Abstract
Purpose
The present investigation was aimed at evaluating the anti-ophidian properties of ethnomedicinal herb Leucas aspera against Indian cobra, Naja naja venom enzymes.
Methods
Methanolic extract of Leucas aspera was evaluated, in vitro, for its ability to inhibit the major enzyme activities of Naja naja venom including protease, phospholipase A2, hyaluronidase and hemolytic factors. The type of phytochemicals present in the extract was analyzed. Also, the major phytoconstituents in the extract was determined by gas chromatography–mass spectrometry (GC–MS).
Results
Venom protease and hyaluronidase activities (two isoforms) were completely (100%) neutralized by the L. aspera methanolic extract at ratio of 1:50 w/w (venom: plant extract) and venom hemolytic activity was also completely neutralized at a ratio of 1:80 w/w by the plant extract. However, the extract failed to neutralize phospholipase A2 activity even at the highest concentration used. Phytochemical analysis revealed the presence of alkaloids, acidic compounds, flavonoids, steroids and cardiac glycosides in the extract. GC–MS analysis indicated that a total of 14 compounds were present in the extract. The major bioactive constituents were found to be 6-octadecenoic acid (32.47%), n-hexadecanoic acid (25.97%), and 17-octadecen-14-yn-1-ol (14.22%) along with the minor constituents, sitosterol (2.45%) and stigmasterol (2%), which was previously reported to exhibit antivenom activity.
Conclusion
The results obtained demonstrate for the first time that the methanolic extract of Leucas aspera possesses anti-venom activity and could be considered as a potential source for the anti-ophidian metabolites.
Keywords: Leucas aspera, Naja naja, Protease, Hyaluronidase, Hemolysis, PLA2
1. Introduction
Since time immemorial man has been dependent on plant sources for food and medicine. Recently, there has been renewed attention and interest in the use of traditional medicine. The World Health Organization (WHO) estimated that nearly 80% of the population depends on herbal medicines to meet primary health care needs due to their low cost and availability [1]. In India there are about 54 million indigenous people of different ethnic groups using medicinal plants for the treatment of various types of ailments, including snakebites [2]. Snakebite is often readily treated by traditional herbal medicines by rural population, especially in tropical and subtropical countries including India.
It is estimated that 1.2 to 5.5 million snakebites occur globally, with 94,000 deaths occurring annually [3]. In India alone, 35,000–50,000 deaths were reported every year [4], [5]. The major victims are agriculturists and their families living in rural areas of the country. Snake venom is complex mixture of enzymes, carbohydrates, proteins and several other small molecules. Among all enzymes present in the venom, proteases, phospholipases, and hyaluronidases are reported to be medically important in developing effective antidotes [6]. Proteases are known to degrade extra cellular matrixes and allow other venom components to access the local tissue. Phospholipases are multifunctional enzymes that degrade membrane phospholipids and release arachidonic acid which is a precursor molecule in causing inflammation by cyclooxygenase (COX) or lipooxygenase (LOX) pathways. Hyaluronidases are spreading factors which facilitate diffusion of venom toxins from the site of bite into general circulation and thereby increase the severity of envenomation. The only treatment available since 1895 is animal derived polyclonal antisnake venom (ASV) therapy. Despite of its wide usage, due to high seasonal and biochemical variations in the venom composition, records indicate that ASV developed against big four venoms is imprecise and less effective [7] in the treatment of snakebite victims. In addition to its high cost, hypersensitive reactions were also reported from the ASV treated victims [8]. Also, the quantity of the ASV produced does not meet the demand. Therefore interest in exploring the snake venom antidotes from plant sources has increased. Hence the present study was aimed to validate the anti-ophidian activity of the commonly used traditional herbal plant Leucas aspera against Naja naja venom enzymes.
2. Materials and methods
2.1. Plant collection
The present investigation was carried out by conducting surveys and questionnaires with tribal people and Irulars living in Irularpatti, Vallimalai, Alamalrengapuram in Vellore district of Tamil Nadu, India and Bangarupalyam, Punganur and Chittoor in Andhra Pradesh, India. Traditional healers, called ‘Vaidyars’ from different indigenous groups were also approached for the documentation of the medicinal plants used in the treatment of snakebite. The plant species collected was identified by a Botanist Dr. Madhavachetty, Department of Botany, Sri Venkateswara University, Tirupati, Andhra Pradesh and a voucher specimen (voucher no: 1200) was deposited in the same department.
2.2. Plant extraction
Five hundred grams of shade dried powdered plant material was extracted with 1000 ml of methanol for 24 h with continuous stirring. The extraction was repeated for 3 days by changing the solvent every 24 h. The extract was concentrated using rotary vacuum evaporator under reduced pressure at 40 °C. Known quantity of the dried extract was subsequently dissolved in 10 mM phosphate buffered saline (PBS pH 7.4). Centrifugation at 10,800 × g (for 10 min) was performed for any insoluble. The clear solution was used for the enzyme inhibition studies. The venom activity in absence of plant extract served as the negative control.
2.3. Venom and chemicals
Lyophilized snake venom (N. naja) was purchased from Irula snake catcher's Industrial co-operative society limited (Chennai, India). The venom was dissolved in 0.9% saline and centrifuged at 675 × g for 10 min and the supernatant was used for the study. Hyaluronic acid was purchased from Sigma Aldrich (US). All other reagents and solvents were of high quality analytical grade and were purchased from SD Fine chemicals/Sisco Research Laboratory Pvt Ltd/HiMedia, Mumbai.
2.4. Caseinolytic inhibition
Protease activity was determined using 1% casein as substrate in 1% agarose plates according to the method described previously [9]. 50 μg of N. naja venom was pre-incubated with various concentrations of plant extract for 1 h at 37 °C. Briefly, the pre-incubated samples were loaded into 3 mm diameter wells of casein-agarose plates (0.1 M Tris–HCl buffer, pH 8.0) and incubated overnight at 37 °C. Plates were then stained with Coomassie Brilliant Blue-R 250 for 1 h and destained with methanol:water:acetic acid (50:40:10). The percentage of protease inhibition was calculated by measuring the zone of clearance in the presence of plant extract. Zone of clearance values obtained for the venom in the absence of the plant extract served as control.
In addition to the plate assay, SDS-PAGE was also performed to evaluate the protease inhibition by the plant extract. Briefly, 50 μg of N. naja venom was pre-incubated with various concentrations of plant extract for 1 h at 37 °C. These samples were further incubated with 15 μl of 1% casein (dissolved (w/v) in 0.1 M Tris–HCl buffer, pH 8.0, 37 °C). After 30 min, the reaction was stopped by adding SDS loading buffer (containing 50 mM Tris HCl pH 6.8, 10% (v/v) glycerol, 2% (w/v) SDS, 100 mM β-mercaptoethanol and 0.1% (w/v) bromophenol blue). SDS-PAGE of the casein treated with venom in presence and absence of plant extract was then performed according to the method described previously [10] using 15% acrylamide gel.
2.5. Phospholipase (PLA2) inhibition
PLA2 inhibition was evaluated using egg yolk as substrate in 1% agarose plates according to the method described by Gutierrez et al. [11]. 50 μg of N. naja venom was pre-incubated with various concentrations of plant extract for 1 h at 37 °C. The pre-incubated samples were then loaded into 3 mm diameter wells of 1% agarose plates containing 0.6% egg yolk and 5 mM CaCl2 followed by overnight incubation at 37 °C. The PLA2 inhibition was calculated by measuring the zone of clearance in the presence and absence of plant extract. PLA2 activity of venom in absence of plant extract served as control.
2.6. Hemolytic inhibition
The hemolytic inhibition assay was carried out as per the method described previously [12], with slight modifications. 100 μg of N. naja venom was pre-incubated with various concentrations of plant extract in a volume of 200 μl PBS for 1 h at 37 °C. Briefly, human erythrocytes and 10 mM PBS (pH 7.4) were mixed (1:8 v/v) and 100 μl of this suspension was incubated with pre-incubated venom samples for 2 h at 37 °C. The reaction was stopped by adding 1 ml of ice cold PBS and centrifuged at 2500 rpm for 10 min at 4 °C. The amount of hemoglobin released in the supernatant was measured at 540 nm.
2.7. Hyaluronidase inhibition assay
Hyaluronidase inhibition assay was performed according to the method of Girish et al. [13] with minor modifications. The substrate gel for zymogram was prepared by incorporating hyaluronic acid at a final concentration of 0.17 mg/ml into 10% SDS-polyacrylamide gel matrix. 100 μg of N. naja venom was pre-incubated with different concentrations of plant extract for 1 h at 37 °C. All samples were prepared under non-reducing conditions for electrophoresis. After electrophoresis, the gel was soaked subsequently in 50 ml of 5% Triton X-100 for 1 h and then in 0.05% Triton X-100 for 30 min followed by distilled water for 1 h. The gel was equilibrated in 0.1 M sodium acetate buffer (pH 5.0) containing 0.15 M NaCl for 48 h at 37 °C. The gel was then washed with distilled water and stained with 0.1% alcian blue solution for 2 h. Destaining was carried out with 5% acetic acid until clear translucent bands appeared against dark blue background.
2.8. Phytochemical analysis
In order to obtain insight into the type of phytochemicals present in the extract, analysis was performed as per the methods described by Kokate et al. [14].
2.9. Gas chromatography–mass spectrometry analysis
Chemical composition of the methanolic extract of L. aspera was analyzed using a GC–MS GCD-HP1800A system (Hewlett-Packard, USA) equipped with a split/splitless capillary injection port. The column size was 30 m × 250 μm. An electron ionization system (Quadruples analyzer; mass range, 10–425 amu) with ionization energy of 70 eV was used for GC–MS detection. Each of these steps was carried out under high vacuum (10−4 to 10−8 Torr). Helium was used as a carrier gas at a constant flow rate of 1 ml/min and an injection volume of 2 μl was employed (split ratio of 10:1). Injector and mass transfer line temperatures were set at 250 °C and 280 °C, respectively and fragments were scanned from 50 to 600 Da. The components of extract were identified by comparison with the NIST database.
3. Results and discussion
Crude extracts of plants are being traditionally used in the treatment of wide variety of diseases including snakebite envenomations. However, in most cases the efficacy of this traditional treatment regimen is unproven. In the present study, the methanolic extract of L. aspera, one of the herbal plants widely used as an antidote by many rural populations was evaluated for its efficacy to inhibit selected enzymes in N. naja snake venom. Snake venom metalloproteases, phospholipase A2 (PLA2) and hyaluronidases are the key enzymes involved in tissue necrosis and extracellular matrix degradation [6], [15], [16]. Thus, inhibition of these enzymes is generally considered to be the rate limiting step in the management of snakebite. Therefore, in the present study, methanolic extract of L. aspera was evaluated for its potential to inhibit these major enzymes.
3.1. Protease inhibition
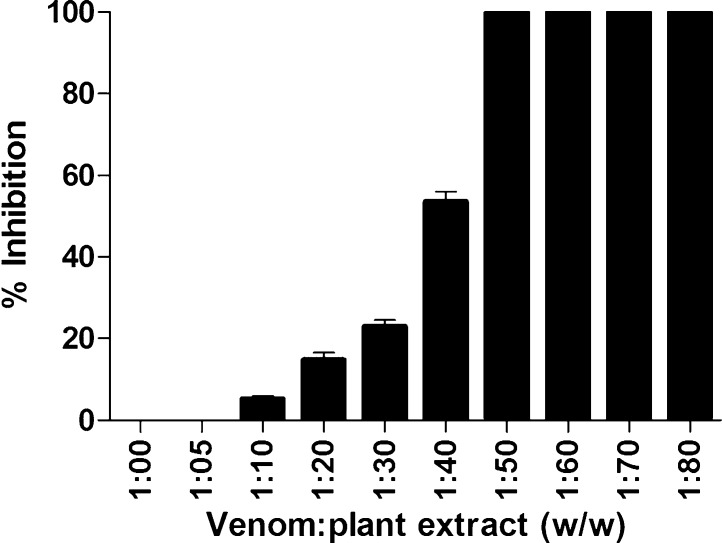
Agar well diffusion assay indicated that the methanolic extract of L. aspera completely inhibited the proteolytic activity of the venom at a ratio of 1:50 (w/w) venom: plant extract (Fig. 1). Also, SDS-PAGE analysis indicated that 50 μg of N. naja venom was sufficient to hydrolyse casein within the incubation period of 30 minutes at 37 °C. Similar to the plate assay results, venom caseinolytic activity was completely inhibited by the plant extract at a ratio of 1:30 w/w venom: plant extract. Fig. 2 demonstrates dose dependent inhibition of venom caseinolytic activity by L. aspera methanolic extract. Snake venom proteases majorly affect hemostasis and cause systemic hemorrhage. The most probable mechanism involved in the inhibition of these proteases by plant extracts could be due to the chelating property of phenolic components present in the extract. It is reported that, phenolic compounds form hydrogen bonds and strongly bind to the histidine residues present in Zn2+ binding motifs of metallo proteases, resulting decrease in the hydrolytic activity of the enzyme [17]. Melo et al. [18] and Soares et al. [19] suggested that the plant extracts would have compounds that bind to divalent metal ions, which are required for enzymatic activities. As the presence of proper metal ion coordination is a pre-requisite for the hydrolytic activity of metalloproteases, any metabolite that can weaken the protease-metal ion interaction will result in inhibition of the proteolytic activity.
Fig. 1.
Dose dependent inhibition of protease activity of N. naja venom by L. aspera methanolic extract. 50 μg of N. naja venom was pre-incubated with various concentrations of the plant extract (w/w) for 1 h at 37 °C. The protease activity was assyed by plate method. Venom protease activity in absence of plant extract was considered as 0% inhibition. Values represent the mean ± S.D of replicates.
Fig. 2.
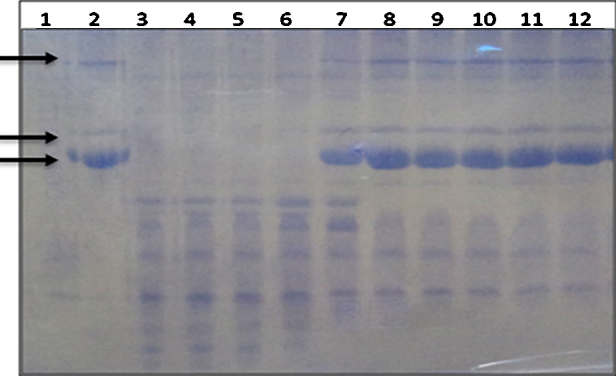
Inhibition of N. naja venom caseinolytic activity by L. aspera methanolic extract. 50 μg of N. naja venom was pre-incubated with various concentrations of plant extract (w/w) for 1 h at 37 °C. The protease activity was assayed in SDS-PAGE method. Lane 1: 50 μg of venom; Lane 2: casein; Lane 3: casein incubated with venom; Lane 4 to 12: samples containing casein incubated with venom in presence of different concentrations of plant extract i.e. 1:05, 1:10, 1:20 to 1:80 (w/w venom: plant extract) respectively. Arrows indicate the major bands of casein. Lane 1–3 served as controls.
3.2. Phospholipase inhibition
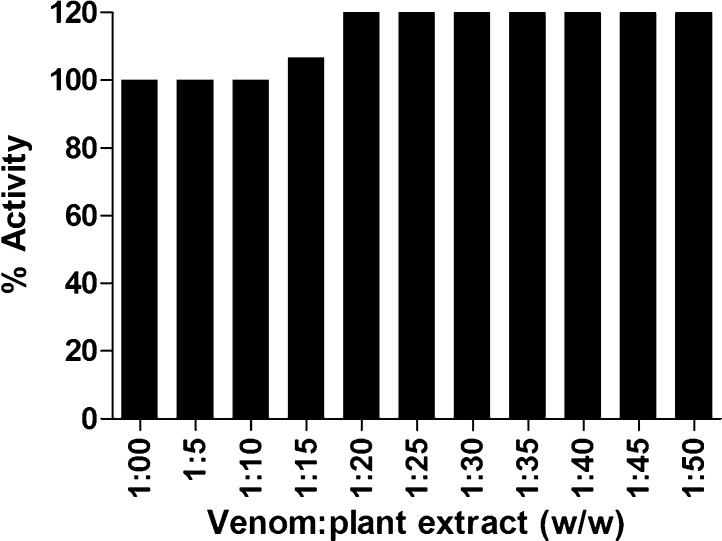
In contrast to protease inhibition, the extract completely failed to neutralize venom PLA2 activity even at highest concentration of extract used (Fig. 3). PLA2 causes cardiotoxicity, myotoxicity, pre or postsynaptic neurotoxicity, edema, hemolysis, hypotension, convulsion, inhibition of platelet aggregation and anticoagulation [20], [21], [22]. Snake venoms are especially rich in group I (Elapidae) and II (Viperidae) PLA2s. These enzymes are Ca2+ dependent and hydrolyse the 2-acyl ester bonds of membrane glycerophospholipids producing free fatty acids and lysophospholipids which are key components of inflammatory process. Timely administration of ASV could neutralize the systemic effects caused by these myotoxic enzymes but provides no protection against local toxicities induced by the venom components. Hence, screening of herbal antidotes to averse snake venom induced toxicity is of great importance in the snakebite management [23]. Hemidesmus indicus root extract had been formerly reported against Daboia russellii and Naja kaouthia snake venom induced toxicities in vivo. Lupeol acetate and 2-hydroxy-4-methoxy benzoic acid isolated from methanolic root extract of H. indicus was demonstrated to have anti-PLA2 activity against viper and cobra venoms [24], [25].
Fig. 3.
Effect of L. aspera methanolic extract on Naja naja venom phosholipaseA2 (PLA2) activity. 50 μg of N. naja venom was pre-incubated with various concentrations of plant extract (w/w) for 1 h at 37 °C. PLA2 activity in absence of plant extract was considered as 100% activity. Values represent the mean ± S.D of replicates.
3.3. Hemolytic inhibition
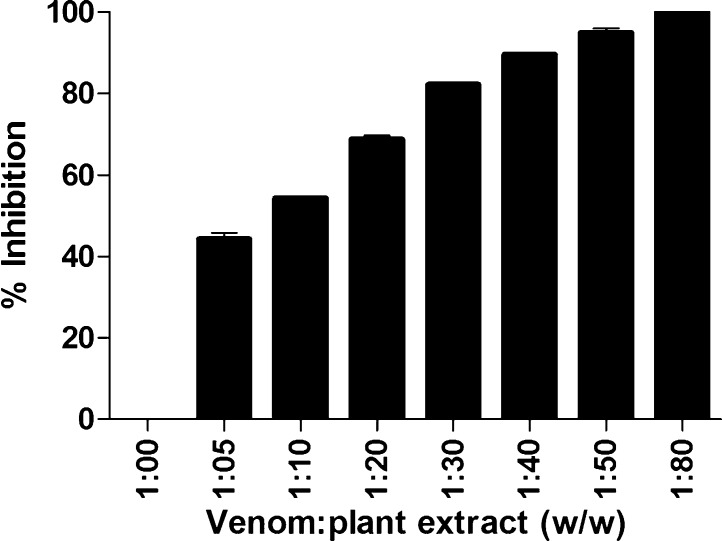
L. aspera methanolic extract inhibited the hemolytic activity of the venom, at a ratio of 1:80 w/w (Fig. 4). Hemolytic activity is another distinct feature of cobra venoms greatly induced by multicomponents including metalloproteases, PLA2, and more specifically, cardiotoxins and cytotoxins of venom [26], [27]. Though, L. aspera did not show inhibition on venom PLA2, it completely protected the hRBC from direct hemolytic activity of N. naja venom. As cardiotoxins and cytotoxins also induce lysis of human erythrocytes [28], it is possible that the phytochemicals in the plant components could inhibit these toxic proteins in the venom.
Fig. 4.
Dose dependent inhibition of N. naja venom hemolytic activity by L. aspera methanolic extract. 50 μg of N. naja venom was pre-incubated with various concentrations of plant extract (w/w) for 1 h at 37 °C. The hemolytic activity in absence of plant extract was considered as 0% inhibition. Values represent the mean ± S.D of replicates.
3.4. Hyaluronidase inhibition
100 μg of N. naja venom exhibited two clear bands of hyaluronidase isomers (in 10% SDS-PAGE zymogram under non reducing conditions). L. aspera exhibited complete inhibition of both the isoforms at a ratio of 1:50 w/w venom-plant extract (Fig. 5). Local tissue damage associated with snakebite envenomation is due to the combined action of metalloproteases along with hyaluronidases and is reported to continue even after antisnake venom (ASV) treatment [29]. Hyaluronidases are a class of endo-β-glycosidases that degrade the hyaluronic acid in the cell wall and facilitate easy diffusion of venom toxins into circulation, which would otherwise take much longer time to diffuse [30], [31].
Fig. 5.

Inhibition of N. naja venom hyaluronidase activity by L. aspera methanolic extract. 100 μg of N. naja venom was pre-incubated with various concentrations of plant extract (w/w) for 1 h at 37 °C. The hyaluronidase activity was assayed in substrate gel SDS-PAGE zymogram under non-reducing conditions. Lane 1 and 8: 100 μg of N. naja venom in absence of plant extract, indicating the presence of two isoforms of hyaluronidase; Lane 2 to 7: 100 μg of venom in presence of different concentrations of plant extract i.e. 1:05, 1:10, 1:25, 1:50, 1:75 and 1:100 (w/w venom: plant extract), respectively, showing dose dependent inhibition of hyaluronidase activity of both the isoforms. Arrows indicate two isoforms of venom hyaluronidase.
3.5. Phytochemical analysis
Qualitative phytochemical analysis revealed the presence of acidic compounds, alkaloids, flavonoids, tannins, saponins and cardiac glycosides as major components in the methanolic extract of L. aspera (Table 1). The anti-venom activity of plants that are widely used against snake venom induced toxicity is majorly due to the presence of various secondary metabolites that bind directly to venom components and neutralizes their effect. Flavonoids and alkaloids isolated from different plant species demonstrated anti-inflammatory and anti-hemorrhagic effects against various snake venoms. Some of them are flavonoid-like (Quercetin, kaempferol, luteolin, myricetin)/alkaloid-like (aristolochic acid and ajmalin) and have revealed strong inhibitory potential against snake venom hyaluronidases [For recent review, 6]. In the present study, L. aspera methanolic extract showed potent inhibition on protease, hemolytic and hyaluronidase activities of N. naja venom. Presence of these active secondary metabolites in the extract could have contributed for plants anti-venom activity.
Table 1.
Qualitative analysis of the phytochemicals present in methanolic extract of L. aspera.
| Phytochemical | Test name | +/− | Phytochemical | Test name | +/− |
|---|---|---|---|---|---|
| Alkali compounds | Litmus paper (Red → Blue) | − | Tannins | Ferric chloride test | + |
| Acidic compounds | Litmus paper (Blue → Red) | + | Proteins | Biuret test | − |
| Alkaloids | Mayers test | + | Steroids | Salkowski test | + |
| Tannic acid test | + | ||||
| Hagers test | + | Triterpenoids | Salkowski test | − | |
| Carbohydrates | Molisch's test | + | Saponins | Froth test | + |
| Fats and fixed oils | Glycerin test | + | Cardiac glycosides | Keller kiliani test | + |
+ Present, − absent.
3.6. GC/MS and qualitative phytochemical analysis
In order to obtain information on the major metabolites present in the extract, GC–MS was performed. Analysis revealed the presence of 6-octadecenoic acid (32.47%), n-hexadecanoic acid (25.97%), 17-octadecen-14-yn-1-ol (14.22%), methyl 13-octadecanoate (5.33%), tetradecanoic acid, 10,13-dimethyl-methyl ester (4.51%), methyl 5-6-octadecadienoate (3.98%), gamma-sitosterol (2.45%), stigmasterol (2%) as the major phytoconstituents. Table 2 describes the phytochemicals identified in the extract along with their molecular weight and formula. Previous reports indicated that tetradecanoic acid binds to catalytic site of (Bothrops neuwiedi) venom PLA2 through hydrogen bonding between ASN27, CYS and HIS47 amino acid residues and also by hydrophobic interactions with neighboring amino acid residues such as LEU2, LEU5, CYS2, TYR21, PRO17, GLY6, LYS7, ALA18, ILE9, LEU111 and PRO113 present in the catalytic site. Such competitive binding of this molecule is expected to inhibit the PLA2 activity of the toxin [32]. Also, hexadecanoic acid was demonstrated to have anti-inflammatory activity (Table 3) through competitive inhibition of PLA2 [33]. However, in the present study the PLA2 activity of N. naja venom was not inhibited by the methanolic extract of L. aspera, even though GC–MS analysis indicate the presence of these fatty acids. Sequence analysis indicated (Gopi and Jayaraman, unpublished results) that the amino acid residues present in binding site of the enzyme are significantly different at these positions and therefore resulted in no inhibition of the enzymatic activity of phospholipase.
Table 2.
Phytochemicals identified in the methanolic extract of L. aspera.
| S. no. | RT | Phytochemical name | Mol. formula | Mol. (wt) | Peak area (%) |
|---|---|---|---|---|---|
| 1 | 14.56 | Heptane-1-methanesulfonic acid,7,7-dimethyl-2-oxo | C10H16O4S | 232 | 1.52 |
| 2 | 14.83 | 3-Methyl-2-(2-oxopropyl) furan | C8H10O2 | 138 | 1.54 |
| 3 | 17.71 | Tetradecanoic acid,10,13-dimethyl-methyl ester | C17H34O2 | 270 | 4.51 |
| 4 | 18.18 | n-Hexadecanoic acid | C16H32O2 | 256 | 25.97 |
| 5 | 19.33 | Methyl 9-cis,11-trans-octadecadienoate | C19H34O2 | 294 | 1.70 |
| 6 | 19.39 | Methyl 13-octadecenoate | C19H36O2 | 296 | 5.33 |
| 7 | 19.61 | Methyl 5-6-octadecqdienoate | C19H34O2 | 294 | 3.98 |
| 8 | 19.84 | 6-Octadecenoic acid | C18H34O2 | 282 | 32.47 |
| 9 | 20.08 | 17-Octadecen-14-yn-1-ol | C18H32O | 264 | 14.22 |
| 10 | 20.23 | 2-(Fecnch-2-yl) fenchane | C20H34 | 274 | 1.36 |
| 11 | 25.48 | [1,1-Bicyclohexyl]-4-carboxylic acid, 4′-pentyl-4-pentylphenyl ester | C29H46O2 | 426 | 1.38 |
| 12 | 28.69 | Stigmasterol | C29H48O | 412 | 2.00 |
| 13 | 29.36 | Gamma-sitosterol | C29H50O | 414 | 2.45 |
| 14 | 29.82 | Benzene,1-methyl-2-(1-methyl-2-methylenecyclopentyl) | C14H18O | 202 | 1.49 |
Table 3.
Phytochemicals identified from methanolic extract of L. aspera by GC–MS and their reported bioactivity.
| S. no. | Phytochemical | Compound nature | Bioactivity | Reference |
|---|---|---|---|---|
| 1 | Heptane-1-methanesulfonic acid,7,7-dimethyl-2-oxo | Sulfonic acid ester | Not reported | – |
| 2 | 3-Methyl-2-(2-oxopropyl) furan | Isoprene | Strong anti-oxidant | [34] |
| 3 | Tetradecanoic acid,10,13-dimethyl-methyl ester | Myristic acid | Anti-inflammatory PLA2 inhibition | [32] |
| 4 | n-Hexadecanoic acid | Saturated fatty acids | Anti-inflammatory PLA2 inhibition | [33] |
| 5 | Methyl 9-cis,11-trans-octadecadienoate | Unsaturated fatty acids | Maintains the cell integrity | [36] |
| 6 | Methyl 13-octadecenoate | Unsaturated fatty acids | Maintains the cell integrity | [36] |
| 7 | Methyl 5-6-octadecqdienoate | Unsaturated fatty acids | Maintains the cell integrity | [36] |
| 8 | 6-Octadecenoic acid | Saturated fatty acids | Synthetic inhibitor of neurotoxins | [35] |
| 9 | 17-Octadecen-14-yn-1-ol | Alcohol | Not reported | |
| 10 | 2-(Fecnch-2-yl) fenchane | Terpenes | Anti-snake venom | [6] |
| 11 | [1,1-Bicyclohexyl]-4-carboxylic acid,4′-pentyl-4-pentylphenyl ester | Esters | Not reported | – |
| 12 | Stigmasterol | Phytosterol | Anti-snakevenom | [37] |
| 13 | Gamma-sitosterol | Phytosterol | Anti-snake venom | [37] |
| 14 | Benzene,1-methyl-2-(1-methyl-2-methylenecyclopentyl) | Not reported | – |
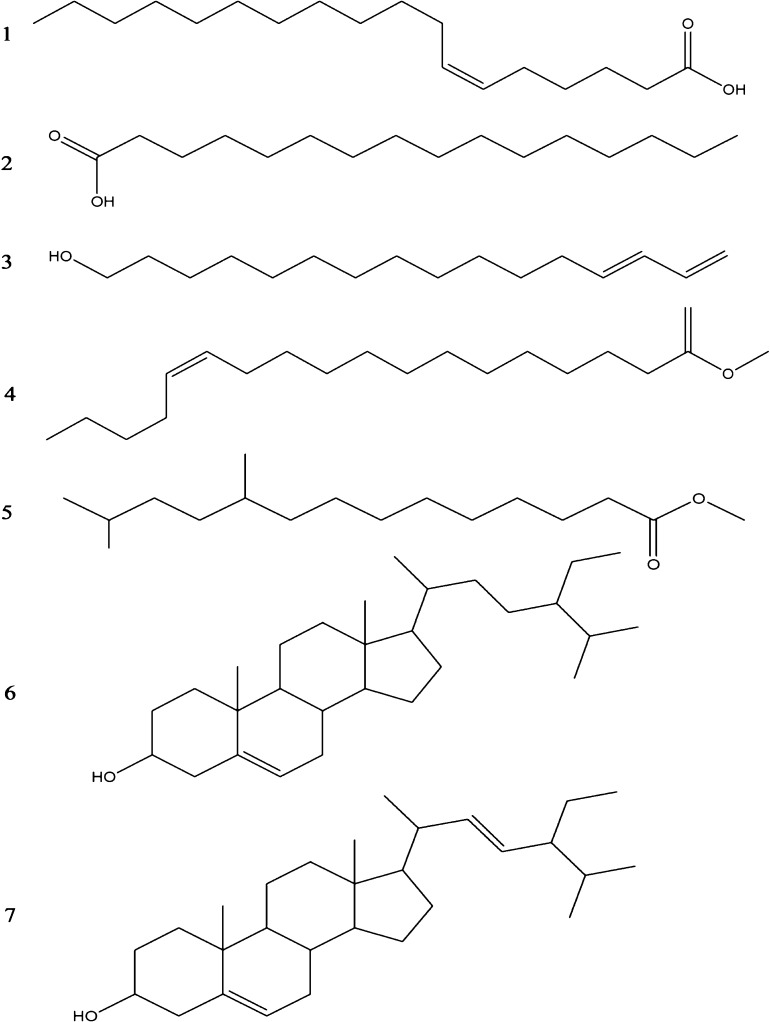
Also, plants having higher the amounts of isoprene derivatives were demonstrated to have strong antioxidant activity by preventing the free radical formation [34]. Antioxidants are known to play an important role in controlling the neurotoxicity. Hence, the presence of isoprene 3-methyl-2-(2-oxopropyl) furan in the L. aspera methanolic extract (Table 3) could help in preventing the oxidative damage caused upon snake envenomations. The derivatives of octadecenoic acid were reported to be very strong synthetic inhibitors of neurotoxins [35]. The unsaturated fatty acids found in the L. aspera methanolic extract would help in maintaining the cell integrity which in-turn prevents the distribution of venom components from the bite site [36]. Apart from isoprene derivatives, octadecanoic acid derivatives in the extract would also help in inhibiting snake venom toxins and their neurotoxicity. Hemorrhage is majorly caused by the combined effect of proteases and hyaluronidases. Sitosterol and stigmasterol were demonstrated to inhibit viper venom induced lethality, neurotoxicity and hemorrhage [37]. In the present study, protease and hyaluroniade activities were completely inhibited in a dose dependent manner. This could be due to the strong anti-venom activity of the phytosterols (sitosterol and stigmasterol) present in the extract. Fig. 6 represents some of the major bioactive components identified from L. aspera methanolic extract. In addition, these phytochemicals were previously demonstrated for their hyaluronidase inhibition activity by either forming an inhibitor-hyaluronic acid complex that restricts the substrate availability to the enzyme or direct binding to the enzyme active site that affects the enzyme activity [38], [39], [40]. Hence, the presence of these multiple bioactive (anti-snake venom) compounds in the extract could have contributed for its efficient antivenom activity in the present study. Based on the finding of the present study, it is demonstrated that methanolic extracts of L. aspera, possess antivenom activity against N. naja venom enzymes and supports their traditional use in herbal medicine against snakebites.
Fig. 6.
Molecular structures of major bioactive phytochemicals from L. aspera methanolic extract identified by GC–MS analysis. (1) 6-octadecenoic acid, (2) n-hexadecenoic acid, (3) 17-octadecen-14-yn-1-ol, (4) methyl-13-octadecenoic acid, (5) tetradecenoic acid, (6) sitosterol, (7) stigmasterol.
Conflict of interest
None.
Transparency document
Footnotes
Available online 29 August 2014
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.toxrep.2014.08.012.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Sandhya S., Sai Kumar P., Vinod K.R., Banji D., Kumar K. Plants as potent antidiabetic and wound healing agents—a review. Hygeia: J. Drug Med. 2011;3:11–19. [Google Scholar]
- 2.Samy R.P., Thwin M.M., Gopalakrishnakone P., Ignacimuthu S. Ethnobotanical survey of folk plants for the treatment of snakebites in Southern part of Tamilnadu, India. J. Ethanopharmacol. 2008;115:302–312. doi: 10.1016/j.jep.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Kasturiratne, Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: a literature analysis and modeling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gutierrez J.M., Williams D., Fan H.W., Warrell D.A. Snakebite envenoming from a global perspective: towards an integrated approach. Toxicon. 2010;56:1223–1235. doi: 10.1016/j.toxicon.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Mohapatra B., Warrell D.A., Suraweera W., Bhatia P., Dhingra N., Jotkar R.M., Rodriquez P.S., Mishra K., Whitaker R., Jha P. Million death study collaborators, snakebite mortality in India: a nationally representative mortality survey. PLoS Negl. Trop. Dis. 2011;5:e1018. doi: 10.1371/journal.pntd.0001018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santhosh M.S., Hemshekhar M., Sunitha K., Thushara R.M., Jnaneshwari S., Kemparaju K., Girish K.S. Snake venom induced local toxicities: plant secondary metabolites as an auxiliary therapy. Mini Rev. Med. Chem. 2013;13:106–123. [PubMed] [Google Scholar]
- 7.Leong P.K., Sim S.M., Fung S.Y., Sumana K., Sitprija V., Tan N.H. Cross neutralization of Afro-Asian cobra and Asian krait venoms by a Thai polyvalent snake antivenom (Neuro Polyvalant Snake Antivenom) PLoS Negl. Trop. Dis. 2012;6:e1672. doi: 10.1371/journal.pntd.0001672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leon G., Herrera M., Segura A., Villalta M., Vargas M., Gutiérrez J.M. Pathogenic mechanisms underlying adverse reactions induced by intravenous administration of snake antivenoms. Toxicon. 2013;76:63–76. doi: 10.1016/j.toxicon.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Okoroma E.A., Garelick H., Abiola O.O., Purchase D. Identification and characterisation of a Bacillus licheniformis strain with profound keratinase activity for degradation of melanised feather. Int. Biodeterior. Biodegrad. 2012;74:54–60. [Google Scholar]
- 10.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Gutierrez J.M., Avila C., Rojas E., Cerdas L. An alternative in vitro method for testing the potency of the polyvalent antivenom produced in Costa Rica. Toxicon. 1988;26:411–413. doi: 10.1016/0041-0101(88)90010-4. [DOI] [PubMed] [Google Scholar]
- 12.Boman H.G., Kaletta U. Chromatography of rattlesnake venom; a separation of three phosphodiesterases. Biochim. Biophys. Acta. 1957;24:619–631. doi: 10.1016/0006-3002(57)90256-1. [DOI] [PubMed] [Google Scholar]
- 13.Girish K.S., Jagadeesha D.K., Rajeev K.B., Kemparaju K. Snake venom hyaluronidase: an evidence for isoforms and extra-cellular matrix degradation. Mol. Cell. Biochem. 2002;240:105–110. doi: 10.1023/a:1020651607164. [DOI] [PubMed] [Google Scholar]
- 14.Kokate C.K., Purohit A.P., Gokhale S.B. 20th ed. Nirali Prakashan; Pune: 2002. Pharmacognosy; pp. 105–109. [Google Scholar]
- 15.Bjarnason J.B., Fox J.W. Hemorrhagic metalloproteinases from snake venoms. Pharmacol. Ther. 1994;62:325–372. doi: 10.1016/0163-7258(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 16.Fox J.W., Serrano S.M.T. Snake toxins and hemostasis. Toxicon. 2005;45:949. [Google Scholar]
- 17.Pithayanukul P., Leanpolcharenchai J., Saparpakorn P. Molecular docking studies and anti-snake venom metalloproteinase activity of Thai mango seed kernel extract. Molecules. 2009;14:3198–3213. doi: 10.3390/molecules14093198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melo P.A., Nascimento M.C., Mors W.B., Suarez-Kurtz G. Inhibition of the myotoxic and hemorrhagic activities of crotalid venoms by Eclipta prostrata extracts and constituents. Toxicon. 1994;32:595–603. doi: 10.1016/0041-0101(94)90207-0. [DOI] [PubMed] [Google Scholar]
- 19.Soares A.M., Ticli F.K., Marcussi S., Lourenço M.V., Januário A.H., Sampaio S.V., Giglio J.R., Lomonte B., Pereira P.S. Medicinal plants with inhibitory properties against snake venoms. Curr. Med. Chem. 2005;12:2625–2641. doi: 10.2174/092986705774370655. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez J.M., Lomonte B. Phospholipase A2 myotoxins from Bothrops snake venoms. Toxicon. 1995;33:1405–1424. doi: 10.1016/0041-0101(95)00085-z. [DOI] [PubMed] [Google Scholar]
- 21.Ownby C.L. Structure, function and biophysical aspects of the myotoxins from snake venoms. J. Toxicol. Toxins Rev. 1998;17:213–238. [Google Scholar]
- 22.Teixeira C., Cury Y., Moreira V., Picolob G., Chaves F. Inflammation induced by Bothrops asper venom. Toxicon. 2009;54:988–997. doi: 10.1016/j.toxicon.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 23.Samy R.P., Gopalakrishnakone P., Chow V.T. Therapeutic application of natural inhibitors against snake venom phospholipase A2. Bioinformation. 2012;8:48–57. doi: 10.6026/97320630008048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alam M.I., Gomes A. Viper venom-induced inflammation and inhibition of free radical formation by pure compound (2-hydroxy-4-methoxy benzoic acid) isolated and purified from Anantamul (Hemidesmus indicus R.BR) root extract. Toxicon. 1998;36:207–215. doi: 10.1016/s0041-0101(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 25.Chatterjee I., Chakravarty A.K., Gomes A. Daboia russellii and Naja kaouthia venom neutralization by lupeol acetate isolated from the root extract of Indian sarsaparilla Hemidesmus indicus R.Br. J. Ethnopharmacol. 2006;106:38–43. doi: 10.1016/j.jep.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 26.Osorio e Castro V.R., Vernon L.P. Hemolytic activity of thionin from Pyrularia pubera nuts and snake venom toxins of Naja naja species: Pyrularia thionin and snake venom cardiotoxin compete for the same membrane site. Toxicon. 1989;27:511–517. doi: 10.1016/0041-0101(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 27.Fletcher J.E., Jiang M.S., Gong Q.H., Yudkowsky M.L., Wieland S.J. Effects of a cardiotoxin from Naja naja kaouthia venom on skeletal muscle: involvement of calcium-induced calcium release, sodium ion currents and phospholipases A2 and C. Toxicon. 1991;29:1489–1500. doi: 10.1016/0041-0101(91)90005-c. [DOI] [PubMed] [Google Scholar]
- 28.Fletcher J.E., Jiang M.S. Possible mechanisms of action of cobra snake venom cardiotoxins and bee venom melittin. Toxicon. 1993;31:669–695. doi: 10.1016/0041-0101(93)90375-s. [DOI] [PubMed] [Google Scholar]
- 29.Girish K.S., Kemparaju K. Inhibition of Naja naja venom hyaluronidase by plant derived bioactive components and polysaccharides. Biochemistry (Mosc) 2005;70:948–952. doi: 10.1007/s10541-005-0207-z. [DOI] [PubMed] [Google Scholar]
- 30.Pithayanukul P., Leanpolchareanchai J., Bavovada R. Inhibitory effect of tea polyphenols on local tissue damage induced by snake venoms. Phytother. Res. 2010;24:S56–S62. doi: 10.1002/ptr.2903. [DOI] [PubMed] [Google Scholar]
- 31.Kemparaju, Girish K.S. Snake venom hyaluronidase: a therapeutic target. Cell Biochem. Funct. 2006;24:7–12. doi: 10.1002/cbf.1261. [DOI] [PubMed] [Google Scholar]
- 32.Delatorre P., Rocha B.A.M., Santi-Gadelha T., Gadelha C.A.A., Toyama M.H., Cavada B.S. Crystal structure of Bn IV in complex with myristic acid: a Lys4 9 myotoxic phospholipase A2 from Bothrops neuwiedi venom. Biochimie. 2011;93:513–518. doi: 10.1016/j.biochi.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Vickers C.E., Possell M., Cojocariu C.I., Velikova V.B., Laothawornkitkul J., Ryan A., Mullineaux P.M., Nicholas Hewitt C. Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ. 2009;32:520–531. doi: 10.1111/j.1365-3040.2009.01946.x. [DOI] [PubMed] [Google Scholar]
- 34.Georgieva D.N., Rypniewski W., Perbandt M., Jain M., Genov N., Betzel C. Crystallization and preliminary X-ray diffraction studies of a toxic phospholipase A2 from the venom of Vipera ammodytes meridionalis complexed to a synthetic inhibitor. Biochim. Biophys. Acta. 2003;1650:1–3. doi: 10.1016/s1570-9639(03)00210-3. [DOI] [PubMed] [Google Scholar]
- 35.Aparna V., Dileep K.V., Mandal P.K., Karthe P., Sadasivan C., Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012;80:434–439. doi: 10.1111/j.1747-0285.2012.01418.x. [DOI] [PubMed] [Google Scholar]
- 36.Gill I., Valiverty R. Polyunsaturated fatty acids, Part 1: Occurrence, biological activities and applications. Trends Biotechnol. 1997;15:401–409. doi: 10.1016/S0167-7799(97)01076-7. [DOI] [PubMed] [Google Scholar]
- 37.Gomes A., Archita S., Ipshita C., Chakravarty A.K. Viper and cobra venom neutralization by β-sitosterol and stigmasterol isolated from the root extract of Pluchea indica Less. (Asteraceae) Phytomedicine. 2007;14:637–643. doi: 10.1016/j.phymed.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 38.Havsteen B., Flavonoids a class of natural products of high pharamacological potency. Biochem. Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 39.Kuppusamy U.R., Das N.P. Inhibitory effects of flavonoids on several venom hyaluronidases. Experientia. 1991;47:1196–1200. doi: 10.1007/BF01918384. [DOI] [PubMed] [Google Scholar]
- 40.Girish K.S., Kemparaju K. Inhibition of Naja naja venom hyaluronidase: role in the management of poisonous bite. Life Sci. 2006;78:1433–1440. doi: 10.1016/j.lfs.2005.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.