Abstract
Purpose:
Palliative care’s role in oncology has expanded, but its effect on aggressiveness of care at the end of life has not been characterized at the population level.
Methods:
This matched retrospective cohort study examined the effect of an encounter with palliative care on health-care use at the end of life among 6,580 Medicare beneficiaries with advanced prostate, breast, lung, or colorectal cancer. We compared health-care use before and after palliative care consultation to a matched nonpalliative care cohort.
Results:
The palliative care cohort had higher rates of health-care use in the 30 days before palliative care consultation compared with the nonpalliative cohort, with higher rates of hospitalization (risk ratio [RR], 3.33; 95% CI, 2.87 to 3.85), invasive procedures (RR, 1.75; 95% CI, 1.62 to 1.88), and chemotherapy administration (RR, 1.61; 95% CI, 1.45 to 1.78). The opposite pattern emerged in the interval from palliative care consultation through death, where the palliative care cohort had lower rates of hospitalization (RR, 0.53; 95% CI, 0.44-0.65), invasive procedures (RR, 0.52; 95% CI, 0.45 to 0.59), and chemotherapy administration (RR, 0.46; 95% CI, 0.39 to 0.53). Patients with earlier palliative care consultation in their disease course had larger absolute reductions in health-care use compared with those with palliative care consultation closer to the end of life.
Conclusion:
This population-based study found that palliative care substantially decreased health-care use among Medicare beneficiaries with advanced cancer. Given the increasing number of elderly patients with advanced cancer, this study emphasizes the importance of early integration of palliative care alongside standard oncologic care.
INTRODUCTION
Over the past decade, the use of palliative care has increased across the United States health-care system, particularly in the field of oncology. The proportion of hospitals with a palliative care program increased from less than a quarter in 2000 to more than two-thirds in 2011.1 Palliative care plays a central role in oncology because of its proven effect, with improved symptoms, quality of life, patient and caregiver satisfaction, improved mood, prolonged survival, and less aggressive care at the end of life.2-8 Current clinical guidelines recommend dedicated palliative care services early in the disease course concurrent with active treatment of patients with advanced cancer.9-11 Cancer remains the second leading cause of death in the United States and often involves intense treatment that draws on substantial patient and economic resources.12,13 With the aging population, there exists a critical public health need for health-care services, such as palliative care, that can improve patients’ quality of life and family satisfaction without straining the existing health-care system.14,15
Aggressive health-care measures at the end of life aim to extend survival, however, have been associated with decreased quality of care.16-18 Previous studies have found less intensive end-of-life health-care use among hospital patients who received palliative care compared with those who did not,19-22 but the relationship between palliative care and end-of-life care in the United States has not been characterized at a population level. Validation of these previous studies in a real-world, population-based setting is critical to demonstrate the practical benefits of palliative care as it is actually implemented. The objective of this study was to define the effect of palliative care consultation on health-care use at the end of life among patients with metastatic prostate, breast, lung, or colorectal cancer.
METHODS
Data
This project used data within the SEER-Medicare linked database. SEER cancer registry data, compiled by the National Cancer Institute, include information from patients from individual cancer registries representing 28% of the US population. Medicare provides federally funded health insurance for people 65 and older. The SEER-Medicare linked database contains Medicare claims data for eligible patients within the SEER registries. This dataset enables the evaluation of health-care delivery and use at a population level over the course of a patient’s diagnosis. The Institutional Review Board of University of California, San Diego, considered this study exempt from review.
Patients
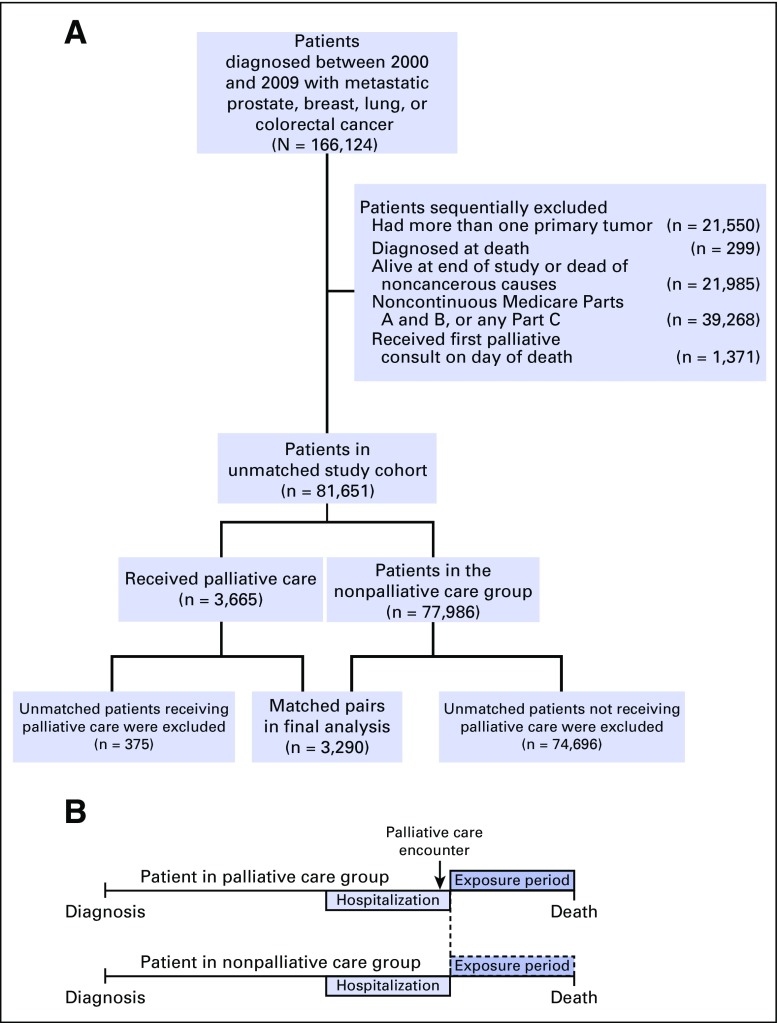
This project focused on the four most commonly diagnosed cancer sites in the United States: prostate, breast, lung, and colorectal cancer.12 We initially identified 166,124 persons with histologically confirmed distant metastatic cancer over the age of 65 years diagnosed between 2000 and 2009. We excluded those who had multiple primary tumors and those who were diagnosed on autopsy or on death certificate only. The goal of the study was to evaluate patterns of care at the end of life among patients dying of cancer. Therefore, we excluded patients who were alive at the end of the study period (December 31, 2010) and those who died of causes other than cancer. We included only patients with complete Medicare claims data, including those with continuous enrollment in Medicare Part A and B from 1 year before diagnosis through death. We also excluded those enrolled in Medicare Part C, because most patients with Part C have it through health maintenance organization plans for which claims data are not readily available. Finally, we excluded patients who received their first palliative care consultation on the day of death, because of lack of time to evaluate patterns of care at the end of life. The prematched study population consisted of 81,651 patients; Figure 1A presents details of the patient-selection process.
Fig 1.
Patient selection criteria. (A) Patient selection and matching. (B) Matching-patient exposure periods.
Matching
To minimize selection bias resulting from nonrandom assignment of palliative care, we used coarsened exact matching23,24 to form two groups balanced on measured covariates. Prior research evaluating patients enrolled in hospice found coarsened exact matching produces a more balanced match compared with other matching strategies, including propensity score matching.23 The “exposure” of interest in this study was consultation with a palliative care provider, defined as the presence of the International Classification of Diseases, version 9 code V66.7, which denotes an “encounter for palliative care.” This palliative care encounter diagnosis code was captured from both inpatient and outpatient Medicare billing claims. Among palliative care encounters during an inpatient hospitalization, the precise date of the encounter was not always available. Typical palliative care encounters often occur toward the end of hospitalizations25; therefore, we assigned the date of palliative care as the last day of hospitalization. The exact date of a palliative encounter was available and used among patients with outpatient palliative care visits.
After dividing our sample into two cohorts on the basis of exposure status, patients were iteratively matched from more to less granular strata on sex, age, place of residence, and year of diagnosis. SEER data do not capture disease severity; therefore, we matched patients on survival measured in months from diagnosis of cancer through death as a proxy for severity of disease.23 The details of the coarsened exact matching process are described elsewhere.24 In brief, the first matching iteration finds exact matches on sex, age, place of residence, year of diagnosis, and number of months from diagnosis to death. Gradually, these matching variables are relaxed into coarser strata to discover additional matched pairs. The maximum levels of coarseness were 5-year age blocks, 3-year diagnosis groups, and 4-month illness duration blocks; place of residence was coarsened from health-care service area to hospital referral region.
The final phase of our matching procedure involved determining a matched exposure period for each pair. In the cohort with a palliative care consultation, the exposure period extended from the day after the palliative care encounter through death. Among patients with a record of multiple palliative care encounters, the first encounter was used as the exposure date. Among the nonexposure cohort of patients, we assigned a pseudo-exposure date (because this cohort did not have a palliative care exposure) and a corresponding pseudo-exposure period that matched the time interval in the exposed matched counterpart (Fig 1B).
End Points Studied
We identified an array of end points for health-care use at the end of life defined previously in the literature.16,23 The specific end points evaluated include visits to the emergency room (ER), hospitalization, intensive care unit (ICU) admission, and hospice use. Other end points evaluated include the use of chemotherapy, initiation of a new chemotherapy agent, and invasive procedures such as venous catheterization, intubation, transfusion of blood products, thoracentesis, lung or liver biopsy, or cardiopulmonary resuscitation. We identified these end points separately in the 30-day window before exposure and again in the interval from exposure through death to better understand the effect of palliative care exposure on health-care use.
Study Covariates
Demographic characteristics acquired from SEER data included age at diagnosis, sex, race, Hispanic ethnicity, marital status, and median household income divided into quartiles, which was calculated from the 2000 US Census favoring census tract data over zip code–level data. Individuals with missing household income (< 0.1%) were grouped into the bottom quartile.26 We implemented the Deyo adaptation of the Charlson Comorbidity Index, which uses inpatient and outpatient Medicare claims data from the year before diagnosis of cancer.27,28 Additional covariates included tumor site, SEER Registry region, urban or rural setting, and year of diagnosis. Health-care delivery in a teaching hospital was defined as any indirect medical education payment during a postdiagnosis hospitalization.
Statistical Analysis
Descriptive statistics were used to illustrate general patient characteristics, stratified by matching group. To evaluate balance between the two cohorts, standardized differences (the difference in group means divided by the common standard deviation)29 were measured for patient covariates. Standardized differences < 0.1 reflect a good balance between matched groups.29 To assess differences in the means of continuous end points, we used paired t tests. The differences in dichotomous end points were assessed with risk ratios and 95% CIs, which were calculated using Mantel-Haenszel standard error estimates stratified on the matched sets. One variable in particular was not balanced between the palliative and nonpalliative cohorts after our matching algorithm. Care at a teaching hospital was higher among patients receiving palliative care compared with control patients (65% v 52%). We used a conditional logistic regression as a sensitivity analysis to verify associations between our exposure and outcomes, adjusting for care at a teaching hospital. The conditional logistic regression results did not vary (data not shown), and we presented only the relative risks for simplicity. We evaluated the effect of timing of the palliative care consultation (measured from death) by assessing for statistical interaction between time of consultation and presence of a palliative care consultation. Significant interactions implied the influence of palliative care varied among those with a palliative care consultation early in diagnosis compared with those with consultations closer to death. All statistical tests were two-sided and P values < .05 were considered significant. Analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
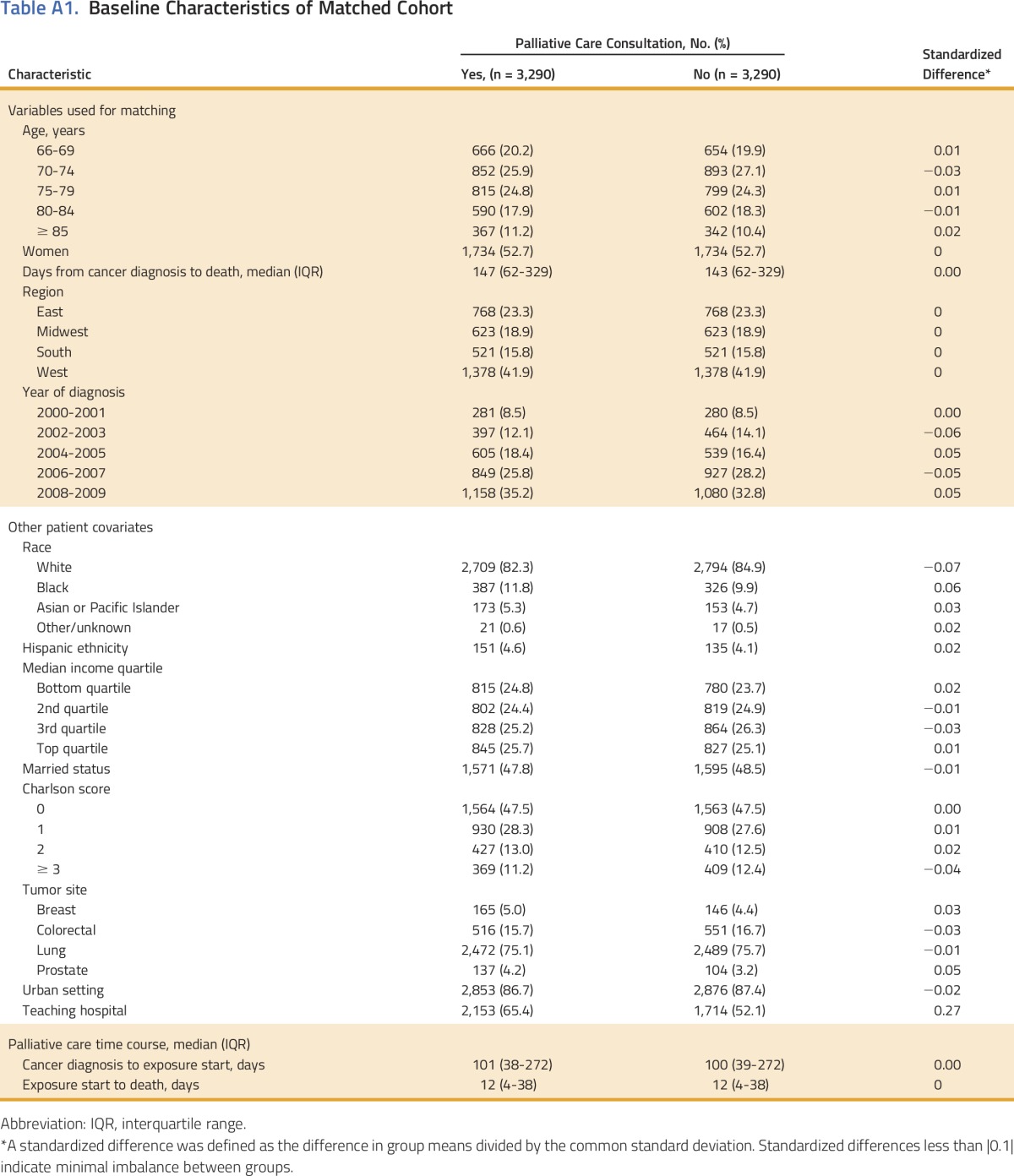
Of 81,651 unmatched patients, 3,665 (4.5%) had received at least one consultation with a palliative care provider. After 108 matching iterations, coarsened exact matching yielded 3,290 matched pairs for a final sample size of 6,580 patients (Fig 1). Appendix Table A1 (online only) demonstrates descriptive characteristics of the matched cohort. All covariables were well balanced between matched groups with the exception of care at a teaching hospital, which was higher among patients with a palliative care encounter (65% v 52%). The median time from diagnosis to death was 4.8 months (interquartile range, 2.1 to 11.0 months). In general, palliative care delivery was more frequent toward the end of the study period, more commonly used among patients with lung cancer, and more prevalent on the West coast. The timing of the palliative care encounter occurred relatively late in a patient’s disease course, with a median time from consultation to death of 12 days (interquartile range, 4 to 38 days).
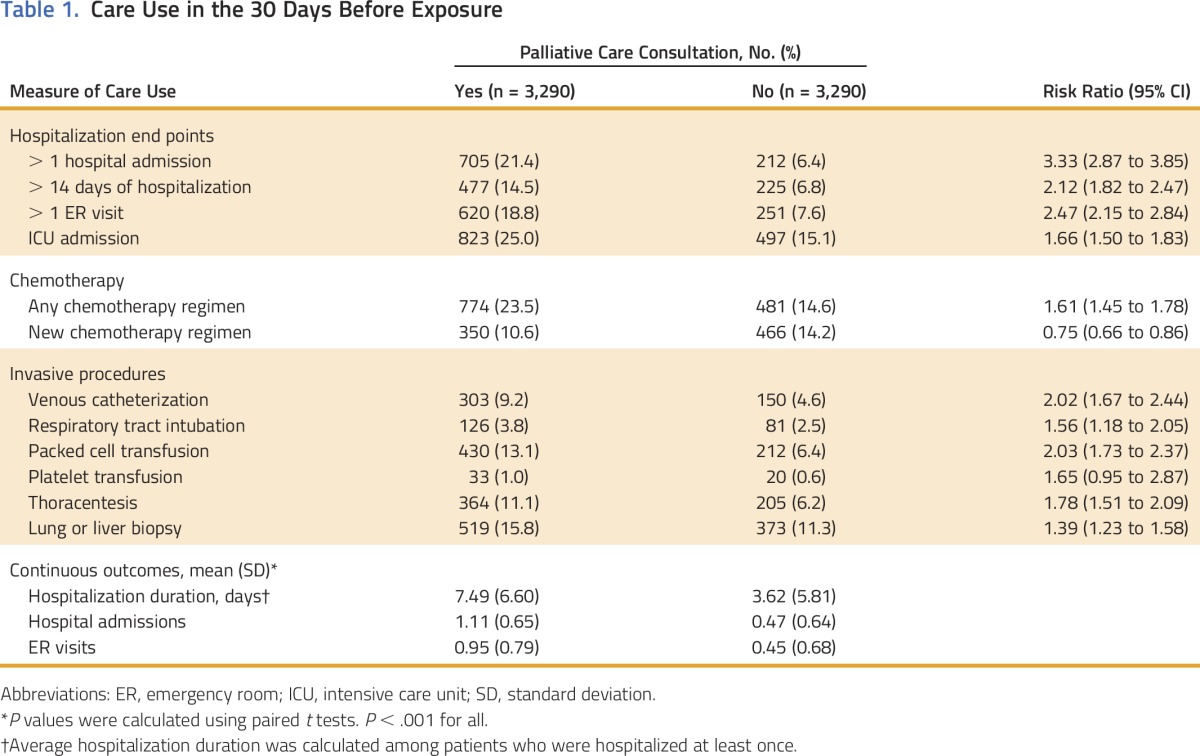
Overall, patients who received palliative care had increased use of most health-care services in the 30 days before consultation compared with the nonpalliative care group (Table 1). The palliative cohort had increased rates of hospitalization, ER visits, ICU admissions, and multiple invasive procedures. The palliative care group had a 61% higher rate of receiving any chemotherapy regimen but a 25% lower rate of starting a new chemotherapy regimen in the 30 days before exposure.
Table 1.
Care Use in the 30 Days Before Exposure
Although patients receiving palliative care had higher rates of health-care use before palliative consultation, this cohort had lower rates of health-care use after palliative consultation compared with the nonpalliative care group (Table 2). Patients receiving palliative care consultation had lower rates of hospitalization, ER visits, ICU admissions, and invasive procedures. The palliative cohort was 54% less likely to receive chemotherapy, and 35% less likely to start a new chemotherapy regimen. The group receiving palliative care was 24% more likely to enroll in hospice and had longer durations on hospice (25.5 days v 21.3 days). Despite the longer average duration of hospice care, patients receiving palliative care were more likely to be admitted to hospice within 3 days of death.
Table 2.
Care Use After Exposure
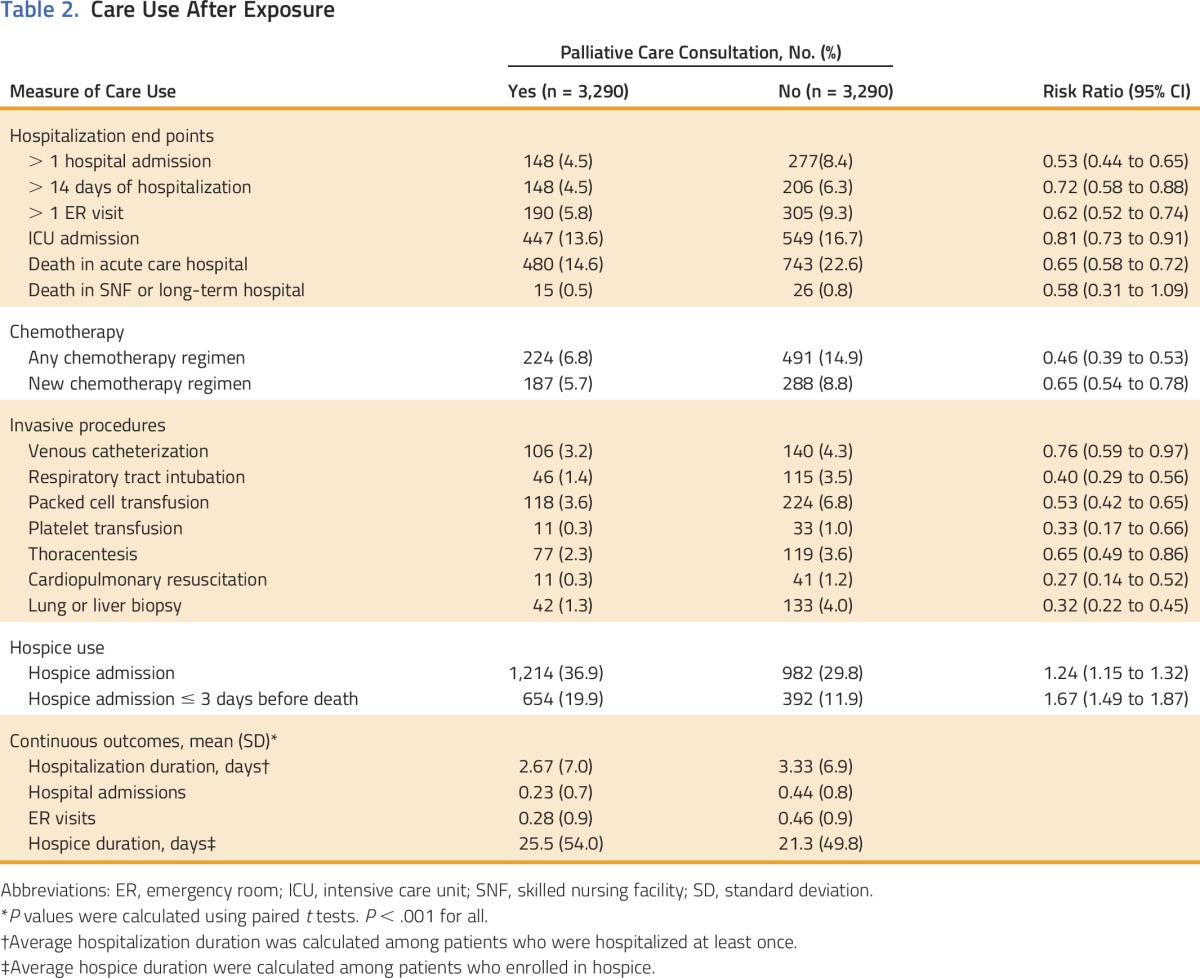
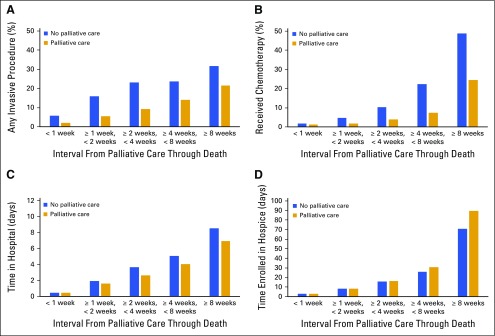
Figure 2 demonstrates the effect of palliative care consultation timing on select markers of health-care use. For several end points, the size of the effect of palliative care depended on the timing of the palliative care consultation, as assessed by the interaction of time and palliative care consultation in each statistical model. Earlier palliative care encounters were associated with greater reductions in chemotherapy use (P < .001), greater reductions in average hospitalization days (P < .05), and increased time enrolled in hospice (P < .001). For example, patients receiving palliative care had a 0.5% absolute decrease in chemotherapy use when palliative consultation occurred in the last week of life versus a 24.3% decrease in chemotherapy use when palliative consultation occurred > 8 weeks from death. Although palliative care consultation did reduce the chances of receiving any invasive procedure, the timing of consultation did not affect the magnitude of effect (P = .90).
Fig 2.
Impact of timing of palliative care consultation on health-care use.
DISCUSSION
The key findings of this study relate to the marked reductions in health-care use at the end of life after exposure to palliative care. These results add to a growing literature demonstrating the effect of palliative care in a large cohort of elderly patients with cancer.30
Overall, our results complement findings of other single-institution studies demonstrating reduced health-care use with palliative care services.19-22 Additionally, the landmark randomized controlled trial by Temel et al2 found early palliative care was associated with improved quality of life, prolonged survival, and less aggressive end-of-life care among patients with metastatic lung cancer. The differences we observed in care use before and after exposure to palliative care concur with the discovery of Morrison et al15 that health-care costs for patients receiving palliative care in eight hospitals reliably declined within 24 to 48 hours after palliative consultation. Essentially, we found palliative care represents an inflection point in patient care, with higher use of health-care services before palliative care consultation and lower use after.
Beyond the overall effect on health-care use, we also found the timing of palliative care consultation differentially influenced use. Earlier encounters with palliative care were associated with larger absolute effects on chemotherapy delivery and hospice duration. This correlates with prior findings that early, versus late, palliative care referrals translate into less intensive care, improved quality outcomes, and cost savings at the end of life for patients with cancer.31 These findings are particularly relevant given the current emphasis of early palliative care integration alongside standard oncologic care in patients with advanced cancer. To date, multiple randomized trials have demonstrated the benefits of early palliative care,2-4,7,8,32 which include improved quality of life, improved mood, decreased aggressiveness of end-of-life care, improved survival, and improved patient and caregiver satisfaction. Importantly, our study does not supersede or replace randomized clinical trials, although it offers additional insight into the real-world influence of palliative care on a diverse cohort of patients with advanced cancer.
This observational study has several limitations worth considering. First, the patient encounter with palliative care was not randomized, and the validity of this study hinges on the accuracy of the matching process. Prior evidence has suggested patients with a higher number of comorbidities have higher cost-saving effect, which we attempted to control for through matching.33 Our matching algorithm provided a good balance on most measured variables; however, the possibility for residual confounding from unmeasured variables remains a potential source of bias. For example, the palliative care cohort had higher use of health-care services before their eventual encounter with palliative care. This implies patients receiving a palliative consultation might have greater disease severity or different health-care preferences compared with patients not receiving palliative care. However, if the palliative cohort had greater disease severity, this would add bias that would lead us to underestimate the effect of palliative care and, therefore, the true effect of palliative care in a more balanced population could be greater than observed in this study. We also cannot determine whether the palliative care consultation resulted from a prepalliative decision to de-escalate care. If patients make the decision to de-escalate care due to adverse effects, or if they exhaust all treatment options, they may seek palliative consultation, which means the decreased use may reflect a natural transition in care that did not come from the influence of the palliative care consultation itself.
Another issue to consider relates to the fact that our exposure of interest (ie, palliative care consultation) could not be validated within Medicare claims data, potentially leading to misclassification. However, we suspect this misclassification would primarily manifest as missed palliative coding among a small fraction of the nonpalliative cohort, as opposed to improper coding among the palliative cohort. This misclassification would bias our results toward the null, and would unlikely affect our overall conclusions. We also note that although we found statistically significant differences between palliative care arms in multiple outcomes, some differences were small in absolute terms and may not represent clinically significant differences. Most of our sample comprised patients with lung cancer, a result of the higher proportion of these patients who present at a metastatic stage compared with other cancers in the study; this may limit the generalizability of our findings to other cancers.
A final limitation relates to the fact that palliative care teams often contain multiple, diverse members including physicians, nurses, social workers, pharmacists, and spiritual counselors. The data in this project lack detail on the frequency, intensity, and granularity of the palliative care consultations. Therefore, we cannot identify the specific aspects of a palliative care team that drives health-care use patterns among patients. Addressing these questions will require probing more detailed datasets.
Despite these limitations, in this representative sample of Medicare patients with advanced cancer, we found patients who received palliative care experienced significantly less aggressive care, lower rates of hospitalization, increased use of hospice, and fewer invasive procedures near the end of life. Given the increasing number of elderly patients with advanced cancer, the findings in this study emphasize the important role palliative care plays in global public health.
ACKNOWLEDGMENT
Supported by National Institutes of Health Grant No. KL2 RR031978 (J.D.M.). This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, Centers for Medicare and Medicaid Services; Information Management Services; and the SEER Program tumor registries in the creation of the SEER-Medicare database.
Appendix
Table A1.
Baseline Characteristics of Matched Cohort
AUTHOR CONTRIBUTIONS
Conception and design: Daniel P. Triplett; James D. Murphy
Collection and assembly of data: Daniel P. Triplett; James D. Murphy
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Effect of Palliative Care on Aggressiveness of End-of-Life Care Among Patients With Advanced Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/journal/jop/site/misc/ifc.xhtml.
Daniel P. Triplett
No relationship to disclose
Wendi G. LeBrett
No relationship to disclose
Alex K. Bryant
No relationship to disclose
Andrew R. Bruggeman
No relationship to disclose
Rayna K. Matsuno
No relationship to disclose
Lindsay Hwang
No relationship to disclose
Isabel J. Boero
No relationship to disclose
Eric J. Roeland
Honoraria: Pfizer
Consulting or Advisory Role: Eisai (Inst), Helsinn Healthcare (Inst), HERON
Research Funding: XBiotech (Inst), AstraZeneca (Inst), Merck (Inst)
Travel, Accommodations, Expenses: Eisai, Teva, Helsinn Healthcare
Heidi N. Yeung
No relationship to disclose
James D. Murphy
No relationship to disclose
REFERENCES
- 1. Center to Advance Palliative Care. Growth of palliative care in U.S. hospitals 2013 snapshot. https://media.capc.org/filer_public/0d/db/0ddbecbc-8dc7-449d-aa50-584960f18880/capc-growth-analysis-snapshot-2013.pdf.
- 2.Temel JS, Greer JA, Muzikansky A, et al. : Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363:733-742, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Temel JS, Greer JA, El-Jawahri A, et al. : Effects of early integrated palliative care in patients with lung and GI cancer: A randomized clinical trial. J Clin Oncol 35:834-841, 2017 [DOI] [PMC free article] [PubMed]
- 4.Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer: The Project ENABLE II randomized controlled trial. JAMA 302:741-749, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greer JA, Pirl WF, Jackson VA, et al. : Effect of early palliative care on chemotherapy use and end-of-life care in patients with metastatic non–small-cell lung cancer. J Clin Oncol 30:394-400, 2012 [DOI] [PubMed] [Google Scholar]
- 6.McNamara BA, Rosenwax LK, Murray K, et al. : Early admission to community-based palliative care reduces use of emergency departments in the ninety days before death. J Palliat Med 16:774-779, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Zimmermann C, Swami N, Krzyzanowska M, et al. : Early palliative care for patients with advanced cancer: A cluster-randomised controlled trial. Lancet 383:1721-1730, 2014 [DOI] [PubMed] [Google Scholar]
- 8.Ferrell B, Sun V, Hurria A, et al. : Interdisciplinary palliative care for patients with lung cancer. J Pain Symptom Manage 50:758-767, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levy MH, Smith T, Alvarez-Perez A, et al. : Palliative care, version 1.2014. Featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 12:1379-1388, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Ferrell BR, Temel JS, Temin S, et al. : Integration of palliative care into standard oncology care: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 35:96-112, 2017 [DOI] [PubMed] [Google Scholar]
- 11.The Quality Oncology Practice Initiative : 2009; http://www.instituteforquality.org/sites/instituteforquality.org/files/QOPI%20Spring%202017%20Measures%20and%20Reporting%20Pathways%20-%20Public%20Website.pdf
- 12.American Cancer Society: Cancer Facts & Figures 2014. Atlanta, GA, American Cancer Society, 2014. [Google Scholar]
- 13.Weinstein MC, Skinner JA: Comparative effectiveness and health care spending--Implications for reform. N Engl J Med 362:460-465, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Choi YS, Billings JA. Changing perspectives on palliative care. Oncology16:515-522, 2002; discussion 522-517. [PubMed]
- 15.Morrison RS, Penrod JD, Cassel JB, et al. : Cost savings associated with US hospital palliative care consultation programs. Arch Intern Med 168:1783-1790, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Earle CC, Landrum MB, Souza JM, et al. : Aggressiveness of cancer care near the end of life: Is it a quality-of-care issue? J Clin Oncol 26:3860-3866, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher ES, Wennberg DE, Stukel TA, et al. : The implications of regional variations in Medicare spending. Part 2: Health outcomes and satisfaction with care. Ann Intern Med 138:288-298, 2003 [DOI] [PubMed] [Google Scholar]
- 18.Teno JM, Mor V, Ward N, et al. : Bereaved family member perceptions of quality of end-of-life care in U.S. regions with high and low usage of intensive care unit care. J Am Geriatr Soc 53:1905-1911, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Back AL, Li YF, Sales AE: Impact of palliative care case management on resource use by patients dying of cancer at a Veterans Affairs medical center. J Palliat Med 8:26-35, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Penrod JD, Deb P, Luhrs C, et al. : Cost and utilization outcomes of patients receiving hospital-based palliative care consultation. J Palliat Med 9:855-860, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Gade G, Venohr I, Conner D, et al. : Impact of an inpatient palliative care team: A randomized control trial. J Palliat Med 11:180-190, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Gonsalves WI, Tashi T, Krishnamurthy J, et al. : Effect of palliative care services on the aggressiveness of end-of-life care in the Veteran’s Affairs cancer population. J Palliat Med 14:1231-1235, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Obermeyer Z, Makar M, Abujaber S, et al. : Association between the Medicare hospice benefit and health care utilization and costs for patients with poor-prognosis cancer. JAMA 312:1888-1896, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iacus SM, King G, Porro G: Multivariate matching methods that are monotonic imbalance bounding. JASA 106:345-361, 2011.
- 25.Kamal AH, Swetz KM, Carey EC, et al. : Palliative care consultations in patients with cancer: A Mayo Clinic 5-year review. J Oncol Pract 7:48-53, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger N: Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health 82:703-710, 1992. [DOI] [PMC free article] [PubMed]
- 27.Charlson ME, Pompei P, Ales KL, et al. : A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40:373-383, 1987 [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA: Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 45:613-619, 1992 [DOI] [PubMed] [Google Scholar]
- 29.Austin PC, Grootendorst P, Anderson GM: A comparison of the ability of different propensity score models to balance measured variables between treated and untreated subjects: A Monte Carlo study. Stat Med 26:734-753, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Jang RW, Krzyzanowska MK, Zimmermann C, et al. Palliative care and the aggressiveness of end-of-life care in patients with advanced pancreatic cancer. J Natl Cancer Inst. 2015;107(3) doi: 10.1093/jnci/dju424. [DOI] [PubMed] [Google Scholar]
- 31.Scibetta C, Kerr K, Mcguire J, et al. : The costs of waiting: Implications of the timing of palliative care consultation among a cohort of decedents at a comprehensive cancer center. J Palliat Med 19:69-75, 2016 [DOI] [PubMed] [Google Scholar]
- 32.Dionne-Odom JN, Azuero A, Lyons KD, et al. : Benefits of early versus delayed palliative care to informal family caregivers of patients with advanced cancer: Outcomes from the ENABLE III randomized controlled trial. J Clin Oncol 33:1446-1452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.May P, Garrido MM, Cassel JB, et al. : Palliative care teams’ cost-saving effect is larger for cancer patients with higher numbers of comorbidities. Health Aff (Millwood) 35:44-53, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]