
Monoamine oxidase (MAO) is a flavin enzyme (EC 1.4.3.4) located in the outer membranes of mitochondria. Of the two isoenzymes, MAO-A has attracted considerable attention because of its relationship to the metabolism of the monoamine neurotransmitter serotonin (5-hydroxytriptamine) [1]. Depletion of serotonin level in the central nervous system (CNS) is suggested to be responsible (at least in part) for the pathogenesis of depressive disorders [2]. There is some evidence that MAO-A activity in the brain is elevated during depression [3]. Therefore, it is likely that elevation of MAO-A activity accelerates serotonin metabolism, thereby resulting in depletion of serotonin in the brain.
MAO-A inhibitors, such as clorgyline, have been used as antidepressants. However, prolonged intake of these drugs may induce so-called “cheese effect,” which is based on the inhibition of MAO-A responsible for detoxification of exogenous amines in the small intestine [4], [5]. Herbal medicines are frequently used for the treatment of depression, along with conventional antidepressants. Several studies have implied the essential role of flavonoids in the antidepressant effect of herbal medicines [6], [7]. In particular, 3,3′,4′,5,7-pentahydroxyflavone, quercetin, as well as its structurally related flavonoids, have been shown to exert antidepressant-like effects in rodent models of depression [8], [9], [10]. Furthermore, in vitro studies have shown that quercetin possesses considerable inhibitory effects on MAO-A activity in mitochondrial fractions obtained from mouse brains [11], [12].
Our previous study revealed that quercetin is a weak (but safe) inhibitor of MAO-A in the modulation of serotonin levels in the brain compared with conventional MAO-A antidepressants because it does not evoke side effects in the small intestine [13]. In addition, we found a remarkable inhibitory effect of a flavone-type flavonoid, luteolin (3′,4′,5,7-tetrahydroxyflavone), on MAO-A activity in mitochondria from mouse brains [13] Ishisaka et al. [14] and Sasaki et al. [15] demonstrated that luteolin also possesses significant antidepressant-like activity in rodent studies. Among flavonoids, flavones and flavonols are suggested to act as multitarget therapeutic tools for protecting the brain [16]. These flavonoids (especially quercetin) are present in fruits and vegetables and consumed daily as ingredients of plant foods. Therefore, the effect of quercetin and luteolin on the MAO-A reaction is interesting for the prevention of depression through foods as well as herbal medicines. Our in vivo study already found that dietary quercetin attenuated MAO-A activity in the brain of mouse [12]. However, exact molecular mechanisms for the action of these flavonoids have not been fully clarified. In particular, their effects on the regulation of MAO-A expression have not been investigated yet.
We wished to elucidate the role of quercetin and luteolin as potential MAO-A modulators in the CNS. We selected SH-SY5Y cells as models of serotoninergic neuronal cells. Then we investigated the effect of quercetin and luteolin on MAO-A activity and protein levels, as well as their cellular uptake and intracellular metabolism.
2. Materials and methods
2.1. Chemicals and reagents
Quercetin, isorhamnetin (3′-O-methyl quercetin), luteolin and diosmetin (4′-O-methyl luteolin) were purchased from Extrasynthese (Genay Cedex, France). Penicillin (5000 U/mL) and streptomycin (5000 μg/mL) were obtained from Invitrogen (Carlsbad, CA, USA). Serotonin was a product of Nacalai Tesque (Kyoto, Japan). Kynuramine and Dulbecco's modified Eagle's medium (DMEM)/Nutrient F-12 Ham (D 8437) were from Sigma-Aldrich (St. Louis, MO, USA). Cell Proliferation Reagent WST-1 was from Roche Diagnostics (Mannheim, Germany). Other reagents were of the highest grade available from commercial sources.
2.2. Cell culture
Human neuroblastoma SH-SY5Y cells were kindly provided by Dr. Makoto Naoi (Aichi Gakuin University, Aichi, Japan). Cell cultures were maintained in DMEM/Nutrient F-12 Ham supplemented with 10% fetal bovine serum, 100 U/mL penicillin and 100 μg/mL streptomycin at 37 °C in a humidified atmosphere of 5% CO2.
2.3. SH-SY5Y cells viability of SH-SY5Y cells
SH-SY5Y cells (1 × 105 cell/well) were plated on 96-well microwell plates. After incubation for 24 h, the medium was replaced with 100 μL of fresh medium containing quercetin, isorhamnetin, luteolin or diosmetin. These test flavonoids were dissolved in dimethyl sulfoxide (DMSO) and added to culture medium at final concentrations 0.1, 1, 10, 50 and 100 μM followed by incubation for a further 24 h. The final concentration of DMSO was ≤0.5%. Cells were treated with 5 μL of Cell Proliferation Reagent WST-1 followed by incubation for 2 h under the same conditions. After shaking of plates thoroughly for 1 min, absorbance at 450 nm was measured. Cell viability was expressed as percent of vehicle-treated control.
2.4. Isolation of mitochondrial fractions from SH-SY5Y cells
Mitochondrial fractions were isolated from SH-SY5Y cells according to the method of [17] with slight modification. Briefly, cells were washed twice with ice-cold 10 mM phosphate-buffered saline (PBS) (pH 7.4) containing 140 mM NaCl and 2.7 mM KCl. Then, they were harvested and centrifuged (1200 × g for 5 min at 4 °C). Cell pellets were resuspended in 800 μL of 10 mM PBS (pH 7.4) containing 320 mM sucrose, and homogenized followed by centrifugation (700 × g for 10 min at 4 °C). Obtained supernatants containing mitochondrial fractions were subjected to a second centrifugation (12,000 × g for 15 min at 4 °C). Supernatants from the second centrifugation were discarded and mitochondrial pellets resuspended with 50 mM PBS (pH 7.4). Concentrations of mitochondrial proteins were determined using a bicinchoninic acid (BCA) Protein Assay kit (Pierce, Rockford, IL, USA).
2.5. Determination of MAO-A activity
MAO-A activity was estimated using kynuramine as MAO-A substrate according to the method described by [18] with slight modification. The reaction mixture contained 50 mM PBS (pH 7.4), 200 μM kynuramine (as a MAO-A substrate) and 0.4 mg/mL mitochondrial protein. The final volume of the reaction mixture was 250 μL. Samples were incubated at 37 °C for 1 h and cooled on ice. Five-hundred microliters of distilled water, 250 μL of 10% ZnSO4 and 50 μL of 1 M NaOH were added. Samples were boiled at 100 °C for 2 min, cooled on ice and centrifuged (1000 × g for 10 min). Supernatants were diluted by 5 times with 1 M NaOH. Fluorescence intensity of the reaction product (4-hydroxylquinoline) was measured with λEx = 310 nm and λEm = 380 nm by a Plate Spectrophotometer (Varioskan Flash; Thermo Scientific, Boston, MA, USA) in standard black 96-well plates. Enzymatic activity was expressed as nmol/min/mg protein.
2.6. Quantitative analyses of flavonoids
For evaluation of cellular uptake and mitochondrial accumulation of flavonoids, SH-SY5Y cells were seeded on 60-mm PLL dishes (1 × 106 cell/dish) or 90-mm dishes (3 × 106 cell/dish), respectively. Quercetin and luteolin dissolved in ethanol (final concentration in the medium, 0.5%) were added at 10 μM. After incubation, cells were washed with ice-cold PBS twice and harvested with 1 mL of the same buffer. Protein concentration was determined using the BCA Protein Assay kit. After centrifugation (1200 × g for 5 min at 4 °C), flavonoids were extracted from cell pellets or culture medium with 100 μL of methanol containing 1% acetic acid by sonication for 1 min using an Astrason XL2020 Ultrasonic Processor (Heat Systems, Farmingdale, NY, USA).
Mitochondrial fractions were obtained from treated cells according to the method described in Section 2.4 and flavonoids were extracted as described above. The protein concentration was determined using the BCA Protein assay kit. Kaempferol was added as an internal standard (final concentration, 5 μM) to determine flavonoid contents in obtained extracts. After centrifugation (20,000 × g for 10 min at 4 °C), samples were used for high-performance liquid chromatography analyses with a column of TSK-gel ODS-80Ts (4.6 × 150 mm; Tosoh, Tokyo, Japan) and a mobile phase of 50% methanol with 0.5% H3PO4 at a flow rate 1 mL/min. Flavonoids were detected at 365 nm using a SPD-20A UV Detector (Shimadzu, Kyoto, Japan).
2.7. Western blotting analyses for MAO-A protein
MAO-A protein in SH-SY5Y cells was detected using western blotting analyses. SH-SY5Y cells were seeded on 60-mm PLL dishes (1 × 106 cell/dish) and, after incubation for 24 h, treated with 10 μM quercetin or luteolin for a further 24 h. Cells were washed twice with ice-cold PBS, harvested and suspended in 20 mM HEPES (pH 7.4) containing 150 mM NaCl, 1 mM ethylenediamine tetra-acetic acid, 1% Triton X100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS) and a Protease Inhibitor Cocktail (Roche). After incubation for 1 h on ice with periodic shaking, the suspension was centrifuged (20,000 × g for 20 min at 4 °C). Protein concentration was determined using the BCA Protein Assay kit.
Extracted proteins were separated by SDS–polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes (Amersham Hybond-P; GE Healthcare, Piscataway, NJ, USA). After blocking with 5% fat-free milk, membranes were treated with antibody against MAO-A (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and anti-rabbit IgG horseradish peroxidase-linked antibody (GE Healthcare). For detection of β-actin on the same membranes, anti-MAO-A antibodies were removed by stripping buffer (Amersham, Buckinghamshire, UK) and membranes treated with mouse anti-β-actin antibody (Sigma-Aldrich). The secondary antibody for β-actin was anti-mouse IgG (GE Healthcare). Protein bands were visualized with Amersham ECL plus Western Blotting Detection Reagents (GE Healthcare) using a LAS-3000 UV Mini Luminescent Image Analyzer (Fujifilm, Tokyo, Japan). MAO-A protein bands were quantified using ImageJ v1.47 (National Institutes of Health, Bethesda, MD, USA) and normalized to that of β-actin.
2.8. Statistical analyses
Significance was analyzed using one-way ANOVA with the Tukey multiple comparison test (p < 0.05) or Student's t-test (p < 0.05).
3. Results
3.1. Cell viability and stability of flavonoids in the medium
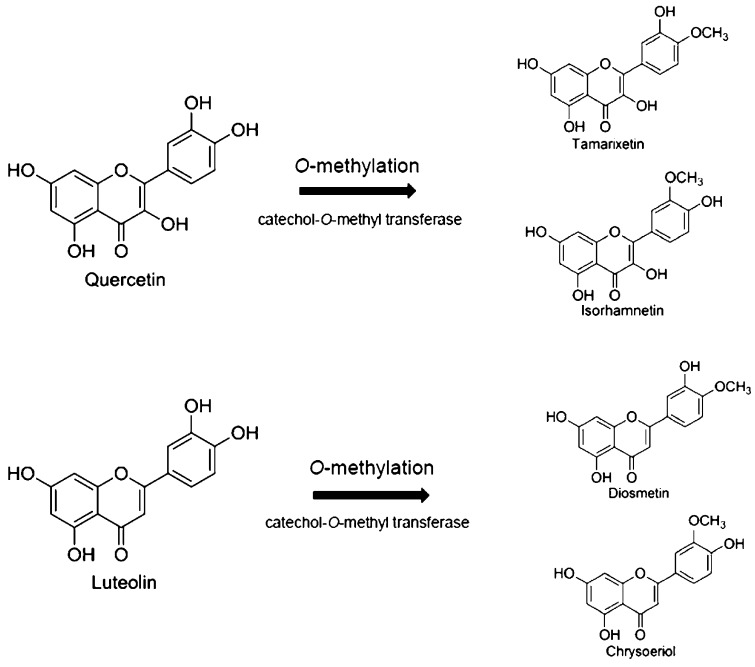
Our previous study [19] demonstrated that quercetin and its O-methylated metabolite accumulated in the brain tissue of quercetin-administered rats. Those data suggested that the catechol group of the B ring of quercetin and luteolin can be subjected to O-methylation by the action of catechol-O-methyl transferase to produce the corresponding O-methylated derivatives tamarixetin (4′-O-methyl quercetin), isorhamnetin (3′-O-methyl quercetin) as well as diosmetin (4′-O-methyl luteolin) and chrysoeriol (3′-O-methyl luteolin), respectively (Fig. 1).
Fig. 1.
Structures of the flavonol quercetin, the flavone luteolin, and their O-methylated metabolites.
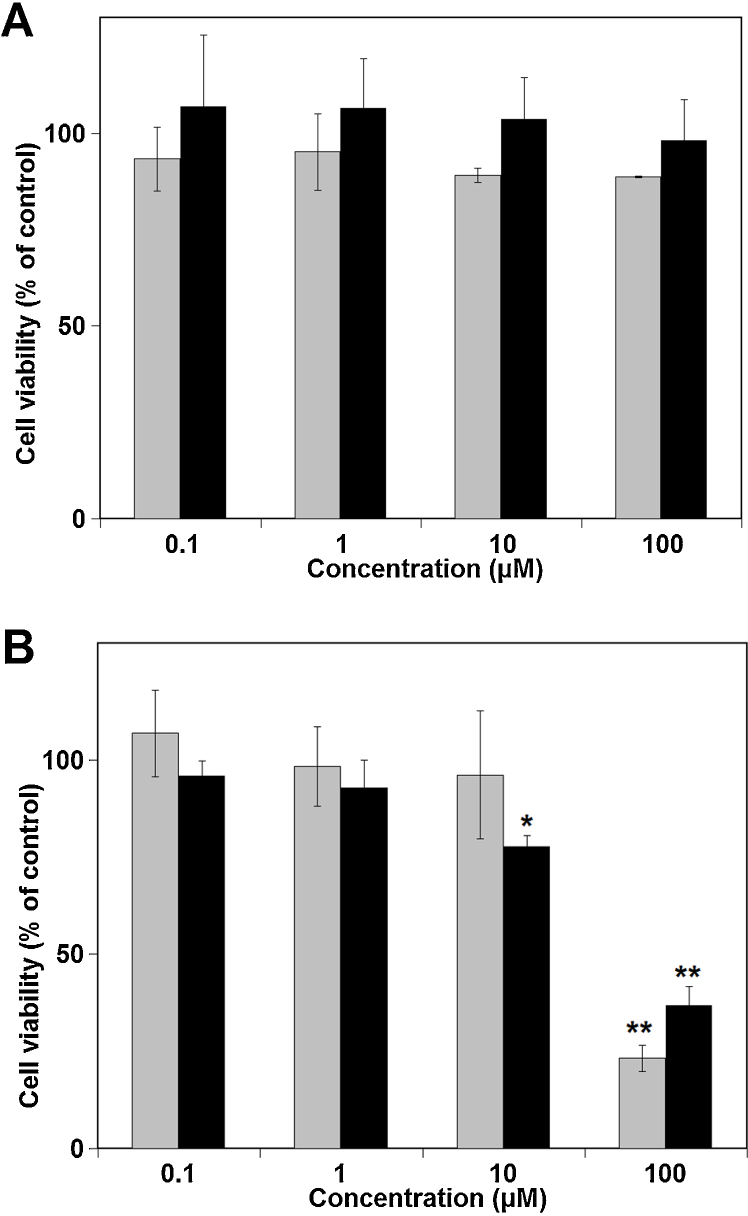
Therefore, we selected isorhamnetin and diosmetin as the representative O-methylated metabolites of quercetin and luteolin, respectively, and their effects on cell viability were compared with those of their non-methylated originals (Fig. 2). Luteolin and diosmetin significantly lowered cell viability by incubation for 24 h at 100 μM (Fig. 2-B), whereas quercetin and its O-methylated metabolite isorhamnetin did not show such lowering effects at ≤100 μM (Fig. 2-A).
Fig. 2.
Effect of quercetin, luteolin and their O-methylated metabolites on the viability of SH-SY5Y cells. SH-SY5Y cells were treated for 24 h with quercetin (A; gray), isorhamnetin (A; black), luteolin (B; gray) or diosmetin (B; black). Cell viability was measured as described in the Materials and methods section. Values were calculated as the percentage of vehicle-treated control and are the mean ± SD from five independent determinations. Significant differences from controls were evaluated by the Student's t-test (*p < 0.01, **p < 0.001).
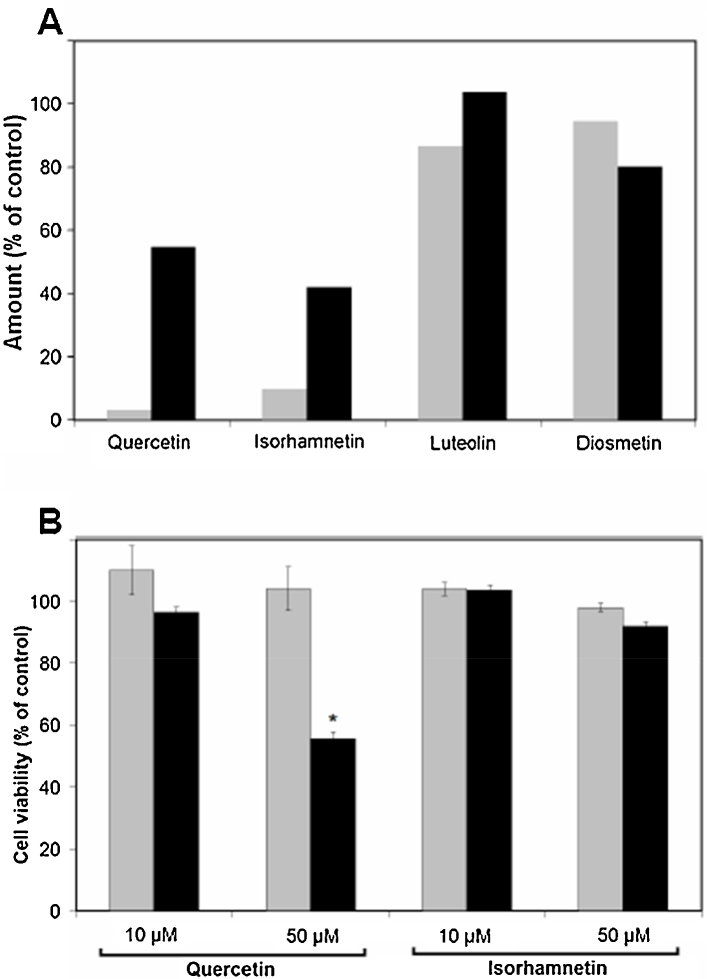
Fig. 3-A shows the effect of ascorbic acid on the stability of quercetin, luteolin and their O-methylated metabolites in cell-free culture medium. In the absence of ascorbic acid, both quercetin and isorhamnetin were unstable in the culture medium, and their amounts were decreased, respectively, to 3% and 10% of initials after 24-h incubation. However, contents of quercetin and isorhamnetin changed to 55% and 42% in the presence of ascorbic acid at 500 μM respectively. Neither luteolin nor diosmetin contents changed irrespective of the presence of ascorbic acid at the same conditions.
Fig. 3.
Effect of ascorbic acid on the stability of quercetin, luteolin and their O-methylated metabolites in the culture medium (A) and cell viability in the presence of flavonols (B). (A) Flavonoids were added to cell-free culture medium without (gray) or with (black) 500 μM ascorbic acid at a final concentration of 10 μM and incubated for 24 h. Values are calculated as percent of zero time-treated control. (B) Cells were treated for 24 h with 10 or 50 μM quercetin or isorhamnetin without (gray) or with (black) 500 μM ascorbic acid. Cell viability was measured as described in the Materials and methods section using Cell Proliferation Reagent WST-1. Values are calculated as the percentage of vehicle-treated (gray bar) or ascorbic acid-treated (black bar) controls and are the mean ± SD from five independent determinations. Significant differences from controls were evaluated by the Student's t-test (*p < 0.05).
This result suggested that flavonols quercetin and isorhamnetin were unstable in the culture medium (and different from the flavones luteolin and diosmetin). Flavonols seem to be liable to auto-oxidation during incubation because ascorbic acid can serve as a hydrophilic antioxidant, which seems to protect them from auto-oxidation in the culture medium. Interestingly, quercetin (50 μM) significantly lowered cell viability in the presence of ascorbic acid (Fig. 3-B), suggesting that (similar to luteolin) it could induce cytotoxicity in SH-SY5Y cells at higher concentrations. It was, therefore, decided that the concentration of quercetin and luteolin should be limited to ≤10 μM for evaluation of their effects on MAO-A activity and protein levels in cells. In contrast to quercetin, isorhamnetin (50 μM) did not show cytotoxic effects even in the presence of ascorbic acid. Hence, different to quercetin, the O-methylated metabolites of quercetin barely induce cytotoxic effects. Ascorbic acid (500 μM) did not affect cell viability (data not shown).
3.2. Distribution of quercetin and luteolin in SH-SY5Y cells and the culture medium
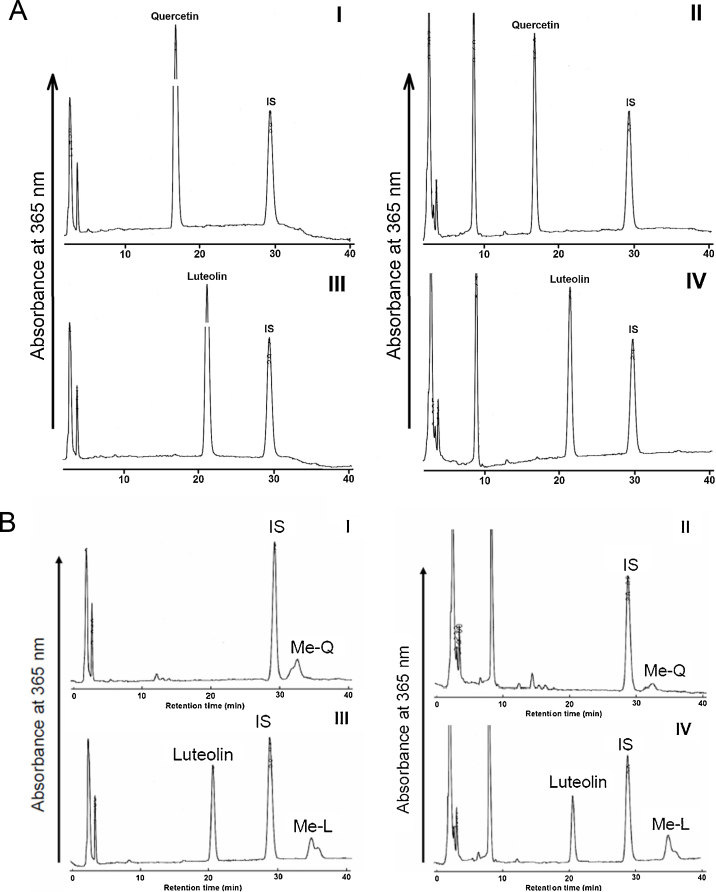
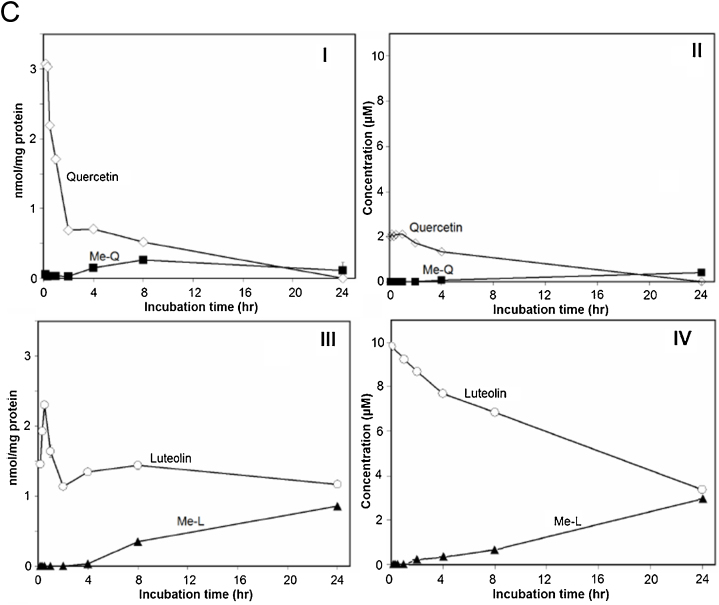
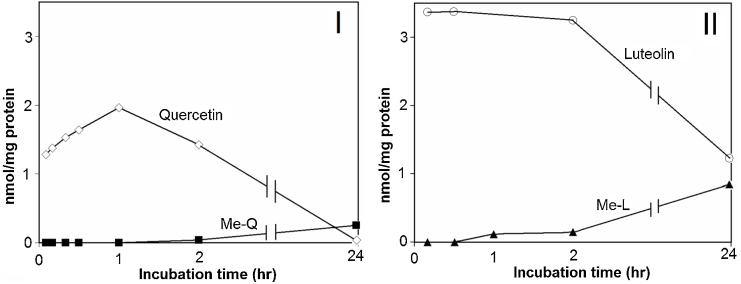
Fig. 4 shows HPLC chromatograms for the extract from cell fractions (I, III) and culture medium (II, IV) after incubation with SH-SY5Y cells for 1 h (A) and 24 h (B). Quercetin appeared in both chromatograms of 1-h incubation (Fig. 4-A, I, II), although it was completely extinguished in the chromatogram of 24-h incubation (Fig. 4-B, I, II). O-Methylated quercetin was only detected in the chromatograms of 24-h incubation (Fig. 4-B, I, II). In contrast, luteolin was detected in cell fractions and culture medium after incubation of both 1 h (Fig. 4-A, III, IV) and 24 h (Fig. 4-B, III, IV). The cellular content of quercetin (3 nmol/mg protein) and luteolin (2.3 nmol/mg protein) reached a maximal level transiently after incubation for 15 and 30 min, respectively (Fig. 4-C). Then, cellular contents of quercetin and luteolin decreased rapidly within 2 h and were maintained at relatively stable levels. Cellular content of quercetin decreased slowly from 2 to 24 h and disappeared completely at 24 h of incubation (Fig. 4-C, I), whereas content of luteolin remained at a relatively higher level and accounted for 1.2 nmol/mg protein after incubation for 24 h (Fig. 4-C, III). In the culture medium, quercetin content decreased rapidly from 10 to 2 μM during the first 15 min of incubation, and then decreased gradually during 24 h until complete disappearance (Fig. 4-C, II), whereas luteolin content in the medium decreased gradually and did not disappear completely even after 24 h of incubation (Fig. 4-C, IV).
Fig. 4.
Distribution of quercetin, luteolin and their O-methylated metabolites in SH-SY5Y cells and culture medium. HPLC chromatograms of quercetin (I, II) and luteolin (III, IV) in SH-SY5Y cell extracts (I, III) and culture medium (II, IV) after incubation for 1 h (A) or 24 h (B); Concentrations of quercetin (I, II), luteolin (III, IV) and their O-methylated derivatives in SH-SY5Y cells (I, III) and culture medium (II, IV) (C). SH-SY5Y cells were treated with 10 μM (final concentration) quercetin or luteolin. Flavonoids were extracted and quantified using HPLC as described in the Materials and methods section.
Quercetin and luteolin were converted to their O-methylated metabolites, in which O-methylation occurred at the catechol group of the ring B (Fig. 1). O-Methylated metabolites were found in both cell fractions and culture medium after incubation of 24 h (Fig. 4-B and -C).
3.3. Accumulation of quercetin and luteolin in mitochondrial fractions
Quercetin and luteolin were rapidly transferred and reached to mitochondria of SH-SY5Y cells within 15 min after treatment with the cultured cell (Fig. 5). Maximal contents of flavonoids in mitochondrial fractions obtained from treated cells were 2 nmol/mg protein for quercetin (incubation for 60 min) and 3.3 nmol/mg protein for luteolin (incubation for 30 min) (Fig. 5). Similar to whole-cell fractions, quercetin disappeared completely from mitochondrial fractions after incubation for 24 h (Fig. 5, I). Luteolin and its O-methylated metabolite were present in mitochondria even after 24 h of incubation at 1.2 and 0.8 nmol/mg protein, respectively (Fig. 5, II).
Fig. 5.
Accumulation of quercetin and luteolin in mitochondrial fractions of SH-SY5Y cells. Cells were seeded on 90-mm dishes and time-dependently treated with 10 μM (final concentration) of quercetin (I) or luteolin (II). Mitochondrial fractions were isolated and flavonoids extracted and quantified using HPLC as described in the Materials and methods section. Quercetin (white diamond), O-methylated metabolites of quercetin (black square), luteolin (white circle), O-methylated metabolites of luteolin (black triangle).
3.4. Effect of ascorbic acid on quercetin content in cell fractions and the culture medium
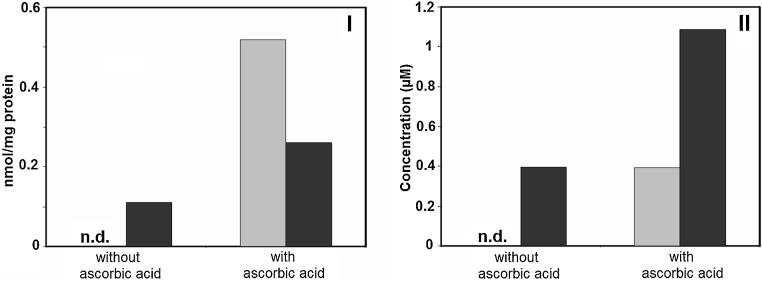
The effects of ascorbic acid on the distribution of quercetin and its O-methylated metabolites in cell fractions and culture medium after incubation for 24 h are shown in Fig. 6. Similarly to the cell-free culture medium (Fig. 3-A), addition of ascorbic acid prevented disappearance of quercetin in the cell fractions and the culture medium. It was apparent that ascorbic acid protected quercetin from auto-oxidation to allow its accumulation in cell fractions and the culture medium upon 24-h incubation. O-Methylated metabolites of quercetin also accumulated at higher levels as compared with those in the absence of ascorbic acid (Fig. 6).
Fig. 6.
Effect of ascorbic acid on the distribution of quercetin and its O-methylated metabolites in cell fractions and culture medium after incubation for 24 h. Concentration of quercetin in cell factions (I) and culture medium (II). Cells were seeded on PLL dishes and treated with 10 μM quercetin for 24 h in the presence or absence of 200 μM ascorbic acid. quercetin (gray bar), O-methylated metabolites of quercetin: (black bar).
3.5. Effects of quercetin and luteolin on MAO-A activity of mitochondrial fractions
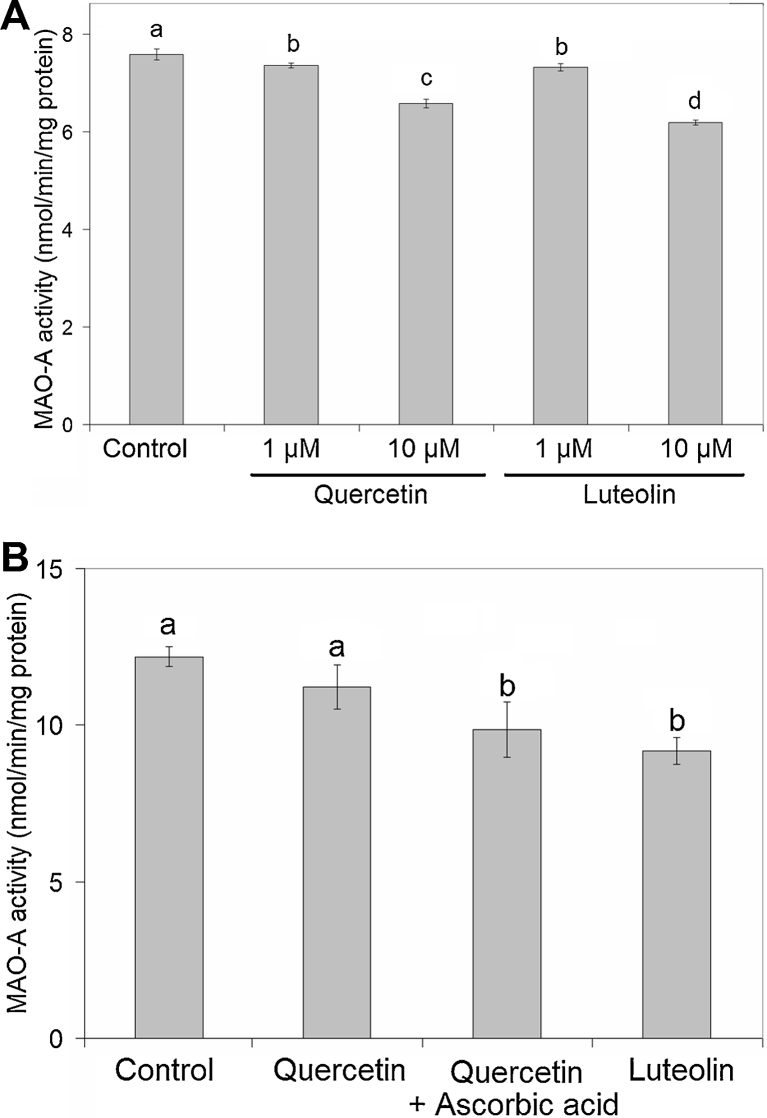
The effects of quercetin and luteolin on MAO-A activity in mitochondrial fractions obtained from cells pretreated with each flavonoid at 1 and 10 μM for 1 h are shown in Fig. 7-A. Quercetin and luteolin lowered MAO-A activity significantly, so both flavonoids were direct inhibitors of the MAO-A reaction in SH-SY5Y cells.
Fig. 7.
Effect of quercetin and luteolin on MAO-A activity in mitochondrial fractions of pretreated SH-SY5Y cells. (A) SH-SY5Y cells were treated with 1 or 10 μM of quercetin or luteolin. After 1 h of incubation, mitochondrial fractions were isolated. MAO-A activity of the fractions was measured as described in the Materials and methods section. (B) SH-SY5Y cells were incubated for 24 h with 10 μM (final concentration) of quercetin in the presence or absence of ascorbic acid at 200 μM, or 10 μM (final concentration) of luteolin. MAO-A activity in mitochondrial fractions was measured as described previously.
Values are the mean ± SD from independent triplicate determinations for each experiment. Significant differences were evaluated by one-way ANOVA with the Tukey multiple comparison test (p < 0.05).
The effects of quercetin and luteolin at 10 μM on MAO-A activity after 24 h of incubation are shown in Fig. 7-B. Luteolin could decrease the MAO-A activity of isolated mitochondria slightly (but significantly). Quercetin exerted an inhibitory effect only in the presence of ascorbic acid.
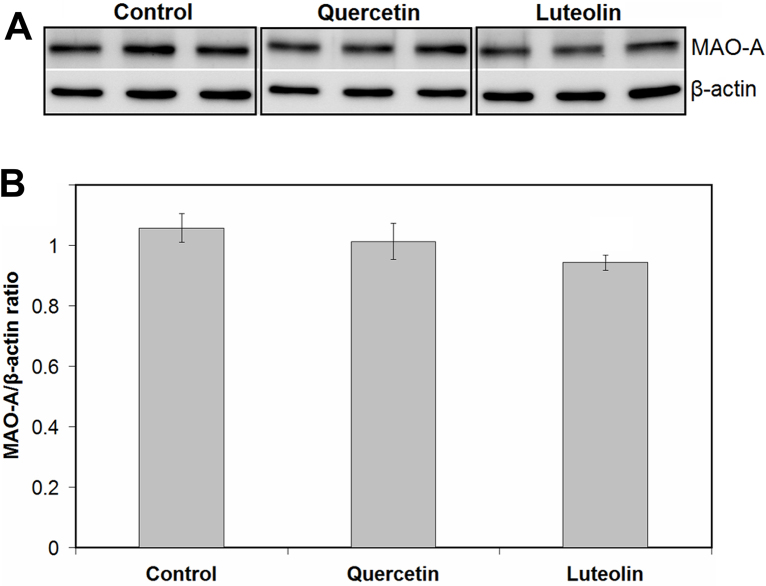
3.6. Effects of quercetin and luteolin on levels of MAO-A protein
Effects of quercetin and luteolin on levels of MAO-A protein in SH-SY5Y cells after 24 h of incubation are shown in Fig. 8. Luteolin did not affect the protein levels of MAO-A. Coexistence of quercetin with ascorbic acid also did not show a significant effect (data not shown).
Fig. 8.
Effects of quercetin and luteolin on levels of MAO-A protein in SH-SY5Y cells. SH-SY5Y cells were treated with 10 μM (final concentration) of quercetin or luteolin for 24 h. Levels of MAO-A protein were quantified using western blotting analyses as prescribed in the Materials and methods section. Density was quantified and normalized to that of β-actin. Values are the mean ± SD from independent triplicate determinations for each experiment. Significant differences were evaluated by one-way ANOVA with the Tukey multiple comparison test (p < 0.05).
4. Discussion
Our previous work demonstrated that luteolin was superior to quercetin with regard to inhibition of MAO-A activity in mitochondrial fractions obtained from mouse brains (though both flavonoids possessed the strongest inhibitory effect among the flavonoids tested [13]). In the present study, we compared the effect of quercetin with that of luteolin on MAO-A activity using the human neuroblastoma SH-SY5Y cell line as a model of serotoninergic neuronal cells because MAO-A (but not MAO-B) is known to be expressed in this cell [20].
First, we estimated the cytotoxicity of quercetin, luteolin and their O-methylated metabolites, isorhamnetin and diosmetin, respectively. Flavonols quercetin and isorhamnetin (3′-O-methyl quercetin) had no effect on cell viability even at 100 μM, but flavones luteolin and diosmetin (4′-O-methyl luteolin) decreased cell viability significantly at 100 μM after 24 h of incubation (Fig. 2). These findings suggested that flavones are more toxic to neuronal cells than flavonols irrespective of free catechol group in the ring B. It could be concluded that lack of a hydroxyl group at the C-3 position is critical for the toxicity of flavonoids to SH-SY5Y cells. The reason for the slight cytotoxicity of flavonols may be derived from instability in the cell-free culture medium (Fig. 3-A). Flavonols are known to be susceptible to auto-oxidation [21]. Quercetin (but not isorhamnetin) was cytotoxic at 50 μM in coexistence with ascorbic acid (Fig. 3-B). Ascrobic acid protected flavonols from auto-oxidative decomposition and allowed them to exert their toxic effect on cells. It is likely that the cytotoxicity of quercetin at higher concentrations derives from quinone formation owing to oxidation of the catechol group [22], [23]. O-Methylation in the ring B may suppress the cytotoxicity of quercetin by withdrawing the catechol group, resulting in prevention of quinone formation. In contrast, it is unlikely that O-methylation in the catechol group of flavones suppresses their cytotoxicity because diosmetin also exerted cytotoxicity at 100 μM, similar to luteolin (Fig. 2-B). It may indicate that the mechanism for the cytotoxicity of flavones lacking a hydroxyl group at the C-3 position is distinct from that of flavonols.
Time-course experiments for cellular uptake of quercetin and luteolin revealed that both flavonoids were immediately incorporated into cells and attained a maximum level within 30 min followed by a rapid decrease (Fig. 4-C). Complete disappearance of quercetin from cell fractions and culture medium after 24 h of incubation because of its susceptibility to auto-oxidation implies that quercetin does not function in the cell after long-term incubation, which is different from the effect of luteolin. However, intracellular accumulation of quercetin was found to be comparable with that of luteolin in the presence of ascorbic acid (Fig. 6). On the basis of time-course experiments shown in Fig. 4-C, accumulation of flavonoids in SH-SY5Y cells may be divided into two stages. In the first stage, flavonoids are attached to cell membranes and/or penetrate into the cytosol rapidly by simple diffusion, thereby leading to maximal accumulation. In the second stage, cellular concentration of flavonoids is modulated by the balance between their efflux and reuptake into cells. This modulation may be well balanced for a long time in the case of luteolin, because it exists in a stable state in cells. Second peak appeared in the time course of cellular luteolin concentration at around 8-h incubation, although the reason of this phenomenon is not known.
O-Methylated metabolites of quercetin and luteolin were found in both cell fractions and culture medium (Fig. 4-B), suggesting that flavonoids are subjected (at least partly) to transformation by catechol-O-methyltransferase, and that the products of this metabolic conversion effluxed from the cell. Reports have suggested that glucuronide and/or sulfate conjugates yield quercetin metabolites in cultured cells [19], [24]. However, metabolites of quercetin/luteolin other than O-methylated derivatives were not found in cell fractions or culture medium (data not shown). Therefore, O-methylation is the main way of metabolic conversion of flavonoids in SH-SY5Y cells. This O-methylation may interfere with the inhibition of MAO-A activity by flavones and flavonols because O-methylated metabolites have been reported to lack the inhibitory effect on MAO-A [13].
The targets of quercetin and luteolin as MAO-A inhibitors in SH-SY5Y cells are mitochondria, because MAO-A is located exclusively in the outer mitochondrial membrane [25]. The present study clearly demonstrates that quercetin and luteolin are transferred into mitochondria immediately after addition to cultured cells (Fig. 5). Fiorani et al. [26] reported that quercetin was readily transported into mitochondrial fractions to exert its function if incubated with Jurkat human T lymphoblast cells. It has also been suggested that quercetin and other structurally related flavonoids prevent production of hydrogen peroxide by targeting mitochondrial particles [26]. Quercetin is likely to be imbedded into the lipid membranes or bound to some molecules located in the membranes resulting in the inhibition of the activity of MAO-A located in mitochondria outer membranes. Furthermore, Jones et al. [27] stated that flavonoids inhibit the process responsible for mitochondrial dysfunction to oppose neurodegeneration. Therefore, mitochondria serve as targets of quercetin and luteolin in these nerve cells.
Initially, luteolin accumulated in mitochondrial fractions at higher concentrations than quercetin, and its content decreased very slowly. Higher accumulation of luteolin in mitochondria initially may be because of its higher hydrophobicity (ClogP of quercetin and luteolin are 1.5 and 2.31, respectively [13]). In addition, luteolin accumulated in mitochondria of SH-SY5Y cells even after 24 h of incubation, even though quercetin disappeared completely in mitochondrial fractions (Fig. 5). We compared the effect of quercetin and luteolin on MAO-A activity in isolated mitochondrial fractions of SH-SY5Y cells pretreated for 1 h and 24 h. Contents of quercetin and luteolin reached to their maximal level in mitochondria during 1 h of incubation and it is therefore likely to attenuate MAO-A activity directly as shown in Fig. 7-A. After incubation for 24 h, quercetin needs ascorbic acid to inhibit mitochondrial MAO-A activity (Fig. 7-B), corresponding to the preventive effect of ascorbic acid on its disappearance in the cell fraction (Fig. 6, I).
Daily consumption of flavonoids originating from plant foods may allow accumulation of these compounds in brain tissue and for them to exert prolonged effects on nerve cells by affecting cell signaling pathways and the resulting gene expression [28], [29], [30]. Therefore, we examined the possibility of quercetin and luteolin modulating MAO-A protein levels after 24 h of incubation with SH-SY5Y cells. Neither quercetin nor luteolin showed any tendency to decrease levels of MAO-A protein in SH-SY5Y cells (Fig. 8). Hence, both flavonoids can attenuate MAO-A activity, but it is unlikely they will affect cellular signaling pathways that result in modulation of MAO-A protein expression.
The concentrations required for inhibition of MAO-A activity in this in vitro study (≤10 μM) are higher than those observed under physiological conditions. However, several evidences based on animal studies have shown that dietary flavonoids can cross the blood–brain barrier and accumulate in brain tissue after long-term feeding [19], [28], [31]. Therefore, long-term intake of quercetin, luteolin and other structurally related flavonoids may modulate mitochondrial MAO-A activity in nerve cells. Flavonoids such as quercetin and luteolin are present in the blood stream and tissues exclusively as their conjugated metabolites (glucuronides and/or sulfates) [32]. However, previously we implied that microglia can deliver the aglycone to nerve cells by deconjugation of conjugated metabolites in the brain [33]. Therefore, quercetin and luteolin could exert their physiological functions in the form of aglycone in brain tissue.
5. Conclusion
The flavonol quercetin and flavone luteolin can move into mitochondria and attenuate MAO-A activity directly in neuronal cells. Stability in the cell medium and distribution in the target site should be taken into account for estimating the modulatory effect of flavonoids on MAO-A activity by interpretation from in vitro cell culture studies. Mechanism underlying the modulation of MAO-A activity in nerve cells by flavonoids needs further evaluation.
Transparency document
Acknowledgments
We thank Dr. Dr. Makoto Naoi at Aichi Gakuin University, Aichi, Japan, for the kind gift of SH-Sy-5Y cells. This work was supported by Japanese Society for the Promotion of Science, Japan, Kakenhi (grant number 252912075).
Footnotes
Available online 6 September 2014
References
- 1.Shih J.C., Chen K., Ridd M.J. Monoamine oxidase: from genes to behavior. Annu. Rev. Neurosci. 1999;22:197–217. doi: 10.1146/annurev.neuro.22.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lang U.E., Borgwardt S. Molecular mechanisms of depression: perspectives on new treatment strategies. Cell Physiol. Biochem. 2013;31:761–777. doi: 10.1159/000350094. [DOI] [PubMed] [Google Scholar]
- 3.Meyer J.H., Ginovart N., Boovariwala A., Sagrati S., Hussey D., Garcia A., Young T., Praschak-Rieder N., Wilson A.A., Houle S. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch. Gen. Psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 4.Wimbiscus M., Kostenko O., Malone D. MAO inhibitors: risks, benefits, and lore. Clevel. Clin. J. Med. 2010;77:859–882. doi: 10.3949/ccjm.77a.09103. [DOI] [PubMed] [Google Scholar]
- 5.Youdim M.B., Riederer P. Dopamine metabolism and neurotransmission in primate brain in relationship to monoamine oxidase A and B inhibition. J. Neural Transm. Gen. Sect. 1993;91:181–195. doi: 10.1007/BF01245231. [DOI] [PubMed] [Google Scholar]
- 6.Dixon Clarke S.E., Ramsay R.R. Dietary inhibitors of monoamine oxidase A. J. Neural. Transm. 2011;118:1031–1041. doi: 10.1007/s00702-010-0537-x. [DOI] [PubMed] [Google Scholar]
- 7.Gong J., Huang J., Ge Q., Chen F., Zhang Y. Advanced research on the antidepressant effect of flavonoids. Curr. Opin. Complement. Altern. Med. 2014;1:1–6. [Google Scholar]
- 8.Butterweck V., Jürgenliemk G., Nahrstedt A., Winterhoff H. Flavonoids from Hypericum perforatum show antidepressant activity in the forced swimming test. Planta. Med. 2000;66:3–6. doi: 10.1055/s-2000-11119. [DOI] [PubMed] [Google Scholar]
- 9.Chakraborty J., Singh R., Dutta D., Naskar A., Rajamma U., Mohanakumar K.P. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in a 3-nitropropionic acid-induced rat model of Huntington's disease. CNS Neurosci. Ther. 2013;20:10–19. doi: 10.1111/cns.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakakibara H., Yoshino S., Kawai Y., Terao J. Antidepressant-like effect of onion (Allium cepa L.) powder in a rat behavioral model of depression. Biosci. Biotechnol. Biochem. 2008;72:94–100. doi: 10.1271/bbb.70454. [DOI] [PubMed] [Google Scholar]
- 11.Chimenti F., Cottiglia F., Bonsignore L., Casu L., Casu M., Floris C., Secci D., Bolasco A., Chimenti P., Granese A., Befani O., Turini P., Alcaro S., Ortuso F., Trombetta G., Loizzo A., Guarino I. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: extraction, biological analysis, and computational study. J. Nat. Prod. 2006;69:945–949. doi: 10.1021/np060015w. [DOI] [PubMed] [Google Scholar]
- 12.Yoshino S., Hara A., Sakakibara H., Kawabata K., Tokumura A., Ishisaka A., Kawai Y., Terao J. Effect of quercetin and glucuronide metabolites on the monoamine oxidase-A reaction in mouse brain mitochondria. Nutrition. 2011;21:847–852. doi: 10.1016/j.nut.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Bandaruk Y., Mukai R., Kawamura T., Nemoto H., Terao J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012;60:10270–10277. doi: 10.1021/jf303055b. [DOI] [PubMed] [Google Scholar]
- 14.Ishisaka M., Kakefuda K., Yamauchi M., Tsuruma K., Shimazawa M., Tsuruta A., Hara H. Luteolin shows an antidepressant-like effect via suppressing endoplasmic reticulum stress. Biol. Pharm. Bull. 2011;34:1481–1486. doi: 10.1248/bpb.34.1481. [DOI] [PubMed] [Google Scholar]
- 15.Sasaki K., El Omri A., Kondo S., Han J., Isoda H. Rosmarinus officinalis polyphenols produce anti-depressant like effect through monoaminergic and cholinergic functions modulation. Behav. Brain Res. 2013;238:86–94. doi: 10.1016/j.bbr.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Dajas F., Andrés A.C., Florencia A., Carolina E., Felicia R.M. Neuroprotective actions of flavones and flavonols: mechanisms and relationship to flavonoid structural features. Cent. Nerv. Syst. Agents Med. Chem. 2013;13:30–35. doi: 10.2174/1871524911313010005. [DOI] [PubMed] [Google Scholar]
- 17.Dounce A.L., Witter R.F., Monty K.J., Pate S., Cottone M.A. A method for isolating intact mitochondria and nuclei from the same homogenate, and the influence of mitochondrial destruction on the properties of cell nuclei. J. Biophys. Biochem. Cytol. 1955;1:139–153. doi: 10.1083/jcb.1.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krajl M. A rapid microfluorimetric determination of monoamine oxidase. Biochem. Pharmacol. 1965;14:1684–1686. doi: 10.1016/0006-2952(65)90025-0. [DOI] [PubMed] [Google Scholar]
- 19.Ishisaka A., Ichikawa S., Sakakibara H., Piskula M.K., Nakamura T., Kato Y., Ito M., Miyamoto K., Tsuji A., Kawai Y., Terao J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic. Biol. Med. 2011;51:1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 20.Inaba-Hasegawa K., Akao Y., Maruyama W., Naoi M. Rasagiline and selegiline, inhibitors of type B monoamine oxidase, induce type A monoamine oxidase in human SH-SY5Y cells. J. Neural Transm. 2013;120:435–444. doi: 10.1007/s00702-012-0899-3. [DOI] [PubMed] [Google Scholar]
- 21.Hodnick W.F., Kalyanaraman B., Pritsos C.A., Pardini R.S. The production of hydroxyl and semiquinone free radicals during the autoxidation of redox active flavonoids. Basic Life Sci. 1988;49:149–152. doi: 10.1007/978-1-4684-5568-7_21. [DOI] [PubMed] [Google Scholar]
- 22.Chedea V.S., Choueiri L., Jisaka M., Kefalas P. o-Quinone involvement in the prooxidant tendency of a mixture of quercetin and caffeic acid. Food Chem. 2012;135:1999–2004. doi: 10.1016/j.foodchem.2012.06.094. [DOI] [PubMed] [Google Scholar]
- 23.Metodiewa D., Jaiswal A.K., Cenas N., Dickancaité E., Segura-Aguilar J. Quercetin may act as a cytotoxic prooxidant after its metabolic activation to semiquinone and quinoidal product. Free Radic. Biol. Med. 1999;26:107–116. doi: 10.1016/s0891-5849(98)00167-1. [DOI] [PubMed] [Google Scholar]
- 24.Terao J., Murota K., Kawai Y. Conjugated quercetin glucuronides as bioactive metabolites and precursors of aglycone in vivo. Food Funct. 2011;2:11–17. doi: 10.1039/c0fo00106f. [DOI] [PubMed] [Google Scholar]
- 25.Alberts R.W., Price D.L. Principles of molecular, cellular and medical neurobiology. eigth ed. Elsevier Academic Press; London: 2012. Basic neurochemistry. [Google Scholar]
- 26.Fiorani M., Guidarelli A., Blasa M., Azzolini C., Candiracci M., Piatti E., Cantoni O. Mitochondria accumulate large amounts of quercetin: prevention of mitochondrial damage and release upon oxidation of the extramitochondrial fraction of the flavonoid. J. Nutr. Biochem. 2010;21:397–404. doi: 10.1016/j.jnutbio.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 27.Jones Q.R., Warford J., Rupasinghe H.P., Robertson G.S. Target-based selection of flavonoids for neurodegenerative disorders. Trends Pharmacol. Sci. 2012;33:602–610. doi: 10.1016/j.tips.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Bieger J., Cermak R., Blank R., de Boer V.C., Hollman P.C., Kamphues J., Wolffram S. Tissue distribution of quercetin in pigs after long-term dietary supplementation. J. Nutr. 2008;138:1417–1420. doi: 10.1093/jn/138.8.1417. [DOI] [PubMed] [Google Scholar]
- 29.Spencer J.P. The interactions of flavonoids within neuronal signaling pathways. Genes Nutr. 2007;2:257–273. doi: 10.1007/s12263-007-0056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Youdim K.A., Shukitt-Hale B., Joseph J.A. Flavonoids and the brain: interactions at the blood–brain barrier and their physiological effects on the central nervous system. Free Radic. Biol. Med. 2004;37:1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 31.Faria A., Meireles M., Fernandes I., Santos-Buelga C., Gonzalez-Manzano S., Dueñas M., de Freitas V., Mateus N., Calhau C. Flavonoid metabolites transport across a human BBB model. Food Chem. 2014;149:190–196. doi: 10.1016/j.foodchem.2013.10.095. [DOI] [PubMed] [Google Scholar]
- 32.Spencer J.P.E., Crozier A. CRC Press; London: 2012. Flavonoids and related compounds: bioavailability and function (oxidative stress and disease) [Google Scholar]
- 33.Mukai R., Kawabata K., Otsuka S., Ishisaka A., Kawai Y., Ji Z.S., Tsuboi H., Terao J. Effect of quercetin and its glucuronide metabolite upon 6-hydroxydopamine-induced oxidative damage in Neuro-2a cells. Free Radic. Res. 2012;46:1019–1028. doi: 10.3109/10715762.2012.673720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.