Abstract
PURPOSE:
The purpose of this study is to determine if phacoemulsification with posterior chamber foldable intraocular lens implantation has a greater impact on the corneal endothelium of type 2 diabetic patients as compared to nondiabetic controls.
MATERIALS AND METHODS:
This study compared the corneal endothelial status in sixty patients with diabetes with good glycemic control and sixty nondiabetic controls before and after (1 week, 1 month, 2 month, and 3 month) uneventful phacoemulsification. Central corneal endothelial cell density, percentage hexagonality, and coefficient of variation were measured using a noncontact specular microscope. Central corneal thickness was taken as a surrogate marker for endothelium functional status.
RESULTS:
Data were age and sex matched. Patients with diabetes showed significantly higher loss in endothelial cell count as compared to nondiabetic controls. Furthermore, the patients with diabetes showed a slower recovery trend in the endothelial healing response as evidenced by lower change in the coefficient of variation. Significant correlation was found between energy used and change in endothelial count as well as coefficient of variation in nondiabetics only.
CONCLUSION:
In spite of good glycemic control, patients with diabetes have significantly more endothelial damage in comparison to nondiabetic controls with similar nuclear grading and phaco energy used. This warrants a more careful use of phaco energy in patient with diabetes.
Keywords: Corneal endothelium, diabetic, phacoemulsification
Introduction
The corneal endothelium is vital for the maintenance of corneal transparency. This is accomplished by its effectiveness in keeping the corneal stroma in a state of continuous dehydration through two main actions – active fluid pump and barrier function. Any compromise in these activities has a direct effect on corneal clarity.
Cataract extraction with phacoemulsification is one of the most common surgical procedures performed today. The corneal endothelium is known to undergo damage during phacoemulsification.[1] The endothelium status is also known to decline with age.[2,3,4,5,6,7,8,9,10,11,12,13,14] With increase in life expectancy as most of the patients undergoing phacoemulsification belong to the elderly age group, endothelial damage during surgery becomes an important factor to consider.
Diabetes mellitus also has been found to be detrimental to the corneal endothelium. Patients with diabetes have been found to have morphologically abnormal endothelium including pleomorphism and polymegethism.[15] Thus, an elderly patient with diabetes undergoing phacoemulsification is particularly vulnerable to greater endothelial damage during surgery. This hypothesis has been supported by few previous studies[15,16,17,18,19,20,21,22] but still remains controversial. Inoue et al.[23] in their study showed that the presence of type 2 diabetes mellitus is irrelevant to any of the parameters of corneal endothelial cells in patients undergoing cataract surgery. In a recent study, Storr-Paulsen et al.[24] have shown that type 2 diabetes has no impact on corneal cell density or morphology in individuals with good glycemic status.
We undertook a prospective interventional study to compare the endothelial status in patients with and without type 2 diabetes mellitus undergoing phacoemulsification with posterior chamber intraocular lens implantation. The endothelial status included cell density, cell size variability, hexagonality, and central corneal thickness (CCT) as a surrogate marker for functional status.
Materials and Methods
Sixty eyes of sixty consecutive patients between 50 and 70 years of age with well-controlled type 2 diabetes mellitus posted for phacoemulsification were selected. Glycosylated hemoglobin (HbA1c) was used as the criteria to assess glycemic status with values <7.0 considered to be good control. Sixty consecutive patients of same age range without diabetes served as controls. Two casual blood glucose tests were taken in accordance with the recommendations of the American Diabetic Association (ADA) to disclose undetected diabetes (ADA 2007). This study was approved by the institutional ethics committee and informed consent was taken from all the participants.
The sample size was calculated taking the significant change in corneal endothelial cell density (ECD), to detect a difference in mean change of its value, to be 112 cells per square millimeter between the two groups, that is, diabetic group, with standard deviation (SD) 211 and nondiabetic group, with SD: 194 (obtained from a previous study)[21] and taking power of study to be 80%, then the sample size comes out to be 53 for each group. Taking a follow-up loss of 10%, the sample size becomes 58.3 (~60) per group, that is, a total of 120 individuals.
Exclusion criteria included HbA1c status >7.0, history of previous ocular surgery, contact lens wear, documented raised intraocular pressure, history of ocular trauma, preoperative endothelial count <1500 cells/mm2, pseudoexfoliation, preexisting corneal pathology, intraocular inflammation, nuclear sclerosis grade IV, and axial length <21 mm.
The detailed preoperative evaluation was done for all patients which included a detailed history, best corrected visual acuity, slit-lamp examination, fundus examination, and Goldmann applanation tonometry. Lens Opacities Classification System III (LOCS III) was used to grade the cataract.
Specular microscopy (using Specular Microscope, SP 3000P, Topcon, Tokyo, Japan, with the IMAGEnet imaging system (version 2.1, Topcon) was done preoperatively and at 1 week, 1 month, 2 month, and 3 month after surgery in all the patients. Three readings were taken and an average of three was finally taken as the result. Extreme variations in values were discarded. The measurements were done by a single evaluator to measure central corneal ECD (cells per square millimeter), variation in size of corneal endothelial cells (coefficient of variation), and percentage of hexagonal cells.
CCT (in micrometers) was measured using standard technique (Pachette 2 ultrasonic pachymeter, DGH 550, DGH Technology Incorporation, Philadelphia, USA.)
All the individuals were operated by a single well-experienced surgeon using a standard technique with the same phacoemulsification equipment (Infiniti vision system; Alcon Laboratories; Inc., Fort Worth, TX, USA) at similar settings.
A standard preoperative regimen included eye drop moxifloxacin hydrochloride 0.5% and ketorolac tromethamine 0.5%, one drop 6 hourly, one day before surgery, and eye drop tropicamide 0.8% and phenylephrine hydrochloride 5.0%, one drop every 15 min 1 h before surgery. Peribulbar anesthesia with 5–6 ml lidocaine hydrochloride 2% + adrenaline 1:200,000 was given before surgery. In all the participants', preoperative pupillary dilatation of around 7–8 mm could be achieved.
A 3.2 mm superotemporal self-sealing clear corneal incision was made just in front of the vascular arcades of the corneoscleral limbus using a calibrated 3.2 mm keratome. Viscoelastic substance (hydroxypropyl methylcellulose 2%) was injected into the anterior chamber. A paracentesis incision of 1 mm was made 60° apart with a 20 gauge microvitreoretinal blade. After the capsulorrhexis, hydrodissection followed by nucleus rotation, nucleotomy was done by a single method, that is, stop and chop technique. After emulsification of nuclear fragments, irrigation aspiration of residual cortical matter was done. A foldable intraocular lens was implanted inside the capsular bag. After this, the removal of the viscoelastic material was done and finally, the incision was hydrated using a 30-gauge cannula. No sutures were applied. The eye was bandaged which was opened next morning. Cumulative dissipated energy (CDE; phaco energy) and pupil size were noted during the intraoperative period.
Postoperative treatment included prednisolone acetate 1% in gradually reducing doses for 4 weeks, tropicamide 0.8%, and phenylephrine hydrochloride 5.0% one drop two times a day for 2 weeks, bromfenac sodium 0.9 mg/ml one drop two times a day for 4 weeks and moxifloxacin hydrochloride 0.5% one drop four times a day for 1 week.
Statistical analysis
Parametric tests (independent t-test) were used for statistical analysis of quantitative variables. The endothelial parameters were analyzed using a linear mixed model with unstructured covariance on repeated measures effect. The trend was compared between groups and also included interaction of the groups with time. All the qualitative variables were analyzed using Chi-square test.
Results
Both the groups were age and sex matched [Table 1]. Mean age was 63.38 years in diabetics and 64 years in the nondiabetic group. Preoperative glycemic control was good as indicated by the mean HbA1c was 6.87 (SD: 0.43). The preoperative endothelial cell status as indicated by the ECD, percentage cell size variability, percentage hexagonality, and CCT, was similar in both groups [Table 2]. The distribution of nuclear grading (according to LOCS III) was similar in both groups.
Table 1.
Age and sex distribution
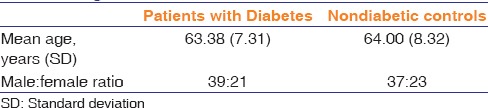
Table 2.
Change in endothelial cell parameters at 3 months' postoperative period
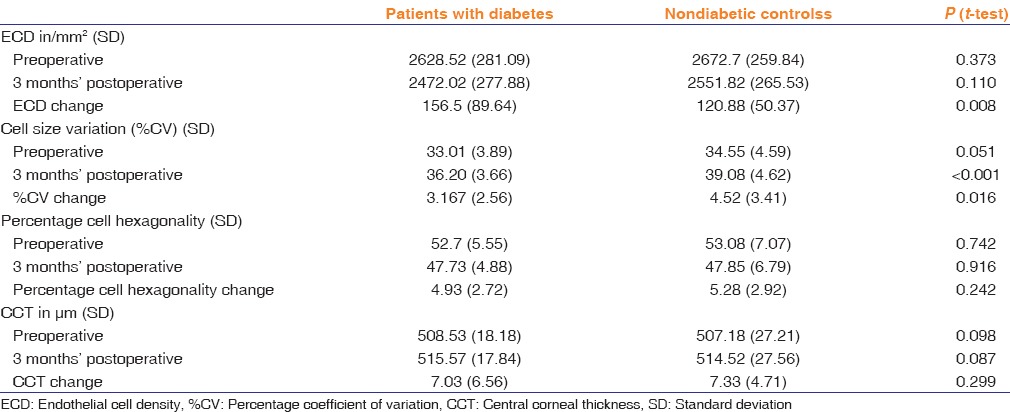
Mean preoperative pupil size in the diabetic group was 7.55 mm and 7.53 mm in the control group. At the end of surgery, there was a slight miosis of average 0.17 mm (SD: 0.05) in the patients with diabetes and 0.09 mm (SD: 0.03) in the control group which was not statistically significant [Table 3]. The average CDE in the diabetic group 18.97 (SD: 8.31) and in the control group 17.95 (SD: 7.12) which was statistically insignificant.
Table 3.
Pupillary aperture
The mean ECD was 2629 (SD: 281) in the patient with diabetes group and 2673 (SD: 260) in the control group. At the end of 3 months, there was a decline of 157 (SD: 90) in the patients with diabetes group and 121 (SD: 50) in the control group. This was statistically significant (P = 0.008) [Table 2]. The trend in ECD was also mapped using the 1 week, 1 month, 2 month, and 3 month values which showed a similar trend in the central endothelial count in both the groups after a steep loss just after phacoemulsification.
Both the groups showed an increase in the percentage coefficient of variation (%CV) at postoperative 3-month follow-up and these values were significantly different (P < 0.001) from preoperative values [Table 2]. The increase was higher in the control group and was statistically significant (P = 0.002). The %CV showed a significantly slower trend of increment in the diabetic group as compared to the control group [Figure 1].
Figure 1.
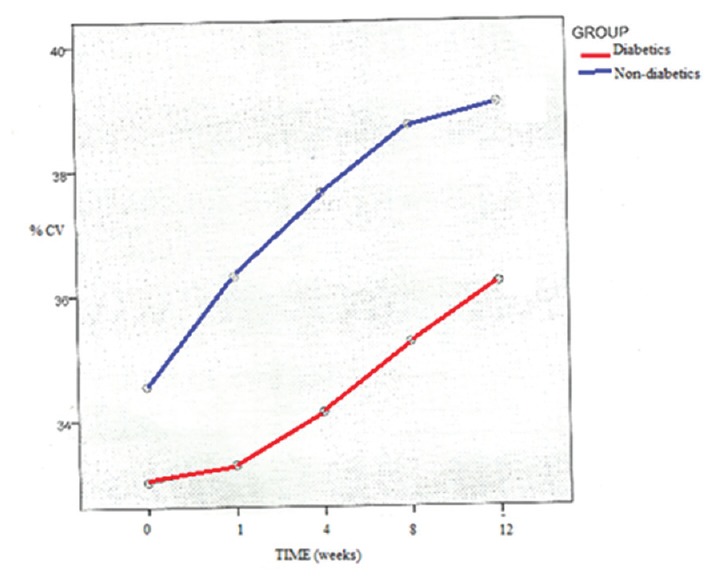
Percentage coefficient of variation with time in weeks (patients with diabetes and nondiabetic controls)
Both the groups showed a decrease in the percentage of hexagonal cells at postoperative 3-month follow-up. No significant difference in either preoperative or postoperative values was found among the two groups (P = 0.742 and 0.916). However, the change in percentage hexagonality was significant within each group (P < 0.001). This change was higher in nondiabetes controls but was statistically not significant (P = 0.242) [Table 2]. The percentage hexagonality showed a steady decline in both the groups with a similar rate of change [Figure 2].
Figure 2.
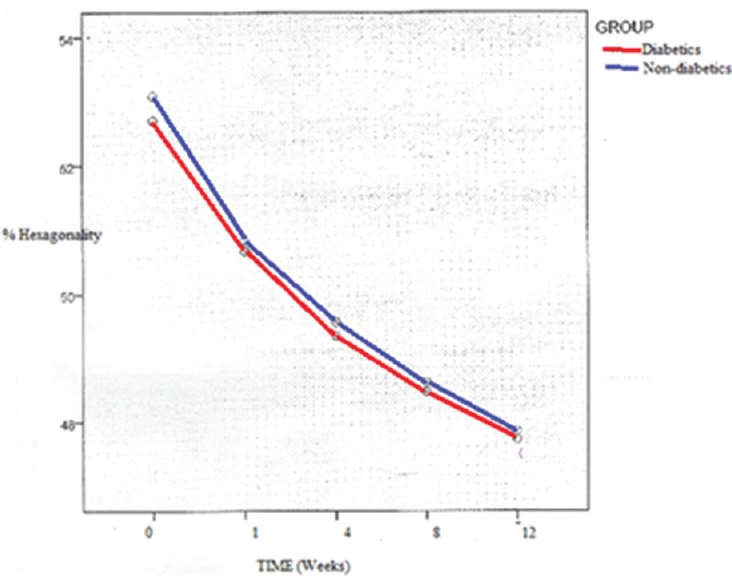
Percentage hexagonality with time in weeks (patients with diabetes and nondiabetic controls)
The CCT increased in the 1st week after surgery which was significantly higher in the diabetic group and then decreased steadily. The difference in the rate of decrease in CCT among the groups was not statistically significant [Figure 3].
Figure 3.
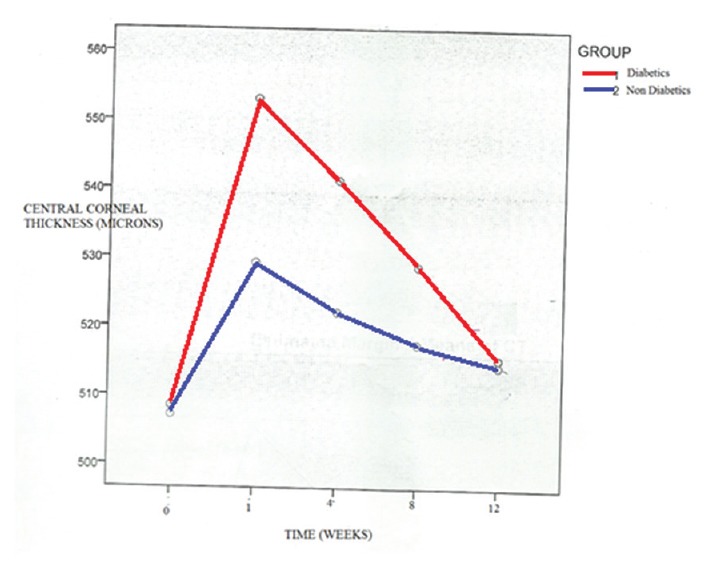
Central corneal thickness with time in weeks (patients with diabetes and nondiabetic controls)
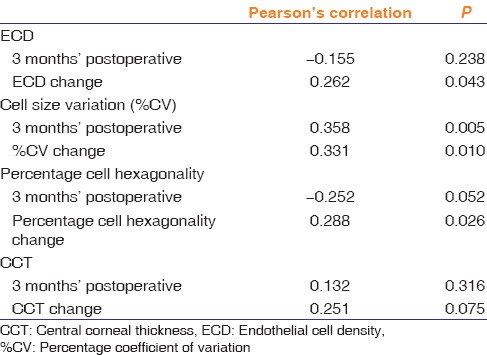
Pearson's correlation studies were done to find out the relationship between the energy used during phacoemulsification and the various endothelial parameters in both the groups. In the diabetic group, the CDE was not found to be significantly correlated to any of the endothelial cell parameters, whereas nondiabetic group showed a significant positive correlation to all the four endothelial cell parameters at 3 month postoperative period [Table 4].
Table 4.
Correlation of endothelial cell parameters with cumulative dissipated energy in nondiabetic controls
Discussion
Several studies have been conducted to study the effect of cataract surgery on the corneal endothelium by various techniques (conventional extracapsular cataract extraction, small incision cataract surgery, and phacoemulsification). All these studies have shown a decline in the endothelial status after surgery. However, there are only a few studies which have compared these changes with patients with diabetes.
This is the second largest sample size for such a type of study, the largest study being the one conducted by Morikubo et al. in 2000–2002, which included 186 eyes (93 in each group).[1]
In our study, we did not include patients with very hard nuclei (grade IV and above) because of high energy required with such hard cataracts. Both the groups had no significant difference between the distributions of nuclear density. This is the first time that nucleus grading is being documented in such a type of study.
CDE is a measure of the total ultrasonic energy used during phacoemulsification. It is calculated automatically by the Alcon Infiniti phacoemulsification unit. In our study, we used only torsional power, keeping the linear mode switched off. Thus, CDE was calculated using the following formula: torsional time × 0.4 × average torsional amplitude.
The average CDE was 18.97 (SD: 8.31) in the diabetic group and 17.95 (SD: 7.12) in the nondiabetic group. This minor difference was statistically not significant (P = 0.473). Such an analysis is unique to our study as previous studies have not quantified the energy used in phacoemulsification.
There was an average 5.95% (SD: 3.08) loss in the ECD in the diabetic group and 4.52% (SD: 1.97) in the controls. This was statistically significant (P = 0.008). Hugod et al. showed a mean loss in ECD of 6.2% in patients with diabetes but only 1.4% in the nondiabetic controls at the end of 3 months after surgery[21] and Morikubo et al. reported the mean loss in ECD to be 3.2% in nondiabetes and 7.2% in patients with diabetes at postoperative 1-month follow-up[1] similar to our finding.
%CV (cell size variability) is an indicator of the uniformity of the size of endothelial cells. High values indicate high levels of pleomorphism. It indicates the activity of the repair and healing mechanism of the endothelium after an insult. Preoperative values were comparable in the diabetics and nondiabetic controls in our study. A significantly higher %CV at 3-month postoperative follow-up (as compared to preoperative values) was seen in both the groups, but the diabetic group showed a significantly lesser change as compared to the nondiabetic group (statistically significant). This demonstrates the slower and weaker recovery of endothelial cells in patient with diabetes. The trend analysis, which revealed a slower rate of change in patient with diabetes, confirms this finding. Hugod et al. also found a decrease in the %CV (33.2 at 3 months as compared to 33.7 preoperatively),[21] but this change was not found to be statistically significant. Studies by Morikubo et al.[1] and Lee et al.[17] also demonstrated nonsignificant differences in the %CV change. This result is unique to our study and supports the theory that the endothelium in patients with diabetes has slower and poor healing response.
Normal corneal endothelial cells have a hexagonal shape forming a regular mosaic. After insult, this morphology is disturbed (pleomorphism) and the percent of hexagonal cells reduce. In our study, decrease in percentage of hexagonal cell was seen in the patient with diabetes as well as nondiabetic controls, but this change was not statistically different. Similar finding was shown by Lee et al.[17] Morikubo et al.[1] also made similar observations but their study had a follow-up of only 1 month which can induce bias as the changes do not stabilize this early. In contrast, Hugod et al.[21] showed a significant decline in the percentage of hexagonal cells among the patients with diabetes only. The possible explanation of this discrepancy between our study and that of Hugod could be due to racial difference in metabolic profile of patients with diabetes and thus the response of corneal endothelial cells to surgical insult could be different.[25,26] Since this is the first Indian study of this design, further studies with larger sample sizes and longer follow-up is required to confirm these findings. Furthermore, the endothelial cells photographed for analysis belonged to the central cornea, thus the changes in morphology may be more toward the periphery which will go undetected with the technique used in our study.
CCT was used as a surrogate marker for endothelial function status. This is because a well-functioning endothelium keeps the corneal stroma in a dynamic state of continuous deturgescence. In our study, the diabetic group showed significantly higher CCT in early postoperative period, but on comparing the change between the two groups later at 3 month, it was not found to be statistically significant. Similar results were found in previous studies.[1,21]
Thus, we see that although the endothelial morphometric parameters show significantly higher deterioration in the patients with diabetes, this does not translate into impaired function. A possible explanation for this could be good glycemic control of all our study participants.
The analysis of correlation between CDE and endothelial status is unique to our study. None of the previous studies have analyzed the relationship between the total ultrasonic energy utilized during phacoemulsification and endothelial parameters in patients with diabetes. Hugod et al.[21] mentioned uniform nuclear densities in their groups, but the same nuclear density does not necessarily translate into similar phaco energy. Among the nondiabetic controls, significant positive correlation was found between CDE and the change in ECD, change in cell size variability, and change in percentage of hexagonal cells at 3 months.
These results indicate that a higher energy used during surgery translates into more endothelial cell loss, more increase in cell size variability, and a greater loss of hexagonality. This seems logically sound as well, because the more is the power delivered to the anterior segment, the more is the magnitude of insult to the endothelium which results in a greater change in the morphometric parameters.
In the patients with diabetes, CDE did not correlate significantly with endothelial morphometric or functional parameters. A possible explanation for this could be that the ultrasound power used during surgery is not the only parameter which affects the endothelium during surgery. Other variables include the amount of irrigation fluid used, total time of surgery, and anterior chamber depth. We have not included these in our study due to logistic and time constraints and they may be functioning as confounding factors. Since there have been no previous studies which have attempted to correlate CDE with endothelial parameters in patients with diabetes, more studies are needed to confirm or deny these findings.
Conclusion
To summarize, our study has found that patients with diabetes show a significantly higher loss of corneal endothelial density after phacoemulsification as compared to nondiabetic controls. Furthermore, a slower and poor healing response was seen in patients with diabetes as evidenced by lesser changes in the coefficient of variation.
These changes were seen even in the presence of good glycemic control, thus other factors might be contributing to the increased vulnerability of endothelial cells in patients with diabetes.
However, these morphometric parameters did not translate in functional deficits as noted by nonsignificant changes in CCT and equivalent visual gain as compared to nondiabetic controls.
Therefore, the corneal endothelium of patients with diabetes does show significantly more damage after phacoemulsification, which warrants a more careful approach during phacoemulsification in these patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Morikubo S, Takamura Y, Kubo E, Tsuzuki S, Akagi Y. Corneal changes after small-incision cataract surgery in patients with diabetes mellitus. Arch Ophthalmol. 2004;122:966–9. doi: 10.1001/archopht.122.7.966. [DOI] [PubMed] [Google Scholar]
- 2.Williams KK, Noe RL, Grossniklaus HE, Drews-Botsch C, Edelhauser HF. Correlation of histologic corneal endothelial cell counts with specular microscopic cell density. Arch Ophthalmol. 1992;110:1146–9. doi: 10.1001/archopht.1992.01080200126039. [DOI] [PubMed] [Google Scholar]
- 3.Sawa M, Tanishima T. The morphometry of the human corneal endothelium and follow-up of postoperative changes. Jpn J Ophthalmol. 1979;23:337–50. [Google Scholar]
- 4.Sturrock GD, Sherrard ES, Rice NS. Specular microscopy of the corneal endothelium. Br J Ophthalmol. 1978;62:809–14. doi: 10.1136/bjo.62.12.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Laule A, Cable MK, Hoffman CE, Hanna C. Endothelial cell population changes of human cornea during life. Arch Ophthalmol. 1978;96:2031–5. doi: 10.1001/archopht.1978.03910060419003. [DOI] [PubMed] [Google Scholar]
- 6.Stefansson A, Müller O, Sundmacher R. Non-contact specular microscopy of the normal corneal endothelium. A statistical evaluation of morphometric parameters. Graefes Arch Clin Exp Ophthalmol. 1982;218:200–5. doi: 10.1007/BF02150095. [DOI] [PubMed] [Google Scholar]
- 7.Yee RW, Matsuda M, Schultz RO, Edelhauser HF. Changes in the normal corneal endothelial cellular pattern as a function of age. Curr Eye Res. 1985;4:671–8. doi: 10.3109/02713688509017661. [DOI] [PubMed] [Google Scholar]
- 8.Majima Y, Nogawa H, Yussa E. The specular microscopic studies of the corneal endothelium. The change with age and the change between pre and post cataract extraction. Acta Soc Ophthalmol Jpn. 1979;83:936–46. [PubMed] [Google Scholar]
- 9.Olsen T. Non-contact specular microscopy of human corneal endothelium. Acta Ophthalmol (Copenh) 1979;57:986–98. doi: 10.1111/j.1755-3768.1979.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 10.Laing RA, Sanstrom MM, Berrospi AR, Leibowitz HM. Changes in the corneal endothelium as a function of age. Exp Eye Res. 1976;22:587–94. doi: 10.1016/0014-4835(76)90003-8. [DOI] [PubMed] [Google Scholar]
- 11.Suda T. Mosaic pattern changes in human corneal endothelium with age. Jpn J Ophthalmol. 1984;28:331–8. [PubMed] [Google Scholar]
- 12.Carlson KH, Bourne WM, McLaren JW, Brubaker RF. Variations in human corneal endothelial cell morphology and permeability to fluorescein with age. Exp Eye Res. 1988;47:27–41. doi: 10.1016/0014-4835(88)90021-8. [DOI] [PubMed] [Google Scholar]
- 13.Ohara K, Tsuru T, Inoda S. Morphometric parameters of corneal endothelial cells. Acta Soc Ophthalmol Jpn. 1987;91:1073–8. [PubMed] [Google Scholar]
- 14.Bourne WM, Kaufman HE. Specular microscopy of human corneal endothelium in vivo. Am J Ophthalmol. 1976;81:319–23. doi: 10.1016/0002-9394(76)90247-6. [DOI] [PubMed] [Google Scholar]
- 15.Schultz RO, Matsuda M, Yee RW, Edelhauser HF, Schultz KJ. Corneal endothelial changes in type I and type II diabetes mellitus. Am J Ophthalmol. 1984;98:401–10. doi: 10.1016/0002-9394(84)90120-x. [DOI] [PubMed] [Google Scholar]
- 16.Busted N, Olsen T, Schmitz O. Clinical observations on the corneal thickness and the corneal endothelium in diabetes mellitus. Br J Ophthalmol. 1981;65:687–90. doi: 10.1136/bjo.65.10.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JS, Lee JE, Choi HY, Oum BS, Cho BM. Corneal endothelial cell change after phacoemulsification relative to the severity of diabetic retinopathy. J Cataract Refract Surg. 2005;31:742–9. doi: 10.1016/j.jcrs.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Su DH, Wong TY, Wong WL, Saw SM, Tan DT, Shen SY, et al. Diabetes, hyperglycemia, and central corneal thickness: The Singapore Malay Eye Study. Ophthalmology. 2008;115:964–8.e1. doi: 10.1016/j.ophtha.2007.08.021. [DOI] [PubMed] [Google Scholar]
- 19.Mathew PT, David S, Thomas N. Endothelial cell loss and central corneal thickness in patients with and without diabetes after manual small incision cataract surgery. Cornea. 2011;30:424–8. doi: 10.1097/ICO.0b013e3181eadb4b. [DOI] [PubMed] [Google Scholar]
- 20.Choo M, Prakash K, Samsudin A, Soong T, Ramli N, Kadir A. Corneal changes in type II diabetes mellitus in Malaysia. Int J Ophthalmol. 2010;3:234–6. doi: 10.3980/j.issn.2222-3959.2010.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 2011;30:749–53. doi: 10.1097/ICO.0b013e31820142d9. [DOI] [PubMed] [Google Scholar]
- 22.Sudhir RR, Raman R, Sharma T. Changes in the corneal endothelial cell density and morphology in patients with type 2 diabetes mellitus: A population-based study, Sankara Nethralaya Diabetic Retinopathy and Molecular Genetics Study (SN-DREAMS, Report 23) Cornea. 2012;31:1119–22. doi: 10.1097/ICO.0b013e31823f8e00. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Tokuda Y, Inoue Y, Amano S, Oshika T, Inoue J. Corneal endothelial cell morphology in patients undergoing cataract surgery. Cornea. 2002;21:360–3. doi: 10.1097/00003226-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Storr-Paulsen A, Singh A, Jeppesen H, Norregaard JC, Thulesen J. Corneal endothelial morphology and central thickness in patients with type II diabetes mellitus. Acta Ophthalmol. 2014;92:158–60. doi: 10.1111/aos.12064. [DOI] [PubMed] [Google Scholar]
- 25.Siegfried CJ, Shui YB, Holekamp NM, Bai F, Beebe DC. Racial differences in ocular oxidative metabolism: Implications for ocular disease. Arch Ophthalmol. 2011;129:849–54. doi: 10.1001/archophthalmol.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rao SK, Ranjan Sen P, Fogla R, Gangadharan S, Padmanabhan P, Badrinath SS. Corneal endothelial cell density and morphology in normal Indian eyes. Cornea. 2000;19:820–3. doi: 10.1097/00003226-200011000-00012. [DOI] [PubMed] [Google Scholar]


