Highlights
-
•
DPPH forms purple color when dissolved in solution and its color is altered to slightly yellowish color with the intervention of rhizome extracts of Curcuma caesia Roxb.at different concentrations showing the scavenging activity against the free radicals generated by DPPH. The scavenging activity of the extracts tested follows the order EECC>MECC>EaECC>AECC.
-
•
Total phenolic and reducing ability were also found to be increased in ethanolic extract as compared to other extracts.
-
•
EECC, MECC, AECC were selected to test the antimutagenic activity against Cyclophosphamide (CP) using Salmonella typhimurium strains TA98 and TA100. S9 mix prepared from male mice liver was used to activate the cyclophosphamide. Cyclophosphamide produces free radicals Phosphoramide and Acrolein on activation with S9 mix causing mutations inside the living system which found to be ammeliorated by the secondary metabolites present in plants. All the extracts tested were found to be non mutagenic.
-
•
CP can also act as positive control for the strain TA98 which was not reported ealier and this can be inhibited by the used of rhizome of Curcuma caesia Roxb. Extract.
Chemical compounds studied in this article: DPPH (PubChem CID: 2735032), Ascorbic acid (PubChem CID: 54670067), Gallic acid (PubChem CID: 370), TCA (PubChem CID: 6421), FeCl3 (PubChem CID: 24380), NaHCO3 (PubChem CID: 516892), KCl (PubChem CID: 4873), l-Histidine (PubChem CID: 6274), d-Biotin (PubChem CID: 171548), α-d-Glucose-6-P (PubChem CID: 439284), Cyclophosphamide (PubChem CID: 2907)
Keywords: Curcuma caesia Roxb., Antioxidant activity, Total phenolic content, Reducing power, Cyclophosphamide, Antimutagenic activity
Abstract
The rhizomes of Curcuma caesia Roxb. (zingiberacea) are traditionally used in treatment of various ailments and metabolic disorders like leukoderma, asthma, tumours, piles, bronchitis, etc. in Indian system of medicine. Considering the importance of natural products in modern phytomedicine, the antioxidant and antimutagenic activities of C. caesia Roxb. rhizome extract and its fractions were evaluated. The ethanolic fraction showed highest antioxidant activity by DPPH assay (86.91%) comparable to ascorbic acid (94.77%) with IC50 value of 418 μg/ml for EECC followed by MECC (441.90 μg/ml) > EAECC(561 μg/ml) > AECC(591 μg/ml). Based on the antioxidant activity, three of the rhizome extracts were evaluated for their antimutagenic properties against indirect acting mutagen cyclophosphamide (CP) using Salmonella typhimurium strains TA98 and TA100. The antimutagenic activity of the extracts against indirect acting mutagen cyclophosphamide in the presence of mammalian metabolic activation system was found to be significant (p < 0.01, p < 0.05). All the extracts showed similar antimutagenicity in dose dependent manner. The total phenolic content as well as reducing ability of the extracts was also determined.
1. Introduction
The emerging concepts of cancer is that the cancer cells are unstable and its unstability is brought about by the documentations of cascade of mutations caused by mutagens and suggested that mutagenesis drives out tumour progression [1]. Mutations results from the side effects of free radicals such as hydrogen peroxides, superoxide anions, and organo peroxides, etc. produced by drugs, ultraviolet radiations, ionising radiations, pollution as well as the endproducts of normal metabolic process of aerobic organisms [2], [3], [4]. The interaction of the free radicals with polyunsaturated fatty acids, nucleotides and disulphide bonds [5] has been implicated as the major factor to cause the oxidation of the biological compounds and eventually leads to mutations [6] and many degenarative diseases like emphysema, cardiovascular, inflammatory diseases, cataracts, etc. [5]. Cellular system has developed many endogenous antioxidants such as superoxide dismutase (SOD), catalase, glutathione, glutathione peroxidases and reductase, and nonenzymatic antioxidants like vitamin E (tocopherols and tocotrienols), vitamin C, etc. [7] to neutralise the free radicals [8]. This has triggered to search for effective antioxidant agents from various sources including plants. Many researchers have investigated that the increase levels of antioxidants present in plants are believed to decrease the oxidative damage and its harmful effects [9]. Synthetic antioxidants such as butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) are preferable but can cause serious ill effects in human health as per recent reports Lobo et al. [10]. The use of plants and medicinal plants has been recommended to combat the effect of free radicals/mutagens because they can induce phase II enzymes reducing the action of initiation, promotion or progression stages of cancer and other degenerative diseases [11], [12], [13], [14]. Also the plants are rich source of secondary metabolites such as flavonoids, phenolics, carotenoids, coumarins, anthraquinones, tannins, terpenoids, saponins that play a prominent role in inhibiting human carcinogenesis and repair the cell mutations [15].
Curcuma caesia Roxb. (black turmeric) is a perennial herb with bluish black rhizomes and it is famous for its medicinal properties. It is recognised as a medicinal herb to possess with various properties such as anti-fungal activity Banerjee and Nigam [16], smooth muscle relaxant and anti-asthmatic activity Arulmozhi et al. [17], bronchodilating activity Paliwal et al. [18], antioxidant activity Mangla et al. [19], anxiolytic and CNS depressant activity, locomotor depressant, anti-convulsant Karmakar et al. [20], anthelmintic activity Gill et al. [21], anti-bacterial activity Rajamma et al. [22], anti-ulcer activity Das et al. [23]. The phytochemical studies of C. caesia revealed the presence of multiple phytoconstituents like essential oils with camphor, ar-turmerone, (Z) ocemene, ar-curcumene,1,8-cineole, elemene, borneol, bornyl acetate, curcumene, etc. [24]. To the best of our knowledge there is no report available on the antimutagenic activity of C. caesia Roxb. Therefore we have selected the rhizome of this plant and evaluated the antioxidant and antimutagenic activity of some of the selected extracts against indirectly acting mutagen cyclophosphamide.
2. Materials and methods
2.1. Plant material collection and extraction
Rhizomes of C. caesia Roxb. were collected in the month of November 2012, from the region of Nambol, Bishnupur District, Manipur, India. The rhizomes were cut into pieces and sun dried. The dried rhizomes were coarsely powdered and 100 g of it was successfully extracted with various solvents starting from least polar solvents to more polar, i.e. from petroleum ether to ethyl acetate, ethanol, methanol and then finally to water through soxhlet at a temperature of 50–60 °C for a period of 12–24 h. The crude extracts of each solvent were dried in water bath and kept for further uses.
2.2. DPPH radical scavenging activity
The quenching of free radical activity of different extracts were determined by spectrophotometric method against 2,2-diphenyl-1-picryl hydrazyl (DPPH) following [25]. 1 ml of each extract of various concentrations (25–800 μg/ml) were mixed with 1 ml of DPPH (0.1 mM) solution prepared in ethanol and incubated in dark for 20 min and absorbance values were recorded at 517 nm. 1 ml of ethanol and 1 ml of ethanolic solution of DPPH (0.2 mM) was taken as control. Similarly 1 ml of ethanolic solution of ascorbic acid (200 μg/ml) was mixed with 1 ml of DPPH ethanolic solution and absorbance values were recorded. The radical scavenging activity was calculated using the following formula:
where Ab is the absorption of the blank and Aa is the absorption of the extract sample.
2.3. Determination of total phenolic contents in the plant extracts
The concentration of phenolics in plant extracts was determined using Folin Ciocalteau method [26] with little modifications. The extracts in the concentration of 1 mg/ml was used in the analysis. The reaction mixture was prepared by mixing 0.5 ml of each extract solution, 2.5 ml of 10% Folin–Ciocalteu's reagent dissolved in water and 2.5 ml of 7.5% NaHCO3. Blank was prepared, containing 0.5 ml ethanol, 2.5 ml 10% Folin–Ciocalteu's reagent dissolved in water and 2.5 ml of 7.5% of NaHCO3. The samples were thereafter incubated at 45 °C for 45 min. The absorbance were determined using spectrophotometer at λmax = 765 nm. The samples were prepared in triplicate for each analysis and the mean value of absorbance was obtained. The same procedure was repeated for the standard solution of gallic acid and the calibration line was constructed. Based on the measured absorbance, the concentration of phenolics was calculated from the calibration line; then the content of phenolics in each extracts was expressed in terms of gallic acid equivalent (mg of GAE/100 g d.w. of extract).
2.4. Reducing power assay
The ability to reduce ferric ions to ferrous ions by the antioxidants present in rhizomes of C. caesia Roxb. was determined by the method of Oyaizu [27] with little modification. From the different concentrations of each extract solutions (200–1000 μg/ml), 1 ml of each was mixed with 2.5 ml of 0.2 M of phosphate buffer (pH = 6.6) and 2.5 ml of 1% potassium ferricyanide. The mixture was heated at 50 °C for 20 min and then cooled followed by the addition of 2.5 ml of 10% TCA and then centrifuged at 3000 rpm for 10 min. 2.5 ml of the supernatant was again mixed with 2.5 ml of distilled water and 0.5 ml of 0.5% FeCl3 and the absorbance was recorded at 700 nm against blank without extract. Increase in the absorbance values shows the increasing reducing ability of the extracts. The entire test was performed in triplicate.
2.5. Bacterial strains
Salmonella typhimurium strains TA98 and TA100 which are histidine-requiring mutants, were kindly provided by IMTECH, Chandigarh, India and are maintained as described by Maron and Ames [28]. The genotypes of the test strains were checked routinely for their histidine requirement, rfa mutatios, UV sensitivity (uvrB mutation). They were stored at −80 °C for further use.
2.6. S9 preparation
S9 is the mitochondrial enzyme mix required for metabolic activation of indirect acting mutagens like cyclophosphamide. The S9 mixture was prepared from male rat liver using the chemicals 1 M glucose-6-phosphate, 0.1 M NADP, 0.2 M phosphate buffer, 0.4 M MgCl2 + 1.65 M KCl (Himedia – India) as described by Maron and Ames [28]. S9 mix was prepared fresh for each assay.
2.7. Salmonella-microsome assay
The bacterial strains were incubated in Nutrient Broth for 16 h at 37 °C in an orbital shaker to obtain a density of 2 × 109 colony forming units (CFU/ml). 0.1 ml of an overnight culture of bacteria and 0.5 ml of sodium phosphate buffer (0.2 M, pH 7.4 for assay without S9) supplemented with 0.2 mM l-histidine and 0.2 mM d-biotin solution containing different concentrations of each extract. They were mixed using votexer for 10 min. The resulting complete mixture was poured on minimal agar plates prepared as described by Maron and Ames [28]. The plates were incubated at 37 °C for 48 h and the revertant bacterial colonies of each plate were counted. Data were collected with a mean ± standard deviation of three experiments (n = 3).
2.8. Antimutagenicity testing
For the experiment with S9 mix, 0.1 ml of overnight grown bacterial cultures were taken followed by the addition of 0.2 mM. Histidine-Biotin solution supplemented with each extracts at different concentrations, were mixed and incubated for 3 min. After incubation 0.1 ml of the CP (500 μg/plate) and 0.5 ml of S9 mix were added. The experiment was performed as mentioned above. Percentage inhibition was calculated using the formula [29].
where R1 is the number of revertants without extracts but with CP, R2 the number of revertants with extracts plus mutagen and SR is the spontaneous revertants i.e. without extracts and mutagen.
2.9. Statistical analysis
The results are presented as the average and S.D. (standard deviations) of three experiments with triplicate plates/dose/experiment. The regression analysis was carried out in Microsoft Excel 2007 between % inhibition of mutagenicity and values of concentrations of the plant extracts. Student's t test was performed to compare the mean values with the positive control.
3. Results
3.1. Antioxidant activity
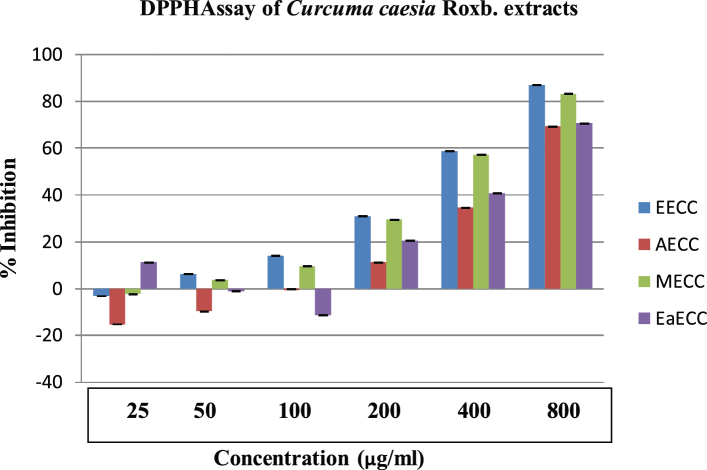
The free radical scavenging activity of the rhizome extracts of C. caesia Roxb. was measured as decolorizing activity following the trapping of the unpaired electron of DPPH as shown in Fig. 1. The fractions showed a varied free radical scavenging activity. The ethanol fraction was found to be the most active free radical scavenger exhibited (86.914% decrease at a concentration of 800 μg/ml) compared to ascorbic acid (94.770%). Likewise the crude methanolic, ethyl acetate and aqueous extract showed scavenging activity with a percent decrease of 83.104%, 70.44% and 69.19%. The IC50 value ranges in the order of 418 μg/ml (EECC) > 441.90 μg/ml (MECC) > 561 μg/ml (EAECC) > 591 μg/ml (AECC), the lowest being the highest antioxidant activity. The ethanolic extract neutralised 50% of free radicals at the concentration of 418 μg/ml (Fig. 2).
Fig. 1.
DPPH method. EECC: ethanolic extract of Curcuma caesia Roxb., MECC: methanolic extract of Curcuma caesia Roxb., EaECC: ethyl acetate extract of Curcuma caesia Roxb., AECC: aqueous extract of Curcuma caesia Roxb.
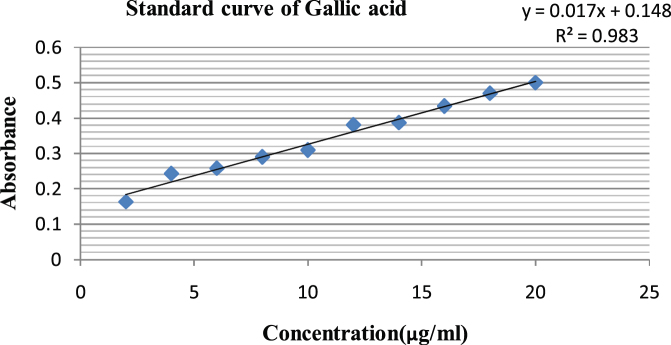
Fig. 2.
Standard curve of gallic acid to find out the total phenolic content.
3.2. Total phenolic content
The total phenolic contents in the examined plant extracts using the Folin Cioclteu's reagent is expressed in terms of gallic acid equivalent (the standard curve: y = 0.0178x + 0.148; R2 = 0.9831). Total phenolic contents in the examined extracts ranged from MECC = 52.11 mg/100 g d.w., EECC = 68.64 mg/100 g d.w., EaECC = 38 mg/100 g d.w., AECC = 4.82 mg/100 g d.w. of the extract. The highest concentration of phenols was measured in ethanolic followed by methanolic, ethyl acetate and aqueous extracts.
The reducing power of C. caesia Roxb. rhizome extracts was dose dependent and is presented in the following table. The maximum absorbance of ethanolic extracts at 1000 μg/ml is more or near to ascorbic acid at 200 μg/ml as given in Table 1. Reducing power methods indirectly evaluates the antioxidant activity (Qureshi et al. [51]) (Table 2).
Table 1.
Reducing power of the extracts.
| Conc (μg/ml) | EECC | MECC | EaECC | AECC |
|---|---|---|---|---|
| 1000 | 2.480 ± 0.010 | 1.639 ± 0.029 | 0.899 ± 0.053 | 0.348 ± 0.023 |
| 800 | 2.277 ± 0.068 | 1.368 ± 0.029 | 0.468 ± 0.028 | 0.275 ± 0.015 |
| 500 | 1.511 ± 0.041 | 0.788 ± 0.005 | 0.333 ± 0.022 | 0.180 ± 0.009 |
| 200 | 0.775 ± 0.002 | 0.372 ± 0.001 | 0.159 ± 0.013 | 0.074 ± 0.002 |
| Ascorbic acid (200 μg/ml) | 2.425 ± 0.03 |
Table 2.
Number of his+ revertants in Salmonella typhimurium strains produced by Curcuma caesia Roxb. extracts against cyclophosphamide.
| Treatment conc. (μg/ml) | TA98 |
TA100 |
||
|---|---|---|---|---|
| −S9 | +S9 | −S9 | +S9 | |
| S.R | 92.66 ± 6.94 | 304 ± 23 | 55 ± 4.54 | 213 ± 13.06 |
| P.C: 50 | 299.66 ± 26.44 | 718 ± 94 | 135.66 ± 19.36 | 652.66 ± 71.2 |
| EECC: 50 | 116.33 ± 18.14 | 395.11 ± 71.2* | 98 ± 10.42 | 398.66 ± 64.8* |
| 500 | 111.33 ± 19.14 | 386.51 ± 74* | 80.33 ± 10.63 | 379.33 ± 63.8* |
| 5000 | 104.33 ± 21.14 | 341.45 ± 93.72* | 71 ± 16.63 | 334.66 ± 86.51* |
| MECC: 50 | 179.66 ± 39.98 | 491.67 ± 98.28* | 126.66 ± 3.39 | 434.33 ± 44.93* |
| 500 | 163.66 ± 47.6 | 487.66 ± 56* | 105.66 ± 5.39 | 412.33 ± 43.4* |
| 5000 | 144.66 ± 13.55 | 401.66 ± 93.14* | 91.66 ± 10.17 | 385.33 ± 74* |
| ECC: 50 | 221.66 ± 15.9 | 596.67 | 131.33 ± 3.86 | 466.33 ± 21.7* |
| 500 | 211.33 ± 22.89 | 562.67 ± 3.5** | 121 ± 5.54 | 449.66 ± 45.7* |
| 5000 | 95.66 ± 31.56 | 479.66 ± 33.15* | 97.66 ± 9.74 | 410.66 ± 51.8* |
The data represented in the table is the mean ± S.D. values of three replicates.
p < 0.01.
p < 0.05.
EECC: ethanolic extract of Curcuma caesia Roxb.; MECC: methanolic extract of Curcuma caesia Roxb.; AECC: aqueous extract of Curcuma caesia Roxb.; P.C: positive control; C.P: cyclophosphamide; S.R: spontaneous revertants.
The increase in the absorbance indicates an increase in reductive ability [30].
Based on the promising antioxidant and reducing activity, ethanolic, methanolic and aqueous extracts were evaluated for their antimutagenic activity by Ames test against indirect acting mutagen cyclophosphamide. All the extracts were found to inhibit in dose dependent manner. Linear relationship between extract dose and antimutagenic response in the case of EECC without S9 is strong in the strain TA98 (r2 = 0.99) followed by TA100 (r2 = 0.97), with S9 it is strong in the strain TA98 (r2 = 0.99) followed by TA100 (r2 = 0.95). At all the doses antimutagenic response was significant at (p < 0.01) against both the strains with a percent mutagenicity decrease from 77.99 to 90.95 for TA98 followed by TA100 with percent antimutagenicity starting from 57.77 to 72.32. Similar trend was followed for methanoilc extract of C. caesia Roxb. Linear relationship between extract dose and antimutagenic response in the case of MECC without S9 is strong in the strain TA98 (r2 = 0.99) followed by TA100 (r2 = 0.97), with S9 it is strong in the strain TA98 (r2 = 0.99) followed by TA100 (r2 = 0.86). At all the doses antimutagenic response was significant at (p < 0.01) with the percent mutagenicity decrease from 54.66 to 76.41 in case of TA98 followed by TA100 with the percent mutagenicity decrease from 49.65 to 60.80 in MECC. The significant level shown was (p < 0.01) for all concentrations 50 μg, 500 μg and 5000 μg. Linear relationship between extract dose and antimutagenic response in the case of AECC without S9 is strong in the strain TA98 (r2 = 0.98) followed by TA100 (r2 = 0.95), with S9 it is same for both the strain TA98 (r2 = 0.95) and TA100 (r2 = 0.95). At the dose of 50 μg of AECC antimutagenic response was insignificant with percent inhibition of 29.30 but at 500 μg the antimutagenic response was significant at (p < 0.05) with percent inhibition of 37.51 and at the dose 5000 μg it was significant at (p < 0.01) (57.57% inhibition) in case of TA98 and in case of TA100 in AECC the significant level shown was (p < 0.01) for all concentrations with the percent mutagenicity decrease from 29.30 to 57.57.
4. Discussion
Considerable attention has been focussed in recent years on the exploration of phytotherapeutic agents for the treatment of oxidative stress and mutation related disorders. The use of medicinal plants is perhaps the oldest method of coping with illness. They can be easily metabolised inside the body without any harmful effects that leads to the phytochemical based remedies [31], [32], [33], [34]. Reactive oxygen intermediates like superoxides, hydrogen peroxides and hydroxyl radicals are known to mediate macromolecular damages by reacting with nucleic acids, proteins as well as various membrane components thus act as direct and indirect initiators of mutagenesis and carcinogenesis [35]. On the basis of this it has been hypothesised that the involvement of antioxidant might be considered as the safest approach in the prevention of process leading to mutagenesis. The chemistry of free radicals is complicated and it caused a major limitation in the identification of free radical scavenging activity. To withstand this problem the potential antioxidant substance are tested in in vitro model and such approaches expand the scope of antioxidant activity. The mechanism that contributes to the antioxidant capacity of phenols and flavanoids include free radical scavenging ability, hydrogen or electron donation ability, chelation of redox active metals ions, modulation of gene expression and interaction with the cell signalling pathways [36]. Therefore, we have examined the rhizome extracts of C. caesia Roxb. for antioxidant activity by DPPH free radical scavenging assay, total phenolic content and reducing power assay. The use of DPPH assay provides an easy and a rapid way to evaluate antioxidants by spectrophotometer [37]. The purple colour of DPPH reduces to light yellow with the intervention of plant extract; the most probable mechanism of action was hydrogen donation by the extracts [38]. Out of the four different extracts of rhizome DPPH radical scavenging activities follows in the order of EECC > MECC > EaECC > AECC with their percentage of inhibition ranging from −15.27 to 86.91%. In fact, the tested extracts are the complex mixtures of several compounds, particularly phenolic compounds which have diverse chemical structures that determine various properties. Rich source of phenolics are of increasing interest nowadays because they retard the oxidative degradation of bio molecules [39]. The chemical structure of phenolic compound which has hydroxyl group attached to benzene ring in its structure provides them the ability to act as free radical scavenger [40]. When reactive oxygen species are present at a certain concentration the bond between O and H is broken. The released hydrogen ion is made available to nucleophilic radicals which subsequently quenched their free radicals [42]. The phenol content of the C. caesia Roxb. extracts was found to be 52.11 mg/GAE for MECC, 68.64 mg/GAE for EECC, 38 mg/GAE for EaECC and 4.82 mg/gGAE for AECC in 100 g of dry weight of the extract in the present study. Literature reviews of Sarangthem and Haokip [41] also confirms that maximum curcuminoids, oil content, flavonoids, phenolics, different important amino acids, protein and high alkaloids are contained in the rhizome of this species. Antioxidants have been reported to act as scavengers of singlet oxygen and free radicals in biological systems [42], [43]. As stated by Oyaizu [27], plant extracts has the reducing ability to transform Fe3+ to Fe+2 and reductones are responsible for it [44]. They have been found to exert antioxidant activity by breaking the free radical chains by donating a hydrogen atom [45]. The reducing power of extracts of C. caesia Roxb. was found to be remarkable and each extract was found to rise as the concentration gradually increases. The reducing power of the extracts follows the order EECC > MECC > EaECC > AECC as shown in the table as well as in the graph.
The antioxidant properties of phytochemicals are linked to their ability to scavenge free radicals generated either endogenously or by exogenous agents. These preventive agents can inhibit the mutation and cancer initiation process by modulating phase I and phase II enzymes, by blocking reactive species either by scavenging, electron donation or through chelation and thus maintains the DNA structure. The inhibition of mutagenesis are grouped into two namely desmutagens and bioantimutagens. It has been hypothesised that bioantimutagens act as second stage inhibitors that blocks the mutagen before they could attack the DNA [46] and bioantimutagenic effect of phytochemicals is determined in co incubation method [47]. The different extracts of C. caesia Roxb. have shown the following order of antimutagenicity EECC > MECC > AECC; against indirect acting mutagen cyclophosphamide (500 μg/plate). The results were based on the number of induced revertant colonies detected. According to Ames et al. [48], a compound is classified as a mutagen if it is able to increase at least twice the number of revertants as compared to spontaneous revertants. Earlier Morffi et al. [49] have reported the antimutagenic activity of Magnifera indica against CP in the strain TA100. Higher mutagenicity was found when CP was activated with S9 but inhibition of this microsomal activity was observed in the presence of rhizome extract. The present results showed the antimutagenic activity in Ames test that may be attributed in part to powerful radical scavenger associated with the extract. According to Negi et al. [50], a compound is found to possess its less antimutagenic activity if its percentage of inhibition is less than 25%, a moderate activity if the percentage inhibition value lies between 25% and 40% and a strong antimutagenicity effect if it is more than 40%. Ethanolic extract reduces the mutagenicity caused by indirect acting mutagen cyclophosphamide by 97.21% and 90.30% respectively in the strains TA98 and TA100 (in the presence of S9) at the highest tested dose (5000 μg/plate) which shows strong antimutagenic activity. From the results it was found that all the extracts showed strong effective antimutagenicity against cyclophosphamide.
5. Conclusion
In conclusion, the present study has shown for the first time that C. caesia Roxb. rhizome extract is a promising source for its antimutagenic compounds. Further studies are needed to isolate the active principles present in it. The present work supported the increasing evidence that the rhizome extract of C. caesia Roxb. plays an important role in cancer chemoprevention, particularly in defending cells from DNA damage induced by oxidative mutagens and by inhibiting CYP enzymes as documented in the present study.
Conflict of interest
The authors have no conflict of interest to this reputed journal.
Acknowledgement
The authors thank Plant Biotechnology Lab, Department of Biotechnology, Assam University, Silchar for providing all the equipments for this research project.
References
- 1.Loeb K.R., Loeb L.A. Significance of multiple mutations in cancer. Carcinogenesis: Int. Canc. Res. 1999;21:379–385. doi: 10.1093/carcin/21.3.379. [DOI] [PubMed] [Google Scholar]
- 2.Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 3.Harman D. Mutation cancer and aging. Lancet. 1961;1:200–201. doi: 10.1016/s0140-6736(61)91371-x. [DOI] [PubMed] [Google Scholar]
- 4.Briviba K., Sies H. Non enzymatic antioxidant defence systems. In: Frei B., editor. Natural Antioxidant in Human Health and Disease. Academic Press; New York: 1994. pp. 107–128. [Google Scholar]
- 5.Machlin L.J., Bendich A. Free radical tissue damage: protective role of antioxidant nutrients. FASEB J. 1987;1(6):441–445. [PubMed] [Google Scholar]
- 6.Devasagayam T.P., Tilak J.C., Boloor K.K., Sane K.S., Ghaskadbi S.S., Lele R.D. Free radicals and antioxidants in human health: current status and future prospects. J. Assoc. Physicians India. 2004;52:794–804. [PubMed] [Google Scholar]
- 7.Yu Y.Y., Kumar V., Bennett M. Murine natural killer cells and marrow graft rejection. Annu. Rev. Immunol. 1992;10:189–213. doi: 10.1146/annurev.iy.10.040192.001201. [DOI] [PubMed] [Google Scholar]
- 8.Ahmad P., Jaleel C.A., Salem M.A., Nabi G., Sharma S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010;30(3):161–175. doi: 10.3109/07388550903524243. [DOI] [PubMed] [Google Scholar]
- 9.Bjelakovic G., Nikolova D., Gluud L.L. Mortality in randomised trials of antioxidant supplements for primary and secondary prevention. J. Am. Med. Assoc. 2007;297:842–857. doi: 10.1001/jama.297.8.842. [DOI] [PubMed] [Google Scholar]
- 10.Lobo V., Patil A., Phatak A., Chandra N. Free radicals antioxidants and functional foods: impact on human health. Pharmacogn. Rev. 2010;4(8):118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hong W.K., Sporn M.B. Recent advances in the chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 12.Tan X.L., Spivack S.D. Dietary chemoprevention strategies for induction of phase II xenobiotic-metabolizing enzymes in lung carcinogenesis: a review. Lung Cancer. 2009;65:129–137. doi: 10.1016/j.lungcan.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kundu J.K., Surh Y.J. Molecular basis of chemoprevention with dietary phytochemicals: redox-regulated transcription factors as relevant targets. Phytochem. Rev. 2009;8:333–347. [Google Scholar]
- 14.Edenharder R., Von Petersdorff I., Rauscher R. Antimutagenic effects of flavonoids, chalcones and structurally related compounds on the activity of 2-amino-3-methylimidazo [4,5-f] quinoline (IQ) and other heterocyclic amine mutagens from cooked food. Mutat. Res. Med. 1993;287:261–274. doi: 10.1016/0027-5107(93)90019-c. [DOI] [PubMed] [Google Scholar]
- 15.Ruan C. Antimutagenic effect of foods and chemoprevention of cancer. Acta Guangxi Med. Coll. 1989;1:68–71. [Google Scholar]
- 16.Banerjee A., Nigam S.S. Antifungal activity of the essential oil of Curcuma caesia Roxb. Indian J. Med. Res. 1976;64(9):1318–1321. [PubMed] [Google Scholar]
- 17.Arulmozhi D.K., Sridhar N., Veeranjaneyulu A., Arora S.K. Preliminary mechanistic studies on the smooth muscle relaxant effect of hydroalcoholic extract of Curcuma caesia. J. Herb. Pharmacother. 2006;6:117–124. doi: 10.1080/j157v06n03_06. [DOI] [PubMed] [Google Scholar]
- 18.Paliwal P., Pancholi S.S., Patel R.K. Pharmacognostic parameters for evaluation of the rhizomes of Curcuma caesia. J. Adv. Pharm. Technol. Res. 2011;2:56–61. doi: 10.4103/2231-4040.79811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mangla M., Shuaib M., Jain J., Kashyap M. In-vitro evaluation of antioxidant activity of Curcuma caesia Roxb. Int. J. Pharm. Sci. Res. 2010;1:98–102. [Google Scholar]
- 20.Karmakar I., Saha P., Sarkar N., Bhattacharya S., Haldar P.K. Neuropharmacological assessment of Curcuma caesia Roxb. rhizome in experimental animal models. Orient. Pharm. Exp. Med. 2011;11:251–255. [Google Scholar]
- 21.Gill R., Kalsi V., Singh A. Phytochemical investigation and evaluation of anthelmintic activity of Curcuma amada and Curcuma caesia: a comparative study. Inventi Impact: Ethnopharmacol. 2011:2011. [Google Scholar]
- 22.Rajamma A.G., Bai V., Nambisan B. Antioxidant and antibacterial activities of oleoresins isolated from nine Curcuma species. Phytopharma. 2012;2:312–317. [Google Scholar]
- 23.Das S., Bordoloi P.K., Phukan D., Singh S. Study of the anti-ulcerogenic activity of the ethanolic extracts of rhizome of Curcuma caesia (eecc) against gastic ulcers in experimental animals. Asian J. Pharm. Clin. Res. 2012;5:200–203. [Google Scholar]
- 24.Pandey A.K., Chowdhary A.R. Volatile constituents of rhizome oil of Curcuma caesia Roxb. from central India. Flavour Frag. J. 2003;18:86463. [Google Scholar]
- 25.Blois M.S. Antioxidant determination by the use of stable free radicals. Nature. 1958;181:1199–2000. [Google Scholar]
- 26.Singleton V.L., Orthofer R., Lamuela-Raventos R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin–Ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 27.Oyaizu M. Studies on product of browning reaction prepared from glucoseamine. Jpn. J. Nutr. 1986;44:307–315. [Google Scholar]
- 28.Maron D.M., Ames B.N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983;113:173–215. doi: 10.1016/0165-1161(83)90010-9. [DOI] [PubMed] [Google Scholar]
- 29.Lakshmi A., Ajith T.A., Jose N., Janardhanan K.K. Antimutagenic activity of methanolic extract of Ganoderma lucidum and its effect on hepatic damage caused by benzo[a]pyrene. J. Ethnopharmacol. 2006;107:297–303. doi: 10.1016/j.jep.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 30.Olayinka A., Aiyegoro A.I., Okoh Preliminary phytochemical screening and in vitro antioxidant activities of the aqueous extract of Helichrysum longifolium DC. BMC Complement. Altern. Med. 2010;10:21. doi: 10.1186/1472-6882-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sangwan N.S., Shanker S., Sangwan R.S., Kumar S. Plant-derived products as antimutagens. Phytother. Res. 1998;12:389–399. [Google Scholar]
- 32.Ma Q., Kineer K. Chemoprotection by phenolic antioxidants. J. Biol. Chem. 2002;277:2477–2484. doi: 10.1074/jbc.M106685200. [DOI] [PubMed] [Google Scholar]
- 33.De Flora S., Ferguson L.R. Overview of mechanisms of cancer chemopreventive agents. Mutat. Res. 2005;11:8–15. doi: 10.1016/j.mrfmmm.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 34.Anetor J.I., Anetor G.O., Udah D.C., Adeniyi F.A.A. Chemical carcinogenesis and chemoprevention: scientific priority area in rapidly industrializing developing countries. Afr. J. Environ. Sci. Technol. 2008;2:150–156. [Google Scholar]
- 35.Halliwell B., Gutteridge J.M.C. The importance of free radicals and catalytic metal ions in human diseases. Mol. Aspects Med. 1985;8:89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- 36.Arouma O.I. Methodological considerations for characterizing potential antioxidant actions of bioactive components in plant foods. Mutat. Res. 2003;523–524:9–20. doi: 10.1016/s0027-5107(02)00317-2. [DOI] [PubMed] [Google Scholar]
- 37.Huang D.J., Ou B.X., Prior R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005;53:1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 38.Prior R.L., Cao G. In vivo total antioxidant capacity: comparison of different analytical methods. Free Radic. Biol. Med. 1999;27:1173–1181. doi: 10.1016/s0891-5849(99)00203-8. [DOI] [PubMed] [Google Scholar]
- 39.Wojdyio A., Oszmianski J., Czemerys R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007;105:940–949. [Google Scholar]
- 40.Clark J. 2007. The Acidity of Phenol, ChemGuide. [Google Scholar]
- 41.Sarangthem K., Haokip M.J. Bioactive components in Curcuma caesia Roxb. grown in Manipur. Bioscan. 2010;1:113–115. [Google Scholar]
- 42.Rice-Evans C.A., Miller N.J., Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–159. [Google Scholar]
- 43.Jorgensen L.V., Madsen H.L., Thomsen M.K., Dragsted L.O., Skibsted L.H. Regulation of phenolic antioxidants from phenoxyl radicals: an ESR and electrochemical study of antioxidant hierarchy. Free Radic. Res. 1999;30:207–220. doi: 10.1080/10715769900300231. [DOI] [PubMed] [Google Scholar]
- 44.Duh P.D., Tu Y.Y., Yen G.C. Antioxidant activity of the aqueous extract of harn jyur (Chrysanthemum morifolium Ramat) Lebensmi-Wiss Technol. 1999;32:269–277. [Google Scholar]
- 45.Gordon M.H. The mechanism of antioxidant action in vitro. In: Hudson B.J.F., editor. Food Antioxidants. Elsevier Applied Science; London: 1990. pp. 1–18. [Google Scholar]
- 46.Ramel C., Alekperov U.K., Ames B.N., Kada T., Wattenberg L.W. Inhibitors of mutagenesis and their relevance to carcinogenesis. Mutat. Res. 1986;168:47–65. doi: 10.1016/0165-1110(86)90021-7. [DOI] [PubMed] [Google Scholar]
- 47.Hung Y.H., Wang Y.J., Chou C.C. Antimutagenic activity of Aspergillus awamori-fermented black soyabean response to stimulated digestive juice treatments and its antimutagenic mechanisms. LWT-Food Sci. Technol. 2009;42:56–62. [Google Scholar]
- 48.Ames B.N., McCann J., Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian microsome mutagenicity test. Mutat. Res. 1975;31:347–364. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 49.Morffi J., Rodeiro I., Hernández S.L., Gonzalez L., Herrera J., Espinosa Aquirre J.J. Antimutagenic properties of Mangifera indica L. stem bark extract and evaluation of its effects on hepatic CYP1A1. Plant Foods Hum. Nutr. 2012;67:223–228. doi: 10.1007/s11130-012-0304-2. [DOI] [PubMed] [Google Scholar]
- 50.Negi P.S., Jayaprakasha G.K., Jena B.S. Antioxidant and antimutagenic activities of pomegranate peel extracts. Food Chem. 2003;80:393–397. [Google Scholar]
- 51.Qureshi N.N., Kuchekar B.S., Logade N.A., Haleem M.A. Antioxidant and hepatoprotective activity of Cordia macleodii leaves. Saud Pharmac. 2009;17:299–302. doi: 10.1016/j.jsps.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]